Open Access Article
Open Access ArticleIn situ transfer of CH3NH3PbI3 single crystals in mesoporous scaffolds for efficient perovskite solar cells†
Yanjun
Guan‡
a,
Mi
Xu‡
a,
Wenhao
Zhang
a,
Da
Li
a,
Xiaomeng
Hou
a,
Li
Hong
a,
Qifei
Wang
a,
Zhihui
Zhang
a,
Anyi
Mei
a,
Min
Chen
 b,
Yuanyuan
Zhou
b,
Yuanyuan
Zhou
 b,
Nitin P.
Padture
b,
Nitin P.
Padture
 b,
Yue
Hu
b,
Yue
Hu
 *a,
Yaoguang
Rong
*a,
Yaoguang
Rong
 *a and
Hongwei
Han
*a and
Hongwei
Han
 a
a
aMichael Grätzel Center for Mesoscopic Solar Cells, Wuhan National Laboratory for Optoelectronics, China-EU Institute for Clean and Renewable Energy, Huazhong University of Science and Technology, Wuhan 430074, Hubei, P. R. China. E-mail: ygrong@hust.edu.cn; yuehu@hust.edu.cn
bSchool of Engineering, Brown University, Providence, RI 02912, USA
First published on 20th November 2019
Abstract
Printable mesoscopic perovskite solar cells are usually fabricated by drop-casting perovskite precursor solution on a screen-printed mesoporous TiO2/ZrO2/carbon triple-layer followed by thermal annealing. They have attracted much attention due to their simple fabrication process and remarkable stability. However, challenges lie in how to achieve complete pore fillings of perovskites in the meso-pores and to obtain high-quality perovskite crystals. Here, we report an in situ crystal transfer (ICT) process based on gas–solid interaction to deposit perovskite CH3NH3PbI3 absorber in the scaffold. CH3NH3PbI3 single crystals are first transformed into a liquid phase via exposure to methylamine gas flow. After complete infiltration into the nano-structured scaffolds, the liquid phase is converted back to the solid phase with reduction of methylamine gas partial pressure, maintaining the high-quality of CH3NH3PbI3 single crystals. Compared with the conventional drop-casting method, the ICT method effectively leads to interconnected morphology and prolongs the charge-carrier lifetime (from ∼37.52 ns to ∼110.85 ns) of the perovskite absorber in the scaffold. As a result, the devices can deliver a power conversion efficiency of 15.89%, which is attributed to the suppressed charge recombination and correspondingly enhanced open-circuit voltage of 0.98 V.
Introduction
The past decade has witnessed a rapid evolution of hybrid organic–inorganic perovskite solar cells (PSCs) as serious contenders to rival the leading photovoltaic technologies with both low cost and high power conversion efficiency (PCE).1–3 The rapid growth of PCE is attributed to many research efforts on device architectures as well as processing techniques, but primarily to optimization of the quality of the perovskite thin films, e.g., high crystallinity and few defects.4–9 To achieve a uniform, high-crystallinity perovskite film, various processing techniques have been explored, such as sequential deposition,8 anti-solvent method,5 vacuum-assisted,10 vapor-assisted11 methods, etc. In parallel to the development of film processing techniques, various device architectures have also been studied. Among them, devices based on a TiO2/ZrO2/carbon triple mesoscopic architecture have attracted attention for the simple fabrication route and lower costs as a result of omitting the hole transport layer (HTL) and replacing the gold back-contact with carbon materials.12 The mesoporous layers are prepared by screen-printing technique followed by sintering. Subsequently, the perovskite precursors are drop-casted into the scaffold. The organic–inorganic halide perovskite then crystallizes through solution via thermal annealing. Due to the difficulty in controlling the crystal growth in such thick layers with mesopores, a large number of grain boundaries and point defects exist in the perovskite. These locations become recombination centers that