Capturing student conceptions of thermodynamics and kinetics using writing
S. A.
Finkenstaedt-Quinn
 a,
A. S.
Halim
b,
G.
Kasner
a,
C. A.
Wilhelm
a,
A.
Moon
c,
A. Ruggles
Gere
d and
G. V.
Shultz
a,
A. S.
Halim
b,
G.
Kasner
a,
C. A.
Wilhelm
a,
A.
Moon
c,
A. Ruggles
Gere
d and
G. V.
Shultz
 *a
*a
aDepartment of Chemistry, University of Michigan, Ann Arbor, Michigan 48109-1055, USA. E-mail: gshultz@umich.edu
bSchool of Pharmacy, University of Michigan, Ann Arbor, Michigan 48109-1055, USA
cDepartment of Chemistry, University of Nebraska, Lincoln, Nebraska 68588-0304, USA
dSchool of Education and Department of Language and Literature, University of Michigan, Ann Arbor, Michigan 48109-1055, USA
First published on 19th May 2020
Abstract
Thermodynamics and kinetics are key topics in the chemistry curriculum that pose challenges to students across a range of educational levels. These struggles arise from the complexity and mixed representations inherent to the topics. Additionally, while thermodynamics and kinetics are related, students struggle to make conceptually correct connections, sometimes seeing them as two separate topics with no relation and other times conflating their meanings and explanatory powers. Herein we captured student conceptions about thermodynamics and kinetics through a Writing-to-Learn activity that utilized peer review and revision to engage students with the concepts by applying them to a real-world context. This study identified whether students focused on the concepts targeted by the assignment and characterized the chemistry content of the peer review feedback. Students’ descriptions of thermodynamics and kinetics content, as well as the relationship between the two and how they connect to the application given in the assignment, improved during the process and suggests that peer review and revision played an important role in supporting students to describe these concepts. When guided by a content-focused peer review rubric, students provided constructive chemistry content-directed feedback. Specifically, analysis of student writing and comments demonstrated the potential of the assignment to engage students in building connections between complexly related topics, including distinguishing between sponteneity and rate and appropriately relating activation energy and rate. Findings from this study suggest that writing can be used to elicit student-specific conceptions of physical chemistry topics and develop students’ explanatory skills of chemistry content even without direct instructor feedback.
Introduction
Thermodynamics and kinetics are important topics for students to understand within the chemistry curriculum and across STEM, with applications in academia, industry, and the health professions (Justi, 2003). Students struggle with these topics due to their complexity as well as the need to translate between various representations. Additionally, with the ubiquity of thermodynamics and kinetics, they can be taught in a range of ways across chemistry, physics, biology, and engineering courses which may lead to further struggles for students as they seek to translate between the discipline-specific foci. As such, engaging students with the content through practices such as Writing-to-Learn that require them to explain the concepts may support their understanding.Thermodynamics and kinetics
Reviews of the education research literature have focused on physical chemistry broadly (Tsaparlis, 2007) and thermodynamics (Goedhart and Kaper, 2003; Bain et al., 2014) or kinetics (Justi, 2003; Bain and Towns, 2016) specifically. In general, physical chemistry courses and the content covered require students to engage with models and mathematical representations of concepts, and students may struggle to utilize these tools in a chemistry context (Tsaparlis, 2007). Mathematical manipulation and interpretation skills have been correlated with student success in physical chemistry across multiple studies (Tsaparlis, 2007; Becker and Towns, 2012; Bain et al., 2014). However, this is an area where many students struggle and students with lower math skills may be at a disadvantage (Bain et al., 2014), indicating a need for interventions that help students translate between mathematical representations and conceptual meaning.Within the topic of thermodynamics specifically, students struggle with the concepts of enthalpy, entropy, and Gibbs free energy even after completing a physical chemistry course (Carson and Watson, 2002). These difficulties can persist as students progress along the undergraduate chemistry track (Bain and Towns, 2018). Regarding enthalpy, students struggle to distinguish enthalpy from change in enthalpy and often lack an understanding of the interplay between heat and work in determining the enthalpy of a reaction (Nilsson and Niedderer, 2014). In particular, students often conflate enthalpy and heat, neglecting to consider work at all (Goedhart and Kaper, 2003; Nilsson and Niedderer, 2014). This confusion is also apparent in student explanations of the mathematical representation of the first law of thermodynamics, where they exhibit difficulty explaining how work and heat relate to the conservation and conversion of energy (van Roon et al., 1994; Hadfield and Wieman, 2010). Students may also relate entropic changes with changes in enthalpy (Carson and Watson, 2002). Beyond this, students find entropy fairly abstract and do not utilize higher order explanations when describing it (Carson and Watson, 2002). Instead, students often rely on the description of entropy as a measure of disorder and struggle to provide scientific explanations (Carson and Watson, 2002; Bennett and Sözbilir, 2007; Bain and Towns, 2018; Abell and Bretz, 2019). Additionally, the concept of Gibbs free energy is particularly abstract for students and difficult for them to define (Carson and Watson, 2002). When determining the change in Gibbs free energy, students often neglect to account for the change in entropy of a reaction, associating exothermic reactions with spontaneity (Thomas and Schwenz, 1998; Wolfson et al., 2014; Bain and Towns, 2018), and ignore standard state requirements for ΔG° (Thomas and Schwenz, 1998; Goedhart and Kaper, 2003; Wolfson et al., 2014).
The literature focused on kinetics spans student learning across the secondary and post-secondary levels (Justi, 2003; Bain and Towns, 2016). At the secondary level, students struggle with translating between particulate explanations of kinetics and macroscopic observable behaviour (Justi, 2003). At the post-secondary level, students have difficulties developing a mathematical understanding of kinetics and translating between mathematical and conceptual models (Justi, 2003; Bain and Towns, 2016; Bain et al., 2018). A common theme across studies was students’ difficulties with the relationship between concentration and temperature with kinetics (Bain and Towns, 2016). Literature also suggests that while most students have a basic understanding of catalysts, i.e. that they increase the rate of a reaction, they do not always understand the mechanism by which catalysts function (Wolfson et al., 2014; Bain and Towns, 2016; Bain et al., 2018).
The literature reports a tendency for students to conflate thermodynamics and kinetics concepts and form incorrect connections between the two (Thomas and Schwenz, 1998; Justi, 2003; Bain et al., 2014; Bain and Towns, 2016). For example, Sözbilir et al. (2010) found that students struggled to differentiate between equilibrium and rate of a reaction. However, more research is needed to examine how students use thermodynamics and kinetics in conjunction.
In general, researchers recommend incorporating a greater focus on the conceptual aspects of thermodynamics (Carson and Watson, 2002; Sözbilir, 2004). Students themselves also suggested that making links between thermodynamics and the real world would help them learn better (Sözbilir, 2004). The aim of this work was to evaluate how students engaged with a Writing-to-Learn assignment where they needed to consider both thermodynamics and kinetics concepts in the context of an authentic scenario. We implemented the assignment and characterized the concepts that students drew on in their written responses as well as during peer review.
Writing-to-Learn
Writing-to-Learn (WTL) is an instructional practice whereby writing is used as a tool to develop content knowledge, critical thinking, and metacognition generally (Bangert-Drowns et al., 2004; Anderson et al., 2015; Klein, 2015; Klein and Boscolo, 2016) and in STEM classrooms specifically (Rivard, 1994; Reynolds et al., 2012; Gere et al., 2019). Additionally, studies have shown that WTL assignments improve scientific literacy and students’ abilities to develop evidence-supported arguments (Wellington and Osborne, 2001; Saul, 2004). These skills are necessary for undergraduate learning in STEM courses, where students are expected to understand large amounts of content as well as develop scientific reasoning. It is specifically the capacity of WTL to support deep conceptual learning and metacognition that makes the practice promising as a way to engage students with challenging concepts such as thermodynamics and kinetics.Within STEM, WTL research encompasses a broad range of assignment types and forms (Rivard, 1994; Reynolds et al., 2012; Gere et al., 2019). Specifically in chemistry, the use of WTL assignments is gaining momentum (Poock et al., 2007; McDermott and Hand, 2013; Russell, 2013; Shultz and Gere, 2015; Finkenstaedt-Quinn et al., 2017; Cox et al., 2018; Logan and Mountain, 2018; Moon et al., 2018; Schmidt-McCormack et al., 2019). Calibrated Peer Review (CPR) is a form of WTL that engages students in evaluating the work of their peers and has been used in a number of chemistry course contexts (Russell, 2013). In one such use, Cox et al. (2018) paired CPR with two writing assignments in an intervention aimed at reducing the acid–base knowledge gap between two student groups. They found that following the intervention, the treatment group, historically a lower performing group, performed either on par or better than the control group on explanations requiring acid–base knowledge (Cox et al., 2018). In a high school chemistry context, McDermott and Hand (2013) found that students who incorporated multimodal representations in writing assignments performed better on a related conceptual assessment, but that only those students who received prompting incorporated various representations. Another subset of the writing assignments described in the chemistry education literature are designed to target both conceptual learning and learning to write in a scientific style (Logan and Mountain, 2018; Rootman-le Grange and Retief, 2018). Following a series of writing assignments targeting ionic compounds and scientific communication, Rootman-le Grange and Retief (2018) found that students were more cognizant of the relationships between various chemistry topics and felt that their science writing skills had improved.
