Transitions between representational levels: characterization of organic chemistry students’ mechanistic features when reasoning about laboratory work-up procedures
Liz
Keiner
 and
Nicole
Graulich
and
Nicole
Graulich
 *
*
Justus-Liebig-University Giessen, Institute of Chemistry Education, Heinrich-Buff-Ring 17, 35392 Giessen, Germany. E-mail: Nicole.Graulich@didaktik.chemie.uni-giessen.de
First published on 20th December 2019
Abstract
Chemists refer to chemical phenomena on different representational levels—macroscopic, symbolic, and submicroscopic—which are directly related and connected to each other. Especially in the laboratory, students have to reason about various mechanistic features at the submicroscopic level and connect them in a meaningful way to make sense of the observable. There is plenty of evidence in chemistry education that students have difficulty connecting the different representational levels when thinking about chemical phenomena. However, current literature provides limited information about the mechanistic features that students activate when reasoning about phenomena and how they transition between the representational levels when in an organic chemistry laboratory. In this study, we performed in-depth analysis of how organic chemistry student teachers (N = 9) explained typical work-up procedures and characterized their activated mechanistic features and transitions between the different representational levels. Our analysis revealed that the students do not activate all features of a mechanism in the same way and construct various explanatory approaches. The findings emphasize the need to explicitly communicate how to connect the macroscopic and submicroscopic levels in a meaningful way in the laboratory. The implications of these findings for research, teaching, and learning to foster meaningful activation of mechanistic features are discussed.
Introduction
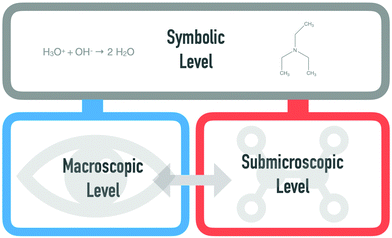
Understanding chemistry often relies on making sense of the invisible and the untouchable (Kozma and Russell, 1997). The first step to approach chemical phenomena is to observe and describe chemical and physical changes, such as colour changes or aggregate states. Besides observable changes, many aspects of the phenomenon are hidden and invisible. This is why chemists go beyond the observable level and use models of the particle level to explain the interaction of entities.Rationalizing chemical phenomena at different levels, namely, the macroscopic, observable level, the submicroscopic, particle level, and the symbolic level, has been one of the most powerful and productive ideas in chemistry education for the past 25 years (Gabel et al., 1992; Johnstone, 1993; Gabel, 1999; Gilbert and Treagust, 2009).
However, it is well documented that students experience difficulties in understanding and representing the nature of matter at these levels (Anderson, 1978; Johnstone, 1982; Ben-Zvi et al., 1986; Gilbert and Treagust, 2009). When students have to use these three levels simultaneously, they often struggle to explain chemical phenomena, especially on the submicroscopic level. Research indicates that students often show difficulties in understanding the submicroscopic level because of its abstractness (Ben-Zvi et al., 1986; Griffiths and Preston, 1992; Kozma and Russell, 1997; Russell et al., 1997; Faulconer et al., 2018) and therefore tend to explain phenomena macroscopically (Gabel, 1999). Especially novice learners often rely on surface features and do not notice meaningful connections between the different levels (Anderson, 1978; Johnstone, 1991; Staver and Lumpe, 1993; Gabel, 1999; Onwu and Randall, 2006; Chandrasegaran et al., 2007).
Johnstone (1982) describes the “mental gymnastics of slipping and sliding from one level to another” (p. 379) as a necessary skill in making sense of chemical phenomena and reducing students’ alternative conceptions in learning chemical concepts. To understand chemical phenomena, students have to understand the role of each level and to translate among the macroscopic (observable), submicroscopic (particles), and symbolic (expression) levels (Gabel and Bunce, 1994; Johnstone, 2000).
Another aspect that must be considered while rationalizing mechanisms of phenomena is discussed by Russ et al. (2008b), who first described physics learners’ explicit use of features of a mechanism, which account for the observable phenomenon. These features include the existence of entities (e.g. atoms or molecules) and their properties (e.g. mass, charge), to determine how different entities interact with each other and the types of activities in which they are engaged (Machamer et al., 2000; Russ et al., 2008b). To fully explain chemical phenomena, students need to shift between the different representational levels and the respective features (Gilbert and Treagust, 2009; Talanquer, 2011; Taber, 2013).
A number of previous studies in science education focused on these features of a mechanism (Bolger et al., 2012; van Mil et al., 2013; Southard et al., 2017; Moreira et al., 2018) or students’ ability to connect the different representational levels (Wu et al., 2001; Ardac and Akaygun, 2004; Tasker and Dalton, 2006; Gilbert and Treagust, 2009).
The laboratory is an essential part of chemistry education (Johnstone, 1991) and provides the unique opportunity to connect theoretical concepts from lectures (submicroscopic and symbolic levels) with experienceable and visible observations (macroscopic level) (Anderson, 1978; Strauss, 1996). However, students often try to rigorously follow a particular step in chemical synthesis, without necessarily knowing what is happening in their flask or connecting the observations to the concepts learned in class. Especially the most popular, and often criticized style of laboratory instruction, the expository or traditional style, focuses on repeating the teacher's instructions and reading the directions from a manual (Tamir, 1977; Domin, 1999). The students are often not forced to reconcile results or confronted with challenges to organize or plan their synthesis (Pickering, 1987). The predominant feature of the expository laboratory is its “cookbook” nature, with often no attention to the planning of the experiment or the interpretation of the experimental results (Tamir, 1977). As a matter of fact, students often acquired very limited and superficial knowledge about the process of synthesis of a chemical compound.
Within the last quarter-century, there has been a shift away from the traditional expository or recipe-based experiments toward inquiry-based (George-Williams et al., 2018), discovery-oriented, or problem-based experiments (Domin, 1999; Costantino and Barlocco, 2019). These approaches, changing recipe-driven organic chemistry laboratory activities into an open-ended, research-based approach, were progressively tested in practice and showed a positive outcome on students’ learning (Domin, 1999; Bertram et al., 2014), as well their laboratory techniques in organic chemistry (Costantino and Barlocco, 2019).
Although these approaches showed improvements in students’ learning, the traditional lab is often prevalent at some universities. To what extend this type of “cook-book” laboratory contributes to students’ understanding of the particulate level of matter and if and how students translate between the representational levels when making sense of their practical work is not yet known. To sustainably improve students’ approaches to make sense of chemical phenomena in the laboratory, we need a detailed understanding of how students change between the macroscopic and submicroscopic levels and which features of a mechanism at which representational level are activated by students to approach typical laboratory problems. For this reason, we analysed students’ reasoning about organic work-up procedures in a qualitative interview study and investigated to what extent explanatory approaches can be deduced with regard to (1) the features of a mechanism and (2) the transitions between representational levels.