inhibit further improvements of the device performance.13 At the same time, large lattice mismatch and non-uniform contact between the perovskite and electron transport layer leads to the distortion of the crystal structure, which produces deep trap-states and reduces the output voltage. Unfortunately, most of the deposition methods that worked effectively in PSCs based on other structures do not work in this triple mesoscopic PSCs. In this structure, the priority is to achieve maximum pore filling of the mesoporous TiO2 with high quality perovskite absorbers for enhanced light harvesting and charge-carrier separation and transport.Previously, we have developed various strategies to improve the perovskite quality in the mesoporous scaffold, such as chemical engineering of the perovskite precursor solution by adding multi-functional additives,14 tuning the crystallization process,15 and employing post-treatments.16 The perovskites with higher crystallinity and reduced trap density have enabled a PCE of over 15% for printable mesoscopic PSCs. In particular, the treatment of MAPbI3-based (MA+ = CH3NH3+) cells with methylamine (MA0) gas16 results in the formation of a liquid intermediate MAPbI3·xMA0, which upon degassing yields perovskite reconstructed crystals with improved crystallinity and a high PCE of 15.2%. Zhou, et al. utilized the same method to improve the crystallinity of MAPbI3·MACl perovskite in order to reduce grain-boundary density.17 Han, et al. used MA0 gas to dissolve MAI and PbI2 into a liquid-state mixture, followed by a soft-cover method for depositing perovskite films.6 It has been proposed that the MA0 gas treatment of MAPbI3 induces rapid defect-healing of perovskite films at room temperature.18–21 In this process the lone-pair electrons on N in the MA0 molecule interact with the octahedral framework of PbI64− in MAPbI3. This causes the MAPbI3 perovskite structure to collapse completely, and forms the liquid phase of MAPbI3·xMA0. It is also likely that this phase is a colloid rather than a pure liquid. Upon removal of the MA0 gas, the MA0 molecule is gradually released from the liquid phase, and the perovskite structure of MAPbI3 is reconstructed.
Considering the phase transitions of MAPbI3 with MA0 gas, we attempted to directly transfer MAPbI3 single crystals into the mesoporous TiO2/ZrO2/carbon scaffold of printable PSCs. MAPbI3 single crystals possess low defect state density, for which the carrier diffusion length can reach 175 ± 25 μm, with a carrier mobility of 164 ± 25 cm2 V−1 s−1.22,23 Inspired by the above work, we report here an in situ transfer process that transforms MAPbI3 single crystals into a liquid phase via exposing them to MA0 gas flow and fills in triple mesoscopic scaffolds. When the liquid phase completely infiltrates into the nano-structured scaffolds and subsequently converts back to solid-state phase, high-quality MAPbI3 crystals form within the scaffold. Owing to the interconnected morphology, and preferred crystal orientation of ICT-process deposited MAPbI3, the devices obtained have a maximum PCE of 15.89%. The perovskite film with low defect density resulted in suppressed charge recombination and correspondingly enhanced open-circuit voltage (VOC). The ICT process is different from conventional solution processes. It does not involve liquid solvent or additional post-annealing processing, thus providing a promising prospect for PSCs application.
Results and discussion
