Open Access Article
Open Access ArticleChemoselective hydrogenation of heteroarenes and arenes by Pd–Ru–PVP under mild conditions†
Chandan Chaudhari*a,
Katsutoshi Sato ab,
Yoshihide Nishidaa,
Tomokazu Yamamotoc,
Takaaki Toriyamad,
Syo Matsumuracd,
Yasuyuki Ikedae,
Kenji Teradae,
Naoya Abee,
Kohei Kusuda
ab,
Yoshihide Nishidaa,
Tomokazu Yamamotoc,
Takaaki Toriyamad,
Syo Matsumuracd,
Yasuyuki Ikedae,
Kenji Teradae,
Naoya Abee,
Kohei Kusuda f,
Hiroshi Kitagawa
f,
Hiroshi Kitagawa f and
Katsutoshi Nagaoka
f and
Katsutoshi Nagaoka *a
*a
aDepartment of Chemical Systems Engineering, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya-464-8603, Japan. E-mail: nagaoka.katsutoshi@material.nagoya-u.ac.jp; chaudhari.chandan.subhash@d.mbox.nagoya-u.ac.jp
bElements Strategy Initiative for Catalysts and Batteries (ESCIB), Kyoto University, Katsura, Kyoto 615-8520, Japan
cDepartment of Applied Quantum Physics and Nuclear Engineering, Kyushu University, Motooka 744, Nishi-ku, Fukuoka 819-0395, Japan
dThe Ultramicroscopy Research Center, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
eFuruya Metal Co., Ltd, Higashitsukuba Niihari Kogyodanchi, 57-4, Sawabe, Tsuchiura City, Ibaraki 300-4104, Japan
fDivision of Chemistry, Graduate School of Science Kyoto University Kitashirakawa-Oiwakecho, Sakyo-ku, Kyoto 606-8502, Japan
First published on 15th December 2020
Abstract
Monometallic (Pd, Ru or Rh) and bimetallic (Pd0.5–Ru0.5) alloy NPs catalysts were examined for the hydrogenation of quinoline. Pd–Ru alloy catalyst showed superior catalytic activity to the traditional Rh catalyst. The characterization of Pd0.5–Ru0.5 catalysts, HAADF-EDX mapping and XPS analysis suggested that the alloy state of PdRu catalysts remained unchanged in the recovered catalyst. Furthermore, the catalyst was highly selective for the hydrogenation of different arenes.
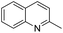
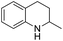
1,2,3,4-Tetrahydroquinolines are important building blocks for the synthesis of fine chemicals, pharmaceuticals and petrochemicals.1–3 Traditionally, 1,2,3,4-tetrahydroquinolines have been synthesized by catalytic cyclization or the Beckman rearrangement method.3–6 Direct hydrogenation of readily available quinoline by molecular hydrogen is a simple and atom-economical route for the hydrogenated quinoline compounds. Nevertheless, the hydrogenation of quinolines reaction suffers from catalyst deactivation caused by the strong interaction between the nitrogen atom of quinoline and active sites of the catalyst. Recently, several heterogeneous (Co, Au, Ru, Pt, Pd and Rh) catalysts have been developed to overcome the catalyst deactivation problem (Table S1†).7–24 Specifically, Rh catalysts exhibits remarkably excellent activity in comparison with other noble metal catalysts but some of them require high temperature (>80 °C)13,21 and/or high pressure (>10 bar).14–21 Later, Rh/AlOOH and Rh nanoparticles (NPs) catalysts have been reported for efficient hydrogenation of quinolines under ambient conditions (1 bar H2 and 25 °C).22–24 Although high selectivity and activity of Rh catalysts, the high cost of Rh make it unfavourable for the industrial purposes. From the economical and environmental point of view, the cost-effective catalyst is necessary for the hydrogenation of quinolines under mild condition.
