An examination of pre-service chemistry teachers’ meaningful understanding and learning difficulties about aromatic compounds using a systemic assessment questions diagram
Gulten
Sendur

Dokuz Eylul University, Buca Faculty of Education, Chemistry Education, Izmir, Turkey. E-mail: gulten.sendur@deu.edu.tr
First published on 29th July 2019
Abstract
In order for students to reach a level of meaningful understanding of chemistry, it is vital that they are able to form accurate relationships between different concepts. In particular, in organic chemistry, identifying intermolecular reactions, considering these reactions as a whole, and defining their results will make important contributions to attaining meaningful understanding. This study aims to explore how pre-service chemistry teachers identify aromatic compound reactions and form associations between them and to discover what kinds of learning difficulties they encounter in forming these associations. In this context, the study, conducted as phenomenographical research, was carried out at a faculty of education in Turkey with 15 pre-service teachers enrolled in the Department of Chemistry Education who had taken the Organic Chemistry 1 and 2 courses. Selected on the basis of purposive sampling, the pre-service chemistry teachers were first asked systemic assessment questions (SAQs) related to aromatic compound reactions. In these questions, the pre-service chemistry teachers were asked to identify 7 molecules in the class of aromatic compounds and complete a diagram by taking into consideration synthesis and reaction conditions. The pre-service chemistry teachers were also asked to indicate two reactions that were not included in the SAQs diagram, together with their reagents and conditions. After completing their responses to the SAQs diagram, individual interviews were held with each of the pre-service chemistry teachers using the think-aloud technique. The research revealed at the end of the quantitative analysis of the data obtained from the SAQs diagram that most of the pre-service chemistry teachers achieved moderate-level scores. At the same time, the qualitative analysis of the data obtained from the SAQs diagram and from the interviews showed that the pre-service chemistry teachers were more successful in identifying and forming associations with the reactions of “nitration” and “sulfonation of aromatic compounds” but had difficulty with the “Friedel–Crafts alkylation,” “oxidation” and “reduction” reactions and with the “bromination of alkenylbenzenes” and the “addition reaction of the double bond of alkenylbenzenes.” Another important finding resulting from this study was that only a few pre-service chemistry teachers were able to identify a new reaction on the SAQs diagram. All of these findings indicate that the pre-service teachers are not very equipped to form meaningful relationships in the context of aromatic compounds, which is one of the basic topics of organic chemistry.
Introduction
The content of organic chemistry encompasses topics that are at the core of the discipline such as organic molecules, stereochemistry and reaction mechanisms, which students will be learning in this course for the first time, and also topics that are familiar to them such as acids–bases, bonds, polarity, and thermodynamic stability, which they studied in their previous basic chemistry, analytical chemistry, inorganic chemistry and physical chemistry classes. At the same time, organic chemistry does not only require chemistry students to understand and interpret concepts, phenomena and reactions, but also calls for a deeper understanding of molecular changes and an inquiry into what these changes can cause (Graulich, 2015). Because of this, students of organic chemistry encounter a myriad of scientific knowledge and symbolic representations that are associated with each other and must consider them within a meaningful structure. This nature of organic chemistry has created many challenges for students at different levels of education, causing them to suffer conceptual challenges (Cruz-Ramirez de Arellano and Towns, 2014; Flynn, 2015). The reasons that have been shown to cause learning difficulties in organic chemistry are the differences in terms and terminology from other branches of chemistry, the stepwise progression of organic chemistry in and of itself, the necessity to consider molecules of many different structural characteristics, the varieties of chemical activity depending on the characteristics of organic molecules, the need to study molecules at a three-dimensional level, abundance of symbolic representations needed in science, and the difficulties involved in understanding the meanings of these representations (Duffy, 2006; Ferguson and Bodner, 2008; Grove et al., 2012; Cruz-Ramirez de Arellano and Towns, 2014). Facing the complex content of organic chemistry, students generally tend to memorize instead of understanding the basic concepts underlying the various rules and, as a result, have to cope with clusters of disconnected bits of knowledge (Anderson and Bodner, 2008; Anzovino and Bretz, 2015; Galloway et al., 2017; Caspari et al., 2018). This clearly points to the important need for creating learning environments that will contribute to students seeking out meaningful learning instead of resorting to memorization in organic chemistry.Ausubel (1968) referred to meaningful learning as the opposite of memorized learning and asserted that meaningful learning can only take place when students form meaningful associations between their pre-knowledge and the new knowledge they are trying to learn. When considered from this perspective, the essence of achieving meaningful learning derives from students’ pre-knowledge of a subject, the meaningful manner in which they are offered new knowledge, and their choosing to connect their pre-knowledge with new information (Ausubel, 1968; Novak, 2002). One of the elements of meaningful learning for students is targeting meaningful understanding by focusing on and understanding conceptual structures (Fyrenius et al., 2005). In this context, meaningful understanding is far beyond simply remembering or retaining what is learned; it encompasses an expansive concept that ranges from making connections between different frames of knowledge to explaining phenomena of daily life on the basis of scientific knowledge (Tzougraki et al., 2014). In other words, meaningful understanding is an indication of the ability to form associations between different types of knowledge (Nieswandt and Bellomo, 2009). Meaningful understanding in the context of chemistry can be defined as the skills needed to make a decision, form associations, state cause and effect relationships, reach a conclusion and make an estimation as to what may occur by making connections with the resulting chemical knowledge (Vachliotis et al., 2014). Meaningful understanding at the same time encompasses the skills needed to access chemical knowledge derived from chemical representations and use this knowledge to create a new chemical representation (Tzougraki et al., 2014). This is particularly of importance in organic chemistry. When the content of organic chemistry is considered, it can be said that when students are able to form relationships between many concepts and treat these relationships in an integrated structure, it is then that they can attain the deep understanding required of them in organic chemistry; in other words, it is then that they can reach a level of meaningful understanding. Looking at the matter from this perspective, assessing students’ meaningful understanding in organic chemistry will also contribute to determining what difficulties they have in learning the different concepts. This can also be helpful to teachers and instructors of organic chemistry in their use of appropriate teaching strategies in the classroom and preventing students from adopting possible alternative conceptions. This is why evaluating students’ meaningful understanding with reliable and valid instruments is of such importance.
A variety of different instruments are used in measuring the extent of meaningful understanding; they include conceptual maps (Novak et al., 2000), structured interviews (Southerland et al., 2000), an open-ended mental model test (Cavallo, 1996), open-ended questions (Nieswandt and Bellomo, 2009) and conceptual questions (Nakhleh and Mitchell, 1993). Hodges and Harvey (2003) stated that standard instruments are useful in determining the learning levels of students with regard to specific concepts or phenomena but that they fall short in assessing the depth of students’ learning. Because of this, Hodges and Harvey (2003) pointed to the importance of applying different methods to assessing the quality of students’ learning in the context of organic chemistry classes. One of the instruments that can be used to assess students’ meaningful understanding is systemic diagrams. Fahmy and Lagowski (2003) devised systemic diagrams on the basis of Ausubel's theory of meaningful learning and they are the fundamental element of the Systemic Approach to Teaching and Learning (SATL). According to the SATL, students’ meaningful understanding of concepts in science will help them learn both the concepts and the relationships between the concepts in an organized manner (Vachliotis et al., 2014). Towards this aim, in the SATL, conceptual systems are organized in the form of closed clusters, where interactions between directly or indirectly associated concepts are considered in what are called “systemic diagrams” (Fahmy and Lagowski, 1999, 2003, 2011). These systemic diagrams used for the purpose of teaching have a subsystem of systemic assessment questions (SAQs) that have a fewer number of concepts and associations between them compared to systemic diagrams. These diagrams are employed to determine and assess the level of students’ meaningful understanding and for this reason are more frequently used in the course of the teaching process (Hrin et al., 2016a). SAQs are created on a systemic diagram where interactive conceptual systems of a cyclic structure are formed (Vachliotis et al., 2011). This structure of SAQs helps students to organize concepts, define the relationships between the concepts and analyze the elements of subsystems, enabling them to synthesize them into one integrated construct (Tzougraki et al., 2014). This has made SAQs one of the instruments that can be used in evaluating the extent of meaningful understanding and learning.
When studies related to SAQs in organic chemistry are reviewed, it can be seen that the research is concentrated in two main groups. The first group includes studies that explore whether SAQs comprise a valid and reliable instrument with which to assess students’ meaningful learning. The study by Vachliotis et al. (2011), for example, is one of the studies probing into the degree of effectiveness of the SAQ diagrams developed to evaluate the meaningful learning of 11th grade students in the context of organic reactions. The researchers reached the conclusion with their item and factor analysis that the SAQ diagrams developed for hydrocarbons, alcohols and carboxylic acids were effective tools that could be used in evaluating students’ meaningful learning. Similarly, in another piece of research by Vachliotis et al. (2014), an exploration was made of whether SAQs were a reliable and valid tool for assessing the meaningful learning of 11th grade students in the context of concepts of organic chemistry. This research showed that the results of both correlation and factor analysis indicated that SAQs developed on the topics of the basic concepts of organic chemistry (classification of organic compounds, IUPAC nomenclature for aliphatic compounds, constitutional isomers of organic compounds, etc.) and aliphatic hydrocarbons (the chemical properties of alkanes, alkenes and alkynes) were effective in assessing meaningful learning. The second group of studies conducted about SAQs involves the examination of whether SAQs used as a teaching tool have an effect on students’ learning and on their higher-level thinking skills. One of the studies in this context was conducted by Hrin et al. (2016a). In their study, which was of experimental design, the researchers selected a group of third-year high school students as their study group and taught the subject of hydrocarbons and halogen derivatives using SAQs, while the control group was provided with traditional instruction. The results of this study revealed that the study group that was exposed to SAQs as a teaching tool was more successful in learning than the students in the control group. In another experimental study conducted by Hrin et al. (2016b), findings parallel to the previous study were reported. Teaching the subject of hydrocarbons and halogen derivatives with SAQs to the high school study group yielded better results than those in the control group; the students in the study group were not only more successful but spent less of an effort in solving the problems. Similarly, in other studies carried out at the high school level, it was found that students supported in learning carboxylic acid and carbonyl compounds with the aid of SAQs were able to reach higher levels of systems thinking (Hrin et al., 2016c; Hrin et al., 2017). Differing from these studies, Hrin et al. (2018) analyzed the systemic synthesis questions that high school students and pre-service chemistry teachers created to understand cognitive constructs in organic chemistry. The research findings ultimately showed that the high school students and the pre-service chemistry teachers had a good level of knowledge about particularly the IUPAC nomenclature of organic compounds and the chemical structures of organic compounds (with the exception of ethers), but that both groups had learning difficulties when it particularly came to reaction mechanisms.
When these studies on the fundamentals of organic chemistry are examined, it can be seen that there is a need for research that will uncover the difficulties students experience when SAQs are used and also, in this context, which alternative conceptions students adopt. Although in their study of the cognitive constructs of pre-service chemistry teachers, Hrin et al. (2018) examined the difficulties the teachers encountered in the systemic synthesis questions they themselves created, the main focus of this study was cognitive constructs. At the same time, because high school students also took part in the sample group of the study, the topics chosen were compatible with the high school organic chemistry syllabus and were limited to the headings of aliphatic hydrocarbons and halogen derivatives, alcohols and ethers. From this perspective, it appears that there is a need for studies that would explore the meaningful understanding and learning difficulties faced by pre-service chemistry teachers, who would be expected to have a defined scope of knowledge in the field, using SAQs with content that is suitable for a university-level chemistry course that covers fundamental topics related to organic molecular classifications in organic chemistry.
There are many topics that would qualify as the building blocks of organic chemistry content. Organic chemistry includes the study of aromatic compounds and their reactions, including electrophilic aromatic substitution that is peculiar to the class of molecules and multi-step synthetic reactions, both of which necessitate the formation of more comprehensive associations between different molecular classes. They have been factors that have made this topic a central element in organic chemistry (Krygowski and Cyranski, 2001; Balaban et al., 2004). Consequently, reaching a level of meaningful understanding in the subject of aromatic compound reactions will, in the natural course of organic chemistry, contribute to the development of multifaceted thinking, forming relationships and problem-solving skills (Şendur, 2019). It is therefore important to determine whether pre-service chemistry teachers form meaningful relationships between aromatic compound reactions and which learning difficulties they experience in this process. In this context, it is believed that this study, in which the aim was to determine the pre-service chemistry teachers’ understanding and learning difficulties with the combination of two techniques (SAQs diagrams and interviews), will make a contribution to the literature on teaching organic chemistry. At the same time, one of the novelties of the study was the fact that the pre-service chemistry teachers were asked to identify aromatic compound reactions.
Purpose and research questions
This study aims to use a SAQs diagram to discover how pre-service chemistry teachers form meaningful relationships between the set of aromatic compounds through the appropriate chemical reactions, and which difficulties they face in doing so. Toward this aim, answers were sought to the following sub-problems:(1) Which aromatic compound reactions did the pre-service chemistry teachers identify correctly and associate with other reactions?
(2) Which learning difficulties do the pre-service chemistry teachers experience in identifying aromatic compound reactions and the relationship between them?
Methods
Since the purpose of this study was to discover how pre-service chemistry teachers formed associations between aromatic compound reactions and which learning difficulties they encountered, the study was conducted with a phenomenographic research approach. Orgill and Bodner (2004) stipulated that phenomenographic studies can be used to define the experiences of individuals with respect to a specific phenomenon, to set forth how they interpret this phenomenon and the different ways in which they understand or conceptualize the phenomenon. In other words, phenomenographic research focuses on how different people conceptualize a specific phenomenon in different ways (Orgill, 2007). The phenomenon examined in phenomenographic research can be a phenomenon, a concept or an incident (Bussey et al., 2013). In this study, the identification of aromatic compound reactions by pre-service chemistry teachers and the relationships they formed was taken as the phenomenon for the research. At the same time, the methodological and theoretical framework of this study is based on personal constructivism. Personal constructivism refers to the way individuals use their existing knowledge to understand and organize new knowledge (Bodner, 1986). In other words, “knowledge is constructed in the mind of the learner” (p. 873). In this context then, the way to understand what an individual knows about a subject is to ask them to directly explain how they formed related concepts in their minds and formed associations (Cruz-Ramirez de Arellano and Towns, 2014). In this study as well, in the interviews held with pre-service chemistry teachers, the aim was to use the think-aloud protocol in particular to discover how they gave meaning to reactions with aromatic compounds and how they formed the necessary associations.Participants
The participants in this study were selected on the basis of the typical case sampling technique, a type of purposive sampling. In this sampling technique, enough information is gathered about a typical situation to inform persons who are not familiar with the situation (Patton, 1987; Lodico et al., 2010). The study participants were 15 pre-service chemistry teachers (11 women, 4 men) in their 5th year who were enrolled in the Chemistry Education Department of a state university in Turkey during the 2016–2017 academic year. The fifteen pre-service chemistry teachers formed the whole group of 5th year students who were enrolled in the Chemistry Education Department.Each of the pre-service teachers participating in the research was given a consent form two weeks prior to the study and the pre-service chemistry teachers were recruited into the study as voluntary participants on the basis of this form. The participants were between the ages of 22 and 24; most were from families of middle-class income. All of the participating pre-service chemistry teachers had taken and passed the Organic Chemistry-1, Organic Chemistry-2 and Organic Structure Determination courses. Their point range in the Organic Chemistry-1 course was 65–92; their point range in the Organic Chemistry-2 course was 63–90. Their point range in the Organic Structure Determination course was 60–85. The content of Organic Chemistry-1 included basic concepts in organic chemistry (resonance, acids–bases, tautomerism, etc.), and basic properties, synthesis and reactions of aliphatic hydrocarbons (alkanes, alkenes, alkynes). The Organic Chemistry-2 content included aromatic hydrocarbons and organic molecule functional groups (alcohols, ethers, aldehydes, ketones, carboxylic acids, esters) and their synthesis and reactions, kinds of reactions and mechanisms (SN1, SN2, E1, E2). The content of the Organic Structure Determination course included the topics of the theories and applications of UV, infrared and NMR. In this context, it was accepted that the pre-service chemistry teachers who made up the group of study participants had enough knowledge to respond to the SAQs diagram on the reactions of aromatic compounds.
