Electrolysis: What textbooks don’t tell us
Hasok
Chang
a,
Katherine
Duncan
 a,
Kihyang
Kim
b and
Seoung-Hey
Paik
a,
Kihyang
Kim
b and
Seoung-Hey
Paik
 *b
*b
aUniversity of Cambridge, Department of History and Philosophy of Science, Cambridge, Cambridgeshire, UK
bChemistry Education, Korea National University of Education, Taesungtapyuonro 250 Heungduk Gu, Chungju, Chungbuk 363-791, Republic of Korea. E-mail: shpaik@knue.ac.kr
First published on 23rd March 2020
Abstract
We present a critical discussion of how chemistry textbooks treat the electrolysis of water and aqueous salt solutions, based on a survey of general chemistry textbooks in English and Korean at secondary and tertiary levels, also informed by the historical background of 19th-century debates. English-language textbooks present various and contradictory accounts of the electrolysis of water; a key point of disagreement is whether hydrogen and oxygen gases originate from pre-existing H+ and OH− ions, or from the direct reduction and oxidation of H2O molecules. School textbooks in South Korea all present the same account, with no indication of alternative views. A vast majority of all texts ignore the possibility that H2 and O2 may result from secondary reactions, which was a standard view in the late 19th century following the works of Daniell and Miller. Concerning the electrolysis of aqueous salt solutions, all texts give oversimplified views of competing reactions based on standard reduction/oxidation potentials. It is understandable that textbooks try to present sufficiently simple pictures that students at each level can handle; however, this should not be done in a way that shuts down questions. We recommend that students should be made aware that textbook accounts are only models, and encouraged to extend their learning beyond the models. The plausibility of our recommendations is shown in a pilot study we conducted with secondary school students in South Korea.
Introduction
The electrolysis of water and aqueous salt solutions is a common and significant subject in chemistry instruction. The experiments are easily performed, the expected results are simple, and the theoretical implications are important. However, those who have thought much about the subject will easily admit that there are many difficult underlying issues. Our inquiry began when we realized that there were some points on which different textbooks gave mutually conflicting accounts. The most salient point of divergence is whether the hydrogen and oxygen gases produced in the electrolysis of water are formed by the reduction and oxidation of pre-existing H+ and OH− ions formed by the spontaneous dissociation of water molecules, or by a direct decomposition of H2O molecules occasioned by the application of electricity. For example, Schmit and Pollard (2016, p. 132) give the story of the oxidation and reduction of OH− and H+ ions, as follows:| at the cathode: 4H+(aq) + 4e− → 2H2(g), |
| at the anode: 4OH−(aq) → 2H2O(l) + O2(g) + 4e−. |
| at the cathode: 4e− + 4H2O → 2H2 + 4OH−, |
| at the anode: 2H2O → O2 + 4H+ + 4e−. |
 | ||
| Fig. 1 The “whole H2O” account of the electrolysis of water, from Pauling and Pauling (1975, p. 357). | ||
Some other textbooks give a mixed picture, in which one reaction is the oxidation or reduction of H2O but the other reaction is the reduction or oxidation of the H+ or OH− ion. For example, Oxtoby et al. (2016, p. 744) give the following account (writing H3O+ instead of H+):
| at the cathode: 2H3O+ + 2e− → H2 + 2H2O, |
| at the anode: 3H2O → (½)O2 + 2H3O+ + 2e−. |
Historical background: the rise and fall of the Daniell–Miller theory of electrolysis
Before entering into our survey of modern textbooks it would be useful to give a brief historical introduction, in order to provide some background and to highlight some basic long-standing questions. Electrolysis was one of the earliest and most exciting phenomena uncovered through Alessandro Volta's invention of the battery, but it was also a difficult one to explain theoretically. In retrospect, that is perfectly understandable. Electrolysis was discovered almost a century before the discovery of the electron, or Svante Arrhenius's conception of ionic dissociation. Many theoretical accounts of electrolysis came and went (Chang, 2012, ch. 2), but a lasting general idea was that there must be electronegative and electropositive components of molecules, which moved to the positive and negative electrodes, respectively. But entering into any level of detail revealed a number of difficulties.Particularly instructive for our purposes is the work of John Frederic Daniell (1790–1845), the first Professor of Chemistry at King's College London. His name remains recognizable through the “Daniell cell” featured ubiquitously in modern lower-level textbooks. He invented that configuration of battery in 1836, and the main thing he did with it was to investigate electrolysis, reaching an important insight that the final products of electrolysis were not necessarily the actual components of the electrolyte. As T. M. Lowry (1929) explained: “When these primary products are liberated, they frequently undergo decomposition or chemical change, giving rise to secondary products by interacting with one another, with the electrode, or with the electrolyte.” For example, “in the case of a solution of sodium chloride, the sodium atoms immediately attack the water, giving caustic soda [NaOH] and hydrogen, whilst the chlorine atoms unite in pairs to form gaseous chlorine.” (Lowry, 1929, p. 321) The same analysis of NaCl electrolysis is also given in J. W. Mellor's classic textbook on inorganic chemistry (Mellor, 1951, p. 497). Any chemists would still accept the general view that there are primary and secondary products of electrolysis. However, when it comes to the electrolysis of water most modern textbooks seem to shy away from this well-established insight.
In a long series of experiments Daniell investigated the electrolysis of aqueous salt solutions. His first breakthrough came with the electrolysis of a strong NaCl solution with a platinum cathode and tin anode (Daniell, 1839, p. 108). Much hydrogen gas was evolved from the cathode, but no oxygen at the anode; instead the tin anode was dissolved, and the amount of tin dissolved was the chemical equivalent of the hydrogen produced at the other side. Daniell now composed a novel interpretation: “chloride of sodium was decomposed … the chlorine of the latter being absorbed by the tin [anode], and the sodium at the [cathode] reacting upon the water, and giving rise to the secondary result of an equivalent of hydrogen: upon this hypothesis, the current must have been transmitted by the chloride of sodium alone, and no water was electrolyzed.” In understanding such accounts we need to keep in mind that the Arrhenius theory of ionic dissociation was still long in the future.
Daniell recognized the possibility that the production of oxygen and hydrogen in the electrolysis of an aqueous solution may not be an indication that water was being electrolyzed (if “electrolyzed” means being decomposed by the direct action of electricity). In a follow-up paper, Daniell (1840, p. 219) concluded that the current flowing through a salt solution could “divide itself” between acting on the water and acting on the dissolved salt, decomposing some of each. This is not a possibility recognized in most modern textbooks. In order to make this idea plausible, he drew an analogy to the “well known fact that the voltaic current will divide itself between two or more metallic conductors in inverse proportion to the resistance which each may offer to its course.”
This view was developed into a more decisive form by Daniell's successor William Allen Miller (1817–1870) (Miller, 1867, pp. 524–525): “the evolution of oxygen and hydrogen during the decomposition of such saline solutions is a secondary action.” If the electrolyte is “a salt of a metal which, like copper or lead, does not decompose water at ordinary temperatures”, then “no hydrogen should be evolved, but the metal itself should appear upon the [cathode]”. And if the anion of the salt is “unable to take hydrogen from water at common temperatures, no oxygen should be emitted.” For example, if a solution of copper chloride or lead chloride is electrolyzed, “the salt is resolved into metallic copper or metallic lead, and chlorine gas, but no oxygen or hydrogen is liberated.”
Miller invoked the well-known fact that “although water, when pure, is scarcely decomposed by the current from 100 [voltaic] cells or upwards, yet it appears instantly to become a good electrolyte on the addition of a few drops of acid, or of solution of a salt of an earth or an alkali”. In other words, Miller held that water by itself was not electrolyzable, but “upon the addition of the salt, it is this body which is decomposed, and the water is then resolved into oxygen and hydrogen by a secondary action in the manner already explained.” Take, for example, the electrolysis of sodium sulphate, which Miller expressed as follows:
| 2Na2SO4 → 2Na2 + 2SO4 (primary action), followed by |
| 2Na2 + 4H2O → 4NaOH + 2H2 (secondary action), and |
| 2H2O + 2SO4 → O2 + 2H2SO4 (secondary action). |
We will call this the “Daniell–Miller view” of the electrolysis of water and aqueous solutions. It introduced a clarity that was lacking before. For one thing, it assigns a precise role to the added electrolyte in the electrolysis of water (unlike many of today's textbooks, as we will show below). It also makes it clear that the electrolysis of water is the electrolysis of an aqueous solution, just at the low-concentration limit.
This view came to be widely accepted for a time, but hardly any trace of it can be found in today's textbooks. The only textbook examined in our survey that features this view is the rather old text by Andrews and Kokes (1962, p. 470), in its discussion of the electrolysis of sodium chloride solution. (In modern specialist literature the production of Na in the electrolysis of an NaCl solution is admitted in exceptional cases; for example, according to Pletcher (2009, pp. 202–203), on a mercury cathode a sodium amalgam (Na–Hg) will form.) Why did the Daniell–Miller view disappear so completely, when it seems to make so much sense? Our best guess is that its demise was a consequence of a distraction caused by Arrhenius's new theory of spontaneous ionic dissociation, first published in 1887. If there are pre-existing H+ and OH− ions in water itself, then it is not necessary to invoke secondary action to explain how H2 and O2 could be produced.
In Max Le Blanc's well-known early textbook of electrochemistry, the excitement of the ascendant Arrhenius theory is palpable (Le Blanc, 1896, p. 267): “This [Daniell–Miller] conception of the process cannot be called simple, and why the assumption of all these secondary reactions which no one has observed, and which are in no sense necessary!” Le Blanc was quite confident about the new direction of thinking: “In electrolysis a primary decomposition of the water takes place.” The actual electrical conductivity is brought about by all the ions in the solution, but at the electrode that action takes place which proceeds most easily, and this is the separation of the hydrogen and hydroxyl ions.” (Le Blanc, 1896, p. 260; emphasis original) To anyone who might worry that there would not be enough pre-existing H+ and OH− ions in water, Le Blanc offered this reassurance: “Lack of hydrogen and hydroxyl ions can never occur, since ions must be immediately generated by the undissociated water, the product of the two ion concentrations always having a definite value.”
In saying that the “ions must be immediately generated by the undissociated water”, Le Blanc ignored the question of how quickly the dissociation of water molecules could happen in order to replenish the supply of the H+ and OH− ions used up in the formation of hydrogen and oxygen gases. If the reduction/oxidation of these ions were a faster process than the spontaneous dissociation of water, then there would be a bottleneck. A century later, Oxtoby et al. (2016, p. 744) state that the rate of the electrolysis of water would be “exceedingly small” unless an electrolyte is added. Indeed, all the classroom teachers and students doing experiments on electrolysis know that an added electrolyte is needed to get water decomposing at a sensible rate at all. As we will show, modern textbooks are split on the exact reaction pathways involved in the electrolysis of water. But all sides seem to have forgotten the possibility that H2 and O2 may be produced by secondary action. The rise and fall of the Daniell–Miller view of electrolysis makes a worthwhile and complex topic for historical research, but in the present paper we will focus on analyzing how modern textbooks deal with phenomena for which it is difficult to give simple and convincing explanations.