Theoretical framework
Representational levels in chemistry and their relationship
Since the introduction of the ‘chemistry triplet’ which is also named ‘Johnstone triangle’ three decades ago (Johnstone, 1982), it has become a powerful tool to conceptualize the challenges and affordances of teaching chemistry. The development of expertise in chemistry is associated with the ability to coordinate and understand the three levels of representation: macroscopic, submicroscopic, and symbolic (Gabel, 1999; Gilbert and Treagust, 2009; Taber, 2013). The macroscopic level is related to the observations of a phenomenon that are tangible and visible in our world. This includes colour changes and observing new products, such as gases, being formed and others disappearing (Treagust et al., 2003). The macroscopic level is the most accessible when conducting experiments in the laboratory. The submicroscopic level represents the particulate level and is used to explain macroscopic phenomena in terms of the movement, organization, or collision of particles, such as electrons, molecules, and atoms.To communicate, chemists commonly use the symbolic level, which includes pictorial, algebraic, physical, and computational forms (e.g. chemical equations, graphs, reaction mechanisms, analogies, and model kits) (Kozma and Russell, 1997; Russell et al., 1997; Treagust et al., 2003). The symbolic level is a supporting tool to visualize things that are invisible in other ways (Harrison and Treagust, 1998; Taber, 2001; Taber, 2009). Taber (2002) describes the symbolic level as a mediator between the macroscopic and submicroscopic levels, which cannot sharply be separated from the other representational levels. Both the macroscopic and the submicroscopic levels can be expressed symbolically. This level is, thus, placed above the two other representational levels, although this does not mean that this level is more important than the others (cf., Fig. 1).
Reasoning about chemical processes
Understanding the basis of phenomena by identifying the cause–effect relationship underlying them is the core of scientific reasoning (Machamer et al., 2000). There has been increased interest in the nature of scientific explanations (Hempel and Oppenheim, 1948; Salmon, 1984; Machamer et al., 2000) and students’ ability to generate them (Talanquer, 2010; Braaten and Windschitl, 2011; Rottman and Keil, 2011; Yeo and Gilbert, 2014). Moreover, in recent years, increased interest in characterizing and fostering students’ ability to generate mechanistic explanations in science education and in chemistry education has arisen (Grotzer, 2003; Russ et al., 2008a, 2008b; Becker et al., 2016; Caspari et al., 2017; Southard et al., 2017; Caspari et al., 2018; Talanquer, 2018a). Mechanistic explanations, as defined in the philosophy of science (Machamer et al., 2000), are characterized by a detailed description of the underlying processes that are responsible for the phenomenon. These types of explanation are based on the identification of relevant entities, and their properties and interactions, that is, the activities in which they are engaged, and their organization in the process. The activities and the organization of core entities are seen as causally responsible for the properties and behaviours of the system as a whole (Machamer et al., 2000). Thus, accounting for a phenomenon is inherently causal from this philosophical perspective. In contrast, educational approaches aim at differentiating students’ accounts of mechanisms in causal, or mechanistic reasoning, or a combination of both (Cooper et al., 2012). This distinction into separate parts appears to be useful to capture students’ reasoning, which can be causal, but not necessarily mechanistic.To recognize the incipient stages of students’ mechanistic reasoning, Russ et al. (2008a, 2008b) proposed a framework that is based on the mechanistic features defined by Machamer et al. (2000) and which has been used in physics education at the primary level. Other groups in biology (Bolger et al., 2012; Southard et al., 2017) and chemistry (Moreira et al., 2018) and explicitly in organic chemistry education (Caspari et al., 2018) have drawn on this idea of mechanistic features to guide their analysis of students’ reasoning.
Krist et al. (2018) recently developed a set of epistemic heuristics that characterizes mechanistic reasoning across science domains. They described that, when one unpacks a phenomenon (macroscopic), (1) the level below the phenomenon is taken into account (submicroscopic), by (2) identifying the factors at this lower level, and (3) connecting them to explain how interactions of these factors give rise to the phenomenon (macroscopic). These epistemic heuristics merge reasoning at different representational levels with the idea of identifying mechanistic features. Unpacking these mechanistic features at different levels has also been focused on for chemistry by Talanquer (2018b), who stated that “the organization of features can take place at various levels, and the properties of a system at a given level often emerge from the properties, interactions, activities, and organization of the subfeatures defined at a sublevel” (p. 1906). Although one can describe a mechanism based on its given mechanistic representation at the symbolic level, that is, expressed by the Lewis structure, causally accounting for the underlying interactions of a mechanism lies at the invisible particulate or molecular level (Sevian and Talanquer, 2014; Becker et al., 2016). Thus, transitioning between the levels is a core competency to progress towards causal mechanistic reasoning.
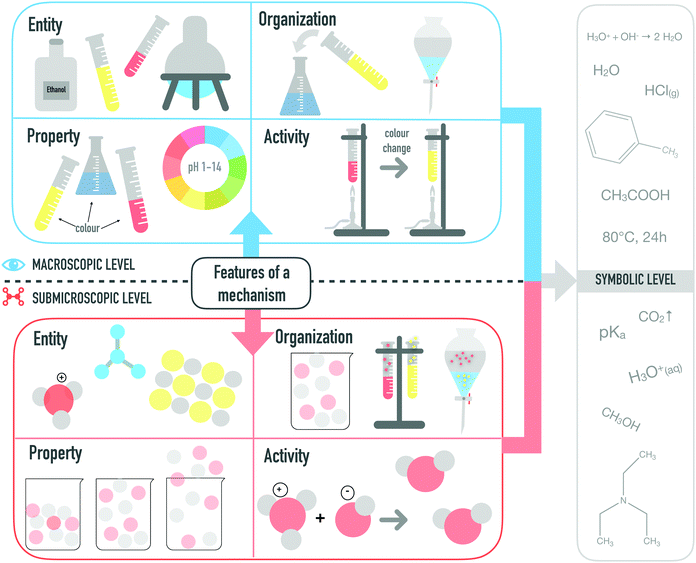
Core mechanistic features at different representational levels
Mechanistic explanations account for the activities and cause/effect relationships that constitute a mechanism by explaining how the core mechanistic features affect each other at the submicroscopic level and give rise to macroscopic observation, a scalar level above. Thus, each mechanistic feature can be conceptualized at the respective representational level (cf.Fig. 2).Entities (E) are the active agents of a system. Entities constitute an essential part of a mechanism and are characterized by their properties (Machamer et al., 2000). An entity on the macroscopic level (EM) is described by the corresponding substance, such as water or toluene. On the submicroscopic level (ES), single particles of an entity are considered, for example, molecules or ions. Symbolically, entities are represented by structural formulas, Lewis structures.
Properties (P) further characterize entities. By knowing them, much can be said about what must have produced them at earlier stages and how they can react in subsequent steps.
They are necessary for the particular mechanism to occur (Russ et al., 2008b). Macroscopic properties (PM) include information about the colour, the visible aggregate state, the observable melting or boiling point, as well as the pH (i.e. indicated by colour of pH paper). On the submicroscopic level (PS), properties include information about the particles that are responsible for features such as the pH, aggregate state, the melting and boiling points, or the density, including intermolecular forces and bonding order. Positive and negative charges, for example, are properties that are represented symbolically in Lewis structures and further characterize the single entity.
