Open Access Article
Open Access ArticleA new hypervalent iodine(III/V) oxidant and its application to the synthesis of 2H-azirines†
Guangtao
Zhang
a,
Yuanxun
Wang
a,
Jun
Xu
a,
Jiyun
Sun
a,
Fengxia
Sun
b,
Yilin
Zhang
c,
Chenglin
Zhang
a and
Yunfei
Du
 *a
*a
aSchool of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China. E-mail: duyunfeier@tju.edu.cn
bCollege of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, China
cC. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, West Virginia 26506-6045, USA
First published on 6th December 2019
Abstract
The reaction of o-nitroiodobenzene and mCPBA in acetic acid was found to afford a novel hypervalent iodine compound, in the structure of which both iodine(III) and iodine(V) moieties coexist. The nitro groups at the ortho phenyl positions were found to be crucial in stabilizing this uncommon structure. This novel hypervalent iodine(III/V) oxidant is proved to be effective in realizing the synthesis of 2-unsubstitued 2H-azirines via intramolecular oxidative azirination, which could not be efficiently achieved by the existing known hypervalent iodine reagents.
Introductions
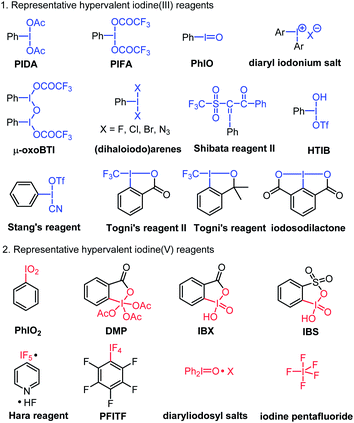
In the past several decades, hypervalent iodine chemistry has witnessed prosperous development in organic synthesis.1 On one hand, organic chemists are making use of the existing hypervalent iodine oxidants to discover new organic reactions.2 On the other hand, many endeavors have been devoted to the development of novel hypervalent iodine oxidants, seeking new versatile applications in important synthetic transformations.3 In this regard, designing novel hypervalent iodine compounds and discovering their new application continue to be an important research topic of hypervalent iodine chemistry.The existing hypervalent iodine reagents can be generally classified into iodine(III) and iodine(V) based on the valence of the iodine atom. The existing hypervalent iodine(III) reagents include phenyliodine diacetate (PIDA), phenyliodine bis(trifloroacetate) (PIFA), iodosylbenzene (PhIO), [hydroxyl(tosyloxy)iodo]benzene (HTIB, Koser reagent),4 PhICl2,5 TolIF2,6 diaryl iodonium salts,7 Togni's reagents,8 μ-oxobis(trifluoroacetoxyiodobenzene) (μ-oxo BTI),9 cyano(trifluoromethylsulfonyloxy)iodobenzene (Stang's reagent),10 1-phenyl-2-(phenyl-λ3-iodaneylidene)-2-((trifluoromethyl)sulfon-yl)ethan-1-one (Shibata reagent II),11 iodosodilactone,1eetc.12 The more widely studied hypervalent iodine(V) regents mainly include 2-iodoxybenzoic acid (IBX),13 Dess–Martin periodinane (DMP),14 Hara reagent,15 pentafluorophenyliodine tetrafluoride (PFITF),16 2-iodoxybenzenesulfonic acid (IBS),17 iodine pentafluoride,18 iodoxybenzene,19 and diaryliodosyl salts20 (Fig. 1). It is evident that all these hypervalent iodine compounds, which bear one single iodine atom center or multinuclear iodine centers (such as polymeric PhIO, μ-oxo BTI, and Zefirov's reagent21), all have a sole valence number of either I(III) or I(V) present in their chemical structure. To the best of our knowledge, there is no report on a hypervalent iodine compound that bears both I(III) and I(V) moieties in its chemical structure. In this communication, we reported such a new hypervalent iodine compound bearing concurrently I(III) and I(V) moieties and its preliminary application in the synthesis of 2-unsubstitued 2H-azirines, which could not be efficiently achieved by the existing known hypervalent iodine reagents.