Scope of textbook surveys
We examined the treatment of electrolysis in general chemistry textbooks at levels ranging from lower secondary school to introductory and intermediate-level university courses. For additional insights, we made a cross-national comparative study. The comparison is basically between England and South Korea, but the situation is somewhat complex, as explained below. It would be interesting to see how textbooks in other national and linguistic contexts compare. Note that we have not systematically surveyed specialist textbooks of electrochemistry, though we have consulted some of them for additional insights.On the English side, the secondary school textbooks we examined were mostly intended for use in England, to support study towards the GCSE and A-level examinations; we took care to include textbooks recommended by each of the various examination boards operating in England. At the university level the textbooks are not so nationally bound; many UK universities freely use texts produced in the United States and other Anglophone countries, and perhaps vice versa. The core of the material we surveyed were textbooks found in the Cambridge University Library system (chiefly the University Library, the Chemistry Departmental Library, and the Betty & Gordon Moore Library). This was not only convenient for us, but also indicates our trust in the collective judgment of the chemical community at a university with a long and venerable tradition in the natural sciences. In total, we examined 24 textbooks at GCSE level, 17 at A-level, and 63 at university level (see Appendix). Even though we do not claim to have made an exhaustive survey, we are confident to have examined a clear majority of the important textbooks in use in England at all three levels.
To give an additional and comparative perspective to this England-focused survey of English-language textbooks, we made a survey of secondary-school chemistry textbooks used in South Korea, all of them written in Korean. The choice of South Korea is meaningful in giving attention to a high-performing nation in the realm of science education in a very different linguistic, cultural and institutional setting from European-origin societies. One important factor is that the Ministry of Education in South Korea exerts a strong control over the national curriculum and the approval of textbooks, and as a consequence there is much more uniformity in the topics covered and their organization, compared to the situation in England.
We analyzed the South Korean textbooks that were written to the specification of the national secondary-school curriculum of 2009. (The national curriculum is revised from time to time, and there was another revision in 2015, but not all of the textbooks answering to the 2015 curriculum have been produced yet.) In the 2009 curriculum electrolysis is covered at three different stages, and we examined all of the approved textbooks at all of those levels. There are currently 9 approved chemistry textbooks for the 8th grade (year 2 of middle school), and 4 each for the 11th and 12th grades (years 2 and 3 of high school, just before university). Even though the middle/high school division in the South Korean secondary school system corresponds very roughly to the GCSE/A-level gradation in England, what is expected of 8th graders in Korea is not quite comparable to what is expected of students taking the GCSE in England, which is roughly equivalent to the end of 10th grade. So our cross-national comparison can only be made sensibly by taking the corpus of secondary-level texts as a whole.
At the university level we did not make a cross-national comparison, since there is no real home-grown tradition of university textbooks of chemistry in South Korea. Generally, university students in South Korea either use imported English-language textbooks, or Korean translations of them. In our survey of English-language textbooks, we have taken care to include all the texts that are in use in Korean translation.
One general remark before we enter into details: especially considering the clear and prominent place that electrolysis occupies in the popular imagination and the scientific common sense concerning electrochemistry, the textbook discussions of electrolysis are often surprisingly sketchy, sometimes even altogether absent. Among the English-language textbooks we surveyed, electrolysis is discussed in 84% of them at university level, 59% at A-level, and 96% at GCSE level. Of those textbooks that do discuss electrolysis, only 52% of the university textbooks, 50% of A-level texts, and 74% of GCSE texts engage specifically with the electrolysis of water. The situation is more uniform in Korean textbooks, all of which treat both topics.
Results and discussion
In the following exposition, we will first discuss how the textbooks treat the electrolysis of water. After that we will discuss how the textbooks treat the electrolysis of aqueous salt solutions. In reality there is no sharp division between the two, since the electrolysis of water is almost always made with an added electrolyte.The sources of hydrogen and oxygen
Our survey of the English-language textbooks revealed a whole range of opinions on the source of hydrogen and oxygen produced in the electrolysis of water. The treatments can be classed into 4 types, as summarized in Table 1.Type 1 is the formation of gases from pre-existing ions. The formation of H2 at the cathode is easy to conceptualize: 2H+ + 2e− → H2. The formation of O2 from OH− at the anode is more complicated, and there are divergent accounts given in different texts. Type 2 is the formation of gases by direct decomposition of H2O. Many textbooks present this picture, in which a whole H2O molecule breaks down as a consequence of receiving or losing an electron, producing H2 or O2. Type 3 is the formation of oxygen by direct decomposition of H2O at the anode, and the formation of hydrogen from pre-existing H+ ions at the cathode. As Tsaparlis (2012; 2019) points out, this picture makes sense especially if the added electrolyte is an acid, providing an abundance of H+ ions in the water; however, the textbooks that give this picture do not mention low pH as a motivation. Type 4, which we have found only in one textbook, is the formation of oxygen from pre-existing OH− ions at the anode and the formation of hydrogen by direct decomposition of H2O at the cathode. In an alkaline environment this picture would make perfect sense.
Aside from the sheer diversity of opinion, it is interesting to note that the majority of the GCSE-level textbooks present Type 1 accounts, while the majority of the university-level textbooks present Type 2 accounts. And the GCSE-level textbooks present either Type 1 or 2, while at university and A-level some are found that present Type 3 or 4. (However, it is not the case that only lower-level texts present Type 1 accounts. We even found one specialist treatise of electrochemistry which favors it (MacInnes, 1961, p. 28): “In pure water, and in solutions of neutral substances, the hydrogen and hydroxyl ions, H+ and OH−, are present in such slight amounts that their presence has, for moderate concentrations of the solute, almost no effect on the conductivity. These small concentrations of the ions of water are, however, in this case [the electrolysis of NaCl solution with inert electrodes] the ones that enter into the electrode reactions to the exclusion of ions present in much higher amounts.”)
In the Korean textbooks the situation is interestingly different. In the 8th grade textbooks the electrolysis of water is covered in two different contexts (Hyun et al., 2013a and b; Im et al., 2013a and b; Lee et al., 2013a and b; Lee et al., 2013c and d; Lee et al., 2013e and f; Lee et al., 2013g and h; Lee et al., 2013i and j; Park et al., 2013a and b; Sin et al., 2013a and b). When electrolysis appears as an illustration of the atomic–molecular theory, by showing that water is a compound, it is simply noted that hydrogen and oxygen gases are produced, without the exact chemical equations. In 4 of the 9 cases, the teachers’ guides accompanying the textbooks do present chemical equations. It is very interesting to note that all of them give Type 2 accounts.
The 8th grade textbooks also discuss electrolysis as the conduction of electricity in order to introduce the concept of ions, but in those sections they only focus on the movement of ions, not the reactions at the electrodes. It is not mentioned that gases are generated at both electrodes. The texts published by the Kumsung press (Lee et al., 2013i, p. 42) and Dusan Donga press (Lee et al., 2013a, p. 45) are good examples, giving diagrams of ions moving towards electrodes but not showing anything about what happens to those ions once they reach the electrodes. So the same phenomenon of electrolysis is presented here in a totally different way from how it is done in the unit on atoms and molecules. The only exception in this regard is the Sinsago textbook (Hyun et al., 2013a, p. 33) and teachers’ guide (Hyun et al., 2013b, p. 70), which note in the unit on ions that different types of products are made, yielding either gases or solid deposits on electrodes. (Note that in South Korea school textbooks are commonly referred to by the name of the publisher, rather than the authors. In many of our citations below we will be mentioning both.)
In the 11th grade textbooks electrolysis is treated in the unit on molecules, in order to teach the role of electrons in chemical bonds (Kim et al., 2011a and b; Noh et al., 2011a and b; Park et al., 2011a and b; Ryu et al., 2011a and b). In these books there seems to be a requirement to arrive at the conclusion that electrons are involved not only in ionic but in covalent bonds, by showing that covalent compounds such as water can be electrolyzed (e.g., Park et al., 2011a, p. 156; Ryu et al., 2011a, p. 135; Noh et al., 2011a, p. 132; Kim et al., 2011a, p. 132). As in the case of 8th grade teachers’ guides, 2 of the 11th grade textbooks and all 4 of the 11th grade teachers’ guides present the Type 2 story about the electrolysis of water.
In the 12th grade textbooks (Kim et al., 2012a and b; Noh et al., 2012a and b; Park et al., 2012a and b; Ryu et al., 2012a and b), electrolysis is treated in the unit on chemical equilibria. All 4 textbooks and teachers’ guides state that whenever oxygen and hydrogen gases are produced in the electrolysis of water or an aqueous solution, this is due to the reduction or oxidation of whole H2O molecules. As we will discuss further below, all of the Korean 12th grade textbooks display a clear awareness that at each electrode there are different possible reactions (reminiscent of Daniell's discussion from 1840). However, there is no mention of the possibility that the formation of hydrogen or oxygen gas may be due to a secondary reaction, or that more than one reaction can be happening at the same time at each electrode.
In summary, the treatment of the electrolysis of water is uniform in all of the Korean textbooks, giving Type 2 accounts. This consistency is useful in orienting the students. However, it is also disturbing: accounts of Types 1, 3, 4 (or at least 1 and 3) are clearly considered as possibilities in some respectable English-language textbooks, but not entertained at all by the Korean textbooks. (One exception is the textbook by Chunjae Education (Noh et al., 2012a, p. 260), which gives a Type 3 account for the photolysis of water, but this is presented in a wholly different section of the book.) It is not clear to us how such uniformity emerged in South Korea, since the directives of the Ministry of Education do not reach down to such fine levels of detail. As for the English-language textbooks, each presents its account as the accepted truth. Then what are we to make of the disagreements? What happens when a student encounters differing stories in going from one level of study to the next, or perhaps in changing schools? Considering the Korean side again, how should we guide the experience of high school students who learn the Type 2 account of the electrolysis of water, who may go on to university and encounter English-language textbooks that give a different account?
