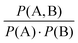What students write about when students write about mechanisms: analysis of features present in students’ written descriptions of an organic reaction mechanism
Field M.
Watts
 a,
Jennifer A.
Schmidt-McCormack
a,
Jennifer A.
Schmidt-McCormack
 b,
Catherine A.
Wilhelm
a,
Ashley
Karlin
c,
Atia
Sattar
c,
Barry C.
Thompson
b,
Catherine A.
Wilhelm
a,
Ashley
Karlin
c,
Atia
Sattar
c,
Barry C.
Thompson
 d,
Anne Ruggles
Gere
e and
Ginger V.
Shultz
d,
Anne Ruggles
Gere
e and
Ginger V.
Shultz
 *a
*a
aDepartment of Chemistry, University of Michigan, Ann Arbor, Michigan 48109, USA. E-mail: gshultz@umich.edu
bChemistry Department, St. Ambrose University, Davenport, Iowa 52803, USA
cWriting Program, University of Southern California, Los Angeles, California 90089-1062, USA
dDepartment of Chemistry and Loker Hydrocarbon Research Institute, University of Southern California, Los Angeles, CA 90089-1661, USA
eDepartment of English Language and Literature and School of Education, University of Michigan, Ann Arbor, Michigan 48109, USA
First published on 10th July 2020
Abstract
Learning to reason through organic reaction mechanisms is challenging for students because of the volume of reactions covered in introductory organic chemistry and the complexity of conceptual knowledge and reasoning skills required to develop meaningful understanding. However, understanding reaction mechanisms is valuable for students because they are useful for predicting and explaining reaction outcomes. To identify the features students find pertinent when explaining reaction mechanisms, we have collected students’ written descriptions of an acid-catalysed amide hydrolysis reaction. Students’ writing was produced during the implementation of Writing-to-Learn assignments in a second semester organic chemistry laboratory course. We analysed students’ written responses using an analytical framework for recognizing students’ mechanistic reasoning, originally developed with attention to the philosophy of science literature. The analysis sought to identify the presence of specific features necessary for mechanistic reasoning belonging to four broad categories: (1) describing an overview of the reaction, (2) detailing the setup conditions required for the mechanism to occur, (3) describing the changes that take place over the course of the mechanism, and (4) identifying the properties of reacting species. This work provides a qualitative description of the variety of ways in which students included these features necessary for mechanistic reasoning in their writing. We additionally analysed instances of co-occurrence for these features in students’ writing to make inferences about students’ mechanistic reasoning, defined here as the use of chemical properties to justify how electrons, atoms, and molecules are reorganized over the course of a reaction. Feature co-occurrences were quantified using the lift metric to measure the degree of their mutual dependence. The quantitative lift results provide empirical support for the hierarchical nature of students’ mechanistic descriptions and indicate the variation in students’ descriptions of mechanistic change in conjunction with appeals to chemistry concepts. This research applies a framework for identifying the features present in students’ written mechanistic descriptions, and illustrates the use of an association metric to make inferences about students’ mechanistic reasoning. The findings reveal the capacity of implementing and analysing writing to make inferences about students’ mechanistic reasoning.
Introduction
Organic chemistry is a challenging subject, largely because of the volume of reaction mechanisms presented in the course, which are especially difficult for students to learn meaningfully. This challenge is due in part to the conceptual nature of the discipline (Anderson and Bodner, 2008; Grove and Bretz, 2012) and is related to the types of problem solving skills required for success in the organic chemistry classroom (Kraft et al., 2010; Graulich, 2015). Previous research has focused on this acknowledged difficulty, including investigations characterizing the use and usefulness of the electron-pushing formalism (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012a; Grove et al., 2012b); research examining students’ use of conceptual reasoning applied to reaction mechanisms (Anzovino and Bretz, 2015; Cooper et al., 2016; Bhattacharyya and Harris, 2018; Caspari et al., 2018a; Petterson et al., 2020); and studies involving restructuring the curricula for general chemistry (Crandell et al., 2018) or organic chemistry (Grove et al., 2008; Flynn and Ogilvie, 2015; Flynn and Featherstone, 2017; Galloway et al., 2017; Webber and Flynn, 2018) to promote students’ understanding of the connections between chemical structure, properties, and reactivity.Understanding how students both describe and explain reaction mechanisms is valuable because of the inherent challenge of learning to use the electron-pushing formalism while connecting steps in a mechanism to conceptual understanding. A means to access students’ descriptions and explanations on a large scale is through students’ writing. Writing-to-Learn (WTL) is a pedagogical practice that instructs students to produce written artefacts of their knowledge, which can serve as a resource for understanding students’ reasoning (Grimberg and Hand, 2009; Moreira et al., 2018; Moon et al., 2019) while serving to promote students’ conceptual understanding (Reynolds et al., 2012; Shultz and Gere, 2015; Finkenstaedt-Quinn et al., 2017; Moon et al., 2018; Gere et al., 2019; Schmidt-McCormack et al., 2019).
The goal of this study is to investigate the mechanistic reasoning used by a large number of students by analysing their written responses to a WTL prompt meant to elicit mechanistic reasoning about a specific reaction mechanism. The first objective of the analysis is to describe the variations in the way students write about the components they found pertinent when describing and explaining the mechanism, coded as features necessary for engaging in mechanistic reasoning. The second objective of the analysis is to identify students’ engagement in mechanistic reasoning by examining the co-occurrences of these features. Note that, although there is no consensus on the definition of mechanistic reasoning (Bhattacharyya, 2013), for the purposes of this study, we conceptualize mechanistic reasoning as the ability to identify the species involved over the course of a reaction (e.g., the starting materials, intermediates, and products), to provide an account for how molecules change over the course of a reaction, and to appeal to chemical properties to justify why these changes occur. This definition aligns with the common features present in the various definitions of mechanistic reasoning identified by organic chemistry faculty (Bhattacharyya, 2013), and this definition aligns with those identified in prior studies (Becker et al., 2016; Cooper et al., 2016; Weinrich and Talanquer, 2016; Moreira et al., 2018). In particular, this definition of mechanistic reasoning requires both the what and how for a reaction—i.e., describing what structural changes occur from starting materials to intermediates to products and how these changes arise from interactions between the involved subcomponents (electrons, atoms, and molecules). This definition also requires justifications for why mechanistic steps occur by appealing to the properties of involved components (e.g., nucleophilicity and electrophilicity). Note that this definition of mechanistic reasoning is distinct from some definitions of causal mechanistic reasoning, which also require an energetic justification for why a reaction proceeds as it does from one step to the next (Caspari et al., 2018a; Caspari et al., 2018b).
Mechanistic reasoning in organic chemistry
Mechanisms are used by organic chemists to explain or predict the outcome of reactions. Because of their usefulness, the organic chemistry curriculum typically involves a study of the mechanisms for each class of reaction presented to students, and problems are often posed assuming students will be able to use mechanisms as a problem-solving tool (Grove et al., 2012a; Grove et al., 2012b). Hence, the ability to reason through a reaction mechanism is a useful skill that can help students achieve success in organic chemistry (Grove et al., 2012a).However, research has shown that many students do not use mechanisms meaningfully and that students often do not value the electron-pushing formalism in the same way as practicing chemists (Grove et al., 2012a; Grove et al., 2012b). Additionally, studies found that students may not conceptualize the electron-pushing formalism to have any physical meaning (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008), though this was shown not to be true in a modified curriculum (Galloway et al., 2017; Webber and Flynn, 2018). Prior research also suggests that students hold a range of intuitions, misconceptions, and understandings regarding fundamental concepts pertaining to organic reaction mechanisms (Cartrette and Mayo, 2011; Anzovino and Bretz, 2016; Cooper et al., 2016; Finkenstaedt-Quinn et al., 2020a; Petterson et al., 2020). Although students might have some conceptual understanding—and are often able to produce correct mechanisms for common reactions—studies have demonstrated that they often lack the ability to connect chemical reasoning to individual steps in a reaction mechanism (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Kraft et al., 2010; Graulich, 2015).
Particular barriers to students’ learning are their approaches to problem-solving, which may be either product- or process-oriented. Product-oriented approaches incorporate reasoning focused on the final product, result, or answer to the problem rather than the process or methods by which the solution is obtained. Process-oriented approaches include model-based reasoning, in which mechanistic explanations are developed using generalized mental models about structure and reactivity (Kraft et al., 2010; Christian and Talanquer, 2012), and are reflected in students’ use of causal or multi-component argumentation to explain chemical reactions (Sevian and Talanquer, 2014; Cooper et al., 2016; Weinrich and Talanquer, 2016; Bodé et al., 2019). Successful process-oriented approaches also include reasoning that demonstrates knowledge of the connections between properties of reacting species (e.g., basicity or nucleophilicity) and the mechanistic steps of a reaction (De Arellano and Towns, 2014). Process-oriented problem-solving requires students to reason about the process of a reaction as opposed to reasoning only about the reactants and products. This type of problem-solving values the usefulness of mechanisms to explain or predict reaction outcomes, and is hence an important skill to develop when learning organic chemistry (Graulich, 2015).
Despite the importance of the process of a mechanism, students often engage in product-oriented problem-solving (Graulich, 2015). This type of problem-solving is evident in students’ drawn mechanisms which often demonstrate a focus on simply illustrating mechanistic steps to arrive at the given product without considering whether or not the steps shown are chemically reasonable (Bhattacharyya and Bodner, 2005; Caspari et al., 2018b; Petterson et al., 2020). Product-oriented strategies include reasoning based on remembered cases or rules that are prompted by the surface features of molecules (Kraft et al., 2010; Christian and Talanquer, 2012; De Arellano and Towns, 2014), and are evident in studies demonstrating students’ use of descriptive or relational argumentation that lacks consideration of multiple components or cause-effect relationships when explaining chemical reactions (Sevian and Talanquer, 2014; Cooper et al., 2016; Weinrich and Talanquer, 2016; Bodé et al., 2019). Additionally, product-oriented strategies are evident in studies illustrating that students do not necessarily consider alternative reaction pathways or the dynamic, rather than static, nature of chemical reactions (Caspari et al., 2018a; Popova and Bretz, 2018). A possible reason that students focus on product- rather than process-oriented problem solving is that general chemistry tends to foster product-oriented strategies, so many of the problem-solving skills students have learned in prior courses do not transfer to organic chemistry (Anderson and Bodner, 2008; Grove and Bretz, 2012).
The disciplinary skills and conceptual knowledge with which students must be proficient while solving mechanistic problems is an additional barrier to learning. Students must have representational competence, and they must engage with many concepts fundamental to understanding mechanisms, including recognizing reactants as acids and bases or as nucleophiles and electrophiles (Graulich, 2015). Because students must access many types of information when working with mechanisms, it can be difficult for them to make connections between what occurs in a mechanism and the chemical explanations underlying each step. This issue of cognitive load has been suggested to contribute to students’ devaluation of mechanisms for problem-solving purposes (Grove et al., 2012a) and is connected to the concern that mechanisms are usually taught in a way that encourages memorization (a product-oriented approach) and discourages chemical understanding (a process-oriented approach) (Galloway et al., 2017). The research in mechanistic reasoning has identified students’ struggles with learning mechanisms, detailing how students solve problems or explain reactions with a focus on the answer rather than using chemical reasoning to understand the process. The literature demonstrates that this lack of engagement is connected to problems of cognitive load and lack of sophisticated chemical understanding. These findings provide space for research-based instructional practices that promote students’ abilities to apply chemical reasoning to reaction mechanisms.
