Open Access Article
Open Access ArticleSynthesis of primary N-arylthioglyoxamides from anilines, elemental sulfur and primary C–H bonds in acetophenones†
Khoa M. Tranab,
Nguyen H. K. Nguyenab,
Thuy T. Buiab,
Tuong A. To ab,
Nam T. S. Phan
ab,
Nam T. S. Phan ab,
Ha V. Le*ab and
Tung T. Nguyen
ab,
Ha V. Le*ab and
Tung T. Nguyen *ab
*ab
aFaculty of Chemical Engineering, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam. E-mail: lvha@hcmut.edu.vn; tungtn@hcmut.edu.vn
bVietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc District, Ho Chi Minh City, Vietnam
First published on 18th December 2020
Abstract
A simple method for coupling of anilines, acetophenones, and elemental sulfur to afford N-arylthioglyoxamides has been developed. Reactions proceeded in the presence of Na2SO3 and DMSO, thus eliminating the need for transition metals and external oxidants. Functionalities such as halogen, ester, methylthio, and heterocycle groups were compatible with the conditions. Electron-poor acetophenones sometimes gave isosteric glyoxamides.
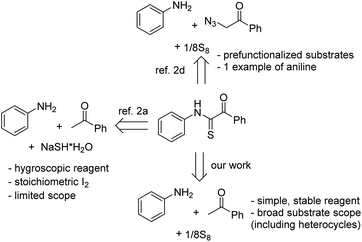
Primary N-arylthioglyoxamides are prominent precursors to afford useful heterocycles.1 Yet, only a few methods for the synthesis of thioglyoxamides have been reported (Scheme 1).2 Wu and co-workers described an I2-mediated coupling of acetophenones, anilines, and sodium hydrosulfide in DMSO to furnish such α-ketothioamides.2a The work suffered from the use of a hygroscopic reagent as well as the formation of a toxic by-product, dimethyl sulfide. An aerobic, iron-catalyzed sulfuration/amination of sp3 C–H bonds in acetophenones with elemental sulfur, a somewhat friendlier agent than sodium hydrosulfide, was later disclosed.2b Notably, the scope of amines was limited to aliphatic compounds. Prefunctionalized, acetophenone-derived molecules such as α-azidoacetophenones or enaminones could be used to couple with elemental sulfur as well.2c,d It should be profitable if a method for coupling of simple, commercial substrates and allowing general scopes of substrates is developed, thus N-arylthioglyoxamides could be obtained feasibly.
Elemental sulfur mediated functionalization of C–H bonds has been extensively studied. Many examples focus on the synthesis of heterocycles.3 Methods for using elemental sulfur to obtain S-containing functionalities are recently presented. Nguyen and co-workers reported a couple examples in which oxidative coupling of benzylic C–H bonds in benzyl amines or those in terminal alkynes with elemental sulfur occurred.4 Such the desired thioamides were affordable via redox-neutral processes involving nitroarenes and activated sp3 C–H bonds in arylacetic acids or picolines.5 Sulfuration of acidic sp2 C–H bonds with elemental sulfur is known.6
Recently, multicomponent reactions that included elemental sulfur for synthesis of S-heterocycles and other synthetically useful functionalities have been presented.7 Herein, we report a method for synthesis of N-arylthioglyoxamides from commercial anilines, acetophenones, and elemental sulfur. No oxidants or transition metals were required. Such thioglyoxamides could be obtained from nitroarenes as amine surrogates, albeit in low yields.
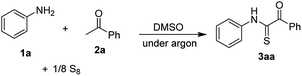
The study was started with the oxidative coupling of aniline 1a, acetophenone 2a, and elemental sulfur. Some results of optimization are presented in Table 1. It should be noted that the desired product was only observed if reactions were run under argon atmosphere. An early attempt to use Na2CO3 base afforded the desired product in 13% yield (entry 1). Switching the base to NaHCO3 increased the yield to 28% (entry 2). Organic, tertiary amine bases such as DABCO (entry 3) and N,N-dimethylpiperazine (entry 4) could be used for the sulfurative coupling, albeit furnishing 3aa in moderate yields. In this reaction, Na2SO3 was a superior base, affording the thioglyoxamide in 60% yield (entry 5). Attempts to vary the use of base including NaOAc and Cs2CO3 were unsuccessful (entries 6 and 7). Lowering the reaction temperature negatively affected the reaction (entry 8). A prominent effect of DMSO amount was observed (entries 9–11). The reaction should be run with 2 mL of DMSO to obtain a reasonable yield of 3aa. No other common solvents such as DMF, 1,4-dioxane, or acetonitrile were able to afford the product. Furthermore, increasing the amount of acetophenone was crucial (entries 12 and 13). If 3 equivalents of 2a was used, the thioglyoxamide 3aa was obtained in 74% yield (entry 13). The reaction progress was carefully monitored by GC (entries 14 and 15). Notably, it could be stopped at 14 h, since no significant improvement of yield was observed after that.