Reaction mechanisms for the electrolysis of water
Only some of the textbooks discuss the specific reaction mechanisms for the formation of oxygen and hydrogen. Once again there are some disagreements among the accounts given by the English-language textbooks, as summarized in Table 2.| Type | Mechanism | University texts | A-level texts | GCSE texts |
|---|---|---|---|---|
| Pre-existing ions (Type 1) | (−) H+ + e− → H, H + H → H2 | Negi and Anand (1985) | ||
| (+) OH− → OH + e−, | ||||
| OH + OH → H2O + 0.5O2 | ||||
| (−) 4H+ + 4e− → 2H2 | Lewis and Berry (2000) | Gallagher and Ingram (2000) | ||
| (+) 4(OH−) → 4(OH) + 4e− | Saunders (2016) | |||
| 4(OH) → 2H2O + O2 | Schmit and Pollard (2016) | |||
| (−) H3O+ + e− → H + H2O | Ramsden (2000) | |||
| 2H → H2 | ||||
| (+) OH− → OH + e− | ||||
| 4OH → O2 + 2H2O | ||||
| (−) 2H+ + 2e− → H2 | Borley (2016) | |||
| (+) 4OH−–4e− → 2H2O + O2 | Honeysett et al. (2006) | |||
| Honeysett et al. (2007) | ||||
| Honeysett et al. (2011) | ||||
| Owen and King (2005) | ||||
| Direct decomposition at both electrodes (Type 2) | (−) 4H2O + 4e− → 2H2 + 4OH− | Blackman et al. (2016) | ||
| (+) 2H2O → O2 + 4H+ + 4e− | Brady and Holum (1993) | |||
| Brady et al. (2000) | ||||
| McMurry and Fay (2000) | ||||
| Petrucci et al. (2017) | ||||
| Zumdahl and Zumdahl (2010) | ||||
| (−) 4H2O + 4e− → 2H2 + 4OH− | Pauling and Pauling (1975) | |||
| (+) 2H2O–4e− → O2 + 4H+ | ||||
| (−) 2H2O + 2e− → H2 + 2OH− | Brady et al. (2012) | |||
| (+) 2H2O → O2 + 4H+ + 4e− | Brown et al. (1994) | |||
| Burrows et al. (2009) | ||||
| Dillard and Goldberg (1978) | ||||
| Kotz et al. (2008) | ||||
| Malone and Dolter (2013) | ||||
| Tro (2015) | ||||
| Ucko (1982) | ||||
| (−) 2H2O + 2e− → H2 + 2OH− | Sisler et al. (1980) | |||
| (+) 6H2O →O2 + 4H3O+ + 4e− | ||||
| Direct decomposition at anode only (Type 3) | (−) 4H+ + 4e− → 2H2 | Andrews and Jelley (2013) | Toh (2016) | |
| (+) 2H2O → O2 + 4H+ + 4e− | Chang (2000) | |||
| Housecroft and Constable (2006) | ||||
| (−) 4H3O+ + 4e− → 2H2 + 4H2O | Olmsted and Williams (2002) | |||
| (+) 6H2O → O2 + 4H3O+ + 4e− | Oxtoby et al. (2016) | |||
| Direct decomposition at cathode only (Type 4) | (−) 2H2O + 2e− → H2 + 2OH− | Verma et al. (2009) | ||
| (+) 2OH− → H2O + 0.5O2 + 2e− | ||||
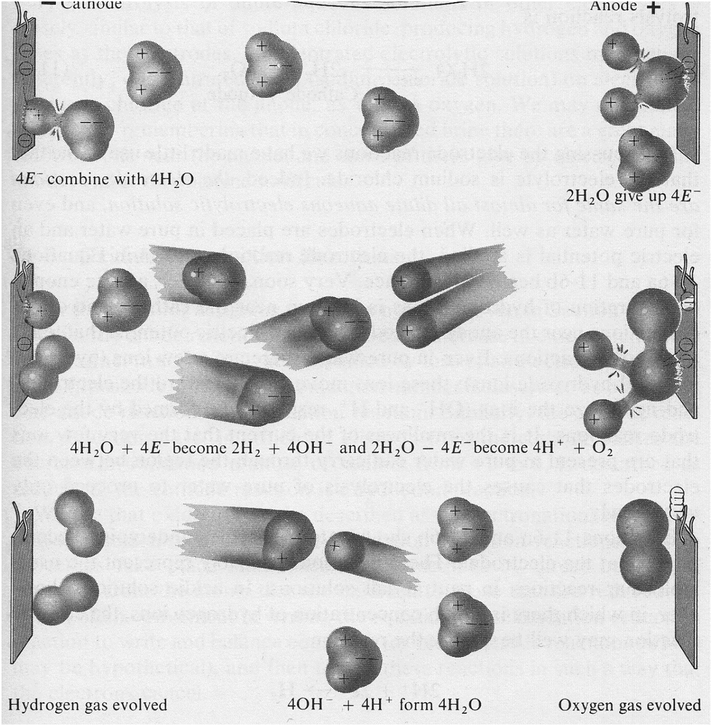
The disagreements are occurring even among accounts belonging to the same type. For example, all Type 1 accounts are agreed that hydrogen ions receive electrons to form hydrogen molecules, but they describe the situation differently. For example, Owen and King (2005, p. 39), Honeysett et al. (2007, p. 139) and others write this as 2H+ + 2e− → H2, while Negi and Anand (1985, p. 491) present it as H+ + e− → H twice, and then H + H → H2. The former authors seem to suggest that two hydrogen ions and two electrons physically come together and form a hydrogen molecule at once. The latter authors seem to suggest that individual neutral hydrogen atoms exist for a time in the solution being electrolyzed, and then they come together pairwise to form hydrogen molecules. Or are they all simply balancing equations and not suggesting actual reaction mechanisms? But in that case, why don’t all Type 1 accounts do as Owen and King do, which would be the simpler solution? Specialist electrochemical treatises point out that the formation of H2 happens because a neutral hydrogen atom can be stable in a state of adsorption on an electrode surface (Pletcher, 2009, Sections 1.1 and 1.7; Bockris and Reddy, 1970, vol. 2, p. 1246). It is interesting to note that Pauling and Pauling (1975) show an adsorbed hydrogen atom in Fig. 1, and Atkins (2011, p. 51) is a rare popular science book that goes into adsorption, though interestingly his renowned university textbooks do not discuss the electrolysis of water.
These questions arise more significantly in the formation of oxygen gas in Type 1 accounts. OH− being not purely oxygen, it is quite impossible to avoid the question of reaction mechanisms. The majority of authors focus on the overall result, to write: 4OH− − 4e− → 2H2O + O2, or 4OH− → 2H2O + O2 + 4e−. This seems to suggest that four hydroxide ions will collide together and lose four electrons, to generate two water molecules and one oxygen molecule in a single step. Anything physically possible will happen, but what is the probability or frequency of the event? Perhaps these authors are just invoking the minimum number of ions and electrons to be able to balance the equation. But some authors do break the process down; Ramsden (2000, p. 238) writes OH− − e− → OH, and then, 4OH → O2 + 2H2O. But is the second step here any more plausible as a mechanism than the one-step account? Ramsden may think that neutral OH can exist freely long enough for four of them to have a chance to come together. The same questions raised so far also pertain to the relevant side of Type 3 and Type 4 accounts.
Very similar kinds of questions can be raised about Type 2 accounts (and the pertinent sides of Type 3 and Type 4 accounts). Interestingly, none of the accounts we have seen postulate any process involving one water molecule losing or gaining one electron. Presumably, this is because such a process would not result in stable products; for example, H2O + e− → H + OH− would involve a self-standing hydrogen atom. We saw that some Type 1 accounts have no problem with that, but it seems that Type 2 authors are not keen to feature unstable products in their equations. But they do not all agree on how many water molecules ought to be involved in the electrolytic dance. The simplest processes involve just two water molecules: 2H2O → O2 + 4H+ + 4e−, and 2H2O + 2e− → H2 + 2OH− (e.g., Kotz et al., 2008, p. 936; Brady and Holum, 1993, p. 771). Brady and Holum (1993, p. 772) then go on to double the latter formula, to present the cathode reaction as 4H2O + 4e− → 2H2 + 4OH−. This way they can balance this equation with the anode-side equation, with four electrons given and taken on the two sides. It seems clear that the needs of equation-balancing are trumping the considerations of reaction mechanisms. When Oxtoby et al. (2016, p. 745) write the anode-side equation as 6H2O → O2 + 4H3O+ + 4e−, they show concern for physical reality in writing H3O+ instead of a naked proton (H+), but not about the physical frequency of the six-molecule coincidence.
Some textbooks do try to go beyond equation-balancing and present a microphysical picture of the processes, as with Pauling and Pauling's attempt shown in Fig. 1. Some of those textbooks also try to present reasoning and evidence in favor of their accounts. One phenomenon commonly invoked in favor of Type 2 accounts is the resulting acidity and alkalinity of the solution on the anode and cathode side respectively. If the cathode-side reaction is 2H2O + 2e−→ H2 + 2OH−, then the solution will obviously go alkaline due to all the OH− ions being added to it; similarly, if the anode-side reaction is 2H2O → O2 + 4H+ + 4e− (or something to that effect) the solution around there will go acidic. Such changes can be shown conveniently by adding some pH indicators to the water. For example, Brady and Holum (1993, p. 772) argue that the direct decomposition of H2O is the correct account by showing the solution of KNO3 turning acidic around the anode and alkaline around the cathode.
These facts are indeed pleasingly consistent with the direct-decomposition account (Type 2). However, it is not clear that they favor the Type 2 account in comparison to other accounts. If we take the Type 1 account postulating the reduction and oxidation of pre-existing ions, here, too, the depletion of H+ ions around the cathode will turn the solution in that vicinity less acidic, and the depletion of OH− ions around the anode will turn it less alkaline there. The observed pH changes do not help us discriminate between the direct-decomposition account and the old Daniell–Miller account, either, because the final products predicted are the same for both theories. For example, for a dilute KOH solution at the cathode:
| Direct-decomposition of water (Brady and Holum): 2H2O + 2e− → H2 + 2OH− (with K+ present but not participating in the reaction). |
| Secondary reaction (Daniell–Miller, modernized): 2K+ + 2e− → 2K, and then 2K + 2H2O → 2KOH + H2, and then 2KOH → 2K+ + 2OH− (with K+ acting as a catalyst). |
One further question can be raised about the Type 2 account of the electrolysis of water. According to the reasoning based on standard electrode potentials, it would seem that the application of a small external potential should be sufficient to force water to take up or lose electrons. Using the standard reduction potentials and the Nernst equation, Oxtoby et al. (2016, p. 745) calculate the “decomposition potential” of pure water to be 1.229 V. Given that, even if we consider overpotentials, why should the application of a reasonably high potential (e.g., from the handy 9 V battery that students may try to use) fail to electrolyze distilled water at a good rate?
Due to all of these questions and other complicating issues, it is actually more difficult to give a neat story at the higher level of discussion. This could be why fewer higher-level texts (A-level and university) discuss the electrolysis of water. It is also interesting to note that none of the GCSE texts that give Type 2 accounts enter into the reaction mechanisms, which would complicate the presentation. In fact the electrolysis of water does not tend to occur as a separate topic in specialist texts on electrochemistry (e.g., Bockris and Reddy, 1970; MacInnes, 1961) or more sophisticated treatments of electrochemistry in general textbooks (e.g.Atkins and de Paula, 2014; Keeler and Wothers, 2013), even though it is of course noted that water molecules can be broken down in the electrolysis of aqueous solutions (e.g. in the brief passage quoted above from MacInnes, 1961).
What is decomposed – the electrolyte or the water?