This study examines student responses to a WTL assignment that was designed based upon the findings of Anderson et al. (2015) and Klein (2015), where students are presented with a realistic scenario and clear learning objectives that guide them to apply specific content knowledge within an authentic context. In addition, students engage in the process of peer review and revision. Similar WTL assignments reported on previously in chemistry focused on Lewis structures, polymer properties, quantum mechanics and spectroscopy, and acid–base chemistry (Shultz and Gere, 2015; Finkenstaedt-Quinn et al., 2017; Moon et al., 2018; Schmidt-McCormack et al., 2019). Incorporating peer review and revision into the WTL process can elicit and remediate misconceptions (Halim et al., 2018) as well as improve student understanding of difficult concepts (Finkenstaedt-Quinn et al., 2017; Moon et al., 2018).
However, not all writing assignments are equal in their capacity to promote learning and we need to understand what specific features of assignments best support particular learning goals (Gere et al., 2019). This research extends beyond previous work by looking at whether the WTL assignment has the potential to engage students in building connections between complexly related chemistry topics, here thermodynamics and kinetics, and provides an analysis of the chemistry content that students focused on when engaging in a content-directed peer review process.
Theoretical framework
The focus of this study is on the capacity of a WTL intervention to engage students in applying their content knowledge to build connections between complex chemistry topics and how students describe the chemistry content. The writing process was supported through students’ interactions with their peers and engagement with physical representations of content in alignment with the learning theories of social constructivism (Ferguson, 2007) and distributed cognition (Klein and Leacock, 2012). Social constructivism posits that learning occurs through the construction of knowledge in a social environment (Ferguson, 2007). As learners obtain new knowledge they incorporate it into their existing knowledge (Bodner, 1986). When the new knowledge is in conflict with the learner's prior knowledge, they must modify or adapt the prior knowledge to develop a cohesive structure of understanding. Specific to social constructivism, learners encounter new knowledge through social interactions, such as with their peers and instructors (Ferguson, 2007). This theory of learning is complemented by distributed cognition, which incorporates the idea that knowledge is held both within individuals, and can be shared between individuals during the knowledge construction process and by external representations such as data representations and textual resources (Nardi, 1996). In distributed cognition, writing acts as an external representation of the knowledge being retrieved by students and can reduce cognitive load during the learning process (Klein and Leacock, 2012). In addition to the act of writing, the WTL intervention described herein supports student learning by incorporating social interactions through peer review and a structured writing prompt.As characterized by Vygotsky (1978), writing can serve as a way for learners to engage with their prior knowledge and articulate it during the act of knowledge construction. As students articulate their existing knowledge to address the concepts, guided by rhetorical elements such as a relevant context and audience, writing allows them to identify gaps in their knowledge (Bereiter and Scardamlia, 1987; Klein and Leacock, 2012). Cognitive perspectives of writing theorize that identifying the gaps creates a cyclic pattern of identifying the gap, retrieving related knowledge, applying the related knowledge, and filling the gap before continuing the writing process (Emig, 1977; Klein et al., 2015). The considerations of audience in the WTL assignment also lead the writers to consider how to present their knowledge and the level of detail necessary in their explanations (Bereiter and Scardamlia, 1987). This can lead to further identification of knowledge gaps and support deep learning, rather than regurgitation of terms learned through rote memorization.
The act of knowledge construction through writing can be supported by a peer review process. The peer review process applied here allows for two forms of learning through social interactions. As students read the initial drafts of their peers it can highlight areas of poor understanding, and students can develop their own understanding by seeing written representations of their peers’ knowledge. Students also receive feedback from their peers who can identify conceptual information that is incorrect or poorly expressed. Further knowledge construction can then occur as students revise their work based off of both what they read and the feedback they received.
Specific to this WTL assignment, students first engage with knowledge housed in two external representations: the assignment description, where students are supplied with a brief article that provides the context to which they will be applying their conceptual understanding, and a graphical representation of catalyst reaction rate data. In accordance with distributed cognition, the article primes student thinking on the target concepts while the graph guides their thinking of kinetics. The writing itself captures students’ knowledge (Klein and Leacock, 2012) and facilitates knowledge construction between peers. During peer review, students may engage with knowledge they are lacking and can also supply their own conceptions of content in the form of written feedback. In addition, the drafts and peer review comments serve as artefacts of students’ knowledge that we as researchers, or instructors, can use to characterize what students know and how they think about the target concepts. While the drafts and peer review comments represent learner knowledge, the peer review comments also serve as indicators of how the social elements lend themselves to a knowledge construction environment.
This work is guided by the following research questions:
(1) In what ways do students engage with the thermodynamics and kinetics concepts targeted by the WTL assignment?
(2) What thermodynamics and kinetics concepts do students focus on during a content-focused peer review process?
Methods
This study included several sources of qualitative data: students’ initial and final drafts for a writing assignment, peer review comments associated with the initial drafts, and interview responses after the writing assignment was completed. The initial and final draft responses were quantitatively transformed using a rubric generated to align with the learning objectives for the assignment. Together these data helped to gauge students’ abilities to describe the target concepts and how their abilities developed through engaging in the WTL process.Setting and participants
This study was conducted at a large, Midwestern university in an introductory physical chemistry course intended for chemistry and chemical engineering majors. Advisory prerequisites included introductory physics, calculus, and introductory organic chemistry. The course had a total of 82 students and two instructors. Students were assessed through problem sets, quizzes, and exams. In addition, there were two WTL assignments incorporated into the course and each section did one of the assignments. The thermodynamics and kinetics assignment discussed here was completed by 39 students. All 39 students completed the initial draft and peer review but only 38 completed the final draft. Of the 39 students who took part in the writing assignment, 35 consented to participate in the study and completed an initial demographics survey. The participants in the study were primarily Caucasian, 19 students were female and 16 were male, the majority were majoring in either biochemistry or chemistry, four were first-generation college students, and eight were non-US born. Eight students indicated that they had encountered the material before, 34 students indicated that they had previous experience with peer review, and all students indicated that they had previous experience with revision. Institutional Review Board approval was obtained to collect student demographic information through surveys, writing responses, and interviews.WTL assignment
The assignment was developed by the research team and one of the course instructors. During the course of the assignment students first responded to a prompt, then participated in peer review, and lastly revised their responses and submitted a second draft (Appendix 1). Students were given one week to complete their initial response, three days to complete peer review, and five days to revise their written response. The assignment focused on applying the principles of thermodynamics and kinetics to the octobot, a soft-bodied robot powered by the decomposition of hydrogen peroxide (Wehner et al., 2016). The writing prompt gave students a scenario where they were asked to write a popular news article focused on how the principles of thermodynamics and kinetics related to the functionality of the octobot developed by Wehner et al. (2016). Students were provided with a link to the article published by Chemical & Engineering News about the octobot and a graph of relative reaction rate data for a range of catalysts as a starting point for their responses (Cybulskis et al., 2016; Everts, 2016). The peer review process involved students giving and receiving anonymous feedback from two to four peers using an online system developed specifically for this use. Students were guided in their feedback by four content-specific questions to which they responded, focusing on (1) enthalpy and entropy; (2) the decomposition reaction; (3) the role of a catalyst on the kinetics; and (4) the laws of thermodynamics (Appendix 1, Student peer review rubric). The rubric questions prompted reviewers to comment on whether the concepts were explained well and to identify which parts were missing or unclear. All components of the writing assignment were graded based on completion instead of content in order to reduce the instructors’ time spent on evaluation and grading.Data collection and analysis
The student drafts were analysed for research purposes by applying a scoring rubric generated based on the learning outcomes for the assignment, where the rubric consisted of three criteria focused on students’ descriptions of thermodynamics, kinetics, and the connection between thermodynamics and kinetics (Appendix 2). Each criterion consisted of four concepts or ideas, where each concept was scored as either zero for absent/incorrect or one for present/correct, so that each criterion could receive a maximum score of four with a total of 12 points for the whole draft. Following initial development of the scoring rubric, it was refined through multiple iterations of applying it to student drafts and discussion between members of the research team. The final rubric was applied simultaneously to students’ initial and final drafts, which had been merged using the track changes functionality in Microsoft Word so that the differences between the two drafts were easily identifiable. The drafts were scored together in this way to minimize scorer error and discrepancy between drafts. Each pair of initial and final drafts was scored individually by two members of the research team, followed by comparing scores and discussing differences until reaching consensus. While scoring the drafts, team members also wrote memos to capture common characteristics they noted in the ways that students wrote about the target content.The sums for each criterion and the total across all three criteria were analysed for differences between drafts using paired t-tests with statistical significance set at 0.05. Additionally, Cohen's d effect sizes were calculated; 0.20–0.49 was considered small, 0.50–0.79 was considered medium, and values greater than or equal to 0.80 were considered large (Cohen, 1987). All statistical analyses were performed using the software package Stata 15 (StatCorp, 2017).
The peer review comments were also thematically analysed by two members of the research team to characterize the content that students included, as well as the level of detail in which they discussed the content (Cohen et al., 2011). Responses to each of the four student peer review rubric questions were analysed separately (N = 376 total peer review comments, with 94 comments per question). For each of the four subsets of responses, one member of the research team initially read through a portion of the comments and identified codes. The initial coding was guided by the peer review questions associated with each subset of reviewer comments (e.g., analysis of the comments responding to the peer review rubric question targeting entropy, enthalpy, and the use of hydrogen peroxide was guided by language students used associated with those ideas). Following this, the researcher discussed the initial codes with another member of the research team. The two members then applied the codes together to a new portion of the comments and discussed any differences or additional codes. They then separately read through and applied the codes to the full subset of peer review comments, compared codes, and discussed any discrepancies until reaching consensus. Each comment was considered a unit of analysis and could receive multiple codes. Additionally, there was some overlap in the codes across the subsets of reviewer comments, in particular those pertaining to enthalpy and entropy and the laws of thermodynamics. Using thematic analysis and deriving a distinct set of codes for each subset of peer review comments allowed us to capture the content that students were discussing during peer review, aligning with our second research question (Appendix 3).
