Open Access Article
Open Access ArticleWidely used catalysts in biodiesel production: a review
Bishwajit
Changmai
a,
Chhangte
Vanlalveni
b,
Avinash Prabhakar
Ingle
c,
Rahul
Bhagat
d and
Samuel Lalthazuala
Rokhum
 *ae
*ae
aDepartment of Chemistry, National Institute of Technology Silchar, Silchar, 788010, India. E-mail: rokhum@che.nits.ac.in
bDepartment of Botany, Mizoram University, Tanhril, Aizawl, Mizoram 796001, India
cDepartment of Biotechnology, Engineering School of Lorena, University of Sao Paulo, Lorena, SP, Brazil
dDepartment of Biotechnology, Government Institute of Science, Aurangabad, Maharashtra, India
eDepartment of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK
First published on 13th November 2020
Abstract
An ever-increasing energy demand and environmental problems associated with exhaustible fossil fuels have led to the search for an alternative renewable source of energy. In this context, biodiesel has attracted attention worldwide as an eco-friendly alternative to fossil fuel for being renewable, non-toxic, biodegradable, and carbon-neutral. Although the homogeneous catalyst has its own merits, much attention is currently paid toward the chemical synthesis of heterogeneous catalysts for biodiesel production as it can be tuned as per specific requirement and easily recovered, thus enhancing reusability. Recently, biomass-derived heterogeneous catalysts have risen to the forefront of biodiesel productions because of their sustainable, economical and eco-friendly nature. Furthermore, nano and bifunctional catalysts have emerged as a powerful catalyst largely due to their high surface area, and potential to convert free fatty acids and triglycerides to biodiesel, respectively. This review highlights the latest synthesis routes of various types of catalysts (including acidic, basic, bifunctional and nanocatalysts) derived from different chemicals, as well as biomass. In addition, the impacts of different methods of preparation of catalysts on the yield of biodiesel are also discussed in details.
1. Introduction
The exponential growth of the world's population coupled with the high standard of living has resulted in a steep increase in energy consumption.1,2 The world's total primary energy consumed (TPEC), which was over 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GW h in the year 2015, is estimated to rise by a triggering 57% in 2050.3 Currently, the transportation of goods and services, which is the major contributor to the global economy, primarily relies on non-renewable fossil fuels. In total primary energy consumption, 80% of the energy consumed is associated with petroleum resources. Amongst these, 54% is consumed in the transportation sector.4 It has been predicted that the energy consumption in the transportation section will increase with an average rate of 1.1% per year. As a result, the high energy consumption of non-renewable petroleum-based fuel to fulfill the increasing energy demand of human society has led to an ecological imbalance, excess greenhouse gas emission, acid rain, global warming and drastic decline in fossil fuel reserves. These negative factors associated with the excessive consumption and exhaustible nature of fossil fuels compel scientific communities to look for an alternative energy source.5,6
000 GW h in the year 2015, is estimated to rise by a triggering 57% in 2050.3 Currently, the transportation of goods and services, which is the major contributor to the global economy, primarily relies on non-renewable fossil fuels. In total primary energy consumption, 80% of the energy consumed is associated with petroleum resources. Amongst these, 54% is consumed in the transportation sector.4 It has been predicted that the energy consumption in the transportation section will increase with an average rate of 1.1% per year. As a result, the high energy consumption of non-renewable petroleum-based fuel to fulfill the increasing energy demand of human society has led to an ecological imbalance, excess greenhouse gas emission, acid rain, global warming and drastic decline in fossil fuel reserves. These negative factors associated with the excessive consumption and exhaustible nature of fossil fuels compel scientific communities to look for an alternative energy source.5,6
Biofuels are an excellent source of energy and widely seen as a potential substitute for fossil fuels. They are prepared from renewable sources, such as plants, municipal wastes, agricultural crops, and agricultural and forestry by-products.7 Over the last few decades, biofuels such as biodiesel have gained significant attention as an alternative fuel in the research field because of its sustainable and environment-friendly nature. Biodiesel has exhibited properties similar to conventional fossil fuels (petro-diesel), and has some properties that are better than petro-diesel, such as high combustion efficiency, high flash point, high cetane number, lower CO2 emission, lower sulfur content and better lubrication.8,9 The high flash point of biodiesel (423 K), as compared to petrodiesel (337 K), makes it non-flammable and non-explosive, resulting in easy and safe handling, storage, and transportation. Additionally, it can be directly used in the automotive engine without any additional alteration.10 It is estimated that biodiesel demand will increase to double or triple by the year 2020.11 In light of this, in the last decades, much attention has been paid to research on biodiesel production with an intension make it more sustainable and economical. An increasing interest in biodiesel is validated by the number of research paper publications in this area, as shown in Fig. 1. Statistical data analysis in Fig. 1 depicted the increasing trend of published research papers in the field of biodiesel. These data were collected in February 2020 from “SciFinder Database” using the keyword “biodiesel”. From a meager 157 publications in the year 1993, it has exponentially increased to 3725 publications during its peak in 2014.
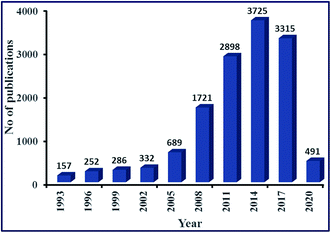 | ||
| Fig. 1 Publications per year for biodiesel during the period 1993 to Feb 2020 (data collected from SciFinder Database). | ||
2. (Trans)esterification
Transesterification or alcoholysis is a process to produce biodiesel in which edible/non-edible oils or triglyceride (TG) and alcohol have undergone nucleophilic reaction to form fatty acid methyl ester (FAME) and glycerol as a byproduct.12 The transesterification reaction is illustrated in Scheme 1. Three sequential reversible reactions occur in the transesterification process: (i) conversion of triglyceride to diglyceride, (ii) diglyceride conversion to monoglyceride, and finally, (iii) monoglyceride conversion to glycerol. An ester is formed in each conversion step; thus, one TG molecule produces three ester molecules. The transesterification reaction can efficiently convert a triglyceride of vegetable oil into FAME, also called biodiesel, as depicted in Scheme 1. However, the esterification reaction, a reaction between carboxylic acids and alcohols to afford esters,13–15 is essential to converting all free fatty acids (FFA) of vegetable oil into biodiesel, as shown in Scheme 2. These transesterification and esterification reactions are usually carried out in the two-pots procedure. Usually, the high FFA content of vegetable oil is first converted to esters (FAME) via esterification reaction by employing an acid catalyst, followed by the transesterification reaction using a basic catalyst to convert triglycerides to FAME. However, (trans)esterification reactions (or simultaneous transesterification and esterification) in one-pot is highly desirable to convert both triglycerides and FFA of vegetable oil (with high FFAs) to FAME to reduce the time and cost of biodiesel production. The different routes to synthesize biodiesel are outlined in Fig. 2.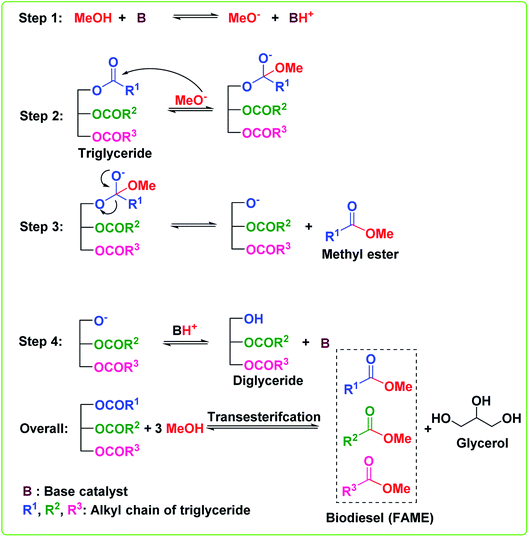 | ||
| Scheme 1 Base-catalyzed reaction mechanism for the transesterification of TGs of vegetable oil to biodiesel. | ||
3. Biodiesel
The American Society for Testing and Materials (ASTM) described biodiesel as a mono-alkyl ester produced from edible/non-edible oils or animal fats.16 Vegetable oils or animal fats comprise mainly triacylglycerol (TAG), which is an ester of fatty acids (FA) and glycerol. The physicochemical properties of vegetable oils and animal fats are greatly influenced by the compositions of the TAG, which further often dictates the quality of biodiesel produced from these resources. FA are classified broadly into two groups: (i) saturated FA, which has carbon–carbon single bonds, and (ii) unsaturated FA, which comprises at least one carbon–carbon double bond. The FA most widely found in vegetable oils are oleic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), palmitic acid (16
1), palmitic acid (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), linoleic acid (18
0), linoleic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), linolenic acid (18
2), linolenic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), stearic acid (18
3), stearic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), palmitoleic acid (16
0), palmitoleic acid (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), myristic acid (14
1), myristic acid (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), and arachidic acid (20
0), and arachidic acid (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0). Besides these FA, a trace amount of phospholipids, tocopherols, carotenes, sulphur compounds, and water are also found in vegetable oils.17,18
0). Besides these FA, a trace amount of phospholipids, tocopherols, carotenes, sulphur compounds, and water are also found in vegetable oils.17,18
4. Feedstocks for biodiesel production
The feedstocks for the production of biodiesel are mainly edible18–20 and non-edible vegetable oils,21–23 waste cooking oils24,25 and animal fats, including tallow,25 yellow grease,26 lard,27 chicken fat28–30 and by-products from the production of omega-3 fatty acids from fish oil.31,32 Algae are another promising feedstock for biodiesel, which have a high potential to replace edible oil due to their availability in a pond, sewage water or in shallow ocean water without dislodging land used for food production.32–34 Worldwide, 31% biodiesel is produced from palm oil, 27% from soybean oil and 20% from rapeseed oil.35 Different countries use various feedstocks based on their local availability. The major feedstocks used in various countries are listed in Table 1. The feedstock cost alone contributed to 75% of the biodiesel cost.36 Thus, the proper selection of feedstocks for biodiesel is necessary to reduce the overall cost of biodiesel production. Ironically, the utilization of edible oils (e.g., sunflower, rape, soy) as feedstocks for biodiesel, called the first-generation biofuels, resulted in a food-versus-fuel problem, and also disturbed the agricultural farmland allocation.27,37 In Malaysia, the edible palm oil price has increased by 70% due to its uses as feedstock in the biodiesel industry.38 In this regard, to mitigate the problem associated with the food-versus-fuel nexus and high cost of first-generation biodiesel, non-edible oils are currently largely targeted as a biodiesel feedstock. Another problem associated with first-generation biofuels is their remarkably higher cost than fossil fuels. Hence, to bring down the cost of biodiesel, the utilization of non-edible oil as biodiesel feedstocks is highly relevant. Non-edible oils of more than 300 species are available in South Asia. India has an abundant amount (approximately 1 million tons per year) of such non-edible oils. Pongamia pinnata (karanja) and Jatropha curcas oils (JCO) were identified as the most promising feedstocks by the Government of India. However, in India's biodiesel program, Jatropha has prominence over karanja due to its lower gestation period. If properly managed, non-edible crops planted in different parts of the world have the potential to reduce our dependence on fossil fuels for energy sources and edible oils as biodiesel feedstocks.| Country | Feedstock |
|---|---|
| India | Jatropha/Pongamia pinnata (karanja)/soybean/rapeseed/sunflower |
| Argentina | Soybeans |
| Brazil | Soybeans/palm oil/castor/cotton oil |
| France | Rapeseed/sunflower |
| Peru | Palm/Jatropha |
| Germany | Rapeseed |
| Spain | Linseed oil/sunflower |
| Italy | Rapeseed/sunflower |
| Turkey | Sunflower/rapeseed |
| Greece | Cottonseed |
| Sweden | Rapeseed |
| Norway | Animal fats |
| China | Jatropha/waste cooking oil/rapeseed oil |
| Indonesia | Palm oil/Jatropha/coconut |
| Japan | Waste cooking oil |
| Malaysia | Palm oil |
| Philippines | Coconut/Jatropha oil |
| Bangladesh | Rubber seed/Pongamia pinnata oil |
| Pakistan | Jatropha oil |
| Thailand | Palm/Jatropha/coconut oil |
| Iran | Palm/Jatropha/castor/algae oil |
| Singapore | Palm oil |
| Ghana | Palm oil |
| Zimbabwe | Jatropha oil |
| Kenya | Castor oil |
| Mali | Jatropha oil |
| UK | Rapeseed/waste cooking oil |
| Ireland | Frying oil/animal fat |
| Canada | Rapeseed/animal fat/soybean oil |
| Mexico | Animal fat/waste oil |
| USA | Soybeans/waste oil/peanut |
| Cuba | Jatropha curcas/Moringa/neem oil |
| Australia | Jatropha/Pongamia/waste cooking oil/animal tallow |
| New Zealand | Waste cooking oil/tallow |
Biodiesel has been widely used as biofuels in the European Union (EU), and 49% of biodiesel was produced from rapeseed oil in 2015 in EU.39 With the increasing uses of waste cooking oil (WCO), recycled vegetable oils and palm oils, the share of rapeseed oil in biodiesel production decreased from 72% in 2008. To reduce our dependency on edible oil and reduce the price of biodiesel, EU has raised the share of WCO to the 2nd position after rapeseed oil in 2015.40 The top five biodiesel producers in EU are Germany, France, Spain, Netherlands, and Poland. Germany is the largest biodiesel producer in EU, and its production capacity increased from 3.2 billion litres in 2010 to 3.8 billion litres in 2014.41
Various types of feedstocks (such as edible plant oils, non-edible oils, waste cooking oils, animal fats, and algal oil) have been considered for the synthesis of biodiesel, and are discussed below.