We now broaden our focus to examine how the textbooks treat the electrolysis of aqueous salt solutions. We will begin with an analysis of the Korean textbooks, because the picture presented there is clear, unified and in many ways insightful. All of the 12th grade textbooks treat electrolysis within the section on chemical equilibria, and they all present similar stories. The 11th grade textbooks, also published by the same companies and the same authors as the 12th grade texts, treat electrolysis under the unit on chemical bonds and molecules, and the discussion of electrolysis there is not uniformly thorough, but they agree largely with the stories given in the 12th-grade books.The Korean textbooks discuss three main categories of substances that are susceptible to electrolysis: molten salts, aqueous salt solutions, and water. Molten salts can be dealt with very straightforwardly. Water and aqueous salt solutions are dealt with in separate sections, and the electrolysis of water uniformly receives Type 2 accounts, as discussed in the previous section. Concerning aqueous salt solutions, these textbooks share the following basic premise: at the cathode there is competition between the reduction of water and the reduction of the cation of the salt, and at the anode there is competition between the oxidation of water and the oxidation of the anion of the salt. See, for example, the Sangsang Academy 12th grade textbook (Kim et al., 2012a, p. 192). Which of these competing reactions “wins” is determined by the standard reduction potentials of the reactions in question, and the outcome may be mixed, with water reacting on one side and the salt ion reacting on the other. Some of GCSE-level English textbooks try to give rules about what will be produced in which situation (e.g., Gallagher and Ingram, 2000, p. 108, and Honeysett et al., 2007, p. 139).
Many pertinent things are ignored in this story: secondary reactions; the possibility that both of the competing reactions may happen at a given electrode, albeit at different rates; the presence of H+ and OH− ions originating from water; the fact that the electrodes will be electrostatically charged, attracting and repelling ions; and the effect of different levels of applied voltage. The authoritative definitiveness of the textbook presentations effectively steers students away from thinking about such things.
On the side of the English textbooks the situation is not fundamentally different, though there is again less uniformity. They do not all share the same thematic structure, either, so the contexts in which electrolysis comes up tend to be more varied as well. One of the most interesting accounts we have found is by Andrews and Kokes (1962, pp. 469–471). Using the case of sodium chloride solution, they give a thoughtful discussion of the possible reactions at each electrode. At the cathode, H+ or Na+ could be reduced, and the final outcome of either reaction would be the production of H2; when they consider what would happen if the reduction of Na+ produces Na, Andrews and Kokes give an account that is essentially the Daniell–Miller view, which sets their text apart from most other modern ones. Their view is cautious (Andrews and Kokes, 1962, p. 470): “The kinetic mechanism of the electrode process is far more complicated than we have indicated here; but regardless of the mechanism, the over-all reaction is represented by this equation”: 2H2O + 2e− → H2 + 2OH−. At the cathode, Andrews and Kokes state that both Cl− and OH− are oxidized, producing chlorine gas and oxygen gas; in dilute solutions the production of oxygen would predominate, with the result that the overall outcome of the electrolysis is the decomposition of water. This is the most sensible discussion that we have found among the textbooks we have surveyed, except that Andrews and Kokes curiously do not consider the direct reduction or oxidation of H2O molecules.
Among all of the general chemistry textbooks we have examined in either language, the most systematic and detailed account of competing reactions in electrolysis is provided by Oxtoby et al. (2016, pp. 744–746), in a “deeper look” section on the “Electrolysis of Water and Aqueous Solutions”. Oxtoby et al. give the basic story based on reduction potentials, and also give calculations on the effect of the concentrations of the various ions based on the Nernst equation. However, even this “deeper look” conceals some tricky issues, presenting an overly simple reliance on the order of reduction potentials to decide which reactions happen. It categorically states that only the most favored reactions will happen, and that a less favored reaction will not happen at all. For example, concerning the electrolysis of a 0.10 M solution of NaCl they state: “O2(g) is generated at the anode, not Cl2(g). Increasing the potential above 1.229 V only increases the rate of the electrolysis reaction. It is not possible to generate sodium and chlorine electrolytically in aqueous solutions of NaCl.” (Oxtoby et al., 2016, p. 745; emphases added) The Kyohaksa 12th-grade textbook (Park et al., 2012a, p. 213) echoes this view, though less rigidly: “At the cathode… the standard reduction potential of the sodium ion (−2.71 V) is lower than that of water (−0.83 V), so it is difficult for the sodium ions to receive electrons.”
Among the many questions that are not addressed by these accounts, we want to highlight just two. First, if the applied voltage is considerably higher than the reduction/oxidation potentials required for enabling all of the competing reactions, shouldn’t all of them happen to some degree, as Andrews and Kokes suggest? What we would want to calculate is the relative frequencies of the different reactions. Oxtoby et al. (2016) do not explain why they say that the less favored reaction cannot happen at all even if the voltage is quite high. A similar question applies to the electrolysis of water: why can’t H2O molecules in pure water receive electrons even if the external voltage applied is very high? Second, the accounts based on standard reduction/oxidation potentials do not take into explicit consideration the electrostatic attraction between a charged electrode and ions of the opposite charge (for a thorough discussion of the electrode–electrolyte interface see Bockris and Reddy, 1970, vol. 2, ch. 7). For example, if the competing reactions at the cathode are the reduction of H2O and the reduction of a metal cation, the electrostatic attraction between the cathode and the cation should make the reduction of the cation more likely. This consideration adds to the plausibility of the Daniell–Miller account of electrolysis, and detracts from the confidence shown in many textbooks in Type 2 accounts of the electrolysis of water. But aren’t the effects of all the relevant factors already included in the empirically determined values of standard potentials? Here it is useful to remember the operational meaning of standard potentials: the measurement setup for these potentials is a Daniell-type voltaic cell with the reference electrode on one side, and the redox reaction in question on the other side. This is not the same as an externally driven electrolytic cell. It is not reasonable to expect that the data gathered in one situation will help us straightforwardly in reasoning about what happens in the other situation.
The confusing state of the textbooks on the electrolysis of aqueous salt solutions can be illustrated quite nicely through the case of NaCl. Brady and Holum (1993, p. 773) acknowledge that there are complicated circumstances that determine which reaction will be dominant. As mentioned above, Oxtoby et al. (2016) state very confidently that oxygen, and no chlorine, will be disengaged at the anode. But Honeysett et al. (2007, p. 140) argue that the outcome depends on the concentration of NaCl, producing oxygen if the concentration is low and chlorine if the concentration is high. Andrews and Kokes (1962, p. 470) reach a more nuanced conclusion, stating that both chlorine and oxygen gas will be produced, but primarily chlorine if the concentration of NaCl is high, and primarily oxygen if concentration is low. The Korean textbooks also engage with this issue. They note that according to the standard potentials, it should be easier to oxidize H2O than to oxidize Cl−; however, if we do the experiment, chlorine gas comes out instead of the oxygen gas that would result from the oxidation of H2O. About why this is so, the Sangsang Academy textbook (Kim et al., 2012a, p. 192) says that it is due to “the ion concentrations and overpotentials”. It adds no further comment on concentrations, and concerning overpotentials it states: “the voltage needed for electrolysis is always higher than what is computed from the standard electrode potentials. We use the term ‘overpotential’ to refer to this phenomenon. If the difference in the standard electrode potentials involved is not large, it is not easy to predict which reaction would actually happen.” The Chunjae 12th grade teachers’ guide (Noh et al., 2012b, p. 191) gives a story of how oxygen bubbles sticking to the anode would increase the overpotential, which does not happen with the production of chlorine. The Visang 12th grade teachers’ guide (Ryu et al., 2012b, p. 236) claims in the main text that the chloride ion will be oxidized in preference to water because that is the order of oxidation potentials, and then corrects itself in a sidebar note and gives the story about overpotentials.
The role of the added electrolyte in the electrolysis of water
Having examined the treatment of the electrolysis of aqueous salt solutions, we can now return more insightfully to the electrolysis of water, which is after all only the electrolysis of very dilute aqueous salt solutions (at least in the typical textbook setup). Now we can ask again: what exactly does the added electrolyte do in the electrolysis of water? Almost no textbooks deny the fact that it is necessary to add a small amount of electrolyte to water in order to enable the electrolysis to happen at a noticeable rate. But we have not found any textbooks that give an entirely convincing account of why this is so, and the electrolyte does not appear anywhere in the formulae given for the electrolysis of water.All of the English GCSE and A-level textbooks that do discuss this issue simply state that adding the electrolyte increases the conductivity of the water, thereby allowing electrolysis to take place. That is, if they discuss the role of the electrolyte at all—only 5 of the GCSE textbooks and 1 of the A-level textbooks do (Gallagher and Ingram, 2000; Holman and Stone, 2001; Roebuck, 2003; Owen and King, 2005; Hoong et al., 2006; Hirsch, 2013). This is also what we find in most university-level textbooks. Negi and Anand (1985, p. 491), for example, claim that the electrolyte (sodium hydroxide in this case) “simply provides the conducting medium”. They do not make clear why the H+ and OH− ions in water cannot themselves do the job of conduction, if the concentration of these ions will be replenished to the 10−7 level just as the existing ions are turned into H2 and O2, as Ramsden (2000, p. 238) confidently states (just as Le Blanc had). For those who give Type 2 accounts, it is not clear why the H+ and OH− ions produced through the oxidation and reduction of H2O cannot do the job of conduction.
The Korean textbooks tend to offer accounts along the same line, too. They are in fact more definitive in their statements, yet quite vague. For example, the Sangsang Academy 11th grade textbook (Kim et al., 2011a, p. 132) writes, “when we cause electricity sufficient for the decomposing-reaction to pass, the covalent bond is broken and water returns to hydrogen and oxygen.” The Chunjae 11th grade textbook (Noh et al., 2011a, p. 132) says: “If/when/as we let the current flow [ ], electrolysis occurs.” Similarly, the Sangsang Academy 12th-grade teachers’ guide (Kim et al., 2012b, p. 215) says: “When we make a current flow through an aqueous solution of an electrolyte or a molten salt, the ions of the electrolyte move toward the electrode of opposite charge to themselves, and engage in oxidation–reduction reactions.” In such presentations the flow of current is presented as if it were a separate event from the movement of ions.
], electrolysis occurs.” Similarly, the Sangsang Academy 12th-grade teachers’ guide (Kim et al., 2012b, p. 215) says: “When we make a current flow through an aqueous solution of an electrolyte or a molten salt, the ions of the electrolyte move toward the electrode of opposite charge to themselves, and engage in oxidation–reduction reactions.” In such presentations the flow of current is presented as if it were a separate event from the movement of ions.
From those presenting Type 2 accounts, including all the Korean textbooks, there is no clear story presented about why the movement of the ions of the added electrolyte should enable the break-up of water molecules. Brady and Holum (1993, pp. 772–773) is the only textbook in our survey in which we have noticed a plausible story about why the electrolyte is necessary. They say its role is “to maintain electrical neutrality in the vicinity of the electrodes.” Without the added electrolyte, if “the electrolysis were to occur anyway, the solution around the anode would become filled with H+ ions, with no negative ions to balance their charge. Similarly, the solution surrounding the cathode would become filled with hydroxide ions with no nearby positive ions. Nature simply does not allow this to happen.” So, for example, the Na+ ions of NaCl do not themselves receive electrons from the cathode, but instead move toward the cathode to balance out the OH− ions being produced in that vicinity. Thereby the Na+ ions assist with the continuing influx of electrons from the electrode to the H2O molecules. This would explain how NaCl can improve conductivity without itself being electrolyzed in the sense of its constituent parts appearing as reaction products.