Activities (A) describe the dynamic changes in a mechanism (Machamer, et al., 2000). Activities are transformations and interactions of entities, in which their set of properties (e.g. change in bonding or colour) changes. Only a few activities (AM) are actually observable macroscopically when physical and chemical changes are visible (e.g. water boiling: observable physical change from liquid water to gaseous water, colour changes, or precipitations). Submicroscopic activities (AS) are physical or chemical changes in which the changes of properties of submicroscopic entities or particles are described (e.g. CO32− ion can react with H+ to form HCO3− anion).
In a mechanistic step, property changes can be approached statically or dynamically (Goodwin, 2003). For example, a static approach could involve reasoning about the presence of a property, for example, a protonated amine, whereas a dynamic approach would involve reasoning about the formation of the property (activity), for example, formation of the positive charge. Previous literature on students’ reasoning has shown that students often tend to consider property changes statically without thinking about the processes that are occurring (Grove et al., 2012).
Organization (O) refers to the spatial and temporal location of entities during a determined activity and its connection to the properties or behaviour of the system. Macroscopic organization (OM) relates to the spatial and temporal organization of macroscopic entities (e.g. phase separation, formation of gas, mixing substances in a flask). Submicroscopic organization (OS) is the spatial and temporal distribution of particles (e.g. the ions present in the solution, particle collision).
Goals and research questions
To support students in activating features of a mechanism and connecting the representational levels in a meaningful way, it is important to investigate which features students activate when reasoning about chemical phenomena and on which representational level they make sense of given phenomena. Based on the theoretical considerations, the study is guided by the following research questions:(1) Which mechanistic features in terms of entities, properties, activities, and organization on which representational level (macroscopic or submicroscopic) do students activate to make sense of chemical phenomena?
(2) What types of transitions are made by students between the mechanistic features of the representational levels?
(3) How do students connect the mechanistic features and transition between the levels when building explanations for chemical phenomena?
To answer these research questions, we performed a qualitative interview study with chemistry student teachers and prompted them to explain chemical phenomena that typically occur in the organic chemistry laboratory.
Methods
Context and participants
The research study was conducted at a German university in August 2018. Students were recruited on a voluntary basis from the practical course “Organic Chemistry Laboratory”. This laboratory is part of the teacher training program for high school teachers and takes place in the semester break of the sixth semester. The organic chemistry laboratory course covers a period of four weeks. The students attend a lecture from 8 to 10 a.m. and work in the laboratory afterwards (10 a.m. to 6 p.m.). Students who participate in the practical course need to successfully complete the module “Organic Chemistry I” beforehand. This lecture provides basic knowledge of organic chemistry and discusses typical reaction mechanisms (e.g. electrophilic addition reaction, nucleophilic substitution, radical polymerization). In the laboratory course, students deal with general laboratory procedures that are used for the isolation and purification (e.g. washing with sodium carbonate, fractional distillation) of given compounds and their synthesis. The general laboratory techniques were provided in advance in a written online manual and should be read by the students on their own. They are not explicitly discussed and explained by the lecturers. The course aims at the synthesis of six given organic compounds which all include various work-up procedures. The experimental protocols of those have an expository style. There is little flexibility in planning or organizing the synthesis, students are strictly tied to the prescribed procedure. The experiments are well proven and there is little chance of anything going wrong in general. Correspondingly, these types of experiments do not explicitly encourage students to think about the individual work-up procedures and to connect the different representational levels with each other during the lab. The course does not include any post-lab activities, besides providing the mechanism and the obtained yield of the synthesis in a lab protocol.Nine chemistry student teachers agreed to participate in the study. They were between 22 and 28 years old (average: 24 years) and were invited via an announcement in a lecture and email. Among the students were four female and five male students. All students who volunteered for this study were provided with information about their rights and the handling of the data; informed consent was obtained from all participants. Institutional Review Board approval is not required at German universities, but the recruitment process followed ethical guidelines and it was clarified that the students could opt out at any time during data collection. All students provided written informed consent to (1) the use of transcripts of their interviews by the research team, (2) the analysis of their data by the research team, and (3) the use of their data, including their drawings, for publication. For this publication, we used pseudonyms for all of the subjects. The interviews were conducted in German and quotes were translated from German to English for this publication.
Data collection
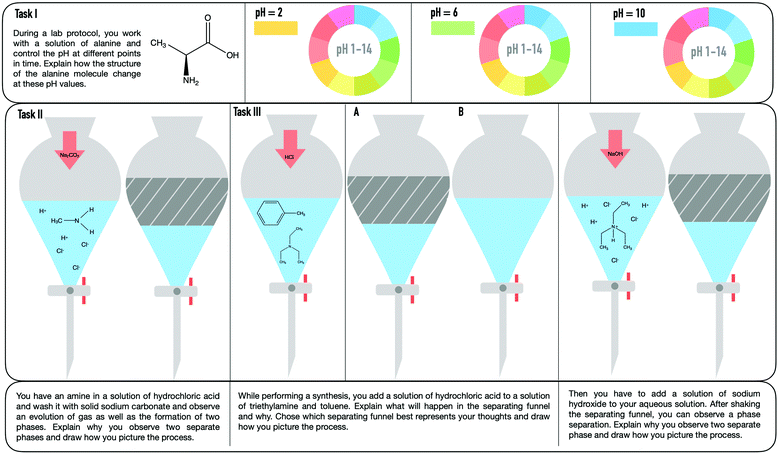
The data collected in this study include transcripts of semi-structured interviews of all students and scans of their worksheets (students’ names were removed). The students were interviewed and asked to think out loud while working on three tasks, which represent typical chemical work-up procedures that occur in an organic chemistry laboratory (cf., Fig. 3). The students were interviewed at the end of their four-week practical course. The interviews lasted between 30 and 45 minutes.Research instrument
Our research instrument consisted of three tasks (cf., Fig. 3) that the students were asked to complete during the interview. Furthermore, the students were asked to create drawings to support their explanations.The tasks involved macroscopic observations of typical work-up procedures in the organic chemistry laboratory. We chose to explicitly prompt students to explain the given macroscopic observations, for incidence, the pH value or phase separation, at the submicroscopic level. The tasks should enable students to transition between the different representational levels and to discuss features on the macroscopic as well as the submicroscopic level. The tasks have different starting points and therefore different possibilities to use and connect the representational levels. Depending on the starting point, it is to be expected that certain features will be mentioned and linked particularly frequently or rarely. For example, in the first task, the students should reason about the structure of alanine at different pH levels. The starting point of the tasks is a macroscopic property (observation of the pH) that should be connected to the entity (symbolic structure of alanine). The other two tasks displayed typical observations of chemical processes that occur during the purification of products. The starting point includes the symbolic representation of entities and the observable macroscopic organization or activity (e.g. phase separation, mixture of substances).