| Entry | Base | DMSO amount, (mL) | 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a ratio 2a ratio |
Yield of 3aa (%) |
|---|---|---|---|---|
| a 1a (0.5 mmol), 2a (0.75 mmol), elemental sulfur (1 mmol, 32 g mol−1), base (1 mmol), DMSO, under argon at 120 °C for 16 h. Yields of 3aa are GC yields using diphenyl ether internal standard. Abbreviations: DiMePi = N,N-dimethylpiperazine.b 100 °C.c 14 h.d 8 h. | ||||
| 1 | Na2CO3 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
13 |
| 2 | NaHCO3 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
28 |
| 3 | DABCO | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
44 |
| 4 | DiMePi | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
34 |
| 5 | Na2SO3 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
60 |
| 6 | NaOAc | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
39 |
| 7 | Cs2CO3 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Trace |
| 8b | Na2SO3 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
47 |
| 9 | Na2SO3 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
61 |
| 10 | Na2SO3 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
68 |
| 11 | Na2SO3 | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
40 |
| 12 | Na2SO3 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
72 |
| 13 | Na2SO3 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
74 |
| 14c | Na2SO3 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
78 |
| 15d | Na2SO3 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
82 |
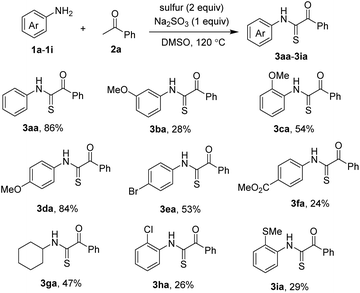
Scope of the reaction with respect to anilines was next studied. The results are shown in Scheme 2. The unsubstituted N-phenylthioglyoxamide 3aa was isolated in good yield. Methoxy-substituted anilines successfully coupled with acetophenone 1a; however, yields of the products were varied. Notably, 3-methoxyaniline was much less active than the other two isomers. Bromo-substituted aniline was a competent substrate, giving the product 3ea that was useful for further modification. Ester functionality was somewhat prone to condition, although isolation of the thioglyoxamide 3fa was still affordable. Aliphatic amines such as cyclohexylamine could be used, affording the desired sulfurative coupling product 3ga in 47% yield. Ortho- chloro and methylthio anilines were compatible with reaction conditions, albeit giving the thioglyoxamides in low yields (3ha, 3ia).
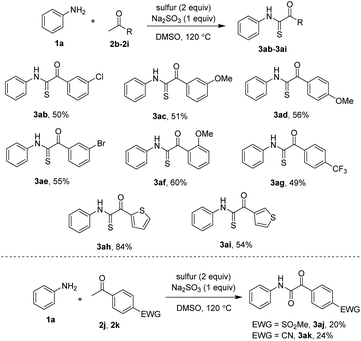
Uses of acetophenones were also investigated (Scheme 3). Meta-substituted substrates coupled with aniline 1a to afford the products in moderate yields (3ab, 3ac, 3ae). Sterically hindered acetophenones such as 2f were still compatible with reaction conditions. 4-Methoxy and 4-trifluoromethyl acetophenones successfully furnished the thioglyoxamides, albeit in moderate yields (3ad, 3ag). Heterocycle-derived acetophenones were competent substrates, affording the reasonable yields of the desired products (3ah, 3ai). The conditions were limited to very electron-poor ketones. In those cases, such as that containing SO2Me (2j) and CN (2k) groups at the para positions to ketones, glyoxamides were the major products (3aj, 3ak).