4.1 Edible plant oils
Soybean oil,42 sunflower oil,43 rapeseed oil,44 and palm oil45 are widely utilized as a biodiesel feedstock in numerous nations, for example, Argentina, Brazil, Indonesia, Europe, US, and Malaysia. At present, an estimated 95% of the worlds' total biodiesel is produced from sunflower oil, rapeseed oil, and palm oil.46 Various types of edible oils exploited as feedstocks for the production of biodiesel are recorded in Table 2.| No. | Edible oil for biodiesel production | Plant source | The botanical name of the plant source |
|---|---|---|---|
| 1 | Sunflower oil | Sunflower | Helianthus annuus |
| 2 | Rapeseed oil | Rape | Brassica napus |
| 3 | Soybean oil | Soybean | Glycine max |
| 4 | Palm oil | Mesocarp of oil palm | Elaeis guineensis |
| 5 | Coconut oil | Coconut | Cocos nucifera |
4.2 Non-edible plant oils
Recently, non-edible plant oils have been increasingly considered as another promising potential feedstock for biodiesel, which is attributable to their high oil content and low cost. In addition, unlike edible oils, it does not pose a ‘food versus fuel’ problem as they can be grown in barren and arid regions, which are not suitable for agriculture. Furthermore, non-edible oil plants can grow under harsh conditions and hardly need any attention. Thus, this reduces the cost involved in cultivation, and potentially reduces the cost of biodiesel.47,48 Some of the commonly investigated non-edible plant oils for biodiesel production include Jatropha curcas, Pongamia glabra (Karanja), Madhuca indica (Mahua), Azadirachta indica (neem), Moringa oleifera (moringa seed), Calophyllum inophyllum, Salvadora oleoides (Pilu), Nicotiana tabacum (tobacco), cottonseed oil, Eruca sativa Gars, terebinth, rubber seed oil, desert date, Acrocomia aculeata (macaúba), Crambe abyssinica (hochst), linseed oil, rubber seed oil, Sapium sebiferum (chinese tallow), Sapindus mukorossi (soapnut), Euphorbia tirucalli (milk bush), Calophyllum inophyllum (polanga oil), jojoba, leather pre-fleshings, apricot seed, Pistacia chinensis (bunge seed), sal oil and Croton megalocarpus. Among all these oil plants, Jatropha curcas, Pongamia glabra (Karanja), Madhuca indica (Mahua), Azadirachta indica (neem) are commercially available and most largely used in biodiesel production.494.3 Waste cooking oil
Biodiesel production from WCO can partially substitute fossil fuels as well as can solve the energy crisis and environmental pollution. Moreover, WCO is cheaper than fresh vegetable oils, consequently, lessening the expense incurred for biodiesel synthesis. WCO can be grouped into two classifications based on their FFA content if the FFA content is >15%. It is then called brown grease; otherwise, it is named ‘yellow grease’. Annually, 1 billion tons of WCO is generated throughout the world. In EU, it is estimated that around 0.7–1 MT WCO were collected per year. Among 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of WCO, around 65
000 tons of WCO, around 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons were collected from the UK alone, basically originating from commercial restaurants and food processing industries. Therefore, the disposal of WCO is a major concern, which otherwise contaminates water and the environment at large. Although some portions of WCO oil were used in the production of soap, major parts of WCO were usually dumped into the river and landfills. In light of this, the production of biodiesel from WCO not only reduced the cost of biodiesel, but also resolved the disposal problem of WCO and minimized environmental pollution.
000 tons were collected from the UK alone, basically originating from commercial restaurants and food processing industries. Therefore, the disposal of WCO is a major concern, which otherwise contaminates water and the environment at large. Although some portions of WCO oil were used in the production of soap, major parts of WCO were usually dumped into the river and landfills. In light of this, the production of biodiesel from WCO not only reduced the cost of biodiesel, but also resolved the disposal problem of WCO and minimized environmental pollution.
4.4 Animal fats
Animal fats are another feedstock for biodiesel production with the potential to reduce the cost of biodiesel. This type of feedstock includes lard, tallow and chicken fat. However, due to the presence of a high quantity of saturated fatty acids, it has some shortcomings both in chemical and physical properties, such as poor cloud point, poor pour point, and so forth. At the same time, its high saturation level has various advantages, such as a high cetane number and high oxidation stability. Moreover, animal fats are more favourable biodiesel feedstocks, as compared to vegetable oils due to their low price.4.5 Algal oil
Currently, microalgae are viewed as one of the most promising feedstocks for the industrial-scale synthesis of biodiesel. Biodiesel production from algal oil is highly sustainable, as several strains of microalgae can double in size within hours. Thus, they have the capacity to create a large number of litres of biodiesel per hectare every year.50 Additionally, as several microalgal strains can be grown on non-arable land in a saline water medium, their mass cultivation does not compete with food production.5. Characterization of catalysts and biodiesel
Several analytical techniques are employed to characterize both catalysts and FAME produced. Each analytical technique will be discussed in the upcoming sections as and when relevant. As a preliminary study, Fourier transform infrared spectroscopy (FT-IR) is usually employed to detect the presence of various functional groups in the catalyst, while X-ray diffraction (XRD) can be employed to investigate the crystallinity and qualitative detection of elements present in the catalyst. The surface morphology, particle size and the structure of the catalysts can be investigated using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The chemical compositions are investigated using energy-dispersive X-ray spectroscopy (EDX). X-ray fluorescence (XRF) is commonly used for the quantitative detection of metal oxides and X-ray photoelectron spectroscopy (XPS) analyses are routinely performed for the quantitative measurement of the elements present in the catalyst, and also provide the chemical state information of the catalyst. The surface area, pore volume and pore diameter are usually measured by Brunauer–Emmett–Teller (BET) analysis, whereas the thermal stability of the catalysts is analyzed using thermogravimetric analysis (TGA). The acidity and basicity of the catalysts are usually investigated using NH3 and CO2 temperature-programmed desorption (TPD) analyses. In addition, the basicity and acidity of the catalyst can be visualized by Hammett indicators tests and acid–base titration methods. Valuable information about the degree of carbonization and/or aromatization of carbonaceous material used as a catalyst can be obtained using solid-state magic-angle spin-nuclear magnetic resonance (MAS NMR). Likewise, the successful conversion of biodiesel feedstocks to FAME is confirmed using different analytical techniques. Usually, NMR analysis is used as a confirmation tool to identify the formation of FAME. Despite not being common, FT-IR analysis can also be used to identify the FAME formation. The chemical components of FAME, along with their respective percentages, are usually identified using gas chromatography-mass spectroscopy (GC-MS) technique. In addition, 1H NMR spectra can be used to give concrete information about the purity of FAME and the percentage conversion of vegetable oil to FAME using the Knothe and Kenar eqn (1).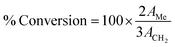 | (1) |
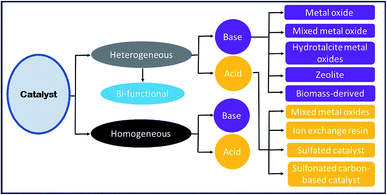
6. Homogeneous catalyst
The homogeneous catalysts utilized for the transesterification reaction are classified into two groups, such as: (i) base catalysts (for example, NaOH and KOH), and (ii) acid catalysts, such as sulphuric, sulphonic, hydrofluoric, and hydrochloric acids.6.1 Base catalyst
Homogeneous base catalysts are most widely investigated in the transesterification of vegetable oil to FAME, as they are cheap and easily accessible. To date, several homogeneous base catalysts have been utilized for the synthesis of FAME, e.g., KOH, NaOH, and NaOCH3, as shown in Table 3. The uses of NaOH and KOH as catalysts showed excellent catalytic activities towards biodiesel production, such as the minimum reaction time and high biodiesel yield, and occurred at ambient temperature and pressure. However, this process has certain limitations, such as water being formed as a byproduct, which reduces the biodiesel yield. Other than KOH and NaOH, sodium methoxide and potassium methoxide give better biodiesel performance, as water is not formed in these processes. An alkaline catalyst is not suitable for the transesterification of vegetable oils with high FFA content (>2 wt%). However, it is fit for refined vegetable oils with low FFA content (ranging from less than 0.5 wt% to less than 2 wt%).| No. | Catalyst | Feedstock | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil (M/O) molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. | |||||
| 1 | KOH | Vegetable oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 25, 40 1, 1, 25, 40 |
51–87 | 51 |
| 2 | KOH | Crude rubber/palm oil | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 55, 300 1, 2, 55, 300 |
98 | 52 |
| 3 | KOH | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 60 1, 1, 60, 60 |
∼96 | 53 |
| 4 | KOH | Roselle oil | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 60, 60 1, 1.5, 60, 60 |
99.4 | 36 |
| 5 | KOH | Rapeseed | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 65, 120 1, 1, 65, 120 |
95–96 | 54 |
| 6 | KOH | Frying oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 120 1, 1, 60, 120 |
72.5 | 55 |
| 7 | KOH | Waste frying oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 65, 60 1, 1, 65, 60 |
96.15 | 56 |
| 8 | KOH | Used olive oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.26, 25, 90 1, 1.26, 25, 90 |
94 | 57 |
| 9 | KOH | Palm kernel | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 60 1, 1, 60, 60 |
96 | 58 |
| 10 | KOH | Duck tallow | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 65, 180 1, 1, 65, 180 |
83.6 | 59 |
| 11 | KOH | Pongamia pinnata | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 90 1, 1, 60, 90 |
92b | 60 |
| 12 | NaOH | Waste cooking oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 50, 90 1, 1, 50, 90 |
89.8b | 23 |
| 13 | NaOH | Waste frying oil | 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.6, 65, 60 1, 0.6, 65, 60 |
98 | 61 |
| 14 | NaOH | Waste frying oil | 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5, 50, 30 1, 0.5, 50, 30 |
96 | 62 |
| 15 | NaOH | Canola oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 45, 15 1, 1, 45, 15 |
98 | 63 |
| 16 | NaOH | Sunflower | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 120 1, 1, 60, 120 |
97.1 | 64 |
| 17 | NaOH | Refined palm oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 30 1, 1, 60, 30 |
95 | 65 |
| 18 | NaOH | Cotton seed oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 60 1, 1, 60, 60 |
97 | 66 |
| 19 | NaOCH3 | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.6, 60, 60 1, 0.6, 60, 60 |
97 | 53 |
| 20 | NaOCH3 | Rice bran | 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.88, 55, 60 1, 0.88, 55, 60 |
83.3 | 67 |
| 21 | NaOCH3 | Waste cooking oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.75, 65, 90 1, 0.75, 65, 90 |
96.6 | 68 |
Dmytryshyn et al.51 examined the transesterification of various vegetable oils, such as canola oil, green seed canola oil from heat-harmed seeds, handled waste fryer oil, and natural waste fryer oil with methanol to afford FAME using the KOH catalyst, and reported a biodiesel yield of 51–87% under the optimum reaction conditions. In another study, KOH was exploited to convert crude rubber oil and palm oil mixture to biodiesel in 98% yield under the optimum reaction conditions. The vegetable oil was esterified using an acid catalyst prior to a base-catalyzed transesterification process, to obtain a low FFA content vegetable oil.52 Similarly, KOH was utilized as a catalyst for the transformation of soybean oil to FAME in 96% yield.53 Roselle oil,34 rapeseed oil,54 frying oil,55,56 used olive oil,57 palm kernel58 and duck tallow59 were also successfully transesterified to FAME using the KOH catalyst. Karmee et al.60 reported the transesterification of Pongamia pinnata to FAME in 92% conversion using the base catalyst KOH. Interestingly, the utilization of tetrahydrofuran (THF) as a co-solvent increased the conversion to 95%.
Meng et al.23 described an exceptionally high activity of NaOH towards biodiesel production from WCO with high FFA in 89.8% conversion under the optimized reaction settings. The high FFA substance of WCO was reduced by a pre-esterification process with sulphuric acid. Similarly, waste cooking/frying oil,61,62 canola oil,63 sunflower oil,64 palm oil65 and cotton seed oil66 were converted to biodiesel using NaOH as a homogeneous catalyst. Furthermore, NaOCH3 (ref. 67 and 68) was evaluated as a catalyst for the transesterification of rice bran oil to FAME by Rashid et al.,67 where 83.3% biodiesel yield was observed in 60 min under the optimum reaction conditions.
6.2 Acid catalyst
Base catalysts are usually preferred over acid catalysts, as they are more reactive and low cost. However, base catalysts may react with the FFA present in the feedstock during transesterification, bringing about soap formation by saponification, which may consume the catalyst and diminish its reactivity. Meanwhile, an acidic catalyst is neutral to the FFA, and thus shows better outcomes for the transesterification or esterification of vegetable oils or fats having a high amount of FFA (≥2 wt%). Generally, acid catalysts are utilized to bring down the FFA content in WCO and animal fats by means of esterification prior to transesterification using a base catalyst.5 Several acids, such as H2SO4, HCl, H3PO4 and sulfonated acids, were mostly utilized for the (trans)esterification of vegetable oils.36 However, acid-catalyzed biodiesel production has some major limitations, such as a slow reaction rate (4000 times slower than the rate of base-catalyzed transesterification), and require a high alcohol-to-oil molar ratio.69–71 Moreover, it has environmental and corrosive related problems.69 Because of these demerits, acid-catalyzed biodiesel synthesis is not very popular and is studied less. Some of the reported literature of acid-catalyzed biodiesel production and their results are listed in Table 4.| No. | Catalyst | Feedstock | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). | |||||
| 1 | H2SO4 | Chicken/mutton tallow | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.25/2.5, 50/60, 1440 1, 1.25/2.5, 50/60, 1440 |
99.01 ± 0.71/93.21 ± 5.07 | 25 |
| 2 | H2SO4 | WCO | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 95, 600 1, 4, 95, 600 |
90 | 70 |
| 3 | H2SO4 | Used frying oil | 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.1, 65, 40 1, 0.1, 65, 40 |
79.3 | 73 |
| 4 | H2SO4 | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 2880 1, 3, 60, 2880 |
98 | 71 |
| 5 | H2SO4 | Zanthoxylum bungeanum | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 60, 80 1, 2, 60, 80 |
98 | 74 |
| 6 | H2SO4 | Tobacco seed oil | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 25 1, 1, 60, 25 |
91 | 75 |
| 7 | C2HF3O2 | Soybean oil | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 M, 120, 300 1, 2 M, 120, 300 |
98.4 | 72 |
Wang et al.70 examined the biodiesel synthesis from WCO and reported a 90% yield. Moreover, Miao et al.72 examined the conversion of soybean oil to biodiesel using trifluoroacetic acid catalyst, and reported 98.4% biodiesel yield at optimal reaction conditions. Similarly, various edible/non-edible oils (such as WCO,73 soybean oil,71 zanthoxylum bungeanum74 and tobacco seed oil75) were used for biodiesel production using sulfuric acid. Moreover, trifluoroacetic acid was utilized as a homogeneous acid catalyst for the esterification/transesterification of soybean oil to biodiesel.72 The catalyst brought about a high biodiesel yield of 98.4% under the optimum reaction conditions. From the above discussion, it was observed that acid-catalyzed esterification/transesterification reactions usually require drastic reaction conditions, such as a high M/O molar ratio, catalyst loading, temperature and long reaction time, as compared to base-catalyzed transesterification reactions.