Data analysis
All interviews were transcribed verbatim and analysed using the coding software MAXQDA. During data analysis, the authors regularly discussed the coding scheme to answer the research questions and fulfil the theoretical framework of the study. To answer our research questions, we analysed the data in three distinct steps (cf., Fig. 4).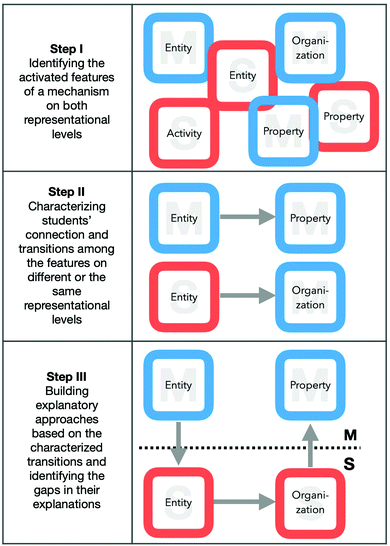 | ||
| Fig. 4 Schematic representation of the analytical steps. The blue squares represent macroscopic features, the red squares submicroscopic features. | ||
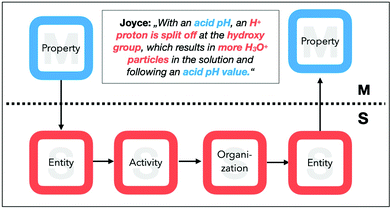
For example, a student explanation may start with the identification of a macroscopic entity (EM: water) and is then connected, for instance, to the description of a property (PS: liquid) or an organization at the submicroscopic level. We further analysed which features are connected on either the macroscopic or the submicroscopic level. For example, Joyce (cf.Fig. 5) temporarily remains on the submicroscopic level in her explanation and concludes from a submicroscopic entity (ES: H+) to a submicroscopic activity (AS: split off) and an organization (OS: more). Afterwards, we combined these connections and transitions into more complex approaches to identify gaps in students’ explanations.
Her explanation starts with reference to a macroscopic property (PM: acid pH), the respective submicroscopic entities (ES: H+), as well the activity (AS: splitting off) and organization (OS: more H3O+) of these entities. In her explanation, she transitions between a macroscopic property (PM: acid pH) and submicroscopic entities (ES: the hydroxy group and the H+). After identifying these submicroscopic entities, she describes an activity, namely, that H+ions are split off to form H3O+particles. She then reasons about the organization of this entity (OS: more in the solution) to infer a statement about the increased acidity of the solution, which is, in our task, a macroscopic property (PM: pH colour).
In all rounds of coding, the first author (PhD candidate with a Master's degree in chemistry education) coded the entire data set. During this analysis, ongoing discussions and reflections with the research group, especially with the second author (professor), were used to ensure that coding decisions faithfully represented the data. Table 1 shows an overview of the final coding rubric for the features of a mechanism at the different representation levels.
| Code | Student examples | Code description |
|---|---|---|
| EntityM | “I still have my amine in hydrochloric acid…and add sodium carbonate” | Entities are described by using the name of the substance or naming a solution of a substance (e.g. amine, hydrochloric acid solution), without reference to the respective molecule or particle. |
| PropertyM | “The picture shows me the pH paper which has a red colour…it is acid” | Properties are described by reference to an observable physical or chemical property (e.g. the colour of a substance, the observable pH value, the aggregate state). |
| ActivityM | “It dissolves in the organic phase and is no longer visible” | Activities are described by referring to an observable physical or chemical change of properties, with no reference to molecules or particles (e.g. an ongoing phase separation). |
| OrganizationM | “…I add sodium carbonate…and have two phases” | Organization of entities is described by referring to the temporal (e.g. stand overnight) and spatial organization (e.g. observation of two separate phases) of macroscopic entities. |
| Code | Student examples | Code description |
|---|---|---|
| EntityS | “… HCl…it means chloride ions” | Entities are described by using the name of the particle or referring to the molecules of a substance (e.g. functional groups, ions, water molecules). |
| PropertyS | “…the amine is deprotonated” | Properties are described by reference to an invisible physical or chemical property (e.g. deprotonated entity, aggregate state). |
| ActivityS | “we have two Na + on one CO 3 2 and the CO 3 − can react with the H + ” | Activities are described by referring to an invisible physical or chemical change of properties, with reference to molecules or particles. |
| OrganizationS | “…in the aqueous phase we have Na + and Cl − ” | Organization of entities is described by referring to the temporal and spatial organization (e.g. more H3O+ ions) of submicroscopic entities. |
Results and discussion
Following our research questions and to facilitate the interpretation and discussion of our results, we divide the following section into three parts. The first part describes students’ activation of the mechanistic features while explaining the three tasks. The second part illustrates students’ connection between the individual features, their transitions made between the representational levels, and the discrepancy between students’ drawings and explanations. The third part discusses students’ explanatory approaches and their identified missing pieces in the chain of reasoning.Which mechanistic features on which representational level do students activate to make sense of chemical phenomena?
In the first part of the analysis, we focused on students’ activated features on each of the two representational levels when reasoning about the given chemical phenomena. All features of a mechanism were activated at least once by a participant during their explanations, but with differing frequencies.Given the fact that naming a property is generally accompanied by a reference to an entity, this leads to a higher number of entities used in students’ explanations. Therefore, we first analysed which features were activated at least once by the students and did not determine the frequency of each activation. Fig. 6 shows an overview of the features activated once by task.
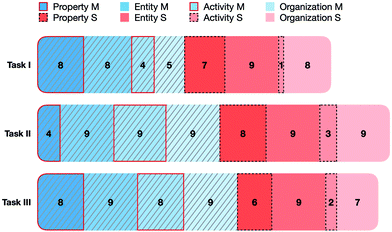 | ||
| Fig. 6 Types of mechanistic features identified in students’ explanations by task and number of students. Blue colours indicate macroscopic and red colours submicroscopic features. | ||
In this figure, nine represents the highest number that can be achieved, which means that this feature was activated by each participant (N = 9) at least once in their explanations. It is not surprising that the feature that was used most at both levels is entities. Almost all participants activated at least one entity in each task, on both the macroscopic and the submicroscopic levels. In this category, students mentioned macroscopic entities, such as water, sodium chloride, acids, or bases, as well as submicroscopic entities like CO2, ions, protons, and functional groups. Furthermore, students often explained the phenomena using the spatial or temporal organization of entities on both levels (e.g. phase separation, evolution of gas, observing colour changes, or the spatial organization of particles), which was usually accompanied by the naming of entities.
Compared with entities and organization, which were used by almost all students, only some students mentioned the properties of entities (illustrated by the black dashed boxes in Fig. 6). Some students specified the properties of the entities on both levels, while other students did not even mention any properties. When referring to the properties of entities, students often stated the property, for instance, a charge, without reference to its formation (e.g. the activity).
This observation relates to the fact that only in a few incidences, especially on the submicroscopic level, were students using activities to explain the phenomena (illustrated by the red boxes in Fig. 6). These results are in accordance with the findings from Moreira et al. (2018), who stated that students’ explanations in their study setting often showed no reference to the activities of entities. They concluded that only at a higher level of sophistication do students’ explanations incorporate activities of one or more features of the system and indicate the change from a static to a more dynamic perspective of the phenomenon. They argue that students who reason or explain at a lower level reason more about static features like entities and properties than about dynamic features such as activities or organization.
In the second part of the analysis, we focused on the connection between the individual features and especially the transitions between the different representational levels. Therefore, we took a closer look at our data and observed different strategies in explaining the chemical phenomena.