MAPbI3 single crystals were synthesized using procedures described in the literature.24 Large-sized single crystals were grown from seed crystals in a supersaturated solution taking up to 2 days. The as-grown single crystals were ground into fine powders for the device fabrication, as illustrated schematically in Fig. 1a. To transfer the MAPbI3 crystals into the mesoporous scaffolds, a homemade prototype reactor was designed, as shown in Fig. 1b and S1.† Briefly, the reactor was firstly pumped to −0.1 MPa pressure to remove the air, and then refilled with MA0 gas with a low pressure of ∼0.015 MPa which was found to be optimum for delivering the highest quality perovskite.25 The whole process was performed at room temperature (RT) ∼25 °C. Once the MA0 gas was refilled into the reactor, the MAPbI3 crystal powders began to turn into the liquid phase MAPbI3·xMA0. As shown in Reaction 1, CH3NH2 gas (g) reacts with the inorganic PbI6-octahedra framework in MAPbI3 perovskite crystals (s) resulting in the collapse of the perovskite structure into the liquid state (l). Within ∼3 minutes of MA0 gas exposure, the mixture of MAPbI3 crystals and liquid-state phase MAPbI3·xMA0 completely converted into transparent colloidal liquid-state phase MAPbI3·xMA0, spreading and penetrating in the mesoporous scaffolds. The treating time refers to the time which transparent colloidal liquid-state phase spreads and penetrates in the mesoporous scaffolds by capillary action or gravity. After full penetration (∼35 minutes) of the colloidal liquid-state phase, the reactor was refilled with N2 flow for over 30 minutes. Upon reduction of MA0 gas partial pressure, the liquid phase slowly transformed back to dark brown MAPbI3, forming high-quality perovskite absorber within the scaffold (confirmed by XRD measurements), as per Reaction 2. More detailed description of the ICT process is included in the experimental section of the (ESI†). Above all, the experimental procedure is easy to perform, as shown schematically in Fig. 1c.| CH3NH3PbI3(s) + xCH3NH2(g) → CH3NH3PbI3·xCH3NH2(l) | (1) |
| CH3NH3PbI3·xCH3NH2(l) → CH3NH3PbI3(s) + xCH3NH2(g) | (2) |
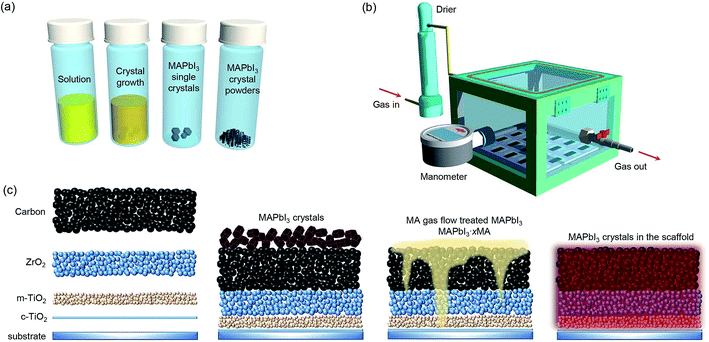 | ||
| Fig. 1 Schematic illustration of the: (a) MAPbI3 single crystals and powders preparation process, (b) ICT reactor (the digital images are shown in Fig. S1†), and (c) ICT process from blank scaffold to perovskites infiltrated scaffold. | ||
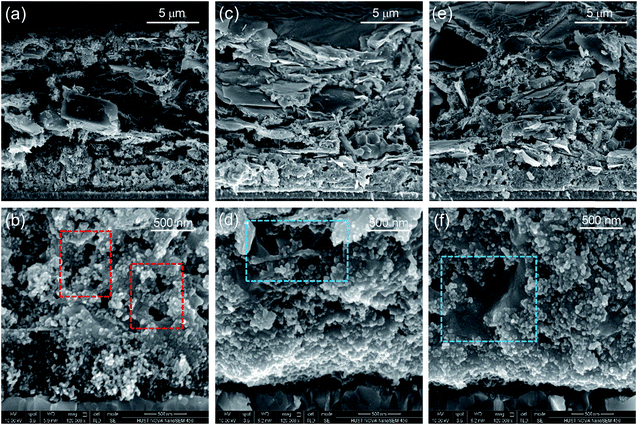
The mesopore filling situation and morphology of the perovskite absorber in the scaffold were characterized by scanning electron microscopy (SEM). As shown in Fig. 2a and b, the drop-casting method resulted in incomplete pore fillings of the perovskite absorber in the mesoporous scaffold due to the fast and uncontrolled crystallization of the perovskite precursors.26 For the ICT process, the perovskite absorber completely filled up the mesopores and formed a compact morphology, as shown in Fig. 2c–f. By prolonging the treating time from ∼9 min (Fig. 2d) to ∼35 min (Fig. 2f), the morphology of the perovskites turned more compact. The top-view SEM images of MAPbI3 films deposited on mesoporous TiO2 by the two methods were shown in Fig. S2.† The drop-casting method resulted in a rough surface with needle-shaped crystals and holes/voids on the MAPbI3 film. In contrast, the perovskite film prepared by the ICT method demonstrates full coverage on the substrate. The distributions of the perovskite absorber in the mesoporous scaffold were further characterized by energy dispersive spectroscopy (EDS) measurements, confirming that all elements are distributed uniformly (Fig. S3†). In addition, less gaps between the perovskite domains and with mesoporous TiO2 layer will lead to less interface combination, which will undoubtedly make contributions to the voltage of the device. Here it should be emphasized that the after transferring to the scaffold, the perovskite single crystals turned into well-connected polycrystalline perovskite networks instead of forming another perovskite single crystal.