7. Heterogeneous catalysts
Although the homogeneous catalyst has its own advantages, such as high reactivity and low cost, its utilization in the production of biodiesel is accompanied by several shortfalls. These shortfalls include the low quality of glycerol produced, the fact that the catalyst cannot be regenerated, and the lengthy process involved in the purification of biodiesel. Thus, the whole process is labor-intensive and uneconomical. Hence, in recent years, the heterogeneous catalyst has attracted immense attention for biodiesel production, as it can be tailored to match specific requirements, and be easily recovered and reused for several cycles of catalytic reaction, thereby potentially bringing down the labor involved and the cost of biodiesel.Unlike homogeneous catalysts, heterogeneous catalysts mostly appear in a solid form; thus, the reaction mixture and the catalyst are in a different phase. In the heterogeneous catalyzed reactions, the catalyst surface is the main site for the reaction to occur.76 The following advantages of utilizing a solid catalyst in transesterification make the process green: (i) the catalyst can be reused, (ii) there is a very minimal amount of wastewater generated during the process, (iii) glycerol separation from the final mixture (glycerol, biodiesel and catalyst) is much easier, and (iv) high purity glycerol is obtained.
Heterogeneous catalysts have several advantages over a homogeneous catalyst, such as simple separation, recyclability and reusability. Moreover, solid catalysts are eco-friendly, less toxic, and have minimum corrosion and reduced energy intake. Thus, solid catalysts provide an efficient and economical pathway for biodiesel production.12,77,78 Heterogeneous or solid catalysts can be grouped into two categories: (i) basic and (ii) acidic heterogeneous catalysts. Nowadays, researchers have developed several heterogeneous catalysts, which can promote esterification and transesterification reactions simultaneously in one reaction vessel (one-pot). These types of catalysts are mostly utilized for biodiesel synthesis from the vegetable oils or animal fats having a high amount of FFA without the requirement of an additional pretreatment step to reduce the FFA content.12
7.1 Base catalysts
In recent years, basic heterogeneous catalysts have been most widely investigated as it can overcome the constraints associated with homogeneous basic catalysts, and shows excellent catalytic activity under mild reaction conditions. However, these catalysts are suitable only for biodiesel feedstock with low FFA content; otherwise, the catalysts will react with the FFA to produce soap by means of the saponification reaction. This makes the separation of biodiesel from glycerol tedious, thereby diminishing the biodiesel yield. Several solid base catalysts reported in the literature, such as the alkaline metal oxides, transition metal oxides, mixed metal oxides, hydrotalcites, zeolites, and biomass-based catalysts, are discussed comprehensively in this section.| No. | Catalyst | Feedstock | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). | |||||
| 1 | CaO | Soybean oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8, 65, 180 1, 8, 65, 180 |
95 | 82 |
| 2 | CaO | Sunflower oil | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 120 1, 3, 60, 120 |
94 | 83 |
| 3 | CaO | Rapeseed oil | 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.7, 60, 160 1, 0.7, 60, 160 |
90 | 84 |
| 4 | SrO | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 70, 30 1, 3, 70, 30 |
95 | 85 |
| 5 | BaO | Palm oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 60 1, 3, 65, 60 |
95.2 | 86 |
Kouzu et al.82 examined the transesterification of soybean oil using the CaO catalyst, and reported a high biodiesel yield of 95% under the optimized reaction conditions. Granados et al.83 found that CaO calcined at 700 °C showed very high activity towards biodiesel production from sunflower oil, and attained 94% biodiesel yield. Furthermore, the transesterification of rapeseed oil was reported by Kawashima et al.,84 where CaO was pretreated with methanol to form Ca(OCH3), which acted as an initiator for the transesterification reaction. A high biodiesel yield of 90% was observed using the optimized reaction conditions. In another work, the SrO-catalyzed transesterification of soybean oil has been reported by Liu et al.85 The catalyst showed excellent activity with a high yield of 95% at 70 °C and 30 min time. The catalyst is highly stable and can be reused for 10 successive cycles.
The ultrasonic-assisted biodiesel synthesis from palm oil was reported using diverse metal oxides, such as CaO, BaO and SrO.86 The activity of the catalyst in ultrasonic-assisted biodiesel synthesis was compared with the traditional magnetic stirring process, and it was found that the ultrasonic process showed 95.2% of yield using BaO within 60 min reaction time, which otherwise take 3–4 h in the conventional stirring process. Similarly, the ultrasonic-assisted transesterification using CaO and SrO resulted in an increase in the biodiesel yield from 5.5% to 77.3% and 48.2% to 95.2%, respectively. These findings show the advantages of using ultrasonication in the field of chemical synthesis, particularly in the field of biodiesel synthesis. The authors also investigated the influence of ultrasonic amplitude on the biodiesel synthesis from palm oil, and observed that 50% ultrasonic amplitude displayed the best result in terms of the biodiesel yield. The catalyst reusability test revealed that the catalytic activity of BaO decreased drastically, especially in the ultrasonic process during the reusability test, which was mainly due to catalyst leaching. The reaction set-up is depicted in Fig. 3.
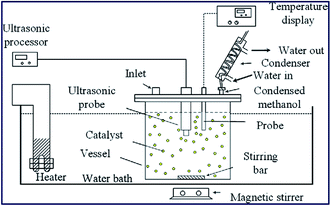 | ||
| Fig. 3 Schematic portrayal of experimental set up for the ultrasonic-assisted transesterification reaction. Reproduced from ref. 86. | ||
| No. | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b w/w. | |||||
| 1 | Cu(II)@chitosan | Soybean oil | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5b, 2, 70, 180 5b, 2, 70, 180 |
88.82 | 90 |
| 2 | Co(II)@chitosan | Soybean oil | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5b, 2, 70, 180 5b, 2, 70, 180 |
94.01 | 90 |
| 3 | SO42−/ZrO2 | Crude palm kernel oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 200, 60 1, 3, 200, 60 |
90.30 | 87 |
| 4 | SO42−/ZrO2 | Crude coconut oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 200, 60 1, 3, 200, 60 |
86.30 | 87 |
| 5 | Mn doped ZnO | Mahua oil | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8, 50, 50 1, 8, 50, 50 |
97 | 91 |
| 6 | Na2MoO4 | Soybean oil | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 120, 180 1, 3, 120, 180 |
95.6 | 92 |
| 7 | Vanadyl phosphate | Soybean oil | 0.88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.5, 180, 60 2, 0.5, 180, 60 |
≥88 | 93 |
Meanwhile, Baskar et al.91 used the Mn-doped ZnO nanomaterial for the conversion of Mahua oil to biodiesel, and observed that the catalyst calcined at 600 °C showed the highest biodiesel yield of 97% under the optimum reaction conditions. The kinetic investigation of the reaction revealed that 181.91 kJ mol−1 activation energy is necessary for biodiesel synthesis from Mahua oil utilizing the Mn-doped ZnO catalyst. The prepared Mn-doped ZnO catalyst was seen as a cluster, and is spherical in shape as depicted in Fig. 4 A. FI-TR analysis was performed to confirm the formation of the biodiesel. Absorption bands at 1744 and 1703 cm−1 demonstrated the CO stretching of the methyl esters in Mahua oil and biodiesel, respectively. The main spectral region that allows for the chemical discrimination between Mahua oil and the produced biodiesel is in the range of 1500–900 cm−1, and is also called known as the fingerprint region. Fig. 4B reveals the symmetric and asymmetric stretching of the alkyl regions at 1376, 1463, 2852, 2922 cm−1, and the CO group of the lactones and esters at 1735 cm−1. Moreover, the stretching band of the CO group of the typical esters at around 1703 cm−1 was observed in Fig. 4C. In light of these FT-IR bands, the product obtained after transesterification of Mahua oil using the Mn-doped ZnO catalyst was confirmed as biodiesel.
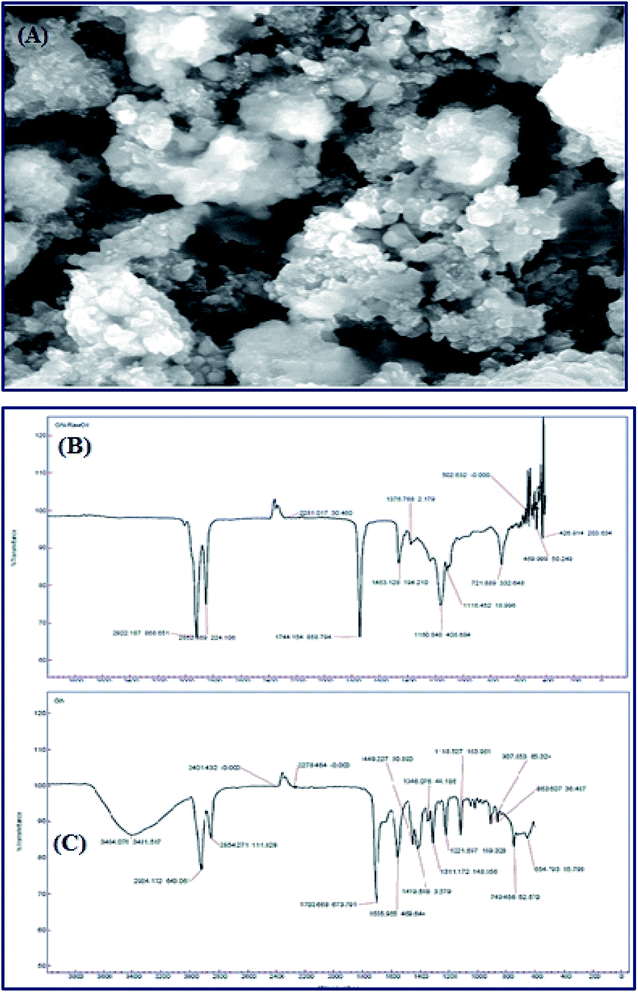 | ||
| Fig. 4 SEM image (A) and FT-IR spectrum (B and C) of Mn-doped ZnO nanomaterial. Reproduced from ref. 92. | ||
Na2MoO4 has been synthesized and investigated as a catalyst in the transesterification of soybean oil by Nakagaki et al.92 The catalyst displayed high activity towards the transesterification reaction, and afforded a biodiesel yield of 95.6%. The high reactivity of the catalyst is due to the acid sites of Mo(VI), which can easily polarize the O–H bond. Correspondingly, Serio et al.93 also reported the high reactivity of the vanadyl phosphate-based catalyst in the biodiesel synthesis from soybean oil. Regardless of the low surface area, the high reactivity of the catalyst is attributed to the structural/surface morphologies. A biodiesel yield of ≥88% was recorded using the optimal reaction conditions. The dehydrated product of the catalyst VOPO4·2H2O can be converted to VOPO4 simply by calcination at 400–500 °C.
| No. | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). | |||||
| 1 | KOH@NaX zeolite | Soybean oil | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 480 1, 3, 65, 480 |
85.6 | 96 |
| 2 | La/zeolite beta | Soybean oil | 14.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.011, 60, 240 1, 0.011, 60, 240 |
48.9 | 97 |
| 3 | Zeolite X | Sunflower oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 60, 420 1, 10, 60, 420 |
95.1 | 98 |
| 4 | CaO@NaY zeolite | Soybean oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 180 1, 3, 65, 180 |
95 | 99 |
| 5 | Ba–Sr/ZSM-5 | Sunflower oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 180 1, 3, 60, 180 |
87.7 | 100 |
| 6 | H4[W12SiO40]@zeolite Hβ | Soybean oil | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.2, 65, 480 1, 0.2, 65, 480 |
95 | 101 |
| 7 | FA/K-X zeolite | Sunflower oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 480 1, 3, 60, 480 |
83.53 | 102 |
| 8 | Sodalite | Soybean oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 65, 120 1, 4, 65, 120 |
95.5 | 103 |
| 9 | KOH/zeolite | Waste sunflower oil | 11.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 50, 120 1, 6, 50, 120 |
96.7 | 104 |
| 10 | La2O3/NaY zeolite | Castor oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 70, 50 1, 10, 70, 50 |
84.6 | 105 |
In 2007, a NaX zeolite loaded with various concentrations of KOH was synthesized and reported as a catalyst in FAME production from soybean oil.96 A catalyst loaded with 10% KOH followed by heating at 393 K for 3 h gave the best result with 85.6% yield under the optimized reaction conditions. Shu et al.97 prepared the La/zeolite beta using La(NO3)3 as a precursor via ion exchange technique, and was exploited in FAME production from soybean oil. They reported that the La/zeolite beta has higher stability and catalytic activity towards FAME production compared to the zeolite beta catalyst. A yield of 48.9% was obtained using the La/zeolite beta under the optimized reaction conditions, such as the 14.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M/O molar ratio, 0.011 wt% catalyst loading, 60 °C and 4 h time. In the year 2008, Ramos et al.98 studied three zeolites, such as mordenite, beta and X, for the conversion of sunflower oil biodiesel. They examined the effect of different loaded/stacked metals on such zeolites. Zeolite X showed the best catalytic activity, as it has a higher number of super basic sites, which is absent in other zeolites. The effect of the binder, sodium bentonite, on the catalytic reactivity of such zeolites was tested, where the X zeolite was agglomerated and thus, the catalytic activity was slightly reduced. A high yield of 93.5% and 95.1% of FAME was obtained at 60 °C with and without binder, respectively. In another report, Wu et al.99 synthesized a series of CaO supported on zeolites, such as NaY, KL and NaZSM-5 via microwave irradiation, and they were utilized in biodiesel synthesis from soybean oil. They reported that the supported CaO showed a better result compared to the naked CaO, as the supported catalyst has a high surface area, porosity and basic strength. Accordingly, the best result was exhibited by the NaY-supported CaO (30% CaO loaded on NaY) under the optimized reaction conditions.