What type of transitions is made by students between the mechanistic features of the representational levels?
While observing and explaining chemical phenomena, it is necessary to activate diverse features of a mechanism and to shift between the macroscopic and submicroscopic levels. In students’ explanations, transitions from the macroscopic to the submicroscopic level (79%) were found much more frequently than from the submicroscopic to the macroscopic level (21%).Fig. 7 illustrates all transitions made by the students (indicated in percentages) between two representational levels per task. The most frequent transition from the macroscopic to the submicroscopic level occurred in task II (31%), followed by task I (25%) and task III (23%). In contrast, from the submicroscopic to the macroscopic level, the highest rates of transition appeared in task III (12%), followed by task II (5%) and task I (4%). The nature of the tasks as well the different initial prompts of the tasks might have influenced the frequency of the respective transitions. Depending on what the task required from the students, different transitions were used by them. However, given the number of students in the cohort and the task selection, the numbers should be interpreted within the context of this study. To better characterize these transitions made by the students, we analysed the single transitions from a macroscopic to a submicroscopic feature and vice versa.
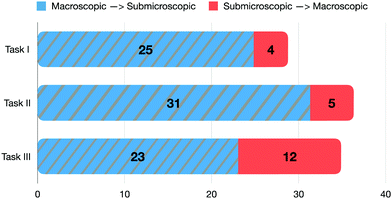 | ||
| Fig. 7 Proportions of the linear transitions relative to all transitions that were made between two representational levels. | ||
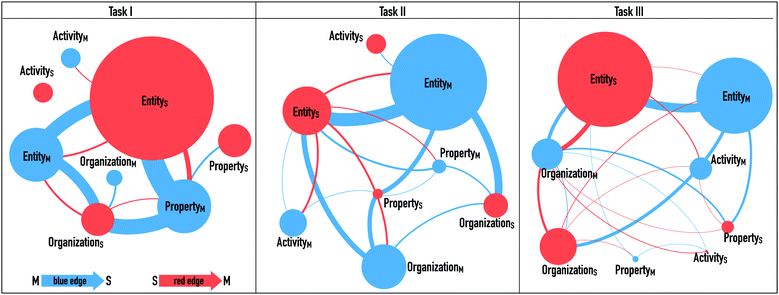
To visualize these transition events, we used the Gephi software (v.0.9.2), which illustrates the connections and transitions students made between the different representational levels in their explanations.
In these network visualizations, nodes (circles) represent the features of a mechanism that we identified during the coding process. The colour of the node represents the respective level of representation (i.e. the blue ones symbolize the macroscopic level and the red ones the submicroscopic level). The size of the circle indicates how often this feature was activated by the students in general. Thus, the larger the circle, the more often the feature was mentioned by the students. The edges (i.e. the lines between two nodes) represent the transitions from one level to another and their thickness indicates how often the transition occurred. A node without edges indicates that this feature was not linked to any other feature in students’ explanations. The colour of the lines between the nodes indicates the respective starting point of the transition; that is, if it is blue, the transition starts from the macroscopic level and ends at the submicroscopic level.
Fig. 8 illustrates the transitions made by the students for each task. Entities were used most frequently and properties and activities less often. The most common transition in general was made from a macroscopic entity to a submicroscopic one (illustrated by the thick nodes). In task I, students also frequently connected a submicroscopic entity or organization to a macroscopic property.
The students rarely connected a macroscopic entity to a submicroscopic activity or property (illustrated by the thin nodes). Especially in task I, none of the students connected a macroscopic feature to a submicroscopic activity or vice versa. In task III, the students connected the activity more frequently with other features, but still very rarely compared with the other transitions. In task I, unsurprisingly, students often related the pH value (macroscopic property) to the presence of H+, OH−, or protons, which were all coded as submicroscopic entities (results in a large node for ES). Some students further used this information to reason about the resulting structure of alanine. Nancy's example shows a successful transition between the macroscopic property and the submicroscopic entity, as she inferred the resulting submicroscopic property of the alanine molecule (Ps: is protonated).
Nancy: “I can observe a blue pH paper…therefore we have a basic pH value which means OH−ions in the solution. The alanine is protonated”.
Other students of the cohort do not incorporate the given alanine structure in their reasoning and rather reason about protons in general. In the following quote, Connie relates the pH value (PM) to the presence of H+ ions (ES).
Connie: “the pH value is…yes…that has something to do with the…eh…with the H+ ions”.
She does not reason about the given alanine and how the molecular structure of alanine changed depending on the given pH, in this case an acidic one.
The following quote from Mike illustrates that students, after relating the macroscopic property to the submicroscopic entity, often struggle to further reason about the correct activities or organization. They sometimes try to mention an activity but name an incorrect one. First, Mike makes a correct deduction from the observable pH value (PM) to the presence of H+ particles (ES). Afterwards, based on his identification of the submicroscopic entities, he erroneously infers a submicroscopic activity (a proton can split off).
Mike: “…if it gets acidic, there should be H+protons in the solution…because of that, a proton can split off and the solution becomes more and more acidic…”.
He explains the pH value by describing an increase in H+ protons in the solution, rather than reasoning about the activity (i.e. protonation) that changes the property (i.e. charge) of the alanine molecule.
Certainly, it is productive to first reflect statically about what types of particles are present at the submicroscopic level, to infer the activities. Interestingly, students often do not further reason about the consequences of the presence of these submicroscopic entities. The transfer to the resulting organization or activity of the entity is often missing. The following quote from task II illustrates another example of a student who struggled to further connect other features to a submicroscopic entity in the given task. Florence interprets the addition of hydrochloric acid at the submicroscopic level (ES: H+and Cl−ions) in the following way:
Florence: “If I add hydrochloric acid…then I add H+ions and Cl−ions and have just an acid…and then I have no idea”.
Florence seems to be aware of the submicroscopic ions, but struggles to make sense of this information and to determine other features of the phenomenon or predict the ongoing reaction behaviour.
Another transition that the students often make, especially in task II, is describing the two observable phases (OM) of the solution and inferring the presence of submicroscopic entities. In the following quote, Nancy locates the entities in the respective phase:
Nancy : “I assume that I have two phases, one organic and one inorganic. In the organic phase is unreacted toluene…while in the aqueous phase I have water, partly H 3 O + and Cl − ”.
She mentions the entities but does not further infer the properties that are responsible for the phase separation. Determining the properties of the entities is a central concept in chemistry for predicting the organization and activity of earlier and subsequent reaction steps.
Activities are among the features that are less used in students’ explanations. Students tend to mention the resulting property of an activity without further verbalizing the activity and the entity that is engaged in this activity. This results in static descriptions of processes.
The following quotes from Mike and Jim (cf.Fig. 9) illustrate two possibilities to describe the consequences (i.e. protonation of the amine) of adding hydrochloric acid to the solution of triethylamine and toluene. The explanation of Mike incorporates an activity (As: attacks the proton) of an entity. Based on this activity, he describes the formation of the submicroscopic property. Jim's explanation is more static. He concludes that the triethylamine molecule is protonated and carries a positive charge (Ps), without mentioning the entity (e.g. protons) and the respective activity that lead to this property. Nevertheless, he is also successful with his explanation (cf.Fig. 9). After explaining the chemical phenomenon, both students draw the protonated amine correctly, even though Jim's explanation does not incorporate its formation. It seems that students achieve similar results (expressed with symbolic representations) even though they activate or omit different mechanistic features in their explanations.