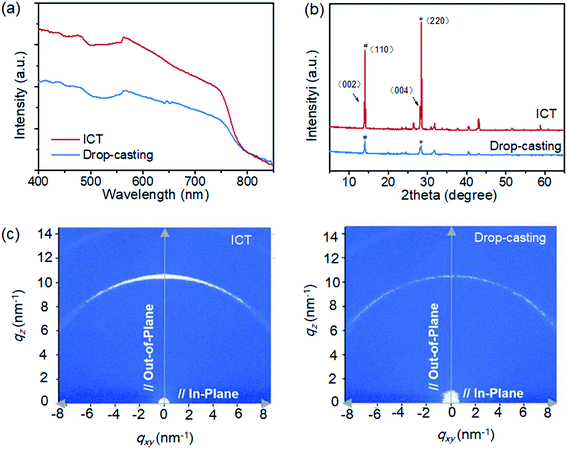
Owing to the improved mesopore filling and compact morphology of the ICT-deposited perovskite absorber in the mesoporous TiO2 scaffold, the absorption was also significantly enhanced, as shown in Fig. 3a. For the crystallinity of the perovskite absorber, the (110) and (220) peak intensities of MAPbI3 deposited using the ICT method are much higher than those obtained using the drop-casting method, as shown in Fig. 3b. Besides, the clear spilt of the (220) and (004) peaks also indicates that the ICT-deposited perovskite adopts a tetragonal phase with higher crystalline quality.24 Furthermore, 2D X-ray diffraction (2D-XRD) images were obtained for directly revealing the texture of the thin films. As seen in Fig. 3c, the (110) reflection of the perovskite film made using the drop-casting method showed a uniform spread along the Debye–Scherrer ring, indicative of random orientations of the grains. However, with the introduction of the ICT method, the Debye–Scherrer ring becomes discrete and a strong (110) texture is observed.27,28 The more anisotropic nature of the perovskite film via the ICT process may contribute positively to the charge-carrier dynamics discussed later.
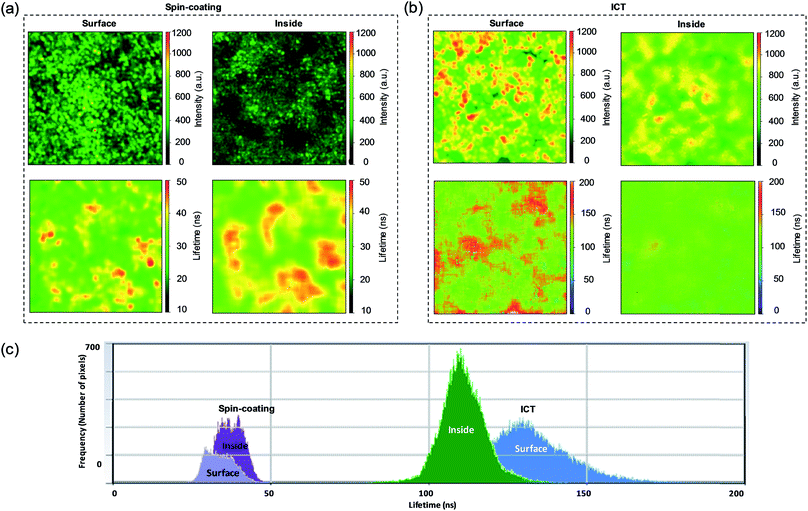
The carrier photoluminescence (PL) behaviour of MAPbI3 perovskite prepared by spin-coating and ICT methods on ZrO2 scaffolds were studied using the ISS Q2 laser scanning microscope in conjunction with FastFLIM, which is a digital frequency domain technique that can simulate both the PL intensity and lifetime of a sample simultaneously. Since a perovskite capping layer usually emerges on top of the mesoporous scaffold, the nature of the perovskite on the surface and within the mesopores is expected to different. However, the high resolution of the laser scanning microscope allows one to separate the perovskite on the surface and that in the mesopores by tuning the focus location. As shown in Fig. 4a, the perovskite tends to crystallize into separate islands in the spin-coating method. Randomly located dark regions with zero intensity exist both on the surface and within the mesopores. These inhomogeneous dark regions indicate extremely low content of perovskite, indicating voids or incomplete pore-fillings. In contrast, the sample prepared by ICT method shows PL with increased intensity (Fig. 4b). These perovskite regions are larger and brighter. The lifetime images are produced by bi-exponential fitting of each pixel and then taking the intensity-weighted average lifetime of the two components. Besides, the lifetime histograms are plotted to compare the difference of lifetime distributions. As shown in Fig. 4c, the average PL lifetime is much enhanced in the films deposited using the ICT method compared with those using spin-coating method. In the ICT method, the crystals tend to grow larger on the surface than in the pores, giving an average PL lifetime of ∼131.29 ns on the surface and ∼110.85 ns in the mesopores. In contrast, for the films prepared using the spin-coating method, the average PL lifetime on the surface is ∼33.80 ns and ∼37.52 ns within the mesopores. The longer lifetime indicates a lower trap state density when ICT method is used to deposit the MAPbI3 film. Conventional PL measurements (Horiba HR800s) were also conducted, as shown in Fig. S4.† The central excitation peak position of the two methods in steady-state PL spectra is blue-shifted from 772 nm for the drop-casting method to 762 nm for the ICT method. This is because the shallow-level defects in the film prepared by the ICT method are reduced, and thus obtain a perovskite layer with high quality.29,30 This is also consistent with the improvement in crystallinity from XRD results.