While the above account is coherent, what would stop the H+ and OH− ions from simply moving away from the regions near the electrodes, as depicted by Pauling and Pauling (1975) in Fig. 1? It seems to us that the old Daniell–Miller account offers a more plausible physical story about the role of the added electrolyte: a cation would be electrostatically attracted to the cathode, and take up an electron from there; an anion would be attracted to the anode, and lose an electron there. Having been neutralized, the particle would easily drift away from the electrode and then react with one of the very abundant water molecules. Clearly the quantum mechanics involved in these reactions would not be trivial to work out, but if these are the main mechanisms by which water is electrolyzed, then the necessity of the added electrolyte is obvious, since the electrolysis cannot get going without the decomposition of the electrolyte (or, more likely, the movement and neutralization of pre-existing ions of the electrolyte).
Having forgotten all about the Daniell–Miller view, the modern textbook-writers seem to have great difficulty coming up with a story as to what exactly the electrolyte does in the electrolysis of water. The Sangsang Academy 12th grade teachers’ guide (Kim et al., 2012b, p. 216) goes so far as to declare that the suitable electrolytes to use for the electrolysis of water are those that “do not participate in the reaction”, listing KNO3, Na2CO3, H2SO4, NaOH, and NaNO3 as examples. The Visang 12th grade teachers’ guide is similar, and clumsier (Ryu et al., 2012b, p. 237): in the electrolysis of NaCl solution, it first calls Na+ a “spectator ion” and then states that NaOH is formed in the reaction! It is striking to observe to what lengths the textbooks go, in order to avoid entertaining the production of H2 and O2 through secondary reactions. For example, the teachers’ guide for the 11th grade Visang textbook (Ryu et al., 2011b, p. 135) makes no mention of Na+ at all in its discussion of what happens in the electrolysis of sodium chloride solution, simply giving the decomposition of water as the cathode-side reaction. So we are no better than those 19th century scientists whom Le Blanc criticized as follows (1896, p. 44): “It was desired to know which really conducts the electricity, the water or the dissolved substance. For a long time this was an open question. One spoke then of ‘water which by the addition of sulphuric acid, for example, becomes a good conductor,’ without having apparently conceived any explanation of the fact.”
Summary of textbook surveys
We conclude the presentation of our textbook survey with a few overall observations with significant pedagogical implications.First, among the English-language textbooks there are significant disagreements in the treatment of electrolysis, at each level of study. This is not a matter of the common progression of accounts in which the teachers might say to the students, “what we taught you last year was wrong.” On the Korean side such disagreement is absent, but it is not clear that the common account given by all the textbooks is the best possible one. Comparing the two, we must ask: is it better to be consistent in giving an inadequate answer, or to disseminate mutually inconsistent stories, each presented as the right answer? Neither is a desirable option.
Second, many textbooks seem preoccupied with the task of balancing equations, without considering the physical mechanisms involved. In many cases it is not made clear whether the reactions implied by the equations are proposed as real, and the plausibility or probability of those reactions is not discussed. Even at the university level the role of the added electrolyte in the electrolysis of water is not considered carefully, and the precise operational meaning of standard reduction potentials is usually not explained.
Third, most of the general chemistry textbooks ignore secondary reactions in the description of the electrolysis of water, and the Daniell–Miller view of electrolysis is almost never mentioned. Its neglect seems to be partly a legacy of the initial over-enthusiasm from a century ago about the then-new Arrhenius theory. After Arrhenius theorized that small amounts of H+ and OH− are automatically ionized in water, there seemed to be no need to explain the role of electrolytes in the electrolysis of water. What may have been a hasty judgment at that time has now turned into unthinking and unjustified neglect.
A pilot study in the classroom
In order to begin the process of applying the insights from our textbook survey to teaching practices, we carried out a pilot study with students at the end of their 10th grade year at the Sejong Academy of Science and Arts, a secondary school for gifted students in South Korea. 23 students volunteered to take part in this study, in which they conducted experiments and answered a questionnaire. The study was designed to allow students to encounter a situation in which experimental results were not in line with the simplified accounts given in their textbooks. The students were asked to perform electrolysis on aqueous NaCl solutions of varying concentrations (1.0 M, 0.50 M or 0.10 M), and using different electrodes (made of graphite or stainless steel). They were divided into 6 groups, each group performing the experiment with a given concentration and a given electrode material, repeating the trial at least three times. In all cases the external voltage applied was 9.0 V, and each run was for 7 minutes. Students recorded the volumes of gases produced at the cathode and the anode, and afterwards stirred the solution and observed its acidity/alkalinity using a universal indicator.Before carrying out the experiments, students were asked to make predictions of results. In this special school students study all of the regular high school chemistry syllabus by their 10th grade, so the students participating in this study had already been given the lessons on electrolysis as discussed in our textbook survey above. Students in our study gave three types of predictions. (1) At the cathode water will be reduced, producing hydrogen gas; at the anode the chloride ion will be oxidized, producing chlorine gas; the gas volume-ratio will be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the solution will be alkaline at the end of the reaction. 14 students (61% of the whole group) gave this type of prediction. (2) At the cathode water will be reduced, producing hydrogen gas; at the anode water will be oxidized, producing oxygen gas; the gas volume-ratio will be 2
1, and the solution will be alkaline at the end of the reaction. 14 students (61% of the whole group) gave this type of prediction. (2) At the cathode water will be reduced, producing hydrogen gas; at the anode water will be oxidized, producing oxygen gas; the gas volume-ratio will be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the solution will be neutral. 8 students (35%) gave this prediction. (3) At the cathode water will be reduced, producing hydrogen gas; at the anode, both water and the chloride ion will be oxidized but mostly water, giving a volume-ratio close to 2
1, and the solution will be neutral. 8 students (35%) gave this prediction. (3) At the cathode water will be reduced, producing hydrogen gas; at the anode, both water and the chloride ion will be oxidized but mostly water, giving a volume-ratio close to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the solution will be alkaline. Only one student gave this prediction.
1, and the solution will be alkaline. Only one student gave this prediction.
The results on gas-volumes were as shown in Table 3. With graphite electrodes, the gas-volume ratios were clearly much greater than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (with the 0.10 M solution, the anode-side volumes were too small to be measured reliably). With stainless steel electrodes no appreciable amounts of gas were produced at the anode, from which the students quickly learned that the electrode material can participate in reactions; this was confirmed easily by the formation of visible precipitates. These results were clearly contrary to the students’ predictions (which had correctly followed their textbook learning).
1 (with the 0.10 M solution, the anode-side volumes were too small to be measured reliably). With stainless steel electrodes no appreciable amounts of gas were produced at the anode, from which the students quickly learned that the electrode material can participate in reactions; this was confirmed easily by the formation of visible precipitates. These results were clearly contrary to the students’ predictions (which had correctly followed their textbook learning).
| Electrode | Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.0 M | 0.50 M | 0.10 M | ||||||||
| First | Second | Third | First | Second | Third | First | Second | Third | ||
| Graphite | Cathode | 5.5 | 5.7 | 5.4 | 3.2 | 2.9 | 3.2 | 0.68 | 0.68 | 0.57 |
| Anode | 0.57 | 0.57 | 0.45 | 0.57 | 0.34 | 0.45 | — | — | — | |
| Stainless steel | Cathode | 4.2 | 4.0 | 4.1 | 2.6 | 2.7 | 2.5 | 0.57 | 0.45 | 0.57 |
| Anode | — | — | — | — | — | — | — | — | — | |
The results on acidity/alkalinity were also complex and intriguing. With graphite electrodes, the 1.0 M and 0.50 M solutions became alkaline, in line with prediction (1) above. This indicated that water was reduced at the cathode producing OH− ions as well as hydrogen gas, but it was not oxidized at the anode (which would have produced H+ ions as well as oxygen gas); at least it must have been mostly Cl− that became oxidized. At 0.10 M the solutions appeared neutral after the reaction, suggesting that the anode-side reaction was predominantly the oxidation of water in this case. Students also observed that the colors of the indicator became more faint as time went by, suggesting the bleaching action of chlorine or chlorine compounds.
After the experimental work we conducted a brief survey asking the students to give us their view on the relation between these experimental outcomes and what they have learned from their textbooks. Choosing among 3 options we gave them, a clear majority (18 students, 78%) responded that the textbooks articulated simple principles or regularities discernible within complex natural phenomena, expressing an appreciation of the value of the textbook account despite the discrepancy with observations. A small number (3 students, 13%) responded that the textbook account was correct and their experimental results were in error. Another small group (2 students, 9%) responded that the experimental results should be trusted and the textbook account may be in error.
We also asked the students’ attitudes towards their experience of this work. Most of them (21 students, 91%) responded that they found these experiments with complex and unexpected results more interesting than those in which the textbook 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gas-volume ratio was obtained (such as the electrolysis of NaOH solutions, which reliably produces the 2
1 gas-volume ratio was obtained (such as the electrolysis of NaOH solutions, which reliably produces the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio even at high concentrations). 15 students (65%) affirmed that they experienced cognitive dissonance from the more complex experiments, yet all of these students also said that the complex experiments were more interesting to them. All students agreed that they have learned from these experiments that it was necessary to consider many variables if they are to predict correctly the results of electrolysis. In free-form answers describing their experience, many students gave indications of sophisticated thinking, for example about the dominance of thermodynamics vs. kinetics, the methods for handling of discrepancies between theory and experiment, the process of adding refinements to theories, and the limits of predictions based on what we already know. Some also expressed the desire for further specific learning, for example about what would happen with other electrode materials, and about the secondary reactions of chlorine.
1 ratio even at high concentrations). 15 students (65%) affirmed that they experienced cognitive dissonance from the more complex experiments, yet all of these students also said that the complex experiments were more interesting to them. All students agreed that they have learned from these experiments that it was necessary to consider many variables if they are to predict correctly the results of electrolysis. In free-form answers describing their experience, many students gave indications of sophisticated thinking, for example about the dominance of thermodynamics vs. kinetics, the methods for handling of discrepancies between theory and experiment, the process of adding refinements to theories, and the limits of predictions based on what we already know. Some also expressed the desire for further specific learning, for example about what would happen with other electrode materials, and about the secondary reactions of chlorine.
At least with these highly motivated and able students, we have confirmed the potential benefits of offering them opportunities for research into the complexity of phenomena, even if such work should go beyond the prescribed syllabus. This would generate much greater potential for learning, compared to the usual experiments in which students learn to produce the results dictated by simplified textbook accounts.
Recommendations
On the basis of our textbook analysis and our pilot study with students, we would like to make four recommendations for the teaching of electrolysis. Our recommendations are broadly consonant with insights provided in Georgios Tsaparlis's study of the teaching and learning of electrochemistry (Tsaparlis, 2012), in particular his advocacy of “active learning” about electrolysis (Tsaparlis, 2019). We also find inspiration in the work of Rosária Justi and John Gilbert (2003) on the use of models in chemical education.(1) It is perfectly understandable that textbooks offer simplified accounts of electrolysis, which is a highly complex phenomenon. However, we recommend that students should be advised clearly that the textbook accounts are models offering theoretical insights, rather than exact predictions for actual experiments. Our recommendation is in line with Justi and Gilbert's general recommendation that “students should: learn about the nature of models and their use as thinking tools; learn about the scope and limitations of specific chemical models; be encouraged to use multiple models for a given phenomenon.” (Justi and Gilbert, 2003, p. 51) Concerning electrolysis in particular, it is important that the students be taught the limitations of reasoning just in terms of standard electrode potentials, and be made aware that a full account of the electrolysis mechanism requires the considerations of kinetics as well as equilibrium thermodynamics.
(2) As Justi and Gilbert suggest in the passage just quoted, the plurality of models is not a problem. We recommend that students and teachers should be helped in reaching a pluralistic understanding of models. Ideally each textbook should be able to offer a variety of models, acknowledging that they all have merit and they all have difficulties. Or at least one model can be given along with a note that there are other plausible ones, too.
(3) In the same vein we can recognize the value of historical knowledge. The explanations that were proposed in the past may disappear from the explanations of modern chemistry, but this does not necessarily mean that the past explanations are wrong. As we have seen through the case of the Daniell–Miller view of electrolysis, a forgotten past model can offer useful questions and answers for modern students. If we invite students into the history of science and give them the opportunity to consider freshly the research questions from the past, it may help them enjoy the true pleasure of doing chemistry.
(4) Recognizing textbook accounts as simplified models can help encourage the more adept and motivated students to engage in creative and critical thinking instead of accepting one explanation as being true. Our pilot study points to clear potential in this direction. We recommend that textbooks and teachers try to move beyond the kind of neat stories that have the effect of shutting down questions that demand complex or sophisticated discussions. Of course, it would be unrealistic to treat all the complications in textbooks for schools and lower-level university courses. However, textbooks and teachers at any level should be prepared to admit that there are important questions that they cannot deal with fully, which constitute subjects for further inquiry either by students with extra initiative or in later years of study. Recognizing the educational value of these questions can foster curiosity about unexplained phenomena and provide an environment for scientific inquiry. We should remember Charles Sanders Peirce's motto: “do not block the way of inquiry.” (Hartshorne and Weiss, 1931, §135)
Conflicts of interest
There are no conflicts to declare.Appendix: English-language textbooks consulted for analysis
| Text | Publisher | Author | Year | Level |
|---|---|---|---|---|
| Chemistry | Oxford University Press | Borley, M | 2016 | GCSE |
| AQA GCSE Science Applied Double Award | Hodder Education | Chenery, S. and Unsworth, S. | 2006 | GCSE |
| Science Uncovered: AQA Additional Science for GCSE Student Book | Heinemann | Clyde, B. | 2006 | GCSE |
| AQA Chemistry for GCSE | Heinemann | Clyde, B. | 2007 | GCSE |
| OCR Gateway GCSE (9-1) Chemistry for Combined Science | HarperCollins | Daniels, A. | 2016 | GCSE |
| New Modular Science for GCSE (No. 1) | Heinemann | Deloughry, W. | 1997 | GCSE |
| Chemistry for AQA: Separate award | Nelson Thornes | Fullick, A. and Fullick, P. | 2001 | GCSE |
| Complete Chemistry | Oxford University Press | Gallagher, R. and Ingram, P. | 2000 | GCSE |
| Chemistry Counts (3rd ed.) | Hodder and Stoughton | Hill, G. | 2002 | GCSE |
| Chemistry (2nd ed.) | Nelson Thornes | Holman, J. and Stone, P. | 2001 | GCSE |
| Gateway Science: OCR Additional Science for GCSE Foundation | Pearson Education | Honeysett, I., Lees, D., Bibby, S. and Macdonald, A. | 2006 | GCSE |
| Revise GCSE – Science | Letts Educational | Honeysett, I., Tear, C. and Poole, E. | 2011 | GCSE |
| Revise GCSE – Additional Science | Letts Educational | Honeysett, I., Tear, C. and Sadler, J. | 2007 | GCSE |
| Interactive Science Textbook 2 | Panpac Education Pte | Hoong, T., Leng, H. and Khang, G. | 2006 | GCSE |
| GCSE AQA Chemistry | Letts and Lonsdale | Horbury, C. | 2006 | GCSE |
| OCR Gateway Chemistry for GCSE Combined Science | Oxford University Press | Saunders, N. | 2016 | GCSE |
| Chemistry at a Glance: Full Chemistry Content of the New GCSE | CRC Press | Owen, R. and King, S. | 2005 | GCSE |
| GCSE Core Science AQA, A Revision Guide – Foundation | Coordination Group Publications | Parsons, R. | 2011 | GCSE |
| Excel Preliminary Chemistry (revised ed.) | Pascal Press | Roebuck, C. | 2003 | GCSE |
| Chemistry for You (2nd ed.) | Nelson Thornes | Ryan, L. | 2001 | GCSE |
| WJEC GCSE Chemistry | Hodder Education Group | Schmit, A. and Pollard, J. | 2016 | GCSE |
| OCR GCSE Sciences Suite B – OCR GCSE Science | Collins | Sherry, C. and Bell, C. | 2006 | GCSE |
| Chemical Reactions (Core Chemistry) | Evans | Walker, D. | 2007 | GCSE |
| The Science Teacher's Activity-A-Day, Grades 5–10: Over 180 Reproducible Pages of Quick, Fun Projects that Illustrate Basic Concepts (pre-GCSE) | Wiley | Walker, P. and Wood, E. | 2010 | GCSE |
| A-level Chemistry Complete Guide (Yellowreef) (4th ed.) | Themis Publishing | Bond, T. and Hughes, C. | 2014 | A-level |
| Edexcel AS Chemistry | Hodder Education Group | Facer, G. | 2005 | A-level |
| Edexcel AS/A2 Chemistry | Hodder Education Group | Facer, G. | 2009 | A-level |
| OCR A-level Chemistry Student Guide: Practical Chemistry | Hodder Education | Henry, N. | 2017 | A-level |
| Hydrogen and Fuel Cells | ABDO | Hirsch, R. | 2013 | A-level |
| Delmar's Standard Textbook of Electricity | Cengage Learning | Herman, S. | 2015 | A-level |
| My Revision Notes: AQA A-Level Chemistry (2nd ed.) | Hodder Education | King, R. | 2015 | A-level |
| AS and A Level Chemistry (3rd ed.) | Longman | Lewis, E. and Berry, M. | 2000 | A-level |
| Chemistry (3rd ed.) | Palgrave Macmillan | Lewis, R. and Evans, W. | 2006 | A-level |
| AQA Chemistry A Level | Oxford University Press | Lister, T. and Renshaw, J. | 2009 | A-level |
| AQA Chemistry: Student Book. A Level (2nd ed.). | Oxford University Press | Lister, T. and Renshaw, J. | 2015 | A-level |
| Chemistry for the IB Diploma: Standard and Higher Level | Oxford University Press | Neuss, G. | 2001 | A-level |
| Cambridge International AS and A Level Chemistry Revision Guide | Cambridge University Press | Potter, J. and Cann, P. | 2015 | A-level |
| A-Level Chemistry (4th ed.) | Oxford University Press | Ramsden, E. | 2000 | A-level |
| Cambridge International AS and A Level Chemistry Coursebook (2nd ed.) | Cambridge University Press | Ryan, L. and Norris, R. | 2014 | A-level |
| A-Level Study Guide Chemistry Ed H2.2 | Step-by-Step International Pte. | Toh, C. | 2016 | A-level |
| Comprehensive Chemistry (Class XI) | Laxmi Publications Pvt | Verma, N., Khanna, S. and Kapila, B. | 2009 | A-level |
| A Textbook of Physical Chemistry (2nd ed.) | Academic Press | Adamson, A. | 1979 | University |
| Physical Chemistry (2nd ed.) | Wiley | Alberty, R. and Silbey, R. | 1997 | University |
| Fundamental Chemistry | Wiley | Andrews, D.H., Kokes, R. J. | 1962 | University |
| Energy Science: Principles, Technologies, and Impacts (2nd ed.) | Oxford University Press | Andrews, J. and Jelley, N. | 2013 | University |
| The Elements of Physical Chemistry (4th ed.) | Oxford University Press | Atkins, P. and de Paula, J. | 2005 | University |
| Atkins' Physical Chemistry (10th ed.) | Oxford University Press | Atkins, P. and de Paula, J. | 2014 | University |
| Chemical Principles: The Quest for Insight, International Edition (5th ed.) | Macmillan | Atkins, P. and Jones, L. | 2010 | University |
| The Elements of Physical Chemistry (3rd ed.) | Oxford University Press | Atkins, P. W. | 2000 | University |
| Physical Chemistry | Cengage Learning | Ball, D. | 2002 | University |
| Electrochemical Methods: Fundamentals and Applications (2nd ed.) | Wiley | Bard, A. and Faulkner, L. | 2000 | University |
| Physical Chemistry | Oxford University Press | Berry, R., Rice, S. and Ross, J. | 2000 | University |
| Introduction to General, Organic and Biochemistry (3rd ed.) | Nelson Education | Bettelheim, F., Brown, W., Campbell, M., Farrell, S. and Torres, O. | 2015 | University |
| Chemistry (3rd ed.) | John Wiley & Sons Australia, Limited | Blackman, A., Bottle, S., Schmid, S., Mocerino, M. and Wille, U. | 2016 | University |
| Chemistry: The Study of Matter and Its Changes | Wiley | Brady, J.E. and Holum, J. | 1993 | University |
| Chemistry: The Molecular Nature of Matter. (6th ed.) | Wiley | Brady, J.E., Jesperson, N.D. and Hyslop, A. | 2012 | University |
| Chemistry: Matter and its changes (3rd ed.) | Wiley | Brady, J., Russell, J. and Holum, J. | 2000 | University |
| Chemistry: Matter and Its Changes – Student Study Guide (2nd ed.). | Wiley | Brady, J. E. and Senese, F. A. | 2008 | University |
| Chemistry for Engineering Students | Cengage Learning | Brown, L. and Holme, T. | 2006 | University |
| Chemistry: The Central Science (6th ed.) | Pearson Education | Brown, T. L., LeMay, E. and Bursten, B. E. | 1994 | University |
| Fundamentals of Chemistry (4th ed.) | Pearson Education International | Burns, R. | 2003 | University |
| Chemistry3: Introducing Inorganic, Organic and Physical Chemistry | Oxford University Press | Burrows, A., Holman, J., Parsons, A., Pilling, G. and Price, G. | 2009 | University |
| General Chemistry: The Essential Concepts (3rd ed.) | McGraw Hill | Chang, R. | 2000 | University |
| Chemistry: Reactions structure and properies (2nd ed.) | Macmillan | Dillard, C. and Goldberg, D. | 1978 | University |
| Concepts of Physical Chemistry | Discovery Publishing House Pvt. | Goel, A. | 2006 | University |
| Fundamentals of Chemistry (5th ed.) | McGraw Hill | Goldberg, D. E. | 2007 | University |
| Essentials of General, Organic, and Biochemistry (2nd ed.) | Macmillan Learning | Guinn, D. | 2014 | University |
| Chemistry for Changing Times (13th ed.) | Pearson | Hill, J., McCreary, T. and Kolb, D. | 2013 | University |
| Chemistry (3rd ed.) | Pearson Education | Housecroft, C. and Constable, E. | 2006 | University |
| Advanced Theoretical Chemistry | Chatto & Windus | Hughes, D. and Maloney, M. | 1964 | University |
| Chemical Structure and Reactivity: An Integrated Approach (2nd ed.) | Oxford University Press | Keeler, J. and Wothers, P. | 2013 | University |
| Principles of Electrochemistry (2nd ed.) | Wiley | Koryta, J., Dvorak, J. and Kavan, L. | 1993 | University |
| Chemistry and Chemical Reactivity (7th ed.) | Cengage Learning | Kotz, J., Treichel, P. and Townsend, J. | 2008 | University |
| College Chemistry | Addison-Wesley | Mahan, B. | 1966 | University |
| University Chemistry | Addison-Wesley | Mahan, B. | 1970 | University |
| Basic Chemistry (9th ed.) | Wiley | Malone, L. and Dolter, T. | 2013 | University |
| Chemistry: Principles and Reactions (7th ed.) | Cengage Learning | Masterton, W. L., Hurley, C. N. and Neth, E. J. | 2011 | University |
| Fundamentals of General, Organic, and Biological Chemistry (4th ed.) | Pearson Education | McMurry, J. and Castellion, M. | 2006 | University |
| Chemistry (3rd ed.) | Pearson Education International | McMurry, J. and Fay, R. | 2000 | University |
| Chemistry: The Molecular Science (5th ed.) | Cengage Learning | Moore, J. W. and Stanitski, C. L. | 2014 | University |
| Physical Chemistry (2nd ed.) | Harcourt Academic Press | Mortimer, R. | 2000 | University |
| Textbook of Physical Chemistry (2nd ed.) | PHI Learning | Moudgil, H. | 2014 | University |
| Principles of Chemistry | W. W. Norton, Inc. | Munowitz, M. and Prigodich, R. | 1999 | University |
| A Textbook of Physical Chemistry | Wiley Eastern | Negi, A. S. and Anand, S. C. | 1985 | University |
| Advanced Problems in Applied Chemistry: Topics in Environmental Chemistry and Related Fields | Taylor & Francis | O'Connor, R. | 2000 | University |
| Chemistry: The Molecular Science (3rd ed.) | Mosby | Olmsted, J. and Williams, G. | 2002 | University |
| Introduction to General, Organic, and Biological Chemistry | Prentice Hall | Ouellette, R. | 1997 | University |
| Principles of Modern Chemistry | Cengage | Oxtoby, D., Gillis, H. and Butler, L. | 2016 | University |
| College Chemistry | W. H. Freeman and Co. | Pauling, L. | 1964 | University |
| Chemistry | W. H. Freeman and Co. | Pauling, L. and Pauling, P. | 1975 | University |
| General Chemistry: Principles and Modern Applications (11th ed.) | Pearson Education | Petrucci, R., Herring, F., Madura, J. and Bissonnette, C. | 2017 | University |
| Principles of Physical Chemistry | Prentice Hall | Raff, L. | 2001 | University |
| Introductory Chemistry | Pearson Benjamin Cummings | Russo, S. and Silver, M. | 2001 | University |
| Electrochemistry for Chemists (2nd ed.) | Wiley | Sawyer, D. T., Sobkowiak, A. and Roberts, J. L. | 1995 | University |
| Chemistry: A Systematic Approach | Oxford University Press | Sisler, H. H., Dresdner, R. and Mooney, W. T. | 1980 | University |
| Chemical Principles in the Laboratory (11th ed.) | Cengage Learning | Slowinski, E., Wolsey, W. and Rossi, R. | 2015 | University |
| Fundamentals of General Chemistry | Prentice Hall | Sorum, C. H. | 1963 | University |
| General, Organic, and Biological Chemistry (6th ed.) | Cengage Learning | Stoker, H. | 2013 | University |
| Introduction to Chemical Principles (11th ed.) | Pearson Education | Stoker, H. | 2013 | University |
| General, Organic, and Biological Chemistry: Structures of Life (5th ed.) | Pearson Higher Education | Timberlake, K. C. | 2016 | University |
| Chemistry: Structure and Properties | Pearson Higher Education | Tro, N. J. | 2015 | University |
| Basics for Chemistry | Elsevier Science | Ucko, D. | 1982 | University |
| Inorganic Chemistry: Theory Practice and Application (2nd ed.) | Royal Society of Chemistry | Zanello, P., Nervi, C. and de Biani, F. | 2012 | University |
| Chemistry (8th ed., International Student Edition) | Cengage | Zumdahl, S. S. and Zumdahl, S. A. | 2010 | University |
Acknowledgements
We would like to thank many colleagues for their very helpful input into the content of this paper, including Rob van Veen, Keith Taber, Peter Wothers, Chris Brackstone, John Suberu, Klaus Ruthenberg, Susan Perkin, Andrea Sella, Daren Caruana, Ju Yeon Park, Andrew Howe and David Oxtoby, as well as seminar audiences at the Faculty of Education and the SCI Seminar Series in Cambridge. We also thank two anonymous referees for CERP for very helpful corrections and critical suggestions. Hasok Chang is grateful to the British Academy for the Wolfson Research Professorship.References
- Andrews D. H. and Kokes R. J., (1962), Fundamental Chemistry, New York: John Wiley & Sons, Inc.
- Andrews J. and Jelley N., (2013), Energy Science: Principles, Technologies, and Impacts, 2nd edn, Oxford: Oxford University Press.
- Atkins P. W., (2011), Reactions: The Private Life of Atoms, Oxford: Oxford University Press.
- Atkins P. and de Paula J., (2014), Atkins' Physical Chemistry, 10th edn, Oxford: Oxford University Press.
- Blackman A., Bottle S., Schmid S., Mocerino M. and Wille U., (2016), Chemistry, 3rd edn, Milton, Qld.: John Wiley & Sons Australia.
- Bockris J. O’M. and Reddy, A. K. N., (1970), Modern Electrochemistry, New York: Plenum Press, vol. 2.
- Borley M., (2016), Chemistry, Oxford: Oxford University Press.
- Brady J. E. and Holum J., (1993), Chemistry: The Study of Matter and Its Changes, New York: Wiley.
- Brady J. E., Russell J. and Holum J., (2000), Chemistry: The Study of Matter and Its Changes, 3rd edn, New York: Wiley.
- Brady J. E., Jesperson N. D. and Hyslop A., (2012), Chemistry: The Molecular Nature of Matter, 6th edn, Hoboken, NJ: Wiley.
- Brown T. L., LeMay E. and Bursten B. E., (1994), Chemistry: The Central Science, 6th edn, London: Prentice-Hall International.
- Burrows A., Holman J., Parsons A., Pilling G. and Price G., (2009), Chemistry: Introducing Inorganic, Organic and Physical Chemistry, Oxford: Oxford University Press.
- Chang H., (2012), Is Water H2O? Evidence, Realism and Pluralism, Dordrecht: Springer.
- Chang R., (2000), Essential Chemistry: A Core Text for General Chemistry, 3rd edn, New York: McGraw-Hill.
- Clyde B., (2006), Science Uncovered: AQA Additional Science for GCSE Student Book, Hirst K. (ed.), Oxford: Heinemann.
- Daniell J. F., (1839), On the Electrolysis of Secondary Compounds, Philos. Trans. R. Soc. London, 129, 97–112.
- Daniell J. F., (1840), Second Letter on the Electrolysis of Secondary Compounds, Philos. Trans. R. Soc. London, 130, 209–224.
- Daniels A., (2016), OCR Gateway GCSE (9-1) Chemistry for Combined Science, London: HarperCollins.
- Deloughry W., (1997), New Modular Science for GCSE (No. 1), Oxford: Heinemann.
- Dillard C. and Goldberg D., (1978), Chemistry: Reactions, Structure and Properties, 2nd edn, New York: Macmillan.
- Fullick A. and Fullick P., (2001), Chemistry for AQA: Separate Award, Cheltenham: Nelson Thornes.
- Gallagher R. and Ingram P., (2000), Complete Chemistry, Oxford: Oxford University Press.
- Hartshorne C. and Weiss P. (ed.), (1931), The Collected Papers of Charles Sanders Peirce, Cambridge, MA: Harvard University Press, vol. 1.
- Hill G., (2002), Chemistry Counts, 3rd edn, London: Hodder & Stoughton.
- Hirsch R., (2013), Hydrogen and Fuel Cells, North Mankato, MN: ABDO Publishing Company.
- Holman J. and Stone P., (2001), Chemistry, 2nd edn, Cheltenham: Nelson Thornes.
- Honeysett I., Lees D., Bibby S. and Macdonald A., (2006), Gateway Science: OCR Additional Science for GCSE Foundation, McDuell B. (ed.), Harlow: Pearson Education.
- Honeysett I., Tear C. and Sadler J., (2007), Revise GCSE – Additional Science, London: Letts Educational.
- Honeysett I., Tear C. and Poole E., (2011), Revise GCSE - Science, London: Letts Educational.
- Hoong T., Leng H. and Khang G., (2006), Interactive Science Textbook 2, Singapore: Panpac Education Pte Ltd.
- Housecroft C. and Constable E., (2006), Chemistry, 3rd edn, Harlow: Pearson Education Limited.
- Hyun J. O., Kim H. S., Yoon H. K., Kang T. W., Choi W. S., Han I. O., Park M. Y., Kim K. S., Ji J. H., Cho H. S., Kim B. I., Kim M. K., Son Y. A. and Lee T. Y., (2013a), Science 2, Seoul: Sinsago.
- Hyun J. O., Kim H. S., Yoon H. K., Kang T. W., Choi W. S., Han I. O., Park M. Y., Kim K. S., Ji J. H., Cho H. S., Kim B. I., Kim M. K., Son Y. A. and Lee T. Y., (2013b), Teachers’ Guide: Science 2, Seoul: Sinsago.
- Im T. H., No S. H., Paik J. M., Lee B. Y., Kang D. H., Jang H. S., Kim J. S., Lee Y. C., Whang I. S., Ko H. D. and Sin M. Y., (2013a), Science 2, Seoul: Visang.
- Im T. H., No S. H., Paik J. M., Lee B. Y., Kang D. H., Jang H. S., Kim J. S., Lee Y. C., Whang I. S., Ko H. D. and Sin M. Y., (2013b), Teachers’ Guide: Science 2, Seoul: Visang.
- Justi R. and Gilbert J., (2003), Models and Modelling in Chemical Education, in Gilbert J. K. et al. (ed.), Chemical Education: Towards Research-based Practice, Dordrecht: Springer, pp. 47–68.
- Keeler J. and Wothers P., (2013), Chemical Structure and Reactivity: An Integrated Approach, 2nd edn, Oxford: Oxford University Press.
- Kim H. J., Kim H. S., Lee B. K., Lee S. M., Lee Y. S., Lee J. H., Lee J. S., Lee H. N. and Cho H. S., (2011a), Chemistry I, Seoul: Sangsang Academy Press.
- Kim H. J., Kim H. S., Lee B. K., Lee S. M., Lee Y. S., Lee J. H., Lee J. S., Lee H. N. and Cho H. S., (2011b), Teachers’ Guide: Chemistry I, Seoul: Sangsang Academy Press.