Following coding of all the peer review responses, the comments were grouped by code within each peer review rubric question. For codes that were applied five or less times, the comments were re-read and it was determined whether there was a code it could be combined with. For example, in the comments associated with the entropy and enthalpy peer review rubric question, the authors initially developed a code to capture reviewer comments which suggested incorporating a discussion relating the increased entropy of the system to an increase in microstates or the number of moles on the product side of the reaction. However, this was only applied three times and so we decided to incorporate it into the code ‘discussion of enthalpy and entropy’ as it is relevant to the content included in that code. The comments for each set of final codes were then read to determine how students were writing about the content captured by each set of codes.
The last source of data consisted of four semi-structured reflective interviews that were conducted after the completion of the writing assignment. Students were recruited via email and were not provided compensation. Each interview took approximately one hour. The interview transcriptions were thematically analysed using NVivo 12 (2018). Each transcript was read through individually by three members of the research team who identified themes related to the research questions and theoretical framework. Using the constant comparison method, two members of the research team re-read the interview transcripts focusing on the agreed upon themes (Corbin and Strauss, 1990). The refined themes entailed (1) interactions with course concepts and (2) the WTL process, with a focus on peer review. Overall, the student interviews indicated that the prompt design and assignment structure functioned as intended. Student pseudonyms are used when presenting the interview analysis.
Results and discussion
To address our research questions, we analysed the student writing—initial drafts, final drafts, and peer review comments—and interviews with students. Our analysis focused on whether students addressed the concepts targeted in the WTL assignment as well as how they engaged with the social components of the assignment.Analysis of content descriptions in student initial and final drafts
Students’ initial and final responses to the WTL prompt were analysed for content aligning with the learning objectives of the assignment. For each set of responses, both the initial and final drafts were scored according to a three-criteria rubric that focused on thermodynamics, kinetics, and the connection between thermodynamics and kinetics, where each criterion was on a scale of 0 to 4 (Appendix 2). These scores were used to quantify students’ abilities to correctly describe the target concepts and to determine if there were improvements in scores between initial and final drafts. Overall, student scores showed meaningful improvement between drafts for all three criteria, along with the total average score, with medium to large effect sizes, indicating that students improved their depictions of or incorporated additional target content (Table 1). The frequencies with which each concept was included in students’ initial and final drafts are presented in the appendix (Appendix 2).| Criteria | Initial (std dev.) | Final (std dev.) | t-Test | Effect sizea |
|---|---|---|---|---|
| Each criteria was scored on a scale of 0 to 4, N = 35.a Cohen's d, ***p ≤ 0.001. | ||||
| Thermodynamics | 1.2 ± 0.91 | 2.5 ± 1.10 | 8.62*** | 1.19 |
| Kinetics | 2.5 ± 1.01 | 3.3 ± 0.84 | 4.74*** | 0.95 |
| Connection | 1.7 ± 0.80 | 2.3 ± 0.79 | 5.88*** | 0.76 |
| Total | 5.4 ± 1.83 | 8.1 ± 1.63 | 10.15*** | 1.56 |
For the thermodynamics criterion, students were scored based on correct descriptions of the first and second laws of thermodynamics, changes in entropy and enthalpy, and Gibbs’ relation to spontaneity (Appendix 2). Students initially scored 1.2 ± 0.91 out of 4. Many students did not include descriptions of the first and second laws of thermodynamics in their initial drafts despite their importance and the prompt's direction to explain how the decomposition reaction followed the laws of thermodynamics. This lack of description led to the low initial draft score on this criterion, as the first and second laws of thermodynamics were two major points on the scoring rubric. However, this criterion showed the greatest improvement after peer review and revision, with a final draft score of 2.5 ± 1.10 out of 4 (p ≤ 0.001, effect size = 1.19, Table 1). The increase in scores may in part be attributable to the fact that two of the peer review rubric questions focused on thermodynamics, one of which specifically targeted the laws of thermodynamics and directed students to incorporate this information. This gain indicates the importance of a peer review rubric with transparent content-focused criteria in supporting the writing process.
Within students’ drafts we identified struggles consistent with previous reports (Carson and Watson, 2002; Bain and Towns, 2018; Abell and Bretz, 2019). Specifically we identified students’ tendencies to neglect to consider the system versus surroundings in thinking about the second law or entropy, and a focus on the change of state, number of moles between reactants and products, or broadly some change in disorder of the system to justify the change in entropy (Carson and Watson, 2002; Bain and Towns, 2018; Abell and Bretz, 2019). In juxtaposition to Carson and Watson (2002), who observed that students did not distinguish between entropy, enthalpy, and Gibbs free energy, we found that students were able to discriminate between the three concepts in their writing. This indicates that the WTL assignment could act as a tool to help students differentiate between the concepts and support students’ transitions to higher levels of conceptions of Gibbs free energy, as they were required to articulate the connections between thermodynamic concepts during the writing process.
In response to the kinetics criterion, students were expected to provide an accurate description of activation energy and its relationship with the rate of a reaction, as well as the role of the catalyst (Appendix 2). Students scored the highest on this criterion for both their initial and final drafts, improving from 2.5 ± 1.01 to 3.3 ± 0.84 out of 4 (p ≤ 0.001, effect size = 0.95, Table 1). Overall, students most often improved their score by adding or expanding on the qualitative reasons for why platinum was chosen over another catalyst. Students’ justification of the use of platinum for this system shows that they were able to apply their understanding of a catalyst to an authentic situation. This could support students in bridging known difficulties in the area of kinetics, specifically connecting mathematical models to conceptual models and particulate-level explanations to macroscopic observations (Justi, 2003; Bain and Towns, 2016). More students also connected the activation energy of a reaction to the rate of the reaction following revision. It is promising that students found it important to include this relationship, as it was not specifically asked for in either the prompt or the peer review rubric and could be indicative of them recognizing that it is through lowering the activation energy that catalysts increase the reaction rate, a concept that some students struggle with (Bain et al., 2018).
Student writing was also scored on how they connected the topics of thermodynamics and kinetics. We evaluated the writing for a discussion of why both thermodynamics and kinetics should be considered to determine the applicability of the reaction, a statement about Gibbs free energy not being impacted by the presence of a catalyst, and a description of how the decomposition reaction led to movement of the octobot (Appendix 2). While this criterion had the smallest improvement in draft scores, with the average increasing from 1.7 ± 0.80 to 2.3 ± 0.79 out of 4 (p ≤ 0.001, effect size = 0.76, Table 1), the improvement still had a medium effect size. The smaller gain between drafts may in part be attributable to the fact that in both drafts most students did not include that Gibbs free energy is not affected by a catalyst. This concept was not articulated explicitly in the WTL prompt; however, we included it in the scoring rubric as it is an important distinction for separating the catalyst from thermodynamics. With so few students including this in their initial drafts (3 out of 35, Appendix 2), the peer review process could only have a minimal impact (as exhibited by only two students adding this in their final drafts, Appendix 2) and so the small gain is not unexpected. While we cannot identify with the data collected herein whether students chose not to include the statement or did not understand the lack of relationship, in a biochemistry context Wolfson et al. (2014) did identify this as a gap in conceptual understanding for students. While not all students explicitly stated that both thermodynamics and kinetics needed to be considered when determining if a reaction would be feasible for the intended application, most did discuss that a reaction could be spontaneous but too slow to be useful. This is promising as considering both topics, while not conflating the concepts included in each, is an area where students are known to struggle (Thomas and Schwenz, 1998; Sözbilir et al., 2010; Bain and Towns, 2016) and demonstrates that in the context of this assignment students were able to distinguish between spontaneity and rate. Additional evidence from the interviews indicates that the assignment did lead students to think more about how thermodynamics and kinetics are related and why one would need to consider both when determining the application of a particular reaction. Trinity said:
I think they stress a lot, that like, the thermo and the kinetics are very like, different and I think writing this I kinda saw how they played together almost. And so I think those like connections were kinda strengthened while writing this.
Students also improved their discussions of how the reaction leads to movement. When addressing the content targeted by this criterion, the majority of students made connections to the macroscopic behaviour of the octobot, focusing on the formation and expansion of gas rather than the work generated by the reaction. This serves as another example of how the context provided by the writing assignment led students to think about the chemistry on a macroscopic level.
Peer review comments
As guided by our theoretical frameworks, where knowledge development is supported by external representations of knowledge and interactions between peers, we posit that the peer review process plays an important role in the WTL assignment. With the focus of this type of WTL assignment on conceptual learning, we wanted to further examine whether peers were providing feedback relevant to chemistry content as well as how they were discussing the content. With this aim, the peer review comments were coded to identify themes in the feedback that students provided when responding to the peer review rubric. The peer review rubric questions asked students to comment on their peers’ discussion of enthalpy and entropy, the decomposition reaction, the catalyst's role in the reaction, and the laws of thermodynamics (Appendix 1, Student peer review rubric). The comments arising from each peer review rubric question were coded separately, as we expected the reviewers to focus on different content for each of the questions, although there was some overlap in the codes used between the four sets. Each set was then analysed for themes across the comments (Appendix 3). Additionally, a group of universal codes arose that applied across all four comment sets, presented in Appendix 3.In general, the peer review comments provided recommendations and were primarily phrased in neutral tones, similar to findings by Finkenstaedt-Quinn et al. (2019). In the majority of the comments, students provided content-focused feedback on their peer's writing, with only a small subset of comments containing no actionable feedback (N = 74 out of 376). Another subset of comments contained feedback about grammar or stylistic features of the writing (N = 32 out of 376), however a majority of those also included feedback on the content (N = 20 out of the 32). Across all four peer review questions, the majority of the comments provided some specificity in their feedback, exemplified by the minimal number of comments that received the ‘more detail’ code instead of a more specific content code (N = 13 out of 376). In line with this finding, the interviews indicated that the peer review rubric successfully directed students towards the target concepts. Jenny discussed how the peer review rubric guided her during the peer review process:
It just made me know what I needed to look for, which was really helpful… It also made the peer review not become me sitting down and editing someone's paper for all the nitty gritty but more content based which is pretty important I guess.