Iodoxybenzenes, as a class of hypervalent iodine(V) oxidants, have found useful applications in organic synthesis.22 For example, in the presence of diaryl selenium can selectively oxidize the allylic position of olefins to form an α,β-unsaturated ketone.22a It can also convert a cyclic ketone compound to an α,β-unsaturated ketone via dehydrogenative oxidation.22c,23 With regard to their synthesis, iodoxybenzenes can be generally prepared through two methods: one is the oxidation of aryl iodine(I) after reacting with an oxidant like peracetic acid (Saltzman's work), oxone (Zhdankin's work), mCPBA (Kropp's work), 3,3-dimethyldioxirane (Serri's work) or potassium peroxomonosulfate (Zhdankin's work),24 while the other is to use aryl iodine(III) as a substrate, which is further oxidized by sodium hypochlorite to form iodoxybenzene via an iodosylbenzene intermediate.24c,25 However, to the best of our knowledge, neither of the two methods reported the synthesis of o-nitroiodoxybenzene, an unusual hypervalent iodine(V) reagent bearing a strong electron-withdrawing ortho-nitro substituent. It was not until 2018 that Powers and coworkers reported that o-nitroiodoxybenzene could be synthesized from o-nitrobenzene mediated by cobalt(II) chloride hexahydrate under an O2 atmosphere (Fig. 2C).26
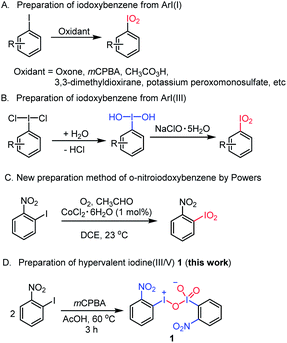 | ||
| Fig. 2 Existing methods for the synthesis of iodoxybenzene and its derivatives vs. the serendipitous access to the first hypervalent iodine(III/V) oxidant. | ||
Results and discussion
At the outset of the study, we were interested to synthesize o-nitroiodoxybenzene according to the literature as shown in Fig. 2A.22 Unfortunately we did not get the desired product (o-nitroiodoxybenzene) by this method. So, we were interested to further optimize the oxidants and the solvents (see ESI, Table 2†). To our surprise, when o-nitroiodobenzene reacted with mCPBA in acetic acid at 60 °C for 3 h, a hypervalent iodine(III/V) 1,27 rather than the expected o-nitroiodoxybenzene, was formed as an orange solid (the structure was undoubtedly confirmed by X-ray analysis) (Fig. 2D). A careful literature survey indicated that this could represent the first hypervalent iodine compound containing both I(III) and I(V) moieties in its chemical structure.The solid state of molecule 1 was established by single-crystal X-ray crystallography (for crystallographic details, see Tables 3–9 in the ESI†). The X-ray data revealed that four crystallographically independent molecules are present in one crystal unit cell. Molecule 1 assembles in an unusual “scissor” manner as a result of intermolecular I(2)⋯O(5) and I(1)⋯O(4) interactions (Fig. 3A). The crystal structure of 1 is uncommon among the known hypervalent iodine structures that have been characterized to date, with a pentavalent I(2) atom and a trivalent I(1) atom coexisting in one molecule via connection of an O(3) atom17,28 (Fig. 3B).
The I(2)–O(3) bond length is 2.447 Å. Analyses of the electron density at BCPs and the Mayer bond order revealed that the I(2)–O(3) bond in molecule 1 was a relatively weak bond compared to the I–O bonds in other hypervalent iodine reagents (see ESI, Fig. S1†).
The I(2)–O(4) bond length and I(2)–O(5) bond length are 1.773 Å and 1.788 Å respectively. The pentavalent I(2) forms an intramolecular I⋯O interaction with one of the nitro oxygen atoms O(6) at a distance of 2.788 Å (Fig. 3B), which was revealed previously.29 In addition, DFT calculations were performed with ORCA 4.1.2 software.30 The comparison of ρbcp and the Mayer bond order of I2–O3 bonds in the hypervalent iodine molecule with or without nitro groups was summarized, which showed that the I2–O3 bond became weaker when the nitro groups in molecule 1 were deleted (see ESI, Fig. S3A†). Multiwfn and VMD programs were used to generate a molecular electrostatic potential surface.31 The electro-positive regions (surface maxima) around I(III) or I(V) atoms displayed electrostatic interaction with the oxygen atoms of nitro groups (see ESI, Fig. S3B†). The calculation results indicated that nitro groups could strengthen I2⋯O3 bonds in molecule 1. Thus, nitro groups played an essential role in molecule 1.
Moreover, the attractive interaction between I(2) and O(6) was demonstrated by the presence of a bond path and a BCP(1) along the bond path in ‘atoms in molecules’ analysis (Fig. 3C). Additionally, the pentavalent I(2) also forms an intermolecular I⋯O interaction with an O(5) atom in another molecule at a distance of 2.534 Å, thereby producing a hexacoordinated complex with pseudooctahedral geometry around iodine atoms (Fig. 3A).