Data collection instruments
The aromatic compound systemic assessment questions diagram (SAQs diagram) and interviews were two data collection instruments in this study. All of the data in this study were collected in the Turkish language and translated into English by the researcher. The reasons all the data were collected in Turkish were that the language of instruction in the university where the study was conducted was Turkish and the English fluency of the pre-service chemistry teachers was not at an adequate level. In fact, Taber (2018) stated that collecting data in English if the participants are not fluent in English would not be appropriate. Because of this, all of the data were then translated into English by the researcher, who is fluent in both Turkish and English. These translations were later reviewed by an organic chemistry expert, a chemistry educator and a native English speaker. In particular, since the data appearing in the transcripts of interviews had a great number of technical terms, the translation was then checked by the organic chemistry expert. Also, the text was given to the chemistry educator and the native English speaker independently of each other for a further check. In addition, the transcripts of the interviews with three pre-service teachers are included as Appendix 3 as an example of the quality of the translations.The aromatic compound SAQs diagram and its application
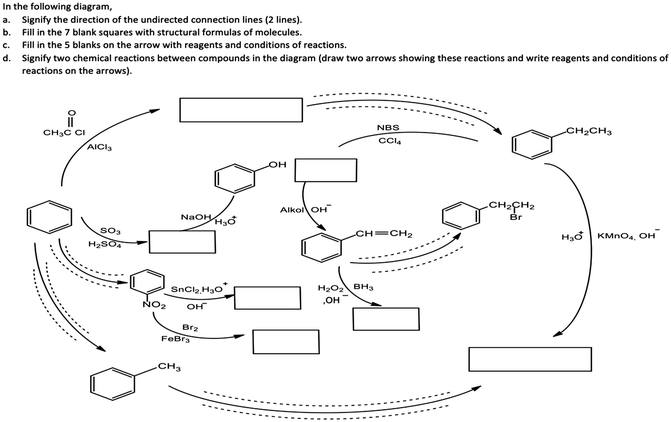
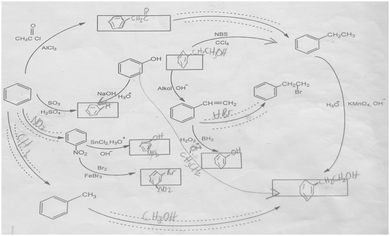
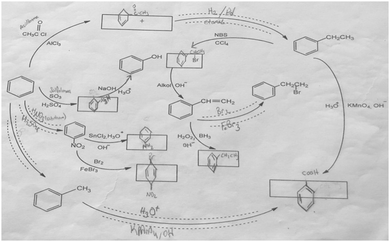
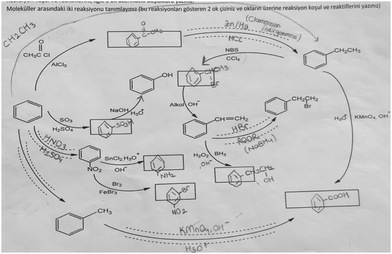
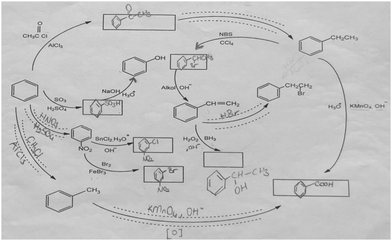
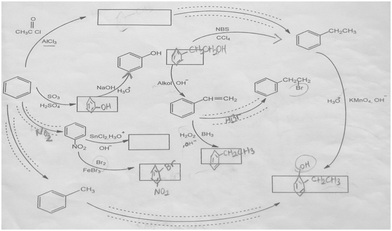
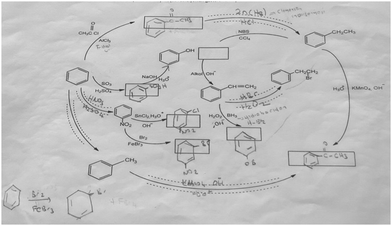
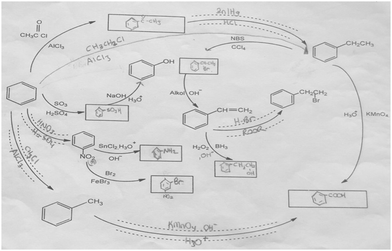
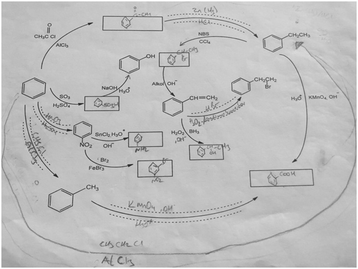
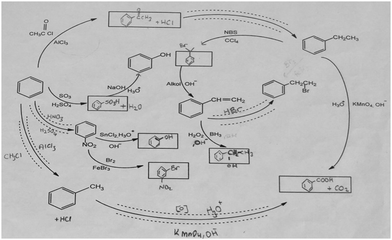
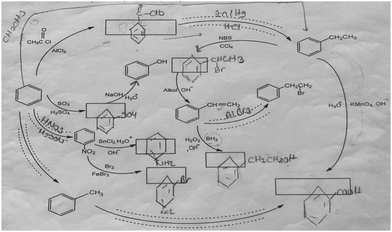
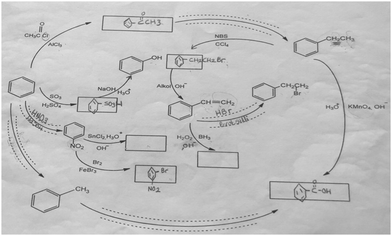
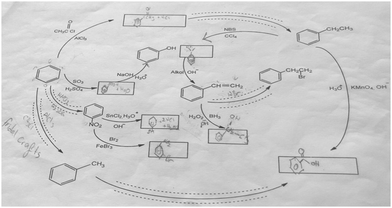
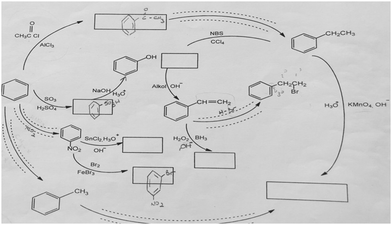
The aromatic compound SAQs diagram, which was one of the data collection instruments in this study, was developed by the researcher to ascertain how pre-service chemistry teachers integrated and associated aromatic compound reactions. The SAQs diagram was drawn up in a semi-completed and structured systematic diagram format (fill in the blanks in the SAQs diagram) and consisted of 14 concepts. In the SAQs diagram, the pre-service chemistry teachers were asked to indicate 5 reactions with conditions and reagents, and 7 molecules classified as aromatic compounds (in terms of their structural formulae). At the same time, the pre-service chemistry teachers were asked to signify two reactions, together with their reagents and conditions, that were not shown in the diagram, and to indicate the direction of the arrow for the two reactions whose directions are not indicated. The SAQs diagram consists of 4 sub-systems related to aromatic compound reactions. They are as follows:Sub-system 1: It contains 5 reactions. They are the Friedel–Crafts acylation reaction, reduction of acetophenone, oxidation of ethylbenzene, Friedel–Crafts alkylation reaction and oxidation of methylbenzene.
Sub-system 2: It contains 2 reactions. They are the sulfonation of benzene and synthesis of phenol from benzenesulphonic acid.
Sub-system 3: It contains 3 reactions. These reactions are nitration of benzene, reduction of nitrobenzene and bromination of nitrobenzene.
Sub-system 4: It contains 4 reactions. They are bromination of ethylbenzene with NBS, synthesis of styrene from (1-bromoethyl)benzene, synthesis of (2-bromoethyl)benzene from styrene (addition of HBr to the double bond in the presence of peroxide), and synthesis of 2-phenylethanol from styrene (hydroboration/oxidation of the double bond).
The SAQs diagram developed for this study is presented in Fig. 1.
The aromatic compound SAQs diagram was developed by the researcher in consultation with two faculty members specialized in organic chemistry. The SAQs diagram that was developed was also implemented outside of the sample group with 5 pre-service chemistry teachers as a pilot study, where the SAQs diagram was tested and revised in terms of comprehensibility and it was determined that a 35 minute allowance for completing the questionnaire was sufficient.
Following the pilot study, the pre-service chemistry teachers in this study were first provided with information about the SAQs diagram and explanations were given on the basis of various examples. After acquainting the pre-service chemistry teachers with how the implementation of the SAQs diagram would be conducted, the actual application was started. The aromatic compound SAQs diagram was completed in 35 minutes.
Aromatic compound SAQs diagram analysis
The SAQs diagram was analyzed both quantitatively and qualitatively. In the quantitative analysis of the SAQs diagram, the scoring was calculated on the basis of the principle that each element in the diagram would contribute equally to constructing the conceptualization (Vachliotis et al., 2011). For this reason, each correct element that needed to be completed on the diagram (the structural formula of the molecule, the direction of the arrow, the reagents and conditions of the reaction, the direction of the arrow for the newly drawn reaction) was given 1 point. The scoring of the SAQs diagram was calculated as follows: 5 points for 5 reactions with noted conditions and reagents; 2 points for signifying the direction of two undirected connection lines; 7 points for filling up the 7 blank boxes with appropriate structural formulas; and 2 + 2 points for signifying 2 unprovided chemical reactions (2 points for the direction of the arrow for the two newly drawn reactions, and 2 points for the reagents and conditions of the newly drawn reactions). According to this scoring approach, the maximum possible score that can be obtained on the SAQs diagram is 18.The answer key of the SAQs diagram is presented in Appendix 1.
The scoring of the SAQs diagram was carried out independently by the researcher and an academic staff member specialized in organic chemistry education, after which these individuals came together to discuss and resolve the points of disagreement. Furthermore, the correlation between the scoring of the evaluators was calculated in order to assess the reliability of the scoring method. For this purpose, the scores given by the two evaluators were tested with the Kolmogorov–Smirnov and Shapiro–Wilk tests to reveal whether the scores displayed a normal distribution. Data for the normality tests are shown in Table 1.
The results of the analysis shown in Table 1 indicated a normal distribution for evaluator 1 and evaluator 2 in both the Kolmogorov–Smirnov test (p values = 0.200 and 0.200 respectively) and the Shapiro–Wilk test (p values = 0.343 and 0.474, respectively) according to the p values. After seeing that the scores showed a normal distribution, the SPSS 15.0 program was used to calculate Pearson's correlation coefficient for the correlation between the scoring of the evaluators.
The mean scores the evaluators gave to the SAQs diagram and the correlation between these scores are shown in Table 2.
It can be seen in Table 2 that the correlation coefficient (r) is 0.997. This coefficient indicates a high and positive correlation between the scoring of the evaluators. In other words, it was understood that the scoring of the SAQs diagram exhibited a high level of reliability.
In the qualitative analysis of the diagram, depending on whether or not the pre-service chemistry teachers correctly completed the reactions asked of them in the SAQs diagram (in the context of the product yielded, the direction of the arrow and the reagents/conditions), the specialist and the researcher conducting the qualitative analysis encoded the answers independently and used Miles and Huberman's (1994) method for calculating the percentage of agreement to find a value of 0.976. Fleiss and Levin (1981) qualified percentages of agreement of 0.75 and over as “excellent”, and on this basis, it can be said that the value calculated in this study showed that there was a high degree of reliability between the codings.
Interviews
After the pre-service chemistry teachers completed the SAQs diagram, individual interviews were held with each using the think-aloud technique. Accordingly, the aim of the interview was first explained to the pre-service chemistry teachers and then information was provided about how the think-aloud protocol would be implemented. The one-on-one interviews held with the pre-service chemistry teachers took 10–15 minutes and the interviews were recorded on a voice recorder. During the interviews, the pre-service chemistry teachers were given the diagrams they completed and asked to explain aloud how they completed the SAQs diagram and how they formed relationships between the reactions. In other words, the interviews aimed to determine how the pre-service chemistry teachers identified the reactions, which conditions they considered, and where they had experienced difficulty. Because this study provided an opportunity for the participants to think about their task out loud, the interviews were held in accordance with the think aloud protocol. The think-aloud protocol is presented in Appendix 2. The researcher made comments or issued reminders only when the pre-service chemistry teachers did not fully explain their thoughts or remained silent. The transcripts of the interviews with three pre-service teachers in Turkish and English are presented in Appendix 3.Interview analysis
The analysis of the data obtained from the interviews was conducted according to “content analysis.” At this stage, the written transcripts of the interviews were reviewed by the researcher and the specialist separately and categories were formed. The categories were determined on the basis of the explanations the pre-service chemistry teachers gave for the reactions on the SAQs diagram, products and reaction conditions/reagents. From these explanations, not only was it possible to discover how the pre-service chemistry teachers formed associations for the reactions in the diagrams but it was also possible to see where they had difficulty. Because of this, the data obtained from the interviews served to find answers to study sub-problems 1 and 2.The categories were discussed one by one and repeatedly until agreement was reached and the final form could be created. Miles and Huberman's (1994) percentage of agreement was calculated to be 0.92. Table 3 displays these categories and their content.
| Category | Category content |
|---|---|
| Type of reaction is right, product is right. | Reaction type stated correctly and product formed according to the right structural formula |
| Type of reaction is right, product is wrong. | Reaction type stated correctly but product formed according to the wrong structural formula |
| Type of reaction is wrong, product is wrong. | Reaction type stated inaccurately and product formed according to the wrong structural formula |
| Type of reaction is unknown, product is wrong. | No explanation provided about reaction type and product formed according to the wrong structural formula |
| Type of reaction is right, reaction conditions/reagent is right. | Reaction type stated correctly and reaction reagent and conditions (e.g., catalyzer, solvent) all written correctly |
| Type of reaction is partially right, reaction conditions/reagent is partially right. | Reaction type not fully correct and reaction reagent and conditions (e.g., catalyzer, solvent) written incompletely |
| Type of reaction is unknown, reaction conditions/reagent is wrong. | No explanation provided about reaction type and reaction reagent and conditions (e.g., catalyzer, solvent) written incorrectly |
| Type of reaction is right, direction of reaction is right. | Reaction type stated correctly and arrow showing direction of reaction drawn correctly |
| Type of reaction is unknown, direction of reaction is wrong. | No explanation provided about reaction type and arrow showing direction of reaction drawn incorrectly |
Ethical issues
The ethical procedures followed in the study are explained in this section. All research work was performed in compliance with the institute's policy on ethics. In order to have the study conform to ethical considerations, all of the pre-service chemistry teachers were informed about the research approximately 2 weeks in advance, after which they were given an information sheet explaining the purpose and process of the study and asked to sign the sheet to indicate their informed consent. The English version of the consent form is presented in Appendix 4. The pre-service chemistry teachers were also informed that they could withdraw from the study at any time they wished to do so and that their responses on the diagram and in the interviews would not be evaluated as part of their grades. After these explanations were given, all of the pre-service chemistry teachers filled out the consent form and indicated that they were participating in the study voluntarily. In fact, Taber (2014) stated that in studies conducted in a university setting, as long as individuals are provided with enough information about the study, they are considered adults who can make an informed decision on whether they wish to participate in the study. Since the age range of the pre-service chemistry teachers participating in this study was 22–24 and because they received adequate information about the research, it is accepted that they gave their clear consent. Additionally, all of the participants were assigned numbers in place of their names and the names of the participants were accordingly removed from the data collection sheets. Consequently, ethical considerations and confidentiality have thus been assured (Fraenkel and Wallen, 2006).Results
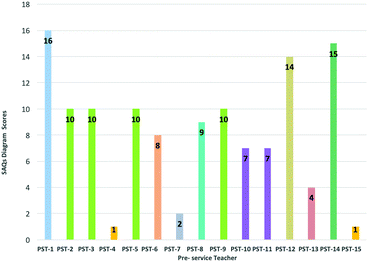
The first aim here was to find the general status of the pre-service chemistry teachers according to their responses to the SAQs diagram and in light of the first sub-problem of the study. For this, a calculation was made of the total scores of the pre-service chemistry teachers on the SAQs diagram; these scores are shown in Fig. 2.The pre-service chemistry teachers were divided into 3 categories (high, moderate and low) according to their scores; the frequencies and percentages pertaining to the pre-service chemistry teachers in these categories are shown in Table 4.
| Category | Score range | f | % | Pre-service chemistry teachers |
|---|---|---|---|---|
| High | 12–18 | 3 | 20.0 | PST-1, PST-12, PST-14 |
| Moderate | 6–11 | 8 | 53.3 | PST-2, PST-3, PST-5, PST-6, PST-8, PST-9, PST-10, PST-11 |
| Low | 0–5 | 4 | 26.7 | PST-4, PST-7, PST-13, PST-15 |
An examination of Table 4 shows that more than half of the pre-service chemistry teachers remained at moderate levels according to their SAQs diagram scores; it was also seen that the percentage of pre-service chemistry teachers at the higher level was at the lowest level among the other categories. Accordingly, it can be said therefore that the pre-service chemistry teachers had some difficulties describing the reactions of aromatic compounds, and forming meaningful relationships between them. In other words, it can be said that they had not reached the desired level of meaningful understanding. A similar situation was noted by Şendur (2019) in the results of a study conducted with pre-service science teachers. In this researcher's study, in which the learning levels of pre-service science teachers regarding electrophilic aromatic substitution reactions were examined in light of SOLO taxonomy, it was found that most of the pre-service science teachers responded at a pre-structural level to these reactions; in other words, they were not sufficiently successful in forming meaningful relationships between aromatic compound reactions.
The data obtained from the qualitative analysis of the 4 sub-systems in the SAQs diagram in my attempt to discover how pre-service chemistry teachers describe the reactions shown on this diagram are described below in order. The results of the descriptive analysis of the first sub-system are presented in Table 5.
| Reaction | Right | Wrong | No answer | |||
|---|---|---|---|---|---|---|
| f | % | f | % | f | % | |
| Synthesis of acetophenone from benzene (Friedel–Crafts acylation) | 12 | 80.0 | 2 | 13.3 | 1 | 6.7 |
| Synthesis of ethylbenzene from acetophenone (reduction of ketones) | 7 | 46.7 | — | — | 8 | 53.3 |
| Synthesis of benzoic acid from ethylbenzene (side-chain oxidation of alkylbenzenes) | 10 | 66.7 | 4 | 26.7 | 1 | 6.7 |
| Synthesis of methylbenzene from benzene (Friedel–Crafts alkylation) | 5 | 33.3 | 1 | 6.7 | 9 | 60.0 |
| Synthesis of benzoic acid from methylbenzene (side-chain oxidation of alkylbenzenes) | 7 | 46.7 | 1 | 6.7 | 7 | 46.7 |
The analysis results shown in Table 5 indicated that 80% of the pre-service chemistry teachers were able to formulate the correct product, acetophenone, as the product of the Friedel–Crafts acylation of benzene. It was seen that the pre-service chemistry teachers providing this response were able to explain both the reaction and the resulting product correctly in their interviews. One of the pre-service chemistry teachers in this category, PST-11 (Fig. 3), provided the following explanations about the diagram in the interview:
PST-11: … I said that this was an acylation reaction. A Friedel–Crafts acylation reaction. Because benzene enters into a reaction with the acyl chloride in the environment. AlCl3was acting as a catalyzer here. I created a ketone, the phenylmethyl ketone, as the product. HCl formed as well. (Type of reaction is right, product is right.)