Using Writing-to-Learn to access students’ mechanistic reasoning
An instructional practice that requires students to engage with mechanisms beyond working with the electron-pushing formalism is Writing-to-Learn (WTL), which involves using writing assignments to engage students with course content. The primary goal of WTL is to foster students’ deeper conceptual understanding (Anderson et al., 2015; Gere et al., 2019). WTL has been implemented in the context of chemistry courses and has been shown to support development of conceptual knowledge and disciplinary reasoning skills (Grimberg and Hand, 2009; Shultz and Gere, 2015; Finkenstaedt-Quinn et al., 2017; Finkenstaedt-Quinn et al., 2019; Moon et al., 2018, 2019; Schmidt-McCormack et al., 2019; Finkenstaedt-Quinn et al., 2020b).WTL can be leveraged in the context of organic chemistry to help students identify the value in utilizing mechanisms to solve problems. Using WTL in this way is motivated by the idea that writing offers a valuable route into the electron-pushing formalism, which prior researchers recognized as a language that students must first learn and understand before being able to use successfully when engaging in reasoning (Grove et al., 2012a; Flynn and Ogilvie, 2015; Flynn and Featherstone, 2017; Galloway et al., 2017). As opposed to problems requiring students to use the electron-pushing formalism—problems which assume that students will implicitly make connections between mechanistic representations and chemical reasoning—writing requires students to explicitly make such connections. This allows researchers to use students’ writing to infer and analyse their reasoning, and for the work of many students to be analysed (as opposed to interview analysis which is typically limited to a small subset of students).
Theoretical framework
This research is grounded in theories of writing as a tool for learning, with particular attention to perspectives on the cognitive processes that occur during writing (Emig, 1977; Klein, 1999; MacArthur and Graham, 2016). These theories not only justify the implementation of WTL pedagogies (Klein, 1999; Klein and Boscolo, 2016), but also serve as a theoretical basis for analysing students’ written work for evidence of mechanistic reasoning. This study is specifically guided by the cognitive process theory of writing originally proposed by Flower and Hayes (1981, 1984) and later revised by Hayes (1996). This theory states that learning occurs when writers must access content knowledge and address content problems to meet their writing goals. Components of the theory include the social environment, the motivation for writing, and the cognitive moves that are made while writing (Hayes, 1996). The theory identifies three cognitive processes—planning, writing, and revising—that occur at every point during the production of a text. These processes occur in the context of the task environment—including the problem or prompt, the text-in-production, and the social environment—and require the writer to access any available knowledge of the topic (Flower and Hayes, 1981). During these processes, the writer must form internal representations of knowledge, translate these representations into language, and evaluate and revise the text being written (Flower and Hayes, 1984). This is where learning can occur, as the writer must explore and consolidate knowledge for the purpose of translating representations into written language.The cognitive process theory of writing provides ground for utilizing students’ written work as an analytical tool for understanding students’ knowledge. Writing a mechanistic description requires students to find or produce the symbolically represented reaction mechanism and to translate it into words, using their knowledge of fundamental chemistry concepts to explain why mechanistic steps occur. While doing this translation, students engage in the recursive process of writing which requires them to explore their knowledge and revisit their ideas. While there is a possibility that students might use appropriate jargon without actually understanding the language they are using (Ferguson and Bodner, 2008), the cognitive process theory posits that when using these words in their writing, students are at least engaging with the related concepts. The analysis of students’ writing relies on the fact that students are given time to decide what information to include and not include. Thus, when a student chooses to include (or, during the process of writing, does not include) some aspect necessary to engage in reasoning, it can provide insight into what content students do and do not find relevant when explaining a reaction mechanism. For these reasons, students’ writing can serve as a useful source of data for understanding students’ reasoning.
Research questions
The present study examines students’ responses to a writing assignment eliciting descriptions of an organic reaction mechanism. The research seeks to address the following questions to demonstrate the use of writing analysis to make inferences about students’ mechanistic reasoning:1. What features necessary for mechanistic reasoning are present in students’ written descriptions of an organic reaction mechanism?
2. How do students write about each feature?
3. What inferences about students’ mechanistic reasoning can be made by analysing co-occurrences of the features necessary for mechanistic reasoning?
Methods
Setting and participants
The study was conducted at a large, Midwestern research university within a second-semester organic chemistry laboratory course (often taken concurrently with the second-semester lecture course). The laboratory course includes a lecture and laboratory component, both of which meet once a week. The lecture is taught by faculty and postdoctoral instructors who describe experiments and procedures, and the laboratory is facilitated by graduate teaching assistants. The coursework requires students to maintain a laboratory notebook, complete three writing assignments (one of which is the focus of this study), and take quizzes for assessment. The three writing assignments made up thirty percent of students’ grades, with each writing assignment contributing ten percent. The participants consisted of the 543 students who received a final score in the course and completed the WTL assignment described below.Writing-to-Learn assignment
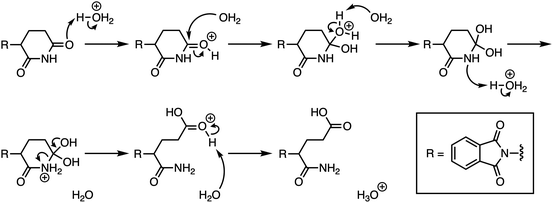
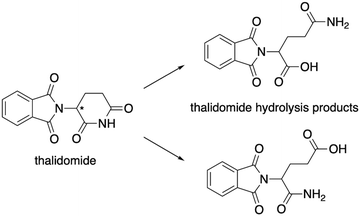
The WTL assignment was the third and final WTL assignment that students completed during the semester. It was developed in collaboration with researchers experienced in designing writing assignments to support meaningful learning and with attention to components of the cognitive process theory of writing (Hayes, 1996; Gere et al., 2019). The relevant prompt components are specified in Fig. 1, with the full prompt reproduced in Appendix 1. The prompt design included consideration of components meant to elicit mechanistic reasoning by describing that thalidomide undergoes acid-catalysed hydrolysis and explicitly illustrating two hydrolysis products. Students were asked to describe the mechanism for the formation of both hydrolysis products and to propose an analog that would prevent the mechanism. For reference, one of the two pathways for the mechanism students were expected to describe is presented in Fig. 2. As students were given starting materials and products, the learning objective for the mechanistic description was for students to demonstrate their reasoning for the reaction mechanism. We limited the focus of this study to students’ descriptions of the amide hydrolysis mechanism.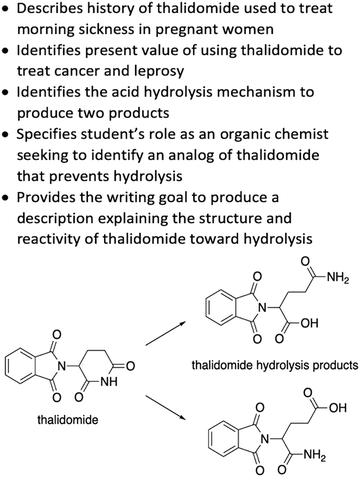 | ||
| Fig. 1 Relevant prompt components and the starting material and products for the reaction students were asked to describe and explain. | ||
Writing-to-Learn implementation
Students’ first drafts were due on a Friday, after which students were randomly assigned to read and provide feedback for three of their peers in a double-blind peer-review by the following Monday. After receiving feedback, students were required to revise and resubmit the assignment by the end of the week. Students were able to ask questions and receive guidance on the assignment from the course writing fellows who were undergraduate students that had previously been successful in the course and were trained to provide feedback on content and writing. Grades for this assignment were determined independently of the present analysis.Data collection
The data collected and analysed from the WTL assignment were students’ final drafts. Before collecting any data, the Institutional Review Board granted approval for the study and the participating students provided consent. Students’ final drafts were the only data source included because students’ revised writing best captures the features they found important to include in their mechanistic descriptions after receiving peer feedback and revising their work. Analysing only the final drafts was done to focus on the writing that best represented students’ knowledge after engaging with the cognitive processes of writing as facilitated by the structured peer-review process.Data analysis
This framework was successfully used in other chemistry education research studies focused on mechanistic reasoning in the context of organic chemistry (Caspari et al., 2018a; Caspari et al., 2018b) and in the context of general chemistry (Moreira et al., 2018). Caspari et al. (2018b) utilized the framework to analyse organic chemistry students’ ability to propose mechanisms while Caspari et al. (2018a) similarly used the framework to analyse students’ construction of accounts relating structural changes to reaction energies, both in interview settings. Moreira et al. (2018) utilized the framework to analyse high school students’ written responses after being given ten minutes to respond to a brief writing assignment eliciting mechanistic explanations of freezing point depression. The present study similarly adapts this framework for recognizing students’ mechanistic reasoning, but differs in that it is focused on written descriptions of the amide acid hydrolysis reaction mechanism. The adaptation of this framework to organic chemistry students’ writing about more complex reaction mechanisms is valuable for understanding how these students think about and understand chemistry principles as applied to organic reactions. Furthermore, this study is differentiated by the WTL process used to promote students’ engagement with the cognitive processes of writing.
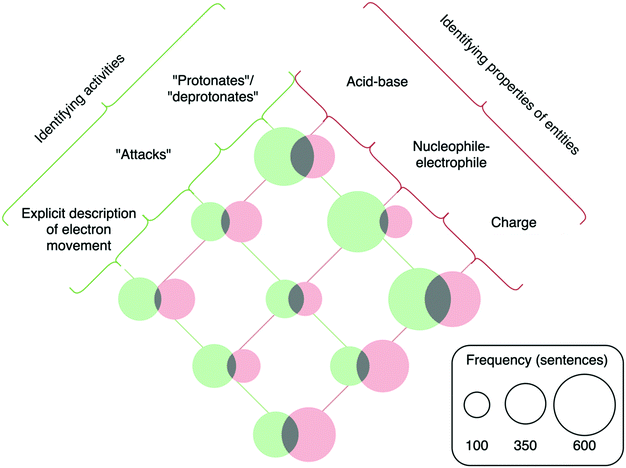
The framework presented by Russ et al. (2008) is centred around entities and activities. Entities are defined as the things which are involved in a mechanism (Machamer et al., 2000; Russ et al., 2008). In terms of organic reaction mechanisms, entities are electrons, atoms, and molecules (Caspari et al., 2018b). Activities are defined as the actions entities take to produce change (Machamer et al., 2000; Russ et al., 2008). For organic reaction mechanisms, activities include the movement of electrons and the breaking and forming of bonds that produces structural change over the course of the mechanism (Caspari et al., 2018b). The original framework described by Russ et al. (2008) included seven hierarchical levels—(1) describing the target phenomenon, (2) identifying setup conditions, (3) identifying entities, (4) identifying activities, (5) identifying properties of entities, (6) identifying organization of entities, and (7) chaining.