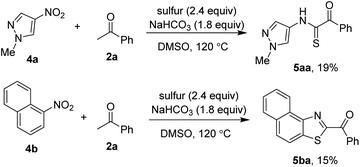
Our group has shown that nitroarenes could be used as the aniline surrogates in sulfur-mediated functionalization of C–H bonds.5 Thus, attempts to couple nitro compounds with acetophenones were investigated. However, low yields of products and limited scope of substrates were obtained. Use a nitro-derived imidazole 4a afforded the thioglyoxamide 5aa in 19% yield. Notably, the 3-aminopyrazole failed to couple with acetophenone. If a hindered 1-nitronaphthalene 4b was reacted with acetophenone, a 2-benzoylbenzothiazole derivative 5ab was isolated. Attempts to develop more efficient conditions for sulfurative coupling of nitroarenes are ongoing.
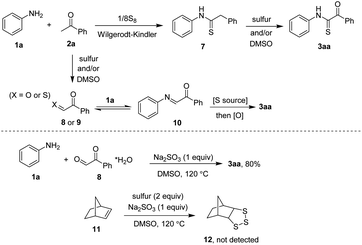
At this moment, two possible pathways were hypothesized as those shown in Scheme 5. Firstly, reaction could occur via a conventional Willgerodt–Kindler coupling of 1a and 2a to afford the thioamide 7.8 This step was in agreement with the result of substrate scope with respect to acetophenones, since electron-poor carbonyl compounds were known to slowly join in the first step.8b Oxidation of 7 in the presence of sulfur and/or co-oxidant DMSO would render the desired thioglyoxamide 3aa. Notably, such the similar pathway was proposed by Nguyen and co-workers.2b,9 An alternative route could begin with the oxidation of sp3 C–H bond in 2a afforded phenylglyoxal 8 or its thio-derived isostere 9. Imine condensation of this intermediate and 1a would furnish 10 followed by a sulfur attack and oxidation to afford 3aa.10 To clarify this pathway, a control condensation of 1a and 8 (as a hydrate) was set up. Since desired product 3aa was obtained in 80% (GC) yield, the oxidation step (2a → 8) could occur during the reaction course. Furthermore, reaction of norbonene 11 with possible trisulfide radical intermediate (10 → 11) was unsuccessful.5b This result implied that the formation of such a strong nucleophilic sulfide could be excluded.
Conclusions
In conclusion, we have developed a general method to couple anilines, acetophenones, and elemental sulfur to afford N-arylthioglyoxamides. The transformation did not need uses of any external oxidants, prefunctionalized substrates, or transition metals. Nitroarenes were also attempted to couple with acetophenones. Control experiments implied the oxidation of α C–H bonds followed by imine condensation or Willgerodt–Kindler-typed sulfuration.Experimental section
General procedure A (for Schemes 2 and 3)
In a typical experiment, an aniline (0.5 mmol), an acetophenone (1.5 mmol), Na2SO3 (126 mg, 1 mmol), elemental sulfur (32 mg, 1 mmol, 32 g mol−1), and DMSO (2 mL) were added to an 8 mL vial equipped with a magnetic stir bar. The vial was flushed with argon for 5 min, capped then placed into a preheated bath (120 °C) and stirred for 14 h. The crude mixture was diluted with ethyl acetate (5 mL) and brine (5 mL). The aqueous phase was extracted with ethyl acetate (3 × 5 mL). Combined organic layers were dried over Na2SO4, filtered, and concentrated under vacuum. The resulting residue was adsorbed onto silica gel (the weight was 10 or 15 times larger than that of the residue) then diluted with hexanes/ethyl acetate to afford the product.General procedure B (for Scheme 4)
In a typical experiment, a nitroarene (0.5 mmol), acetophenone 2a (108 mg, 0.9 mmol), NaHCO3 (75 mg, 0.9 mmol), elemental sulfur (38 mg, 1.2 mmol, 32 g mol−1), and DMSO (1.2 mL) were added to an 8 mL vial equipped with a magnetic stir bar. The vial was flushed with argon for 5 min, capped then placed into a preheated bath (120 °C) and stirred for 14 h. The crude mixture was diluted with ethyl acetate (5 mL) and brine (5 mL). The aqueous phase was extracted with ethyl acetate (3 × 5 mL). Combined organic layers were dried over Na2SO4, filtered, and concentrated under vacuum. The resulting residue was adsorbed onto silica gel (the weight was 10 or 15 times larger than that of the residue) then diluted with hexanes/ethyl acetate to afford the product.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Viet Nam National Foundation for Science and Technology Development (NAFOSTED) is acknowledged for supporting this research under project code 104.01-2019.354 (for Ha V. Le).Notes and references
- (a) X. Feng, Q. Wang, Z.-B. Huang and D.-Q. Shi, RSC Adv., 2014, 4, 54719 Search PubMed; (b) M. Li, G.-R. Cao, J.-W. Zhao, L.-R. Wen, Y.-J. Liu, Y.-M. Xia and S.-Z. Zhu, Tetrahedron Lett., 2009, 50, 6247 Search PubMed; (c) X.-J. Mu, J.-P. Zou, R.-S. Zeng and J.-C. Wu, Tetrahedron Lett., 2005, 46, 4345 Search PubMed.
- (a) H.-Z. Li, W.-J. Xue and A.-X. Wu, Tetrahedron, 2014, 70, 4645 Search PubMed; (b) T. B. Nguyen and P. Retailleau, Green Chem., 2017, 19, 5371 Search PubMed; (c) L. Gan, Y. Gao, L. Wei and J.-P. Wan, J. Org. Chem., 2019, 84, 1064 Search PubMed; (d) P. Yu, Y. Wang, Z. Zeng and Y. Chen, J. Org. Chem., 2019, 84, 14883 Search PubMed; (e) Z. Wang, H. Xie, F. Xiao, Y. Guo, H. Huang and G.-J. Deng, Eur. J. Org. Chem., 2017, 1604 Search PubMed.
- For recent reviews, see: (a) T. B. Nguyen, Adv. Synth. Catal., 2020, 362, 3448 Search PubMed; (b) S. Liu, G.-J. Deng and H. Huang, Synlett, DOI:10.1055/s-0040-1707217.
- (a) T. B. Nguyen, M. Q. Tran, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2014, 16, 310 Search PubMed; (b) T. B. Nguyen, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2012, 14, 4274 Search PubMed.
- (a) N. T. Do, K. M. Tran, H. T. Phan, T. A. To, T. T. Nguyen and N. T. S. Phan, Org. Biomol. Chem., 2019, 17, 8987 Search PubMed; (b) T. H. Ho, H. H. K. Le, T. A. To, T. T. Nguyen and N. T. S. Phan, Bull. Chem. Soc. Jpn., 2020, 93, 783 Search PubMed.
- For selected examples, see: (a) J. -R. Zhang, L. -Z. Zhan, L. Wei, Y. -Y. Ning, X. -L. Zhong, J. -X. Lai, L. Xu and R. -Y. Tang, Adv. Synth. Catal., 2018, 360, 533 Search PubMed; (b) J.-C. Deng, Y.-C. Gao, Z. Zhu, L. Xu, Z.-D. Li and R.-Y. Tang, Org. Lett., 2019, 21, 545 Search PubMed; (c) T. Guo, X.-N. Wei, M. Zhang, Y. Liu, L.-M. Zhu and Y.-H. Zhao, Chem. Commun., 2020, 56, 5751 Search PubMed.
- For selected reviews, see: (a) L. M. Ramos, M. O. Rodrigues and B. A. D. Neto, Org. Biomol. Chem., 2019, 17, 7260 Search PubMed; (b) S. Zhi, X. Ma and W. Zhang, Org. Biomol. Chem., 2019, 17, 7632 Search PubMed . Recent examples:; (c) S. Shi, P. Zhang, C. Luo, S. Zhuo, Y. Zhang, G. Tang and Y. Zhao, Org. Lett., 2020, 22, 1760 Search PubMed; (d) R. Semwal, C. Ravi, S. Saxena and S. Adimurthy, J. Org. Chem., 2019, 84, 14151 Search PubMed.
- (a) D. L. Priebbenow and C. Bolm, Chem. Soc. Rev., 2013, 42, 7870 Search PubMed; (b) K. Okamoto, T. Yamamoto and T. Kanbara, Synlett, 2007, 17, 2687 Search PubMed.
- An independent synthesis of intermediate 7 will be attempted.
- For a consideration of such the sulfur attack to imine, see: W.-J. Xue, Y.-Q. Guo, F.-F. Gao, H.-Z. Li and A.-X. Wu, Org. Lett., 2013, 15, 890 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedures, characterization of the products. See DOI: 10.1039/d0ra08740h |
| This journal is © The Royal Society of Chemistry 2020 |