In general, the geometry of the trivalent I(1) center is consistent with that of the reported o-nitrophenylhydroxyiodonium molecule, with a strong interaction formed between the iodine atom and one of the oxygen atoms from the nitro group on the ortho-position of the phenyl ring.29 In molecule 1, the distance between I(1) and O(2) in the nitro group (2.638 Å) is slightly longer than that in the o-nitrophenylhydroxyiodonium molecule (2.510 Å), but is much shorter than the sum of the respective vdW radii (3.530 Å), indicating a strong interaction between the iodine atom and nitro group.29 Similar to the attractive interaction between I(2) and O(6), I(1) and O(2) interaction was also found with the presence of a bond path and a BCP(2) (Fig. 3C). The computed electrostatic potential surfaces of molecule 1 showed the electrostatic attraction between hypervalent iodine atoms I(1) or I(2) (surface maxima) and nitro oxygen atoms O(2) or O(6) (surface minima) (Fig. 3D and S1†). The I(1)–O(3) bond is 1.861 Å in length, which is shorter than that (1.901 Å) observed in a o-nitrophenylhydroxyiodonium molecule.29 Moreover, the 5-membered ring O(2)–N(1)–C(3)–C(4)–I(1) is nearly coplanar with a small dihedral angle N(1)–O(2)–I(1)–C(4) of about 3.55°. The bond angle O(2)–I(1)–O(3) is about 164.44° and distorted from optimal T-shaped geometry same as that in the o-nitrophenylhydroxyiodonium molecule (Fig. 3B). The trivalent I(1) also maintains an intermolecular I⋯O interaction with an O(4) atom from another molecule at a distance of 2.696 Å, creating a tetracoordinated complex with pseudo-square-planar geometry about iodine (Fig. 3A).32
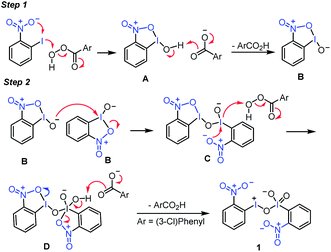
A possible reaction mechanism was proposed for the formation of this new hypervalent iodine(III/V) compound 1 (Fig. 4). First, the reaction of o-nitroiodobenzene with mCBPA afforded the iodine(III) intermediate A, in which the nitro substituent at the ortho position of o-nitroiodobenzene played a crucial auxiliary role. With the abstraction of a proton, A was converted to I(III) species B. Next, the intermolecular reaction between two molecules of B led to the formation of the iodine(III/III) intermediate C. Similarly, assisted by the “free” adjacent nitro group, C was further oxidized by mCPBA to give the iodine(III/V) intermediate D. Finally, D was converted to the titular iodine(III/V) 1via the removal of one proton.
Next, we were interested to investigate the applicability of this newly discovered hypervalent iodine(III/V) oxidant. As our research interest focuses on constructing heterocyclic skeletons using various hypervalent iodine regents,1b,33 we first investigated whether this novel hypervalent iodine(III/V) oxidant could be used to realize conversions that are not readily achievable by other hypervalent iodine reagents.
2H-Azirines34 are an important class of N-containing heterocycles and have received extensive attention because of their occurrence in natural products and application as building blocks in various useful transformations. Several synthetic strategies, including the classical Neber reaction,35 pyrolysis or photolysis of vinyl azide,36 elimination of aziridine derivatives,37 the Swern oxidation reaction,38 intramolecular ring contraction reactions39 and intermolecular aziridination,40 have been reported for the synthesis of this privileged class of heterocycles.41 In 2009, we found that the reaction of enamine substrates with PIDA in DCE could enable a convenient intramolecular azirination, leading to the formation of 2H-azirine compounds in satisfactory to good yield42 (Fig. 5a). However, substrate scope study indicated that this method could not be applied to β-unsubstituted enamines bearing a β-methoxycarbonyl group (E = CO2Me), albeit such a type of substrate could be converted to trifluoroethoxylated 2H-azirines through reaction with PhIO in trifluoroethanol (TFE), via an oxidative trifluoroethoxylation followed by an azirination reaction43 (Fig. 5b). Since β-unsubstituted 2H-azirines bearing an ester moiety are basic skeletons commonly seen in naturally occurring (S)-(+)-dysidazirin44 compounds, it is equally desirable to develop a method that can efficiently achieve the direct intramolecular azirination of β-unsubstituted enamines.44 To our delight, we found that this newly discovered hypervalent iodine(III/V) is able to realize this expected azirination, demonstrating the unusual properties of this hypervalent iodine oxidant (Fig. 5c).45
