Plasma-assisted nitrogen fixation in water with various metals†
Abstract
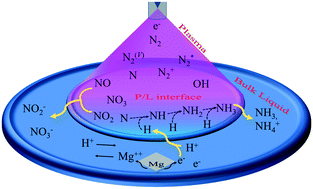
In this study, nitrogen fixation in water was achieved using an atmospheric pressure non-thermal nitrogen plasma jet generated with an AC driven source of a 10 kV (peak) power supply with a repetition frequency of 33 kHz. Plasma has been proposed for the excitation of nitrogen gas, which can be readily converted to ammonia, nitrite, nitrate, and hydrogen ions in plasma-activated water. In addition, some easily accessible metals such as Mg, Al, Zn, and Cu were immersed separately in the water. These immersed metals oxidized in the plasma-activated water and the released electron reduced the hydrogen ion into a hydrogen atom. The evolved hydrogen again reduced the plasma-excited nitrogen into ammonia. In our experiment, the pH value and ammonia synthesis rate were higher with Mg owing to its higher oxidation capacity than other metals. The reduction of hydrogen ions with the help of metals not only controls the pH of PAW but also increases the ammonia synthesis rate by providing an additional hydrogen donor. Our studies provide a new direction for ongoing research in the field of sustainable nitrogen fixation.


 Please wait while we load your content...
Please wait while we load your content...