A group of 13.3% of the pre-service chemistry teachers created the wrong product in this reaction. In the review of the responses, it was seen that PST-7 wrote another ketone, the dimethyl ketone, instead of acetophenone, and that PST-15 wrote something similar to acetophenone, but this product formed according to the wrong structural formula.
In the interview notes collected from PST-7, it was observed that although the pre-service chemistry teacher explained the type of reaction correctly, the wrong product had been formed. PST-7's interview notes (Fig. 4) were as follows:
PST-7: … I thought that this was acylation reaction and there was something that could form a ketone. Then I looked at the reaction conditions. I looked at the reagents above the arrow and said that a ketone would form from this. I connected the methyl groups to the carbonyl group and formed the dimethyl ketone. (Type of reaction is right, product is wrong.)
In the interview, another pre-service chemistry teacher, PST-15, was not able to explain the type of reaction and it was also seen that the formation of the product was wrong. PST-15's interview notes (Fig. 5) were as follows:
PST-15: … I thought chlorine would break. I don’t remember the rule but I could guess what would happen. I connected the ring to the other group that remained after chlorine. That's how I formed the product. I don’t think I did it exactly… (Type of reaction is unknown, product is wrong.)
In the second reaction that included obtaining ethylbenzene from acetophenone in the first sub-system of the SAQs diagram, in other words, the reduction of ketones, a very high percentage of the pre-service chemistry teachers (53.3%) left this part blank. This may be considered an indication that the pre-service chemistry teachers were not able to sufficiently identify the type of reaction. The pre-service chemistry teachers who responded correctly to this reaction produced ethylbenzene, from two different reduction reactions. One of these pre-service chemistry teachers, PST-5, chose to write the catalytic reduction reaction of the aryl alkyl ketones with Pd/C. In the interview with this pre-service chemistry teacher, it was seen that PST-5 was successful in identifying the reaction correctly, and the pre-service chemistry teacher could also explain exactly what conditions were necessary for aryl alkyl ketone reduction. PST-5's interview notes (Fig. 6) were as follows:
PST-5: … After I formed this ketone, I put a reduction reaction here. The carbonyl group transforms into –CH2. That's why there was a catalytic reduction reaction with the catalyzer palladium and so I formed ethylbenzene. (Type of reaction is right, reaction conditions/reagent is right.)
The other pre-service chemistry teachers responding correctly to this reaction wrote down the Clemmensen reduction, which is known to be a reduction reaction of ketones with amalgamated zinc (Zn(Hg)) under acidic conditions. One of the pre-service chemistry teachers who provided this response, PST-12 (Fig. 7), offered the following explanations about the diagram in the interview:
PST-12: …After I obtained the ketone, I came to this stage. Then I said that a reduction reaction would be necessary for this conversion and so I did the Clemmensen reduction. The environment had HCl and also zinc and mercury. This way, the carbonyl group was reduced and I could produce alkyl benzene. (Type of reaction is right, reaction conditions/reagent is right.)
Table 5 shows that a large percentage of the pre-service chemistry teachers (66.7%) responded correctly to the question on the synthesis of benzoic acid from ethylbenzene, a side-chain oxidation of the alkylbenzenes. It was seen that the pre-service chemistry teachers in their interview responses were able to correctly form benzoic acid through the oxidation of the alkylbenzenes containing hydrogen in the benzylic carbon atom. One of the pre-service chemistry teachers who provided this response, PST-2 (Fig. 8), offered the following explanations about the diagram in the interview:
PST-2: … There's an oxidation reaction here. Ethylbenzene is oxidized… In other words, it's like this. There's hydrogen in the benzylic carbon atom; in this case KMnO4is the oxidizing agent and ethylbenzene is oxidized. In the end, benzoic acid is formed. (Type of reaction is right, product is right.)
Of the pre-service chemistry teachers, 26.7% were not able to correctly respond to the side-chain oxidation reaction of alkylbenzenes. When the responses of the pre-service chemistry teachers giving the wrong responses were examined in the diagram, it could be seen that they wrote down different types of alcohol, phenol or acetophenone as the product. The responses of the pre-service chemistry teachers in their interview were evaluated in two different categories. Three pre-service chemistry teachers took their place in one of these categories: these individuals were unable to identify the type of reaction and came up with the wrong product. One of them, PST-7 (Fig. 4), gave the following explanations:
PST-7: … So I formed alcohol here… I added the hydroxyl group in the conditions of reaction… type of reaction … I don’t know…
Researcher: Why did you attach the hydroxyl group to this carbon atom? Could you please explain this for us?
PST-7: I added the hydroxyl group because the carbon atom closer to the ring was more stable. (Type of reaction is unknown, product is wrong.)
Another pre-service chemistry teacher in the same category was similarly unable to identify the type of reaction and formed the wrong product. In the response this pre-service chemistry teacher provided, what was striking was that the pre-service chemistry teacher identified the phenol derivative organic compound as an alcohol because of the hydroxyl group. This response is important because it indicates that the pre-service chemistry teacher was unable at the same time to differentiate between alcohol and phenol. The interview notes of PST-4 (Fig. 9) were as follows:
PST-4: … There are potassium permanganate and hydroxyl here. Since this is the hydroxyl group, I said I could get alcohol from this and I attached the hydroxyl to the carbon atom next to the ethyl group. This way, alcohol was formed.
Researcher: Could you explain this in terms of type of reaction?
PST-4: … I don’t know but I bonded them together because I saw the hydroxyl group. (Type of reaction is unknown, product is wrong.)
With regard to this reaction, where ethylbenzene was oxidized and formed benzoic acid, it was seen that one of the pre-service chemistry teachers (PST-10) expressed the type of reaction correctly, but formed a ketone as a product. Because of this, PST-10 fell into the second category in the analysis of the interview responses, that is, “type of reaction is right, product is wrong.” PST-10's interview notes (Fig. 10) were as follows:
PST-10: … There is oxidation here. Potassium permanganate causes the oxidation and a ketone is formed. Ethylbenzene, which is an alkylbenzene, was oxidized and a phenylmethyl ketone was formed. (Type of reaction is right, product is wrong.)
In the results of the analysis seen in Table 5, it is noted that a large percentage (60.0%) of the pre-service chemistry teachers omitted the reagent and catalyzer parts of the synthesis of methylbenzene from benzene, comprising Friedel–Crafts alkylation. This finding can be interpreted to mean that the pre-service chemistry teachers had difficulty formulating how special reactions such as Friedel–Crafts, in particular, progressed depending on the reaction reagents and conditions. A group of 33.3% of the pre-service chemistry teachers was able to write down the appropriate reagent and catalyzer. It was noted in their interviews that these pre-service chemistry teachers were able to correctly express both the type of reaction and the reaction conditions. One of the pre-service chemistry teachers in this category, PST-1 (Fig. 11), said the following:
PST-1: … I alkylated benzene. For this, I thought of Friedel–Crafts alkylation. This was a reaction created from methyl chloride and the catalyst aluminum chloride, which produced methylbenzene. (Type of reaction is right, reaction conditions/reagent is right.)
Only one of the pre-service chemistry teachers (PST-15) used the wrong reagent for this reaction. In the interview with this pre-service chemistry teacher, it was observed that the pre-service chemistry teacher could not identify the reaction correctly but only looked at the reactant and the product of the reaction to decide that the methyl group had to be the reagent; in other words, the pre-service chemistry teacher had no knowledge of the type of reaction or the way the reaction came about. The explanations PST-15 (Fig. 5) gave were as follows:
PST-15: … Methylbenzene formed from benzene; in other words, methyl attached to benzene. So I said that benzene should be in a reaction with methyl. One of the hydrogens in the benzene broke off and attached to the methyl.
Researcher: Then as far as the type of reaction is concerned, what kind of a reaction is this? Can you explain?
PST-15: … I don’t really know what kind of a reaction it is. I only know that the hydrogen in the benzene broke off and bonded with the methyl group. (Type of reaction is unknown, reaction conditions/reagent is wrong.)
In the results of the analysis in Table 5, it can be seen that close to half of the pre-service chemistry teachers (46.7%) were unable to write down the appropriate reagent and reaction conditions in connection with the methylbenzene oxidation reaction, which is another example of the side-chain oxidation of alkylbenzenes. This may mean that the pre-service chemistry teachers had difficulty in structuring the reaction reagents and conditions and did not quite understand that methylbenzene, as ethylbenzene, would form benzoic acid by oxidizing under the same conditions because it carried benzylic hydrogen. In this context, it can be seen from their interview transcripts that PST-9 was one of the pre-service chemistry teachers who wrote down that the oxidation of ethylbenzene resulted in benzoic acid but could not, however, write down the appropriate reagent and reaction conditions responsible for the formation of benzoic acid from methylbenzene (Appendix 3). PST-9 thought that the oxidation of ethylbenzene was based not on the aromatic structure carrying benzylic hydrogen but on the presence of KMnO4 under the reaction conditions. This can mean that the pre-service teacher had difficulty identifying the type of reaction when reagents were not found in the reaction.
In the interview findings from the interviews with the pre-service chemistry teachers who wrote down the reagents and conditions of the reaction correctly, it was seen that the reaction was expressed as an oxidation reaction. One of the pre-service chemistry teachers in this category, PST-14 (Fig. 12), provided the following explanations about the diagram in the interview:
PST-14: … After methylbenzene was formed, I said it would have to be oxidized to form benzoic acid. Potassium permanganate produces the oxidation. Of course, here the benzylic carbon atom in the methylbenzene can oxidize into benzoic acid because it contains hydrogen. (Type of reaction is right, reaction conditions/reagent is right.)
Only one of the pre-service chemistry teachers (PST-15) wrote down the wrong reagent for this reaction. In the discussions with this pre-service chemistry teacher, it was seen that the pre-service chemistry teacher did not know the type of reaction, as in the example of obtaining methylbenzene from benzene, and that the pre-service chemistry teacher tended to write down the reagent by looking at the product formed. PST-15 (Fig. 5) said the following:
PST-15: … I looked at the product and I said alcohol would be formed so alcohol should be in the reaction. There was already CH3in the ring; that meant that I should add CH2OH as alcohol… But I don’t exactly know what to write down as a type of reaction. (Type of reaction is unknown, reaction conditions/reagent is wrong.)
Table 6 shows the findings for the second sub-system of the SAQs diagram. In this system, which consists of two reactions, the first reaction is the sulfonation of benzene; here the pre-service chemistry teachers were expected to write down the formula for benzene sulfonic acid as the product of this reaction. It can be seen from Table 5 that a large percentage (66.7%) of the pre-service chemistry teachers were able to correctly come up with benzene sulfonic acid. In the findings from the discussions with these pre-service chemistry teachers, it was seen that they were able to correctly express both the type of reaction and the product. One of the pre-service chemistry teachers in this category, PST-3 (Fig. 13), said the following in the discussions about the diagram:
| Reaction | Right | Wrong | No answer | |||
|---|---|---|---|---|---|---|
| f | % | f | % | f | % | |
| The synthesis of benzene sulfonic acid from benzene (sulfonation of benzene) | 10 | 66.7 | 5 | 33.3 | — | — |
| The synthesis of phenol from benzene sulfonic acid (indicating the direction of the arrow) | 10 | 66.7 | 5 | 33.3 | — | — |
PST-3: … This is the sulfonation of benzene. Because we have H2SO4and SO3, that is, fuming sulphuric acid. Under this reaction, benzene turns into benzene sulfonic acid. This is a substitution reaction. This reaction was peculiar to aromatic structures such as benzene… (Type of reaction is right, product is right.)
From the wrong answers that the pre-service chemistry teachers gave in connection with the sulfonation of benzene, it was seen that these responses could be divided into two categories. The first category of responses was when, although the type of reaction was known, groups such as –SO2 and –SO4 were bonded to the aromatic rings but the wrong sulfonation products were written down. In the second category of responses, not only was the type of reaction not known, but the wrong molecules (phenol, water, benzene) were formed as products. One of the pre-service chemistry teachers responding in the first category, PST-9 (Fig. 14), provided the following explanations about the diagram in the interview:
PST-9: …This was the sulfonation of benzene. That's why I attached the SO4group to the ring.
Researcher: Why the SO4group? Can you explain?
PST-9: Because it forms a reaction with sulphuric acid. So SO4came from there. (Type of reaction is right, product is wrong.)
From PST-9's explanations, it can be seen that the pre-service chemistry teacher tended to form the product of the reaction by looking at the reagent. In other words, it is understood that PST-9 had not completely comprehended the progress of the reaction, the breaking and formation of bonds, or the mechanism of the electrophilic aromatic substitution reaction.
One of the wrong responses to the reaction in the second category was given by PST-4. Not only did PST-4 (Fig. 9) not provide an explanation for the sulfonation of benzene, but phenol was written down as the product. This pre-service chemistry teacher made the following explanations in the interview:
PST-4: … There is hydrogen sulphide in the reaction and also alkene. That's why I created alcohol. In other words, I attached the hydroxyl group to the ring.
Researcher: What kind of a reaction was there between the alkene and hydrogen sulphide to produce alcohol? Could you please explain this for us?
PST-4: Well, benzene is an alkene, and in the presence of hydrogen sulphide, alcohol is obtained when water is added. I can’t remember the exact name of the type of reaction right now. (Type of reaction is unknown, product is wrong.)
From PST-4's explanations, it was seen that the pre-service chemistry teacher was unable to identify the sulfonation of benzene and also have some alternative conceptions about the basic notions of organic chemistry. For example, the pre-service chemistry teacher categorized benzene as an alkene instead of an aromatic compound and defined phenol as an alcohol. A striking aspect of PST-4's explanations was that the pre-service chemistry teacher identified sulphuric acid as hydrogen sulphide. This finding indicates that this pre-service chemistry teacher did not have a sufficient knowledge of inorganic compounds. At the same time, it was also seen that PST-4, without paying attention to the reaction conditions and reagents involved, applied the synthesis of alcohol by adding water to alkenes in the presence of acid. In the second reaction in the subsystem, the pre-service chemistry teachers were asked to indicate the direction of the reaction related to the synthesis of phenol from benzene sulfonic acid. All of the pre-service chemistry teachers who correctly identified the sulfonation of benzene also drew the direction of the reaction correctly. One of the pre-service chemistry teachers in this category, PST-6 (Fig. 15), provided the following explanations about the diagram in the interview:
PST-6: … I thought it would be the synthesis of phenol from benzene sulfonic acid. That's why I drew the arrow's direction toward the phenol. I remember this as being a special reaction. First, sodium salt was formed and then phenol was obtained from acidification. (Type of reaction is right, direction of reaction is right.)
In the wrong responses about this reaction, the answer PST-15 gave was particularly noteworthy. PST-15 drew the direction of the reaction wrong and also defined phenol as an alcohol. The explanations PST-15 (Fig. 5) gave were as follows:
PST-15: …There must be alcohol here, I said to myself. The –OH group broke off from the alcohol and hydrogen took its place. That's because there is hydrogen in the reaction. That's why I drew the direction of the reaction toward the side opposite the alcohol.
Researcher: Can you explain to us the type of reaction this was?
PST-15: … I don’t know.(Type of reaction is unknown, direction of reaction is wrong.)
This explanation by the pre-service chemistry teacher showed that the pre-service chemistry teacher did not have sufficient knowledge about the types of reactions and also that there was some difficulty in identifying organic molecules. It was also seen that the pre-service chemistry teacher did not exactly know the structural formula of benzene.
Table 7 shows an analysis of the responses of the pre-service chemistry teachers regarding the three reactions in the third subsystem of the SAQs diagram. In the nitration of benzene, one of these reactions, it was discovered that 73.3% of the pre-service chemistry teachers were able to correctly state that benzene reacted with a mixture of nitric and sulphuric acid. From the interview findings, it was understood that the pre-service chemistry teachers had correctly identified both the type of reaction and the reagents/conditions of the reaction.
| Reaction | Right | Wrong | No answer | |||
|---|---|---|---|---|---|---|
| f | % | f | % | f | % | |
| Synthesis of nitrobenzene from benzene (nitration of benzene) | 11 | 73.3 | 3 | 20.0 | 1 | 6.7 |
| Synthesis of aniline from nitrobenzene (reduction of nitrobenzene) | 5 | 33.3 | 6 | 40.0 | 4 | 26.7 |
| Synthesis of m-bromonitrobenzene from nitrobenzene (bromination of nitrobenzene) | 13 | 86.7 | 2 | 13.3 | — | — |
One of the pre-service chemistry teachers, PST-8 (Fig. 16), said the following in the discussions about the diagram:
PST-8: … This reaction was a nitration reaction. For this, we put nitric acid and sulphuric acid into a reaction. This is because we can obtain the –NO2ion from the reaction with nitric acid and sulphuric acid, and next steps in the reaction, we formed nitrobenzene from the substitution reaction.(Type of reaction is right, reaction conditions/reagent is right.)