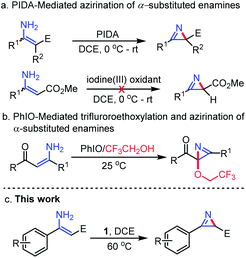 | ||
| Fig. 5 Existing methods for the construction of 2H-azirine skeletons mediated by hypervalent iodine reagents. | ||
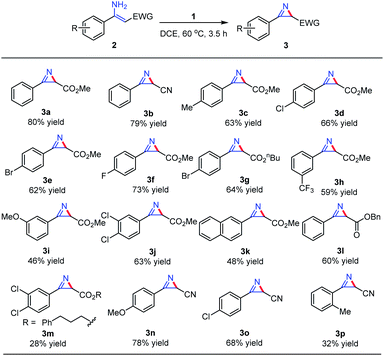
The initial reactivity assay employed a simple α-substituted enamine 2a as the model substrate to optimize the reaction parameters. The results are summarized in Table 1. First, when substrate 2a was treated with iodine(III/V) 1 in DCE at room temperature for 5 h, only a trace amount of the desired product 3a was obtained (Table 1, entry 1). To our delight, elevating the reaction temperature to 60 °C led to the formation of product 3a in a much higher yield of 47% (Table 1, entry 2). Solvent screening revealed that compared to the protic solvents such as MeOH and AcOH, aprotic solvents, especially DCE, were found to be ideal for this specific reaction, as the yield of the product could reach 80% (Table 1, entries 3–9). We also investigated the dosage of the oxidant and the best result was obtained when 0.7 equivalent of the oxidant was added (Table 1, entry 8). Further attempts to improve the reaction outcome by using Lewis acids as an additive proved to be unfruitful (Table 1, entries 10 and 11). Also, in light of our previous work and structural analysis of 1, we attempted to complete the synthesis of 2H-azirines in a one-pot protocol. Unfortunately, no 2H-azirine product was observed under these conditions, probably because the strong oxidizing power of mCPBA preferentially acts on the model substrate (Table 1, entry 12). Further tuning of the reaction by reacting o-nitroiodobenzene with mCPBA in AcOH, followed by a reaction with substrate 2a, only led to the formation of the desired product 3a in 26% yield (Table 1, entry 13). These results implied that the one-pot protocol utilizing the in situ generation of compound 1 for efficient synthesis of 2H-azirines was not feasible.
| Entry | Oxidant (equiv.) | Additive | Solvent | Temp. (°C) | Time [h] | Yieldb [%] |
|---|---|---|---|---|---|---|
| a Reaction conditions: 2a (1.0 mmol), 1 (0.7 mmol), DCE (5 mL), and stirred at 60 °C for 3.5 h. b Isolated yield. c 2a (1.0 mmol), o-nitroiodobenzene (2.0 mmol), mCPBA (2.2 mmol), and AcOH (5 mL). d o-Nitroiodobenzene (2.0 mmol), mCPBA (2.2 mmol), AcOH (5 mL), stirred at 60 °C for 3 h, and then added 2a (1.0 mmol) and stirred at the same temperature for another 2.5 h. e 2.0 equiv. of the additive was used. | ||||||
| 1 | 1 (0.5) | None | DCE | rt | 5 | Trace |
| 2 | 1 (0.5) | None | DCE | 60 | 3.5 | 47 |
| 3 | 1 (0.7) | None | EtOAc | 60 | 4 | 57 |
| 4 | 1 (0.7) | None | Toluene | 60 | 4 | 70 |
| 5 | 1 (0.7) | None | MeCN | 60 | 4 | 53 |
| 6 | 1 (0.7) | None | MeOH | 60 | 3.5 | 19 |
| 7 | 1 (0.7) | None | PhCl | 60 | 3.5 | 55 |
| 8 | 1 (0.7) | None | DCE | 60 | 3.5 | 80 |
| 9 | 1 (0.7) | None | AcOH | 60 | 3 | 55 |
| 10e | 1 (0.7) | BF3·Et2O | DCE | 60 | 3 | 76 |
| 11e | 1 (0.7) | TBSOTf | DCE | 60 | 3 | 73 |
| 12c | 1 (0.7) | None | AcOH | 60 | 2 | N.D. |
| 13d | 1 (0.7) | None | AcOH | 60 | 5.5 | 26 |
With the optimized conditions in hand, substrate scope study was carried out by using this newly discovered hypervalent iodine(III/V) reagent on a series of α-substituted enamines. It was found that a variety of substituents on the phenyl ring could be well tolerated during the reaction. With regard to the substituent effect of the R group, substrates bearing electron-withdrawing groups or electron-donating groups at the para-position of the phenyl ring both gave the corresponding products in good yields (2b–g, 2i–o), with the substrates bearing electron-withdrawing groups affording a better outcome (2d–2fvs.2c). A lower yield was obtained for substrates containing substituents at the meta position of the phenyl ring (2h–i), while for the substrate bearing an ortho-substituent methyl group on the phenyl ring, the desired 2H-azirine product was only afforded in a much lower yield (3p) (Table 2).
