Student assumptions and mental models encountered in IR spectroscopy instruction
Lyniesha Chanell
Wright
 * and
Maria Theresa
Oliver-Hoyo
* and
Maria Theresa
Oliver-Hoyo

Department of Chemistry, North Carolina State University, USA. E-mail: lcwrigh2@ncsu.edu
First published on 4th December 2019
Abstract
The mental models students have after engaging in an activity designed to teach infrared (IR) spectroscopy without reliance on IR absorption tables, were characterized. Qualitative analysis of semi-structured interviews, through open coding, allowed the classification of the mental models as Molecules as Dynamic (MAD), Bonds as Dynamic (BAD), Molecules as Static (MAS), External Energy (EE), and Internal Energy (IE). Assumptions students have about structure, dynamics, and spectra when solving IR spectra were identified and grouped as intuitive, valid, and spurious. A connection was found between participants with more sophisticated mental models and those who used multi-variate reasoning. Participants were also more likely to be successful when they compared spectra. The results of the analysis suggest IR spectroscopy should be taught through a conceptual lens to guide learning about the interaction of energy and matter.
Introduction
Since reasoning in organic chemistry requires deciphering between multiple variables to make sense of chemical properties, it has been recommended to provide explicit instruction requiring students to combine multiple concepts to reason about molecular structures and chemical behaviour (Bhattacharyya, 2006; Kraft et al., 2010). In response to this recommendation, a physical model and activity were designed to guide students to reason through multiple concepts in order to find meaning in structural representations (Wright and Oliver-Hoyo, 2019). The instructional materials facilitate (a) reasoning by directing students to compare structures and analyse how those differences will be reflected on an IR spectrum and (b) discussions of molecular properties such as bond order, polarity, dipole, reduced mass, and abundance as they relate to the stretching and bending of molecules when induced with IR light. Multiple concepts were incorporated into the instructional materials with the aim to promote the use of appropriate assumptions and heuristics and refute those that are not domain-specific. It was shown that students successfully used the model and activity to extract how those properties influence the position and intensity of peaks in the spectra of molecules (Wright and Oliver-Hoyo, 2019). This current analysis further uncovers the assumptions and mental models students used to predict and explain peaks on spectra. The following research questions were addressed:1. What assumptions and mental models were expressed by students that completed the activity?
2. What is the relationship between participants’ mental models and processes when analysing spectra and structures?
In order to address these research questions, a mental model framework (Briggs et al., 2011) and two assumption classification schemes (Maeyer and Talanquer, 2013; Cullipher and Sevian, 2015) were used to guide in the analysis of the rich data obtained. Ultimately, the study aimed at characterizing mental models students hold when studying IR spectroscopy in order to better understand assumptions held when interpreting IR spectra and be able to identify and prevent barriers students may encounter.
Mental models
There is a large emphasis in the literature about understanding student mental models across a variety of domains (Gentner and Gentner, 1983; Bhattacharyya, 2006; Ealy and Hermanson, 2006; Kraft et al., 2010; Strickland et al., 2010; McClary and Talanquer, 2011; Cullipher and Sevian, 2015). Mental models, as defined by Johnson-Laird, are considered the basis of cognition: describing the role of objects and the structure of events (1983). They have been described as dynamic internal representations that are constructed when faced with a problem, but also can be stored in long-term memory and applied when given a specific task (McClary and Talanquer, 2011). Internal mental models can provide predictive explanatory power for understanding interactions (Gentner, 1983). Alternative conceptions of or inaccurate connections between chemical phenomena can lead to underdeveloped or limited mental models (McClary and Talanquer, 2011; Cooper et al., 2013).Briggs and colleagues developed an explanatory mechanism of mental models to assess inquiry-based teaching methods (2011). Their framework categorizes responses as referents, relations, and results to make sense of an experience or chemical phenomena. Referents are symbols or units that students use to articulate knowledge. The connection between referents produces relations while the application of relations, in a given context, produces results. While the framework was designed to qualitatively assess inquiry-based teaching, it has also been used to identify student mental models when engaging in a sense-making activity (Larson et al., 2012). This framework aided in the analysis of our data to identify the terms and ideas students connect and how they are applied in order to understand the underlying assumptions held.
Assumptions control the development of mental models about concepts or the phenomena at hand. They are constraints, skeletal in nature, that guide cognitive processes (Sevian and Stains, 2015). Our analysis looks at the assumptions students use in the process of interpreting IR spectra in order to characterize their mental models.
Assumptions about bonding and IR
IR vibrational frequencies differ as a result of changes in bond properties. Therefore, if students have an appropriate understanding of bonding concepts, it can be used to generalize and predict aspects of an IR spectrum. However, when reasoning about bonding, studies have shown that:(a) Undergraduate students may consider molecules as a collection of distinct atoms acting independently (Bhattacharyya and Bodner, 2005; Cullipher and Sevian, 2015).
(b) Novice students tend to interpret a molecule by assuming additive properties instead of emergent properties (Talanquer, 2008; Bhattacharyya, 2014).
(c) Students often overlook the dynamic features of bonds (Bhattacharyya, 2014; Cullipher and Sevian, 2015; Graulich, 2015).
If students struggle with making meaning from structures, the ability to understand structure–property relations or predict how they will behave in reactions becomes a conceptual challenge.
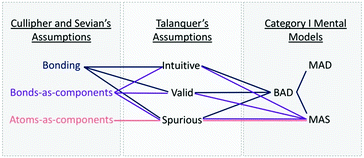
Cullipher and Sevian conducted a study to identify the implicit assumptions students have when reasoning about molecular structures as they relate to IR spectra (Cullipher and Sevian, 2015). They used eye-tracking and think-aloud interviews to search for primary thinking patterns across first, second, and final year undergraduates in addition to graduate students. They found that students reasoned based on three primary thinking patterns and their assumptions were classified as “atoms-as-components,” “bonds-as-components,” or “bonding.” “Atoms-as-components” participants thought about molecules as a collection of atoms with an inclusion or lack of atoms influencing the spectral appearance. “Bonds-as-components” participants considered molecules as a collection of atoms and static bonds or as a series of separate functional groups that appear differently on a spectrum. “Bonding” participants considered molecules as more dynamic with varying intramolecular forces between regions of molecules that influence how energy interacts with the molecule to impact spectral appearance.
As expected, the researchers observed that with increased experience in chemistry, student's assumptions became more scientifically normative. For example, while 75% first-year students held “atoms-as-components” assumptions, 72% of second year students held “bonds-as-components” assumptions and all graduate participants reached “bonding” assumptions. Their implications for chemistry educators included purposefully using instruction that allows students to reach bonding assumptions earlier as it may facilitate more advanced reasoning that can be transferred to other problems in chemistry (Cullipher and Sevian, 2015). Our work builds upon this analysis as the instructional materials were designed to reinforce underlying principles about bonding in the context of IR and to target documented invalid assumptions. For example, students have stated “larger peaks resulted from atoms with a higher atomic weight” when reasoning about IR and bonding (Cullipher and Sevian, 2015). This assumption was addressed in our activity by focusing on how mass and other properties correctly influence peaks on a spectrum (Wright and Oliver-Hoyo, 2019). The assumptions student's hold as a result will be related to the mental models they use to reason about structures as it relates to IR spectroscopy.
Assumptions can guide reasoning to enhance and constrain learning; therefore, using the common sense model (Talanquer, 2006), they have been categorized into three general groups. Intuitive assumptions are justifications stated when applying intuitive knowledge of the properties or behaviours. Valid chemical assumptions are justifications based on established and acceptable chemical principles. Spurious chemical assumptions are invalid ideas about chemical properties and behaviours. This categorization guides the analysis in identifying what assumptions aid effective reasoning about IR. Each of the three implicit assumptions identified by Cullipher and Sevian can contain intuitive, valid, and spurious assumptions. Connor and Shultz recently identified invalid chemical assumptions, similar to the spurious chemical assumptions, that constrain organic chemistry student's reasoning when viewing IR and 1H NMR spectra (2019). As we looked at the assumptions students hold for the “atoms-as-components,” “bonds as components,” and “bonding” assumptions, we will also describe the intuitive, valid, and spurious chemical assumptions uncovered.
When analysing the spectra without the presence of IR tables, the participants of our study were probed to predict and justify peaks based on their understanding of molecular and chemical principles. We aim to describe the mental models students held when analysing spectra by considering the assumptions that construct them.
Methodology
Participants
Participants were undergraduate students enrolled in an organic laboratory and simultaneously taking their first semester of organic chemistry, at a large public university in the USA. None of the students had exposure to IR spectroscopy prior to completing the laboratory activity. Of the 86 students who completed the IR laboratory, 29 volunteered to be interviewed within one week of having engaged in the lab, in order to capture the reasoning and rationalization students use to make meaning of IR spectroscopy. The volunteers were considered a representative sample of the entire group as both the demographics and activity scores showed no significant differences between the group of volunteers and students completing the IR lab.Data collection
One-on-one, semi-structured, think-aloud interviews were conducted with the 29 volunteers. Additionally, notes were taken by the interviewer to capture what the participants were doing when presented with the questions. According to our IRB guidelines, permission was obtained from the participants to audio record their responses and consent forms were signed to utilize their responses. Upon completion, all interviews were transcribed for qualitative analysis. The interview questions were designed to solicit participant's assumptions and mental models when reasoning about bonding as it relates to IR spectroscopy. The interview questions, shown in the appendix, required students to explain, predict, and sequence concepts when analysing spectra which are characteristics of sense-making activities (Briggs et al., 2011). The molecules for the interview were chosen based on case comparisons, in which the students are discerning between similar and different features and their effect on a spectrum (Alfieri et al., 2013). Part one of the interview probed for student understanding of the concepts and part two aimed to identify how students applied their assumptions and reasoned when problem-solving with IR spectra.Data analysis
Qualitative analysis rooted in grounded theory, was used to identify emergent themes and patterns from the data through open coding (Corbin and Strauss, 1990). Each participant was given an identifier to ensure anonymity. The interviews were transcribed and uploaded into ATLAS.ti software (https://atlasti.com/), a desktop application used for qualitative data analysis of text, graphical, audio, and visual data. The interviews were analysed line-by-line for common terms and phrases participants used. In order to categorize and compare codes, constant comparative analysis was used (Glasser and Strauss, 1967). The explanatory methods of mental models aided in the creation of the coding scheme. Terms the participants used to make sense of information were identified as referents. When students made connections between referents it was categorized as a relation. Relations that were applied to spectra were assigned as results (Briggs et al., 2011). For example, bond order and bond strength were two referents commonly used. A relation that could be established is that the greater the bond order, the stronger the bond. An example of a result is if a participant stated the greater the bond order the higher the wavenumber. These relations and results were identified and grouped as intuitive, valid, or spurious assumptions and were used to identify the participants’ underlying assumptions (Maeyer and Talanquer, 2013).Three additional codes emerged from the data reflecting the process students used when answering the interview questions. Participants who verbalized assumptions articulated how a concept related to the spectra. Participants who predicted assumptions used the concepts to describe how a bond is reflected on a spectrum (without having the spectrum present). When assumptions were applied during the labelling of peaks on a spectrum, the process was coded as utilized.
Peer debriefing and negative case analysis were used to improve the credibility and trustworthiness of the data (Lincoln and Guba, 1985). The first author consistently dialogued with two other researchers to discuss interpretations of the data and remove biases. Negative case analysis was used to confirm emerging patterns. Validation of the coding scheme was established through interrater reliability. An independent-rater analysed 28% of the interviews. Upon discussion, an agreement of 94% was achieved (Campbell et al., 2013).
Results
Assumptions
The interviews provided the participants with an opportunity to express their assumptions when predicting or identifying peaks on spectra. Referents were used to identify assumptions and make sense of participants’ mental models. Some referents were explicitly asked about, or targeted, during the interview while other referents emerged without prompting. With an interest in student mental models, only the emerging referents were quantified. As shown in Table 1, the most frequent emerging referents included energy, implicit hydrogens, and electronegativity. These referents represent the items most accessible to students when answering questions.| Targeted referents | ||
| Bond order | Change in dipole | Reduced mass |
| Bond polarity | Abundance | Transmittance |
| Wavenumber | Intensity | Position |
| Additional referents (percent occurrence) | ||
| Energy (83%) | Implicit hydrogens (76%) | Electronegativity (72%) |
| Bend (45%) | Stretch (41%) | Strength (38%) |
| U-shaped peaks (38%) | Lone pairs (28%) | Hybridization (17%) |
| Light (14%) | Functional groups (14%) | Formal charge (7%) |
| Fingerprint region (10%) | Hydrogen bonding (7%) | Length (3.5%) |
There were thirteen underlying assumptions that were held by at least ten percent of the participants. These assumptions are grouped based on three features of IR: molecular structure, molecular dynamics, and the relation to spectra. The assumptions are ordered by their level of sophistication, with the highest level being the most sophisticated. The assumptions identified about molecular structure (Table 2) and dynamics (Table 3) show a linear progression with an increased level of sophistication. As an example, participant S7S10 held a valid assumption at the highest level of sophistication about molecular structure considering that bonds have energy and differences in polarity impact the energy when he stated, “And so, the one that's going to be the most polar of these three probably would be farthest to the left at the IR spectrum because I think it's going to take more energy put in the bond because the bonds are probably going to be a little bit stronger if they're polar, it's going to put more energy in it in order to vibrate it.” However, when it came to the dynamics, this participant fell at level 2. During the interview this participant did not discuss how bonds can have many vibrational modes. Most participants held advance assumptions about molecular structure while their understanding dynamics was more naïve.
| Assumptions about molecular structure | |||
|---|---|---|---|
| Level | Underlying assumptions | Examples | % of participants |
| 3 | The differences in electronegativity between atoms vary the bond strength and energy. (V) | Polarity is a difference in electron distribution. (V) | 76 |
| C–H is a strong bond because of a large dipole. (S) | 24 | ||
| The more polar the bond the stronger the bond. (V) | 13 | ||
| The more lone pairs in a molecule the more polar and the greater the energy. (S) | 13 | ||
| 2 | Bonds have energy. Differences in bond order vary the energy. (V) | The greater the bond order the more energy in that bond. (I) | 79 |
| 1 | Molecules are made of atoms linked by bonds that take up space. Differences in bond order vary bond strength. (V) | The greater the bond order the stronger the bond. (I) | 82 |
| Assumptions about dynamics | |||
|---|---|---|---|
| Level | Underlying assumptions | Examples | % of participants |
| 4 | Vibrations can result in a change in dipole. (V) | The more symmetry in a molecule the less change in its dipole. (V) | 10 |
| 3 | There are different vibrational modes. (V) | Some bonds stretch and other bend. (V) | 38 |
| 2 | Bonds have one vibrational mode. (S) | The C–H bonds will all vibrate at a high wavenumber. (S) | 45 |
| 1 | Atoms or fragments are removed in IR. (S) | An intense peak requires more energy to remove it. (S) | 7 |
While discussing structure and dynamics the participants would identify with only one level of sophistication. However, this was not the case with the assumptions about spectra. When reasoning about spectra, not only was a linear progression of sophistication not evident, but participants could have multiple assumptions. In Table 4, the underlying assumptions are classified into two groups. The first describes the influence of energy; some participants had no mention of energy or light while others believed that external energy or light influences the spectral peaks. The second category describes how molecular structure or dynamics influenced spectral peaks.
| Underlying assumptions about spectra | Examples | % of participants | |
|---|---|---|---|
| Assumptions involving energy | Energy applied to the system determines if there is a peak on the spectrum. (V) | Energy is required for vibrations. (V) | 79 |
| Greater the bond order the more energy needed to vibrate. (I) | 27 | ||
| Energy in the bond determines if there is a peak on the spectrum. (S) | Energy of the bond causes vibrations. (S) | 21 | |
| Energy does not play a role in IR. (S) | NA | 21 | |
| Assumptions involving molecular properties | Vibrations of a bond cause peaks to appear on a spectrum. Different vibrations produce different peaks. (V) | Stretching peaks are further left on the spectrum, while bending peaks are further right. (V) | 38 |
| Peaks appear based on implicit properties of mass and/or polarity. (V) | The greater the reduced mass the lower the wavenumber. (V) | 65 | |
| The greater the polarity the greater the wavenumber. (V) | 62 | ||
| Mass of the entire structure determines where the peaks are. (S) | 14 | ||
| The greater the RM the lower the intensity. (S) | 14 | ||
| Peaks appear based on explicit properties of bond order and/or abundance (V) | Greater the bond order the greater the wavenumber. (V) | 72 | |
| Greater the abundance the greater the intensity. (V) | 69 | ||
| The greater the bond order the lower the intensity. (S) | 14 | ||
| The greater the abundance the greater the number of peaks. (S) | 10 | ||
It is important to note, that while participants may have a valid underlying assumption it may have been expressed through examples that were spurious, valid or intuitive. For example, when asked why the peak labelled as carbon–hydrogen was intense, participant S3S13 stated, “Um, I mean they do have a dipole, right? Because carbon is a lot more electronegative than hydrogen. So they do have a dipole. It's just not as strong.” This participant, like others, demonstrates the valid underlying high-level molecular structure assumption that “differences in electronegativity increase bond strength” even though her statement about a carbon–hydrogen bond having a large dipole is spurious (Table 2).
Cullipher and Sevian found that 14% of second year students had “atoms-as-components,” 14% had “bonding” assumptions, and 72% held “bonds-as-components” assumptions (2015). Using Cullipher and Sevian's categorization, we found no participants with only “atoms-as-components” assumptions, 21% held “bonds-as-components” assumptions, and 79% of the participants held “bonding” assumptions. Both “bonds-as-components” and “bonding” assumptions groups had intuitive, valid, and spurious assumptions, as shown in Table 5 and Fig. 1. Overall, there were more “bonding” assumptions than any other category. These results strongly suggest that targeting concepts explicitly (as designed in our instructional materials) aided student's in reasoning at the most sophisticated level.
| Assumption category | Participant quotes | Assumption |
|---|---|---|
| Atoms-as-components assumption | ||
| Spurious | “…since they all have oxygen, which is usually a very polar substance…” –S6S8 | Atoms have molecular properties. (S) |
| Bonds-as-components assumptions | ||
| Intuitive | “Um, the energy absorbed, uh, will be, will need to be stronger to for the double bond.” –S6S2 | Bonds have energy. Differences in bond order impact the energy. (V) |
| Valid | “…like the more bonds there are, of that certain type, stronger the like peak would be or something because they're all like on the same reading” –S2S9 | Peaks appear based on explicit properties of bond order and/or abundance. (V) |
| Spurious | “It would have a high number, yeah It takes more effort to remove a group.” –S8S2 | Fragments are removed in IR. (S) |
| Bonding assumptions | ||
| Intuitive | “So, the harder, the more energy it takes to vibrate the bond the further to the left they are going to be” –S1S2 | Energy applied to the system determines if there is a peak on the spectrum. (V) |
| Valid | “Well, stretching takes more energy. So, I think stretching would be higher up. And bending takes less energy, so it would be further down.” –S1S9 | Vibrations of a bond causes peaks to appear on a spectrum. Different vibrations produce different peaks. (V) |
| Spurious | “The larger the polarity, the further to the right of the spectrum, it will be. So, for things like carbon hydrogen, where the polarities not as large, that's why their peaks are around 3000, somewhere around that” –S5S1 | Peaks appear based on implicit properties of mass and/or polarity. (V) |
However, some participants with the “bonding” assumptions were more successful at interpreting IR spectra than others. We wanted to identify a distinction between those that were more successful and those that were not. In reviewing the statements categorized as “bonding,” it was evident that the intuitive assumptions dealt heavily with interactions between energy and matter. Both valid and spurious assumptions had evidence of the participants describing the electronic distribution of bonds as a static entity or the movement of the molecule as a result of the interaction of energy and matter. This mixture of static and dynamic “bonding” assumptions confirmed the need to distinguish these in order to better characterize their mental models.
Mental models
There were five prominent mental models that emerged from the data (Table 6). Since problem solving with IR involves (1) understanding molecular and chemical properties and (2) understanding how the interaction of light with molecules can influence those properties, the mental models were grouped into two categories. Category I entails the structural and dynamic nature of molecules and how those properties are reflected on an IR spectrum. Three “mobility-focused” mental models were identified: Molecules are Dynamic (MAD), Molecules are Static (MAS), and Bonds are Dynamic (BAD). Given that energy was the most common emergent referent, category II involves energetic considerations of molecular vibrations. The two mental models in this category were identified as External Energy (EE) and Internal Energy (IE). Both of these mental models are associated with the first set of underlying assumptions about spectra (Table 4). The MAD and MAS mental models have associated underlying assumptions connecting molecular properties to spectra (Table 4). The BAD mental model is associated with the assumptions about molecular structure (Table 2).| Mental models | Description | Underlying assumptions | % of participants | |
|---|---|---|---|---|
| Category 1 | MAD | Bonds and atoms stretch and bend. Based on the structure and symmetry, there are changes in dipoles that result in the different peaks on a spectrum. | • Peaks appear based on bond vibrations. Different vibrations produce different peaks. | 38 |
| MAS | Each bond type is the same and will result in one peak. | • Peaks appear based on implicit properties of mass and/or polarity. | 62 | |
| • Peaks appear based on explicit properties of abundance and bond order. | ||||
| BAD | The differences in electronegativity, based on the atoms present, can change the polarity of a bond. Different bond polarities will appear differently on a spectrum. | • The differences in electronegativity between atoms vary the bond strength and energy. | 76 | |
| Category 2 | EE | Energy must be put into the system to for the structure to vibrate and to see peaks on the spectrum. | • Energy applied to the system determines if there is a peak on the spectrum. | 79 |
| IE | The energy of the system, stored in the bonds, causes the molecule to vibrate resulting in various peaks on the spectrum | • Energy in the bond determines if there is a peak on the spectrum. | 21 | |
The majority of participants held assumptions connected to a combination of multiple mental models. Mental models are complex transitory constructs and it is not uncommon for students to use a variety of mental models. For example, McClary and Talanquer's participants demonstrated multiple mental models contingent upon the task or feature when analysing acid strength (2011). Similar to our analysis, Tümay used a combinatory, or modular approach to represent the mental models held by prospective chemistry teachers about vapor pressure (2014). This approach allowed them to present the findings in a clear and practical manner enabling educators and researchers to use the combinations to predict potential responses.
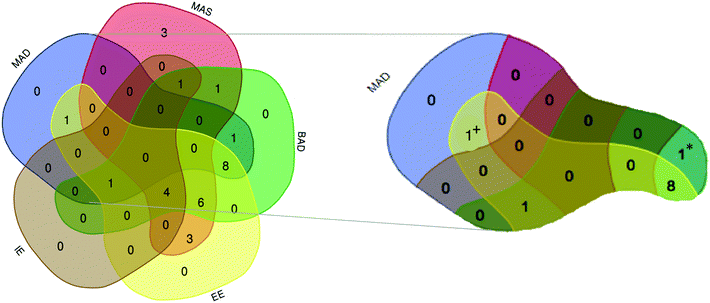
Given that the overlap of these mental models represents how students interpreted spectra, the Venn diagram in Fig. 2, was constructed and used to visualize the intersection of mental models. For example, a total of 11 students manifested the MAD mental model. Only one participant overlapped the only the MAD and BAD models (1* on Fig. 2). Another participant expressed MAD and EE models (1+ on Fig. 2) while 8 participants expressed MAD, BAD, and EE. Let's discuss each model.
“C–C [bonds] will not, um, show up in the spectra because of the lack of changing dipole when the molecule vibrates.”
The participants in this group recognized the differences of molecular vibrations as a result of changes in physical properties and they were more successful at identifying peaks on a spectrum. They also described how differences in structure and symmetry may cause a change in dipole resulting in different peaks on a spectrum. The unique referents used by these participants were stretch and bend.
Participants who viewed molecules as static had an incomplete understanding of IR and used the inaccurate assumption that each peak is a bond type. Therefore, these participants struggled to label the peaks on the spectrum. For example, participant S4S9 knew that carbon–carbon single bonds didn’t appear on the spectra but used it to label a peak anyway.
S4S9: “Okay. So, I guess first I'll just start off by like, like what bonds we have. So like C–H, C–C bonds, C double bonded to an oxygen and, C single bond O, yeah that's good on that one. So, see I would most likely label C–H bond this 2934 because that also has the lowest reduced mass. So that would make it or that would push it farther to the left on, on the spectrum and yeah, because it's the most abundant bond would have like a strong intensity reading. And then next between the C–Cs, like I guess they technically wouldn't. Would they show?
Interviewer: Why are you debating whether or not they would show?
S4S9: Oh yea, due to the fact that they don't have like a dipole. If like between the four of them, I would say it would most likely be the 1469 and C double O bond or, C double bonded to the O would probably be the 1716… And then 1119 would say most likely be the C–C bond. And the reason its intensity is so high is because there is a there… Um, so we have one, two, three, four, four C–C bonds. So that's why the intensity would be where it is on the spectrum.
If this student would have considered the concept of multiple vibrational modes, perhaps he may have been more successful at identifying and justifying peaks.
“Well the electronegativity of something changes where the electron density is and that changes a lot of things. So, these, these groups right here, these oxy, these alcohol groups are quite polar so, they can be drawing electron density away from the rest of the molecule and potentially weakening those bonds. Weakening or strengthening…”
These participants were also more likely to reference lone pairs on atoms when describing which atoms were more electronegative. However, a few participants, like S6S2, used lone pairs to make a spurious assumption stating: “The more lone pairs an atom has, the more polar the molecule is.”
“Uh, just it requires a lot of energy is what I remember. So, because it requires a lot of energy, it's going to be a big spike there… The, uh, the single bonds require less energy. So, they're further to the right, if you had a double bond or a triple bond it would require more energy, so it'd be further to the left spectrum.”
When describing the impact of energy, only a few participants stated that energy was proportional to wavenumber. The majority, like participant S5S1 above, predicted the location of peaks because of what may require less energy, but were not clear about how this related to wavenumber.
“My kinda gut instinct is that because the (double) bonds have a high amount of energy that would show in terms of intensity more than anything else… Oh, and because the bond energy is greater in the alcohol group, it's further along in the spectrum.”
Similarly, to EE, the participants used energy to describe the intensity of peaks.
S1S1: I’m going to pick the 1730 as the double bonded oxygen Then there is carbon–carbon bonds and carbon–hydrogen bonds. Hmmmm, I’m going to say the 2934 is the carbon–hydrogen bonds and the 1469 is the carbon–carbon bonds. Or! They could both be carbon–hydrogen bonds. One is a stretch and one is a bend. Didn’t you say something about like hydrogen doesn’t show up?
Interviewer: Um, there are things that don’t show up in IR spectra. What is a qualification to have, do you remember the qualification to have a peak in IR?
S1S1: Does it have to have like a dipole… So, then they are both carbon–carbon bonds. Because hydrogen doesn’t have a dipole. Right? No. I don’t think so. It's just like a little positive charge, like floating around.
Interviewer: What is a dipole to you?
S1S1: Isn’t it like, okay all I can think of is in like the lecture videos. She’ll (the professor) give like a molecule and like half of it is red and the other half is blue because one side is more electronegative than the other. Like the Bromine is pulling this way so yea. That's what I think of. Wait, so then the carbon–carbon bonds wouldn’t show up.
Interviewer: Why?
S1S1: No, that doesn’t make sense than either. Because they, I don’t know. Because they’re the same. Carbon–hydrogen would show up because one is more electronegative than the other. Same with carbon and oxygen. They are both carbon–hydrogen bonds. This one's a stretch (points to the peak around 2900). This one's a bend (points to peak around 1400). It's the difference in how the molecules are acting when the light is transmitted through the bonds. That's what it is.
An interesting example is participant S5S1 who used the MAD–BAD–EE–IE mental models. He reasoned similarly to participant S1S1, but he alternated between considering the energy of the bond and the energy applied to the bonds causing vibrations.
S5S1: …because it's a single bond it, it's going to require or it's going to be on the lower side of the spectrum to the left just because the double bond and the triple bond. They have more energy in them, so they go further to the right.
Interviewer: What about the intensity of the carbon–hydrogen?
S5S1: Um, I’m not sure about that
Interviewer: What about the carbon–oxygen double bond, why it has its intensity?
S5S1: Uh, just it requires a lot of energy is what I remember. So, because it requires a lot of energy, it's going to be a big spike there.
Those with MAS–BAD–EE (6 participants), MAS–BAD–IE (1 participant), and the MAS–BAD–EE–IE (4 participants) mental models had similar difficulties when problem solving about IR spectra because they connected each bond in the molecule to only one peak in the spectrum, a characteristic of the MAS mental model. The main distinction is in their rationale of how energy plays a role. Most of the participants who referenced internal energy said it was a result of bond order as shown by the exchange below:
Interviewer: You mentioned that a triple bond has a higher wavenumber than a double bond. Why do you say that?
S7S2: I think it was energy increases to the left, have more energy in carbon–carbon triple bond than a single bond. Um, yes, it's a stronger bond. There's more electrons in it, but if it were excited, which one would, have more of an effect on IR spectrum, I’m not sure…
In this context the ability to describe whether external or internal energy influenced the vibrations of the molecule did not limit participants’ ability to analyse spectra. Nonetheless, understanding what bond energy means and whether or not a triple bond has higher energy than a double bond is of significance when understanding reactivity and kinetics.
There were three participants who held MAS–EE mental models. None of these participants had success labelling peaks aside from carbon–hydrogen stretches because they did not consider polarity or different vibrations that result in changes in dipoles. When these participants discussed the effects of external energy it was in relation to bond order or reduced mass. Even with those valid assumptions, their analysis was limited without considering how other components played a role. Participant S6S2 demonstrates this in the dialogue below:
S6S2: Yea, so, like a carbon–oxygen …the oxygen only is bonded twice, like it only has two lone pairs or like, I think. Yeah, I think so. Um, so, then compared to the let's say number two, which has like a carbon double bond oxygen. So, the double bond on the carbon–oxygen would be stronger than the single bond on the carbon–oxygen, if that makes sense.
Interviewer: Got it, and how does that, the double, the double bond being stronger than a single bond impact the spectrum, again?
S6S2: It impacts it because the um, the energy absorbed I think is the right word. Um, the energy absorbed, uh, will be, will need to be stronger to for the double bond, therefore it would have a bigger dip.
Five participants did not use energy to reason at all. Three of the participants only used the MAS mental model, one used the MAS–BAD mental models and the fourth held MAD–BAD mental models. These participants considered the concepts as a list of items to check off; the more concepts a molecule has, the higher the wavenumber or the more intense the peak. Participant S3S7, who held the MAD–BAD, demonstrates this when he says,
“Well, my guess is um, since this left, so it would be higher and is since it's a double bond and it has dipole moment. So that's two factors playing into it, sort of make it more shifted to the left.”
The other participants had a similar method of justifying their answers, but they did not include change in dipole or polarity as factors. This strategy of more concepts greater wavenumber was unsuccessful in efficiently identifying and justifying peaks on a spectrum.
Mental models and assumption categories
As shown in Fig. 3, it is notable that all 6 “bonds-as-components” participants expressed the MAS mental model. “Bonding” participants discussed characteristics related to all three mobility-focused mental models (22 for BAD with 11 also having MAS, and the other 10 having MAD mental models). Participant S2S10 articulated the intramolecular properties needed for “bonding” assumptions and expressed the MAD and BAD mental models when she said,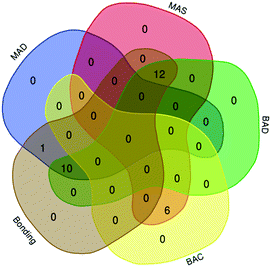 | ||
| Fig. 3 Venn diagram showing the overlap of the category I, mobility-related, mental models and the bonds-as-components (BAC) and bonding assumptions. | ||
“Um this 1461 mmm. Let's see. We only have carbon–oxygen, carbon–hydrogen, and carbon–carbon, there… I guess, okay, that's (peak at 2800) going to be the stretching and then the C–H bending maybe would be the 1461… The more polar, the more um, intense the peak is going to be, so you know, um for that carbon–oxygen it's going to be intense.”
There were participants who held “bonding” assumptions that still viewed molecules as being static, like participant S3S13 who discussed intramolecular forces associated with “bonding” assumptions, albeit incorrectly, when he stated,
“Um, I mean they do have a dipole, right? Because carbon is a lot more electronegative than hydrogen. So, they do have a dipole,”
and also viewed each bond as static by saying,
“the bonds that exist within the structure are C double bond, O, C–C, and C–H. So, I guess each of these has to be a different peak.”
The participants with MAS mental model and “bonding” assumptions considered a dipole a permanent property of a bond like polarity. Therefore, the identification of the MAD and MAS mental models showed two groups within the “bonding” assumptions groups and enabled us to distinguish those that have a more accurate representation of molecules and their dynamic nature from those who do not. It is the participants who exhibit an understanding of the dynamics of bond and molecular interactions as a result of the interaction of energy and matter who were most successful at interpreting the spectra.
Processes
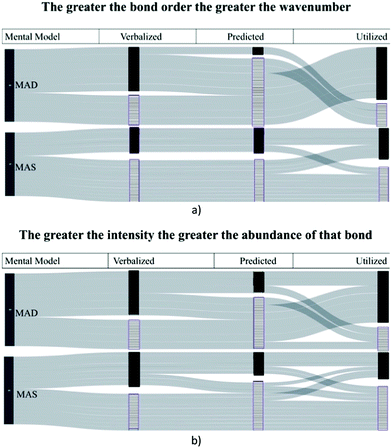
One way to address our second research question was to identify if there was consistency between concepts stated by participants when making predictions about spectra (during part one of the interview) and concepts used by participants when analysing spectra (part two). The least convoluted way to address this question was by comparing the two mental models that did not overlap but included all participants, MAD and MAS. Sankey diagrams were used to visualize how participants connected their verbalizations to their predictions and utilization. Sankey diagrams are flow diagrams where the width of the lines is proportional to the quantity of flow (Schmidt, 2008). They have been used in chemistry to visualize change in student representations of intermolecular forces (Williams et al., 2015). Fig. 4 depicts participants’ responses when discussing valid assumptions (expressed in the titles of each diagram). Each horizontal band represents one participant; the vertical solid bar represents those who expressed the principle and the vertical striped bar represents those who did not at different points in the interview. The diagrams illustrate if a participant was able to verbalize a concept when analysing the structural representation, predict where a bond may appear on the spectrum as a result of the concept, or effectively utilize a concept when comparing spectra to structures.When observing the flow of participant responses, it was evident that there were instances when participants did not verbalize or predict a concept, but later correctly utilized that concept to explain peaks on a spectrum. It seems the “triggering” occurred when the exercise involved comparing spectra. Four participants were only able to incorporate concepts when viewing and comparing the spectra (Fig. 4a). This “triggering” resulted from comparing peaks within and between spectra and occurred to a greater degree with those with the MAD mental model over the MAS. Prior to viewing the spectra, participant S1S9 could not state how the abundance of the bonds would influence the spectra. But after identifying the carbon–hydrogen stretch when comparing two spectra, he noticed a difference in the peak size and a difference in the number of carbon–hydrogens on the structures and said:
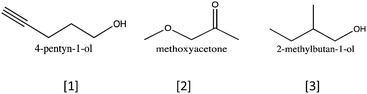
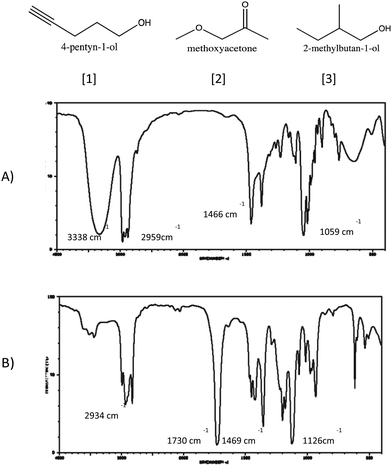
“So maybe (the intensity) has to do with the number carbon–hydrogen bond. So, (4-pentyn-1-ol) would have like… seven, and (2-methylbutan-1-ol) like… eleven. This one has more carbon–hydrogen bonds than in that one. So, that's the way I'm going to reason. And say that (Structure A) is (2-methylbutan-1-ol).”
Comparing spectra and structures aided participants in discriminating between multiple concepts related to molecular structure and spectra in their justifications. This finding is supported by the case comparison technique from analogical learning. It has been found that cases, or purposely designed experiences, that are simultaneously or sequentially compared, promote students to search for commonalities by aligning target features and can ultimately result in engaging with the material in a meaningful way (Alfieri et al., 2013; Graulich and Schween, 2018).
Another way to look at participants’ processes was to record the number of concepts incorporated when solving IR spectra. Multi-variate thinking, as defined by Bhattacharyya, is coordinating the effects of multiple variables (2014). This is contrary to the One-Reason Decision Making (ORDM) inductive judgement heuristic (Talanquer, 2014). Both reasoning strategies were evident by participants during the interviews. Participant S6S13 only used reduced mass to evaluate the spectra saying,
“Carbon–hydrogen has the lowest reduced mass than the rest of them… so that's why I assigned it to 3338 cm −1 . And then, I pretty much just assigned it in order of reduced mass.”
Even when asked about other concepts, participants who used ORDM either stated they don’t know how another concept could impact the spectrum, or they could verbalize a relation or assumption, but not be able to reason about how it applies to spectra.
Six of the 29 participants used ORDM and notably none of them held MAD mental models (Fig. 5). The participants who held a static mental model were also more likely to rely on one concept. The majority of participants engaged in multi-variate decision making by alternating and sequencing the concepts to make sense of the spectra. Participant S4S9, who had a MAS mental model, sequenced multiple concepts as shown below:
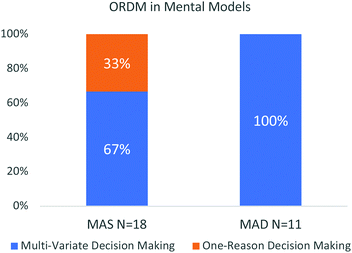 | ||
| Fig. 5 A distribution showing the students who used the MAS and MAD mental models and their use of ORDM. | ||
“Okay. So, I guess first I'll just start off by like, like what bonds we have? So, like C–H and C–C bonds C double bonded to an oxygen… the most abundant bond on here would be the C–H. So, see I would most likely label C–H bond this 2884 because that also has the lowest reduced mass. So that would make it or that would push it farther to the left on, on the spectrum and yeah, because it's the most abundant bond would have like a strong intensity reading.”
Conclusions and implications
Mental models represent perceived components of a system, their properties, relations, behaviours, and functions (Johnson-Laird, 1983; Tümay, 2014). The purpose of this study was to characterize mental models participants held after engaging in targeted instruction on IR. To do so, the assumptions participants had were identified. Their assumptions about IR have three dimensions: molecular structure and molecular dynamics (which had a linear progression of sophistication), and spectra. These assumptions aided in the identification of five mental models; Molecules are Dynamic (MAD), Molecules are Static (MAS), Bonds are Dynamic (BAD), External Energy (EE) and Internal Energy (IE). MAD and MAS were the only mental models that participants did not expressed simultaneously. Participants that discussed the ability of a molecule to stretch or bend (MAD), described how intramolecular forces placed a role (BAD), and recognized energy is required to have a vibration (EE) were the most successful at identifying and justifying peaks on an IR spectrum.When it came to the process of solving IR spectra, our data showed that the majority of the participants used multi-variate decision making and that there is a correlation between the sophistication of the mental model and the use of the ORDM. Those with MAD mental models did not use ORDM while 33% of participants with MAS mental models used ORDM. Therefore, it is encouraged to teach spectroscopy by incorporating multiple variables, as those that reasoned considering multiple variables were more likely to be successful with spectral analysis.
The findings of this study have important implications for teaching IR. We found that the participants were best able to explain the concepts related to spectra when they compared spectra and the structures. This strongly suggests that students were triggered by cues leading them to utilize appropriate assumptions to find meaning from the information contained in spectra. This agrees with research that suggest that instruction that uses specifically designed case comparisons and promotes students to discern between features can aid in interconnected knowledge (Graulich et al., 2012; Graulich and Schween, 2018).
Referencing intuitive variables like bond strength and energy can be effective in teaching about the interaction of energy and matter as it relates to IR, as the referent energy was used frequently by our participants to aid in their explanations. However, we caution the usage of this term and advocate for instruction to be explicit about how energy is defined in a specific context or students will apply it arbitrarily or incorrectly. This study supports the need of explicit instruction about energy being proportional to wavenumber on the x-axis while the amount of that wavenumber or energy that is absorbed is related to the intensity. Analogously, when reasoning about atomic spectra, Körhasan and Wang found that energy, particularly of photons, was essential in understanding atomic spectra but their students with unscientific mental models often confused spectral lines with energy levels (2016).
In our case, we identified underlying assumptions and used them to aid in characterizing the mental models. While participants may have demonstrated valid underlying assumptions, these were often expressed through spurious statements. Though it is important to have students deciphering between multiple variables, it is just as important that they reason with valid assumptions at a high level of sophistication in a domain specific context. The intuitive, valid, and spurious assumptions identified here can be further used to design effective instructional approaches that will promote more sophisticated mental models. This study shows that if students are able to view molecules as dynamic and to consider the influence of external forces, students would be better equipped to label and interpret IR spectra without memorizing wavenumbers or relying on absorption tables. Through this study we were able to identify mental models used by students when solving IR spectra and their assumptions that explain why peaks appear the way they do on a spectrum. We propose that having students reason about IR spectra and structure–property relations from a conceptual lens will promote a dynamic perspective when thinking about molecular properties and engaging in other spectroscopy techniques.
Conflicts of interest
There are no conflicts to declare.Appendix 1: Example interview questions
Part one
The following three structures have different IR spectra.(a) How are the bond types in each substance different from the bonds in the others?
(b) Explain how reduced mass can assist in distinguishing between the structures.
(c) Explain how bond order can assist in distinguishing between the structures.
(d) Explain how bond polarity can assist in distinguishing between the structures.
(e) How does a change in dipole assist in distinguishing between the structures?
(f) How does the abundance of bonds assist in distinguishing between the structures?
(g) What approximate peak positions do you expect will help distinguish the substances?
(h) What approximate peak intensities do you expect will help distinguish the substances?
Part two
The two spectra below correspond with two of the three compounds. Identify which compound corresponds with each structure by relating the structure’ to the spectra's peak position and peak intensity and justify your answers.Acknowledgements
We thank the organic chemistry lab director, Maria Gallardo-Williams, for her assistance in conducting this study. We also want to acknowledge the students who participated and Briley Humphrey for assisting with interrater reliability.References
- Alfieri L., Nokes-Malach T. J., and Schunn C. D., (2013), Learning Through Case Comparisons: A Meta-Analytic Review, Educ. Psychol., 48(2), 87–113.
- Bhattacharyya G., (2006), Practitioner development in organic chemistry: how graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7(4), 240–247.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Briggs M., Long G., and Owens K., (2011), Qualitative Assessment of Inquiry-Based Teaching Methods, J. Chem. Educ., 88(8), 1034–1040.
- Campbell J. L., Quincy C., Osserman J., and Pedersen O. K., (2013), Coding In-depth Semistructured Interviews, Sociol. Methods Res., 42(3), 294–320.
- Connor M. C., Finkenstaedt-Quinn S. A., and Shultz G. V., (2019), Constraints on organic chemistry students’ reasoning during IR and 1 H NMR spectral interpretation, Chem. Educ. Res. Pract., 20, 522–541.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An Investigation of College Chemistry Students' Understanding of Structure-Property Relationships, J. Res. Sci. Teach., 50(6), 699–721.
- Corbin J. and Strauss A., (1990), Grounded Theory Methodology: Procedures, Canons, and Evaluative Criteria, Qual. Sociol., 13(1), 3–21.
- Cullipher S. and Sevian H., (2015), Atoms versus Bonds: How Students Look at Spectra, J. Chem. Educ., 92(12), 1996–2005.
- Ealy J. B. and Hermanson J., (2006), Molecular Images in Organic Chemistry: Assessment of Understanding in Aromaticity, Symmetry, Spectroscopy, and Shielding, J. Sci. Educ. Technol., 15(1), 59–68.
- Gentner D., (1983), Structure Mapping: A Theoretical Framework for Analogy, Cognit. Sci., 7(2), 155–170.
- Gentner D. and Gentner D. R., (1983), Flowing Waters or Teeming Crowds: Mental Models of Electricity, in Gentner D. and Stevens A. L. (ed.), Mental Models, Hillsdale, NJ: Lawrence Erlbaum Associates, pp. 99–129.
- Glasser B. and Strauss A., (1967), The Constant Comparative Method of Qualitative Analysis, The Discovery of Grounded Theory: Strategies for Qualitative Research, New Bunswick and London: AldineTransaction, pp. 101–117.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Graulich N. and Schween M., (2018), Concept-Oriented Task Design: Making Purposeful Case Comparisons in Organic Chemistry, J. Chem. Educ., 95(3), 376–383.
- Graulich N., Tiemann R., and Schreiner P. R., (2012), Heuristic chemistry—a qualitative study on teaching domain-specific strategies for the six-electron case, Chem. Educ. Res. Pract., 13(3), 337–347.
- Johnson-Laird P. N., (1983), Mental Models, Cambridge: Cambridge University Press.
- Körhasan N. D. and Wang L., (2016), Students’ mental models of atomic spectra, Chem. Educ. Res. Pract., 17(4), 743–755.
- Kraft A., Strickland A. M., and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- Larson K. G., Long G. R., and Briggs M. W., (2012), Periodic Properties and Inquiry: Student Mental Models Observed during a Periodic Table Puzzle Activity, J. Chem. Educ., 89(12), 1491–1498.
- Lincoln Y. and Guba E., (1985), Naturalistic Inquiry, Thousand Oaks, CA: Sage Publications.
- Maeyer J. and Talanquer V., (2013), Making Predictions About Chemical Reactivity: Assumptions and Heuristics, J. Res. Sci. Teach., 50(6), 748–767.
- McClary L. and Talanquer V., (2011), College Chemistry Students’ Mental Models of Acids and Acid Strength, J. Res. Sci. Teach., 48(4), 396–413.
- Schmidt M., (2008), The Sankey Diagram in Energy and Material Flow Management: Part 1: History, J. Ind. Ecol., 12(1), 82–94.
- Sevian H. and Stains M., (2015), Uncovering Implicit Assumptions: a Large-Scale Study on Students’ Mental Models of Diffusion, Res. Sci. Educ., 45(6), 807–840.
- Strickland A. M., Kraft A., and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students’ mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11(4), 293–301.
- Talanquer V., (2006), Commonsense Chemistry: A Model for Understanding Students’ Alternative Conceptions, J. Chem. Educ., 83(5), 811–816.
- Talanquer V., (2008), Students’ Predictions About the Sensory Properties of Chemical Compounds: Additive Versus Emergent Frameworks, Sci. Educ., 92(1), 96–114.
- Talanquer V., (2014), Chemistry education: Ten heuristics to tame, J. Chem. Educ., 91(8), 1091–1097.
- Tümay H., (2014), Prospective chemistry teachers’ mental models of vapor pressure, Chem. Educ. Res. Pract., 15(3), 366–379.
- Williams L. C., Underwood S. M., Klymkowsky M. W., and Cooper M. M., (2015), Are Noncovalent Interactions an Achilles Heel in Chemistry Education? A Comparison of Instructional Approaches, J. Chem. Educ., 92(12), 1979–1987.
- Wright L. C. and Oliver-Hoyo M. T., (2019), Supporting the Teaching of Infrared Spectroscopy Concepts Using a Physical Model, J. Chem. Educ., 96(5), 1015–1021.
| This journal is © The Royal Society of Chemistry 2020 |