In examining the wrong responses the pre-service chemistry teachers offered for the nitration of benzene, it was found that they had taken the NO2 group as the reagent. This result indicated that the pre-service chemistry teachers did not fully understand the mechanisms of electrophilic aromatic substitution reactions and, in this context, could not identify the reagents but tended to write down the reagent by looking at the product of the reaction. One of the pre-service chemistry teachers offering this response, PST-13 (Fig. 17), said the following in the discussions about the diagram:
PST-13: … Here, the nitro group bonded with the benzene. So I bonded the NO2group because the product has that in it. This way, I got the product.
Researcher: Can you give us the type of reaction or its name?
PST-13: … I don’t exactly know the type of reaction… (Type of reaction is unknown, reaction conditions/reagent is wrong).
It can be seen in Table 7 that in the reaction in which the aniline as an arylamine is formed through the reduction of nitrobenzene with SnCl2, only 33.3% of the pre-service chemistry teachers were able to respond correctly. One of the pre-service chemistry teachers who did respond correctly, PST-5 (Fig. 6), offered the following explanation:
PST-5: … Nitrobenzene is reduced with this reaction. This is its reduction in the presence of SnCl2. I think this was a special reaction and aniline was formed. (Type of reaction is right, product is right.)
In this reaction, 40.0% of the pre-service chemistry teachers responded incorrectly, while 26.7% could not answer at all, showing that they did not sufficiently understand the nitrobenzene reduction and the formation of aniline. Indeed, when the pre-service chemistry teachers’ wrong responses were reviewed on the diagram, it was found that they had bonded the reagents of the reaction or the catalyzing ions (OH−, H3O+) to the ring. For instance, PST-10 and PST-2 bonded the Cl atom in the reagent SnCl2 to nitrobenzene's meta position and wrote down m-chloronitrobenzene in the nitrobenzene reduction. One of these pre-service chemistry teachers, PST-2 (Fig. 8), explained this as follows:
PST-2: …I thought that this was like the chlorination of nitrobenzene. One hydrogen broke off and so I bonded the chlorine atom instead.
Researcher: But why did you bond the chlorine atom in that position? Did you have a special reason? Could you please explain this for us?
PST-2: I bonded it to its meta position because the nitro group is a group that attracts electrons, and its direction was in the meta position. That's why I bonded the chlorine atom to the meta.(Type of reaction is wrong, product is wrong.)
These explanations by PST-2 showed that the pre-service chemistry teacher accepted the reduction reaction of nitrobenzene as the chlorination reaction of aromatic compounds. This indicates that the pre-service chemistry teachers could not fully understand the reactions of aromatic compounds and had difficulty differentiating types of reactions by taking into consideration reagents and conditions.
Another striking point in the responses of the pre-service chemistry teachers who answered incorrectly to the formation of aniline through the reduction of nitrobenzene was that they wrote down the product as phenol or a derivative of phenol. In the discussions with the pre-service chemistry teachers providing these responses (PST-3, PST-7 and PST-15), it was found that the pre-service chemistry teachers wrote down these answers because of the –OH− ion in the reaction. One of these pre-service chemistry teachers (PST-3) (Fig. 13) provided these explanations in the interview:
PST-3: … We have nitrobenzene, and OH−ion in the reaction. Then I said that the OH–ion would replace the nitro group.
Researcher: Can you tell us the type of reaction then?
PST-3: I think it's a substitution reaction. Because the nitro group replaces the OH–ion. (Type of reaction is wrong, product is wrong.)
One of the most striking among the wrong responses the pre-service chemistry teachers gave to this reaction belonged to PST-8. This pre-service chemistry teacher bonded the tin atom (Sn) in SnCl2 to the ring and interpreted this as if it were an electrophilic aromatic substitution. This pre-service chemistry teacher's (Fig. 16) explanation was as follows:
PST-8: … I think this is a substitution. In other words, the tin atom in the reaction replaced the hydrogen in the ring. So this kind of structure formed.
Researcher: But why did you bond the tin atom to that position? Was there a special reason?
PST-8: … Well, I remembered that the nitro group has a meta directing effect. That's why the second group was bonded with the meta.(Type of reaction is wrong, product is wrong.)
From the third reaction in Table 7, which is nitrobenzene bromination producing m-bromonitrobenzene, it can be seen that a very high percentage of 86.7% of the pre-service chemistry teachers were able to provide this correct answer. One of these pre-service chemistry teachers, PST-14 (Fig. 12), explained this reaction on the diagram as follows:
PST-14: …There is a bromination of nitrobenzene here. The nitro group attracts electrons, and because of this, it is meta-directing. Because of this, bromine was bonded to a meta position. (Type of reaction is right, product is right.)
An explanation of the responses of the pre-service chemistry teachers who gave wrong answers to this reaction showed that there were two different types of responses. The first belonged to PST-7 (Fig. 4), who wrote that the nitro group and the bromine replaced and formed bromobenzene as the product. This pre-service chemistry teacher's explanations in the discussions were as follows:
PST-7: …I remembered that bromine is more selective. That's why I replaced the nitro group with the bromine.
Researcher: Then what can you say about the type of reaction? Can you explain?
PST-7: I would say this is a reaction where bromine replaced by nitro.(Type of reaction is wrong, product is wrong.)
These explanations by PST-7 show that the pre-service chemistry teacher did not exactly understand how an electrophilic aromatic substitution reaction would occur, especially in an aromatic ring when there was a substituent involved and also did not comprehend the directing effect of the substituent in the reaction. Another who provided a wrong response was PST-5 (Fig. 6). In the discussions, this pre-service chemistry teacher was able to correctly identify the reaction, but because there was an error in understanding the directing effect of the nitro group, the product formed was wrong. PST-5's explanations were as follows:
PST-5: … There is a bromination reaction here. I bonded bromine to the structure.
Researcher: Why did you bond it to this position? Could you please explain this for us?
PST-5: The nitro groups donate electrons to the ring. That's why it is para-directing.
Researcher: Then what can you say about what type of reaction this is? Could you please explain this for us?
PST-5: An aromatic compound is brominating. It's a bromination reaction. This is a substitution reaction that specifically occurs in aromatic compounds.(Type of reaction is right, product is wrong.)
Table 8 shows an analysis of the reactions that the pre-service chemistry teachers had to think about in terms of many perspectives on the SAQs diagram. The main reason these reactions were shown on the SAQs diagram was that, among the aromatic compound reactions, they were reactions that were related to side-chain halogenation and to additions to the double bond of alkenylbenzenes. In these reactions, which are in the fourth subsystem of the diagram, the pre-service chemistry teachers particularly had to consider the synthesis and reactions of the alkenes and plan how a transition would be made between the reactions. This stimulated the pre-service chemistry teachers to think about the reactions from all angles and to find an association between them.
| Reaction | Right | Wrong | No answer | |||
|---|---|---|---|---|---|---|
| f | % | f | % | f | % | |
| Side-chain bromination of ethylbenzene (indicating the direction of the arrow) | 10 | 66.7 | 3 | 20.0 | 2 | 13.3 |
| Synthesis of (1-bromoethyl)benzene from ethylbenzene (side-chain bromination of ethylbenzene) | 9 | 60.0 | 4 | 26.7 | 2 | 13.3 |
| Synthesis of (2-bromoethyl)benzene from styrene (adding HBr to the double-bond in the presence of peroxide) | 5 | 33.3 | 9 | 60.0 | 1 | 6.7 |
| Synthesis of 2-phenylethanol from styrene (hydroboration/oxidation of the double bond) | 3 | 20.0 | 10 | 66.7 | 2 | 13.3 |
It is seen that the percentages of correct answers of the pre-service chemistry teachers in Table 8 are lower when compared to the correct answers to the other reactions (Tables 5–7). This can be interpreted as an indication that the pre-service chemistry teachers were challenged in associating aromatic compounds with classifications of different organic molecules within an integrated structure.
In the first reaction in this system, the pre-service chemistry teachers were asked to indicate the direction of the side-chain bromination reaction of ethylbenzene with NBS. It can be understood from Table 8 that 66.7% of the pre-service chemistry teachers were able to indicate the direction of this reaction but 20.0% made a wrong indication. The pre-service chemistry teachers that incorrectly indicated the direction of the reaction (PST-4, PST-7 and PST-15) also put down the side-chain bromination product incorrectly. In this reaction, where ethylbenzene reacted with NBS in a benzylic bromination and (1-bromoethyl)benzene was formed, 60.0% of the pre-service chemistry teachers were able to write down the correct product. These pre-service chemistry teachers were also able to correctly indicate the type of reaction as well as explain that this product was formed because of benzylic stability. At the same time, all of these pre-service chemistry teachers indicated the direction of the reaction correctly. One of these pre-service chemistry teachers (PST-11) (Fig. 3) provided these explanations in the interview:
PST-11: … I called this bromination. I remember that NBS is a source of bromine. That's why this side-chain bromination reaction occurred.
Researcher: Why did you bond the bromine to the carbon atom? Can you explain?
PST-11: It could have been the other carbon atom too but the benzylic carbon atom is more stable. There is resonance stability here, that's why.(Type of reaction is right, product is right; type of reaction is right, direction of reaction is right.)
An examination of the wrong responses of the pre-service chemistry teachers to this reaction showed that they could be collected in two categories. In the first category, the pre-service chemistry teacher (PST-6) defined the reaction correctly, but because the stability of the benzylic position was not taken into account, the bromine was attached to the other carbon atom and the wrong product was obtained. The explanation of the pre-service chemistry teacher (Fig. 15) was as follows:
PST-6: …This is bromination. I bonded the bromine to the structure of the alkyl instead of the hydrogen. In other words, the hydrogen broke off and bromine filled its place.
Researcher: But why did you attach the bromine to the carbon atom? Can you explain?
PST-6: …I don’t exactly know why.(Type of reaction is right, product is wrong; type of reaction is right, direction of reaction is right.)
In the other category, the responses of the pre-service chemistry teachers PST-4, PST-7, and PST-15 not only incorrectly defined the type of reaction but also wrote down the direction of the reaction and the product incorrectly. The pre-service chemistry teachers PST-4 and PST-15 formed the alcohol molecule as the product by considering the OH− ion in the next reaction. That both pre-service chemistry teachers showed the same tendency in the other reactions in the diagram (e.g., Friedel–Crafts alkylation, side-chain oxidation) showed that they had not completely understood the reactions peculiar to aromatic compounds and radical reactions. The explanations PST-4 (Fig. 9) provided in the interview were as follows:
PST-4: … I said that there should be alcohol here because there is a hydroxyl ion on top of the arrow. I then remembered that when CCl4is removed from alcohol, the alcohol disappears. In other words, I said, there was a transition into the alkyl structure and so I obtained ethylbenzene. That's why I put the arrow in the direction of the ethylbenzene.
Researcher: So what can you tell us about the type of reaction?
PST-4: …I think this was an elimination reaction.(Type of reaction is wrong, product is wrong; type of reaction is wrong, direction of reaction is wrong.)
Another pre-service chemistry teacher who gave the wrong response to the reaction, PST-7, thought the side-chain bromination reaction was a product of alkane and added sodium to the aromatic structure. It was understood from the explanations of the pre-service chemistry teachers that they had not fully comprehended Wurtz synthesis and had classified alkylbenzene as an alkane, and in the molecule they wrote in the diagram, it was seen that the number of bonds of the carbon atom had not been considered. The explanations of PST-7 (Fig. 4) were as follows:
PST-7: …I thought there was a transition into the alkane structure in this reaction. I thought of Wurtz synthesis. That's why I added sodium.
Researcher: What made you decide on Wurtz synthesis? Could you please explain this for us?
PST-7: … In other words, the product has the structure of an alkane. Alkane is formed when there is halide in the reaction. And the presence of CCl4in the reaction shows this.(Type of reaction is wrong, product is wrong; type of reaction is wrong, direction of reaction is wrong.)
It can be said from Table 8 that in the 4th subsystem of the SAQs diagram one of the reactions that the pre-service chemistry teachers gave the least right answers to was obtaining (2-bromoethyl)benzene from styrene. In this reaction, the pre-service chemistry teachers were expected to write down the addition of HBr to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the presence of peroxide. As for the pre-service chemistry teachers who were able to come up with the appropriate reagent and reaction conditions, their comments in the interviews showed that they were able to explain that there was a radical mechanism in the reaction and that the addition was made not according to Markovnikov's rule but according to the mechanism of an anti-Markovnikov reaction. One of these pre-service chemistry teachers, PST-12 (Fig. 7), explained this as follows:
C double bond in the presence of peroxide. As for the pre-service chemistry teachers who were able to come up with the appropriate reagent and reaction conditions, their comments in the interviews showed that they were able to explain that there was a radical mechanism in the reaction and that the addition was made not according to Markovnikov's rule but according to the mechanism of an anti-Markovnikov reaction. One of these pre-service chemistry teachers, PST-12 (Fig. 7), explained this as follows:
PST-12: …Here I looked at the reactant and the product of the reaction. To see what happened. The double bond between the carbon atoms has broken and hydrogen and bromine have bonded with the carbon atoms. Then I looked at which carbon atom this double bond of the hydrogen and bromine bonded to. The opposite of what was expected has happened. In other words, if it had been according to Markovnikov's rule, the hydrogen atom would have bonded with the double-bond carbon atom that had the most hydrogen. I saw that just the opposite had happened here. In other words, this was an anti-Markovnikov reaction. So I thought HBr should be added to the double bond in the presence of peroxide.
Researcher: Then why were there no additions to the reaction according to Markovnikov? What kind of a change took place? Can you explain?
PST-12: Normally, if there had been an addition according to Markovnikov, the reaction would have taken place with the formation of a carbocation. Here the mechanism of the reaction changed in the presence of peroxide and the reaction took place with the formation of radicals. The order of the steps changed, in other words.(Type of reaction is right, reaction conditions/reagent is right.)
In the review of the wrong responses the pre-service chemistry teachers gave regarding this reaction, it was seen that a large part of the pre-service chemistry teachers (PST-2, PST-3, PST-4, PST-8, PST-11, PST-13, PST-15) said that the reaction was the addition of HBr to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, but in this, they explained that the reaction had occurred in line with Markovnikov's rule, which showed that they had not considered the presence of the peroxide in the reaction. This finding indicated that the pre-service chemistry teachers tended to complete the reactions by rote without understanding the mechanism, and the process of the reaction. Indeed, this can be more clearly seen from the explanations of PST-13 (Fig. 17):
C bond, but in this, they explained that the reaction had occurred in line with Markovnikov's rule, which showed that they had not considered the presence of the peroxide in the reaction. This finding indicated that the pre-service chemistry teachers tended to complete the reactions by rote without understanding the mechanism, and the process of the reaction. Indeed, this can be more clearly seen from the explanations of PST-13 (Fig. 17):
PST-13: … Actually we see the addition of hydrogen bromide here as in an alkene reaction.
Researcher: So can you explain this addition reaction?
PST-13: … The carbon atom to which the bromine is added is the primary carbon atom; the other one, the one to which hydrogen is added, is the secondary carbon atom. Because the secondary carbon atom is more stable, the hydrogen is added to the secondary carbon atom, and the bromine is added to the primary atom. That's to say that this was how it worked according to Markovnikov's rule.
Researcher: Can you explain Markovnikov's rule for us, please?
PST-13: According to this rule, we bonded the hydrogen to the secondary or tertiary carbon atom and the halogen to the less stable carbon atom. (Type of reaction is partially right, reaction conditions/reagent is partially right.)
From these explanations, we can not only see PST-13's mistake in applying Markovnikov's rule, but it is also noted that the pre-service chemistry teachers incorrectly interpreted the stability of the carbocation in this process, showing that they were challenged in understanding the mechanism of the electrophilic addition of alkenes.
Another two wrong responses in the context of this reaction were received from two pre-service chemistry teachers (PST-9, PST-5), who chose the wrong reagents (AlBr3, Br2/FeBr3) and identified the type of reaction as the addition of bromine. In the review of the reagents the pre-service chemistry teachers referred to, it can be seen that they used the reagents and catalyzers in the electrophilic aromatic substitution reactions. This indicates that the pre-service chemistry teachers were unable to differentiate between the electrophilic aromatic substitution reactions and the electrophilic addition reactions of alkenes. Indeed, PST-5's (Fig. 6) explanations are testament to this:
PST-5: … I added bromine to break the double bond.
Researcher: Could you please explain this reaction? Why did you write Br2and FeBr3?
PST-5: Well, FeBr3works as a catalyser. At the same time, Br2provides bromine. This bromine atom bonds to the double bond. (Type of reaction is wrong, reaction conditions/reagent is wrong.)
It can also be seen from these explanations that the pre-service chemistry teacher did not fully understand the addition of Br2, which is one of the electrophilic addition reactions of alkenes.
Of all the reactions on the SAQs diagram, the reaction for which the pre-service chemistry teachers provided the least number of right answers was the synthesis of 2-phenylethanol from styrene, which involves the hydroboration/oxidation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Only 20.0% of the pre-service chemistry teachers were able to correctly respond to this reaction, which takes place with the addition of water, according to anti-Markovnikov's rule. A review of the pre-service chemistry teachers’ responses indicated that they were able to correctly identify the type of reaction together with the steps involved. One of these pre-service chemistry teachers, PST-1 (Fig. 11), explained this as follows:
C bond. Only 20.0% of the pre-service chemistry teachers were able to correctly respond to this reaction, which takes place with the addition of water, according to anti-Markovnikov's rule. A review of the pre-service chemistry teachers’ responses indicated that they were able to correctly identify the type of reaction together with the steps involved. One of these pre-service chemistry teachers, PST-1 (Fig. 11), explained this as follows:
PST-1: … Here we have BH3and H2O2. I said this was a hydroboration reaction. Actually, there's the addition of water to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/i_char_e001.gif) C bond, but in this reaction, water was added according to anti-Markovnikov. That is, in the C
C bond, but in this reaction, water was added according to anti-Markovnikov. That is, in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/i_char_e001.gif) C bond, I attached hydrogen to the one with less hydrogen and I attached the –OH group to the other carbon atom.