The coding scheme adapted from this framework, located in Appendix 2, Table 1 and detailed in the results and discussion, was developed by deductively coding for features expected in students’ writing for each level of the hierarchy and open coding for additional features present in students’ writing. Early in the coding process, the authors decided to code on a sentence-level grain size with the allowance that all appropriate codes would be applied to each sentence. This grain-size was chosen so we would be able to analyse what features were present, how frequently they appeared, and how often they co-occurred with other features. The coding frame began with the first sentence in a students’ response in which a code could be applied and ended when the response shifted to answering another part of the prompt.
| Category | Code name | Code name (shortened) | Definition | Exemplars |
|---|---|---|---|---|
| Describing the target phenomenon | Overview of hydrolysis | over | The sentence provides a broad description of the hydrolysis reaction. | “One reaction of thalidomide is an acid hydrolysis reaction” |
| “Thalidomide is a compound which, when undergoing an acid hydrolysis reaction, can form two constitutionally isomeric products.” | ||||
| “Hydrolysis is the breakdown of a compound which proceeds as a result of water reacting with a carbonyl group.” | ||||
| Identifies two reaction pathways | idpath | The sentence identifies that the initial protonation and nucleophilic attack can occur at two carbonyls, which leads to two different products. | “Two different hydrolysis products can be made based on which carbonyl gets attacked, but the mechanism is the same.” | |
| “The same general mechanism occurs when the other carbonyl is first protonated” | ||||
| “This hydrolysis reaction can occur with either one of the carbonyl groups present on the ring.” | ||||
| Identifying setup conditions | Specifies reaction medium—acidic | acid | The sentence identifies the acidic environment or conditions. Simply stating that the mechanism was an acid hydrolysis reaction does not suffice, as “acid hydrolysis” is the name of the reaction and does not itself indicate an awareness of the reaction occurring in acidic media | “Acid present in solution” |
| “Acidic environment” | ||||
| “Acidic conditions” | ||||
| Specifies reaction medium—aqueous | aq | The sentence identifies the aqueous environment or conditions. | “Aqueous environment” | |
| “Water in solution” | ||||
| “Presence of water” | ||||
| Specifies reaction medium—body | body | The sentence identifies that the reaction is occurring in the body. | “In the body” | |
| “In the blood” | ||||
| Specifies the carbonyls involved | carb | The sentence specifies which carbonyls on the thalidomide molecule are involved in the reaction. | “Carbonyl in the 6-membered ring” | |
| “Carbonyl that is closest to the stereocenter” | ||||
| “Furthest away from the aromatic ring” | ||||
| Description of connectivity | conn | The sentence includes a depiction of the connectivity of the starting materials, intermediates, or products. This code was not applied when only the word “intermediate” was used, as simply stating that an intermediate is present gives no indication of connectivity. | “The nitrogen atom that is part of the imide group is attached to a hydrogen atom” | |
| “The Thalidomide molecule has two amide groups” | ||||
| “…creating a hydroxyl group” | ||||
| “At this moment, we have a neutral tetrahedral intermediate.” | ||||
| Identifying activities | Explicit electron movement | exp | The sentence uses the word “electrons” or phrase “lone pair” as the subject of a phrase when describing the movement of electrons. | “Electrons from one of the oxygens then move…” |
| “The lone pair then comes back down to reform the double bond…” | ||||
| Implicit electron movement—entity focused | entity | The sentence uses a word or phrase other than “electrons” or “lone pair” as the subject of a phrase when describing the movement of electrons, with any verb besides “attacks.” | “One of the hydroxyl substituents forms a double bond…” | |
| Implicit electron movement—“attacks” | att | The sentence uses a word or phrase other than “electrons” or “lone pair” as the subject of a phrase when describing the movement of electrons, with the verb “attacks.” | “Water then attacks…” | |
| Implicit electron movement—protonates-deprotonates | prot | The sentence uses some variation of the word “protonates” or “deprotonates” to describe a mechanistic step. This code was not applied when variations of these words were used to describe a structural feature (e.g. “the protonated oxygen”). | “The hydronium ion protonates…” | |
| “A water molecule deprotonates…” | ||||
| Implicit electron movement—double bond movement | dbm | The sentence refers to the movement of double bonds rather than the movement of electrons. | “This pushes the double bond up onto the oxygen…” | |
| Implicit electron movement—passive electron pushing | epush | The sentence uses a phrase that passively describes the movement of electrons (in the sense that the subject of the phrase is something other than the electrons or atoms/molecules involved in the mechanism). | “Electron pushing results in…” | |
| “The oxygen in the water molecule then attacks the carbon in the carbonyl, which, through electron pushing, forms a tetrahedral intermediate…” | ||||
| Identifying properties of entities | Changes in bonding—bond breaking and making | bbm | The sentence uses language to indicate that bonds are being broken or formed in the process of a mechanistic step. | “The bond between the nitrogen and carbon breaks” |
| “A lone pair from the oxygen reforms the carbonyl double bond.” | ||||
| Changes in bonding—ring opening | ring | The sentence explicitly describes thalidomide's ring structure being broken or opened in the mechanism. | “The ring then opens” | |
| “Breaking the ring” | ||||
| Changes in bonding—nitrogen leaving | nitro | The sentence explicitly refers to the nitrogen-carbon bond breaking as the nitrogen acting as a leaving group. | “Eliminates the nitrogen” | |
| “Kicking out the nitrogen” | ||||
| “The nitrogen group leaves” | ||||
| Acid–base | ab | The sentence refers to a reactant acting as an acid or a base or refers to a mechanistic step as an acid–base reaction. This code was not applied when the phrase “acid hydrolysis” appeared; students needed to have included language relating to acid–base chemistry in connection to entities acting in the mechanism. | “An acid protonates…” | |
| “The carbonyl group will then be deprotonated by the conjugate base of the original acid…” | ||||
| “…either carbonyls are protonated through an acid/base reaction…” | ||||
| Nucleophile-electrophile | nuc | The sentence refers to the identify of reacting species as nucleophiles or electrophiles when describing a mechanistic step. | “Then, water, acting as a nucleophile, attacks the electrophilic carbon” | |
| “Electrophilic means it is extremely attracted to electrons.” | ||||
| Charge | charge | The sentence refers to the creation or neutralization of formal charges when describing a mechanistic step. | “The oxygen is then deprotonated to neutralize the charge…” | |
| “The water would attack that positively charged carbonyl group.” | ||||
| “The positive oxygen activates the carbonyl making the carbon a partial positive.” | ||||
| Resonance | res | The sentence justifies a mechanistic step by referring to the resonance structures of the reacting molecules. | “The positive charge on the oxygen atom is stabilized through resonance” “The resonance form of this molecule results in a positive charge…” | |
| “The electrons from the double bond resonate onto the oxygen” | ||||
| Electronegativity | eneg | The sentence justifies a mechanistic step by referring to the electronegativity of the reacting atoms. | “…because nitrogen is more electronegative, the lone pair falls on the nitrogen atom” | |
| “This increases the net inductive effect on the associated carbonyl carbon since it makes the oxygen more electron deficient.” |
We conducted the initial coding (which included deductive and open coding in tandem) on a randomly selected subset of student responses, using constant comparative analysis to ensure all features were represented in the coding scheme and to clarify coding definitions (Corbin and Strauss, 1990; Nowell et al., 2017). The first and second authors worked in conjunction to develop the coding definitions, and other members of the research team with knowledge of mechanistic reasoning in organic chemistry assisted with further refinements. Improvements made to the coding scheme included incorporating codes developed from the open coding into the appropriate level of the hierarchical coding scheme. For example, in our deductive coding we did not include students’ descriptions of the connectivity of starting materials and reaction intermediates, but it was a feature present in many responses. Thus, this feature of students’ writing was included in the open coding and later integrated into the identifying setup conditions category of the hierarchical coding scheme. The choice was made to expand what was included within the setup conditions category beyond what was expected, as descriptions of connectivity relate the organization of atoms bonded together. This aligns with the setup conditions category, as specific connectivity is a requirement for particular mechanistic steps to occur. Furthermore, the way students wrote about and described connectivity during the course of the mechanism aligned with this category of the coding scheme, as their descriptions for products of one mechanistic step operationally served as the setup conditions for the next mechanistic step in the reaction. We combined and reorganized other codes from the deductive and open coding into the adapted coding scheme in a similar fashion. Additionally, we determined that some aspects of the original framework were not appearing in students’ writing at the sentence level and thus we did not incorporate these into the coding scheme. The process of developing the coding scheme continued until saturation was reached (Miles et al., 2014). In total, we coded 163 responses, representing 30% of the entire dataset.
The finalized coding scheme included four broad categories corresponding to four levels of the original framework that reflect the features necessary for engaging in mechanistic reasoning: (1) describing the target phenomenon, (2) identifying setup conditions, (3) identifying activities, and (4) identifying properties of entities. Codes relating to general descriptions of hydrolysis or the two reaction pathways leading to the two hydrolysis products were placed in the category of describing the target phenomenon. The identifying setup conditions category included codes relating to specifying the reaction medium or describing the structure or connectivity of starting materials, intermediates, and products. The third category, identifying activities, included codes relating to descriptions of electron movement or descriptions of bonds being broken or formed. The final category included the properties of entities—such as being acidic or basic, nucleophilic or electrophilic, or formally charged—that students identified in their mechanistic explanations. To illustrate the application of the coding scheme, two example student responses, with the applied codes indicated, are provided in Appendix 3, Fig. 14.
We did not include the third level of the original hierarchy, identifying entities, in the adapted coding scheme because the relevant entities (electrons, atoms, and molecules) were inherently coded for in other categories of the coding scheme. In other words, students never simply identified the entities without also describing their properties or the activities in which they were engaged. We also did not include the final two levels of the original framework—identifying organization of entities and chaining. Identifying the organization of entities was not included because of the category's focus exclusively on the spatial organization of entities as they are interacting during a mechanistic step, a feature which did not present itself in the students’ writing. It is possible that whether or not students attend to the organization of entities depends on the mechanism—for instance, it might be present in mechanisms where there is a difference in stereochemical outcome depending upon the spatial organization of molecules as they interact (e.g., a unimolecular elimination reaction), or where spatial orientation during a mechanistic step might be described (e.g., the backside attack during a bimolecular substitution reaction). Chaining, defined as an explanation of how each mechanistic step leads to the next or why steps occur in the order that they do (Russ et al., 2008), did not appear distinctly in student responses aside from the ordering of mechanistic steps. There was little variety in the ordering of mechanistic steps in students’ writing, and analysing chaining was not an insightful avenue of analysis in the present study due to this uniformity. It is likely that chaining pertains primarily to non-written descriptions of mechanisms in which students are proposing unknown mechanisms, or to written descriptions when students do not have the opportunity to refer to outside resources or revise their assignments after peer-review. Notably, chaining was the focus of the coding scheme presented by Caspari et al. (2018b), in which students were proposing familiar and unfamiliar mechanisms during an interview. It is also possible that chaining was not identified due to the sentence-level grain size for coding, as chaining requires recognizing connections between mechanistic steps that might only be apparent across multiple sentences. Though chaining was likely present in students’ thought processes regarding the hydrolysis mechanism, it was not necessarily identifiable in the conducted analysis.