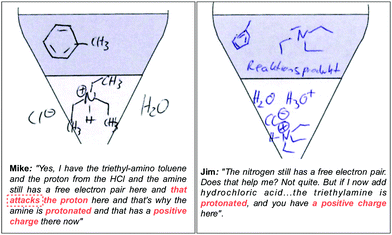 | ||
| Fig. 9 Two students’ answers with the corresponding drawings. The left side represents a more dynamic explanation, the right side a static one. | ||
Discrepancy between students’ drawings and explanations
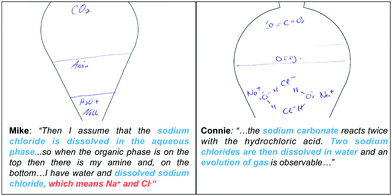
Another interesting point that emerged when characterizing the activation of mechanistic features is the discrepancy between students’ symbolic representations and their verbalization. Students rarely differentiated between submicroscopic particles (e.g. water molecules) and the corresponding substance (e.g. water) using explicit terminology. Some students exemplified what they meant, for example, dissolved sodium chloride, which means Na+and Cl−.Besides students’ difficulties in explicitly naming the particles, their drawing and their verbalization often express different representational levels. Mike interprets the given information in task II (i.e. presence of sodium chloride in water) on the macroscopic level (PM: dissolved in the aqueous phase), but does not further visualize the hydration process on the particle level (cf., Fig. 10 on the left side).
Connie, in contrast, mostly uses descriptions of macroscopic entities, such as sodium carbonate, hydrochloric acid, sodium chlorides, and water, and a macroscopic activity, here the evolution of gas, in her verbal explanation and does not seem to switch to the particle level. However, her symbolic drawing visualizes the process of hydrating the sodium ions at the submicroscopic level (cf., Fig. 10 on the right side). This discrepancy between students’ drawings and their verbalization warrants attention when interpreting students’ use of symbols in their drawings. A chemical formula symbolically represents an entity and the number of atoms of each element in a compound, but is not reserved either for submicroscopic or for macroscopic entities. In some cases, students clearly use symbols to represent a submicroscopic entity (cf. Connie), whereas others use symbols to represent macroscopic entities (cf. Mike).
How do students connect the mechanistic features and transition between the levels when building explanations for chemical phenomena?
After analysing students’ transitions between the levels, we were interested in their overall explanatory approaches, that is, how they relate several features of different representational levels and if there are shortcuts in their reasoning. Therefore, we used their explanations and visualized their chain of reasoning. The following explanatory approach from Nancy highlights how she connects the features to build her answer for task I (Fig. 11). She first uses the amino acid (EM) and further specifies the functional groups (ES) of this entity, for example, the carboxyl and amino groups. Subsequently, she directly infers the property (PS: being deprotonated) of the carbonyl function and connects it to the red colour of the pH paper (PM). Her explanatory approach shows that she takes a shortcut to express the entity and the activity that causes the change in the property. Her shortcut was common for most of the students; when expressing an acid–base reaction, at least in task I, this was often performed by describing the property.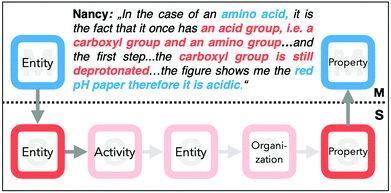 | ||
| Fig. 11 Nancy's static explanatory approach from task I (dark coloured features) and an exemplary, more dynamic approach. | ||
Although it may be common language to express an acid–base reaction using the term deprotonated or protonated, this covers the actual property change, which is the change in charge, and as well the reaction partner. Automatically, the explanation given by Nancy shows a static perspective on the underlying reaction.
The explanation given by Joyce (cf., Fig. 12) is more dynamic, as she focuses on the activities of the process in task I.
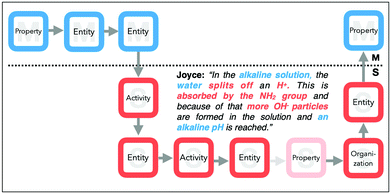 | ||
| Fig. 12 Joyce's explanatory approach (dark coloured features) and her missing piece of the submicroscopic property. | ||
She starts with describing the observable basic pH value (PM) and mentions afterwards a macroscopic entity (water), which generates (AS: can split off) other submicroscopic entities (ES: H+protons). These protons can further be absorbed (AS) by the NH2group (ES). At this point, she directly infers the submicroscopic entities and their organization (OS: more OH−particles), but she does not further close the chain by mentioning the resulting property of the described activity (e.g. the protonated NH2 group). Her focus is on the hydroxide ions (ES) in solution that influence the observable alkaline pH value (PM).
However, because naming an entity and the associated property is a central concept for explaining chemical phenomena, filling in these missing pieces is crucial for instruction.
The next quote from Mike (Fig. 13) illustrates some missing pieces in his explanation. He starts with naming triethylamine (EM) and concludes afterwards directly with the submicroscopic property (PS: is charged), without mentioning the formation of that property. At this point, his missing piece with regard to a dynamic explanation is the activity. Further, because of the charge, Mike infers the dissolving process (AS) in the aqueous phase (OM).
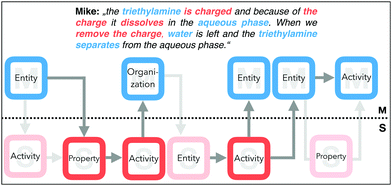 | ||
| Fig. 13 Mike's explanatory approach (represented by the grey lines and the dark coloured features) with the missing pieces. | ||
Afterwards, he talks about removing the charge (AS) and the remaining water, whereby he infers from the triethylamine directly to the separation of two phases (AM). He does not mention the entities or any properties (e.g. deprotonated, the polarity, or the interactions between the particles) that could be responsible for the observation of two phases.
The presented explanatory approaches indicate that students are readily able to keep track of the entities, on both the submicroscopic and macroscopic levels, but connecting them to their respective property and activity is often not verbalized in their explanations. This shows that the causal connection between the mechanistic features is often lacking. Students’ use of descriptive mechanistic instead of causal mechanistic explanations appears to be a common finding not only in the present work, but also in various study settings (Moreira et al., 2018; Caspari and Graulich, 2019; Crandell et al., 2019).
Conclusions and implications
Successfully understanding work-up procedures and chemical phenomena in the laboratory entails the ability to explain them by making use of multiple mechanistic features at the macroscopic and submicroscopic levels. Therefore, the central goal of this study was to identify students’ identified features of a mechanism on the macroscopic and submicroscopic levels and to characterize students’ transitions between these representational levels while explaining observations that can be made during organic synthesis (e.g. change in pH value, evolution of gas, phase separation). Relating observations and interactions of entities on the particle level is crucial to understanding the goal of a synthetic step, as well as interpreting unexpected observations that might occur during synthesis.Our analysis revealed that the students used all mechanistic features on both representational levels to construct a wide range of explanations for the given work-up procedure. However, the frequency and the link between these mechanistic features varied. Students could easily identify entities on both the representational levels but used activities or properties less in their explanations. Analysing the use of these features in their explanatory approaches revealed that the causal link between them was often missing. Students have no problems identifying the entities; however, they struggle to further describe the activity that gives rise to these entities or the properties that are responsible for earlier or subsequent reaction steps. The explanations thus often include a description of the static target phenomenon, for example, stating entities or property, without referring to the dynamic part of the reaction that gives rise to relevant, macroscopic or submicroscopic, properties. Similar to the results of previous research (Grove et al., 2012; Caspari et al., 2018), students’ explanations are thus often static, focusing on the starting material and product and less on the process of the underlying reaction. Submicroscopic changes are often not observable macroscopically in typical organic synthesis and work-up procedures. This lack of observable property changes may reinforce students’ static perspective on the process and indicates that instructors need to focus more on the dynamic aspects of a seemingly static process.
With regard to previous studies that used mechanistic features to analyse students’ scientific explanations (Russ et al., 2008a; Moreira et al., 2018), one can also observe a hierarchy of identifying mechanistic features. Identifying major entities in a system and then inferring their relevant properties, on the macroscopic and submicroscopic levels, are initial steps in the process of explaining and predicting reaction behaviour. Adding activities and organization of entities to reason about the spatial and temporal organization of entities and their interactions follows, but requires higher abstraction of the process and is less used by students (Moreira et al., 2018). As students may not effortlessly and constantly consider each mechanistic feature, supporting students to explicitly identify and connect the features may improve their mechanistic explanations.
Another finding that presented itself throughout the data was the notable discrepancy between students’ verbalization of entities and their actual drawings. This was often due to an inaccurate use of terms when verbally solving the tasks. Students, for example, verbalize an entity on the macroscopic level, for example, talking about chlorine, while their drawing showed the negatively charged chloride ion. This looseness in using the respective terminology of the representational level, mixing substances and particles, is well reported (Barke and Büchter, 2018) and makes it difficult to fully capture students’ understanding. This observation reminds us as educators of the importance of communicating accurately on these levels. Teachers sometimes tend to use shortcut statements in teaching and may thus unconsciously cause misconceptions or foster imprecise descriptions (Barke and Büchter, 2018). In particular, instructors should pay attention to how they verbalize processes and help students become aware of their language use. Future research is needed to shed light on this important factor in the learning process.
The findings of this study are in line with previous studies that showed that students in general have difficulties in connecting observable phenomena on the macroscopic level with the corresponding theoretical concept on the submicroscopic level (Anderson, 1978; Johnstone, 1991; Gabel et al., 1992; Treagust et al., 2003; Gilbert and Treagust, 2009). This study further adds an in-depth analysis of the mechanistic features that students used in their explanations on these representational levels. The findings show that although students’ transition between the representational levels in their explanations, their verbalization of each level is often vague and the causal links between the mechanistic features are missing. This study in this traditional laboratory setting further emphasizes the need to develop learning environments and instructional materials for laboratory education that focus on the transition between the representational levels, and the relationship between observations of phenomena and the submicroscopic level, as already claimed in various previous studies (Harrison and Treagust, 2000; Chittleborough and Treagust, 2008; Chandrasegaran et al., 2011). Instructional approaches should explicitly prompt students to attend to the mechanistic features of a phenomenon and differentiate between the two representational levels to close these gaps in their explanations and help students to understand on which level they are building their explanations and which mechanistic features are missing to close the gaps. Building on this, the difficulties that students, in this traditional laboratory, had in making sense of the given work-up procedures show that an explicit focus on connecting the macroscopic and submicroscopic levels, not only for the main mechanism of the synthesis, but also for the various steps before and after the main reaction, might be helpful for students to understand the goals of each step of chemical synthesis. Problem-based laboratories, in contrast, encourage students to think much more about the work-up procedures of the synthesis and require spontaneous behaviour and action of the students (Bertram et al., 2014; Costantino and Barlocco, 2019).
Limitations
The conclusions drawn here should be considered with caution given some inherent limitations of this work. This was a qualitative study with a small number of volunteer student teachers. Thus, we can only describe how this particular group of students explained the given chemical phenomena. More investigations are needed to evaluate whether our analysis is robust and productive by diverse types of student when asked to construct explanations for a wide range of phenomena in different contexts.Additionally, these students were asked to complete a task in an individual interview setting, which was likely quite different from the environment in the laboratory. On one hand, the research instruments and environment may have prompted some students to pay closer attention to the information provided than they would normally do under typical conditions. On the other hand, our research instrument and approach may have constrained students’ reasoning and as a consequence limited the conclusions of this work.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication represents a feature of the first author's doctoral (Dr rer. nat.) thesis at the Faculty of Biology and Chemistry, Justus-Liebig-University Giessen, Germany. We thank all students who participated in the study. We also thank the Graulich research group for fruitful discussions about the research and especially Prof. Dr. Richard Göttlich for his support.References
- Anderson G. W., (1978), The Playfair Collection and the teaching of chemistry at the University of Edinburgh, BRILL, pp. 1713–1858.
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia-based instruction that emphasizes molecular representations on students' understanding of chemical change, J. Res. Sci. Teach., 41, 317–337.
- Barke H.-D. and Büchter J., (2018), Laboratory jargon of lecturers and misconceptions of students, Afr. J. Chem. Educ., 8, 28–38.
- Becker N., Noyes K. and Cooper M., (2016), Characterizing Students’ Mechanistic Reasoning about London Dispersion Forces, J. Chem. Educ., 93, 1713–1724.
- Ben-Zvi R., Eylon B. and Silberstein J., (1986), Is an Atom of Copper Malleable? J. Chem. Educ., 63, 64.
- Bertram A., Davies E. S., Denton R., Fray M. J., Galloway K. W., George M. W., Reid K. L., Thomas N. R. and Wright R. R., (2014), From cook to chef: facilitating the transition from recipe-driven to open-ended research-based undergraduate chemistry lab activities, New Dir. Teach. Phys. Sci., 10, 26–31.
- Bolger M. S., Kobiela M., Weinberg P. J. and Lehrer R., (2012), Children's mechanistic reasoning, Cognit. Instruct., 30, 170–206.
- Braaten M. and Windschitl M., (2011), Working toward a stronger conceptualization of scientific explanation for science education, Sci. Educ., 95, 639–669.
- Caspari I. and Graulich N., (2019), Scaffolding the structure of organic chemistry students' multivariate comparative mechanistic reasoning, Int. J. Phys. Chem. Educ., 11, 31–43.
- Caspari I., Weinrich M. L., Graulich N. and Sevian H., (2017), This mechanistic step is “productive”: organic chemistry students’ backward-oriented reasoning, Chem. Educ. Res. Pract., 19, 42–59.