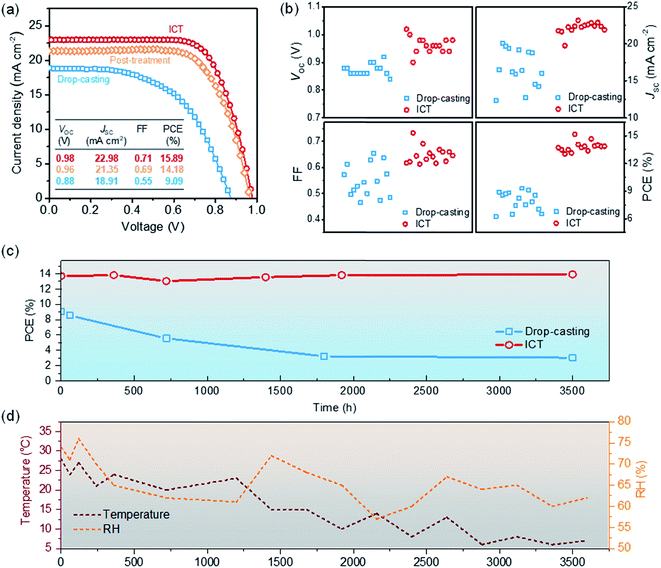
The photovoltaic performance parameters are illustrated in Table 1 in consistent with the cross-sectional SEM results above. The devices prepared by the drop-casting method achieve an average efficiency of 7.95% and a voltage of only 0.87 V. Whereas, using the ICT method, with a MA-gas-treatment duration of ∼9 minutes, PCE up to 9.45% is obtained. The short-circuit current density (JSC) is effectively improved because of the low density of gross defects such as voids in the mesoporous film. In addition, the quality of the perovskite film using the ICT method is also significantly higher than that using the drop-casting method. When the MA0 gas treatment time is ∼35 minutes, the perovskite film becomes continuous and dense (Fig. 2e and f), increasing the average JSC to 22.69 mA cm−2. It is worth mentioning that due to better crystal quality, the carrier recombination in the device is reduced, and the average VOC of the device is increased to 0.96 V.
| V OC (V) | J SC (mA cm−2) | FF | PCE (%) | |
|---|---|---|---|---|
| Drop-casting | 0.87 ± 0.03 | 16.75 ± 0.36 | 0.55 ± 0.08 | 7.95 ± 1.20 |
| ICT (∼9 min) | 0.88 ± 0.02 | 19.19 ± 2.84 | 0.56 ± 0.09 | 9.45 ± 1.18 |
| ICT (∼30 min) | 0.96 ± 0.04 | 22.18 ± 0.71 | 0.65 ± 0.08 | 13.83 ± 1.34 |
In particular, we compared representative J–V curves of the champion cells fabricated using three methods in Fig. 5a. The simple one-step drop-casting method can only obtain a PCE of 9.09%. According to our results for the pure MAPbI3 as the light absorber in triple-mesoscopic PSCs, the efficiency can be improved to over 13% by using a sequential deposition method or optimizing the solvents.26,31 Previously, we have developed a MA0 gas post-treatment method to enhance the performance of MAPbI3 based cells.20 The MA0 gas can make the morphology of the perovskite more uniform and compact. Correspondingly, an average efficiency of over 14% was achieved (champion efficiency of 15.26%). Notably, this post-treatment method can only improve the morphology of the perovskite in the scaffold, but not enhancing the mesopore filling ratio. In addition, it might be difficult for MA0 gas to reach the bottom of the scaffold and interact with the perovskite absorber there. For the ICT method, the perovskite crystals are able to adequately interact with MA0 gas, then liquify and infiltrates in the scaffold. Correspondingly, an efficiency of 15.89% was achieved with an impressively high VOC of 0.98 V, an enhanced JSC of 22.98 mA cm−2, and FF of 0.71. The distribution of the VOC, JSC, FF and PCEs for 15 devices is illustrated in Fig. 5b. Compared with drop-casting method, ICT method showed better reproducibility, especially in terms of VOC and JSC, and achieved a VOC of up to 0.98 V. The incident photon-to-current conversion efficiency (IPCE) spectra of the devices are illustrated in Fig. S5.† It is confirmed that the increased JSC is due to the enhanced light harvesting ability from 400 nm to 800 nm. Furthermore, the higher IPCE between 500 nm and 800 nm can be also related to the suppressed recombination reactions.