1 M/O molar ratio, 0.011 wt% catalyst loading, 60 °C and 4 h time. In the year 2008, Ramos et al.98 studied three zeolites, such as mordenite, beta and X, for the conversion of sunflower oil biodiesel. They examined the effect of different loaded/stacked metals on such zeolites. Zeolite X showed the best catalytic activity, as it has a higher number of super basic sites, which is absent in other zeolites. The effect of the binder, sodium bentonite, on the catalytic reactivity of such zeolites was tested, where the X zeolite was agglomerated and thus, the catalytic activity was slightly reduced. A high yield of 93.5% and 95.1% of FAME was obtained at 60 °C with and without binder, respectively. In another report, Wu et al.99 synthesized a series of CaO supported on zeolites, such as NaY, KL and NaZSM-5 via microwave irradiation, and they were utilized in biodiesel synthesis from soybean oil. They reported that the supported CaO showed a better result compared to the naked CaO, as the supported catalyst has a high surface area, porosity and basic strength. Accordingly, the best result was exhibited by the NaY-supported CaO (30% CaO loaded on NaY) under the optimized reaction conditions.
The strontium nanocatalyst supported on ZSM-5 by the incipient wetness impregnation method was prepared and applied in biodiesel synthesis from sunflower oil.100 The authors reported the effect of the calcination temperature and Sr/ZSM-5, Ba–Sr/ZSM-5 mass ratios. Ba–Sr/ZSM-5 (Ba 4 wt% to the Sr weight and Sr 6 wt% to the ZSM-5 weight) exhibited the best performance with 87.7% yield under optimal conditions. In the meantime, Narkhede et al.101 synthesized a series of 12-tungstosilicic acid, SiW12 (10–40 wt%) impregnated on zeolite Hβ, and applied it in biodiesel synthesis from soybean oil. Interestingly, the SEM image of the 30% SiW12/Hβ (Fig. 5b) is similar to the pure zeolite Hβ (Fig. 5a), and revealed that the framework structure of Hβ was retained even after the impregnation of SiW12. This suggested that SiW12 was homogeneously distributed in the framework structure of the Hβ zeolite. They reported a 95% yield of FAME under the optimized reaction conditions.
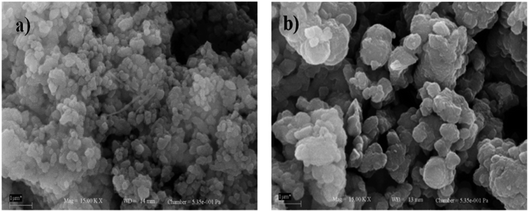 | ||
| Fig. 5 SEM micrographs of (a) Hβ and (b) 30% SiW12/Hβ. Reproduced from ref. 101. | ||
In 2012, Babajide et al.102 synthesized a zeolite derived from fly ash and then ion-exchanged with K to form the FA/K-X zeolite, which was then applied in biodiesel synthesis from sunflower oil. They reported a high yield of 83.53% under the optimized reaction conditions. Similarly, Manique et al.103 prepared zeolite (sodalite) derived from coal fly ash via the hydrothermal process, and utilized in biodiesel synthesis from soybean oil. The developed sodalite has a definite surface area of 10 m2 g−1. They also reported a maximum conversion of 95.5% soybean oil using the optimized reaction conditions. Recently, Al-Jammal et al.104 prepared zeolite derived from zeolite tuft, followed by the impregnation of a series of KOH solutions (1–6 M), and heated at 80 °C for 4 h to form the KOH/zeolite catalyst. Finally, it was utilized in biodiesel synthesis from waste sunflower oil. The catalyst (1–4 M) KOH/zeolite exhibited a biodiesel yield of 96.7% under the reaction conditions: 11.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M/O molar ratio, catalyst amount of 6 wt% w.r.t. oil, 50 °C temperature and reaction time of 2 h.
1 M/O molar ratio, catalyst amount of 6 wt% w.r.t. oil, 50 °C temperature and reaction time of 2 h.
In the same vein, Du et al.105 developed La2O3 impregnated on the NaY zeolite catalyst having a spherical shape of 3–5 mm size, and utilized it in biodiesel synthesis from castor oil. In addition, they explored the impact of the calcination temperature in the range of 600–1000 °C on the biodiesel yield, and observed that the catalyst calcined at 800 °C showed the best result. They also revealed that the incorporation of the surfactant improved the dispersion of La2O3 and the pore size of the zeolite. The XRD patterns of the pure zeolite NaY and the catalyst La2O3/NaY zeolite calcined in the temperature range of 600–1000 °C are displayed in Fig. 6. The XRD patterns of the pure zeolite (Fig. 6a) and the catalyst calcined at 600 °C (Fig. 6b) and 800 °C (Fig. 6c) are almost the same, and revealed that the crystallinity of the zeolite NaY does not change upon the incorporation of La2O3. However, on increasing the temperature to 1000 °C, the XRD pattern (Fig. 6e) showed no characteristic peaks of zeolite, suggesting that at high calcination temperature, the crystallinity of the zeolite is lost.
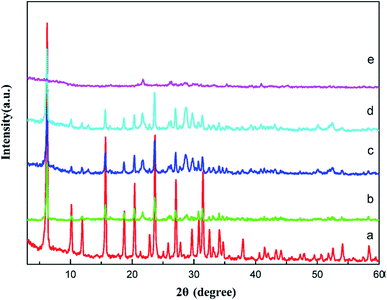 | ||
| Fig. 6 XRD pattern of pure zeolite (a), La2O3/NaY-600 (b), La2O3/NaY-800 (c), S–La2O3/NaY-800 (d), La2O3/NaY-1000 (e). Reproduced from ref. 105. | ||
| No. | Catalyst | Feedstock | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. c NR: not reported. | |||||
| 1 | KI@Al2O3 | Soybean oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 65, 480 1, 2, 65, 480 |
96 | 108 |
| 2 | K@KOH@Al2O3 | Rapeseed oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 60, 60 1, 4, 60, 60 |
84.52 | 110 |
| 3 | K@γ-Al2O3 | Soybean oil | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10.6, 60, 60 1, 10.6, 60, 60 |
96.4 | 111 |
| 4 | KOH/La–Ba–Al2O3 | Microalgae | NR, 25, 60, 180 | 97.7b | 112 |
| 5 | CaO@Al2O3 | Nannochloropsis oculata | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 50, 240 1, 2, 50, 240 |
97.5 | 113 |
| 6 | CaO@Al2O3 | Palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 65, 300 1, 6, 65, 300 |
98.64 | 114 |
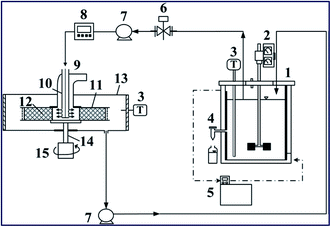 | ||
| Fig. 7 RPB experimental apparatus utilized for the heterogeneously catalyzed transesterification reaction. Components: (1) CSTR reactor; (2) stirrer; (3) thermocouples; (4) sample port; (5) thermostat; (6) control valve; (7) pumps; (8) flow-meter; (9) RPB reactor; (10) stationary liquid distributor; (11) packed-bed rotator; (12) K/g-Al2O3 catalyst; (13) housing case; (14) rotor shaft; (15) motor. Reproduced from ref. 111. | ||
Zhang et al.112 synthesized a KOH-impregnated modified alumina catalyst for biodiesel synthesis from microalgae oil. First, the alumina was modified with lanthanum and barium to increase its surface area, ensure that it possessed the desired pore volume and pore distribution, and finally impregnate KOH on the modified alumina to form the desired catalyst. They reported that the condition of 25% KOH (w.r.t. modified alumina) impregnated on modified alumina and calcined at 550 °C for 4 h showed the best activity towards the transesterification reaction with 97.7% biodiesel yield under the ideal reaction conditions. Umdu et al.113 synthesized CaO@Al2O3via the sol–gel method and conducted a transesterification reaction of microalgae (Nannochloropsis oculata) oil to produce biodiesel. The catalyst has higher reactivity than the bare CaO, which was almost inactive towards transesterification of the desired microalgae. The alumina was loaded with 80 wt% (w.r.t. Al2O3) Ca(NO3)2·4H2O and calcined at 500 °C for 6 h to form 80 wt% CaO@Al2O3 that possessed the highest catalytic activity with 97.5% biodiesel yield. In addition, Zabeti et al.114 synthesized a CaO@Al2O3 catalyst using calcium acetate via calcination at 718 °C for biodiesel synthesis from palm oil. They have used the Response Surface Methodology (RSM) in association with the Central Composite Design (CCD) to determine the optimum reaction conditions, such as the M/O molar ratio, catalyst amount, reaction temperature and reaction time. A biodiesel yield of 98.64% was obtained under the optimum reaction conditions.
Apart from alumina, there are several materials that are used as a catalyst support, such as SiO2, ZrO2 and activated carbon (AC) (Table 9). In 2010, Samart et al.115 conducted the transesterification reaction using CaO impregnated on a mesoporous SiO2 catalyst for FAME production. They also investigated the influence of the CaO amount, and reported that 15 wt% CaO (w.r.t. SiO2) loading showed the maximum yield of 95.2%. In addition, the synthesis of FAME from palm oil using a CaO impregnated on a bimodal meso–macroporous SiO2 support catalyst was reported by Witoon et al.116 They investigated the influence of CaO loading and pellet size on the biodiesel conversion, and also compared with the unimodal SiO2-supported CaO catalyst. CaO in 40 wt% CaO@SiO2 was highly aggregated on the surface of the mesoporous SiO2, and hence increases the surface basicity. In contrast, CaO in 30 wt% CaO@SiO2 was highly dispersed inside the mesopore of the silica support. Accordingly, 40 wt% CaO@SiO2 showed higher FAME yield compared to 30 wt% CaO@SiO2. They also reported that the catalyst with a pellet size of 335 μm showed a maximum yield of 92.45%. Moreover, Wu et al.117 reported on catalysts consisting of three different potassium compounds (KAc, K2CO3 and K2SiO3) impregnated on mesoporous SiO2, such as AlSBA-15 and SBA-15, for the production of FAME from JCO. Three potassium salts with different concentrations were impregnated on AlSBA-15 and SBA-15, and it was found that the basicity lies in the order of 35 wt% K2SiO3@AlSBA-15 > 35 wt% K2CO3@AlSBA-15 > 35 wt% KAc@AlSBA-15. Thus, 30 wt% K2SiO3 showed the highest yield of 95.7% under the optimized reaction conditions.
| No. | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). | |||||
| 1 | CaO/SiO2 | Soybean oil | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 480 1, 5, 60, 480 |
95.2 | 115 |
| 2 | CaO/SiO2 (bimodal) | Palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 240 1, 5, 60, 240 |
94.15 | 116 |
| 3 | K2SiO3@AlSBA- | Jatropha oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15.30, 60, 180 1, 15.30, 60, 180 |
95.7 | 117 |
| 4 | KOH/AC | Corn oil | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.75, 62.5, 60 1, 0.75, 62.5, 60 |
92 | 118 |
| 5 | CaO/AC | WCO | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NR, 60, 480 1, NR, 60, 480 |
94 | 119 |
| 6 | CaO/AC | Vegetable oil | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111, 120, 420 111, 120, 420 |
>90 | 120 |
| 7 | KF/AC | WCO | 8.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 175, 60 1, 3, 175, 60 |
83 | 121 |
| 8 | KOH/AC | Palm oil | 24; 1, 30.3, 64.1, 60 | 98.03 | 122 |
| 9 | K2CO3@KFA | Rapeseed oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 120 1, 3, 65, 120 |
99.6 | 123 |
| 10 | KOH@AC | WCO | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NR, 60, 120 1, NR, 60, 120 |
86.3 | 124 |
| 11 | CaO@AC | Palm oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5.5, 190, 81 1, 5.5, 190, 81 |
80.98 | 125 |
| 12 | KAc/AC | Bitter almond oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.50, 65, 150 1, 2.50, 65, 150 |
93.21 | 126 |
| 13 | KF/CaO/AC | Soybean oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.1, 65, 20 1, 2.1, 65, 20 |
99.9 | 127 |
| 14 | Ag@ZnO | Palm oil | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 60, 60 1, 10, 60, 60 |
96 | 128 |
| 15 | KOH/AC | WCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 120 1, 3, 60, 120 |
96.65 | 129 |
The concept of the AC-based catalyst is an attempt towards the development of a novel alternative to homogeneous alkaline in the form of a heterogeneous catalyst. These kinds of catalysts have pulled in a lot of consideration from the scientific community because the uses of carbon as catalysts not only makes them reusable in the production process, but also greatly reduces the formation of the soap and increases the glycerol purity.118 To date, different kinds of activated carbon-based catalysts have been developed and successfully exploited in biodiesel production, and some of them are briefly discussed here (Table 18). Narowska et al.118 proposed the development of a novel carbon-based catalyst to replace the alkaline homogeneous catalyst as a solid catalyst, which has the potential to be reused multiple times, eliminating various limitations associated with other traditional catalysts. In this context, the authors demonstrated the preparation of FAME from corn oil via transesterification utilizing KOH supported on an activated carbon catalyst. The result showed that the highest yield (92 wt%) of FAME was recorded using optimal reaction conditions. These findings indicated that activated carbon-supported catalysts can be promisingly employed in the transesterification of the waste corn oil using methanol.
Previously, Buasri et al.119 reported on calcium oxide impregnated on the AC catalyst in the synthesis of highly pure FAME from waste cooking palm oil through the continuous transesterification of FFA. After the optimization of various reactions, a maximum FAME yield (94%) was accomplished. In another study, Konwar et al.120 also synthesized AC-supported calcium oxide from the Turbonilla striatula shell. Furthermore, their applicability as a catalyst has been investigated in biodiesel synthesis from vegetable oil. It was reported that the catalyst displayed more than 90% oil conversion under the optimized reaction conditions. Moreover, this approached is economically viable due to the easy recoverability of the catalyst. The catalyst was utilized for five progressive reaction cycles with minimum activity loss.
Hameed et al.121 examined a solid catalyst KF supported on AC for biodiesel synthesis from WCO. They designed a composite rotatable reactor to optimize the reaction parameters, and obtained 83% methyl ester yield. In 2010, Baroutian et al.122 studied FAME synthesis in a packed bed membrane reactor (PBMR) from palm oil using a solid catalyst of KOH supported on AC generated from palm shell (Fig. 8). They also investigated the impact of the reaction parameters using RSM. The highest biodiesel yield of 98.03% was reported using the catalyst with optimized reaction conditions. In addition, Li et al.123 reported the in situ synthesis of K2CO3@KFA via mixing of K2CO3 and kraft lignin (KF), followed by calcination at 800 °C, and utilized the catalyst in biodiesel synthesis from rapeseed oil. They also investigated the influence of the reaction parameters on the FAME production, and reported a maximum yield of 99.6% under the optimized reaction conditions.