C bond, I attached hydrogen to the one with less hydrogen and I attached the –OH group to the other carbon atom.
Researcher: What is the function of H2O2in this reaction? Can you explain?
PST-1: Firstly, there is a reaction of BH3in the reaction. It forms a structure with borane. Then oxidation occurs with H2O2. (Type of reaction is right, product is right.)
A high percentage (66.7%) of the pre-service chemistry teachers gave wrong answers for this reaction. Some of the pre-service chemistry teachers providing these wrong responses (PST-2, PST-3, PST-8, PST-14) identified the type of reaction correctly but drew the addition reaction according to Markovnikov's rule, thus yielding the wrong product. One of these pre-service chemistry teachers, PST-2 (Fig. 8), explained this as follows:
PST-2: … I said there was hydroboration here.
Researcher: What do you mean by hydroboration? And how did you understand that this was hydroboration? Can you explain?
PST-2: I understood it from the BH3. The π bond between the carbon atoms in the hydroboration reaction breaks, and hydrogen and hydroxyl bond with the carbon atoms.
Researcher: Can you explain why you attached the hydrogen and hydroxyl groups to the carbon atoms?
PST-2: I attached the hydrogen to the carbon atom with more hydrogen and I attached the one with less to the –OH group. I wrote this down according to Markovnikov's rule.(Type of reaction is right, product is wrong.)
PST-10 and PST-11 correctly identified the hydroboration reaction but there were those that wrote down wrong products, such as phenol. The explanations of these pre-service chemistry teachers show that they did not fully comprehend the hydroboration reaction in particular. One of them, PST-11 (Fig. 3), gave the following explanations:
PST-11: … This reaction could be found in the alkenes. The reaction started out with BH3. Hydroboration. The π bond between the C bond breaks in the reaction and so we attach the hydroxyl group in the reaction to the carbon atom in the ring.
Researcher: Then what happened to the double-bond carbon atoms?
PST-11: …Well, the π bond broke. I think the carbon atoms transferred to another molecular structure(Type of reaction is right, product is wrong.)
A group of pre-service chemistry teachers (PST-4, PST-5, PST-7) identified the type of reaction as the hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and indicated ethylbenzene as the product. These results show that besides the hydroboration reaction the pre-service chemistry teachers also did not fully understand the catalytic hydrogenation, a reduction reaction of the C
C bond and indicated ethylbenzene as the product. These results show that besides the hydroboration reaction the pre-service chemistry teachers also did not fully understand the catalytic hydrogenation, a reduction reaction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. One of the pre-service chemistry teachers, PST-4 (Fig. 9), gave the following explanation:
C bond. One of the pre-service chemistry teachers, PST-4 (Fig. 9), gave the following explanation:
PST-4: … I went into the alkane structure here. For this, I had to add hydrogen atoms in BH3to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/i_char_e001.gif) C bond. So the π bond between the carbon atoms was broken.
C bond. So the π bond between the carbon atoms was broken.
Researcher: What can you then say about the type of reaction?
PST-4: I would say that this is the reaction where hydrogen is added to a double-bond. (Type of reaction is wrong, product is wrong.)
One of the pre-service chemistry teachers providing wrong reactions here, PST-15, was unable to identify the type of reaction and wrote down phenol as the product, which the pre-service chemistry teacher thought was alcohol. As in the previous reactions, this pre-service chemistry teacher chose to write down the product by looking at the conditions of reaction, not taking into consideration the type of reaction or the mechanism. The explanations PST-15 (Fig. 5) gave were as follows:
PST-15: … I said alcohol would form here. Because the reaction contains the OH–ion. I attached this to the ring. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond structure broke, in other words.
C bond structure broke, in other words.
Researcher: Can you explain to us the type of reaction this was?
PST-15: … I wasn’t able to define exactly which type of reaction this was.(Type of reaction is not known, product is wrong.)
The pre-service chemistry teachers were also asked to indicate the two reactions between molecules that were not shown on the diagram, writing out their reaction reagents and conditions. The results of the analysis showed that this section was the part on the diagram for which the pre-service chemistry teachers provided the least number of responses. Only six of the pre-service chemistry teachers were able to identify a reaction on the diagram. While 3 of these reactions were written out with the right reagents and reaction conditions, 2 had the wrong reagents and conditions. One of the reactions written out was scientifically incorrect. The reactions indicated by the pre-service chemistry teachers on the SAQs diagram are shown in Table 9.
| Reaction | Type of reaction | Pre-service chemistry teacher |
|---|---|---|
| a Pre-service chemistry teachers wrote down the reagents and conditions of the reaction incorrectly. b The reaction the pre-service chemistry teacher wrote down is scientifically incorrect. | ||
| Synthesis of ethylbenzene from benzene | Friedel–Crafts alkylation | PST-1, PST-14, PST-9,a PST-12a |
| Synthesis of ethylbenzene from styrene | Catalytic hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond C bond |
PST-7 |
| Synthesis of 2-phenylethanol from phenol | Scientifically incorrect reaction | PST-15b |
The explanations given by PST-12 and PST-9 about these reactions on the SAQs diagram were particularly striking. These pre-service chemistry teachers said in their interviews that they did not know what the Friedel–Crafts alkylation reaction was and that they only looked at the product and chose the ethyl group as the reagent. The explanations PST-9 (Fig. 14) gave were as follows:
PST-9: … I said here that ethylbenzene could also be formed from benzene. That's why I made the direction of the reaction go towards ethylbenzene.
Researcher: Then how did you choose the reagent for this reaction? What was your reasoning?
PST-9: To tell the truth, I thought if the ethyl group is in this product, then the input must be the same too. That's why I put ethyl into the reaction.
Researcher: Then what can you say about what type of reaction this is?
PST-9: … Well, I couldn’t really say. (Type of reaction is not known, reaction conditions/reagent is wrong.)
In line with the second sub-problem of the research, first, the pre-service chemistry teachers’ learning difficulties related to aromatic compound reactions were determined, and then the alternative conceptions that appeared as part of these learning difficulties were looked into. In this context, it was found that the two main areas of learning difficulty that the pre-service chemistry teachers exhibited were “classifying organic molecules” and “the breaking/formation of bonds and the reaction mechanism.”
In the context of the learning difficulty related to the “classifying organic molecules,” it was seen that the pre-service chemistry teachers had a hard time correctly classifying some organic molecules. For example, PST-4 identified benzene as an alkene and phenol as an alcohol and explained the reactions in this light, which showed that the pre-service chemistry teacher did not fully understand the concepts of aromatic compounds and alcohols. In fact, many other research findings have pointed to how students misinterpret reactions because they classify organic molecules incorrectly (Şendur and Toprak, 2013a; Ealy, 2018). In particular, in the case of classifying phenol as an alcohol, the pre-service chemistry teachers focused only on the –OH group in identifying alcohol, which can be interpreted as the result of their inability to completely differentiate between enol and alcohol. It has been shown in the literature that students and pre-service chemistry teachers think that the presence of the –OH group is enough for a molecule to be alcohol and their classification of phenol as alcohol has been mentioned in many research findings (Potgieter and Davidowitz, 2011; Şendur and Toprak, 2013b; Akkuzu and Uyulgan, 2016). Similarly, as with PST-4, classifying benzene as an alkene and ethylbenzene as an alkane indicates that the pre-service chemistry teacher not only is unable to structure the concept of aromaticity but is also challenged in differentiating hydrocarbons from aromatic hydrocarbons. Indeed, Ealy and Hermanson (2006) reported that the concept of aromaticity is hard for students to grasp and that students do not have adequate knowledge in this subject. Table 10 shows alternative conceptions the pre-service chemistry teachers exhibited in the context of their learning difficulties with respect to classifying organic molecules.
| Learning difficulty | Alternative conceptions | Pre-service chemistry teachers |
|---|---|---|
| Classifying organic molecules | Benzene is an alkene | PST-4, PST-7 |
| Phenol is an alcohol | PST-4, PST-7, PST-15 | |
| Ethylbenzene is an alkane | PST-4, PST-7 |
In the context of the learning difficulty related to the “breaking/formation of bonds and the reaction mechanism”, situations that resulted from the pre-service chemistry teachers’ inadequate knowledge about the nature and mechanism of reactions were considered. A similar learning difficulty was reported by Hrin et al. (2018). Some of the alternative conceptions with respect to this were related to Friedel–Crafts alkylation and acylation. For example, PST-7 wrote down that when benzene reacts with acetylchloride in the presence of AlCl3, dimethyl ketone is formed; this shows that the pre-service chemistry teacher did not take into account the steps when benzene acts as an electron donor in the Friedel–Crafts acylation reaction that starts with the electron attack on the acetyl cation, which is an electrophile. A similar situation came up in the Friedel–Crafts alkylation reaction. PST-15 wrote down methyl as the reagent in the reaction to obtain methylbenzene from benzene, while PST-12 and PST-9 used ethyl as a reagent in obtaining ethylbenzene from benzene. In all three situations, the pre-service chemistry teachers’ choosing the alkyl group as a reagent that reacts with benzene by looking at the product showed that in the first step of the Friedel–Crafts alkylation reaction they did not focus on the fact that the reaction occurred within a certain mechanism that depended on the formation of a carbocation from alkyl halide. It may be said that lying at the base of the pre-service chemistry teachers’ having difficulty with both the Friedel–Crafts alkylation and Friedel–Crafts acylation reactions was also the fact that they could not identify the electrophile in the electrophilic aromatic substitution reactions and the role of the electrophile in these reactions, and could not understand how the electrophile was formed.
Another learning difficulty that the pre-service chemistry teachers encountered with regard to the breaking/formation of bonds and reaction mechanisms was related to the side-chain oxidation reaction of alkyl benzenes. Two points were ascertained in the pre-service chemistry teachers’ responses. The first of them was that the pre-service chemistry teachers PST-4, PST-7, and PST-15 wrote down the alcohol molecule as a product by only looking at the OH− ion in the reaction without considering the type of reaction. This indicates that the pre-service chemistry teachers did not take the existence of an oxidizing agent such as KMnO4 in the reaction into consideration and could not therefore interpret how this substance affected the mechanism of the reaction. Another reason for this was that the pre-service chemistry teachers were unable to exactly differentiate between a reagent and a solvent and based their interpretations on types that they encountered frequently such as OH–. In fact, Galloway et al. (2018) stated that students make only a surface interpretation of reactions without considering the interactions between the chemical substances that take part in organic reactions. At the same time, the researchers pointed out that students can focus on certain properties of reagents in a reaction but they cannot discuss how these properties affect the reaction mechanism. The second situation that came up with regard to types of reactions was, as with PST-10, the reaction type was identified as oxidation and the product of oxidation as ketones. The reason for this was that the students had not completely understood the mechanism behind the oxidation of the benzylic radical.
Another indication of the learning difficulty the pre-service chemistry teachers had with understanding the breaking/formation of bonds and the reaction mechanism in electrophilic aromatic substitution reactions appeared in nitration and sulfonation of benzene reactions. In the reaction of the nitration of benzene, the fact that the pre-service chemistry teachers PST-4, PST-13, and PST-15 chose the NO2 group as the reagent showed that they did not understand the first step of the reaction where nitronium was formed to act as an electrophile or the role of an electrophile in the reaction. In other words, in the nitration of benzene, the pre-service chemistry teachers did not consider that the electrophilic nitronium ion was attacked by the π electron system of the benzene ring. A similar situation was seen in the sulfonation of benzene reaction. In this reaction and differing from nitration, the pre-service chemistry teachers were asked to write down the product of the reaction. In their responses, it was seen that the pre-service chemistry teachers PST-9 and PST-11 bonded the SO2 or SO4 groups to the aromatic ring and wrote this down as the product. This showed that the pre-service chemistry teachers were unable to identify the reactive electrophile in the reaction. Fundamental to both reactions was the inability of the pre-service chemistry teachers to fully structure the concept of electrophiles in their minds and, related to this, their failure to understand and interpret the process of the reaction. Indeed, Cruz-Ramirez de Arellano and Towns (2014) called attention to the fact that students generally had difficulty with assessing the electrophilic/nucleophilic structure that formed the basics of understanding reaction mechanisms. Anzovino and Bretz (2015), too, in research they conducted with organic chemistry students, reported that students tended to first identify the reaction mechanism and then determine the nucleophile and electrophile in the reaction. The researchers noted that when students based their thinking on the wrong mechanism, they failed to consider the chemical feasibility of the mechanism of a reaction and identified nonexistent intermediate products as nucleophiles or electrophiles. The main reason for this was that, instead of acquiring an in-depth understanding of nucleophilic and electrophilic behaviors and related properties and functionalities, the students memorized the material.
Some other learning difficulties the pre-service chemistry teachers displayed in the context of the breaking/formation of bonds and reaction mechanisms were related to the bromination of alkylbenzenes and the addition reaction of the double bond of alkenylbenzenes. In these reactions in particular, this situation was caused by the inability of the pre-service chemistry teachers to exactly differentiate between reactions based on radical or ionic reaction mechanisms and their failure to assess products formed in the context of their mechanisms. For example, PST-6 expressed ethylbenzene's reaction with NBS correctly as side-chain bromination, but when writing down the product, the pre-service chemistry teacher could not form the right product because of not taking into consideration the stability of the benzylic radical. This indicates that the pre-service chemistry teacher tended to write down the product without considering the mechanism in this reaction that displayed a radical mechanism. In fact, many studies in the literature point to the fact that reaction mechanisms are not too clear for students and they focus on the properties of products or reactants instead of on the mechanism of the reaction (Bhattacharyya and Bodner, 2005; Rushton et al., 2008; Bhattacharyya 2014; Hrin et al., 2018). Another example of this has to do with the synthesis of (2-bromoethyl)benzene from styrene. For this reaction, the pre-service chemistry teachers are expected to respond by saying this is an anti-Markovnikov reaction and thus represents the addition of HBr to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the presence of peroxide. The pre-service chemistry teachers PST-2, PST-3, PST-4, PST-8, PST-11, PST-13 and PST-15, however, answered by identifying the reaction according to Markovnikov's rule, perhaps because they tended to generalize this rule unnecessarily. Indeed, Graulich and Bhattacharyya (2017) said that, instead of considering reactions mechanistically, some students have taken Markovnikov's rule to be limited to superficial changes in the structure. At the same time, the fact that the pre-service chemistry teachers explained this reaction in terms of Markovnikov's rule may also be a result of their failure to consider the radical mechanism of this reaction.
C bond in the presence of peroxide. The pre-service chemistry teachers PST-2, PST-3, PST-4, PST-8, PST-11, PST-13 and PST-15, however, answered by identifying the reaction according to Markovnikov's rule, perhaps because they tended to generalize this rule unnecessarily. Indeed, Graulich and Bhattacharyya (2017) said that, instead of considering reactions mechanistically, some students have taken Markovnikov's rule to be limited to superficial changes in the structure. At the same time, the fact that the pre-service chemistry teachers explained this reaction in terms of Markovnikov's rule may also be a result of their failure to consider the radical mechanism of this reaction.
One of the reactions to consider in the context of the learning difficulties the pre-service chemistry teachers faced with regard to the breaking/formation of bonds and reaction mechanisms is hydroboration/oxidation, which is an addition reaction of the double bond of alkenylbenzenes. In this reaction, in which water is added to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond according to anti-Markovnikov, some of the pre-service chemistry teachers (PST-2, PST-3, PST-8, PST-14) made their explanations according to Markovnikov's rule. This may have been caused by the fact that the pre-service chemistry teachers had not understood that the reaction had progressed on the basis of a mechanism that did not involve the formation of a carbocation. Graulich and Bhattacharyya (2017) said that the different mechanisms (radical, ionic) involved in C
C bond according to anti-Markovnikov, some of the pre-service chemistry teachers (PST-2, PST-3, PST-8, PST-14) made their explanations according to Markovnikov's rule. This may have been caused by the fact that the pre-service chemistry teachers had not understood that the reaction had progressed on the basis of a mechanism that did not involve the formation of a carbocation. Graulich and Bhattacharyya (2017) said that the different mechanisms (radical, ionic) involved in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond reactions make it difficult for students to adopt a mechanistic approach to these reactions. Researchers have therefore emphasized that it is important to teach mechanistic arrangements in addition to reaction mechanisms so that students can develop a mechanistic approach to understanding organic reactions. With respect to the hydroboration/oxidation of styrene, one of the alternative concepts the pre-service chemistry teachers adopted was considering the reaction as the hydrogenation of the C
C bond reactions make it difficult for students to adopt a mechanistic approach to these reactions. Researchers have therefore emphasized that it is important to teach mechanistic arrangements in addition to reaction mechanisms so that students can develop a mechanistic approach to understanding organic reactions. With respect to the hydroboration/oxidation of styrene, one of the alternative concepts the pre-service chemistry teachers adopted was considering the reaction as the hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Some of the pre-service chemistry teachers (PST-4, PST-5, PST-7) considered this reaction hydrogenation and formed ethylbenzene, showing that they had not fully understood the metal catalyzers used in this reaction or the different reaction mechanisms progressing from other reactions. Indeed, Graulich and Bhattacharyya (2017) asserted that due to the typical dipolar mechanism in the hydrogenation reaction, students are likely to be challenged in categorizing this reaction. Table 11 displays the alternative conceptions the pre-service chemistry teachers were seen to adopt as evidence of their learning difficulties related to the breaking/formation of bonds and reaction mechanisms.