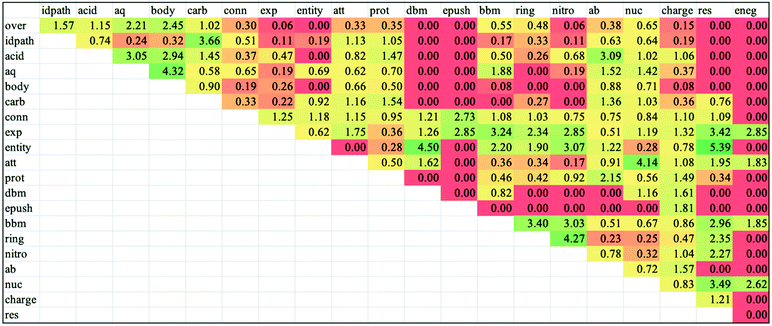
Lastly, we examined the co-occurrences of codes to develop a more detailed understanding of how students were reasoning through the acid hydrolysis mechanism. To do this, we calculated a metric called lift, an association rule which measures the degree of dependence between two items, for each pair of codes. These values are useful to determine which pairs of codes were appearing together more or less than probabilistically expected. Lift is defined as
Results and discussion
The results from analysing students’ written descriptions of the hydrolysis reaction are drawn from the application of the coding scheme adapted from Russ et al. (2008), specifically by examining the prevalence and co-occurrences of codes within students’ responses. The codebook is structured with four broad categories, each containing codes that indicate the specific features of students’ writing corresponding to each category. These categories relate to the different components necessary for mechanistic reasoning present across the set of responses. We first report the percentages of responses in which each of the broad categories appears. Next we provide a detailed description of each category, focusing on the codes used to support claims made throughout the section. Lastly, we include an analysis of the co-occurrences of codes to make inferences about students’ mechanistic reasoning for the acid hydrolysis mechanism.What features are present in students’ written mechanistic descriptions?
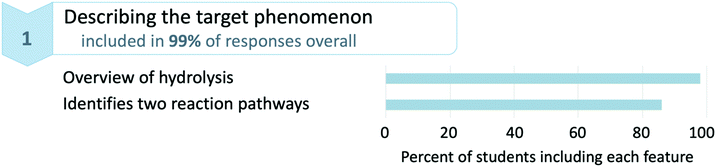
To examine the features present in students’ written descriptions, we first observed how often each of the four broad categories of the coding scheme appeared in responses across the dataset. For these categories, 99% of responses included at least one description of the target phenomenon, 96% included an indication of setup conditions for the mechanism, 100% included a description of an activity taking place over the course of the mechanism, and 95% included an identification of the properties of entities. The high percentages of students incorporating each of these components necessary for mechanistic reasoning in their response indicates that the assignment, in general, successfully elicited descriptions of the acid hydrolysis mechanism. Since the majority of these features were present across responses, these values also suggest that the majority of students likely engaged in some form of mechanistic reasoning, which was the objective of the WTL assignment.How do students write about the features present in their mechanistic descriptions?
Next we describe and provide examples of codes to illustrate how students appealed to each category of a mechanistic description. The reported percentages indicate the proportion of students including particular features in their response at least once. The full coding scheme, with definitions and examples for every code, can be found in Appendix 2.Students identified the two reaction pathways by stating an explanation, however minimal, of why two products were formed—such as “Two different hydrolysis products can be made based on which carbonyl gets attacked, but the mechanism is the same.” Note that this example was also coded with providing an overview of hydrolysis, as it also states that there are two hydrolysis products. Students’ responses might also have included language suggestive of the existence of multiple reaction pathways without explicitly making the connection to the two hydrolysis products, as in statements such as “This hydrolysis reaction can occur with either one of the carbonyl groups present on the ring.” Notably, 14% of students did not make reference to the two reaction pathways leading to the different hydrolysis products identified in the writing assignment. This suggests that some students are not considering or placing enough importance on alternative, essentially equivalent, reaction pathways even when the results of these pathways are presented to them.
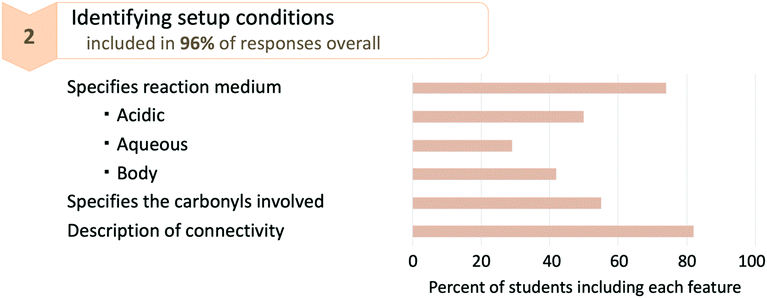
Students described the acidic reaction medium by including phrases such as the “acid present in solution,” the “acidic environment” or the “acidic conditions.” Students similarly described the aqueous reaction conditions. As shown in Fig. 4, 74% of responses incorporated at least one of the codes relating to the reaction conditions—and of that 74%, only 50% identified the reaction as occurring in acidic conditions and only 29% identified the reaction as occurring in aqueous conditions. From these percentages, it is clear that not all students are recognizing the value of identifying the reaction conditions in their mechanistic descriptions despite the importance of reaction conditions for understanding a mechanism.
Students specified the carbonyls involved by identifying the location on thalidomide where the hydrolysis reaction was taking place. They did this by providing some spatial description to identify which of the four carbonyls was reacting, such as “carbonyl in the 6-membered ring” or “carbonyl that is closest to the stereocenter” or “furthest away from the aromatic ring.” This code only appeared in 55% of responses, suggesting that nearly half of the students did not pay sufficient attention to differentiating the reactive and non-reactive carbonyls.
Many students provided a description of the connectivity for the starting materials, intermediates, or products of the reaction. Descriptions of connectivity ranged from being relatively detailed (e.g. “the nitrogen atom that is part of the imide group is attached to a hydrogen atom”) to including only reference to a functional group (e.g., “the Thalidomide molecule has two amide groups” or “…creating a hydroxyl group”). Students also included more general descriptions of connectivity such as “At this moment, we have a neutral tetrahedral intermediate.” Descriptions of connectivity for the starting materials and intermediates are considered setup conditions for the mechanism, as such descriptions help the reader identify the connectivity required for each step of the mechanism to take place.
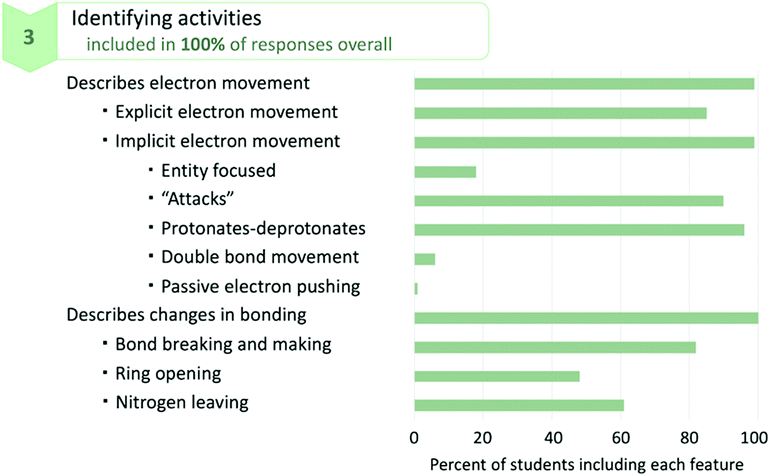
Students described electron movement both explicitly and implicitly. Explicit descriptions included students’ reference to “electrons” or “lone pairs” when describing the movement of electrons. Implicit descriptions were those which did not explicitly refer to electrons, and were subdivided into codes for descriptions (a) focusing on the entity, (b) using variations of the word “attacks,” (c) using variations of the words “protonates” and “deprotonates,” (d) suggesting the movement of a double bond, and (e) mentioning passive electron pushing. Students’ descriptions of entity-focused implicit electron movement included instances when the subject of a sentence describing a mechanistic step was something other than electrons (e.g., “One of the hydroxyl substituents forms a double bond…”). Students’ use of the word “attacks” is a special case of this code in which the subject of the sentence was something other than electrons and the verb of the sentence was “attacks” (e.g., “Water then attacks…”). Students also described mechanistic steps using variations of the words “protonates” or “deprotonates.” Descriptions indicating the movement of double bonds were those which described the movement of a pi bond rather than the movement of electrons in a pi bond. The code for electron pushing was applied when students passively described electron movement, in the sense of identifying something other than the entity involved in the mechanism performing the action (e.g., “The oxygen in the water molecule then attacks the carbon in the carbonyl, which, through electron pushing, forms a tetrahedral intermediate…”). Despite its infrequent appearance, this code remained in the codebook because it was an artefact of students’ language use aligning with prior findings in the literature which suggest that students find the electron pushing formalism to be simply an academic exercise with little physical meaning (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008). It is promising that the potentially more problematic codes for descriptions of implicit electron movement appeared infrequently.
Explicit descriptions of electron movement were present in 85% of responses, while at least one of the codes for implicit descriptions of electron movement was present in 99% of responses. That a majority of students explicitly referred to electrons is a promising finding, indicating that the WTL assignment encouraged students to make connections between mechanistic steps and the movements of electrons. This suggests that, during the process of writing, students are attentive to the physical meaning of mechanistic steps, as opposed to prior studies that have shown students to not associate physical meaning when using the electron-pushing formalism (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008). However, 15% of students did not, in any sentence of their mechanistic description, identify the movement of electrons to describe a mechanistic step, while nearly every student included implicit descriptions of electron movement. Note that nothing is inherently wrong with implicit descriptions of electron movement; these descriptions simply do not indicate with certainty whether students are conceptualizing mechanistic steps as occurring due to the movement of electrons. It is notable that the most common codes for implicit electron movement are those for using variations of the words “attacks,” “protonates,” and “deprotonates,” as practicing chemists and instructors frequently use these words when describing mechanisms. This provides evidence that students are using appropriate language when describing mechanistic steps.
The other set of codes categorized as identifying activities included descriptions of changes in bonding, as indicated in Fig. 5. Students commonly did this using phrases such as “the bond between the nitrogen and carbon breaks” or “A lone pair from the oxygen reforms the carbonyl double bond.” These descriptions can be thought of as a counterpoint to the aforementioned code for descriptions of connectivity in that this code was applied to active descriptions of changes in connectivity while the other code was applied to descriptions of connectivity before or after mechanistic steps. Students largely included descriptions of bonds being broken or formed, but 18% of responses contained no explicit description of this. Many students also referred to surface features of molecules to describe changes in bonding for the ring-opening step, with 48% of responses describing changes in bonding as a ring opening and 61% of responses describing changes in bonding as the nitrogen leaving. It is not necessarily incorrect to describe changes in bonding in terms of these surface features; however, it does suggest that some students may be overlooking the fundamental changes occurring in mechanisms—the bonds being broken and formed—in favour of paying attention to the more obvious surface features (such as the ring opening or nitrogen leaving, changes in bonding which result in obvious structural change).