Solid-solution alloy method is a promising way for the synthesis of alloy NPs which offers an opportunity to control the electronic state of alloy by changing the composition ratio and/or different combinations of alloy in this method. In search of a cheap alternative for Rh catalyst, Pd and Ru are important metals because both metals are relatively cheaper than Rh metal and their well-known activity for hydrogenation reactions. We believe that Pd0.5–Ru0.5 solid solution alloy NPs could be alternative for the traditional Rh catalyst. However, a solid-solution alloy of Pd and Ru is a challenging task due to the immiscibility of Pd and Ru in bulk state.25,26 Recently, our research group led by H. Kitagawa has reported the first successful example of Pd0.5–Ru0.5 NPs via a chemical reduction method using a nano-size effect which showed almost similar catalytic activity than those of Rh for CO oxidation and automobile exhaust purification.27,28 As part of our continuing interest in the applications of Pd–Ru as pseudo Rh catalyst, we herein report that chemoselective hydrogenation of heteroarenes and arenes under mild condition.
In preliminary experiments, we carried out the hydrogenation of quinoline to 1,2,3,4-tetrahydroquinoline as model reaction (Table 1). Monometallic Pd and Ru catalysts were inactive for hydrogenation of quinoline (entries 1 and 2). In previous reports, our group has shown that the electronic state and consequently, catalytic activity of monometallic Pd and Ru could be modified by making Pd0.5–Ru0.5 alloy catalyst.27,28 We found the similar trend using Pd0.5Ru0.5 catalyst and it showed full conversion with 95% yield of 1,2,3,4-tetrahydroquinolines (entry 3). The effect of the solvent showed that the reactivity of Pd0.5Ru0.5 was improved using methanol as solvent. Probably, methanol provides the better dispersion of Pd0.5–Ru0.5 NPs in the reactions and/or high solubility of hydrogen gas in methanol. Next, we examined the activity of previously reported Rh catalyst. Although, the particle size of Rh and Pd0.5–Ru0.5 was similar, the moderate yield of tetrahydroquinoline was obtained in the presence of Rh–PVP (entry 7). Due to the high amount of PVP, the catalytic activity of Rh was slightly declined via less interaction between quinoline and metal sites.29
| Entry | Catalyst | Particle sizeb (nm) | Conv. (%) | GC yield (%) |
|---|---|---|---|---|
| a Reaction conditions: quinoline (1 mmol), catalyst (2 mol%), CH3OH (1 mL), 5 bar H2, 25 °C, 6 h, CH3OH.b Determined by STEM analysis.c THF.d Toluene.e 1,4-Dioxane.f Ref. 38. | ||||
| 1 | Ru–PVP | 8.4 ± 2.4 | 13 | 13 |
| 2 | Pd–PVP | 4.8 ± 0.8 | 41 | 32 |
| 3 | Pd0.5Ru0.5–PVP | 5.6 ± 1.6 | 99 | 95 |
| 4c | Pd0.5Ru0.5–PVP | 5.6 ± 1.6 | 63 | 60 |
| 5d | Pd0.5Ru0.5–PVP | 5.6 ± 1.6 | 45 | 40 |
| 6e | Pd0.5Ru0.5–PVP | 5.6 ± 1.6 | 21 | 13 |
| 7 | Rh–PVP | 5.4 ± 1.0f | 66 | 66 |
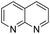
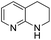
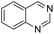
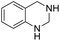
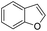
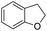
The substrate scope of Pd0.5–Ru0.5PVP catalyst was explored under mild reaction conditions (5 bar H2 and 25 °C). As summarized in Table 2, quinolines with different functional groups converted to corresponding tetrahydroquinolines. The catalyst showed reduction of the heteroarene ring only. Pd0.5–Ru0.5 was also active for the hydrogenation of biologically important heterocyclic compounds such as 1,8-naphthyridine and quinazoline. O-Containing heterocycle, benzofuran was also transformed to 1,2-dihydrobenzofuran with high yield (80%).