- Kim H. J., Kim H. S., Lee B. K., Lee S. M., Lee Y. S., Lee J. H., Lee J. S., Lee H. N. and Cho H. S., (2012a), Chemistry II, Seoul: Sangsang Academy Press.
- Kim H. J., Kim H. S., Lee B. K., Lee S. M., Lee Y. S., Lee J. H., Lee J. S., Lee H. N. and Cho H. S., (2012b), Teachers’ Guide: Chemistry II. Seoul: Sangsang Academy Press.
- Kotz J., Treichel P. and Townsend J., (2008), Chemistry and Chemical Reactivity, 7th edn, Boston, MA: Cengage Learning.
- Le Blanc M., (1896), The Elements of Electrochemistry, translated from the German by Whitney W. R., London: Macmillan and Co., Ltd.
- Lee J. S., Kim S. H., Kim H. R., Kim H. S., Park K. T., Park M. S., Park S. Y., Park Y. O., Bae M. J., Song S. J., Lee S. Y., Lim H., Jung D. H. and Hong J. Y., (2013a), Science 2, Seoul: Dusan Donga.
- Lee J. S., Kim S. H., Kim H. R., Kim H. S., Park K. T., Park M. S., Park S. Y., Park Y. O., Bae M. J., Song S. J., Lee S. Y., Lim H., Jung D. H. and Hong J. Y., (2013b), Teachers’ Guide: Science 2, Seoul: Dusan Donga.
- Lee K. S., Kim S. J., Kim T. I., An H. S., Choi M. H., Kim H. S., Kim H. K., Cho K. S., Oh H. S., Bae M. J., Lee J. W., Ryu H. K. and Choi G. S., (2013c), Science 2, Seoul: Mirae-n.
- Lee K. S., Kim S. J., Kim T. I., An H. S., Choi M. H., Kim H. S., Kim H. K., Cho K. S., Oh H. S., Bae M. J., Lee J. W., Ryu H. K. and Choi G. S., (2013d), Teachers’ Guide: Science 2, Seoul: Mirae-n.
- Lee M. W., Jang B. G., Ryu S. H., Cho Y. G., Noh S. G., Lee J. H., Chang C. H., Lee B.Y., Kang H. J., Han E. G., Lee S. J., Kim Y. G. and Lee Y. J., (2013e), Science 2, Seoul: Chunjae.
- Lee M. W., Jang B. G., Ryu S. H., Cho Y. G., Noh S. G., Lee J. H., Chang C. H., Lee B.Y., Kang H. J., Han E. G., Lee S. J., Kim Y. G. and Lee Y. J., (2013f), teachers’ Guide: Science 2, Seoul: Chunjae.
- Lee S. I., Kim Y. H., Nam K. S., Hwang S. Y., Kim Y. K., Kwon O. S., Kim K. T., No D. G., Park R. W., Shin S. J., Paik S. Y., Yeo J. Y., Cho B. J., Jeon B. H. and Kim C. W., (2013g), Science 2, Seoul: Jihaksa.
- Lee S. I., Kim Y. H., Nam K. S., Hwang S. Y., Kim Y. K., Kwon O. S., Kim K. T., Noh D. G., Park R. W., Shin S. J., Paik S. Y., Yeo J. Y., Cho B. J., Jeon B. H. and Kim C. W., (2013h), Teachers’ Guide: Science 2, Seoul: Jihaksa.
- Lee S. M., Chae K. P., Nam K. W., Noh T. H., Seo I. H., Kang S. J., Kim Y. S., Khum J. H., Moon K. W., Lee M. W., Kwon S. M. and Son Y. W., (2013i), Science 2, Seoul: Kumsung.
- Lee S. M., Chae K. P., Nam K. W., Noh T. H., Seo I. H., Kang S. J., Kim Y. S., Khum J. H., Moon K. W., Lee M. W., Kwon S. M. and Son Y. W., (2013j), Teachers’ Guide: Science 2, Seoul: Kumsung.
- Lewis E. and Berry M., (2000), AS and A Level Chemistry, 3rd edn, London: Longman.
- Lowry T. M., (1929), A Class Book of Physical Chemistry, London: Macmillan and Co., Ltd.
- MacInnes D. A., (1961), The Principles of Electrochemistry, New York: Dover.
- Malone L. and Dolter T., (2013), Basic Chemistry, 9th edn, New York: Wiley.
- Masterton W. L., Hurley C. N. and Neth E. J., (2011), Chemistry: Principles and Reactions, 7th edn, Belmont, CA: Brookes/Cole.
- McMurry J. and Fay R., (2000), Chemistry, 3rd edn, Belmont, CA.: Pearson Education International.
- Mellor J. W., (1951), Mellor's Modern Inorganic Chemistry, revised and edited by Parkes G. D., London: Longmans, Green and Co.
- Miller W. A., (1867), Elements of Chemistry: Theoretical and Practical, Part 1. Chemical Physics, 4th edn, London: Longmans, Green, Reader, and Dyer.
- Negi A. and Anand S., (1985), A Textbook of Physical Chemistry, New Delhi: Wiley Eastern.
- Noh T. H., Choi S. S., Kang S. J., Lee S. Y., Bae B. I., Go S. Y., Joo Y. and Choi S. Y., (2011a), Chemistry I, Seoul: Chunjae.
- Noh T. H., Choi S. S., Kang S. J., Lee S. Y., Bae B. I., Go S. Y., Joo Y. and Choi S. Y., (2011b), Teachers’ Guide: Chemistry I, Seoul: Chunjae.
- Noh T. H., Choi S. S., Kang S. J., Lee S. Y., Bae B. I., Go S. Y., Joo Y. and Choi S. Y., (2012a), Chemistry II, Seoul: Chunjae.
- Noh T. H., Choi S. S., Kang S. J., Lee S. Y., Bae B. I., Go S. Y., Joo Y. and Choi S. Y., (2012b), Teachers’ Guide: Chemistry II, Seoul: Chunjae.
- Olmsted J. and Williams G., (2002), Chemistry: The Molecular Science, 3rd edn, St. Louis, MO: Mosby.
- Owen R. and King S., (2005), Chemistry at a Glance: Full Chemistry Content of the New GCSE, Boca Raton, FL: CRC Press.
- Oxtoby D., Gillis H. and Butler L., (2016), Principles of Modern Chemistry, 8th edn, Boston, MA: Cengage.
- Park H. S., Jeong D. Y., Shin H. S., Kim J. H., Hur S. I., Cho S. Y., You S. N., Lee H. W., Kim J. Y., Lee S. J., Choi B. S., Kang S. C. and Oh S. W., (2013a), Science 2, Seoul: Kyohaksa.
- Park H. S., Jeong D. Y., Shin H. S., Kim J. H., Hur S. I., Cho S. Y., You S. N., Lee H. W., Kim J. Y., Lee S. J., Choi B. S., Kang S. C. and Oh S. W., (2013b), Teachers’ Guide: Science 2, Seoul: Kyohaksa.
- Park J. S., Youn Y., Jung J. O., Cho E. M. and Ryu S. K., (2011a), Chemistry I, Seoul: Kyohaksa.
- Park J. S., Youn Y., Jung J. O., Cho E. M. and Ryu S. K., (2011b), Teachers’ Guide: Chemistry I, Seoul: Kyohaksa.
- Park J. S., Youn Y., Jung J. O., Cho E. M. and Ryu S. K., (2012a), Chemistry II, Seoul: Kyohaksa.
- Park J. S., Youn Y., Jung J. O., Cho E. M. and Ryu S. K., (2012b), Teachers’ Guide: Chemistry II, Seoul: Kyohaksa.
- Pauling L. and Pauling P., (1975), Chemistry, San Francisco: W. H. Freeman and Co.
- Petrucci R., Herring F., Madura J. and Bissonnette C., (2017), General Chemistry: Principles and Modern Applications, 11th edn, Toronto: Pearson Education.
- Pletcher D., (2009), A First Course in Electrode Processes, 2nd edn, Romsey, Hants: The Electrochemical Consultancy.
- Ramsden E., (2000), A-Level Chemistry, 4th edn, Oxford: Oxford University Press.
- Roebuck C., (2003), Excel Preliminary Chemistry, Revised edn, Glebe, NSW: Pascal Press.
- Ryu H. I., Kim C. S., Lee G. P., Lee J. B., Park S. B., Kang S. G., Kim Y. Y. and Lee H. G., (2011a), Chemistry I, Seoul: Visang.
- Ryu H. I., Kim C. S., Lee G. P., Lee J. B., Park S. B., Kang S. G., Kim Y. Y. and Lee H. G., (2011b), Teachers’ Guide: Chemistry I, Seoul: Visang.
- Ryu H. I., Kim C. S., Lee G. P., Lee J. B., Park S. B., Kang S. G., Kim Y. Y. and Lee H. G., (2012a), Chemistry II, Seoul: Visang.
- Ryu H. I., Kim C. S., Lee G. P., Lee J. B., Park S. B., Kang S. G., Kim Y. Y. and Lee H. G., (2012b), Teachers’ Guide: Chemistry II, Seoul: Visang.
- Saunders N., (2016), OCR Gateway Chemistry for GCSE Combined Science, Oxford: Oxford University Press.
- Schmit A. and Pollard J., (2016), WJEC GCSE Chemistry, London: Hodder Education Group.
- Sin Y. J., Jin M. S., Han M. J., Lee G. Y., Jung E. Y., Kang J. C., Kang S. J., Shon J. W., Bae Y. H., Lee B. W., Im H. Y. and Ha E. S., (2013a), Science 2, Seoul: Chunjae.
- Sin Y. J., Jin M. S., Han M. J., Lee G. Y., Jung E. Y., Kang J. C., Kang S. J., Shon J. W., Bae Y. H., Lee B. W., Im H. Y. and Ha E. S., (2013b), Teachers’ Guide: Science 2, Seoul: Chunjae.
- Sisler H. H., Dresdner R. and Mooney W. T., (1980), Chemistry: A Systematic Approach, Oxford: Oxford University Press.
- Toh C., (2016), A-Level Study Guide Chemistry Ed H2.2, Singapore: Step-by-Step International Pte. Ltd.
- Tro N. J., (2015), Chemistry: Structure and Properties, Global edn, Harlow: Pearson Higher Education.
- Tsaparlis G., (2012), Electrolysis, Electrolytes, and Galvanic Cells, in Taber K. S. (ed.), Teaching Secondary Chemistry, 2nd edn, London: Association for Science Education/Hodder Education, pp. 253–278.
- Tsaparlis G., (2019), Teaching and Learning Electrochemistry, Isr. J. Chem., 59, 478–492.
- Ucko D., (1982), Basics for Chemistry, New York: Academic Press.
- Verma N., Khanna S. and Kapila B., (2009), Comprehensive Chemistry XI, New Delhi: Laxmi Publications Pvt Limited.
- Walker D., (2007), Chemical Reactions (Core Chemistry), London: Evans Brothers.
- Zumdahl S. S. and Zumdahl S. A., (2010), Chemistry, 8th edn, International Student Edition, New York: Cengage Learning.
| This journal is © The Royal Society of Chemistry 2020 |