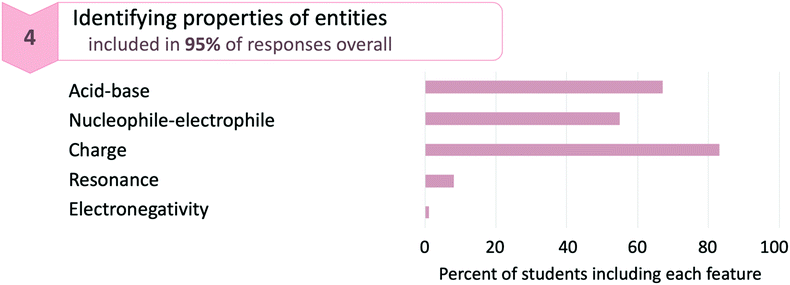
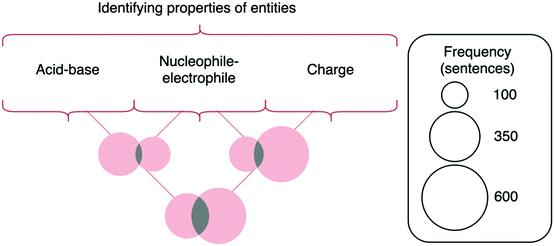
As illustrated in Fig. 6, only 55% of responses appealed to the properties of reacting molecules as nucleophiles or electrophiles, which is a fundamental property for explaining an acyl transfer mechanism. Instead, more students (67%) appealed to the properties of molecules as acids or bases. This is not surprising, as many of the reaction steps are protonations and deprotonations. Furthermore, acid–base chemistry is a topic that is introduced in general chemistry, so students in organic chemistry are likely more familiar with thinking of molecules in terms of acids and bases than in terms of nucleophiles and electrophiles. An even higher percentage of students (83%) appealed to the charged nature of reacting species. Again, this is not surprising since charges are explicit, surface features of molecules that change during the mechanism and are perhaps the simplest way for students to connect the movement of electrons to the properties of molecules. The relative percentages of students appealing to these three different properties of molecules aligns with prior studies in which students were found to rely on charges when considering mechanisms (Anzovino and Bretz, 2015; Galloway et al., 2017; Graulich and Bhattacharyya, 2017; Caspari et al., 2018a).
The remaining codes in the category—identifying resonance or electronegativity—appeared less frequently. Students identified resonance by applying the concept either correctly (e.g., “The positive charge on the oxygen atom is stabilized through resonance”), somewhat correctly (e.g., “The resonance form of this molecule results in a positive charge…”), or incorrectly (e.g., “The electrons from the double bond resonate onto the oxygen”). Some responses also appealed to the electronegativity of atoms to describe electron density. It is somewhat surprising that few students identified resonance or electronegativity, as prior studies have shown that students often use these concepts to guide their mechanistic thinking (Ferguson & Bodner, 2008). However, it is unclear whether this is due to the specific mechanism students described or the nature of producing a written mechanism.
Overall, the results for the first two research questions (summarized by the complete coding scheme in Appendix 2 and the appearance and frequency data in Appendix 4, Table 2) indicate that while most students are including the components necessary for mechanistic reasoning as identified in the adapted coding scheme, there is considerable variety in how students include each of these components. Furthermore, despite promisingly high percentages of students appealing to each level of the coding scheme, the results draw attention to the codes within each category for which fewer students are incorporating particular components necessary for mechanistic reasoning.
| Category/code | Appearancea (%) | Frequencyb | Meanc | St. dev.c |
|---|---|---|---|---|
| a Percent of responses in which the code, or any code within the category, appears at least once (N = 163 responses). b Number of sentences to which the code was applied (N = 1497 sentences). c Statistic for the frequencies, across the set of responses in which the code appeared. | ||||
| Describing the target phenomenon | 99 | |||
| Overview of hydrolysis | 98 | 402 | 2.51 | 1.20 |
| Identifies two reaction pathways | 86 | 214 | 1.52 | 0.67 |
| Identifying setup conditions | 96 | |||
| Specifies reaction medium | 74 | |||
| Acidic | 50 | 133 | 1.62 | 0.87 |
| Aqueous | 29 | 59 | 1.23 | 0.51 |
| Body | 42 | 88 | 1.29 | 0.62 |
| Specifies the carbonyls involved | 55 | 132 | 1.47 | 0.69 |
| Description of connectivity | 82 | 274 | 2.04 | 1.21 |
| Identifying activities | 100 | |||
| Describes electron movement | 99 | |||
| Explicit electron movement | 85 | 263 | 1.88 | 0.84 |
| Implicit electron movement | 99 | |||
| Entity focused | 18 | 37 | 1.23 | 0.50 |
| “Attacks” | 90 | 205 | 1.40 | 0.65 |
| Protonates-deprotonates | 96 | 581 | 3.72 | 1.22 |
| Double bond movement | 6 | 9 | 1.00 | 0.00 |
| Passive electron pushing | 1 | 2 | 1.00 | 0.00 |
| Describes changes in bonding | 100 | |||
| Bond breaking and making | 82 | 202 | 1.52 | 0.78 |
| Ring opening | 48 | 85 | 1.08 | 0.27 |
| Nitrogen leaving | 61 | 132 | 1.33 | 0.55 |
| Identifying properties of entities | 95 | |||
| Acid–base | 67 | 233 | 2.14 | 1.16 |
| Nucleophile–electrophile | 55 | 143 | 1.61 | 0.86 |
| Charge | 83 | 414 | 3.04 | 1.54 |
| Resonance | 8 | 15 | 1.15 | 0.38 |
| Electronegativity | 1 | 4 | 2.00 | 1.41 |
What inferences about students’ mechanistic reasoning can be made by analysing co-occurrences of the features necessary for mechanistic reasoning?
In addition to what features were present in students’ responses and how frequently these features appeared, we also examined the frequencies in which codes co-occurred with one another. We did this to make inferences about how students were engaging in mechanistic reasoning in their written explanations of the acid hydrolysis mechanism, specifically by examining how students combined properties of entities with the activities during the mechanism. In order to assess which pairs of codes were co-occurring in a meaningful way, we calculated the lift for each pair as described in the methods. The lift values and co-occurrence frequency data for all pairs of codes are presented in Appendix 5, Fig. 15 and 16. From examination of the co-occurrence data, particular themes arose that are each supported by specific lift values and sets of Venn diagrams. Each of these themes are described below.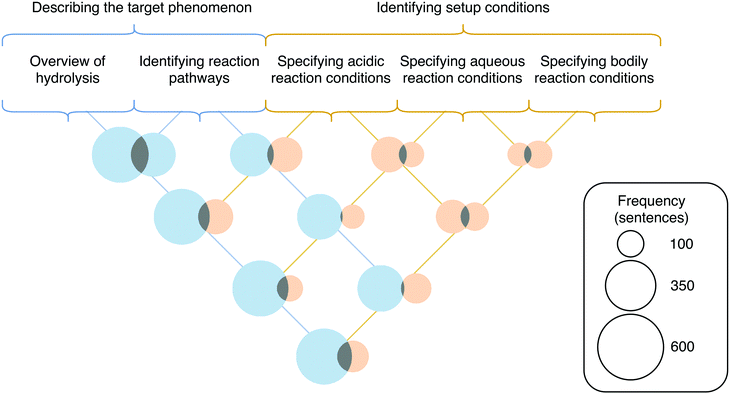 | ||
| Fig. 7 Venn diagrams between codes for describing the target phenomenon and identifying setup conditions. Overlaps indicate the number of sentences in which both codes in the pair appear together. | ||
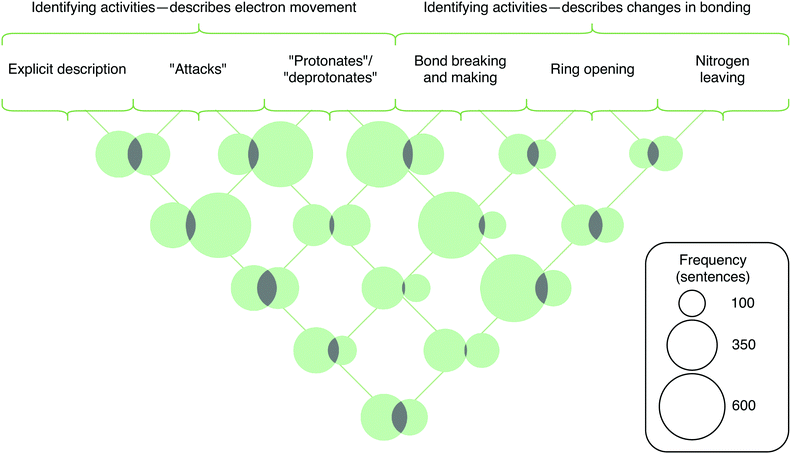
There are similar trends between codes in the third category of the coding scheme (describing activities), with some notable co-occurrences as illustrated in Fig. 8. First, explicit descriptions of electron movement had high lift with the code for implicitly describing electron movement with the word “attacks” (1.75). This is an artefact of when students used the word “attacks” followed by an explicit depiction of electron movement—such as the case when a nucleophile attacks an electrophilic carbonyl followed by the movement of the pi electrons onto the carbonyl oxygen. Explicit descriptions of electron movement also had high lift with the three codes related to the formation or breaking of bonds (2.34, 2.85, and 3.24). This finding aligns with prior research that has found students to be able to describe changes in bonding using electron movement (Galloway et al., 2017). In contrast, the codes for implicit descriptions of electron movement—using the word “attacks,” “protonates,” or “deprotonates”—had lift values below 1.0 for the codes related to the formation of bonds. This suggests that students’ writing does not reflect that bonds are formed or broken in the processes of nucleophilic attacks, protonations, or deprotonations. Unsurprisingly, there were high lift values (3.40, 3.03, and 4.27) between the three codes related to the forming and breaking of bonds, as students often explicitly described the fact that bonds were being broken or made in conjunction with describing the surface feature changes of the ring opening or nitrogen leaving.
Notably, the lift values were generally below 1.0 for codes in the first and second categories of the coding scheme paired with codes in the third and fourth categories. This result shows that the codes related to describing mechanistic activities (the third category) and identifying properties of entities (the fourth category) are largely independent of the codes for describing the target phenomenon (the first category) and identifying the setup conditions (the second category). The lift values below 1.0 provide further evidence for the hierarchical nature of students’ mechanistic descriptions, as students included features from the first two categories alongside features from the last two categories less than expected by chance.