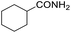
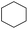
Hydrogenation of arenes to saturated compounds is an important transformation for petrochemical and pharmaceutical industries. For instance, hydrogenation of benzoic acid to cyclohexanecarboxylic acid, it is used for the synthesis of pharmaceutical drugs such as ansatrienin.30 Hydrogenation of benzoic acid has been reported by using supported and polymer stabilized metal nanoparticles.31–38 Among them, Rh catalyst demonstrated the excellent activity for the hydrogenation of benzoic acid. The scope of Pd0.5–R0.5 catalyst was examined for different arene hydrogenation for the hydrogenation of benzoic acid. The scope of Pd0.5–R0.5 catalyst was examined for different arene hydrogenation under solvent-free condition (Table 3). Pd0.5–Ru0.5–PVP catalyst was able to hydrogenate benzoic acid with high yield. In previous reports, the role of solvent was explained for the selective hydrogenation of benzoic acid. The selectivity for cyclohexanecarboxylic acid was controlled using polar solvent (water). The byproduct (benzyl alcohol) was obtained using less polar solvent (cyclohexane). On the other hand, Pd0.5–Ru0.5–PVP catalyst showed the high activity in solvent-free conditions. Hydrogenation of benzene and its derivative were also studied under optimized conditions. The high yields of the corresponding hydrogenated products was obtained in the presence of the Pd0.5–Ru0.5 catalyst.
| Entry | Arene | Product | Conv. (%) | GC yield (%) | |
|---|---|---|---|---|---|
| a Reaction conditions: substrate (1 mmol), catalyst (2 mol%), 10 bar H2, 150 °C, 24 h. | |||||
| 1 | 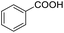 |
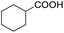 |
99 | 94 | |
| 2 | 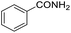 |
 |
99 | 92 | |
| 3 | 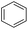 |
 |
99 | 99 | |
| 4 | 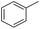 |
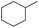 |
95 | 95 | |
| 5 | 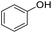 |
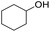 |
99 | 92 | |
| 6 | 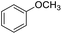 |
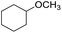 |
80 | 60 | |
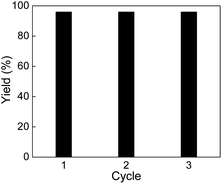
The reusability of Pd0.5–Ru0.5 catalyst was investigated using optimized condition (5 bar H2 and 25 °C) for the hydrogenation of quinoline. The catalyst was washed by acetone multiple times after completion of reaction and separated by centrifugation. The recovered catalyst was dried under vacuum at 40 °C for 12 h and reused without further purification. The catalyst was reused three times without loss in activity (Fig. 1).
To study morphological changes, the fresh and recovered catalyst after the 1st cycle was analysed by high-angle annular dark field. The reconstructed overlaying image of fresh Pd0.5–Ru0.5 catalyst revealed that Pd and Ru atom was distributed equally in the particle which indicated the formation of Pd0.5Ru0.5 alloy via homogeneous mixing at atomic-level. Dealloying and/or aggregation were not observed in Pd0.5–Ru0.5 (Fig. 2). Next, the electronic state of fresh and used Pd0.5–Ru0.5 catalyst was examined by XPS (Fig. 3). In our previous reports, the binding energy of Pd0.5–Ru0.5 was shifted positively in 3d5/2 (334.5 eV) from 3d5/2 (334.30 eV) of monontellic Pd and negatively in 3P3/2 (460.6 eV) from 3P3/2 (461.4 eV) of monometallic Ru.39 Such shifting was also observed in fresh and used Pd0.5–Ru0.5 catalyst. From these results, we concluded that the electronic state of used catalysts was not changed significantly after 1st cycle.