In order to explore the underlying mechanisms responsible for the excellent performance of the device, electrochemical impedance spectroscopy of devices under the bias of 0 to −0.8 V in dark was tested. Fig. S6† shows the Nyquist plots of devices made using the two different methods at a bias of −0.5 V. The inset is the circuit employed to fit the plots. There is only one arc in the figure32 which represents the charge transfer resistance (RCT) of the whole device. With the increase of the applied bias voltage, RCT of the device fabricated using the ICT method drops slowly. On the one hand, the density of defects in the perovskite layer is expected to be lower because of the enhanced crystallinity in the device prepared using the ICT method. On the other hand, the perovskite single crystals fill up the mesoporous structure assisted by the MA0 gas with improved interface contact between the carbon electrode and the mesoporous TiO2 layer, thus the carrier accumulation and recombination at the interface is expected to be lower. This may provide an explanation of the high VOC in devices fabricated using the ICT method.
Notably, Hinsch, et al. has reported a similar molten-salt approach for depositing perovskite absorbers in the mesoporous scaffold.33 MAPbI3 powders were used as the perovskite source and acetonitrile (ACN) was used to tune the viscosity of the precursor. A high voltage of 1 V was obtained, although the overall PCE was only 12.6%. This work also emphasized the key concept of our method. We employed MAPbI3 single crystals as the perovskite sources instead of normal perovskite powders. It is believed that the enhanced performance is due to the high quality of the perovskite crystals, and the ICT process can maintain the excellent properties after transferring them into the mesoporous scaffold.34
Besides the efficiency, the stability of the cells fabricated with ICT method was also improved. The cells were fabricated in ambient air and continuously monitored in laboratory environment without encapsulations for up to 3500 hours (Fig. 5c). The temperature was within the range of 4–35 °C, and the relative humidity (RH) was between 55% and 75%. The PCE of the device prepared using the drop-casting method decreased from the initial 9.09% to 5.89% after 1000 h. After 3500 h, the PCE of the device dropped to 3%. Under the same conditions, the efficiency of the cells prepared using ICT method was stable. Previously, we have investigated the ambient stability of triple mesoscopic PSCs based on MAPbI3 under different RH.15 The high RH may lead to the decomposition of the perovskite absorber and causes severe performance degradation. Here for the cells fabricated by ICT method, the performance is relative insensitivity to the environment even with RH of over 70%. It is proposed that such enhanced ambient stability is due to the high crystallinity and compact morphology of perovskite absorber in the mesoporous scaffold. For further practical applications, the stability under continuous illumination or high temperature may be more important, although the high ambient stability benefits the mass-production of this PV technology. Unfortunately, for pure MAPbI3, the inevitable release of I2 may make it challenging to fabricate long-term stable PSCs.35 Employing mixed-cations perovskites or interface modifications seems a promising route to achieve long-term stability.36–38 Here for the ICT method, it can only transfer pure phase perovskites in the mesoporous scaffold at present. If other cations can be properly incorporated, or the interface can be tuned, higher stability under more harsh conditions may be expected.