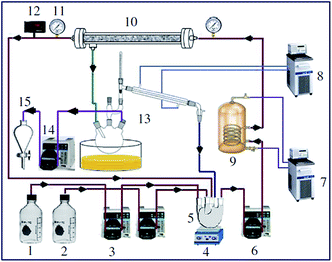 | ||
| Fig. 8 Schematic diagram of PBMR for FAME synthesis. Components: (1) palm oil; (2) methanol; (3) crude material siphon; (4) magnetic stirrer; (5) blending vessel; (6) flowing siphon; (7) boiling water flowing; (8) water chiller; (9) wound thermal exchanger; (10) ceramic membrane; (11) pressure check; (12) temperature indicator; (13) methanol recuperation unit; (14) siphon; (15) isolating funnel. Reproduced from ref. 122. | ||
Furthermore, Buasri et al.124 conducted a synthesis process, where a solution of KOH was mixed with activated carbon (AC) originated from coconut shell to form KOH@AC, and used this catalyst in biodiesel synthesis from WCO. The authors claimed that the synthesized catalyst has extraordinary catalytic reactivity, and showed 86% biodiesel yield under the optimized reaction conditions. Similarly, Wan et al.125 examined a solid base catalyst CaO@AC for FAME synthesis from palm oil. RSM was utilized to investigate the impact of the reaction parameters on biodiesel synthesis. A maximum yield of 80.98% was reported under the optimal reaction conditions, and also claimed that the catalyst can retain its activity even after two cycles. Recently, Fadhil et al.126 conducted a transesterification reaction of bitter almond oil to produce biodiesel using KAc impregnated on activated carbon originated from the waste of polyethylene terephthalate. A maximum yield of 93.21% with high purity was reported. The authors claimed that the catalyst showed excellent reactivity towards biodiesel synthesis compared to other reported solid base catalysts, as the catalyst showed a very high yield in very suboptimal reaction conditions. Moreover, according to the authors, the catalyst has great stability as it can be reused for 6 cycles.
Liu et al.127 examined a solid base catalyst KF/CaO/AC calcined at 500 °C for 5 h for the conversion of soybean oil to biodiesel. The authors claimed that the main catalytic role was played by K2O and KCaF3, which are present in the catalyst. The catalyst demonstrated a high yield of 99.9% in only 20 min. Nonetheless, they reported that the catalyst is highly sensitive towards the water contents in methanol and oleic acid. Therefore, it is necessary to use anhydrous oil and methanol to overcome this problem. In conclusion, from all of these above-mentioned studies, a collective inference can be drawn that the activated carbon-based catalysts will be the next-generation novel alternative to traditionally available catalysts for the efficient transesterification of different oils.
In the meantime, the application of zinc oxide-supported silver nanoparticles (ZnO@Ag NPs) as a solid catalyst for the conversion of palm oil to FAME was reported by Laskar et al.128 The transformation of palm oil to FAME was confirmed using NMR analysis and 10 components of FAME were identified using GC-MS technique, with methyl octadecanoate (C18:0) being the major component. A mixture with different ratios of Ag on ZnO were prepared, where 10 wt% ZnO@Ag was found to be the most active catalyst producing 96% FAME under the optimum reaction conditions. In the recent past, Taslim et al.129 also demonstrated the efficacy of low-cost AC-based catalysts developed from candlenut shells (an agricultural waste) through the impregnation of KOH for biodiesel production from WCO. The results obtained have shown a yield of biodiesel up to 96.65% using the optimized reaction conditions.
| No. | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b NR = not reported. | |||||
| 1 | Mg–Al HT | Sunflower oil | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 60, 480 1, 2, 60, 480 |
92 | 132 |
| 2 | Mg–Al HT | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 65, 240 1, 1.5, 65, 240 |
90.5 | 133 |
| 3 | Mg–Al HT | WCO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 80, 150 1, 1.5, 80, 150 |
95.2 | 134 |
| 4 | Mg/Al–CO3 | Microalgae oil | 6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.7, 66, 240 1, 1.7, 66, 240 |
90.3 | 135 |
| 5 | K/Mg–Al HT | Palm oil | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, 100, 360 1, 7, 100, 360 |
86.6 | 136 |
| 6 | Zn–Al HT | Soybean oil | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NR, 140, 60 1, NR, 140, 60 |
76 | 137 |
| 7 | KF/Ca–Al | Palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 300 1, 5, 65, 300 |
97.98 | 138 |
| 8 | Mg–Al HT | Poultry fat | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 120, 120 1, 10, 120, 120 |
75 | 139 |
| 9 | Mg–Al HT | Jatropha oil | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 160, 240 1, 5, 160, 240 |
93.4 | 140 |
| 10 | Zn5(OH)8(NO3)2·2H2O | Palm oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 140, 120 1, 2, 140, 120 |
96.5 | 141 |
Zeng et al.133 reported on Mg–Al hydrotalcite with various Mg/Al molar ratios, and used them as a heterogeneous catalyst for the transesterification of soybean oil. The hydrotalcite calcined at 773 K and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg-to-Al molar ratio exhibited the highest catalytic activity with 90.5% conversion of oil. Recently, Ma et al.134 investigated a heterogeneous catalyst Mg–Al hydrotalcite in the production of biodiesel from WCO. They mentioned that the catalyst with a Mg/Al molar ratio of 3
1 Mg-to-Al molar ratio exhibited the highest catalytic activity with 90.5% conversion of oil. Recently, Ma et al.134 investigated a heterogeneous catalyst Mg–Al hydrotalcite in the production of biodiesel from WCO. They mentioned that the catalyst with a Mg/Al molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and calcined at 500 °C has a high surface area, excellent crystallinity and mesoporous structure, and subsequently showed excellent activity. They also reported 95.2% FAME yield under the optimized reaction condition. In the same manner, Zeng et al.135 prepared Mg/Al–CO3 with a Mg/Al molar ratio of 4
1 and calcined at 500 °C has a high surface area, excellent crystallinity and mesoporous structure, and subsequently showed excellent activity. They also reported 95.2% FAME yield under the optimized reaction condition. In the same manner, Zeng et al.135 prepared Mg/Al–CO3 with a Mg/Al molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via urea method, and compared their structures and catalytic activities with those prepared by co-precipitation for the biodiesel synthesis from microalgae oil. They studied the crystal size and surface basicity of all of the prepared hydrotalcites, and reported that the crystal size of the hydrotalcites prepared using the urea method is greater than the as-synthesized ones. They also reported that the mixed oxide of the hydrotalcite prepared via urea method showed the highest catalytic reactivity with the maximum conversion of 90.30%.
1 via urea method, and compared their structures and catalytic activities with those prepared by co-precipitation for the biodiesel synthesis from microalgae oil. They studied the crystal size and surface basicity of all of the prepared hydrotalcites, and reported that the crystal size of the hydrotalcites prepared using the urea method is greater than the as-synthesized ones. They also reported that the mixed oxide of the hydrotalcite prepared via urea method showed the highest catalytic reactivity with the maximum conversion of 90.30%.
Furthermore, the Mg–Al hydrotalcite loaded with 1.5% K was prepared and used as a catalyst for the synthesis of biodiesel from palm oil.136 A maximum 86.6% yield was reported using the optimized reaction conditions. They also studied the effect of the synthesized biodiesel on six types of elastomers, such as NBR, HNBR, NBR/PVC, acrylic rubber, co-polymer FKM, and terpolymer FKM, which are commonly found in the fuel system. For testing, the elastomers were immersed in B10 (10% biodiesel in diesel) and found that only terpolymer FKM and co-polymer FKM showed a slight change in the properties. Thus, it was concluded that B10 is compatible with the diesel engines without any modification. In another work, Liu et al.137 prepared Zn–Al hydrotalcite within the temperature range of 413–773 K to form dehydrated Zn–Al hydrotalcite and Zn–Al mixed oxides, and used both catalysts in the transesterification reaction in a fixed-bed reactor. The OH groups in the dehydrated Zn–Al are responsible for the high basicity of the catalyst. However, the Mn+–O2− pairs and isolated O2− anions are the main basic sites in the Zn–Al metal oxides. Furthermore, they compared the catalytic activity of both dehydrated Zn–Al HT and Zn–Al oxides, and found that the dehydrated HT calcined at 473 K showed the highest catalytic activity and stability towards biodiesel synthesis with a maximum yield of 76% at 140 °C for 1 h. Similarly, a heterogeneous base catalyst, KF/Ca–Al was developed for the biodiesel production from palm oil.138 The catalyst was prepared from layered double hydroxides of Ca–Al, where the introduction of KF enhanced the catalytic activity. It was observed that 100 wt% loading of KF decreased the particle size of the catalyst, as shown by the SEM image of KF/Ca–Al (Fig. 9). The authors also reported a biodiesel yield of 97.14% under the optimized reaction conditions. Besides, biodiesel production from poultry fats was reported by using a solid base catalyst, Mg–Al hydrotalcite.139 The influence of the calcination temperature for the preparation of the catalyst was investigated, and it was disclosed that the catalyst calcined at 550 °C showed the maximum catalytic activity. Moreover, the authors detailed that the rehydration of the catalyst before the transesterification reaction and preferential adsorption of TAGs on the surface of the catalyst reduced the catalytic activity.
 | ||
| Fig. 9 SEM image of KF/Ca–Al. Reproduced from ref. 138. | ||
Helwani et al.140 synthesized a Mg–Al hydrotalcite via combustion method using saccharose for biodiesel synthesis from JCO. The SEM image of the catalyst calcined at 850 °C displays a lamellar microstructure with closely packed flakes (Fig. 10). The catalyst calcined at 850 °C and recrystallized with 20% saccharose fuel showed the best reactivity with 75.2% biodiesel conversion under the optimized reaction conditions. A layered double hydroxide of zinc hydroxide nitrate was also reported for FAME synthesis from palm oil.141 The catalyst showed excellent reactivity towards the transesterification reaction with 96.5% biodiesel yield.
 | ||
| Fig. 10 SEM image of Mg–Al HT calcined at 850 °C. Reproduced from ref. 140. | ||
| No. | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, Catalyst loading (wt%), temperature (°C), reaction time (min). | |||||
| 1 | CaO–CeO2 | Rapeseed oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 60, 600 1, 10, 60, 600 |
90 | 144 |
| 2 | La2O3/ZrO2 | Sunflower oil | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 21, 200, 300 1, 21, 200, 300 |
84.9 | 145 |
| 3 | TiO2–MgO | WCO | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 160, 360 1, 10, 160, 360 |
92.3 | 146 |
| 4 | SrO/SiO2 | Olive oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 10 1, 5, 65, 10 |
95 | 147 |
| 5 | SrO/CaO | Olive oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 20 1, 5, 65, 20 |
95 | 147 |
| 6 | TiO2–ZnO | Palm oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 14, 60, 300 1, 14, 60, 300 |
92 | 148 |
| 7 | ZnO–La2O3 | Waste oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.3, 200, 180 1, 2.3, 200, 180 |
96 | 149 |
| 8 | CaO–ZnO | Palm kernel oil | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 60, 60 1, 10, 60, 60 |
>94 | 150 |
| 9 | MgO–ZrO2 | Soybean oil | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 150, 360 1, 3, 150, 360 |
99 | 151 |
| 10 | ZrO2@SiO2 | Stearic acid | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 120, 180 1, 10, 120, 180 |
48.6 | 152 |
| 11 | SiO2/ZrO2 NP | Soybean oil | 6.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.8 mmol, 50, 180 1, 2.8 mmol, 50, 180 |
96.2 ± 1.4 | 153 |
| 12 | MgO–CaO | Sunflower oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5, 60, 60 1, 2.5, 60, 60 |
92 | 154 |
Kawashima et al.144 investigated various calcium-containing catalysts (CaTiO3, CaMnO3, Ca2Fe2O5, CaZrO3, and CaO–CeO2) in the biodiesel production from rapeseed oil. Among these, CaO–CeO2 showed excellent results (approximately 90% yield) with high stability compared to the other calcium-containing heterogeneous catalysts under the optimized reaction conditions. The catalyst can be reused for 7 times with a high yield of >80% each time. Sun et al.145 also prepared a La2O3-loaded ZrO2 catalyst by varying the La2O3 amount from 7 to 28 wt%, and investigated for the synthesis of biodiesel. The conditions of 21 wt% La2O3 loading on ZrO2 and calcination at 600 °C demonstrated the highest catalytic activity towards biodiesel production from sunflower oil. The authors proposed a model for the preparation of the catalyst, where La(NO3)3 was impregnated on the surface of ZrO2, followed by drying to form a film of La(NO3)3, which upon calcination forms the La2O3/ZrO2 composite, resulting in a decrease in the particle size due to the t/m phase transition (Fig. 11). A high oil conversion of 96% and 84.9% FAME yield was observed under optimal reaction conditions. They reported an excellent activity of the catalyst prepared by 21 wt% loaded La2O3 and calcined at 600 °C.
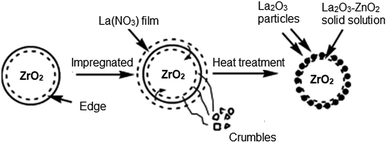 | ||
| Fig. 11 Proposed model for the solid-state reaction on the catalyst surface. Reproduced from ref. 145. | ||
Wen et al.146 obtained the TiO2–MgO catalyst via the sol–gel method, and employed it in the FAME synthesis from WCO. Substitution of Ti to the Mg lattice led to defects in the surface of the catalyst, and enhanced both the activity and stability of the catalyst. It was revealed that the catalyst with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ti to Mg molar ratio, and calcined at 923 K is the most active one in FAME synthesis. A biodiesel yield of 92.3% was observed when utilizing the catalyst MT-1-923 and the optimal reaction conditions. Similarly, SrO/SiO2 and SrO/CaO have been synthesized, and their catalytic activity was compared with naked SrO in transesterification of olive oil by Chen et al.147 Although the naked SrO showed very good catalytic activity and afforded 82% yield in just 15 min, the biodiesel yield shrank to 68.9% when the reaction was performed for 3 h. They reported that the reason for the unusual decrease in biodiesel yield was due to a reverse reaction between FAME and glycerol, which showed that the catalyst not only catalyzed the forward reaction, but also catalyzed the reverse reaction. In contrast, modification of SrO with SiO2 and CaO provided excellent activity, as well as high stability. They observed that around 95% conversion was obtained at 65 °C using SrO/SiO2 and SrO/CaO in 10 and 20 min, respectively. However, they reported that on decreasing the reaction temperature to 45 °C, SrO/CaO showed only 20.20% conversion as compared to SrO/SiO2, which showed 76.9% conversion. Thus, SrO/SiO2 displayed better reactivity towards the transesterification of olive oil than SrO/CaO, and possessed high tolerance to the water content and FFA of the biodiesel feedstocks.