- Caspari I., Graulich N. and Kranz D., (2018), Resolving the complexity of organic chemistry students’ reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19, 1117–1141.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2007), The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students’ ability to describe and explain chemical reactions using multiple levels of representation, Chem. Educ. Res. Pract., 8, 293–307.
- Chandrasegaran A., Treagust D. F. and Mocerino M., (2011), Facilitating high school students' use of multiple representations to describe and explain simple chemical reactions, J. Aus. Sci. Teach., 57, 13–20.
- Chittleborough G. and Treagust D., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38, 463–482.
- Cooper M., Cox E. L. and Grove N., (2012), Does Mechanistic Thinking Improve Student Success in Organic Chemistry? J. Chem. Educ., 89, 850–853.
- Costantino L. and Barlocco D., (2019), Teaching and Undergraduate Organic Chemistry Laboratory Course with a Tailored Problem-Based Learning Approach, J. Chem. Educ., 96, 888–894.
- Crandell O. M., Kouyoumdjian H., Underwood S. M. and Cooper M. M., (2019), Reasoning about Reactions in Organic Chemistry: Starting It in General Chemistry, J. Chem. Educ., 96, 213–226.
- Domin D. S., (1999), A review of laboratory instruction styles, J. Chem. Educ., 76, 543–547.
- Faulconer E. K., Griffith J. C., Wood B. L., Acharyya S. and Roberts D. L., (2018), A comparison of online and traditional chemistry lecture and lab, Chem. Educ. Res. Pract., 19, 392–397.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76, 548–554.
- Gabel D., Briner D. and Haines D., (1992), Modelling with magnets: a unified approach to chemistry problem solving, Sci. Teach., 59, 58–63.
- Gabel D. L. and Bunce D. M., (1994), Handbook of research on science teaching and learning, vol. 11, pp. 301–326.
- George-Williams S. R., Soo J. T., Ziebell A. L., Thompson C. D. and Overton T. L., (2018), Inquiry and industry inspired laboratories: the impact on students’ perceptions of skill development and engagements, Chem. Educ. Res. Pract., 19, 583–596.
- Gilbert J. K. and Treagust D. F., (2009), in Gilbert J. K. and Treagust D. F. (ed.), Multiple representations in chemical education, Dordrecht: Springer, vol. 4, pp. 333–350.
- Goodwin W., (2003), Explanation in organic chemistry, Ann. N. Y. Acad. Sci., 988, 141–153.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29, 611–628.
- Grotzer T. A., (2003), Learning to understand the forms of causality implicit in scientifically accepted explanations, Stud. Sci. Educ., 39, 74.
- Grove N. P., Cooper M. M. and Cox E. L., (2012), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Educ., 89, 850–853.
- Harrison A. G. and Treagust D. F., (1998), Modelling in science lessons: are there better ways to learn with models? Sch. Sci. Math., 98, 420–429.
- Harrison A. G. and Treagust D. F., (2000), A typology of school science models, Int. J. Sci. Educ., 22, 1011–1026.
- Hempel C. G. and Oppenheim P., (1948), Studies in the logic of explanation, Philos. Sci., 15, 135–175.
- Johnstone A. H., (1982), Macro- and microchemistry, Sch. Sci. Rev., 64, 377–379.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem., J. Comput. Assis. Learn., 7, 75–83.
- Johnstone A. H., (1993), The Development of Chemistry Teaching: a changing response to changing demand, J. Chem. Educ., 70, 701–705.
- Johnstone A. H., (2000), Teaching of chemistry- logical oder psychological? Chem. Educ. Res. Pract., 1, 9–15.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Krist C., Schwarz C. V. and Reiser B. J., (2018), Identifying Essential Epistemic Heuristics for Guiding Mechanistic Reasoning in Science Learning, J. Learn. Sci., 28, 160–205.
- Machamer P., Darden L. and Craver C. F., (2000), Thinking about mechanisms, Philos. Sci., 67, 1–25.
- Moreira P., Marzabal A. and Talanquer V., (2018), Using a mechanistic framework to characterise chemistry students’ reasoning in written explanations, Chem. Educ. Res. Pract., 20, 120–131.
- Onwu G. O. M. and Randall E., (2006), Some aspects of students’ understanding of a representational model of the particulate nature of matter in chemistry in three different countries, Chem. Educ. Res. Pract., 7, 226–239.
- Pickering M., (1987), What goes on in students' heads in lab? J. Chem. Educ., 64, 521–523.
- Rottman B. M. and Keil F. C., (2011), What matters in scientific explanations: effects of elaboration and content, Int. J. Cognit. Sci., 121, 324–337.
- Russ R. S., Coffey J. E., Hammer D. and Hutchison P., (2008a), Making Classroom Assessment More Accountable to Scientific Reasoning: A Case for Attending to Mechanistic Thinking, Sci. Educ., 93, 875–891.
- Russ R. S., Scherr R. E., Hammer D. and Mikeska J., (2008b), Recognizing Mechanistic Reasoning in Student Scientific Inquiry: A Framework for Discourse Analysis Developed From Philosophy of Science, Sci. Educ., 92, 499–525.
- Russell J. W., Kozma R. B., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts, J. Chem. Educ., 74, 330–334.
- Salmon W. C., (1984), Scientific explanation and the causal structure of the world, Princeton: Princeton University Press.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15, 10–23.
- Southard K. M., Espindola M. R., Zaepfel S. D. and Bolger M. S., (2017), Generative mechanistic explanation building in undergraduate molecular and cellular biology, Int. J. Sci. Educ., 39, 1795–1829.
- Staver J. R. and Lumpe A. T., (1993), A content analysis of the presentation of the mole concept in chemistry textbooks, J. Res. Sci. Teach., 30, 321–337.
- Strauss M. J., (1996), Mistaking the Map for the Territory, J. Coll. Sci. Teach., 25, 408–412.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ. Res. Pract., 2, 123–158.
- Taber K., (2002), Chemical misconceptions: prevention, diagnosis and cure, London: Royal Society of Chemistry.
- Taber K. S., (2009), Challenging misconceptions in the chemistry classroom: resources to support teachers, Educ. Quim., 13–20.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Talanquer V., (2010), Exploring Dominant Types of Explanations Built by General Chemistry Students, Int. J. Sci. Educ., 32, 2393–2412.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Talanquer V., (2018a), in Education Research and Practice in Asia-Pacific and Beyond, Singapore: Springer, pp. 39–52.
- Talanquer V., (2018b), Importance of Understanding Fundamental Chemical Mechanisms, J. Chem. Educ., 95, 1905–1911.
- Tamir P., (1977), How are the laboratories used? J. Res. Sci. Teach., 14, 311–316.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7, 141–159.
- Treagust D. F., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25, 1353–1368.
- van Mil M. H., Boerwinkel D. J. and Waarlo A. J., (2013), Modelling molecular mechanisms: a framework of scientific reasoning to construct molecular-level explanations for cellular behaviour, Sci. Educ., 22, 93–118.
- Wu H. K., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38, 821–842.
- Yeo J. and Gilbert J. K., (2014), Constructing a scientific explanation—A narrative account, Int. J. Sci. Educ., 36, 1902–1935.
| This journal is © The Royal Society of Chemistry 2020 |