Conclusions
We have demonstrated the use of the ICT method based on gas–solid interaction to deposit MAPbI3 within mesoporous scaffolds. In this process, MAPbI3 single crystals placed on top of the scaffolds are first transformed into a liquid phase via exposing them to MA0 gas flow. After complete infiltration into the scaffolds, the liquid phase is converted back to solid phase, forming high-quality MAPbI3 crystals with minimum volume shrinkage within the scaffold. As a result, the PSCs fabricated using the ICT method showed a PCE approaching 16%, which is attributed to the suppressed charge recombination and the correspondingly enhanced VOC. We believe it is a promising technique for future upscaling manufacturing high quality perovskite thin films and related optoelectronics devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (Grant No. 21702069 and 91733301), the Fundamental Research Funds for the Central Universities, the Science and Technology Department of Hubei Province (No. 2017AAA190), the 111 Project (No. B07038), the Program for HUST Academic Frontier Youth Team (2016QYTD06), and the Double First-Class Research funding of China-EU Institute for Clean and Renewable Energy (No. ICARE-RP-2018-SOLAR-001 and ICARE-RP-2018-SOLAR-002). We thank the Analytical and Testing Center of Huazhong University of Science and Technology (HUST) for performing various characterization and measurements. M. C., Y. Z., and N. P. P. acknowledge the funding from the National Science Foundation (Grants OIA-1538893) and the Office Naval Research (Grant N00014-17-1-2232). The authors would also thank Dr Yan Guan and Dr Yuansheng Sun for performing the PL measurements.References
- Y. Rong, Y. Hu, A. Mei, H. Tan, M. I. Saidaminov, S. I. Seok, M. D. McGehee, E. H. Sargent and H. Han, Science, 2018, 361, eaata8235 CrossRef PubMed.
- J.-P. Correa-Baena, M. Saliba, T. Buonassisi, M. Grätzel, A. Abate, W. Tress and A. Hagfeldt, Science, 2017, 358, 739 CrossRef CAS PubMed.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Nat. Photonics, 2019, 13, 460–466 CrossRef CAS.
- W. Tress, N. Marinova, T. Moehl, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Gratzel, Energy Environ. Sci., 2015, 8, 995–1004 RSC.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS.
- H. Chen, F. Ye, W. Tang, J. He, M. Yin, Y. Wang, F. Xie, E. Bi, X. Yang, M. Grätzel and L. Han, Nature, 2017, 550, 92–95 CrossRef CAS.
- X. Li, D. Bi, C. Yi, J.-D. Décoppet, J. Luo, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Science, 2016, 353, 58–62 CrossRef CAS.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS.
- Y. Zhou, O. S. Game, S. Pang and N. P. Padture, J. Phys. Chem. Lett., 2015, 6, 4827–4839 CrossRef CAS.
- X. Zhang, G. Zhou, P. Shi, H. Du, T. Lin, J. Teng and F. S. Chau, Opt. Lett., 2016, 41, 1197–1200 CrossRef CAS.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- A. Mei, X. Li, L. Liu, Z. Ku, T. Liu, Y. Rong, M. Xu, M. Hu, J. Chen, Y. Yang, M. Grätzel and H. Han, Science, 2014, 345, 295–298 CrossRef CAS.
- Y. Rong, L. Liu, A. Mei, X. Li and H. Han, Adv. Energy Mater., 2015, 5, 1501066 CrossRef.
- Y. Hu, Z. Zhang, A. Mei, Y. Jiang, X. Hou, Q. Wang, K. Du, Y. Rong, Y. Zhou, G. Xu and H. Han, Adv. Mater., 2018, 30, 1705786 CrossRef.
- Y. Rong, X. Hou, Y. Hu, A. Mei, L. Liu, P. Wang and H. Han, Nat. Commun., 2017, 8, 14555 CrossRef.
- L. Hong, Y. Hu, A. Mei, Y. Sheng, P. Jiang, C. Tian, Y. Rong and H. Han, Adv. Funct. Mater., 2017, 27, 1703060 CrossRef.
- F. Ji, S. Pang, L. Zhang, Y. Zong, G. Cui, N. P. Padture and Y. Zhou, ACS Energy Lett., 2017, 2, 2727–2733 CrossRef CAS.
- Z. Zhou, Z. Wang, Y. Zhou, S. Pang, D. Wang, H. Xu, Z. Liu, N. P. Padture and G. Cui, Angew. Chem., Int. Ed., 2015, 54, 9705–9709 CrossRef CAS.