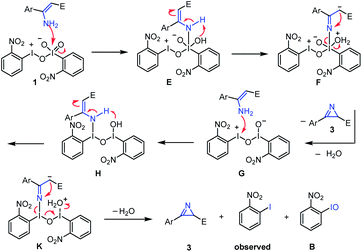
We also proposed a possible reaction pathway for the formation of a 2H-azirine product using this novel hypervalent iodine(III/V) oxidant (Fig. 6). First, the reaction of the enamine substrate with hypervalent iodine(III/V) 1 afforded enamine intermediate Evia the formation of an N–I(V) bond.46 Then enamine E was isomerized into imine F, which underwent intramolecular azirination to give 2H-azirine product 3, with the concurrent generation of hypervalent iodine(III/III) G. Following the reaction of this intermediate G with one more molecule of the enamine substrate, enamine intermediate H was formed via the formation of an N–I(III) bond. Similarly, enamine intermediate H was converted of 2H-azirine product 3via isomerization and intramolecular azirination, with the formation of o-nitro iodobenzene and o-nitro iodosylbenzene as by-products. It is worth noting that o-nitro iodosylbenzene is not stable under the conditions and was converted to o-nitro iodobenzene, which can be supported by the result of a control experiment: when a solution of o-nitro iodosylbenzene47 in DCE was heated at 60 °C for 8 h, o-nitroiodobenzene was obtained in a yield of 80% (Fig. 7).
Conclusions
In summary, we have reported for the first time a new hypervalent iodine oxidant containing both I(III) and I(V) moieties in its chemical structure. Computational experiments were carried out to reveal that the nitro group at the ortho phenyl position is crucial in stabilizing this new structure. This novel hypervalent iodine(III/V) oxidant can be applied to the synthesis of 2-unsubstitued 2H-azirines, which cannot be successfully achieved by other traditional hypervalent iodine reagents. Further application of this hypervalent iodine(III/V) oxidant is currently under study in our lab.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We acknowledge the National Natural Science Foundation of China (#21472136) for financial support. Computational support was provided by the Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase) under Grant No. U1501501.Notes and references
- (a) L. F. Silva Jr and B. Olofsson, Nat. Prod. Rep., 2011, 28, 1722 RSC; (b) A. Yoshimura and V. V. Zhdankin, Chem. Rev., 2016, 116, 3328 CrossRef CAS PubMed; (c) R. M. Moriarty, J. Org. Chem., 2005, 70, 2893 CrossRef CAS; (d) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (e) J. Tian, W. C. Gao, D. M. Zhou and C. Zhang, Org. Lett., 2012, 14, 3020 CrossRef CAS PubMed; (f) U. Farid and T. Wirth, Angew. Chem., Int. Ed., 2012, 51, 3462 CrossRef CAS PubMed; (g) N. O. Ilchenko, M. Hedberg and K. J. Szabo, Chem. Sci., 2017, 8, 1056 RSC; (h) Y. Tamura, T. Yakura, Y. Shirouchi and J. Haruta, Chem. Pharm. Bull., 1986, 34, 1061 CrossRef CAS; (i) M. W. Justik, in PATAI'S Chemistry of Functional Groups [online], John Wiley & Sons, Ltd, New York, 2009, pp. 1–88 Search PubMed.
- (a) J. D. Haupt, M. Berger and S. R. Waldvogel, Org. Lett., 2019, 21, 242 CrossRef CAS PubMed; (b) L. Wang, H. Li and L. Wang, Org. Lett., 2018, 20, 1663 CrossRef CAS PubMed; (c) S. R. Kandimalla, S. P. Parvathaneni, G. Sabitha and B. V. S. Reddy, Eur. J. Org. Chem., 2019, 1687 CrossRef; (d) I. Colomer, C. Batchelor-McAuley, B. Odell, T. J. Donohoe and R. G. Compton, J. Am. Chem. Soc., 2016, 138, 8855 CrossRef CAS PubMed; (e) D. Bhattacherjee, S. Ram, A. S. Chauhan, Yamini, Sheetal and P. Das, Chem.–Eur. J., 2019, 25, 5934 CrossRef CAS PubMed.