1 Ti to Mg molar ratio, and calcined at 923 K is the most active one in FAME synthesis. A biodiesel yield of 92.3% was observed when utilizing the catalyst MT-1-923 and the optimal reaction conditions. Similarly, SrO/SiO2 and SrO/CaO have been synthesized, and their catalytic activity was compared with naked SrO in transesterification of olive oil by Chen et al.147 Although the naked SrO showed very good catalytic activity and afforded 82% yield in just 15 min, the biodiesel yield shrank to 68.9% when the reaction was performed for 3 h. They reported that the reason for the unusual decrease in biodiesel yield was due to a reverse reaction between FAME and glycerol, which showed that the catalyst not only catalyzed the forward reaction, but also catalyzed the reverse reaction. In contrast, modification of SrO with SiO2 and CaO provided excellent activity, as well as high stability. They observed that around 95% conversion was obtained at 65 °C using SrO/SiO2 and SrO/CaO in 10 and 20 min, respectively. However, they reported that on decreasing the reaction temperature to 45 °C, SrO/CaO showed only 20.20% conversion as compared to SrO/SiO2, which showed 76.9% conversion. Thus, SrO/SiO2 displayed better reactivity towards the transesterification of olive oil than SrO/CaO, and possessed high tolerance to the water content and FFA of the biodiesel feedstocks.
In the recent past, Madhuvilakku et al.148 developed a TiO2–ZnO nanocatalyst and utilized it in the FAME synthesis from palm oil. The arrangement of deformities on the catalyst surface as a result of the substitution of Ti on the Zn grid improved the reactivity and stability of the prepared catalyst. They recorded that 92% biodiesel yield was acquired under the optimized reaction conditions. Similarly, a series of ZnO–La2O3 catalysts have been examined in the biodiesel synthesis from waste oil by Yan et al.149 Incorporation of La promoted the dispersion of ZnO and improved the acidic-basic sites, thereby increasing the catalytic activity towards both transesterification and esterification reactions. The molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn to La showed the highest activity towards biodiesel production. A high yield of 96% was reported under the optimal reaction conditions. The authors also reported that the catalyst could endure FFA and water contents, and thus allowed for the direct conversion of waste oil to FAME. In another work, the transesterification of palm kernel oil to produce biodiesel was also reported using a mixed metal oxide solid base catalyst CaO–ZnO.150 Upon incorporation of Zn to the CaO phase, the particle size of the catalyst decreased and reduced the calcination temperature required for the decomposition of carbonates to their oxides. The lowering of the calcination temperature for the decomposition of CaCO3 upon the incorporation of Zn can be explained by the particle size reduction coupled with a loss of H2O and CO2 from the zinc carbonate. The schematic representation for the decomposition of CaCO3 and formation of CaO–ZnO mixed metal oxides is displayed in Scheme 3. It is well known that decarbonisation is a reversible process, which mostly depends on atmospheric CO2, particle size and composition. The dissociation of CO2 normally occurs in the outer surface (Scheme 3A). Moreover, upon calcination, the evolved CO2 may form a layer on the surface of the material during the continuous disjunction of inner particles, generating a possibility for recarbonation of CaO to CaCO3 (Scheme 3B). However, incorporation of ZnCO3 resulted in the formation of voids due to its decomposition to zinc oxide. The resulting voids facilitated heat transfer to the interior particles and evaporation of the gaseous compounds. Moreover, due to the small particle size of CaO–ZnO, the diffusion distance of CO2 decreased, and thus the calcination temperature also decreased.
1 Zn to La showed the highest activity towards biodiesel production. A high yield of 96% was reported under the optimal reaction conditions. The authors also reported that the catalyst could endure FFA and water contents, and thus allowed for the direct conversion of waste oil to FAME. In another work, the transesterification of palm kernel oil to produce biodiesel was also reported using a mixed metal oxide solid base catalyst CaO–ZnO.150 Upon incorporation of Zn to the CaO phase, the particle size of the catalyst decreased and reduced the calcination temperature required for the decomposition of carbonates to their oxides. The lowering of the calcination temperature for the decomposition of CaCO3 upon the incorporation of Zn can be explained by the particle size reduction coupled with a loss of H2O and CO2 from the zinc carbonate. The schematic representation for the decomposition of CaCO3 and formation of CaO–ZnO mixed metal oxides is displayed in Scheme 3. It is well known that decarbonisation is a reversible process, which mostly depends on atmospheric CO2, particle size and composition. The dissociation of CO2 normally occurs in the outer surface (Scheme 3A). Moreover, upon calcination, the evolved CO2 may form a layer on the surface of the material during the continuous disjunction of inner particles, generating a possibility for recarbonation of CaO to CaCO3 (Scheme 3B). However, incorporation of ZnCO3 resulted in the formation of voids due to its decomposition to zinc oxide. The resulting voids facilitated heat transfer to the interior particles and evaporation of the gaseous compounds. Moreover, due to the small particle size of CaO–ZnO, the diffusion distance of CO2 decreased, and thus the calcination temperature also decreased.
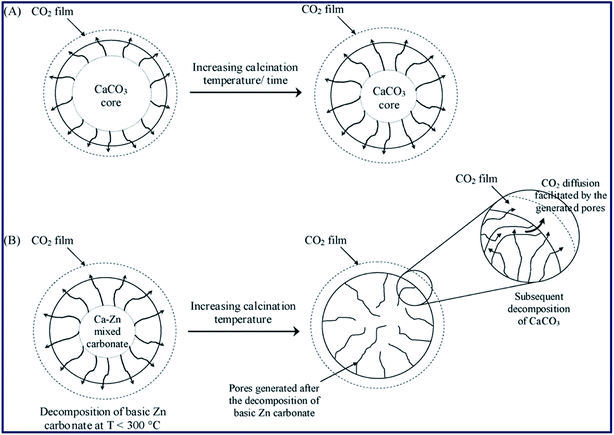 | ||
| Scheme 3 Proposed models for CaCO3 decomposition to CaO (A) and mixed precipitate of Ca–Zn (B). Reproduced from ref. 150. | ||
Among solid base catalysts, solid ZrO2 catalysts became popular because of their environmentally benign nature and economic viability for biodiesel production. To date, different types of ZrO2 catalysts have been developed for use in biodiesel production. In this line, Su et al.151 synthesized microporous solid base MgO–ZrO2 composites and utilized them as effective heterogeneous catalysts in biodiesel synthesis. They claimed that such microporous catalysts are of great significance as the presence of porous materials in the preparation of these catalysts provided the ability to interact with atoms, ions, and molecules.
Recently, Ibrahim et al.152 examined the influence of different support materials like Al2O3, Fe2O3, TiO2 and SiO2 on the physicochemical properties and efficacy of the ZrO2 solid catalysts commonly used in biodiesel synthesis. From the results obtained, it was revealed that ZrO2 supported on SiO2 showed the highest conversion rate due to a comparatively high surface area and a high number of Lewis acid sites. In another study, Faria et al.153 developed a nanosized catalyst mixed metal oxides SiO2/ZrO2 catalyst prepared via sol–gel strategy, and examined its reactivity in the synthesis of biodiesel from soybean oil. It was observed that this catalyst displayed promising reactivity and gave 96.2 ± 1.4% biodiesel yield after 3 h of reaction time. In addition, the catalyst can be reused for 6 progressive cycles with little drop in activity. In 2008, Albuquerque et al.154 synthesized MgO–CaO mixed metal oxides with different Mg/M (M = Al or Ca) molar ratios, and used it as a highly active catalyst for the transformation of sunflower oil to biodiesel in 92% yield under the optimized reaction conditions. The highest activity towards the transesterification reaction was found for a bulk Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca molar ratio of 3.8, whereas bare CaO was found to afford a lower yield of biodiesel under the same reaction conditions. The authors attributed this interesting activity to the higher BET surface area of the MgO–CaO mixed metal oxide (12.8 m2 g−1), in comparison to CaO (1.2 m2 g−1).
Ca molar ratio of 3.8, whereas bare CaO was found to afford a lower yield of biodiesel under the same reaction conditions. The authors attributed this interesting activity to the higher BET surface area of the MgO–CaO mixed metal oxide (12.8 m2 g−1), in comparison to CaO (1.2 m2 g−1).
7.1.7.1 Waste shells. Although several of the chemically synthesized heterogeneous catalysts mentioned earlier show promising and comparatively high biodiesel yield, their synthesis routes are sometimes complicated, expensive, chemically wasteful, time consuming and non-economical. Therefore, with the growing high demand for renewable energy, there is a need to search for an ideal heterogeneous catalyst that is easy to synthesize, non-toxic, low cost, widely available, biodegradable and eco-friendly in nature, yet exhibits high catalytic activity in biodiesel production. In light of this, the utilization of CaO (derived from the high-temperature calcination of waste shells containing CaCO3) has been a front-runner in recent times. The use of waste shells as a source of CaO not only make the whole production of biodiesel sustainable, but also solved the problem associated with the waste disposal of huge quantities of waste shell generated due to human consumption.
7.1.7.1.1 Eggshell. Various eggshell-derived heterogeneous catalysts are available for the transformation of edible/non-edible oils to FAME, as listed in Table 12. For the first time, CaO originated from chicken eggshell calcined at 1000 °C was utilized for biodiesel synthesis by Wei et al.161 Biodiesel yield greater than 95% was obtained. They calcined the eggshell at different temperatures from 200 °C to 1000 °C, and then tested their efficacy for the transformation of soybean oil to FAME. They observed that those calcined above 800 °C were the most active catalysts, where the XRD spectra display a crystalline CaO (Fig. 12). Samples calcined at 700 °C for 2 h contain CaCO3 as the principal constituent and CaO as a minor one; hence, a medium yield (90%) was obtained. Calcinations below 600 °C did not result in the formation of CaO; hence, low catalytic activity was observed (<30% biodiesel yield). Hence, CaO in the catalyst is the principal basic constituent, which led to the high reactivity of the catalyst. From this experiment, it is suggested that waste shells have to be calcined at a temperature of at least 800 °C for 2 h to fully convert CaCO3 to CaO, a highly basic catalyst.
| No. | Catalyst source | Catalyst | Feedstock | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. c NR = not reported, WCPO = waste cooking palm oil. | ||||||
| 1 | Chicken eggshell | CaO | Soybean oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 180 1, 3, 65, 180 |
>95 | 161 |
| 2 | Chicken eggshell | CaO | Soybean oil | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, 57.5, 120 1, 7, 57.5, 120 |
93 | 216 |
| 3 | Chicken eggshell | CaO | Soybean oil | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 65, 180 1, 10, 65, 180 |
90 | 163 |
| 4 | Chicken eggshell | CaO | Soybean oil | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 60, 180 1, 4, 60, 180 |
91 | 164 |
| 5 | Ostrich eggshell | CaO | Karanja oil | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5, 65, 150 1, 2.5, 65, 150 |
95 | 165 |
| 6 | Chicken eggshell | CaO | WCO | 22.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3.5, 65, 330 1, 3.5, 65, 330 |
91 | 166 |
| 7 | Chicken eggshell | CaO | WCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 65, 120 1, 1.5, 65, 120 |
94 | 167 |
| 8 | Chicken eggshell | CaO | WCO | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 65, 120 1, 2, 65, 120 |
NR | 168 |
| 9 | Chicken eggshell | CaO | WFO | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 180 1, 3, 65, 180 |
95.05 | 169 |
| 10 | Chicken eggshell | CaO | WCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 60, 60 1, 1.5, 60, 60 |
96.23 | 191 |
| 11 | Chicken eggshell | CaO | WCO | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 60, 240 1, 4, 60, 240 |
100 | 217 |
| 12 | Chicken eggshell | CaO | WCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 60 1, 5, 65, 60 |
94.52b | 172 |
| 13 | Chicken eggshell | CaO | WCO | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 60, 50 1, 1.5, 60, 50 |
96.07 | 173 |
| 14 | Chicken eggshell | CaO | WCO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 30 1, 3, 60, 30 |
97.50 | 174 |
| 15 | Chicken eggshell | CaO | WCO | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 165 1, 5, 65, 165 |
87.8 | 175 |
| 16 | Chicken eggshell | CaO | WCO | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 65, 420 1, 6, 65, 420 |
75.92 | 218 |
| 17 | Chicken eggshell | CaO | Palm oil | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 60, 90 1, 10, 60, 90 |
>90 | 176 |
| 18 | Chicken eggshell | CaO | Palm oil | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15, 900 W, 4 1, 15, 900 W, 4 |
96.7 | 177 |
| 19 | Chicken eggshell | CaO | Palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 60, 120 1, 10, 60, 120 |
94.1 | 178 |
| 20 | Chicken eggshell | CaO | Palm oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, NR, 30 1, 5, NR, 30 |
95 | 179 |
| 21 | Chicken eggshell | CaO | Rape seed oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 180 1, 3, 60, 180 |
96 | 180 |
| 22 | Chicken eggshell | CaO | Rapeseed oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 60, 60 1, 4, 60, 60 |
95.12 | 181 |
| 23 | Chicken eggshell | CaO | Sunflower oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 180 1, 3, 60, 180 |
96 | 182 |
| 24 | Chicken eggshell | CaO | Sunflower oil | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 3 1, 5, 60, 3 |
83.2 | 183 |
| 25 | Chicken eggshell | CaO | Sunflower oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 240 1, 3, 60, 240 |
97.75 | 219 |
| 26 | Chicken eggshell | CaO | Sunflower oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 60, 180 1, 2, 60, 180 |
100 | 185 |
| 27 | Chicken eggshell | CaO | JCO | 81, 2, 65, 150 | 90 | 186 |
| 28 | Chicken eggshell | CaO | Microalgae Chlorella vulgaris | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.39, 70, 180 1, 1.39, 70, 180 |
92.03 | 187 |
| 29 | Chicken eggshell | CaO | Microalgae | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.7, 70, 216 1, 1.7, 70, 216 |
86.41 | 188 |
| 30 | Chicken eggshell | CaO | Micro algae/S. armatus | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.61, 75, 240 1, 1.61, 75, 240 |
90.44 | 189 |
| 31 | Chicken eggshell | CaO | Chicken fat | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8.5, 57.5, 300 1, 8.5, 57.5, 300 |
90.41 | 190 |
| 32 | Chicken eggshell | CaO | Catfish oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 60, 60 1, 1.5, 60, 60 |
87.77 | 191 |
| 33 | Chicken eggshell | CaO | Helianthus annuus L oil | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5, 65, 120 1, 2.5, 65, 120 |
99.2 | 192 |
| 34 | Chicken eggshell | CaO | Cotton oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 180 1, 3, 60, 180 |
98.08 | 193 |
| 35 | Chicken eggshell | CaO | C. sativa oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 65, 120 1, 1, 65, 120 |
97.2 | 194 |
| 36 | Chicken eggshell | CaO | C. inophyllum L oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3.88, MW, 12.47 1, 3.88, MW, 12.47 |
98.90 | 195 |
| 37 | Chicken eggshell | CaO/W/Mo | WCO | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 70, 120 1, 2, 70, 120 |
96.2 | 196 |
| 38 | Chicken eggshell | CaO/anthill | WCO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 120 1, 5, 60, 120 |
70 | 197 |
| 39 | Chicken eggshell | CaO/Zn | WCO | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 240 1, 5, 65, 240 |
96.74 | 198 |
| 40 | Chicken eggshell | CaO/KF/Fe3O4 | WCO | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 65, 120 1, 6, 65, 120 |
97 | 199 |
| 41 | Chicken eggshell | CaO/SiO2 based on PEFB | WCO | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8, 60, 90 1, 8, 60, 90 |
96 | 200 |
| 42 | Chicken eggshell | Mo–Zr/CaO | WCPO | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 80, 180 1, 3, 80, 180 |
90.1 | 201 |
| 43 | Chicken eggshell | ZnO/CaO | JCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 60 1, 5, 65, 60 |
98.2 | 164 |
| 44 | Chicken eggshell | CaO NPs | JCO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 90, 120 1, 2, 90, 120 |
98 | 202 |
| 45 | Chicken eggshell | Ky(MgCa)2xO3 | Palm oil | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5.53, 65, 273 1, 5.53, 65, 273 |
88 | 203 |
| 46 | Chicken eggshell | CaO/SiO2 | Palm oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 9, 65, 480 1, 9, 65, 480 |
80.21 | 204 |
| 47 | Chicken eggshell | CaO/SiO2 | Palm oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 120 1, 3, 60, 120 |
87.5 | 205 |
| 48 | Chicken eggshell | CaO/Rice husk | Palm oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, 65, 240 1, 7, 65, 240 |
91.5 | 206 |
| 49 | Chicken eggshell | CaO/Coconut waste | Palm oil | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 180 1, 5, 65, 180 |
81 | 207 |
| 50 | Chicken eggshell | Li/CaO | Nahor oil | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 240 1, 5, 65, 240 |
94 | 208 |
| 51 | Chicken eggshell | CaO/Zn | Eucalyptus oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 150 1, 5, 65, 150 |
93.2 | 209 |
| 52 | Chicken eggshell | CaO/KF/Fe3O4 | Neem oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 65, 120 1, 6, 65, 120 |
97 | 199 |
| 53 | Chicken eggshell | CaO/fly ash | Soybean oil | 6.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 70, 300 1, 1, 70, 300 |
96.97 | 210 |
| 54 | Chicken eggshell | CaO/KF | Soybean oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 65, 120 1, 2, 65, 120 |
99.1 | 211 |
| 55 | Chicken eggshell | Na/CaO | Madhuca indica oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 120 1, 5, 60, 120 |
81.1 | 212 |
| 56 | Ostrich eggshell | CaO | Palm oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8, 60, 60 1, 8, 60, 60 |
92.7 | 213 |
| 57 | Duck eggshell | CaO | SODD | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 60, 80 1, 10, 60, 80 |
94.6 | 2 |
| 58 | Quail eggshell | CaO | Palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 65, 120 1, 1.5, 65, 120 |
98 | 214 |
| 59 | Quail eggshell/crab shell | CaO | Jatropha oil | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, MW, 5 1, 4, MW, 5 |
94 | 215 |
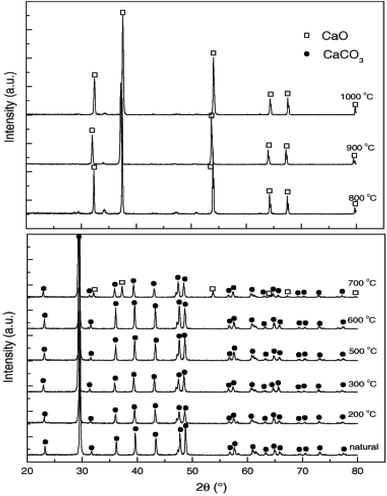 | ||
| Fig. 12 XRD patterns of natural eggshell and the materials obtained by calcining natural eggshell in the range of 200–1000 °C. Reproduced from ref. 161. | ||
In recent years, CaO derived from eggshell has been widely investigated in the transformation of various edible/non-edible oils, such as soybean oil,162–164 karanja oil,165 WCO,166–175 palm oil,176–179 rapeseed oil,180,181 sunflower oil,182–185 JCO,186 microalgae oil,187–189 chicken fat,190 catfish oil,191Helianthus annuus L oil,192 cotton oil193 and sativa oil194 for FAME production. In 2014, Niju et al.172 examined a highly active modified chicken eggshell derived CaO catalyst for the synthesis of FAME from WFO. The authors reported that highly reactive CaO can be obtained from eggshells via calcination–hydration–dehydration treatment. While the FAME conversion was only 67.57% for the commercial CaO catalyst, CaO obtained from the eggshell calcined at 900 °C followed by hydration and dehydration at 600 °C (eggshell-CaO-900-600) gave 94.52% conversion under the optimized reaction conditions. Calcination followed by hydration and dehydration greatly increased the surface area of the eggshell-derived CaO as compared to those obtained with the only calcination. The high activity of the modified CaO (eggshell-CaO-900-600) is attributed to the high surface area (8.6401 m2 g−1) compared to both commercial CaO (3.0022 m2 g−1) and eggshell derived-CaO calcined at 900 °C (eggshell-CaO-900) (3.7262 m2 g−1). The basicity of the modified catalyst lies in the region 12.2 < H_ < 15.0. Fig. 13b depicts the SEM image of CaO generated from the calcination–hydration–dehydration treatment of eggshells (i.e., egg shell-CaO-900-600), which shows a honeycomb-like porous surface. However, in the case of eggshell-CaO-900, a rod-like structure with microporous particles (size ranging from 1.29 to 2.0 μm) was observed (Fig. 13a).
 | ||
| Fig. 13 SEM image of (a) eggshell-CaO-900 and (b) eggshell-CaO-900-600. Reproduced from ref. 172. | ||
In another work, waste chicken fat obtained from a slaughterhouse was converted to FAME using calcined chicken eggshell catalyst under microwave irradiation (Fig. 14).190 Esterification was carried out to lessen the FFA content of the chicken oil below 1 mg KOH per g of oil, followed by transesterification to yield FAME. A flow diagram of the biodiesel production using chicken eggshell as a catalyst is presented in Fig. 15. Optimization of the transesterification process parameters by response surface methodology was performed.
 | ||
| Fig. 14 Microwave-assisted synthesis of FAME using an eggshell catalyst. Reproduced from ref. 190. | ||
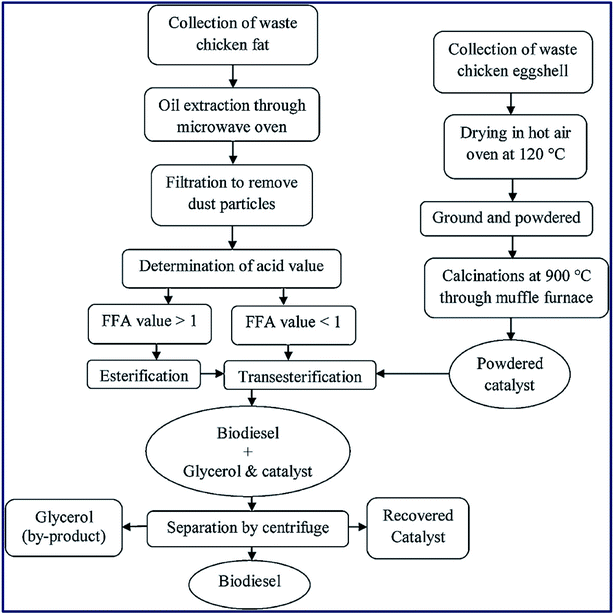 | ||
| Fig. 15 Flow diagram of biodiesel production utilizing chicken eggshell catalyst. Reproduced from ref. 190. | ||
Similarly, Helianthus annuus L oil was converted to FAME using eggshell-derived CaO.192 The preparation route of CaO starting from the shell is presented in Fig. 16. Under the optimized reaction conditions, 99.2% of the FAME yield was achieved. The catalyst is stable up to the fourth cycle, where 87.8% yield was observed.
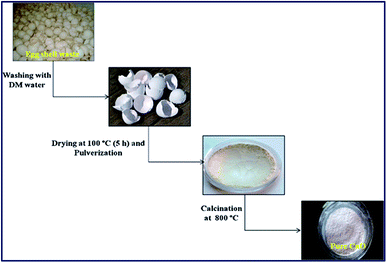 | ||
| Fig. 16 Schematic layout for eggshell-originated CaO synthesis. Reproduced from ref. 192. | ||
Earlier, Ansori et al.195 reported a chicken shell-derived CaO catalyzed synthesis of FAME from C. inophyllum L oil under a microwave (MW) irradiation. Initially, the oil FFA content was pre-esterified utilizing H2SO4, which was then transesterified by utilizing the CaO catalyst (originated from chicken shell), and they reported 98.90% FAME yield in 12.47 min. In another work, Mansir et al.196 examined the application of the W/Mo/CaO catalyst, where tungsten and molybdenum were impregnated on CaO derived from waste eggshell, for the transformation of WCO via a concerted esterification/transesterification to produce FAME in a one-pot process. Moreover, the authors investigated the influence of W and Mo loading on CaO in its catalytic activity, and found that catalytic activity increased when the wt% of W was higher than the wt% of Mo over the range of 0.3–0.7%. A maximum yield of 96.2% was reported under the optimum reaction conditions using 0.6 W/0.4 Mo/CaO. In addition, several studies in the literature are available for the transesterification of WCO having FFA content in the range of 4–7.1% to produce the methyl ester using various eggshell-derived CaO catalysts impregnated with acidic and basic compounds. Examples of such catalysts are CaO/anthill,197 CaO/Zn,198 CaO/KF/Fe3O4,199 CaO/SiO2 based on palm empty fruit bunch (PEFB),200 and Mo–Zr/CaO.201
In 2015, Joshi et al.164 synthesized various metal oxides, for example, ZnO, MnO2, Fe2O3 and Al2O3 impregnated on CaO derived from eggshell via calcination at 900 °C, and exploited these catalysts in the conversion of non-edible JCO to FAME. Among all of the mixed metal oxides, the surface area and pore volume of ZnO–CaO were highest and thus showed an excellent 95.2% JCO conversion. The authors also reported that the catalyst is very stable towards the transesterification of JCO, and can be reused for 4 cycles. Similarly, Teo et al.202 synthesized CaO NPs derived from Gallus domesticus eggshell via precipitation method, and utilized it for the conversion of JCO to give FAME with 97% yield under the optimal reaction conditions. The TEM images and particle size distribution of the waste eggshell of Gallus domesticus derived nano-CaO catalyst is displayed in Fig. 17(a–c), which revealed that the particles were regular spheroidal shape and the average particle diameter is 16–27 nm. Fig. 17d displays the basicity measurement of the catalyst and commercial CaO using CO2-TPD technique. All CaO catalysts showed a broad desorption peak owing to the existence of the strong basic strength. The desorption peaks of both catalysts observed over the temperature ranging from 550 to 700 °C are attributed to the super-basic characteristics of the nanoparticles.