- S. R. Raga, Y. Jiang, L. K. Ono and Y. Qi, Energy Technol., 2017, 5, 1750–1761 CrossRef CAS.
- Q. Wang, W. Zhang, Z. Zhang, S. Liu, J. Wu, Y. Guan, A. Mei, Y. Rong, Y. Hu and H. Han, Adv. Energy Mater., 2019 DOI:10.1002/aenm.201903092.
- Y. Zhou and N. P. Padture, ACS Energy Lett., 2017, 2, 2166–2176 CrossRef CAS.
- Q. Dong, Y. Fang, Y. Shao, P. Mulligan, J. Qiu, L. Cao and J. Huang, Science, 2015, 347, 967–970 CrossRef CAS.
- Z. Chen, Q. Dong, Y. Liu, C. Bao, Y. Fang, Y. Lin, S. Tang, Q. Wang, X. Xiao, Y. Bai, Y. Deng and J. Huang, Nat. Commun., 2017, 8, 1890 CrossRef.
- Y. Liu, Z. Yang, D. Cui, X. Ren, J. Sun, X. Liu, J. Zhang, Q. Wei, H. Fan, F. Yu, X. Zhang, C. Zhao and S. Liu, Adv. Mater., 2015, 27, 5176–5183 CrossRef CAS.
- M. Long, T. Zhang, H. Zhu, G. Li, F. Wang, W. Guo, Y. Chai, W. Chen, Q. Li, K. S. Wong, J. Xu and K. Yan, Nano Energy, 2017, 33, 485–496 CrossRef CAS.
- J. Chen, Y. Xiong, Y. Rong, A. Mei, Y. Sheng, P. Jiang, Y. Hu, X. Li and H. Han, Nano Energy, 2016, 27, 130–137 CrossRef CAS.
- Z. Wang, Q. Lin, F. P. Chmiel, N. Sakai, L. M. Herz and H. J. Snaith, Nat. Energy, 2017, 2, 17135 CrossRef CAS.
- Y. Zong, Y. Zhou, M. Ju, H. F. Garces, A. R. Krause, F. Ji, G. Cui, X. C. Zeng, N. P. Padture and S. Pang, Angew. Chem., Int. Ed., 2016, 55, 14723–14727 CrossRef CAS.
- D. W. de Quilettes, S. M. Vorpahl, S. D. Stranks, H. Nagaoka, G. E. Eperon, M. E. Ziffer, H. J. Snaith and D. S. Ginger, Science, 2015, 348, 683–686 CrossRef CAS PubMed.
- Y. Shao, Z. Xiao, C. Bi, Y. Yuan and J. Huang, Nat. Commun., 2014, 5, 5784 CrossRef CAS PubMed.
- T. Liu, L. Liu, M. Hu, Y. Yang, L. Zhang, A. Mei and H. Han, J. Power Sources, 2015, 293, 533–538 CrossRef CAS.
- H. Chen, Z. Wei, H. He, X. Zheng, K. S. Wong and S. Yang, Adv. Energy Mater., 2016, 6, 1502087 CrossRef.
- L. Wagner, S. Chacko, G. Mathiazhagan, S. Mastroianni and A. Hinsch, ACS Energy Lett., 2018, 3, 1122–1127 CrossRef CAS.
- D.-N. Jeong, D.-K. Lee, S. Seo, S. Y. Lim, Y. Zhang, H. Shin, H. Cheong and N.-G. Park, ACS Energy Lett., 2019, 4, 1189–1195 CrossRef CAS.
- S. Wang, Y. Jiang, E. J. Juarez-Perez, L. K. Ono and Y. Qi, Nat. Energy, 2016, 2, 16195 CrossRef.
- M. Saliba, T. Matsui, K. Domanski, J.-Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J.-P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Science, 2016, 354, 206–209 CrossRef CAS.
- G. Grancini, C. Roldán-Carmona, I. Zimmermann, E. Mosconi, X. Lee, D. Martineau, S. Narbey, F. Oswald, F. De Angelis, M. Graetzel and M. K. Nazeeruddin, Nat. Commun., 2017, 8, 15684 CrossRef CAS.
- N. Arora, M. I. Dar, A. Hinderhofer, N. Pellet, F. Schreiber, S. M. Zakeeruddin and M. Grätzel, Science, 2017, 358, 768–771 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc04900b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |