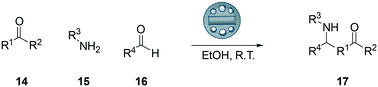Open Access Article
Open Access ArticleDesign and development of 3D printed catalytically-active stirrers for chemical synthesis†
Matthew R.
Penny
and
Stephen T.
Hilton
 *
*
UCL School of Pharmacy, 29-39 Brunswick Square, London WC1N 1AX, UK. E-mail: s.hilton@ucl.ac.uk
First published on 19th March 2020
Abstract
In this present study, we describe the novel design, preparation and evaluation of catalyst-impregnated stirrer beads for chemical synthesis. Using a low-cost SLA 3D printer and freeware design software, a high surface area holder for a magnetic stirrer bead was developed and 3D printed containing p-toluenesulfonic acid. The devices were used to efficiently catalyze Mannich reactions in excellent yields and it was demonstrated that the devices can be re-used up to 5-times with excellent reproducibility.
Although conceptualized almost 30 years ago, three-dimensional (3D) printing has received growing attention due to its innovative use across scientific disciplines.1 Expiration of printer patents and reductions in the prices of 3D printers have helped boost its applications in non-engineering research. As such, rapid developments in 3D printing include bioprinting for tissue growth,2 the creating of microfluidic,3 and medical devices4 as well as pharmaceutics5 and bespoke laboratory equipment have taken place.6 All discoveries have been underpinned by the inherent nature of 3D printing which focuses on its ability to facilitate the iterative rapid prototyping of designs at low cost. Synthetic chemistry has benefited from 3D printing with the introduction of customized reactors for organic and inorganic synthesis. The ability to fabricate a reactor in a matter of hours, and easily modify if necessary, offers synthetic chemists greater control over optimization as well as the possibility for catalysis.7 Further benefits of 3D printing have come from increases in sensing technology and sensors in particular, where in-line analysis of reactions can readily be incorporated into designed flow paths.8 Due to the clear advantages and potential of 3D printing, recent research in the group has focused on the development of large bespoke molecular model kits for chemistry,9 low-cost devices for tissue slicing and crystallography,10 the printing of models to aid in the visualization of infection in dental models11 and low-cost bespoke polypropylene reactors for continuous flow chemistry as well as a novel low-cost continuous flow system designed to fit stirrer hotplates and a continuous flow electrochemistry system.12
As a result of our developments in 3D printing and its clear advantages, we decided to explore the potential of 3D printing to improve the catalysis of batch reactions in synthetic chemistry. We were particularly intrigued by the concept of developing a 3D printed device containing an impregnated catalyst, which could be simply removed from the reaction once complete. This could then be easily washed and reused for further reactions. This is clearly analogous to that of the immobilization of homogeneous and heterogeneous catalysts, which have provided clear benefits for chemical synthesis, due to the ease with which they can be removed from a flask post-reaction. This in turn, simplifies subsequent isolation and purification procedures.13 The synthetic utility of organocatalysts, particularly with respect to stereochemistry, has been reported extensively and their translation to solid support has already been described as well as reports of 3D printed variants.14
Despite their obvious potential, supported reagents have only been selectively used in synthetic chemistry as they still need to be weighed prior to their addition and use in chemical reactions, thus reducing their practical utility. We therefore sought to develop a new approach where the device containing the catalyst would be integral to the reaction itself (Fig. 1).
 | ||
| Fig. 1 Concept design of a catalytically active stirrer device. Left) Pictographic of a traditional batch reaction. Right) Initial design of a stirrer surrounding device. | ||
As such, we decided to explore batch reactions and how these could be catalyzed. One commonality across all batch reactions, is that they are mixed internally, either via an internal magnetic stirrer/follower or via externally powered reactor paddles. As such, we wanted to explore whether the stirrer/follower itself could be made to be catalytically active via the incorporation of a catalytically active surround. This would then enable facile catalysis of the reaction and provide ready reusability of the device. The device would therefore already form part of a normal synthetic reaction workflow with the clear and obvious advantage of removing the step of the weighing out and addition of a catalyst.
We envisaged that the device surrounding the stirrer/follower could be designed using computer aided design (CAD) software and printed using a 3D printer. Catalysts could be impregnated into the printed device in much the same way as colors are incorporated into colored plastics. Impregnating the input material of a 3D printer with a catalyst would allow us to not only generate complex objects but also provide facile control over the amount of catalyst in the device and its subsequent reaction. This would allow for incorporation of a range of catalysts which could be applied to a wide array of synthetic chemistry. We elected to apply this concept via stereolithography (SLA) printing, which offered greater accuracy and reproducibility than the more prevalent filament deposition modelling (FDM) printers and more importantly, it would also be simpler to combine a catalyst with a suitable photopolymerizable resin.
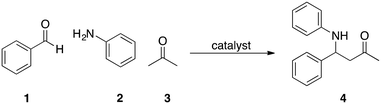
We now report on the results of our initial studies to develop catalytic devices for synthetic batch chemistry following our initial disclosure.15 We decided to first develop a device with para-toluene sulfonic acid (pTsOH) as catalyst for our initial investigations into this area of research as it has found widespread use in synthetic chemistry and would provide ready proof of principle of catalysis. In order to explore the initial efficacy of our pTsOH impregnated devices, it was decided to explore their utility in the Mannich reaction of benzaldehyde 1, aniline 2 and acetone 3 to give the addition product 4. The Mannich reaction has been widely reported in the literature with a plethora of catalysts, which facilitate efficient as well as diastereo- and stereoselective syntheses of the α-amino ketones.16 Solid supported pTsOH has previously been used in the Mannich reaction as well as impregnated into 3D printed cuvettes to catalyse the Mannich reaction and would therefore provide ready proof of principle of our device (Scheme 1).17
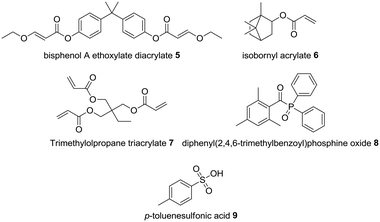
Given the proprietary nature of photopolymerizable resins typically used with commercial SLA printers, we developed our own resin formulation for use with the Formlabs Form 1+ 3D printer. A resin was required that would harden at the same rate as the commercial acrylate resin that would also well provide reasonable chemical resistance. Adapting a known formulation,18 bisphenol A ethoxylate diacrylate 5 (50%) was selected as the bifunctional oligomer and isobornyl acrylate 6 (33%) as monomer to aid solvation of the organocatalyst. Trimethylolpropane triacrylate 7 (15%) was used as crosslinker and diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide 8 as photoinitiator (2%) and pTSOH 9 as catalyst (5%) (Fig. 2).
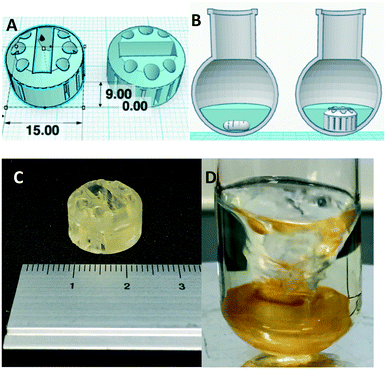
With a suitable resin formulation thus prepared, the efficacy was explored using a stirrer housed within solidified resin impregnated with 5% pTsOH. In a simple experiment, an ordinary magnetic stirrer/follower was placed inside a disposable syringe (5 mL), 0.8 mL of resin added and the mixture polymerized by exposure to ambient light. The solid cylindrical device obtained after removal of the syringe was then used to stir the Mannich reaction between benzaldehyde 1, aniline 2 and acetone 3 in water at room temperature, the results of which are shown (Fig. 3 and Table 1).
Pleasingly on completion of the reaction, the Mannich addition product was obtained in an excellent 81% yield, clearly demonstrating the potential of this approach. The reaction was also repeated in ethanol as solvent and cyclohexanone as the ketone as there was some degradation of the stirrer device in the water/acetone solvent mixture, which was attributed to the anticipated destructive swelling of acetone with 3D printed resins.7a The addition product from the reaction between cyclohexanone, aniline and benzaldehyde was again obtained in a good albeit slightly reduced yield (51%, Table 1, entry 2), which again clearly demonstrated the potential of the stirrer device to catalyse the Mannich reaction.
As the proof of concept had proven successful, attention turned towards the 3D printing of the stirrer device to catalyze the Mannich reaction. Cognizant of the fact that polymer supported reactions have a large surface area to promote reaction, we set about investigating alternative designs to our initial concept to increase the surface area/throughput over the device. Inspired by a commercially available overhead stirrer,19 we designed a small stirrer bead/follower holder with a high surface area that would also create turbulence when rotating, resulting in a high flow of reaction mixture over the surface of the stirrer. The web-based CAD freeware program Tinkercad was used to create an .stl (surface tessellation language) file for the device, which contains a central holder for a magnetic follower. It was designed with vertical columns and horizontal gaps to increase fluid turbidity in the reaction once it is rotating, which results in a high throughput over the device surface. The stirrer device was printed in the pTsOH resin using the Formlabs Form1+ SLA printer and a small magnetic follower secured inside and the design is shown (Fig. 4).
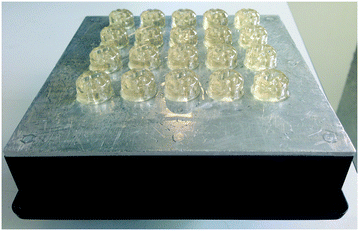
Once designed, the stirrer device was printed using the pre-prepared 5% containing pTsOH resin using a Formlabs Form1+ SLA printer with the stirrers printed directly on the build plate using no supports and a small magnetic follower was secured inside post printing (Fig. 5). Multiple copies of the stirrer were printed on the same build plate at 100-micron accuracy to provide sufficient quantities for subsequent reactions.
Following printing of the pTsOH containing large surface area 3D printed device, they were washed with IPA to remove excess resin and air dried prior to curing for a further 24 hours in ambient light. Their efficacy in catalyzing the Mannich reaction between cyclohexanone, aniline and benzaldehyde was explored by the reactions being carried out with 1.5 equivalents of cyclohexanone and 1 equivalent of both aniline 2 and benzaldehyde 1 and control reactions were carried out in conjunction with the initial experiment as shown (Scheme 2 and Table 2).
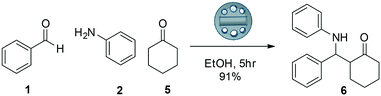 | ||
| Scheme 2 Mannich reaction between benzaldehyde 1, aniline 2 and cyclohexanone 5 catalysed by a pTsOH impregnated 3D printed device. | ||
When the 3D printed stirrer was used in the Mannich reaction with cyclohexanone 5, the reaction was complete in 5 hours and gave the product in an excellent 91% yield as a 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 diastereomeric ratio (syn/anti). The background reaction using a stirrer also gave the product in a reduced yield of 18%. Use of free added pTsOH also gave good yield of the product in 5 hours albeit in a slightly lower yield. With the promising results thus obtained, we next carried out a range of reactions to try to understand the scope of the pTsOH impregnated stirrer to catalyse the Mannich reaction between a range of ketones, aldehydes and anilines and the results are shown (Scheme 3, Table 3).
67 diastereomeric ratio (syn/anti). The background reaction using a stirrer also gave the product in a reduced yield of 18%. Use of free added pTsOH also gave good yield of the product in 5 hours albeit in a slightly lower yield. With the promising results thus obtained, we next carried out a range of reactions to try to understand the scope of the pTsOH impregnated stirrer to catalyse the Mannich reaction between a range of ketones, aldehydes and anilines and the results are shown (Scheme 3, Table 3).
| Entry | Ketone R1⋯R2 | Amine [R3] | Aldehyde [R4] | Product | Time [h] | Yield [%] | dr syn/anti | |
|---|---|---|---|---|---|---|---|---|
All reactions were carried out at room temperature in ethanol using a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ketone 1 ratio of ketone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) amine amine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aldehyde. aldehyde.
|
||||||||
| 1 | –(CH2)5– | Ph | Ph | 6 | 5 | 91 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
|
| 2 | –(CH2)5– | Ph | 4-NO2C6H4 | 18 | 5 | 89 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 64 |
|
| 3 | –(CH2)5– | Ph | 4-MeOC6H4 | 19 | 5 | 71 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
|
| 4 | –(CH2)5– | Ph | 4-FC6H4 | 20 | 5 | 85 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 68 |
|
| 5 | –(CH2)5– | Ph | 4-ClC6H4 | 21 | 5 | 70 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
|
| 6 | –(CH2)5– | 4-FC6H4 | Ph | 22 | 5 | 84 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 71 |
|
| 7 | –(CH2)5– | 4-CF3C6H4 | Ph | 23 | 5 | 60 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
|
| 8 | –(CH2)5– | 4-CF3OC6H4 | Ph | 24 | 5 | 87 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
|
| 9 | –(CH2)5– | 4-ClC6H4 | Ph | 25 | 5 | 72 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
|
| 10 | –(CH2)5– | 4-CF3OC6H4 | 4-NO2C6H4 | 26 | 5 | 91 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
|
| 11 | CH2 | Ph | Ph | Ph | 27 | 24 | 52 | N/A |
| 12 | CH2 | CH3 | Ph | Ph | 4 | 3 | 65 | N/A |
As can be seen in Table 3, all of the reactions carried out with the 5% pTsOH impregnated stirrer devices gave good to excellent yields of the Mannich addition products. Following the reaction, the stirrer device was simply removed from the reaction and washed with a little ethanol allowing facile removal of the device from the reaction clearly demonstrating the potential of this approach. In the case of the reaction with acetone (Table 3, entry 12) the reaction was complete after 3 hours and gave good yield of the addition product (65%). In the case of acetophenone (entry 11, Table 3), the Mannich reaction proved slower and was therefore left for 24 hours until all the starting material had been consumed giving the addition product in 52% yield. Electron donating and electron withdrawing groups were well tolerated on the aniline and the aldehyde, giving good yields for all reactions.
Following successful demonstration of the 3D printed catalytic stirrer device to generate Mannich adducts in good yield, attention turned towards investigations as to whether they could be used for sequential reactions. We were intrigued as to the nature of the printed stirrer containing pTsOH and whether pTsOH was being released from the printed object during the reaction or whether the reaction occurred on the acidic surface. As can be seen from the SEM image, the printed device is characterized by a contiguous surface and the printing striation layers can clearly be seen (Fig. 6). We estimated that there is approximately 0.065 g of catalyst per device, with up to 8% of this total mass of catalyst available for reaction (ESI†).17b
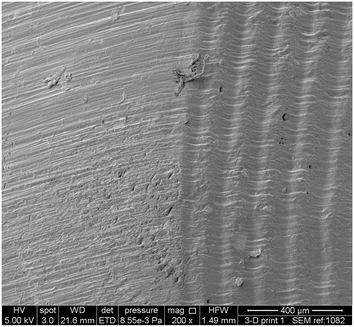 | ||
| Fig. 6 Scanning electron microscope image of the surface of the 3D printed pTsOH impregnated device showing the solid surface striation lines of 3D printing. | ||
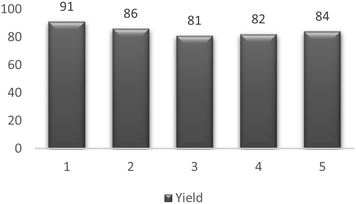
We reasoned that were pTsOH simply released from the device in the reaction, then subsequent reactions with the same device would lead to rapid reductions in yield as the printed surface lost pTsOH. Whereas, if the reaction occurs on the surface, then there should be little or no reduction in yield. As such, a stirrer bead containing pTsOH was printed and used for five subsequent reactions between cyclohexanone 5, aniline 2 and benzaldehyde 1 and the results are shown in the following table (Table 4).
Following the reactions shown above, it can be seen that there is a small reduction in yield between the first and second use of the 3D printed stirrer device but that it can be then used for a further 3 times with little loss of yield and as such, can be successfully used for a series of reactions. Whilst it appears there is a small amount of surface loss of pTsOH as evidenced from the reduction in yield from entry one to two, subsequent reactions show a stabilization in yield indicating that the reaction occurs on the surface of the reactor bead and is not simply a case of pTsOH leaching into the reaction. As such, it demonstrates the potential of these 3D printed devices to be used for multiple reactions.
Conclusions
In summary, we have developed a novel 3D printed stirrer bead holder with an impregnated organocatalyst that can be used in organic synthesis. The successful application of this stirrer to the synthetically useful Mannich reaction illustrates the utility of our approach. This, together with the reusability of the stirrer and operational simplicity, offers synthetic chemists the practical benefits of solid supported synthesis combined with the adaptability of 3D printing and fits within the traditional synthetic chemistry workflow. Further reaction exploration, catalyst scope and mechanistic insights will be reported in due course.Conflicts of interest
M. R. P. and S. T. H. are authors of a patent related to this research.15
Notes and references
- C. W. Hull, US4575330, 1986 CrossRef CAS PubMed; B. C. Gross, J. L. Erkal, S. Y. Lockwood, C. Chen and D. M. Spence, Anal. Chem., 2014, 86, 3240–3253 CrossRef CAS PubMed.
- (a) M. Nakamura, S. Iwagana, C. Henmi, K. Arai and Y. Nishiyama, Biofabrication, 2010, 2, 014110 CrossRef CAS PubMed; (b) B. Derby, Science, 2012, 338, 921–926 CrossRef CAS PubMed.
- (a) C. I. Rogers, K. Qaderi, A. T. Woolley and G. P. Nordin, Biomicrofluidics, 2015, 9, 016501 CrossRef PubMed; (b) G. Comina, A. Suska and D. Filippini, Lab Chip, 2013, 14, 424–430 RSC; (c) G. Comina, A. Suska and D. Filippini, Lab Chip, 2013, 14, 2978–2982 RSC; (d) K. G. Lee, K. J. Park, S. Seok, S. Shin, D. H. Kim, J. Y. Park, Y. S. Heo, S. J. Lee and T. J. Lee, RSC Adv., 2014, 4, 32876–32880 RSC; (e) A. H. Au, W. Huynh, L. F. Horowitz and A. Folch, Angew. Chem., Int. Ed., 2016, 55, 3862–3881 ( Angew. Chem. , 2016 , 128 , 3926–3946 ) CrossRef CAS PubMed; (f) C. M. B. Ho, S. H. Ng, K. H. H. Li and Y.-J. Yoon, Lab Chip, 2015, 15, 3627–3637 RSC.
- R. J. Morrison, S. J. Hollister, M. F. Nieder, M. G. Mahani, A. H. Park, D. K. Mehta, R. G. Ohye and G. E. Green, Sci. Transl. Med., 2015, 7, 285ra64 CrossRef PubMed.
- (a) A. Goyanes, A. B. M. Buanz, A. W. Basit and S. Gaisford, Int. J. Pharm., 2014, 476, 88–92 CrossRef CAS PubMed; (b) S. A. Khaled, J. C. Burley, M. R. Alexander, J. Yang and C. J. Roberts, Int. J. Pharm., 2015, 494, 643–650 CrossRef CAS PubMed; (c) A. Goyanes, P. R. Martinez, A. Buanz, A. W. Basit and S. Gaisford, Int. J. Pharm., 2015, 494, 657–663 CrossRef CAS PubMed; (d) B. Wijnen, E. J. Hunt, G. C. Anzalone and J. M. Pearce, PLoS One, 2014, 9, e107216 CrossRef PubMed.
- (a) J. M. Pearce, Science, 2012, 337, 1303–1304 CrossRef CAS PubMed; (b) T. H. Lücking, F. Sambale, S. Beutel and T. Scheper, Eng. Life Sci., 2015, 15, 51–56 CrossRef.
- (a) A. J. Capel, S. Edmondson, S. D. R. Christie, R. D. Goodridge, R. J. Bibb and M. Thurstans, Lab Chip, 2013, 13, 4583–4590 RSC; (b) V. Dragone, V. Sans, M. H. Rosnes, P. J. Kitson and L. Cronin, Beilstein J. Org. Chem., 2013, 9, 951–959 CrossRef CAS PubMed; (c) P. J. Kitson, R. J. Marshall, D. Long, R. S. Forgan and L. Cronin, Angew. Chem., Int. Ed., 2014, 53, 12723–12728 ( Angew. Chem. , 2014 , 126 , 12937–12942 ) CrossRef CAS PubMed; (d) P. J. Kitson, M. D. Symes, V. Dragone and L. Cronin, Chem. Sci., 2013, 4, 3099–3103 RSC; (e) P. J. Kitson, M. H. Rosnes, V. Sans, V. Draone and L. Cronin, Lab Chip, 2012, 12, 3267–3271 RSC; (f) A. Ambrosi and M. Pumera, Chem. Soc. Rev., 2016, 45, 2740–2755 RSC; (g) S. Rossi, R. Porta, D. Brenna, A. Puglisi and M. Benaglia, Angew. Chem., Int. Ed., 2017, 56, 4290–4294 ( Angew. Chem. , 2017 , 129 , 4354–4358 ) CrossRef CAS PubMed; (h) P. J. Kiton, S. Glatzel, W. Chen, C.-G. Lin, Y.-F. Song and L. Cronin, Nat. Protoc., 2016, 11, 920–936 CrossRef PubMed; (i) A. J. Capel, R. R. Rimington, M. P. Lewis and S. D. R. Christie, Nat. Rev. Chem., 2018, 2, 422–436 CrossRef.
- (a) V. Sans, L. Porwol, V. Dragone and L. Cronin, Chem. Sci., 2015, 6, 1258–1264 RSC; (b) J. S. Mathieson, M. H. Rosnes, V. Sans, P. J. Kitson and L. Cronin, Beilstein J. Nanotechnol., 2013, 4, 285–291 CrossRef PubMed; (c) M. D. Symes, P. J. Kitson, J. Yan, C. J. Richmond, G. J. T. Cooper, R. W. Bowman, T. Vilbrandt and L. Cronin, Nat. Chem., 2012, 4, 349–354 CrossRef CAS PubMed; (d) G. Scotti, A. M. E. Nilsson, M. Haapala, P. Pöhö, G. B. Gennäs, J. Yli-Kauhaluoma and T. Kotiaho, React. Chem. Eng., 2017, 2, 299–303 RSC; (e) A. J. Capel, A. Wright, M. J. Harding, G. W. Weaver, Y. Li, R. A. Harris, S. Edmonson, R. D. Goodridge and S. D. R. Christie, Beilstein J. Org. Chem., 2017, 13, 111–119 CrossRef CAS PubMed; (f) T. Monaghan, M. J. Harding, R. A. Harris, R. J. Friel and S. D. R. Christie, Lab Chip, 2016, 16, 3362–3373 RSC.
- M. Penny, Z. Jing Cao, B. Patel, B. Sil dos Santos, C. Asquith, B. R. Szulc, Z. X. Rao, Z. Muwaffak, J. P. Malkinson and S. T. Hilton, J. Chem. Educ., 2017, 94, 1265–1271 CrossRef CAS.
- (a) A. L. Tyson, S. T. Hilton and L. C. Andreae, Int. J. Pharm., 2015, 494, 651–656 CrossRef CAS PubMed; (b) S. K. Talapatra, M. R. Penny, S. T. Hilton and F. Kozielski, J. Appl. Crystallogr., 2019, 52, 171–174 CrossRef CAS.
- (a) S. A. Mohammed, M. E. Vianna, M. R. Penny, S. T. Hilton, N. Mordan and J. C. Knowles, Dent. Mater., 2016, 32, 1289–1300 CrossRef PubMed; (b) S. A. Mohammed, M. E. Vianna, M. R. Penny, S. T. Hilton, N. Mordan and J. C. Knowles, MicrobiologyOpen, 2017, 00, e00455 CrossRef PubMed.
- (a) Z. X. Rao, B. Patel, A. Monaco, Z. J. Cao, M. Barniol-Xicota, E. Pichon, M. Ladlow and S. T. Hilton, Eur. J. Org. Chem., 2017, 6499–6504 CrossRef CAS; (b) C. G. W. Van Melis, M. R. Penny, A. D. Garcia, A. Petti, A. P. Dobbs, S. T. Hilton and K. Lam, ChemElectroChem, 2019, 6, 4144–4148 CrossRef CAS; (c) M. R. Penny, Z. X. Rao, B. F. Peniche and S. T. Hilton, Eur. J. Org. Chem., 2019, 23, 3783–3787 CrossRef.
- (a) Z. Wang, G. Chen and K. Ding, Chem. Rev., 2009, 109, 322–359 CrossRef CAS PubMed; (b) B. Clapham, T. S. Reger and K. D. Janda, Tetrahedron, 2001, 57, 4637–4662 CrossRef CAS; (c) M. Benaglia, A. Puglisi and F. Cozzi, Chem. Rev., 2003, 103, 3401–3430 CrossRef CAS PubMed.
- (a) M. Gruttadauria, F. Giacalone and R. Noto, Chem. Soc. Rev., 2008, 37, 1666–1688 RSC; (b) X. Wang, Q. Guo, X. Cai, S. Zhou, B. Kobe and J. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 2583–2587 CrossRef CAS PubMed; (c) A. S. Díaz-Marta, C. R. Tubío, C. Carbajales, C. Fernández, L. Escalante, E. Sotelo, G. Guitián, V. L. Barrio, A. Gil and A. Coelho, ACS Catal., 2018, 8, 392–404 CrossRef.
- S. T. Hilton, M. R. Penny, B. S. Dos Santos and B. Patel, Br. Pat., GB201604322D0, 2016 Search PubMed.
- (a) J. M. M. Verkade, L. J. C. van Hemert, P. J. L. M. Quaedflieg and F P. J. T. Rutjes, Chem. Soc. Rev., 2008, 37, 29–41 RSC; (b) D. Zareyee and H. Alizadeh, RSC Adv., 2014, 4, 37941–37946 RSC.
- (a) S. Iimura, D. Nobutou, K. Manabe and S. Kobayashi, Chem. Commun., 2003, 1644–1645 RSC; (b) J. S. Manzano, Z. B. Weinstien, A. S. Sadow and I. I. Slowing, ACS Catal., 2017, 7, 7567–7577 CrossRef CAS.
- E. Napadensky and H. Gothait, US2002008333, 2002 Search PubMed.
- http://www.silverson.com/us/products/ultramix-mixers4, accessed December 2019.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9re00492k |
| This journal is © The Royal Society of Chemistry 2020 |