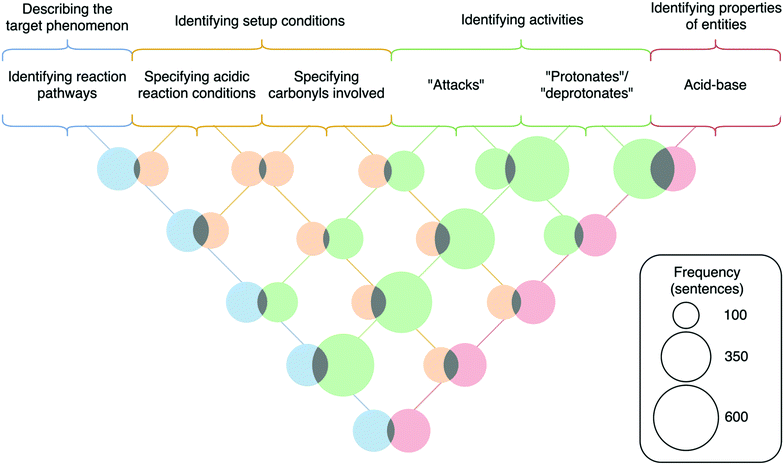
The code for specifying the carbonyls involved in the reaction had high lift values with three other codes—identifying the acidic conditions (1.45), using the words “protonates” or “deprotonates” (1.54), and identifying entities as acids or bases (1.36). There were similarly high lift values between the other combinations of these codes (ranging from 1.47 to 2.15). The relationships between these codes show that students are making the logical connections between the acidic medium and the protonation steps in the mechanism—particularly the protonation of one of the two carbonyls that leads to one of the final products. This result differs from prior research by Caspari et al. (2018b) and Petterson et al. (2020), in which students did not verbalize alternative mechanistic steps that lead to alternative reaction pathways. This finding suggests that the WTL assignment, which included clear expectations to explain the formation of two products, elicited students’ consideration of the alternative mechanistic pathways that they might not have considered otherwise.
Another observation is that the code for using the word “attacks” is relatively independent of the codes for identifying the reaction pathway or specifying the carbonyls involved (lift of 1.13 and 1.16, respectively). This independence is notable in light of the two ways students chose to identify the divergence in the reaction that leads to two products. The first, which the co-occurrence data suggests students did with more frequency, was to identify the divergence at the first step of the reaction—the protonation of one of the two carbonyls (e.g., “…the final product is determined by which oxygen is initially protonated” or “Depending on which amide is originally protonated, two hydrolysis products can form”). However, an alternative way that some students identified the divergence in the reaction was by considering which protonated carbonyl served as the electrophile in the nucleophilic attack by water (e.g., “The other hydrolysis product forms when water attacks the other carbonyl” or “The hydrolysis product depends on which carbonyl group on the 6-membered ring is attacked.”). While the divergence at the protonation step is reflective of how this reaction mechanism might be drawn to show the formation of two products, the divergence at the step of nucleophilic attack suggests a potentially more nuanced understanding of the dynamic equilibrium between protonated and deprotonated species in acidic media, as the protonation step is likely to be more easily reversible than the nucleophilic attack. Hence, the lower co-occurrence between the codes for using the word “attacks” and identifying the two reaction pathways suggests that more students are writing the descriptions for alternative mechanisms as the individual mechanisms would be drawn, rather than locating within the description the most likely point of divergence. This result could indicate that some students do not have a full conceptual understanding of the dynamic nature of reactions, especially when reactions lead to similar products. The difference between these two descriptions could indicate differences in whether students perceive reactions to be occurring stepwise or in a more dynamic manner, a possibility that has emerged in other studies (Galloway et al., 2017).
Furthermore, the set of co-occurrences between identifying the acidic conditions, using “protonates” or “deprotonates,” and identifying entities as acids or bases (with lift values ranging from 1.47 to 2.15) illustrates that students did make the connection between the acidic medium and the presence of a molecule acting as an acid to perform a protonation. This finding suggests that students engaged in reasoning that connected the acidic setup conditions to the molecules being in a protonated state through the mechanism of an acid–base reaction. Notably, there is no dependence between the acidic conditions code and the charge explanation code (lift of 1.06). This may be an artefact of students not making the conceptual connection between acidic environments and the presence of positively charged species. However, we might expect students to apply rule-based reasoning to directly make this connection using the rule that positive charges are associated with acidic reaction conditions, similar to students’ rule-based-reasoning described in prior studies (Kraft et al., 2010; Christian and Talanquer, 2012; De Arellano and Towns, 2014). Hence, this result may suggest that the WTL assignment facilitated reasoning reflective of process-oriented rather than product-oriented problem-solving.
The lift values between different properties of entities and explicit descriptions of electron movement are also notable. While the lift values between explicit descriptions of electron movement and identifying nucleophiles/electrophiles or charges are slightly above 1.0 (1.19 and 1.32, respectively), the lift between explicit descriptions of electron movement and identifying acids/bases is below 1.0 (0.51). These values reveal a modest dependence between describing explicit electron movement and identifying entities by either their nucleophilicity/electrophilicity or charge. However, the overlap between explicit electron movement and identifying acids/bases is less than expected due to chance—meaning that when students identified acids/bases they were less likely to accompany that identification with explicit descriptions of electron movement (and vice versa). This finding suggests that students are appealing to Brønsted–Lowry acid–base theory more than they are appealing to Lewis acid–base theory, aligning with prior research regarding students’ application of different acid–base theories (Cartrette and Mayo, 2011; Schmidt-McCormack et al., 2019; Petterson et al., 2020). The lack of appeal to Lewis acid–base theory is valuable to recognize in students’ writing, as the Lewis theory is a concept necessary for mechanistic reasoning (Bhattacharyya, 2013) and students who use Lewis acid–base theory are more successful at mechanism tasks (Cooper et al., 2016; Dood et al., 2018). In addition, the percent of overlap between explicit descriptions of electron movement and the identification of properties of entities is the largest for identifying charges. Together, these findings suggest that students are able to connect explicit—as opposed to implicit—descriptions of electron movement with more accessible or surface-level reasoning (identifying charges or using Brønsted–Lowry acid–base theory) as opposed to reasoning with more sophisticated concepts (identifying nucleophiles/electrophiles or using Lewis acid–base theory). Such a focus on surface features of reactants has been shown to engender rule- or case-based reasoning, and might be reflective of students’ product-oriented approaches to problem-solving (Kraft et al., 2010; Christian and Talanquer, 2012; De Arellano and Towns, 2014).
Lastly, among the three most prevalent codes for the identifying properties of entities, the lift values are less than 1.0 for identifying nucleophilicity and electrophilicity in conjunction with both other commonly identified properties (acidic/basic and charge). The overlaps between these codes are presented in Fig. 11. These co-occurrences indicate that identifying nucleophiles and electrophiles occurs most commonly with the absence of identifying other properties of entities, matching findings from prior research in which few students made connections between acids/bases and nucleophiles/electrophiles (Cartrette and Mayo, 2011). However, there is a high lift value (1.57) between identifying acids and bases and identifying charges, indicating that these constructs frequently occur together. This lift value provides further support for the hypothesis that students are more comfortable identifying the more familiar construct of charge or using Brønsted–Lowry acid–base theory—and even use them to complement each other. On the other hand, when students do identify nucleophiles and electrophiles, it is much less likely to be accompanied with identification of other properties of entities. This finding may reflect students’ abilities to engage in integrated multicomponent reasoning only with certain properties of entities (i.e., being able to use charge and acid/base character simultaneously), but that these abilities are limited when considering properties such as nucleophilicity or electrophilicity (Sevian and Talanquer, 2014; Weinrich and Talanquer, 2016; Bodé et al., 2019).
Conclusions
We have described the analysis of student responses to a WTL assignment designed to elicit mechanistic descriptions of an acid hydrolysis reaction. Our study was guided by an analytical framework for discourse analysis grounded in the philosophy of science literature. Responses were coded for the presence of features necessary for mechanistic reasoning within the broad categories of describing the target phenomenon, specifying setup conditions, identifying activities, and identifying properties of entities. Our goal for coding was to provide a rich description of how students incorporated these features in their descriptions of the reaction mechanism. The second aspect of this research identified how these features co-occurred to make inferences about students’ mechanistic reasoning. This analysis furthers our understanding of the way students think about reaction mechanisms in the context of a specific reaction. It has shown that, in general, the assignment successfully elicited complete mechanistic descriptions, as most students appealed to each level of components necessary for mechanistic reasoning as described by the coding scheme adapted from Russ et al. (2008), with 85% of students explicitly describing the movement of electrons. Additionally, trends in the co-occurrence data—in which codes within the same category or from neighbouring categories generally co-occurred more often compared to codes from more separated categories—provided support for the hierarchical ordering of the components necessary for engaging in mechanistic reasoning.A number of findings arose from analysis of the frequency and co-occurrence data presented which identify the features students did (or did not) engage with during the process of writing. First, there were notable percentages of responses that did not incorporate some of the important features of a description for the mechanism. Some students (26%) did not specify the reaction medium, indicating that these students are not recognizing the importance of the reaction conditions as they pertain to reaction mechanisms. Additionally, some students (14%) did not consider the two reaction pathways, even though the assignment explicitly requested an explanation for the formation of two products. For those students who did consider the two reaction pathways, there was evidence to suggest different interpretations of where the reaction diverged. Many students indicated the divergence at the first mechanistic step, while fewer students indicated the divergence at a later (more chemically reasonable) step, suggesting differences in students’ understanding of the dynamic nature of reactions when considering multiple reaction pathways.
Perhaps most notable is that 45% of students made no reference to the reacting species as nucleophiles or electrophiles. In general, identifying charges was more prevalent than identifying properties of entities that allow for more sophisticated conceptual reasoning such as identification of nucleophiles and electrophiles or acids and bases. Furthermore, compared to other properties of entities, identifying nucleophilicity and electrophilicity occurred less often in conjunction with identifying other properties. The findings also showed that students more often made connections between charges and explicit descriptions of electron movement compared to other properties of entities. Explicit descriptions of electron movement were also frequently connected to descriptions of bonds being broken and formed, but this connection was not present for implicit descriptions of electron movement. In addition, when describing changes in the mechanism, identifying the properties of entities more frequently accompanied descriptions of electron movement than descriptions of changes in bonding. Another finding that presented itself throughout the data was that many students were using appropriate language to describe mechanistic steps. Students commonly used the word “attacks” when describing a nucleophilic attack and used variations of “protonates” or “deprotonates” in reference to acid–base reactions. This suggests that students were making appropriate connections between concepts across different categories of the coding scheme. Taken together, the findings from this research identify how students were engaging in mechanistic reasoning by revealing how students used or did not use different properties of entities in conjunction with descriptions of the activities and changes occurring over the course of the mechanism.
Limitations
This research is limited by a variety of factors. First, the generalizability of the results are limited by the context in which the research was conducted. Data was collected only from a single, selective institution. Students’ mechanistic descriptions are likely influenced by their backgrounds, their instructors, and other factors which vary with institution. Specifically, the language used by instructors and the emphasis placed on particular aspects of mechanistic reasoning may influence students’ written mechanistic descriptions.The results are also limited by the data collected and the analytical framework. Since we only analysed students’ final drafts, the findings are limited to the evidence of students’ reasoning demonstrated in their written work after the peer-review process. Some aspects of students’ understanding may not be captured by examining their writing, and students’ actual ability to reason through mechanisms could be greater or less than suggested by their writing. Also, the framework used to analyse students’ writing did not assess the accuracy or correctness of the written mechanisms. Hence, the framework is limited to characterizing how students include the features necessary for mechanistic reasoning as opposed to whether or not their written mechanism is correct. The analysis is also limited in that no external measures of students’ mechanistic reasoning were administered, so the research cannot suggest the efficacy of the WTL assignment to develop the capacity for reasoning.
Another limitation is that the framework was applied to a specific prompt eliciting students’ mechanistic descriptions of a specific reaction mechanism. Descriptions of other reaction mechanisms might produce different results in terms of the prevalence of particular features; furthermore, writing to describe other reaction mechanisms might prompt students to incorporate additional features not included in the present analytical framework. Additionally, elements of prompt design likely influence the way students write about mechanisms. In particular, the features necessary for mechanistic reasoning not present in students’ writing (e.g., identifying organization of entities) could be due to the specific mechanism or prompt examined in this study. The absence of these features could alternatively be an artefact of translating a mechanism into writing. This distinction is unclear and would require further research.
Implications
Implications for teaching
There are a number of implications for practice stemming from this work. First, this research presents a WTL assignment that successfully elicited detailed mechanistic descriptions, which, as suggested by the cognitive process theory of writing, can support students’ learning. Additionally, the findings suggest that the language students use to write about mechanisms—and, tangentially, the way students think about mechanisms—is reasonably accurate and thus potentially influenced by the language instructors use when describing mechanisms. For example, students frequently used the word “attacks” to describe a nucleophilic attack, but it is not certain that students understand the implicit electron movement described when they write that a nucleophile “attacks” an electrophile. Therefore, it is important to be as explicit as possible that these words being used to describe mechanistic steps—words like “attacks” and “protonates”—are words that are implicitly describing the movement of electrons. Furthermore, it may be valuable for instructors to use words that more accurately represent molecular behaviour—for example, replacing the word “attacks” with “collisions” when describing interactions between nucleophiles and electrophiles.Building upon this observation, it is vital that instructors connect mechanistic steps to the underlying chemical properties driving mechanisms. The findings in this study suggest that students are able to say what is happening but not always able to explain why things are happening. This tendency suggests that instructors need to emphasize the appropriate use of fundamental chemistry concepts students should be thinking of when considering reaction mechanisms. In particular, instructors can place more focus on considering the nucleophilicity and electrophilicity of reacting species as a way to describe the flow of electrons in each step of a mechanism; this concept is perhaps the most fundamental way that practicing chemists think about mechanisms, but it was less common among students’ written explanations in comparison to considerations of charges or acid–base chemistry.
In addition to carefully modelling for students all components of a mechanistic description when presenting a mechanism in class, further implications for practice could be to incorporate these components into mechanism questions on assignments or assessments. The four categories of features in students’ mechanistic descriptions provide a natural scaffold for engaging students in mechanistic reasoning; these could be presented in the text accompanying a mechanism problem or could be made into problems themselves. For example, a problem asking students to provide a mechanism might include components where the student must identify the reaction conditions or describe the relevant properties of molecules driving particular mechanistic steps in addition to providing the electron-pushing diagram. Incorporating such questions into a problem will emphasize for students the components of a mechanism that practicing chemists are considering—the reaction medium, alternative reaction pathways, the properties of entities, etc.—as opposed to only emphasizing for students the electron-pushing formalism itself.
Implications for research
Prior research has identified differences in students’ reasoning (Sevian and Talanquer, 2014; Cooper et al., 2016; Weinrich and Talanquer, 2016; Bodé et al., 2019), including identification of the hierarchical relationships between components of a mechanistic description (Moreira et al., 2018). The present research is the first study to use the lift metric to empirically demonstrate this hierarchical relationship between components. Furthermore, this study used lift to analyse a large set of written data to make inferences about students’ mechanistic reasoning. This is valuable because it has allowed for the investigation of students’ mechanistic reasoning at a larger scale, which in prior studies has been investigated using think-aloud interviews with limited numbers of participants. Generally, lift is a metric that can be applied in other settings to examine co-occurrences between codes in a qualitative coding scheme. It is applicable to any coding scheme in which multiple codes may be applied to a single unit of analysis and is valuable for identifying when code co-occurrences occur more or less than expected by chance. Hence, lift could be useful in analysing coding results for any number of research studies utilizing a coding scheme.Studies by Moon as well as Moreira examined students’ writing to understand their reasoning (Moon et al., 2019) and mechanistic reasoning (Moreira et al., 2018) in general chemistry and high school chemistry settings. This study expands on this work to examine students’ responses to a WTL prompt eliciting explanations of an organic reaction mechanism. The methods presented in this study provide a route to access students’ reasoning using qualitative methods to identify features in students’ responses followed by a quantitative method to make inferences about their reasoning. This methodology could be used in similar studies of students’ mechanistic reasoning to afford further insights. For instance, more specific coding of entities (e.g., specific functional groups) and their properties and activities could allow researchers to specifically characterize how students construct structure–property relationships. Such efforts could identify the sophistication of students’ mechanistic reasoning by recognizing if students connect properties to function or simply associate specific structural features with particular mechanistic activities. This may be especially insightful in situations where students are proposing an unknown mechanism without access to outside resources, where they would be required to use these relationships to determine reaction progress. Furthermore, analysing student writing, as opposed to their use of symbolic notation, could be applied to similar WTL activities engaging students in tasks of describing other organic reaction mechanisms. Doing so would broaden our understanding of how students reason through mechanisms and develop our understanding of the relationship between reaction type (e.g., hydrolysis versus substitution) and students’ use of components necessary for engaging in mechanistic reasoning.
Additional studies are also needed to further explore the application of this framework in other contexts, with attention to variables such as institution, prompt design, instructors’ use of language, and students’ prior experience with organic chemistry. These variables, among others, may influence students’ mechanistic descriptions. Beyond this, future research could include examining the effect of peer-review and revision on students’ mechanistic descriptions by applying the framework to students’ first and final drafts and examining changes in the presence of each feature of mechanistic reasoning. Another future direction could involve further examination of the data to identify if there are differences in mechanistic reasoning between students. For example, the features present in students’ writing may correlate to their success in the course or relate to other factors linked to student performance. If this is the case, such writing assignments could be utilized as a tool for providing formative assessment to students in order to develop their mechanistic reasoning skills.
Conflicts of interest
There are no conflicts to declare.Appendix 1. The Writing-to-Learn assignment
Thalidomide: a pharmaceutical Jekyll and Hyde
Thalidomide was widely used after World War II as a sedative and later as a treatment for morning sickness. Unfortunately, it was only after widespread use that it was discovered that thalidomide causes very serious side effects – in particular, birth defects such as phocomelia (limb malformation). The drug was banned in 1962 and these events resulted in important changes to the way the FDA approves drugs.Despite the inherent dangers, thalidomide is now used for treatment of serious diseases, such as cancer and leprosy, when the benefit of treatment outweighs the inherent risks. It is now understood that thalidomide exists as two enantiomers; one is a teratogen and the other has therapeutic properties. Rapid racemization occurs at body pH and both enantiomers are formed at roughly an equal mixture in the blood, which means that even if only the useful isomer is used, both will form once introduced in the body. Furthermore, both enantiomers are subject to acid hydrolysis in the body and produce hydrolysis products that may or may not be teratogens depending on their structure. The structure of Thalidomide and two Thalidomide hydrolysis products are shown below in Fig. 12.
You are an organic chemist collaborating with a team of other researchers from USC with the goal of testing Thalidomide analogs for cancer treatment. An analog is a compound that is very similar to the pharmaceutical target that has small structural differences. For example, m-cresol (shown in Fig. 13 below) is an analog of phenol. Your goal will be to design a structural difference that will make the Thalidomide analog less reactive toward hydrolysis than Thalidomide. Your analogs will be tested for the inhibition of a pro-inflammatory protein mediator, which in elevated levels may be responsible for symptoms associated with the early stages of HIV.
Although Thalidomide is warranted for treatment of some diseases, it would be preferable to identify an analog that has similar therapeutic qualities without the potentially devastating side effects. It is known that Thalidomide is easily hydrolyzed, and it has been proposed that one of the biologically active species may be one of the two possible hydrolysis products shown above. Thus it is important to propose analogs that are not readily hydrolyzed.
Your research team is drafting a grant proposal for the National Institute of Health. You must contribute a 500–750 word description explaining the structure and reactivity of thalidomide toward hydrolysis and the structural differences in proposed analogs that will make them inert to hydrolysis. The committee who will review the proposal is likely to be made up of scientists from disciplines including biology, chemistry and medicine. While they are experts in their own field, they may not be knowledgeable about organic chemistry, racemization, hydrolysis, or NMR spectroscopy.
When writing, you should consider the following:
1. Design one compound (thalidomide analog) that should be a pro-inflammatory protein mediator inhibitor. Explain.
2. Explain why it is important that thalidomide analogs do not have acidic protons at their stereocenters.
3. Explain the mechanism for acid hydrolysis of thalidomide to form the two hydrolysis products in Fig. 12.
4. Describe how you would monitor hydrolysis of thalidomide by NMR.
5. Set the tone of your piece by placing your description in the context of the larger goal of developing a safer drug for the treatment of cancer patients.
6. You should consider carefully which organic chemistry terms you use and when you define or explain them. Remember, your collaborators are relying on you to clearly communicate your plan so that they can write a competitive proposal for funding from the NIH.
NOTE: you can choose to include drawings of either the mechanism or of your proposed analog. However, given your audience, your written explanation should be sufficient such that your proposed analog can be understood without the drawing. Your grade will be solely determined based on what you wrote.
Appendix 2. Coding scheme
Appendix 3. Sample responses and application of coding scheme
Appendix 4. Appearance rate and frequency data
Appendix 5. Co-occurrence and lift data
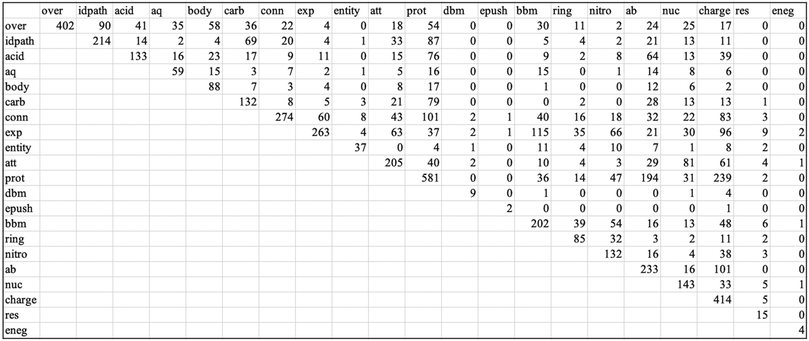 | ||
| Fig. 15 Co-occurrence frequency data for all codes. The values indicate the total number of sentences for which each pair of codes appeared together. | ||
Acknowledgements
The authors would like to thank the Keck Foundation and the University of Michigan Third Century Initiative for funding. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE 1256260. The authors would additionally like to thank Solaire Finkenstaedt-Quinn, other members of the Shultz group, and Arthur Miranda for discussions related to the preparation of this manuscript.References
- Anderson T. L. and Bodner G. M., (2008), What can we do about “Parker”? A case study of a good student who didn’t “get” organic chemistry, Chem. Educ. Res. Pract., 9(2), 93–101.
- Anderson P., Gonyea R. M., Anson C. M., and Paine C., (2015), The Contributions of Writing to Learning and Development: Results from a Large-Scale Multi-institutional Study, Res. Teach. Engl., 50(2), 199–235.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students’ ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16(4), 797–810.
- Anzovino M. E. and Bretz S. L., (2016), Organic chemistry students’ fragmented ideas about the structure and function of nucleophiles and electrophiles: a concept map analysis, Chem. Educ. Res. Pract., 17(4), 1019–1029.
- Becker N., Noyes K., and Cooper M., (2016), Characterizing Students’ Mechanistic Reasoning about London Dispersion Forces, J. Chem. Educ., 93(10), 1713–1724.
- Bhattacharyya G., (2013), From source to sink: Mechanistic reasoning using the electron-pushing formalism, J. Chem. Educ., 90(10), 1282–1289.
- Bhattacharyya G. and Bodner G. M., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82(9), 1402.
- Bhattacharyya G. and Harris M. S., (2018), Compromised Structures: Verbal Descriptions of Mechanism Diagrams, J. Chem. Educ., 95(3), 366–375.
- Bodé N. E., Deng J. M., and Flynn A. B., (2019), Getting Past the Rules and to the WHY: Causal Mechanistic Arguments When Judging the Plausibility of Organic Reaction Mechanisms, J. Chem. Educ., 96(6), 1068–1082.
- Cartrette D. P. and Mayo P. M., (2011), Students’ understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12(1), 29–39.
- Caspari I., Kranz D., and Graulich N., (2018a), Resolving the complexity of organic chemistry students’ reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19(4), 1117–1141.
- Caspari I., Weinrich M. L., Sevian H., and Graulich N., (2018b), This mechanistic step is “productive”: organic chemistry students’ backward-oriented reasoning, Chem. Educ. Res. Pract., 19(1), 42–59.
- Christian K. and Talanquer V., (2012), Modes of reasoning in self-initiated study groups in chemistry, Chem. Educ. Res. Pract., 13(3), 286–295.
- Cooper M. M., Kouyoumdjian H., and Underwood S. M., (2016), Investigating Students’ Reasoning about Acid-Base Reactions, J. Chem. Educ., 93(10), 1703–1712.
- Corbin J. and Strauss A., (1990), Grounded Theory Research: Procedures, Canons, and Evaluative Criteria, Qual. Sociol., 13(1), 3–21.
- Crandell O. M., Kouyoumdjian H., Underwood S. M., and Cooper M. M., (2018), Reasoning about Reactions in Organic Chemistry: Starting It in General Chemistry, J. Chem. Educ., 96(2), 213–226.
- De Arellano D. C.-R. and Towns M. H., (2014), Students’ understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15(4), 501–515.
- Dood A. J., Fields K. B., and Raker J. R., (2018), Using Lexical Analysis to Predict Lewis Acid-Base Model Use in Responses to an Acid-Base Proton-Transfer Reaction, J. Chem. Educ., 95(8), 1267–1275.
- Emig J., (1977), Writing as a Mode of Learning, Coll. Compos. Commun., 28(2), 122–128.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113.
- Finkenstaedt-Quinn S. A., Halim A. S., Chambers T. G., Moon A., Goldman R. S., Gere A. R., and Shultz G. V., (2017), Investigation of the Influence of a Writing-To-Learn Assignment on Student Understanding of Polymer Properties, J. Chem. Educ., 94(11), 1610–1617.
- Finkenstaedt-Quinn S. A., Snyder-White E. P., Connor M. C., Gere A. R., and Shultz G. V., (2019), Characterizing Peer Review Comments and Revision from a Writing-to-Learn Assignment Focused on Lewis Structures, J. Chem. Educ., 96(2), 227–237.
- Finkenstaedt-Quinn S. A., Watts F. M., Petterson M. N., Archer S. R., Snyder-White E. P. and Shultz G. V., (2020a), Exploring Student Thinking about Addition Reactions, J. Chem. Educ., DOI:10.1021/acs.jchemed.0c00141.
- Finkenstaedt-Quinn S. A., Halim A. S., Kasner G., Wilhelm C. A., Moon A., Gere A. R. and Shultz G. V., (2020b) Capturing student conceptions of thermodynamics and kinetics using writing, Chem. Educ. Res. Pract., 10.1039/c9rp00292h.
- Flower L. and Hayes J. R., (1981), A Cognitive Process Theory of Writing, Coll. Compos. Commun., 32(4), 365–387.
- Flower L. and Hayes J. R. R., (1984), Images, Plans, and Prose: The Representation of Meaning in Writing, Writ. Commun., 1(1), 120–160.
- Flynn A. B. and Featherstone R. B., (2017), Language of mechanisms: exam analysis reveals students’ strengths, strategies, and errors when using the electron-pushing formalism (curved arrows) in new reactions, Chem. Educ. Res. Pract., 18(1), 64–77.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before reactions: a mechanistic approach to the organic chemistry curriculum based on patterns of electron flow, J. Chem. Educ., 92(5), 803–810.
- Galloway K. R., Stoyanovich C., and Flynn A. B., (2017), Students’ interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18(2), 353–374.
- Gere A. R., Limlamai N., Wilson E., MacDougall Saylor K., Pugh R., and Saylor K. M., (2019), Writing and Conceptual Learning in Science: An Analysis of Assignments, Writ. Commun., 36(1), 99–135.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: How do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Graulich N. and Bhattacharyya G., (2017), Investigating students’ similarity judgments in organic chemistry, Chem. Educ. Res. Pract., 18(4), 774–784.
- Grimberg B. I. and Hand B., (2009), Cognitive pathways: analysis of students’ written texts for science understanding, Int. J. Sci. Educ., 31(4), 503–521.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: from rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13(3), 201–208.
- Grove N. P., Hershberger J. W., and Bretz S. L., (2008), Impact of a spiral organic curriculum on student attrition and learning, Chem. Educ. Res. Pract., 9(2), 157–162.
- Grove N. P., Cooper M. M., and Cox E. L., (2012a), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Educ., 89(7), 850–853.
- Grove N. P., Cooper M. M., and Rush K. M., (2012b), Decorating with arrows: Toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Hayes J. R., (1996), A New Framework for Understanding Cognition and Affect in Writing, in Levy C. M. and Ransdell S. (ed.), The Science of Writing: Theories, Methods, Individual Differences, and Applications, Mahwah, New Jersey: Lawrence Erbaum Associates, pp. 1–27.
- Kirilenko A. P. and Stepchenkova S., (2016), Inter-Coder Agreement in One-to-Many Classification: Fuzzy Kappa, PLoS One, 11(3), e0149787.
- Klein P. D., (1999), Reopening Inquiry into Cognitive Processes in Writing-To-Learn, Educ. Psychol. Rev., 11(3), 203–270.
- Klein P. D. and Boscolo P., (2016), Trends in Research on Writing as a Learning Activity, J. Writ. Res., 7(3), 311–350.
- Kraft A., Strickland A. M., and Bhattacharyya G., (2010), Reasonable reasoning: Multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- MacArthur C. A. and Graham S., (2016), Writing Research from a Cognitive Perspective, in MacArthur C. A., Graham S., and Fitzgerald J. (ed.), Handbook of Writing Research, New York, NY: Guilford, pp. 24–40.
- Machamer P., Darden L., and Craver C. F., (2000), Thinking about Mechanisms, Philos. Sci., 67(1), 1–25.
- McHugh M. L., (2012), Interrater reliability: the kappa statistic, Biochem. Med., 22(3), 276–282.
- Merceron A. and Yacef K., (2008), Interestingness Measures for Association Rules in Educational Data, in Proceedings of the First International Conference on Educational Data Mining, pp. 57–66.
- Miles M. B., Huberman A. M., and Saldana J., (2014), Qualitative data analysis: a methods sourcebook, 3rd edn, Los Angeles, CA: Sage.
- Moon A., Zotos E., Finkenstaedt-Quinn S., Gere A. R., and Shultz G., (2018), Investigation of the role of writing-to-learn in promoting student understanding of light–matter interactions, Chem. Educ. Res. Pract., 19(3), 807–818.
- Moon A., Moeller R., Gere A. R., and Shultz G. V., (2019), Application and testing of a framework for characterizing the quality of scientific reasoning in chemistry students’ writing on ocean acidification, Chem. Educ. Res. Pract., 20(3), 484–494.
- Moreira P., Marzabal A., and Talanquer V., (2018), Using a mechanistic framework to characterise chemistry students’ reasoning in written explanations, Chem. Educ. Res. Pract., 20(1), 120–131.
- Nowell L. S., Norris J. M., White D. E., and Moules N. J., (2017), Thematic Analysis: Striving to Meet the Trustworthiness Criteria, Int. J. Qual. Methods, 16(1), 1–13.
- Petterson M. N., Watts F. M., Snyder-White E. P., Archer S. R., Shultz G. V., and Finkenstaedt-Quinn S. A., (2020), Eliciting student thinking about acid-base reactions via app and paper-pencil based problem solving, Chem. Educ. Res. Pract. 10.1039/C9RP00260J.
- Popova M. and Bretz S. L., (2018), It's only the major product that we care about in organic chemistry: An analysis of students’ annotations of reaction coordinate diagrams, J. Chem. Educ., 95(7), 1086–1093.
- QSR International Pty Ltd., (2018), NVivo qualitative data analysis software (Version 12).
- Reynolds J. A., Thaiss C., Katkin W., and Thompson R. J., (2012), Writing-to-learn in undergraduate science education: a community-based, conceptually driven approach, CBE Life Sci. Educ., 11(1), 17–25.
- RStudio Team, (2018), RStudio: Integrated Development for R.
- Russ R. S., Scherr R. E., Hammer D., and Mikeska J., (2008), Recognizing mechanistic reasoning in student scientific inquiry: A framework for discourse analysis developed from philosophy of science, Sci. Educ., 92(3), 499–525.
- Schmidt-McCormack J. A., Judge J. A., Spahr K., Yang E., Pugh R., Karlin A., et al., (2019), Analysis of the role of a writing-To-learn assignment in student understanding of organic acid-base concepts, Chem. Educ. Res. Pract., 20(2), 383–398.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: A learning progression on chemical thinking, Chem. Educ. Res. Pract., 15(1), 10–23.
- Shultz G. V. and Gere A. R., (2015), Writing-to-Learn the Nature of Science in the Context of the Lewis Dot Structure Model, J. Chem. Educ., 92(8), 1325–1329.
- Webber D. M. and Flynn A. B., (2018), How Are Students Solving Familiar and Unfamiliar Organic Chemistry Mechanism Questions in a New Curriculum? J. Chem. Educ., 95(9), 1451–1467.
- Weinrich M. L. and Talanquer V., (2016), Mapping students’ modes of reasoning when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 17(2), 394–406.
| This journal is © The Royal Society of Chemistry 2020 |