C bond. Some of the pre-service chemistry teachers (PST-4, PST-5, PST-7) considered this reaction hydrogenation and formed ethylbenzene, showing that they had not fully understood the metal catalyzers used in this reaction or the different reaction mechanisms progressing from other reactions. Indeed, Graulich and Bhattacharyya (2017) asserted that due to the typical dipolar mechanism in the hydrogenation reaction, students are likely to be challenged in categorizing this reaction. Table 11 displays the alternative conceptions the pre-service chemistry teachers were seen to adopt as evidence of their learning difficulties related to the breaking/formation of bonds and reaction mechanisms.
| Learning difficulty | Alternative conceptions | Pre-service chemistry teacher(s) |
|---|---|---|
| Breaking/formation of bonds and reaction mechanisms | Benzene's reaction with acetylchloride in the presence of AlCl3 yields dimethyl ketone (as a Friedel–Crafts acylation reaction) | PST-7 |
| Benzene's reaction with methyl yields methylbenzene (as a Friedel–Crafts alkylation reaction) | PST-15 | |
| Benzene's reaction with ethyl yields ethylbenzene (as a Friedel–Crafts alkylation reaction) | PST-9, PST-12 | |
| Phenylmethylketone forms as a result of ethylbenzene oxidation (as a side-chain oxidation of alkylbenzenes) | PST-10 | |
| Phenylmethylketone forms as a result of methylbenzene oxidation (as a side-chain oxidation of alkylbenzenes) | PST-10 | |
| Alcohol forms as a result of ethylbenzene oxidation (as a side-chain oxidation of alkylbenzenes) | PST-4, PST-7, PST-15 | |
| Nitrobenzene forms as a result of benzene's reaction with NO2 | PST-4, PST-13, PST-15 | |
| As a product of the sulfonation of benzene, the SO2 or SO4 groups attach to the aromatic ring | PST-9, PST-11 | |
| (2-Bromoethyl)benzene forms from the reaction of ethylbenzene with NBS | PST-6 | |
| As a result of the reaction of styrene with HBr, according to Markovnikov's rule, (2-bromoethyl)benzene is formed | PST-2, PST-3, PST-4, PST-8, PST-11, PST-13, PST-15 | |
In the hydroboration of styrene, addition of water to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is a reaction that occurs according to Markovnikov's rule C bond is a reaction that occurs according to Markovnikov's rule |
PST-2, PST-3, PST-8, PST-14 | |
The hydroboration of styrene is the hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and ethylbenzene is formed C bond and ethylbenzene is formed |
PST- 4, PST-5, PST-7 |
Conclusions and implications
To achieve meaningful understanding in organic chemistry, the reactions between molecules must be identified, and then these reactions must be considered as a whole and relationships must be correctly drawn between them. This study sought to reveal how pre-service chemistry teachers associate aromatic compound relationships on a SAQs diagram, exploring whether these relationships are meaningful and which learning difficulties cause pre-service chemistry teachers to make mistakes in considering these reactions.The study showed that in the distribution of the scores the pre-service chemistry teachers received from the SAQs diagram (Fig. 2 and Table 4), the highest percentage was 53.3% at a moderate level, followed by 26.7% at a low level. This result indicates that the pre-service chemistry teachers had difficulty with aromatic compound reactions and were challenged in forming meaningful relationships between them.
The qualitative data collected from the SAQs diagrams and the interviews in this study were helpful in detailing how the pre-service chemistry teachers associated the aromatic compound reactions and which learning difficulties they encountered. The data revealed that the pre-service chemistry teachers generally were more successful in some reactions in which they were asked to form products considering reagents/catalyzers in the reaction (such as Friedel–Crafts acylation, ethylbenzene oxidation, sulfonation of benzene, bromination of nitrobenzene) as compared to other reactions (writing down the reagent/catalyzer, indicating a reaction that has not been defined in the diagram). At the foundation of this may lie the reality that the pre-service chemistry teachers are used to their familiar habit of focusing on the product in their chemistry courses (Graulich, 2015). Notwithstanding that the pre-service chemistry teachers had a higher achievement rate in these reaction types, it was also understood from the study that they had some striking learning difficulties. In particular, it was seen that the pre-service chemistry teachers tended to write down the product of a reaction in the context of the “breaking/formation of bonds and the reaction mechanism” without considering the mechanism, thus arriving at the wrong product. This was especially noticeable in the reactions of the sulfonation of benzene, the side-chain oxidation of ethylbenzene, and the hydroboration/oxidation of styrene (Table 11). Bhattacharyya and Bodner (2005) noted that even master's level students focused on the structural properties of the reactants and the products and neglected the reaction mechanism that caused a substance to transform into another. It has furthermore been pointed out in many studies that the curved arrows used in reaction mechanisms do not mean much to students at different learning levels or that they have not adequately understood the basic chemical concepts that lie at the foundation of a reaction mechanism (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Friesen, 2008; Weinrich and Sevian, 2017). When considered from this perspective, it can be said that ensuring that reaction mechanisms and the concepts at the foundations of these mechanisms are fully understood will raise the awareness of students about why mechanisms are important in organic chemistry, and contribute to meaningful understanding of organic chemistry (Bhattacharyya and Bodner, 2005; Raker and Towns, 2012a, 2012b).
The data gathered from the study show that the pre-service chemistry teachers had particular difficulty with the reactions for which they were given the reactant and the product and then asked to write down the reagents and reaction conditions (such as obtaining ethylbenzene from acetophenone, obtaining methylbenzene from benzene, obtaining benzoic acid from methylbenzene). These reactions required that pre-service chemistry teachers think of reaction types, conditions, and mechanisms of the reaction step by step (Eticha and Ochonogor, 2015; Sloop, Tsoi and Coppock, 2016). In other words, instead of memorization, what was expected of the pre-service chemistry teachers was to effectively use problem-solving strategies to make a full chemical inquiry (Bhattacharyya and Bodner 2005; Austin et al., 2015). It was clearly understood that the pre-service chemistry teachers were unable to implement these strategies when they were unable to indicate the two reactions between the molecules on the diagram. Only three of the pre-service chemistry teachers in the study were able to exactly indicate a reaction on the diagram. One of these reactions was Friedel–Crafts alkylation, which was shown on the diagram as a reaction between other molecules. In short, only one pre-service chemistry teacher (PST-7) was able to write a different reaction than what was shown in the diagram, putting down the product ethylbenzene from styrene as a result of the catalytic hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. This clearly showed that the pre-service chemistry teachers had difficulty in designing a plan for the synthesis of organic molecules. Stated differently, it was seen in the SAQs diagram that the pre-service chemistry teachers had difficulty forming relationships between the organic molecules by considering their structures.
C bond. This clearly showed that the pre-service chemistry teachers had difficulty in designing a plan for the synthesis of organic molecules. Stated differently, it was seen in the SAQs diagram that the pre-service chemistry teachers had difficulty forming relationships between the organic molecules by considering their structures.
In light of the data derived from this study, it is believed that organic chemistry courses may include some approaches such as the one Sloop et al. (2016) suggested, the Synthesis Scaffold Approach (SSA) that will provide pre-service chemistry teachers with the opportunity to consider multiple aspects of a reaction occurring between different aromatic compounds and develop problem-solving approaches that will help them arrive at the correct conclusion. At the same time, asking the pre-service chemistry teachers to prepare SAQ diagrams for different organic molecules will help the pre-service chemistry teachers to consider organic molecules in an integrated manner.
One of the findings of the study was that the pre-service chemistry teachers could not exactly make the distinction between the substances written on the arrows in the reactions and define which one was a reagent, which was a catalyzer and which was a solvent. This caused the pre-service chemistry teachers to add the wrong substances to reactions in the beginning and to thus form the wrong products. When considered from this perspective, it can be particularly helpful if, when pre-service chemistry teachers are learning about the reactions of aromatic compounds, they learn to classify reactions through the use of various card applications and, as Galloway et al. (2018) suggested, to have the names of the solvents written in parentheses to distinguish them. It was observed that the pre-service chemistry teachers were unable to correctly classify certain organic compounds. This created a barrier for the pre-service chemistry teachers in structuring the reactions between molecules. In this context, treating the subject of hydrocarbons and other organic molecular classifications with functional groups with alternative measuring/assessment tools (structured grids, concept maps, etc.) prior to learning about electrophilic aromatic substitution reactions would contribute to determining the deficiencies experienced in these topics. The most striking results of the study were the pre-service chemistry teachers’ alternative conceptions emerging from the deficiencies in their knowledge of reaction mechanisms. It is believed in this context that it is important to create learning environments in which reaction mechanisms and the concepts that lie at the foundations of these mechanisms (electrophiles, nucleophiles, carbocations, curved arrows, etc.) are compared to other types of mechanisms (SN1, SN2, E1, E2, etc.) in discussions where pre-service chemistry teachers can take part with their peers.
Limitations of the study
There were two fundamental limitations in this study. The first was that the SAQs diagram prepared contained a concentration of electrophilic aromatic substitutions and the side-chain reactions of alkylbenzenes. In light of this, it can be said that if the diagram had included another sub-system of nucleophilic aromatic substitution reactions, it would have been possible to observe whether the pre-service chemistry teachers could form meaningful relationships between reactions on a wider scale. The reactions included in the SAQs diagram were limited to the side-chain reactions of alkylbenzenes, the bromination of alkylbenzene, and the formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the side-chain with an elimination reaction, followed by the addition of HBr to the C
C bond in the side-chain with an elimination reaction, followed by the addition of HBr to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the presence of peroxide and hydroboration/oxidation reactions. In particular, including in the diagram the addition of water to the C
C bond in the presence of peroxide and hydroboration/oxidation reactions. In particular, including in the diagram the addition of water to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the presence of acid, synthesis of alcohol by oxymercuration/demercuration, the formation of a diol via the reaction of the C
C bond in the presence of acid, synthesis of alcohol by oxymercuration/demercuration, the formation of a diol via the reaction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond with OsO4 and NaHSO3 and other reactions may contribute to urging the pre-service chemistry teachers to explore the different structural relationships and differences in the different reactions and thus gain a deeper understanding. The second limitation to the study was that the learning difficulties and alternative conceptions of the pre-service chemistry teachers were noted in the interviews using the think aloud method and only in the context of the way they identified the reactions in the SAQs diagram and their explanations as to what they considered in their thinking process. With respect to this, it will be beneficial to ask the pre-service chemistry teachers, particularly in the interviews, to indicate the reaction mechanisms (including the steps and the intermediates) by showing the electron flow using curved arrows and to define structures like electrophiles, nucleophiles and radicals in the reactions, thereby providing a clearer picture of their learning difficulties.
C bond with OsO4 and NaHSO3 and other reactions may contribute to urging the pre-service chemistry teachers to explore the different structural relationships and differences in the different reactions and thus gain a deeper understanding. The second limitation to the study was that the learning difficulties and alternative conceptions of the pre-service chemistry teachers were noted in the interviews using the think aloud method and only in the context of the way they identified the reactions in the SAQs diagram and their explanations as to what they considered in their thinking process. With respect to this, it will be beneficial to ask the pre-service chemistry teachers, particularly in the interviews, to indicate the reaction mechanisms (including the steps and the intermediates) by showing the electron flow using curved arrows and to define structures like electrophiles, nucleophiles and radicals in the reactions, thereby providing a clearer picture of their learning difficulties.
Conflicts of interest
There are no conflicts to declare.Appendix 1: Answer key
Appendix 2: Think-aloud protocol
Dear Pre-service Teacher:With this interview, I would like you to explain how you filled out the systemic assessment questions (SAQs) diagram, how you defined the reactions in the diagram and what you considered in this process. Toward this aim, please
(1) Tell us, thinking out loud, where you started to fill out the diagram and, step-by-step, how you defined which reactions, and how you filled out the blanks (the direction of the arrow, completing the reagents/conditions, writing down the product of the reaction and indicating two new reactions).
(2) Tell us, thinking out loud, what you considered when forming associations between the reactions; please tell us about what went through your mind, even though it may seem very simple to you.
(3) Tell us out loud especially what types of reactions are in the diagram.
(4) At any time when you remain silent or are unable to explain yourself fully, you will be asked questions and you may be asked to think aloud.
Please note: This interview will be recorded with your consent.
Thank you for your participation.
Assoc. Prof. Dr Gülten ŞENDUR
Appendix 3: Interview transcripts of the pre-service chemistry teachers categorized into three different levels (high, moderate and low) according to their SAQs diagram scores (English and Turkish)
| Turkish | English |
|---|---|
| *R: researcher. **PST-1: pre-service chemistry teacher at a high level. ***PST-4: pre-service chemistry teacher at a low level. ****PST-9: pre-service chemistry teacher at a moderate level. | |
| A: Merhaba…(kimya öğretmen adayının ismi), görüşme protokolünü okudun ama tekrar bazı açıklamalarda bulunmak istiyorum. Senden istediğim bu diyagramı adım adım nasıl düşünerek tamamladığını açıklaman. Yani diyagrama nereden başladın? Tepkimeleri nasıl tanımladın? Nelere odakladın? Tepkimelerin türü ne? Bunları sesli olarak düşünmeni ve ifade etmeni istiyorum. | *R: Hello… (pre-service chemistry teacher's name), you read the interview protocol but I would like to repeat some of the instructions to you. What I want from you is to explain to me, step by step, how you thought through the diagram and completed it. In other words, where did you start the diagram? How did you describe the reactions? What did you focus on? What kinds of reactions were they? I’d like you to think aloud and express your thoughts to me. |
| ÖA-1: Ben diyagrama benzenden başladım ve yukarıya doğru gittim. Baktım açil klorür ve alüminyum klorür var. Bu Friedel–Crafts açilleme tepkimesi dedim ve ketonu oluşurdum. Sonra baktım etilbenzen oluşmuş. O zaman dedim keton indirgenecek. Keton indirgenmesi için tepkime olarak Clemmensen aklıma geldi. Asidik ortamda çinko ve civa oluyordu. Sonra devam ettim. Etilbenzen, KMnO4‘lü ortamda tepkimeye sokulmuş. O zaman dedim yükseltgenme ve benzoik asit oluşuyor, onu yazdım. Sonra, benzenin alt kısmındaki tepkimelere geçtim. Benzeni alkilledim. Bunun için, Friede-Crafts alkillemesini düşündüm. Bu tepkime, alüminyum klorür katalizörlüğü ve metil klorür ile gerçekleşti ve metilbenzen oluştu. Metilbenzenden, benzoik asit oluşumunu ise etilbenzende ki gibi yükseltgenme dedim ve aynı maddeleri yazdım. | **PST-1: I started off the diagram with benzene and went upwards. I saw that there were acyl chloride and aluminum chloride. I said that this was a Friedel–Crafts acylation reaction and so I formed the ketone. Then I saw that ethylbenzene had formed. Then I said the ketone would be reduced. I remembered Clemmensen reduction for ketone reduction. Zinc and mercury exist in acidic condition. Then I continued. Ethylbenzene was put into a reaction in the presence of KMnO4. Then I said oxidation and benzoic acid form so I wrote these down. Then I went on to the reactions below the benzene. I alkylated benzene. For this, I thought of Friedel–Crafts alkylation. This was a reaction created from methyl chloride and the catalyst aluminum chloride, which produced methylbenzene. I said the formation of benzoic acid from methylbenzene was oxidation just as in ethylbenzene and so I wrote the same substances. |
| A: Neden yükseltgenme dedin? Neye göre dedin? Açıklar mısın? | R: Why did you say oxidation? What did you base this on? Could you please explain this for us? |
| ÖA-1: …Hmmm. Şey vardı… Benzilik hidrojeni olan alkil benzenler KMnO4’lü ortamda yükseltgenerek, benzoik asit oluşuyordu. Burada da etilbenzende olduğu metilbenzende de benzilik hidrojen var o zaman aynı tepkime yani yükseltgenme dedim. | PST-1: …Hmmm. Well, there was… Alkyl benzenes with benzylic hydrogen oxidize in the condition with KMnO4 and benzoic acid is formed. As in ethylbenzene, methylbenzene contains benzylic hydrogen so I said it was the same reaction and called it oxidation. |
| A: Anladım. Devam edebilirsin. | R: I see. Please go on. |
| ÖA-1: Sonra, diyagramın iç kısmına geçtim. Baktım nitrobenzen oluşmuş. Benzenin nitrolanması dedim, Bunun için nitrik asit ve sülfürik asit kullanıyordu. Sonra buradan devam ettim. Bromlanma var dedim. Ama burada aklıma yönlendirici gruplar geldi hemen. NO2 meta yönlendiriciydi. | PST-1: Then, I went on to the inner part of the diagram. I saw that nitrobenzene had formed. I said this was the nitration of benzene and that's why it was using nitric acid and sulphuric acid. And I continued from there. I said that there was bromization. But then I suddenly remembered the directing groups. NO2 is a meta directing group. |
| A: Neden açıklayabilir misin? | R: Can you explain why? |
| ÖA-1: Şey, NO2 grubu halkadan elektron çekiyor o yüzden meta yönlendiricisi. | PST-1: Well, the NO2 group withdraws electrons from the ring and that's why it's meta directing. |
| A: Peki devam edebilirsin. | R: Ok, you can go on. |
ÖA-1: Bu yüzden ben bromu meta pozisyonuna bağladım. Sonra nitrobenzenin diğer tepkimesine geçtim buda indirgenme tepkimesiydi. Sn’den anladım. Nitrobenzen, aniline indirgendi. Böylelikle bu tarafı tamamladım ve benzenin sülfollanmasına geçtim. Burada sülfolanma dedim çünkü SO3 ve H2SO4 var. Benzensülfonik asiti oluşturdum. Sonrasında da okun yönünü fenole doğru yaptım. Çünkü bu tepkime fenol eldesi diye hatırlıyorum. Yani o şekilde aklımda kalmış özel bir tepkimeydi. Fenole doğru çizdim. Sonrasında da, NBS’li olan kısma geçtim. NBS, brom kaynağı idi ve yer değiştirme tepkimesi olacak etil grubu hidrojeni ile. Ama benzilik hidrojen kararlıydı; o yüzden, onunla bromu yer değiştirdim. Okun yönünü de Bromlu ürüne doğru yaptım. Sonrasında C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bağı içeren moleküle ve ürüne baktım, π bağı kopmuş ve Hidrojen ve Brom katılmış. O halde ikili bağa HBr katılması dedim ama Markovnikov kuralına uygun değil. Hidrojen atomu, hidrojeni az olan ikili bağ C atomuna katılmış dedim. O yüzden anti-Markovnikov olmalı bu yüzden peroksitli ortamda HBr yazdım. Sonra ise son tepkimeye geçtim. Burada, BH3 ve H2O2 ‘ye sahibiz. Bu hidroborasyon tepkimesi dedim. Açıkçası, bu tepkimede C C bağı içeren moleküle ve ürüne baktım, π bağı kopmuş ve Hidrojen ve Brom katılmış. O halde ikili bağa HBr katılması dedim ama Markovnikov kuralına uygun değil. Hidrojen atomu, hidrojeni az olan ikili bağ C atomuna katılmış dedim. O yüzden anti-Markovnikov olmalı bu yüzden peroksitli ortamda HBr yazdım. Sonra ise son tepkimeye geçtim. Burada, BH3 ve H2O2 ‘ye sahibiz. Bu hidroborasyon tepkimesi dedim. Açıkçası, bu tepkimede C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bağına su katılıyor, su anti-Markovnikova göre katılıyor. Yani, C C bağına su katılıyor, su anti-Markovnikova göre katılıyor. Yani, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bağında daha az hidrojeni olana hidrojeni bağladım ve –OH grubunu da diğer karbon atomuna bağladım. C bağında daha az hidrojeni olana hidrojeni bağladım ve –OH grubunu da diğer karbon atomuna bağladım. |
PST-1: That's why I bonded the bromine to a meta position. Then I went on to the other reaction of nitrobenzene and this was the reduction reaction. I understood this when I saw the Sn. Nitrobenzene was reduced to aniline. So I finished this side and went on to the sulfonation of benzene. I called this sulfonation because there was SO3 and H2SO4. I formed benzenesulfonic acid. Then I put the arrow in the direction of the phenol. Because I remembered this reaction as a synthesis of phenol. I mean, that was the special reaction that I remembered. I drew the arrow toward the phenol. Then I went on to the part with the NBS. NBS was the source of bromine and this was the substitution reaction with the hydrogen of the ethyl group. But benzylic hydrogen was stable, and that's why I substituted bromine for that. I drew the direction of the arrow toward the bromine product. Then I looked at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C molecule and the product, the π bond had broken off and hydrogen and bromine had been added on. Then I said that addition of HBr to the double bond but it wasn’t consistent with Markovnikov's rule. So I said that the hydrogen atom was added to the double bond C atom where there was less hydrogen. And I thought this is why this should fit the anti-Markovnikov rule and so I wrote HBr in the presence of peroxide. Then I came to the last reaction. Here we have BH3 and H2O2. I said this was a hydroboration reaction. Actually, there's the addition of water to the C C molecule and the product, the π bond had broken off and hydrogen and bromine had been added on. Then I said that addition of HBr to the double bond but it wasn’t consistent with Markovnikov's rule. So I said that the hydrogen atom was added to the double bond C atom where there was less hydrogen. And I thought this is why this should fit the anti-Markovnikov rule and so I wrote HBr in the presence of peroxide. Then I came to the last reaction. Here we have BH3 and H2O2. I said this was a hydroboration reaction. Actually, there's the addition of water to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, but in this reaction, water was added according to anti-Markovnikov. That is, in the C C bond, but in this reaction, water was added according to anti-Markovnikov. That is, in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, I attached hydrogen to the one with less hydrogen and I attached the –OH group to the other carbon atom. C bond, I attached hydrogen to the one with less hydrogen and I attached the –OH group to the other carbon atom. |
| A: H2O2’nin bu tepkimedeki fonksiyonu nedir? Açıklayabilir misin? | R: What is the function of H2O2 in this reaction? Can you explain? |
| ÖA-1: Önce, tepkimede BH3’ün bir tepkimesi var. Boranlı bir yapı oluşturuluyor. Sonrasında ise, H2O2 ile yükseltgenme oluyor. | PST-1: Firstly, there is a reaction of BH3 in the reaction. It forms a structure with borane. Then oxidation occurs with H2O2. |
| A: Peki, diyagram üzerinde yeni iki tepkime göstermeni istemiştim. Bir tane göstermişsin sanırım. Bu tepkimeyi açıklar mısın? | R: All right, I’d asked you to show me two new reactions in the diagram. I think you just showed me one. Could you please explain this reaction? |
| ÖA-1: İşin açıkçası ben sadece 1 tane yazabildim. Başka da göremedim. Oda benzenden, etilbenzen eldesi. Friedel–Crafts alkillemesi. Etil klorürü, AlCl3 katalizörlüğünde tepkimeye soktum. | PST-1: I’m afraid I could only write one down. I couldn’t see any other. This was a synthesis of ethylbenzene from benzene. Friedel–Crafts alkylation. I put the ethylchloride into the reaction where the catalyzer was AlCl3. |
| A: Merhaba… (öğretmen adayının ismi), görüşme protokolünü okudun ancak bazı açıklamalar yapmak istiyorum. Senden istediğim bu diyagramı adım adım nasıl düşünerek tamamladığını açıklaman. Yani diyagrama nereden başladın? Tepkimeleri nasıl tanımladın? Nelere dikkate ettin? Tepkimelerin türü ne? Bunları sesli olarak düşünüp, ifade etmeni istiyorum. | R: Hello… (pre-service teacher's name), you read the interview protocol but I would like to repeat some of the instructions to you. What I want from you is to explain to me, step by step, how you thought through the diagram and completed it. In other words, where did you start the diagram? How did you describe the reactions? What did you pay attention to? What kinds of reactions were they? I’d like you to think aloud and express your thoughts to me. |
| ÖA-4: Diyagrama baktığımda aslında benzenden başlamam lazım sanırım dedim. Ama ben bu tepkimeleri hatırlayamadım. | ***PST-4: When I look at the diagram, I think I should really start with benzene. But I couldn’t remember these reactions. |
| A: Hangi tepkimeleri açıklar mısın? | R: Which reactions? Can you explain? |
| ÖA-4: AlCl3 olan tepkime sonrasında etilbenzenin sentezlendiği tepkimeyi bilemedim. | PST-4: I couldn’t figure out the reaction after the AlCl3 reaction where ethylbenzene was synthesized. |
| A: Peki devam et bakalım. | R: OK, please continue. |
| ÖA-4: Sonra devam ettim. Potasyum permanganat ve hidroksil var burada. Bu hidroksil grubu olduğu için, bundan alkol elde edebilirim dedim ve hidroksil grubunun etil grubunun yanındaki karbon atomuna bağladım. Bu şekilde alkol oluştu. | PST-4: So I went on. There are potassium permanganate and hydroxyl here. Since this is the hydroxyl group, I said I could get alcohol from this and I attached the hydroxyl to the carbon atom next to the ethyl group. This way, alcohol was formed. |
| A: Tepkime türü olarak bunu açıklayabilir misin? | R: Could you explain this in terms of type of reaction? |
| ÖA-4: … Bilmiyorum fakat onları birbirine bağladım çünkü hidroksil grubunu gördüm. | PST-4: … I don’t know but I bonded them together because I saw the hydroxyl group. |
| A: Peki kaldığın yerden devam edebilirsin | R: OK, you can continue from where you left off. |
| ÖA-4: Diyagramın altındaki tepkimeleri de bilemedim. Yani metilbenzenin oluşumunda ortamda ne olmalı, sonrasında gene ortamda ne olmalı bilemedim. Bu yüzden diyagramın iç kısmındaki tepkimelere geçiş yaptım. Tepkimede (Sülfolanma tepkimesini göstererek) hidrojen sülfür ve ayrıca alken var. Bu yüzden alkol oluşturdum. Yani, hidroksil grubunu halkaya bağladım. | PST-4: And I couldn’t figure out the reactions at the bottom of the diagram. In other words, what should be in the environment in the formation of methylbenzene, and then what should be in the environment afterwards. That's why I passed on to the reactions in the inside part of the diagram. There is hydrogen sulphide in the reaction and also alkene (indicating the sulfonation reaction). That's why I created alcohol. In other words, I attached the hydroxyl group to the ring. |
| A: Alken ile hidrojen sülfür arasında alkol oluşumunu sağlayan ne tür bir tepkime var? Bunu bize açıklayabilir misin? | R: What kind of reaction was there between the alkene and hydrogen sulphide to produce alcohol? Could you please explain this for us? |
| ÖA-4: Şey. Benzen bir alkendir ve hidrojen sülfür varlığında, su eklendiğinde alkol elde edilir. Şu an tepkime türünün tam adını hatırlayamıyorum. | PST-4: Well, benzene is an alkene, and in the presence of hydrogen sulphide, alcohol is obtained when water is added. I can’t remember the exact name of the type of reaction right now. |
| A: Peki devam edebilirsin. | R: OK, you can go on. |
| ÖA-4: Sonrasında okun yönünü yukarı doğru çizdim. | PST-4: Then I drew the direction of the arrow upwards. |
| A: Peki bu iki madde aynı değil mi? Bu tepkime nasıl oldu? Okun yönü neden öyle? | R: OK, but aren’t these two substances the same? How did this reaction come about? Why is the arrow going in that direction? |
| ÖA-4: … Hmm evet. Bilemiyorum. | PST-4: …Hmm, you’re right. I don’t really know. |
| A: Peki devam edebilirsin. | R: OK, you can go on. |
| ÖA-4: Sonra tekrar benzene döndüm, NO2 grubu bağlanmış o halde ben de NO2 grubu ile tepkimeye girer dedim ve onu reaktif olarak yazdım. Sonrasında bromlama tepkimesi vardı. Bromu bağladım. Bromu bağlarken meta pozisyonuna bağladım çünkü NO2 grubu meta yönlendiricisiydi. Kalay ile olan tepkimeyi bilmedim. O yüzden geçtim. Sonrasında etilbenzenin oluştuğu tepkimeye geçiş yaptım. Bir önceki tepkimeye baktım. Alkol olmalı dedim çünkü okun üstünde hidroksil iyonu var. Sonra CCl4, alkolden uzaklaştırıldığında alkolün yok olduğunu hatırladım. Yani, alkil yapısına geçiş var dedim ve böylelikle etilbenzeni elde ettim. Bu yüzden de, oku etilbenzen yönüne koydum. | PST-4: Then I went back to the benzene. The NO2 group was attached so I said a reaction would be caused with the NO2 group and I put that down as a reagent. Then there was a bromization reaction. I attached the bromine. When attaching the bromine, I attached it to the meta position because the NO2 group is meta directing. I couldn’t figure out the reaction with tin. That's why I skipped this. Then I went on to the reaction where ethylbenzene formed. I looked at the previous reaction. I said that there should be alcohol here because there is a hydroxyl ion on top of the arrow. I then remembered that when CCl4 is removed from alcohol, the alcohol disappears. In other words, I said, there was a transition into the alkyl structure and so I obtained ethylbenzene. That's why I put the arrow in the direction of the ethylbenzene. |
| A: Peki, tepkimenin türü hakkında bize ne söyleyebilirsin? | R: So what can you tell us about the type of reaction? |
| ÖA-4:…Eliminasyon tepkimesi olduğunu düşünüyorum. | PST-4: …I think this was an elimination reaction. |
| A: Tepkimelere kaldığın yerden devam edebilirsin. | R: OK, you can continue with the reactions from where you left off. |
ÖA-4: Sonrasında tekrar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bağına katılma olmuş dedim. Ürüne baktım. HBr katılmış dedim. Hidrojen bir karbon atomuna, brom diğer karbon atomuna katılmış. C bağına katılma olmuş dedim. Ürüne baktım. HBr katılmış dedim. Hidrojen bir karbon atomuna, brom diğer karbon atomuna katılmış. |
PST-4: Then I said that something else was added to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. I looked at the product. I said that HBr had been added. Hydrogen had been added to a carbon atom and bromine had been added to the other carbon atom. C bond. I looked at the product. I said that HBr had been added. Hydrogen had been added to a carbon atom and bromine had been added to the other carbon atom. |
| A: Peki bu katılma tepkimesini neye göre yaptın? | R: So what was the basis of your addition reaction? |
| ÖA-4: Markovnikov kuralına uygun oldu. Hidrojen bir karbon atomuna, brom diğer karbon atomuna katıldı. | PST-4: It was in line with Markovnikov's rule. Hydrogen was added to a carbon atom and the bromine was added to the other carbon atom. |
| A: Peki diğer tepkimeye geçecek olursan. | R: So, please go on to the other reaction. |
ÖA-4: Burada alkan yapısına girdim. Bunun için, BH3’deki hidrojen atomlarını C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bağına eklemek zorundaydım Böylece, karbon atomları arasındaki π bağı koptu. C bağına eklemek zorundaydım Böylece, karbon atomları arasındaki π bağı koptu. |
PST-4: I went into the alkane structure here. For this, I had to add hydrogen atoms in BH3 to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. So the π bond between the carbon atoms was broken. C bond. So the π bond between the carbon atoms was broken. |
| A: Tepkime türü ile ilgili olarak ne söyleyebilirsin? | R: What can you then say about the type of reaction? |
| ÖA-4: Hidrojenin, bir çifte bağa katıldığı bir tepkime olduğunu söyleyebilirim. | PST-4: I would say that this is the reaction where hydrogen is added to a double-bond. |
| A: Diyagram üzerinde iki yeni tepkime göstermeni istemiştim. Bunlar ile ilgili ne söyleyebilirsin? | R: I’d asked you to show me two new reactions in the diagram. What can you say about those? |
| ÖA-4:…Hmm… ben bu tepkimeleri bulamadım. Baya zor geldi. | PST-4: …Hmm… I couldn’t find these reactions. That was hard for me. |