- (a) J. Kalim, T. Duhail, T.-N. Le, N. Vanthuyne, E. Anselmi, A. Togni and E. Magnier, Chem. Sci., 2019, 10, 10516 RSC; (b) G.-X. Li, C. A. Morales-Rivera, F. Gao, Y. Wang, G. He, P. Liu and G. Chen, Chem. Sci., 2017, 8, 7180 RSC; (c) J. Qurban, M. Elsherbini, H. Alharbia and T. Wirth, Chem. Commun., 2019, 55, 7998 RSC; (d) A. Yoshimura, M. T. Shea, C. L. Makitalo, M. E. Jarvi, G. T. Rohde, A. Saito, M. S. Yusubov and V. V. Zhdankin, Beilstein J. Org. Chem., 2018, 14, 1016 CrossRef CAS PubMed; (e) Y. Wang, H. Yuan, H. Lu and W.-H. Zheng, Org. Lett., 2018, 20, 2555 CrossRef CAS.
- Z. Begum, G. Bhavani, B. Sridhar and B. V. S. Reddy, Synthesis, 2018, 50, 4089 CrossRef CAS.
- P. Ajda, J. Markus, S. Stojan, Z. Marko, I. Jernej and J. A. Gladysz, J. Org. Chem., 2009, 74, 3133 CrossRef PubMed.
- J. Hu, B. Xing and C. Ni, Angew. Chem., Int. Ed., 2018, 57, 9896 CrossRef PubMed.
- J. L. Dektar and N. P. Hacker, J. Org. Chem., 1990, 55, 639 CrossRef CAS.
- (a) S. Kyrill, K. Raffael and T. Antonio, J. Org. Chem., 2010, 40, 7678 Search PubMed; (b) S. Kawamura, D. Sekine and M. Sodeoka, J. Fluorine Chem., 2017, 203, 115 CrossRef CAS; (c) E. Patrick, G. Sebastian and T. Antonio, Chem.–Eur. J., 2010, 12, 2579 Search PubMed.
- (a) J. Gallos, A. Varvoglis and N. W. Alcock, J. Chem. Soc., Perkin Trans. 1, 1985, 1985, 757 RSC; (b) T. Dohi, T. Uchiyama, D. Yamashita, N. Washimi and Y. Kita, Tetrahedron Lett., 2011, 52, 2212 CrossRef CAS.
- P. J. Stang, B. L. Williamson and V. V. Zhdankin, J. Am. Chem. Soc., 1991, 113, 5870 CrossRef CAS.
- Y.-D. Yang, A. Azuma, E. Tokunaga, M. Yamasaki and N. Shibata, J. Am. Chem. Soc., 2014, 44, 8782 Search PubMed.
- (a) S. V. Kohlhepp and T. Gulder, Chem. Soc. Rev., 2016, 45, 6270 RSC; (b) T. Hokamp, L. Mollari, L. C. Wilkins, R. L. Melen and T. Wirth, Angew. Chem., Int. Ed., 2018, 57, 8306 CrossRef CAS; (c) M. Bedin, A. Karim, M. Reitti, A. C. C. Carlsson, F. Topic, M. Cetina, F. F. Pan, V. Havel, F. Al-Ameri, V. Sindelar, K. Rissanen, J. Grafenstein and M. Erdélyi, Chem. Sci., 2015, 6, 3746 RSC; (d) W. C. Agosta, Tetrahedron Lett., 1965, 6, 2681 CrossRef.
- P. J. Stevenson, A. B. Treacy and M. Nieuwenhuyzen, J. Chem. Soc., Perkin Trans. 2, 1997, 589 RSC.
- D. B. Dess and J. C. Martin, J. Org. Chem., 1983, 15, 4155 CrossRef.
- S. Hara, M. Monoi, R. Umemura and C. Fuse, Tetrahedron, 2012, 68, 10145 CrossRef CAS.
- W. Breuer and H. J. Frohn, J. Fluorine Chem., 1987, 34, 443 CrossRef CAS.
- I. A. Mironova, P. S. Postnikov, R. Y. Yusubova, A. Yoshimura, T. Wirth, V. V. Zhdankin, V. N. Nemykin and M. S. Yusubov, Beilstein J. Org. Chem., 2018, 14, 1854 CrossRef CAS.
- T. Birchall, R. D. Myers, H. D. Waard and G. J. Schrobilgen, Inorg. Chem., 1982, 21, 1068 CrossRef CAS.
- A. R. Katritzky, J. K. Gallos and H. D. Durst, Magn. Reson. Chem., 1989, 27, 815 CrossRef CAS.
- F. M. Beringer and P. Bodlaender, J. Org. Chem., 1968, 33, 2981 CrossRef CAS.
- N. S. Zefirov, V. V. Zhdankin, V. V. Dan'kov and A. S. Koz'min, J. Org. Chem. USSR, 1984, 20, 401 Search PubMed.
- (a) D. H. R. Barton and D. Crich, Tetrahedron, 1985, 41, 4359 CrossRef CAS; (b) R. Gleiter and G. Mueller, J. Org. Chem., 1988, 53, 3912 CrossRef CAS; (c) T. Iida, S. Nishida, F. C. Chang, T. Niwa, J. Goto and T. Nambara, Chem. Pharm. Bull., 1993, 41, 763 CrossRef CAS.
- H. Künzer, G. Sauer and R. Wiechert, Tetrahedron, 1989, 45, 6409 CrossRef.
- (a) N. Soldatova, P. Postnikov, A. A. Troyan, A. Yoshimura, M. S. Yusubov and V. V. Zhdankin, Tetrahedron Lett., 2016, 57, 4254 CrossRef CAS; (b) J. G. Sharefkin and H. Saltzman, Org. Synth., 1963, 43, 65 CrossRef CAS; (c) R. I. Davidson and P. J. Kropp, J. Org. Chem., 1982, 47, 1904 CrossRef CAS; (d) N. Taneja and R. K. Peddinti, Tetrahedron Lett., 2016, 57, 3958 CrossRef CAS; (e) A. Bravo, F. Fontana, G. Fronza, F. Minisci and A. Serri, Tetrahedron Lett., 1995, 36, 6945 CrossRef CAS; (f) A. Watanabe, K. Miyamoto, T. Okada, T. Asawa and M. Uchiyama, J. Org. Chem., 2018, 83, 14262 CrossRef CAS; (g) M. S. Yusubov, A. A. Zagulyaeva and V. V. Zhdankin, Chem.–Eur. J., 2009, 15, 11091 CrossRef CAS.
- (a) R. Bell and K. J. Morgan, J. Chem. Soc., 1960, 1960, 1209 RSC; (b) M. S. Yusubov, K. W. Chi, J. Y. Park, R. Karimov and V. V. Zhdankin, Tetrahedron Lett., 2006, 47, 6305 CrossRef CAS; (c) A. A. Mironova, V. V. Orda, L. M. Yagupolskii and I. I. Maletina, Synthesis, 1983, 1983, 456 CrossRef; (d) L. Troiangautier, J. D. Winter, P. Gerbaux and C. Moucheron, J. Org. Chem., 2013, 78, 11096 CrossRef CAS.
- A. Maity, S.-M. Hyun, A. K. Wortman and D. C. Powers, Angew. Chem., Int. Ed., 2018, 57, 7205 CrossRef CAS.
- (a) Compound 1 tends to decompose into o-nitroiodobenzene if stored in air at room temperature. It is necessary to have compound 1 stored under a N2 atmosphere and at lower temperature (<3 °C); (b) It is worth noting that compound 1 is relatively stable in MeOH, as the use of MeOH/CH2Cl2 for column purification does not bring about any obvious decomposition. We tried to dissolve compound 1 in ethanol, p-methoxybenzyl alcohol, and p-chlorophenylethanol. However, the poor solubility of 1 allowed it to be well stored in these alcoholic solvents at room temperature for more than one day. However, an obvious decomposition of compound 1 into o-nitroiodobenzene was observed if it was dissolved in benzyl alcohol and stirred for 10 minutes at room temperature.
- M. S. Yusubov, D. Y. Svitich, A. Yoshimura, V. N. Nemykin and V. V. Zhdankin, Chem. Commun., 2013, 49, 11269 RSC.
- V. A. Nikiforov, V. S. Karavan, S. A. Miltsov, S. I. Selivanov, E. Kolehmainen, E. Wegelius and M. Nissinen, ARKIVOC, 2003, 6, 191 Search PubMed.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73 Search PubMed.
- (a) T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580 CrossRef CAS; (b) W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33 CrossRef CAS.
- We carried out structural remodeling of all-iodine(III) and all-iodine(V) agents and performed DFT calculations. The results indicated that the values of ρbcp and Mayer bond order of the I2⋯O3 bond of the mixed valent agent molecule 1 were significantly higher than those of all-iodine(III) and all-iodine(V) agents. Therefore, it could exist in the unusual mixed valent form 1. Please see ESI, Fig. S2† for details.
- (a) I. Tellitu and E. Dominguez, Synlett, 2012, 23, 2165 CrossRef CAS; (b) W. Thomas, Angew. Chem., Int. Ed., 2005, 36, 3656 Search PubMed; (c) E. A. Merritt and B. Olofsson, Angew. Chem., Int. Ed., 2009, 48, 9052 CrossRef CAS.
- (a) T. W. Miller, E. W. Tristram and F. J. Wolf, J. Antibiot., 1971, 24, 48 CrossRef CAS; (b) E. O. Stapley, D. Hendlin, M. Jackson, A. K. Miller, S. Hernandez and J. M. Mata, J. Antibiot., 1971, 24, 42 CrossRef CAS.
- (a) D. F. Morrow, M. E. Butler and E. C. Y. Huang, J. Org. Chem., 1965, 30, 579 CrossRef CAS; (b) D. J. Cram and M. J. Hatch, J. Am. Chem. Soc., 1953, 75, 33 CrossRef CAS; (c) P. W. Neber and A. V. Friedolsheim, Justus Liebigs Ann. Chem., 1926, 449, 109 CrossRef CAS; (d) P. W. Neber and A. Uber, Justus Liebigs Ann. Chem., 1928, 467, 52 CrossRef CAS.
- G. Smolinsky, J. Org. Chem., 1962, 27, 3557 CrossRef CAS.
- (a) J. C. Guillemin, J. M. Denis, M. C. Lasne and J. L. Ripoll, Tetrahedron, 1988, 4, 4447 CrossRef; (b) F. A. Davis, G. V. Reddy and H. Liu, J. Am. Chem. Soc., 1995, 117, 3651 CrossRef CAS; (c) R. G. Kostyanovskii, G. K. Kadorkina, S. V. Varlamov, I. I. Chervin and I. K. A. Romero-Maldonado, Chem. Heterocycl. Compd., 1988, 24, 387 CrossRef.
- L. Gentilucci, Y. Grijzen, L. Thijs and B. Zwanenburg, Tetrahedron Lett., 1995, 36, 4665 CrossRef CAS.
- R. R. Sauers, L. M. Hadel, A. A. Scimone and T. A. Stevenson, J. Org. Chem., 1990, 55, 4011 CrossRef CAS.
- (a) Y. Zhang, X. Zhao, C. Zhuang, S. Wang, D. Zhang-Negrerie and Y. Du, Adv. Synth. Catal., 2018, 360, 2107 CrossRef CAS; (b) J. Sun, X. Zhen, H. Ge, G. Zhang, X. An and Y. Du, Beilstein J. Org. Chem., 2018, 14, 1452 CrossRef CAS.
- (a) F. Palacios, A. M. O. de Retana, E. M. de Marigorta and J. M. de los Santos, Eur. J. Org. Chem., 2001, 2001, 2401 CrossRef; (b) F. Palacios, A. M. O. de Retana, E. M. de Marigorta and J. M. de los Santos, Org. Prep. Proced. Int., 2002, 34, 219 CrossRef CAS.
- X. X. Li, Y. F. Du, Z. D. Liang, X. K. Li, Y. Pan and K. Zhao, Org. Lett., 2009, 11, 2643 CrossRef CAS.
- X. Q. Sun, Y. R. Lyu, D. Zhang-Negrerie, Y. F. Du and K. Zhao, Org. Lett., 2013, 15, 6222 CrossRef CAS PubMed.
- C. E. Salomon, D. H. Williams and D. J. Faulkner, J. Nat. Prod., 1995, 58, 1463 CrossRef CAS.
- We also investigated other commonly used iodine(III) and iodine(V) reagents including PIDA, PIFA, PhIO, IBX, DMP and PhIO2 to see if they are also effective for this azirination process. The results implied that the reactivity of iodine(III/V) 1 is superior to that of the other iodine(III) or (IV) reagents. See the ESI† for details.
- An alternative mechanistic pathway involving the formation of a C–I intermediate was also provided in the ESI.† It is worth noting that the DFT calculations supported a mechanistic pathway involving the formation of an I–N intermediate. See the ESI† for details.
- T. Baba, S. Takahashi, Y. Kambara, A. Yoshimura, V. N. Nemykin, V. V. Zhdankin and A. Saito, Adv. Synth. Catal., 2017, 359, 3860 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data of all new compounds and NMR spectra. CCDC 1950436. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc05536c |
| This journal is © The Royal Society of Chemistry 2020 |