The prevalence of specific, content-focused feedback shows promise for students’ abilities to participate in the knowledge building process scaffolded by the WTL assignment for difficult topics in chemistry such as thermodynamics and kinetics.
A subset of comments contained suggestions to add more quantitative information to the text (‘quantitative’) or discuss the meaning behind the quantitative elements provided in the text (‘linking values to meaning’). These comments were primarily associated with the entropy and enthalpy rubric question (N = 21 out of the 34 ‘quantitative’ codes and N = 20 out of the 26 ‘linking values to meaning’ codes), which is somewhat surprising as each of the four rubric questions could be associated with quantitative discussion in students’ drafts. This may indicate that students found quantitative information more relevant in discussions pertaining to thermodynamics.
The peer review comments contained a small number of examples where the reviewer was either correcting a student or where they provided incorrect information themselves. Of the ten comments where the reviewer identified incorrect content in the drafts that they read, eight were typos and mathematical errors rather than conceptual. The remaining two related to the functionality of the catalyst. The incorrect feedback that reviewers provided was relatively minor and not about fundamental concepts (N = 4 out of the 7 ‘reviewer incorrect’ codes). The cases where the reviewer commented on incorrect content but did not identify that it was wrong were more problematic (N = 3 out of the 7 ‘reviewer incorrect’ codes). Specifically they did not identify incorrect statements about how the octobot violates the first law of thermodynamics or that a catalysed reaction will run perpetually. Considered in light of the usefulness of peer review, these results are promising and in line with results from Halim et al. (2018). The minimal number and severity of reviewer errors is a positive outcome as it indicates a minimal risk of incorrect content being propagated by students engaging in the peer review process. However, the low number of reviewers who commented on incorrect content in the drafts they read may be problematic if it allows errors to continue unchecked through the WTL process, especially as within our data set there were only two sets of comments where two reviewers commented on the same incorrect idea.
| Themes | Exemplar |
|---|---|
| Expand on the discussion of enthalpy and entropy | Also, discussing the change in gas moles could strengthen the argument about increase in entropy. |
| Discuss Gibbs free energy and spontaneity | What could be added, however, is a discussion of Gibbs free energy. This is a good concept that ties both your enthalpy and entropy together into a discussion of spontaneity. |
| Provide more quantitative information | Explained how the decomposition results in an exothermic and exergonic reaction. It was easy to understand, maybe include the values for change in entropy and enthalpy. |
| Link thermodynamic values to their meaning | I think it would be beneficial to include the values of the entropy and enthalpy, and the effects it has on Gibbs energy and what it means in this reaction. |
| Justify hydrogen peroxide as the fuel | I think that the argument could be made stronger by focusing also on why hydrogen peroxide was chosen as a fuel instead of some other chemical that also can undergo a decomposition reaction. |
The reviewer comments that focused on enthalpy and entropy generally contained feedback that their peers should define enthalpy or entropy, explain them in relation to the hydrogen peroxide decomposition reaction, or elaborate on the importance of these concepts when considering how the octobot functions. While these comments used primarily generic language, a small subset of comments went into more detail about the chemical considerations that should be included in the descriptions of enthalpy and entropy.
Another set of comments covered Gibbs free energy. These focused on either explaining the concept of Gibbs free energy or pushed students to connect it more clearly to the change in enthalpy and entropy of the reaction. A number of these contained suggestions that students include a better discussion of how thermodynamic considerations drive the spontaneity of the reaction. The comments in response to this peer review rubric question indicate that reviewers are thinking about the connections between enthalpy, entropy, and Gibbs free energy. As the literature indicates that students struggle to form distinct conceptions of these concepts (Carson and Watson, 2002), reviewer comments directing students to articulate the connections may support them to construct that knowledge and provides further evidence of students’ abilities to distinguish between the concepts in the context of this assignment.
As mentioned previously, a subset of the reviewer comments in response to this rubric question contained suggestions to provide more quantitative information or tie the values or signs provided in students’ initial drafts to some chemical meaning. In the comments coded as ‘quantitative’, reviewers often suggested including the values for entropy, enthalpy, or Gibbs free energy, or that the Gibbs free energy equation should be included in students’ drafts. In some of the comments, reviewers went further and suggested that students provide a more qualitative chemical explanation for thermodynamic values, such as explaining the signs of the enthalpy and entropy of the reaction or connecting the values to spontaneity. While the focus on providing quantitative information about the thermodynamics of the reaction may be due to how the content is often presented to students, it is promising that some of the reviewers suggested that students go into the underlying chemistry behind the values. Comments such as these may be especially beneficial to support conceptual learning as students are known to have difficulty connecting quantitative values to their chemical meaning (Bain et al., 2014).
The last code applied to the comments in response to the first student peer review rubric question was ‘justification for fuel.’ In these comments, reviewers suggested students incorporate or expand on why the hydrogen peroxide decomposition reaction was chosen for the octobot. A number of these comments contained suggestions to add an explicit discussion of the choice of fuel. These comments ranged from general to more detailed language, with specific statements that the student should connect the reaction choice to its spontaneity or the favourable values of the enthalpy and entropy of the reaction. Another portion of the reviewers suggested justifying the choice of decomposition reaction. The comments about the fuel indicate that students are reflecting on how the chemical considerations tie to the realistic applications of the reaction. By incorporating this into the peer review process, some of those students who had not previously thought about the implications were directed to do so by their reviewers.
| Themes | Exemplar |
|---|---|
| Explain the physical causes of motion | The fact that gas has more volume than liquid hydrogen peroxide should be mentioned, so that it can be clear that the change in volume is what drives the movement in the robot's arms. |
| Explain how energy causes the motion | I do think that a connection to the negative Gibbs' energy would make this explanation even stronger, connecting the engineering to the chemistry we have learned. From where exactly is the energy to power the robot coming? |
| Explain the reaction itself in more detail | I think the only thing that is missing is possibly a discussion of how exactly the reaction itself is initiated and possible ways that the reaction can be controlled or if it's simply spontaneous and random in how it moves. |
In this set of peer review comments, there was a range in how students were using the term ‘power’, from applying it to the motion of the octobot to the work of the reaction. The different ways students used this term exemplifies the blurring between disciplinary and colloquial usage of scientific terms, where none of the students used the term ‘power’ in a way that aligns with its definition in the physical sciences. The improper use of such terms by students in chemistry contexts has previously been characterized generally (Song and Carheden, 2014), and specifically within thermodynamics (Thomas and Schwenz, 1998). In this case, students may have also used the term ‘power’ due in part to the wording of the peer review rubric question. This is another indicator of how the language in the WTL prompts may guide student responses and thinking. Due to the ambiguity of what students meant when using the word ‘power’, we grouped together comments focused on power and movement.
The reviewer comments overarchingly focused on what causes the octobot to move, generally prompting students to expand on why or how the octobot moves or to define related terms, such as pneumatics. Some comments focused on using energetics to explain the octobot movement. For example, some focused on how the chemical energy or negative change in Gibbs free energy was converted into mechanical energy, whereas others also incorporated the idea of work in relation to energy and motion. Reviewer suggestions to incorporate a discussion of work are promising as students are known to neglect this concept (Goedhart and Kaper, 2003; Nilsson and Niedderer, 2014). A few reviewers also suggested that a discussion of the energy source of the octobot should be tied to the explanation about why it is not a perpetual motion machine.
Some reviewers were more focused in their comments on the physical forces responsible for moving the arms of the robot. These comments directed the students to consider how gas formation, in some cases focusing specifically on the resultant increase in pressure, contributes to the robot moving. These comments show that during the peer review process students were able to address a range of aspects related to how the decomposition reaction creates motion in the octobot, focusing both on energetic and macroscopic sources. Responses to this rubric prompt demonstrate that students had different perspectives on what they believed to be important focal points for explaining the motion. As each student received an average of three peer review comments per rubric question, each serving as an external representation of their peers’ knowledge, they could have been exposed to a range of ideas. This may impact the process of knowledge construction as it would necessitate a negotiation between the various perspectives and student's own schema of the topic.
The final subset of the reviewer comments for this rubric question focused on the reaction specifically rather than the motion of the robot. Many of these comments described a need to provide more detailed information about the reaction, referencing thermodynamic considerations again but also including suggestions to explain how the reaction is initiated or that gas was produced during the course of the reaction. It is interesting that peers touched on thermodynamics in these comments despite that content being addressed by the first rubric question, which may be tied to the higher gains on the thermodynamics criterion in the students’ drafts.
| Themes | Exemplar |
|---|---|
| Compare catalysts and justify platinum catalyst | The explanation of why platinum is a good catalyst is thorough but the writer should also put talk about why the catalyst enzyme and sodium iodide are not as good of choices for catalysts. |
| Relate the catalyst more strongly to reaction kinetics | The author describes the reasoning behind choosing platinum very well, but needs a deeper description about catalysts and how they affect the kinetics of a reaction. |
| Relate the context to why a catalyst is needed | You do need to explain why this specific reaction we are presented is so slow that we need to use a catalyst in the first place. |
| Describe activation energy or rate of a reaction | Regarding activation energy, it would be helpful if you provided a definition of what activation energy exactly is. |
The largest subset of constructive comments pertained to the choice of platinum for the catalyst used in the octobot. These comments were a mix between peers suggesting that students add a general explanation for why platinum was chosen and justifying the use of platinum compared to other catalysts, with more belonging to the latter grouping. There was a particular emphasis on comparing platinum specifically to sodium iodide and the catalase enzyme. The emphasis in the peer review comments on comparing platinum to the other catalysts, specifically catalase, also aligned with the number of students who added a comparison of catalysts to their revised drafts and demonstrates how peer review can lead to substantive changes during revision. The focus on comparing catalysts was most likely motivated by the graph provided in the prompt of the relative decomposition rates of hydrogen peroxide with various catalysts (Cybulskis et al., 2016). We found evidence of this in the student interviews where participants discussed how the prompt guided the content they included in their drafts. One student, Pete, noted how the provided graph was helpful when writing about the kinetics of the reaction:
I think what really helped me out was looking at the graph, because I feel like, for a lot of the peer reviews I did, they didn’t explain the importance of the catalyst. When you see two billion times that, why wouldn’t you use it? You know?
Thus, analysis of both the peer review comments and interviews indicate that incorporating this external representation in the prompt successfully directed students towards considering how catalysts can differentially impact reaction rate and how the choice of catalyst may be dependent on the application.
Peers also suggested providing a better description of how catalysts impact the kinetics of a reaction. These comments ranged from broad statements suggesting the student connect the catalyst to kinetics more explicitly to providing more detail about why a catalyst is needed for this particular context. During the interview, Trinity discussed how the peer reviews focused on connecting the catalyst to the context helped her with the kinetics portion of the assignment.
It took me a little bit to understand the catalyst and why they chose platinum. The peer reviews kinda helped me with that too, to understand why an enzyme was not useful.
Often, the comments focused on explicitly connecting the catalyst to kinetics directed students to incorporate or expand on their existing discussions of the rate of a reaction or the activation energy of a reaction. For example, some peers recommended defining or adding in a discussion of reaction rate or activation energy. In some cases, these suggestions directed students to tie the reaction rate or activation energy to the catalyst, either focusing on how the catalyst impacts those kinetic elements or to compare the values with/without a catalyst or between catalysts. More peers focused on the rate of the reaction rather than activation energy and only a small number suggested directly linking the impact of the catalyst on the activation energy to the rate of the reaction. This is interesting, as we noted an increase in the number of students who connected activation energy to reaction rate in their second drafts, which may indicate that getting feedback related to either reaction rate or activation energy was enough to lead students to be more explicit about the connection between the two.
Overall, the comments in response to this rubric question indicated that students were thinking about the role that catalysts play in mediating reaction kinetics and how catalysts apply to this specific application. This indicates that the details built into the prompt extended into shaping the comments that reviewers provided. These results are promising, especially as students are known to struggle with kinetics in general and catalysts in particular (Justi, 2003; Bain and Towns, 2016). The minimal number of comments focusing on the tie between activation energy and the catalyst is perhaps expected as students struggle with this concept (Bain et al., 2018), but it is promising that some reviewers directed students toward it and it supports using peer review to promote knowledge construction mediated by social interactions.
| Themes | Exemplar |
|---|---|
| Incorporate a greater discussion of the laws of thermodynamics | A lot of ideas you talked about contained the idea of laws of thermodynamics, but you never talked about it explicitly. |
| Focus on how the laws of thermodynamics relate to the octobot | You don't say much about the specific laws of thermodynamics. I think you should include how the laws help power the robot, especially the first and second laws. |
| Relate the laws to thermodynamics concepts (e.g., Gibbs free energy, change in entropy) | You could probably use the first law to help bolster the discussion of Gibbs' energy, specifically the energy lost by the reaction must be used to power the robot through conservation of energy. |
The other subset of actionable comments with respect to this question focused on how the laws of thermodynamics applied to the octobot system. Many of these comments contained suggestions that students generally explain how the robot follows the laws of thermodynamics without being more directive. Often reviewers pointed students towards the first and second laws. In more specific comments, reviewers suggested relating the laws to why the octobot can propel itself and why the reaction is spontaneous. A subset of comments specifically suggested incorporating the first law into a discussion of the internal energy of the system or why the octobot is not a perpetual motion machine. Comments targeting the second law of thermodynamics also contained suggestions to relate it to the change in entropy of the reaction.
These comments demonstrate that, when prompted, students were able to draw connections between the laws of thermodynamics and the functionality of the octobot. However, students had differing ideas of which laws should be used to explain the different forces driving the octobot. The reviewers provided fairly actionable comments considering that a minimal number of students discussed the laws of thermodynamics in their initial drafts and the increased amount of content related to the laws in the revised drafts was captured in our scoring of that criterion. The extent of the additions may indicate that the peer review rubric can serve as a secondary source to direct students towards learning goals.
However, it is worth noting that despite the distinct focus of the rubric question, a number of the peer review comments touched upon content related to previous peer review rubric questions rather than providing feedback about the laws. This may indicate that by the fourth peer review question students were experiencing cognitive strain or that they felt it was their last chance to provide feedback on the drafts they had read and so used the space to focus on elements they had not previously addressed. This last is exemplified by the fact that almost half of the comments coded as ‘grammar/stylistic’ fell under this rubric question. Alternatively, they may have felt less comfortable providing feedback on the laws of thermodynamics and instead commented on other content. The range in comments captured in response to this peer review rubric question has implications for peer review rubric design, indicating that we need to consider how the number and phrasing of peer review rubric questions impact student responses.
Limitations
Our analysis did not specifically track the presence of incorrect student content in writing samples, so we did not quantify what subset of student errors were actually identified through the peer review process or whether alternative conceptions were propagated through the WTL process. Work by Halim et al. (2018) in an introductory biology course, which characterizes this across four WTL assignments designed similarly to that presented in this study, indicated minimal propagation of alternative conceptions. However, similar work is merited in a chemistry context as the results may not be transferable. One of the aims of this WTL assignment was to support students in developing a correct understanding of the interplay between thermodynamics and kinetics. Our analysis indicates that students did improve in their ability to describe this connection, but more explicit direction to students in the prompt or peer review rubric could further support this. Lastly, the scoring rubric for the writing was not aligned with the peer review rubric, which limited the connections we could draw between gains in the drafts and related peer review comments.Conclusions and implications
This study focused on a WTL assignment designed to support student understanding of thermodynamics and kinetics in an introductory physical chemistry course. When viewed in conjunction, our data sources—writing, peer review comments, and student interviews—all indicate that the assignment functioned as designed and that students successfully engaged with the targeted chemistry content. The external resources (i.e., peer review rubric, graph, and C&EN article) served to direct students towards target content and helped to guide their writing about the content. Students were further supported by the social elements incorporated into the assignments, successfully focusing on content during the peer review process.The writing analysis demonstrated gains in students’ abilities to describe and explain concepts pertaining to thermodynamics and kinetics, with meaningful improvements between drafts of the assignment. In their drafts, students correctly described thermodynamics and kinetics concepts that previous research has demonstrated they have difficulty with. Additionally, students successfully related thermodynamics and kinetics principles to the functionality of the octobot. Our findings indicate that this assignment can be used as one method of providing students with more opportunities to think about the conceptual aspects of thermodynamics and kinetics as well as relate the mathematical representations to the underlying concepts.
The peer review process exposed students to various perspectives or ways of explaining the chemistry content that they then needed to negotiate when revising their drafts. Considered as a whole, the peer review comments in response to all four rubric questions indicate that students were thinking about the concepts targeted by the assignment and can provide substantive and actionable feedback to their peers. Overall, students were distinguishing between the concepts within thermodynamics and providing qualitative discussions of the kinetics of the reaction. They also demonstrated thinking about how the chemistry content they learned relates to macroscopic changes and linked quantitative values to physical outcomes. Not only were reviewers focusing on difficult concepts in their comments, but they were also directing students to articulate the relationships both within and between thermodynamics and kinetics. The focus on the content during the social interactions mediated by peer review suggests that the peer review rubric and process can support students to engage in a knowledge transformation process in alignment with the theories of social constructivism and distributed cognition. This supports the ability of the peer review rubric and social interactions to guide students’ focus during the revision process on chemistry content.
This research has implications both for instructors and future research. With regards to content, our analysis indicates that instructors should provide a deeper conceptual emphasis on entropy as student responses indicated a surface level understanding, both in terms of how to explain entropy and in differentiating between entropy of the system versus the surroundings. Focusing more on the relationships that do and do not exist between thermodynamics and kinetics, specifically that Gibbs free energy is not related to the rate of a reaction, may also be warranted. Additionally, the WTL assignment detailed herein can be used during instruction for multiple purposes. Incorporating this assignment can address the need identified by prior studies to engage students in more qualitative discussion of thermodynamics and kinetics (Carson and Watson, 2002; Sözbilir, 2004). With the prevalence of alternate conceptions students have related to thermodynamics (Bain et al., 2014) and kinetics (Justi, 2003; Bain and Towns, 2016), instructors can use student responses to this WTL assignment as a means of identifying individualized conceptions that students hold. Both the draft responses and peer review comments can provide information about how students think about the chemistry content and can inform future instructional decisions. Feedback falling into the categories of reviewer errors or reviewers identifying incorrect content are both useful sources of information for instructors on student difficulties with the chemistry content. Incorporating this assignment towards the end of the semester would allow instructors to identify if there are lingering issues that they need to address in students’ understanding of and ability to connect the concepts within thermodynamics and kinetics. Our analysis also provides additional evidence about the utility of a concept-focused peer review process as an instructional tool to support students in building content knowledge through social interactions. For instructors interested in utilizing this or similar assignments in their course, the peer review process provides students with a source of feedback that can mitigate the instructor workload generally associated with incorporating writing.
While more research is needed, the WTL assignment design described herein shows potential for helping students make connections between chemistry topics. Further research into the use of discipline-specific language is warranted, where WTL assignments have potential to be a way to explore how students code-switch between disciplinary and colloquial meanings of words and what role this plays in their understanding of chemistry content. Additional research on the peer review and revision process, as well as on the features of the writing prompt, could inform the development of more effective WTL assignments. Specifically, future research could focus on how students negotiate multiple perspectives when the chemistry content is presented in different ways during peer review and how different comments guide the revision process. Considered as a whole, the results presented herein indicate that WTL shows promise as an instructional practice that can be used to guide student learning about mathematical and complexly related concepts within chemistry.
Conflicts of interest
There are no conflicts to declare.Appendices
Appendix 1: thermodynamics and kinetics WTL assignment components
Thermodynamics and kinetics WTL assignment
You contribute science articles to the popular science and technology magazine, Wired. After reading the recent article published in Chemical & Engineering News (Available through the American Chemical Society—see link below) about the “octobot”, you decide that it would be a great public interest story to write an article about. Your editor agrees, but is worried that people will think the “octobot” is a perpetual motion machine since they see no obvious power source. To prevent this misconception, your editor wants you to focus the article on the thermodynamics and kinetics of the hydrogen peroxide decomposition that powers the robot. In your article, explain how the decomposition reaction runs the robot, discussing how the changes in energy and entropy follow the laws of thermodynamics. Use the data below to justify the fuel (hydrogen peroxide) and catalyst (platinum) that were chosen in designing this robot (Fig. 1).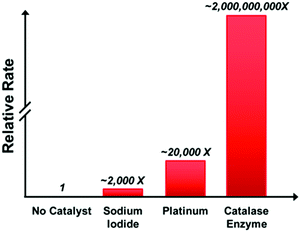 | ||
| Fig. 1 Relative H2O2 decomposition rates (arbitrary units) by different catalysts (adapted from Cybulskis, et al., 2016). | ||
For the decomposition of H2O2:
ΔH = −196.1 kJ mol−1
ΔS = 125.76 J mol−1
E a = 75 kJ mol−1 (without Pt catalyst)
E a = 49 kJ mol−1 (with Pt catalyst)
Items to keep in mind
• Your goal with this article is to explain the thermodynamics behind the octobot• The audience for this magazine has varied scientific backgrounds, but all possess interest in scientific developments
• External references are not required, but if they are used they should be cited using MLA format
• Since you are writing an article in a magazine available online and in print, you should take care to edit and proofread your article
• Your article should be a minimum of 350 words
References
• http://cen.acs.org/articles/94/web/2016/08/Octopus-look-alike-first-robot.html?utm_Source=Newsletter&utm_medium=Newsletter&utm_campaign=CEN• http://chemed.chem.purdue.edu/demos/main_pages/22.13.html
• Using a Hands-On Hydrogen Peroxide Decomposition Activity to Teach Catalysis Concepts to K-12 Students, J. Chem. Educ., 2016, 93(8), pp. 1406–1410. DOI: http://10.1021/acs.jchemed.5b00946
Student peer review rubric
• Read the brief more slowly keeping the rubric in mind.
• Highlight the pieces of texts that let you directly address the rubric prompts in your online responses.
• In your online responses, focus on larger issues (higher order concerns) of content and argument rather than lower order concerns like grammar and spelling.
• Be very specific in your responses, referring to your peer's actual language, mentioning terms and concepts that are either present or missing, and following the directions in the rubric.
• Use respectful language whether you are suggesting improvements to or praising your peer.
• There should be an explanation of how the hydrogen peroxide decomposition serves to power the robot. Which aspects are explained clearly? Which aspects were missing or unclear?
• There should be a justification for the use of the catalyst and the role it plays in the reaction kinetics. What is explained thoroughly? What needs to be covered in more detail?
• This article should include a discussion of how the robot follows the laws of thermodynamics. What is described well? Which parts are difficult to understand?
Revision prompt and guidelines
• Re-read the rubric and consider what a complete and effective response would include, noting what you do not fully address.
• Make a list of effective content you noticed in the writing of your peers.
• Read and summarize the feedback you received from your peers.
• With these things in mind, re-read your draft and mark places where you can improve the content.
• Revise and submit your response.
• There should be an explanation of how the hydrogen peroxide decomposition serves to power the robot.
• There should be a justification for the use of the catalyst and the role it plays in the reaction kinetics.
• This article should include a discussion of how the robot follows the laws of thermodynamics.
Appendix 2: student draft scoring rubric
| Criteria overview | Criteria rubric points | Number of drafts with each concept | |
|---|---|---|---|
| Initial | Final | ||
| Each criterion rubric point was scored as 0 (missing or incorrect) or 1 (present and correct) for each concept, for a total of 0–4 points per criteria and 0–12 points per full draft. N = 35. | |||
| Criteria 1: thermodynamics | |||
| This draft should include a discussion of the enthalpy and entropy change associated with hydrogen peroxide decomposition and why hydrogen peroxide was chosen as fuel. | Connects spontaneity or favorability to Gibbs free energy and discusses the sign of Gibbs free energy relative to enthalpy and entropy (includes signs of enthalpy and entropy, sign must be correct). | 19 | 28 |
| Includes and correctly defines the first law of thermodynamics and connects to octobot reaction/movement (does not need to mention the system). | 9 | 23 | |
| Includes/correctly defines 2nd law of thermodynamics, specify system being universe or isolated system and change is greater than or equal to zero. | 7 | 17 | |
| Discusses what enthalpy of the reaction (exothermic or heat or work) and entropy of the reaction (measure of disorder/microstates) indicate about the reaction. | 8 | 18 | |
| Criteria 2: kinetics | |||
| There should be an explanation for the use of the catalyst in the hydrogen peroxide decomposition, explaining the role it plays in the reaction kinetics, and how this impacts the movement of the robot. | Discusses how the catalyst lowers the activation energy. | 29 | 34 |
| Discusses how the activation energy relates to the rate of reaction. | 17 | 25 | |
| Includes a qualitative discussion of why a specific catalyst was chosen (comparison of reaction rates or availability/suitability). | 15 | 27 | |
| Uses relevant numerical data (including relative rates) to support why the platinum catalyst was chosen. | 25 | 31 | |
| Criteria 3: connection | |||
| The draft should include a discussion of how thermodynamics and kinetics are related and how decomposition reaction results in movement of the robot. | Relates energetics and catalyst, i.e. Gibbs free energy does not change when a catalyst is added to the system but activation energy and rate do. | 3 | 5 |
| Explicitly states that the reaction is governed by both thermodynamics and kinetics. | 19 | 19 | |
| States that a reaction can be spontaneous/thermodynamically favourable but too slow to be useful OR that a reaction occurs but is inefficient. | 23 | 30 | |
| Explains how the decomposition reaction translates to movement of the robot (reaction forms gases which increase pressure/expansion and creates movement; can use pneumatic for pressure change). | 14 | 26 | |
Appendix 3: peer review comment coding scheme
| Code | Definition | Exemplar |
|---|---|---|
| Entropy and enthalpy | ||
| Enthalpy and entropy | Reviewer suggests incorporating or expanding the concepts of enthalpy, entropy, or both. | Gibbs free energy was explained well for the reasoning that the reaction was spontaneous but enthalpy and entropy are just mentioned as part of the Gibbs free energy equation without an explanation as to what those two concepts are. |
| Gibbs free energy | Reviewer suggests the student add in a discussion of Gibbs free energy, connect it to enthalpy and entropy, or go into what the value means in this context. | Yes, it was explained well. I would say for some readers who may not know, it would be good to tell more about Gibbs free energy, how you know its negative, etc. |
| Spontaneity/favourability | Reviewer suggests incorporating a discussion of spontaneity or connecting it to the entropy, enthalpy, and/or Gibbs free energy of the reaction. | The article mentions the change in enthalpy and change in entropy and how that affects the change in Gibbs energy is explained well. However, the student could benefit in explaining why these values are positive and negative and what that means for the spontaneity of the reaction. |
| Justification for fuel | Reviewer suggests providing more justification of hydrogen peroxide as the fuel source, why the specific reaction was chosen, or adding general information about the reaction (e.g., chemical equation, reactants and products). | The student has incorporated a nice discussion of enthalpy, entropy, and gibbs free energy, and how this relates to the power supply of the robot. What is missing is a justification as to why hydrogen peroxide was chosen as the fuel source over, say, another reagent that results in a spontaneous reaction. This component could be discussed right after the author states, “The answer lies in the simple chemical hydrogen peroxide, H2O2” |
| Decomposition reaction | ||
| Physical causes of motion | Reviewer suggests adding more to the description of how pneumatics, pressure, gas formation, or volume change creates motion. | The article mentioned creation of moles of gas leading to a buildup in pressure that serves to power the arms of the robot but failed to go into deeper detail than that. An explanation of what exactly pressure is and how that would move the arm would improve the article. Beyond that the explanation was clear and concise. |
| Energy causing motion | Reviewer suggests adding more to the description of how energy, Gibbs free energy, or work creates motion; or an explanation that it is not a perpetual motion machine because energy causes motion. | Although they discuss the energy transferred from the first law of thermodynamic is used to power the octobot, they didn't discuss how the chemical energy converts to mechanical energy to allow it to function. |
| Reaction causing motion | Reviewer suggests adding more to the description of how the reaction, or its products, creates motion. | In this aspect, your paper was a little vague. You talk about how the reaction is spontaneous, but what about the reaction causes the octobot to move? Think about the products of the decomposition of hydrogen peroxide and the how the octobot utilizes these products to function. |
| Energy to power | Reviewer suggests the student relate the energy of the reaction to the ability to power the robot. | I thought that the explanation of how the reaction actually powers the robot was well done (paragraph one and three). I do think that a connection to the negative Gibbs' energy would make this explanation even stronger, connecting the engineering to the chemistry we have learned. From where exactly is the energy to power the robot coming? |
| Physical (volume/gas expansion) to power | Reviewer suggests the student explain how the change in volume between gas and liquid creates movement in the robot. | The explanation for how the reaction causes the robot to move is there but it is a little unclear that the change in pressure due to the increase in gas mentioned is part of the explanation of how the reaction powers the octobot. Other than that the explanation is clear and thorough. |
| Reaction to power | Reviewer suggests adding more information about how the decomposition reaction, or its products, powers the robot. | The exact way that the hydrogen peroxide decomposition serves to power the robot was missing from the discussion of the octobot, it was never talked about how the reaction actually forms gas which is routed to different areas to power the robot. |
| Reaction | Reviewer suggests that the student incorporate more about the reaction itself or include that the reaction is exothermic. | There is an explanation; however, the description of the decomposition reaction is wrong. A free oxygen atom is not formed; that is not a stable form of oxygen. The balanced equation is 2H2O2→ 2H2O + O2. It is these two gases that ultimately power the robot. |
| Catalyst | ||
| Catalyst to kinetics | Reviewer suggests that the student discuss how the catalyst impacts reaction kinetics. | I think this is decently well covered, but it is lacking that the platinum doesn't actually partake in the reaction; this part makes it seem like platinum is a reactant rather than a catalyst. Also, explanation of what a catalyst can do to a reaction in general might be helpful. |
| Decomposition rate | Reviewer suggests adding more to the discussion of the rate of the reaction (with or without the catalyst). | You mentioned the purpose of the catalyst clearly, but I think it might help just a bit to mention how fast/slow the reaction would proceed without any catalyst involved. |
| Compare catalysts and justify platinum catalyst | Reviewer suggests the student compare the platinum catalyst to the other possible catalysts presented in the prompt (e.g., sodium iodide, catalase) and give specifics as to why platinum was chosen (e.g., durability, conditions, rate increase). | The working of a catalyst in decreasing the activation energy of a reaction in order to increase reaction rate is discussed thoroughly. You should discuss why the platinum catalyst was chosen over other possible catalysts such as sodium iodide or catalase in this discussion. By doing so, you will be able to compare the benefits of platinum and explain why platinum was chosen for this design. |
| Activation energy | Reviewer suggests including a discussion of activation energy in relation to catalyzed and/or uncatalyzed reactions. | There is definitely a great justification for the use of the catalyst and the role it plays in reaction kinetics, but it is lacking in depth. The discussion should compare the use of a the platinum catalyst to the enzymatic catalyst and there should be a clear cut explanation of why this platinum catalyst was chosen. Also, the rate of reaction and the activation energies should be explained further concerning how exactly the catalyst works and also what exactly it means to lower the activation energies as the audience of the article may not know explicitly how this could affect the reaction kinetics. |
| Laws of thermodynamics | ||
| Laws | Reviewer suggests adding more to the discussion of the laws of thermodynamics, using general terms. | No, this part was lacking, no specific laws were mentioned or obviously pointed out. |
| 1st law | Reviewer suggests adding the definition or discussion of the first law of thermodynamics. | While ideas of H, S, and G were used, there was not a specific use of the first, second, or third laws of thermodynamics. You could probably use the first law to help bolster the discussion of Gibbs' energy, specifically the energy lost by the reaction must be used to power the robot through conservation of energy. |
| 2nd law | Reviewer suggests adding a definition or discussion of the second law of thermodynamics. | You give a great definition of the first law of thermodynamics in that you explain how “energy is always conserved”. You also explain how the Octobot's reaction does not violate this law. However, you can also discuss the second law's concept of increasing entropy in the universe. |
| 3rd law | Reviewer suggests adding a definition or discussion of the third law of thermodynamics. | You only mentioned the first and second law, but try to include the other laws in your discussion. |
| Apply laws | Reviewer suggests adding more to the discussion of how the laws of thermodynamics are applied to the octobot system, using general terms. | You don't say much about the specific laws of thermodynamics. I think you should include how the laws help power the robot, especially the first and second laws. |
| Apply 1st law | Reviewer suggests adding a definition or discussion of how the first law of thermodynamics applies to the octobot system. | It is touched briefly that the second law is followed, however, the paper could benefit from also incorporating the first law and its implications to the octobot. With the prompt talking about the 'perpetual motion machine' problem, it would be able to counter that and better address the prompt. |
| Apply 2nd law | Reviewer suggests adding a definition or discussion of how the second law of thermodynamics applies to the octobot system. | You do mention that the reaction follows the second law of thermodynamics, but you state it very briefly. I think you could add a few more sentences going into greater detail as to how/why it follows the second law. Other than that, I did not find any parts difficult to understand. |
| Apply thermodynamics to the octobot | Reviewer suggests adding more to the description of thermodynamics and/or how they apply to the octobot (not using 'laws' terminology). | The article includes a discussion about thermodynamics and the spontaneity of hydrogen peroxide decomposition. However, it seems to be lacking a connection between the chemical thermodynamics and the physical movement of the robot. |
| Entropy increasing | Reviewer suggests adding more to the discussion of entropy of the universe increasing (not using second law terminology). | The writer mentions both the first and second laws of thermodynamics but could expand on how the decomposition reaction contributes to the increase in entropy. The explanation about the first law of thermodynamics is very clear and thorough. |
| Universal | ||
| Grammar/stylistic | Reviewer comments on spelling, punctuation, units, or structural issues that do not improve the content. | You did talk about the how enthalpy and entropy change associated with hydrogen peroxide decomposition; however, your idea is lost within the paragraph. If you split up the paragraphs, your ideas will be communicated more effectively. |
| Student incorrect | Reviewer identifies and tries to correct incorrect content in their peer's writing. | There is an explanation; however, the description of the decomposition reaction is wrong. A free oxygen atom is not formed; that is not a stable form of oxygen. The balanced equation is 2H2O2→ 2H2O + O2. It is these two gases that ultimately power the robot. |
| Reviewer incorrect | Reviewer incorrectly tries to correct content in their peer's writing. | I am not sure if I read that the octobot needs the water to operate. I do know that the octobot needs oxygen, but not sure about the water. However, this student did explain that the decomposition powers the robot and that work is being done. |
| Quantitative | Reviewer suggests adding equations or values to a students’ conceptual discussion. | Explained how the decomposition results in an exothermic and exergonic reaction. It was easy to understand, maybe include the values for change in entropy and enthalpy. |
| Linking values to meaning | Reviewer suggests connecting the values provided in the draft to what they mean chemically (e.g., meaning behind signs). | A discussion of enthalpy and entropy were indeed included: “This coupled with the negative? H (−196.1 kJ mol−1) and positive? S (125.76 J mol−1) values the decomposition reaction already has means that the reaction is highly favorable and always spontaneous.” Good! But, I was left with a few questions… What do these values mean? Why is the enthalpy change negative, and the entropy change positive? Is this why this specific reaction was chosen? The ideas are present, but they need to be made more clear. |
| Sufficient | Reviewer does not provide any actionable feedback. | The piece went through each law of thermodynamics and pointed out how the robot obeyed each law. This part was well described and clear. |
| More detail | Reviewer asks for more explanation for the rubric point, using general terms. | The decomposition was explained fairly well but it could've been more specific. |
Appendix 4: interviewee writing scores
| Pete | Jenny | Trinity | Leo | |||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| Thermodynamics | 2 | 3 | 1 | 4 | 1 | 2 | 1 | 3 |
| Kinetics | 4 | 4 | 4 | 4 | 2 | 3 | 2 | 4 |
| Connection | 3 | 3 | 1 | 1 | 2 | 2 | 2 | 3 |
| Total | 9 | 10 | 6 | 9 | 5 | 7 | 5 | 10 |
Acknowledgements
The authors would like to acknowledge the National Science Foundation (DUE 1524967) for funding and the University of Michigan Third Century Initiative for funding, and the students who agreed to participate in this study.References
- Abell T. N. and Bretz S. L., (2019), Macroscopic observations of dissolving, insolubility, and precipitation: General chemistry and physical chemistry students’ ideas about entropy changes and spontaneity, J. Chem. Educ., 96, 469–478.
- Anderson P., Anson C. M., Gonyea R. M. and Paine C., (2015), The contributions of writing to learning and development: Results from a large-scale multi-institutional study, Res. Teach. Engl., 50, 199–235.
- Bain K. and Towns M. H., (2016), A review of research on the teaching and learning of chemical kinetics, Chem. Educ. Res. Pract., 17, 246–262.