| A: Merhaba… (öğretmen adayının ismi), görüşme protokolünü okudun ancak bazı açıklamaları tekrar yapmak istiyorum. Anlaşılmayan bir nokta kalmasın diye. Senden istediğim bu diyagramı adım adım nasıl düşünerek tamamladığını açıklaman. Yani diyagrama nereden başladın? Nelere dikkat ettin? Tepkimelerin türü ne? Tepkimeleri nasıl tanımladın? Bunları sesli olarak düşünüp, ifade etmeni istiyorum. | R: Hello… (pre-service teacher's name), you read the interview protocol but I would like to repeat some of the instructions to you. Just so everything is understood. What I want from you is to explain to me, step by step, how you thought through the diagram and completed it. In other words, where did you start the diagram? What did you pay attention to? What kinds of reactions were they? How did you describe the reactions? I’d like you to think aloud and express your thoughts to me. |
| ÖA-9: Ben benzenden başlayarak diyagramın yukarı kısmını tamamladım öncelikle. Yani benzen var AlCl3 ‘lü tepkime Friedel–Crafts açilleme dedim ve keton oluşturdum. Ketondandan sonra, etilbenzenli yapıya geçiş için indirgenme olmalı dedim. Bunun için çinko, civa ve hidroklorik asitli ortamda indirgenme tepkimesini yazdım. Sonrada ise KMnO4’lü tepkimede şeyyy… dedim. Yükseltgenme tepkimesi ve benzoik asiti oluşturdum. | ****PST-9: I first started from the benzene and completed the upper part of the diagram. In other words, there was benzene so I said the AlCl3 reaction was a Friedel–Crafts acylation reaction and I formed the ketone. After the ketone, I said there had to be reduction for it to turn into a structure with ethylbenzene. For this I wrote down a reduction reaction in the presence of zinc, mercury and hydrochloric acid. Then I called the KMnO4’ reaction hmmm… It was an oxidizing reaction and so I formed benzoic acid. |
| A: Peki bu tepkimeyi nasıl belirledin ve neden ürün olarak olarak onu yazdın? | R: But how did you identify this reaction and why did you write it down as the product? |
| ÖA-9: KMnO4’ü gördüğümde yükseltgenme dedim. Etilbenzen yükseltgendiğinde benzoik asit oluşur diye düşündüm. | PST-9: When I saw the KMnO4 I said this was oxidation. I thought that when ethylbenzene was oxidized, benzoic acid formed |
| A: Peki kaldığın yerden devam edebilirsin. | R: OK, you can continue from where you left off. |
| ÖA-9: Sonra tekrar benzene döndüm ama benzenden, metilbenzen eldesi için hangi maddeler gerekli bilemedim. Aynı şekilde metilbenzenden de benzoik asit eldesinin yazamadım. | PST-9: Then I went back to the benzene but I didn’t know which substances were needed to obtain methylbenzene from benzene. In the same way, I couldn’t write down how to obtain benzoic acid from methylbenzene. |
| A: Tepkime türü olarak bir şey diyebilecek misin? Metilbenzenden benzoik asit eldesi için peki? | R: Can you say something about the kind of reaction? About synthesis of benzoic acid from methylbenzene? |
| ÖA-9: Bilemiyorum. | PST-9: I don’t know. |
| A: Peki devam edebilirsin. | R: OK, you can go on. |
| ÖA-9: Sonrasında, H2SO4’lü tepkimeye geçtim. Bu benzenin sülfolanması. Bu nedenle, SO4 grubunu halkaya bağladım. | PST-9: I then went on to the reaction with H2SO4. This was the sulfonation of benzene. That's why I attached the SO4 group to the ring |
| A: Neden SO4 grubu? Açıklayabilir misin? | R: Why the SO4 group? Can you explain? |
| ÖA-9: Çünkü sülfürik asit ile olan tepkimeden oluşur. SO4 oradan geldi. | PST-9: Because it forms a reaction with sulphuric acid. So SO4 came from there. |
| A: Peki devam edebilirsin. | R: OK, you can go on. |
| ÖA-9: Sonra okun yönünü çizdim OH’lı yapıya doğru ama tepkimeyi tam bilemedim. Tam emin değilim. Ardından nitrobenzene geçtim, benzenin nitrolanma tepkimesi dedim ve HNO3/H2SO4’ ü yazdım. Baktım ardından bromlama tepkimesi gelmiş halkaya brom bağladım. | PST-9: Then I drew the direction of the arrow toward the structure with OH but I couldn’t know the reaction exactly. I’m not really sure. Then I moved on to the nitrobenzene and said this was the nitration of benzene so I wrote HNO3/H2SO4. Then I saw that the bromization reaction came next so I bonded it to the ring |
| A: Bromu neden o poziyona bağladın? Açıklayabilir misin? | R: Why did you tie the bromine to that position? Can you explain? |
| ÖA-9: Nitrodan dolayı. Çünkü o metaya yönlendiriyor. | PST-9: Because of the nitro. It directs it to the meta. |
| A: Peki devam edebilirsin. | R: OK, you can go on. |
| ÖA-9: Sonrasında da SnCl2’li tepkimeye geçtim. Bu tepkime indirgenme diye hatırlıyorum. NO2 grubu NH2 grubuna indirgendi. Ardından etilbenzene döndüm. NBS’yi görünce yan zincir bromlanması dedim ve bromu hidrojen atomu ile yer değiştirdim. | PST-9: Then I moved on to the SnCl2 reaction. I remembered that this was a reduction reaction. The NO2 was reduced to an NH2 group. Then I turned to the ethylbenzene. When I saw the NBS, I said this was a side-chain bromination and so I exchanged the bromine and the hydrogen atom. |
| A: Neden bromu oraya bağladın? Açıklar mısın? | R: Why did you bond the bromine there? Could you please explain this for us? |
| ÖA-9: Orası daha kararlı çünkü diğer karbon atomu o kadar kararlı değil | PST-9: That position was more stable and the other carbon atom was not that stable. |
| A: Peki devam edebilirsin. | R: Ok, you can go on. |
ÖA-9: Okun yönünü de o ürün olan bromlu yapıya doğru çizdim. Sonra C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bağına baktım brom katılmış dedim. AlBr3’ü yazdım. C bağına baktım brom katılmış dedim. AlBr3’ü yazdım. |
PST-9: I drew the direction of the arrow toward the structure with the bromine. Then I looked at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and said that the bromine had been added. I wrote down AlBr3. C bond and said that the bromine had been added. I wrote down AlBr3. |
| A: Peki AlBr3’ü neden kullandın? Açıklayabilir misin? | R: So why did you use the AlBr3? Can you explain? |
| ÖA-9: Brom kaynağı ondan | PST-9: It's the source of the bromine, that's why. |
| A: Peki, ikili bağ karbon atomlarına neler bağlandı açıklayabilir misin? | R: So, could you tell us what were bonded to the double-bond carbon atoms? |
| ÖA-9: Baktığımda bir brom bağlı, AlBr3;’ten geliyor; diğerinde hidrojen bağlanmış…Hmm. O nerden geldi? Bilemiyorum | PST-9: I saw that one bromine was bonded to it, deriving from AlBr3; the other was the hydrogen…Hmmm. Where did that come from? I don’t know. |
| A: Peki, devam et öyleyse. | R: OK, so go on. |
| ÖA-9: Ardından diğer tepkime. Biliyorum bunu BH3’lü olan hidroborasyon tepkimesiydi. İkili bağa su katılıyordu. Hatta anti-Markovnikova göre, Hidrojen hidrojeni az olan ikili bağ karbon atomuna, OH hidrojeni çok olana katıldı | PST-9: And then I went to the other reaction. I knew this. This was the hydroboration reaction with BH3’. Water was being added to the double-bond. In fact, according to anti-Markovnikov, hydrogen was added to the double-bond carbon atom with less hydrogen, whereas the OH was added to one with more hydrogen. |
| A: Peki aynı zamanda diyagram üzerinde iki yeni tepkime göstermenizi istemiştim. Sen bir tane göstermişsin. Açıklayabilir misin? | R: All right, I’d also asked you to show me two new reactions in the diagram. You have shown me one. Can you explain? |
| ÖA-9: Onu şöyle yaptım. Burada etilbenzen, benzenden de oluşturulabilir dedim. Bu yüzden, tepkime yönünü etilbenzene doğru yönlendirdim. | PST-9: This is what I did. I said here that ethylbenzene could also be formed from benzene. That's why I made the direction of the reaction go towards ethylbenzene. |
| A: Öyleyse, bu tepkime için reaktifi nasıl seçtin? Sebebin neydi? | R: Then how did you choose the reagent for this reaction? What was your reasoning? |
| ÖA-9: Gerçeği söylemek gerekirse, etil grubu üründe ise, aynısının girişte de olması gerektiğini düşündüm. Bu yüzden tepkimeye etili koydum. | PST-9: To tell the truth, I thought if the ethyl group is in this product, then the input must be the same too. That's why I put ethyl into the reaction. |
| A: Öyleyse, tepkimenin türünün ne olduğu ile ilgili ne söyleyebilirsin? | R: Then what can you say about what type of reaction this is? |
| ÖA-9:…Yani …. Gerçekten söyleyemedim. | PST-9: … Well, I couldn’t really say. |
Appendix 4: English version of the consent form
References
- Akkuzu N. and Uyulgan M. A., (2016), An epistemological inquiry into organic chemistry education: exploration of undergraduate students’ conceptual understanding of functional groups, Chem. Educ. Res. Pract., 17(1), 36–57.
- Anderson T. L. and Bodner G. M., (2008), What can we do about Parker? A case study of a good student who didn’t “get” organic chemistry, Chem. Educ. Res. Pract., 9(2), 93–101.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students’ ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16(4), 797–810.
- Austin A. C., Ben-Daat H., Zhu, M., Atkinson R., Barrows N. and Gould I. R., (2015), Measuring student performance in general organic chemistry, Chem. Educ. Res. Pract., 16(1), 168–178.
- Ausubel D. P., (1968), Educational psychology: a cognitive view, New York: Holt, Rinehart and Winston.
- Balaban A. T., Oniciu D. C. and Katritzky A. R., (2004), Aromaticity as a cornerstone of heterocyclic chemistry, Chem. Rev., 104(5), 2777–2812.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), It gets me to the product: how students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63(10), 873–878.
- Bussey T. J., Orgill M. and Crippen K. J., (2013), Variation theory: a theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14(1), 9–22.
- Caspari I., Weinrich M. L., Sevian H. and Graulich N., (2018), This mechanistic step is “productive”: organic chemistry students’ backward-oriented reasoning, Chem. Educ. Res. Pract., 19(1), 42–59.
- Cavallo A. M. L., (1996), Meaningful learning, reasoning ability, and students’ understanding and problem solving of topics in genetics, J. Res. Sci. Teach., 33(6), 625–656.
- Cruz-Ramirez de Arellano D. and Towns M., (2014), Students understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15(4), 501–515.
- Duffy A. M., (2006), Students’ ways of understanding aromaticity and electrophilic aromatic substitution reactions, Doctoral dissertation, San Diego: University of California.
- Ealy J., (2018), Analysis of students’ missed organic chemistry quiz questions that stress the importance of prior general chemistry knowledge, Educ. Sci., 8(2), 42, DOI:10.3390/educsci8020042.
- Ealy J. and Hermanson J., (2006), Molecular images in organic chemistry, J. Sci. Educ. Technol., 15(1). 59–68.
- Eticha A. T. and Ochonogor C. E., (2015), Assessment of Undergraduate Chemistry Students’ Difficulties in Organic Chemistry, ISTE International Conference Proceedings, Unisa Press, retrieved from http://uir.unisa.ac.za/handle/10500/19962?show=full.
- Fahmy A. F. M. and Lagowski J. J., (1999), The use of a systemic approach in teaching and learning chemistry for the 21st century, Pure Appl. Chem., 71(5), 859–863, retrieved from http://media.iupac.org/publications/pac/1999/pdf/7105x0859.pdf.
- Fahmy A. F. M. and Lagowski J. J., (2003), Systemic reform in chemical education: an international perspective, J. Chem. Educ., 80(9), 1078–1083.
- Fahmy A. F. M. and Lagowski J. J., (2011), The systemic approach to teaching and learning (SATL): a 10 year review, Afr. J. Chem. Educ., 1(1), 29–47.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113.
- Fleiss J. L. and Levin B. L., (1981), Statistical methods for rates and proportions, New York: Wiley.
- Flynn A. B., (2015), Structure and evaluation of flipped chemistry courses: organic & spectroscopy, large and small, first to third year, English and French, Chem. Educ. Res. Pract., 16(2), 198–211.
- Fraenkel J. R. and Wallen N. E., (2006), How to design and evaluate research in education, 5th edn, New York: McGraw-Hill Publishing.
- Friesen J. B., (2008), Saying what you mean: teaching mechanisms in organic chemistry, J. Chem. Educ., 85(11), 1515–1518.
- Fyrenius A., Bergdahl B. and Silen C., (2005), Lectures in problem-based learning—Why, when and how? An example of interactive lecturing that stimulates meaningful learning, Med. Teach., 27(1), 61–65.
- Galloway K. R., Stoyanovich C. and Flynn A. B., (2017), Students’ interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18(2), 353–374.
- Galloway K. R., Leung M. W. and Flynn A. B., (2018), Patterns of reactions: a card sort task to investigate students’ organization of organic chemistry reactions, Chem. Educ. Res. Pract., 20(1), 30–52.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Graulich N. and Bhattacharyya G., (2017), Investigating students’ similarity judgments in organic chemistry, Chem. Educ. Res. Pract., 18(4), 774–784.
- Grove N. P., Cooper M. M. and Rush K. M., (2012), Decorating with arrows: toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Hodges L. C. and Harvey L. C., (2003), Evaluation of student learning in organic chemistry using the solo taxonomy, J. Chem. Educ., 80(7), 785–787.
- Hrin T. N., Milenković D. D. and Segedinac M. D., (2016a), The effect of systemic synthesis questions [SSynQs] on students’ performance and meaningful learning in secondary organic chemistry teaching, Int. J. Sci. Math. Educ., 14(5), 805–824.
- Hrin T. N., Fahmy A. F. M., Segedinac M. D. and Milenković D. D., (2016b), Systemic synthesis questions [SSynQs] as tools to help students to build their cognitive structures in a systemic manner, Res. Sci. Educ., 46(4), 525–546.
- Hrin T. N., Milenković D. D., Segedinac M. D. and Horvat S., (2016c), Enhancement and assessment of students’ systems thinking skills by application of systemic synthesis questions in the organic chemistry course, J. Serb. Chem. Soc., 81(12), 1455–1471.
- Hrin T. N., Milenković D. D., Segedinac M. D. and Horvat S., (2017), Systems thinking in chemistry classroom: the influence of systemic synthesis questions on its development and assessment, Think. Skills Creat., 23, 175–187.
- Hrin T. N., Milenković D. D. and Segedinac M. D., (2018), Diagnosing the quality of high school students’ and pre-service chemistry teachers’ cognitive structures in organic chemistry by using students’ generated systemic synthesis questions, Chem. Educ. Res. Pract., 19(1), 305–318.
- Krygowski T. M. and Cyranski M. K., (2001), Structural aspects of aromaticity, Chem. Rev., 101(5), 1385–1419.
- Lodico M. G., Spaulding D. T. and Voegtle K. H., (2010), Methods in Educational Research From Theory to Practice, 2nd edn, San Francisco: Wiley.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis: an expanded sourcebook, London: Sage.
- Nakhleh M. B. and Mitchell R. C., (1993), Concept learning versus problem solving: there is a difference, J. Chem. Educ., 70(3), 190–192.
- Nieswandt M. and Bellomo K., (2009), Written extended-response questions as classroom assessment tools for meaningful understanding of evolutionary theory, J. Res. Sci. Teach., 46(3), 333–356.
- Novak J. D., (2002), Meaningful learning: the essential factor for conceptual change in limited or inappropriate propositional hierarchies leading to empowerment of learners, Sci. Educ., 86(4), 548–571.
- Novak J. D., Mintzes J. J. and Wandersee J. H., (2000), Epilogue: on ways of assessing science understanding, in Mintzes J. J., Wandersee J. H. and Novak J. D. (ed.), Assessing science understanding. A human constructivist view, San Diego: Academic Press, pp. 355–374.
- Orgill M., (2007), Phenomenography, in Bodner G. M. and Orgill M. (ed.), Theoretical frameworks for research in chemistry/science education, Upper Saddle River, NJ: Pearson, pp. 132–151.
- Orgill M. and Bodner G., (2004), What research tells us about using analogies to teach chemistry, Chem. Educ. Res. Pract., 5(1), 15–32.
- Patton M. Q., (1987), How to use qualitative methods in evaluation, Newbury Park, CA: Sage.
- Potgieter M. and Davidowitz B., (2011), Preparedness for tertiary chemistry: multiple applications of the chemistry competence test for diagnostic and prediction purposes, Chem. Educ. Res. Pract., 12(2), 193–204.
- Raker J. R. and Towns M. H., (2012a), Designing undergraduate-level organic chemistry instructional problems: seven ideas from a problem-solving study of practicing synthetic organic chemists, Chem. Educ. Res. Pract., 13(3), 277–285.
- Raker J. R. and Towns M. H., (2012b), Problem types in synthetic organic chemistry research: implications for the development of curricular problems for second-year level organic chemistry instruction, Chem. Educ. Res. Pract., 13(3), 179–185.
- Rushton G., Hardy R. C., Gwaltney K. and Lewis S., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9(2), 122–130.
- Sloop J. C., Tsoi M. Y. and Coppock P., (2016), Benefits of using a problem-solving scaffold for teaching and learning synthesis in undergraduate organic chemistry, International Journal for the Scholarship of Teaching and Learning, 10(1), 8, DOI:10.20429/ijsotl.2016.100108.
- Southerland S. A., Smith M. U. and Cummins C. L., (2000), “What do you mean by that?” Using structured interviews to assess science understanding, in J. J. Mintzes, J. H. Wandersee and J. D. Novak (ed.), Assessing science understanding. A human constructivist view, San Diego: Academic Press, pp. 71–93.
- Şendur G., (2019), Investigation of pre-service science teachers’ learning in organic chemistry according to SOLO taxonomy: the case of aromatic compound reactions, Elementary Education Online, 18(2), 642–662.
- Şendur G. and Toprak M., (2013a), The role of conceptual change texts to improve students’ understanding of alkenes, Chem. Educ. Res. Pract., 14(4), 431–449.
- Şendur G. and Toprak M., (2013b), An analysis of prospective teachers’ understanding levels and misconceptions in the subjects of organic chemistry: the case of alcohols, Necatibey Faculty of Education Electronic Journal of Science and Mathematics Education, 7(1), 264–301.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving ‘human subjects’, Chem. Educ. Res. Pract., 15(2), 109–113.
- Taber K. S., (2018), Lost and found in translation: guidelines for reporting research data in an ‘other’ language, Chem. Educ. Res. Pract.,19(3), 646–652.
- Tzougraki C., Salta K. and Vachliotis T., (2014), Development and evaluation of a systemic assessment framework in organic chemistry, Afr. J. Chem. Educ, 4(2), 101–121.
- Vachliotis T., Salta K., Vasiliou P. and Tzougraki C., (2011), Exploring novel tools for assessing high school students’ meaningful understanding of organic reactions, J. Chem. Educ., 88(3), 337–345.
- Vachliotis T., Salta K. and Tzougraki C., (2014), Meaningful understanding and systems thinking in organic chemistry: validating measurement and exploring relationships, Res. Sci. Educ., 44(2), 239–266.
- Weinrich M. L. and Sevian H., (2017), Capturing students’ abstraction while solving organic reaction mechanism problems across a semester, Chem. Educ. Res. Prac., 18(1), 169–190.
| This journal is © The Royal Society of Chemistry 2020 |