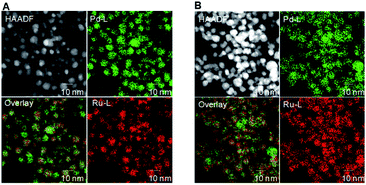 | ||
| Fig. 2 HAADF-STEM images Pd-L and Ru-L STEM-EDX maps and reconstructed overlay images of fresh (A) and recovered (B) Pd0.5–Ru0.5–PVP catalyst. | ||
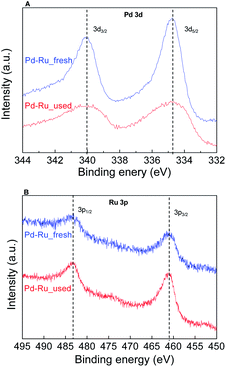 | ||
Fig. 3 XPS spectra for Pd0.5–Ru0.![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif) fresh (blue) and Pd0.5–Ru0. fresh (blue) and Pd0.5–Ru0.![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif) used (red) with Pd 3d (A) and Ru 3p (B). used (red) with Pd 3d (A) and Ru 3p (B). | ||
The superiority of Pd0.5–Ru0.5 alloy over Pd and Ru can be explained by unique electronic state. Our group has used first-principles methods to study the electronic states of Pd, Ru, Rh, and the Pd0.5–Ru0.5 alloy. Interestingly, we have found that the electronic state of the Pd–Ru alloy differs from those of the parental Pd or Ru metal. This result has indicated that a new electronic state is produce by alloying Pd and Ru. The density of state of the Pd0.5–Ru0.5 alloy is similar to that of Rh.
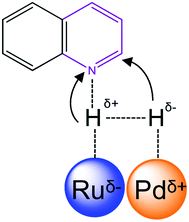
Furthermore, Pd0.5–Ru0.5 alloy shows the electron transfer from Pd to Ru and produced the partial positive (δ+) charge at Pd and partial negative charge (δ−) at Ru. This unique electronic state with bifunctional sites of Pd0.5–Ru0.5 alloy made it favorable for chemoselective for hydrogenation of quinoline. In case of monometallic Pd or Ru catalyst, the strong adsorption of quinoline might be responsible for low conversion of quinoline.
It is well-known that the hydrogenation of organic functional group such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N proceeded via heterolytic cleavage of H2. Such cleavage of H2 yielded to H+ and H− using metal and support/ligand.8,37 In colloidal Pd0.5Ru0.5 catalyst, the heterolytic cleavage of hydrogen proceeded using bifunctional sites of Pd0.5–Ru0.5 catalysis (Scheme 1). The better H2 cleavage over bifunctional sites of Pd0.5Ru0.5 catalyst may have been responsible for the higher catalytic activity of the Pd0.5–Ru0.5 catalyst for the hydrogenation of quinoline.
N proceeded via heterolytic cleavage of H2. Such cleavage of H2 yielded to H+ and H− using metal and support/ligand.8,37 In colloidal Pd0.5Ru0.5 catalyst, the heterolytic cleavage of hydrogen proceeded using bifunctional sites of Pd0.5–Ru0.5 catalysis (Scheme 1). The better H2 cleavage over bifunctional sites of Pd0.5Ru0.5 catalyst may have been responsible for the higher catalytic activity of the Pd0.5–Ru0.5 catalyst for the hydrogenation of quinoline.
In summary, we have developed a successful example of Pd0.5–Ru0.5 NPs for the hydrogenation of N-,O-heteroarenes and arenes. This catalyst exhibited the higher activity than Rh catalyst which was extensively studied for the hydrogenation of quinoline. A parametric study demonstrated that methanol was the best solvent for the hydrogenation of heteroarenes, whereas solvent-free conditions facilitated for the hydrogenation of arenes. The catalyst was recycled three times recycled without loss in activity. It is likely that Pd0.5–Ru0.5 NPs could be efficient catalysts for the hydrogenation of nitroarenes and synthesis of N-heterocycles. Currently, we are exploring this possibility.
Author contributions
Chandan Chaudhari: designing of research, data collection, analysis and writing-editing. Katsutoshi Sato: data curation and validation. Yoshihide Nishida: data collection. Katsutoshi Nagaoka: designing of research, data collection, analysis and writing-editing. Yasuyuki Ikeda, Kenji Terada and Naoya Abe: catalyst preparation. Tomokazu Yamamoto, Takaaki Toriyama and Syo Matsumura: HAADF-STEM analysis. Kohei Kusuda and Hiroshi Kitagawa: XPS analysis and catalyst design.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the ACCEL program, Japan Science and Technology Agency (JST), JPMJAC1501.Notes and references
- R. T. Shuman, P. L. Ornstein, J. W. Paschal and P. D. Gesellchen, J. Org. Chem., 1990, 55, 738 Search PubMed.
- A. R. Katritzky, S. Rachwal and B. Rachwal, Tetrahedron, 1996, 52, 15031 Search PubMed.
- V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Chem. Rev., 2011, 111, 7157 Search PubMed.
- R. Omar-Amrani, A. Thomas, E. Brener, R. Scneider and Y. Fort, Org. Lett., 2003, 5, 2311 Search PubMed.
- T. Kubo, C. Katoh, K. Yamada, K. Okano, H. Tokuyama and T. Fukuyama, Tetrahedron, 2008, 64, 11230 Search PubMed.
- K. Maruoka, T. Miyazaki, M. Ando, T. Matsumura, S. Sakane, K. Hattori and H. Yamamoto, J. Am. Chem. Soc., 1983, 105, 2831 Search PubMed.
- J. Hervochon, V. Dorcet, K. Junge, M. Beller and C. Fischmeister, Catal. Sci. Technol., 2020, 10, 4820 Search PubMed.
- D. Ren, L. He, L. Yu, R.-S. Ding, Y.-M. Liu, Y. Cao, H.-Y. He and K.-N. Fan, J. Am. Chem. Soc., 2012, 134, 17592 Search PubMed.
- L. Zhang, X. Wang, Y. Xue, X. Zeng, H. Chen, R. Li and S. Wang, Catal. Sci. Technol., 2014, 4, 1939 Search PubMed.
- H. Konnerth and M. H. G. Prechtl, Green Chem., 2017, 19, 2762 Search PubMed.
- M. M. Dell'Anna, V. F. Capodiferro, M. Mali, D. Manno, P. Cotugno, A. Monopoli and P. Mastrorilli, Appl. Catal., A, 2014, 481, 89 Search PubMed.
- L. Bai, X. Wang, Q. Chen, Y. Ye, H. Zheng, J. Guo, Y. Yin and C. Gao, Angew. Chem., Int. Ed., 2016, 55, 15656 Search PubMed.
- G.-Y. Fan and J. Wu, Catal. Commun., 2013, 31, 81 Search PubMed.
- H. Mao, X. Liao and B. Shi, Catal. Commun., 2011, 16, 210 Search PubMed.
- H.-Y. Jiang and X.-X. Zheng, Appl. Catal., A, 2015, 499, 118 Search PubMed.
- M. Niu, Y. Wang, P. Chen, D. Du, J. Jiang and Z. Jin, Catal. Sci. Technol., 2015, 5, 4746 Search PubMed.
- A. Karakulina, A. Gopakumar, İ. Akçok, B. L. Roulier, T. LaGrange, S. A. Katsyuba, S. Das and P. J. Dyson, Angew. Chem., 2016, 128, 300 Search PubMed.
- M. N. Shaikh, Md. A. Aziz, A. N. Kalanthoden, A. Helal, A. S. Hakeem and M. Bououdinac, Catal. Sci. Technol., 2018, 8, 4709 Search PubMed.
- F. Martinez-Espinar, P. Blondeau, P. Nolis, B. Chaudret, C. Claver, S. Castillón and C. Godard, J. Catal., 2017, 354, 113 Search PubMed.
- A. Karakulina, A. Gopakumar, Z. Fei and P. J. Dyson, Catal. Sci. Technol., 2018, 8, 5091 Search PubMed.
- Z. Luo, Y. Min, D. Nechiyil, W. Bacsa, Y. Tison, H. Martinez, P. Lecante, I. C. Gerber, P. Serpa and M. R. Axet, Catal. Sci. Technol., 2019, 9, 6884 Search PubMed.
- I. S. Park, M. S. Kwon, K. Y. Kang, J. S. Lee and J. Park, Adv. Synth. Catal., 2007, 349, 2039 Search PubMed.
- I. S. Park, M. S. Kwon, N. Kim, J. S. Lee, K. Y. Kang and J. Park, Chem. Commun., 2005, 349, 5667 Search PubMed.
- V. Mévellec and A. Roucoux, Inorg. Chim. Acta, 2004, 357, 3099 Search PubMed.
- N. Zarkevich, T. Tan and D. Johnson, Phys. Rev. B, 2007, 75, 104203 Search PubMed.
- S. N. Tripathi, S. R. Bharadwaj and S. R. Dharwadkar, J. Phase Equilib., 1993, 14, 638 Search PubMed.
- K. Kusada, H. Kobayashi, R. Ikeda, Y. Kubota, M. Takata, S. Toh, T. Yamamoto, S. Matsumura, N. Sumi, K. Sato, K. Nagaoka and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 1864 Search PubMed.
- K. Sato, H. Tomonaga, T. Yamamoto, S. Matsumura, N. D. B. Zulkifli, T. Ishimoto, M. Koyama, K. Kusada, H. Kobayashi, H. Kitagawa and K. Nagaoka, Sci. Rep., 2016, 6, 28265 Search PubMed.
- G.-H. Han, S. H. Lee, M. Seo and K.-Y. Lee, RSC Adv., 2020, 10, 19952 Search PubMed.
- S. M. Patton, T. A. Cropp and K. Reynolds, Biochemistry, 2000, 39, 7595 Search PubMed.
- X. Xuan, M. Tang, M. Li, H. Li and Y. Wang, ACS Catal., 2014, 4, 3132 Search PubMed.
- X. H. Lu, Y. Shen, J. He, R. Jing, P. P. Tao, A. Hu, R. F. Nie, D. Zhou and Q. H. Xia, Mol. Catal., 2018, 444, 53 Search PubMed.
- T. Maegawa, A. Akashi, K. Yaguchi, Y. Iwasaki, M. Shigetsura, Y. Monguchi and H. Sajiki, Chem.–Eur. J., 2019, 15, 6953 Search PubMed.
- T. Harada, S. Ikeda, Y. H. Ng, T. Sakata, H. Mori, T. Torimoto and M. Matsumura, Adv. Funct. Mater., 2008, 18, 2190–2196 Search PubMed.
- H. Wang and F. Zhao, Int. J. Mol. Sci., 2007, 8, 628–634 Search PubMed.
- G. Bai, X. Wen, Z. Zhao, F. Li, H. Dong and M. Qiu, Ind. Eng. Chem. Res., 2013, 52, 2266 Search PubMed.
- M. Tang, S. Mao, M. Li, Z. Wei, F. Xu, H. Li and Y. Wang, ACS Catal., 2015, 5, 3100 Search PubMed.
- C. Chaudhari, H. Imatome, Y. Nishida, K. Sato and K. Nagaoka, Catal. Commun., 2019, 126, 55 Search PubMed.
- Md. S. Kutubi, K. Sato, K. Wada, T. Yamamoto, S. Matsumura, K. Kusuda, K. Kobayashi, H. Kitagawa and K. Nagaoka, ChemCatChem, 2015, 7, 3887 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09981c |
| This journal is © The Royal Society of Chemistry 2020 |