- Bain K. and Towns M. H., (2018), Investigation of undergraduate and graduate chemistry students’ understanding of thermodynamic driving forces in chemical reactions and dissolution, J. Chem. Educ., 95, 512–520.
- Bain K., Moon A., Mack M. R. and Towns M. H., (2014), A review of research on the teaching and learning of thermodynamics at the university level, Chem. Educ. Res. Pract., 15, 320–335.
- Bain K., Rodriguez J.-M. G. and Towns M. H., (2018), Zero-order chemical kinetics as a context to investigate student understanding of catalysts and half-life, J. Chem. Educ., 95, 716–725.
- Bangert-Drowns R. L., Hurley M. M. and Wilkinson B., (2004), The effects of school-based writing-to-learn interventions on academic achievement: A meta-analysis, Rev. Educ. Res., 74, 29–58.
- Becker N. and Towns M., (2012), Students' understanding of mathematical expressions in physical chemistry contexts: An analysis using Sherin's symbolic forms, Chem. Educ. Res. Pract., 13, 209–220.
- Bennett J. M. and Sözbilir M., (2007), A study of Turkish Chemistry Undergraduates' understanding of entropy, J. Chem. Educ., 84, 1204.
- Bereiter C. and Scardamlia M., (1987), The psychology of written composition, Mahwah, NJ: Lawrence Erlbaum Associates, Inc.
- Bodner G. M., (1986), Constructivism: A theory of knowledge, J. Chem. Educ., 63, 873.
- Carson E. M. and Watson J. R., (2002), Undergraduate students' understandings of entropy and Gibbs free energy, Univ. Chem. Educ., 6, 46–51.
- Cohen J., (1987), Statistical power analysis for the behavioral sciences, Hillsdale, New Jersey: Lawrence Erlbaum Associates, pp. 24–27.
- Cohen L., Manion L. and Morrison K., (2011), Chapter 30 – Coding and content analysis, Routledge, pp. 559–573.
- Corbin J. M. and Strauss A., (1990), Grounded theory research: Procedures, canons, and evaluative criteria, Qual. Sociol., 13, 3–21.
- Cox C. T., Poehlmann J. S., Ortega C. and Lopez J. C., (2018), Using writing assignments as an intervention to strengthen acid–base skills, J. Chem. Educ., 95, 1276–1283.
- Cybulskis V. J., Ribeiro F. H. and Gounder R., (2016), Using a hands-on hydrogen peroxide decomposition activity to teach catalysis concepts to K-12 students, J. Chem. Educ., 93, 1406–1410.
- Emig J., (1977), Writing as a Mode of Learning, College Composition and Communication, 28, 122–128.
- Everts S., (2016), This ‘octobot’ is self-powered, untethered, and entirely soft, 94, 12–13.
- Ferguson R. L., (2007), in Bodner G. M. and Orgill M. (ed.), Theoretical frameworks for research in chemistry/science education, Upper Saddle River, NJ: Pearson Prentice Hall, ch. 2, pp. 28–49.
- Finkenstaedt-Quinn S. A., Halim A. S., Chambers T. G., Moon A., Goldman R. S., Gere A. R. and Shultz G. V., (2017), Investigation of the influence of a writing-to-learn assignment on student understanding of polymer properties, J. Chem. Educ., 94, 1610–1617.
- Finkenstaedt-Quinn S. A., Snyder-White E. P., Connor M. C., Gere A. R. and Shultz G. V., (2019), Characterizing peer review comments and revision from a writing-to-learn assignment focused on Lewis structures, J. Chem. Educ., 96, 227–237.
- Gere A. R., Limlamai N., Wilson E., MacDougall Saylor K. and Pugh R., (2019), Writing and conceptual learning in science: an analysis of assignments, Writ. Commun., 36, 99–135.
- Goedhart M. J. and Kaper W., (2003), in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical education: Towards research-based practice, Dordrecht: Springer Netherlands, pp. 339–362.
- Hadfield L. C. and Wieman C. E., (2010), Student interpretations of equations related to the first law of thermodynamics, J. Chem. Educ., 87, 750–755.
- Halim A. S., Finkenstaedt-Quinn S. A., Olsen L. J., Gere A. R. and Shultz G. V., (2018), Identifying and remediating student misconceptions in introductory biology via writing-to-learn assignments and peer review, CBE – Life Sci., 17, ar28.
- Justi R., (2003), in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical education: Towards research-based practice, Dordrecht: Springer Netherlands, pp. 293–315.
- Klein P. D., (2015), Mediators and moderators in individual and collaborative writing to learn, J. Writ. Res., 7, 201–214.
- Klein P. D. and Boscolo P., (2016), Trends in research on writing as a learning activity, J. Writ. Res., 7, 311–350.
- Klein P. D. and Leacock T. L., (2012), in Berninger V. W. (ed.), Past, present, and future contributions of cognitive writing research to cognitive psychology, New York: Psychology Press, ch. 6, pp. 133–152.
- Klein P. D., Arcon N. and Baker S., (2015), in MacArthur C. A., Graham S. and Fitzgerald J. (ed.), Handbook of writing research, 2nd edn, New York: The Guilford Press, ch. 16.
- Logan K. and Mountain L., (2018), Writing instruction in chemistry classes: Developing prompts and rubrics, J. Chem. Educ., 95, 1692–1700.
- McDermott M. A. and Hand B., (2013), The impact of embedding multiple modes of representation within writing tasks on high school students’ chemistry understanding, Instruct. Sci., 41, 217–246.
- Moon A., Zotos E., Finkenstaedt-Quinn S., Gere A. R. and Shultz G., (2018), Investigation of the role of writing-to-learn in promoting student understanding of light–matter interactions, Chem. Educ. Res. Pract., 19, 807–818.
- Nardi B. A., (1996), Studying context: A comparison of activity theory, situated action models, and distributed cognition, Cambridge, MA: MIT Press.
- Nilsson T. and Niedderer H., (2014), Undergraduate students' conceptions of enthalpy, enthalpy change and related concepts, Chem. Educ. Res. Pract., 15, 336–353.
- NVivo 12, (2018), NVivo qualitative data analysis software.
- Poock J. R., Burke K. A., Greenbowe T. J. and Hand B. M., (2007), Using the science writing heuristic in the general chemistry laboratory to improve students' academic performance, J. Chem. Educ., 84, 1371.
- Reynolds J. A., Thaiss C., Katkin W. and Thompson R. J., (2012), Writing-to-learn in undergraduate science education: A community-based, conceptually driven approach, CBE life Sci. Educ., 11, 17–25.
- Rivard L. P., (1994), A review of writing to learn in science: Implications for practice and research, J. Res. Sci. Teach., 31, 969–983.
- Rootman-le Grange I. and Retief L., (2018), Action research: Integrating chemistry and scientific communication to foster cumulative knowledge building and scientific communication skills, J. Chem. Educ., 95, 1284–1290.
- Russell A. A., (2013), in Trajectories of chemistry education innovation and reform, Washington, DC: American Chemical Society, vol. 1145, ch. 9, pp. 129–143.
- Saul E., (2004), Crossing Borders in Literacy and Science Instruction: Perspectives on Theory and Practice.
- Schmidt-McCormack J. A., Judge J. A., Spahr K., Yang E., Pugh R., Karlin A., Sattar A., Thompson B. C., Gere A. R. and Shultz G. V., (2019), Analysis of the role of a writing-to-learn assignment in student understanding of organic acid–base concepts, Chem. Educ. Res. Pract., 20, 383–398.
- Shultz G. V. and Gere A. R., (2015), Writing-to-learn the nature of science in the context of the Lewis dot structure model, J. Chem. Educ., 92, 1325–1329.
- Song Y. and Carheden S., (2014), Dual meaning vocabulary (DMV) words in learning chemistry, Chem. Educ. Res. Pract., 15, 128–141.
- Sözbilir M., (2004), What makes physical chemistry difficult? Perceptions of Turkish chemistry undergraduates and lecturers, J. Chem. Educ., 81, 573.
- Sözbilir M., Pınarbaşı T. and Canpolat N., (2010), Prospective chemistry teachers’ conceptions of chemical thermodynamics and kinetics, Eurasia J. Math., Sci Technol. Ed., 6, 111–120.
- StatCorp, (2017), Stata Statistical Software: Release 15.
- Thomas P. L. and Schwenz R. W., (1998), College physical chemistry students' conceptions of equilibrium and fundamental thermodynamics, J. Res. Sci. Teach., 35, 1151–1160.
- Tsaparlis G., (2007), in Advances in teaching physical chemistry, American Chemical Society, vol. 973, ch. 7, pp. 75–112.
- van Roon P. H., van Sprang H. F. and Verdonk A. H., (1994), ‘Work’ and ‘Heat’: on a road towards thermodynamics, Int. J. Sci. Educ., 16, 131–144.
- Vygotsky L. S., (1978), Mind in society, Cambridge, MA: MIT Press.
- Wehner M., Truby R. L., Fitzgerald D. J., Mosadegh B., Whitesides G. M., Lewis J. A. and Wood R. J., (2016), An integrated design and fabrication strategy for entirely soft, autonomous robots, Nature, 536, 451.
- Wellington J. and Osborne J., (2001), Language and Literacy in Science Education.
- Wolfson A. J., Rowland S. L., Lawrie G. A. and Wright A. H., (2014), Student conceptions about energy transformations: progression from general chemistry to biochemistry, Chem. Educ. Res. Pract., 15, 168–183.
| This journal is © The Royal Society of Chemistry 2020 |