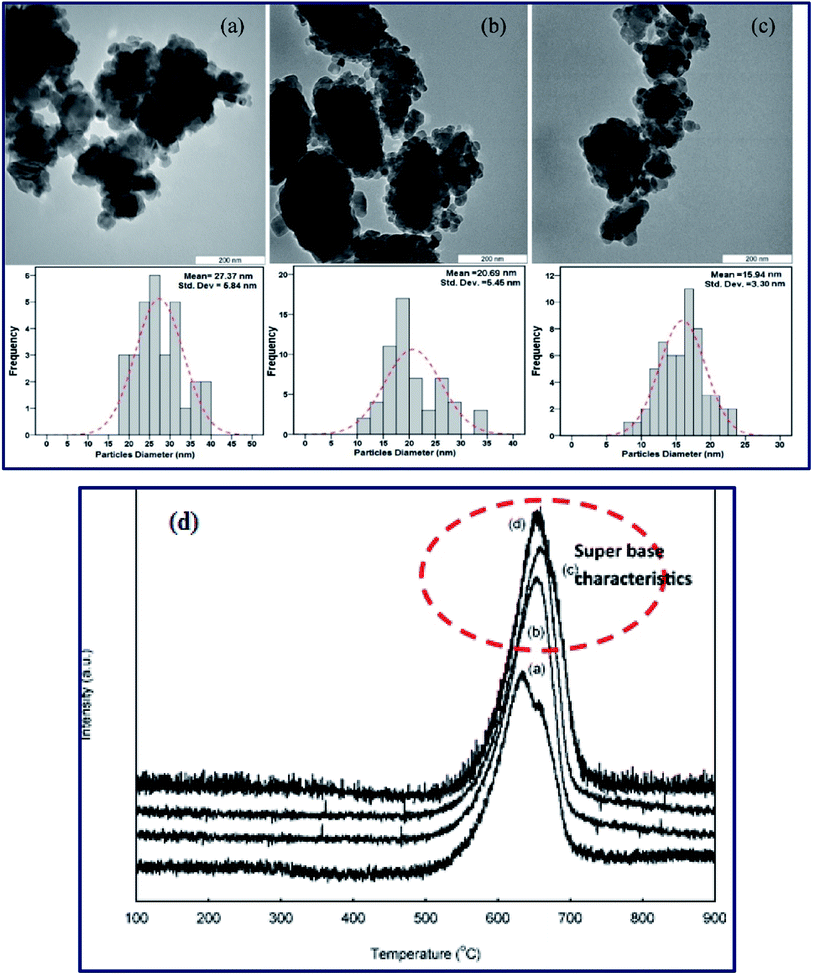 | ||
| Fig. 17 TEM images and particle size distributions of the surfactant assistant CaO nanocatalysts: after 40 min (a), after 80 min (b), and after 120 min (c). CO2 desorption performance commercial of CaO (a), and nano CaO catalysts: after 40 min (b), after 80 min (c), and after 120 min (d). Reproduced from ref. 202. | ||
In 2011, Olutoye et al.203 reported a mixed metal solid catalyst, where Mg(NO3)2 and KNO3 were impregnated on CaO originated from eggshell, and exploited it in the transformation of palm oil to FAME. The authors made three sets of a catalyst by changing the loading amount of Mg(NO3)2 and KNO3 on CaO with wt% ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and investigated their influence on the transesterification reaction. They reported that the catalyst with wt% ratio of 6
1.5, and investigated their influence on the transesterification reaction. They reported that the catalyst with wt% ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed the maximum yield of 85.8%. In addition, several works are reported in the literature regarding the transesterification of palm oil using chicken shell-derived CaO modified solid catalysts, such as CaO/SiO2 (ref. 204 and 205) and CaO/rice husk.206 Recently, Sulaiman et al.207 successfully synthesized a mixture of calcined coconut waste and egg waste for the transformation of palm oil to biodiesel. The authors employed RSM based on CCD to study the ideal reaction conditions: coconut waste/eggshell waste ratio, M/O molar ratio, catalyst amount, reaction temperature and reaction time. After a successful investigation, they reported that 5
1 showed the maximum yield of 85.8%. In addition, several works are reported in the literature regarding the transesterification of palm oil using chicken shell-derived CaO modified solid catalysts, such as CaO/SiO2 (ref. 204 and 205) and CaO/rice husk.206 Recently, Sulaiman et al.207 successfully synthesized a mixture of calcined coconut waste and egg waste for the transformation of palm oil to biodiesel. The authors employed RSM based on CCD to study the ideal reaction conditions: coconut waste/eggshell waste ratio, M/O molar ratio, catalyst amount, reaction temperature and reaction time. After a successful investigation, they reported that 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt% ratio of coconut waste/eggshell waste showed the maximum yield of 81% under the optimal reaction conditions.
1 wt% ratio of coconut waste/eggshell waste showed the maximum yield of 81% under the optimal reaction conditions.
In another work, A Li-doped CaO catalyst derived from eggshell was examined for the transformation of nahor oil to produce FAME by Boro et al.208 They measured the FFA content in the nahor oil and found 15 mg KOH per g. Due to this high FFA contents, a two-step process was investigated. First, an esterification was performed using sulfuric acid to bring down the FFA amount to <1, followed by transesterification reaction using the Li/CaO catalyst. They also examined the impact of Li doping on the conversion of oil to FAME, and reported a maximum 94% conversion when the Li doping was 2 wt%. Recently, Rahman et al.209 modified CaO derived from chicken eggshell with transition metals, such as Zn and Cu, and applied the catalyst in the transformation of eucalyptus oil to FAME. The authors reported that the surface area and basicity of Zn/CaO are higher than the Cu/CaO, therefore Zn/CaO showed better results with 93.2% FAME yield. Moreover, the impregnation of Zn on CaO improved the stability of the catalyst and can be used for 7 consecutive cycles. In another report, a magnetically recoverable KF-modified CaO derived from eggshell was prepared and employed in the transformation of neem oil to FAME.199 The author reported that the primary advantage of the catalyst is that the catalyst circumvented the saponification reaction. Therefore, the transesterification of neem oil (FFA content 4.2%) can proceed through the one-step process, and 94.5% FAME can be achieved.
In 2010, a novel eggshell originated CaO impregnated with fly ash was reported for the transesterification of soybean oil to form FAME. The influence of CaO loading was studied by the authors, and it was found that 30 wt% CaO loading showed a maximum yield of 96.97%. Moreover, CaO supported on fly ash enhanced the catalyst reusability and reactivity compared to the neat eggshell originated CaO.210 In addition, a KF modified CaO originated from eggshell was examined for the transformation of soybean oil to FAME. The modified catalyst has higher basicity than the neat CaO due to the addition of KOH in the process.211 Recently, Chowdhury et al.212 synthesized a Na-doped CaO derived from chicken eggshell, and exploited it in the transesterification of Madhuca indica oil. A two-step process was employed as the oil has 45% of FFA content. They first esterified the oil using 5 wt% sulfuric acid to lessen the FFA content of the oil, followed by transesterification using Na-doped CaO catalyst. To study the influence of the reaction parameters on the transformation of oil to biodiesel, the Taguchi approach was used, where they observed that the M/O molar ratio and the reaction temperature have the highest impact, and the reaction time has minimal impact on the transformation of oil to FAME. In 2014, Chen et al.213 demonstrated the synthesis of FAME from palm oil using CaO catalyst derived from ostrich egg-shell via ultrasonication. They compared the production of biodiesel using both mechanical stirring and ultrasonication process, and reported that the latter case showed higher yield (92.7%). Moreover, the catalyst can be used for 8 consecutive cycles. A transesterification process for soybean oil deodorizer distillate (SODD) to produce FAME was reported using CaO derived from the duck eggshell. They measured the FFA content of SODD and found 53.2%. Therefore, to overcome the saponification problem, the oil was pre-esterified with sulfuric acid and then the transesterification was performed for the pre-esterified SODD oil using the CaO catalyst to produce FAME with an overall yield of 94.6%.2 In addition, CaO derived from quail eggshell was utilized for the transformation of palm oil214 and JCO215 to biodiesel in high yield.
7.1.7.1.2. Mollusk shell and other seashells. Mollusk shell and other seashell-derived solid catalysts have been widely investigated in the transformation of edible/non-edible oils to produce biodiesel, and are listed in Table 13. Examples include a basic solid catalyst developed by the impregnation of KI on the calcined oyster shell, which was utilized in the transformation of soybean oil to FAME.116–119 The authors reported that the impregnation and calcination increased the surface area to an extent of 32-fold, and therefore increased the catalytic activity. The main disadvantages of the catalyst are the reusability factor and higher loading of KI.220 In addition, there are various studies in the literature, where neat CaO derived from oyster shell was utilized for the transformation of soybean oil to FAME221 and microwave-assisted (800 W) biodiesel synthesis from jatropha oil.222 Recently, a basic heterogeneous catalyst was developed from the river snail shell by calcination at 800 °C for 4 h. The catalyst was employed for the transesterification of WCO for biodiesel production. They performed KOH titration and found that the FFA content in the WCO is 0.3%. Therefore, direct transesterification was carried out and 98.19% yield was achieved under the optimal reaction conditions.223 Elsewhere, other reports are also available where CaO derived from calcined river snails were used for the transesterification of various edible/non-edible oils, for example, palm oil,224 soybean oil225 and WFO.226
| No. | Catalyst source | Catalyst | Feedstock | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. c w/w. d PFAD = palm fatty acid distillate. | ||||||
| 1 | Oyster shell | CaO/KI | Soybean | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mmol g−1, 50, 240 1, 1 mmol g−1, 50, 240 |
79.5 | 220 |
| 2 | Oyster shell | CaO | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 25, 65, 300 1, 25, 65, 300 |
73.8 | 221 |
| 3 | Oyster and Pyramidella shells | CaO | Jatropha oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, MW, 6 1, 4, MW, 6 |
93 | 222 |
| 4 | River snail shell | CaO | WCO | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 60 1, 3, 65, 60 |
92.5b | 223 |
| 5 | River snail shell | CaO | Palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 90 1, 5, 65, 90 |
98.5 | 224 |
| 6 | River snail shell | CaO | Soybean oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3c, 65, 180 1, 3c, 65, 180 |
98 | 225 |
| 7 | River snail shell | CaO | WFO | 6.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 60, 420 1, 2, 60, 420 |
87.28 | 226 |
| 8 | Snail shell | CaO/KBr/kaolin | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 65, 120 1, 2, 65, 120 |
98.5 | 227 |
| 9 | Snail shell | CaO | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, RT, 420 1, 3, RT, 420 |
98 | 228 |
| 10 | Snail shell | CaO | WFO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 60 1, 3, 60, 60 |
96 | 229 |
| 11 | Snail shell | CaO | WCO | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 9, 60, 180 1, 9, 60, 180 |
84.14 | 230 |
| 12 | Snail shell (S. canarium) | CaO | WCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 240 1, 3, 65, 240 |
83.5 | 231 |
| 13 | Snail shell | Nano-CaO | H. wightiana oil | 12.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.892, 61.6, 145.154 1, 0.892, 61.6, 145.154 |
98.93 | 232 |
| 14 | Snail shell | CaO | A. africana seed oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 55, 65 1, 1.5, 55, 65 |
85 | 233 |
| 15 | Mussel/cockle/scallop shell | CaO | Palm oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 65, 180 1, 10, 65, 180 |
95 | 234 |
| 16 | Mussel shell (Perna varidis) | C/CaO/NaOH | Palm oil | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7.5, 65, 180 1, 7.5, 65, 180 |
95.12 | 235 |
| 17 | Mussel shell | CaO/KOH | Castor oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 60, 180 1, 2, 60, 180 |
91.17 | 236 |
| 18 | Mussel shell | CaO | Soybean oil | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 12, 60, 480 1, 12, 60, 480 |
94.1 | 237 |
| 19 | Mussel shell | CaO | Soybean oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 65, 180 1, 4, 65, 180 |
>98b | 238 |
| 20 | Fresh water mussel shell | CaO | Chinese tallow oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 70, 90 1, 5, 70, 90 |
97.5 | 239 |
| 21 | Mussel/clamp/oyster | CaO | Camelina sativa oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 65, 120 1, 1, 65, 120 |
95/93/91 | 240 |
| 22 | Angel wing shell | CaO | N. oculata (microalgae) oil | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 9, 65, 60 1, 9, 65, 60 |
84.11 | 241 |
| 23 | Angel wing shell | CaO–SO4 | PFAD | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 80, 180 1, 5, 80, 180 |
98b | 242 |
| 24 | Clamshell | CaO | Palm oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 65, 120 1, 1, 65, 120 |
98 | 243 |
| 25 | Short necked clam (O. orbiculata) shell | CaO | JCO | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 65, 360 1, 4, 65, 360 |
93 | 244 |
| 26 | Clamshell (M. mereterix) | CaO | WFO | 6.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 180 1, 3, 60, 180 |
> 89 | 245 |
| 27 | White bivalve clamshell | CaO | WFO | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8, 65, 180 1, 8, 65, 180 |
95.84 | 246 |
| 28 | Venus clam (Tapes belcheri S.) | CaO | Palm oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 360 1, 5, 65, 360 |
97 | 247 |
| 29 | Abalon shell | CaO | Palm oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, 65, 150 1, 7, 65, 150 |
96.2 | 248 |
| 30 | T. jourdani shell | CaO | Palm oil | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 80, 420 1, 10, 80, 420 |
99.33b | 249 |
| 31 | A. cristatum shell | CaO | Palm oil | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 360 1, 3, 60, 360 |
93 | 250 |
| 32 | Cockleshell | CaO | Palm oil | 0.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4.9, reflux, 180 1, 4.9, reflux, 180 |
99.4 | 251 |
| 33 | Obtuse horn shell | CaO | Palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, reflux, 360 1, 5, reflux, 360 |
86.75 | 252 |
| 34 | Biont (turtle) shell | CaO/KF | Rape seed oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 70, 180 1, 3, 70, 180 |
97.5 | 253 |
| 35 | Turbonilla striatula shell | CaO | Mustard oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65 ± 5, 360 1, 3, 65 ± 5, 360 |
93.3 | 254 |
| 36 | Turbonilla striatula shell | CaO/Ba | WCO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 65, 120 1, 1, 65, 120 |
> 98b | 255 |
| 37 | Chicoreus brunneus shell | CaO | Rice bran oil | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.4, 65, 120 1, 0.4, 65, 120 |
93 | 256 |
| 38 | Shrimp shell | CaO/KF | Rape seed oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5, 65, 180 1, 2.5, 65, 180 |
89.1b | 257 |
| 39 | P. erosa seashells | Nano-CaO | Jatropha oil | 5.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.02, RT, 133.1 1, 0.02, RT, 133.1 |
95.8 | 258 |
| 40 | Crab shell (S. tranquebarica) | CaO | Sunflower oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8, 95, 75 1, 8, 95, 75 |
94.2 | 259 |
| 41 | Crab shell | CaO/Na-ZSM-5 | Neem oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15, 75, 360 1, 15, 75, 360 |
95 | 260 |
| 42 | Crab shell (S. serrata) | CaO | Palm oil | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 150 1, 5, 65, 150 |
98.8 | 261 |
| 43 | Crab shell | CaO | Karanja oil | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5, 65, 120 1, 2.5, 65, 120 |
94 | 262 |
In 2016, Liu et al.227 developed a solid catalyst, where KBr was loaded on calcined snail shell and kaoline mixture, followed by activation of the catalyst via calcination at 500 °C for 4 h, and applied the catalyst in the transformation of soybean oil to FAME. They also investigated the effect of the loading of KBr and the wt% ratio of the snail shell/kaoline mixture on biodiesel yield. It was found that the catalyst showed a maximum yield of 98.5% when the KBr loading and wt% ratio of the snail shell/kaoline were 40 wt% and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The mixing of the snail shell and kaoline together provides the catalyst with extra stability compared to their pure form.227 In addition, Laskar et al.228 developed a solid basic catalyst CaO derived from a calcined snail shell for the conversion of soybean oil to biodiesel. Under the ideal reaction states, 98% biodiesel yield was achieved. It is reported that at 400–600 °C calcination temperature, CaCO3 of the snail shell was transformed to calcite. When the calcination temperature was further increased to 700 and 800 °C, a minor and major component of CaO was achieved, which was later completely transformed into CaO at 900 °C calcination temperature. Fig. 18 reveals that 100% transformation of CaCO3 into CaO can be achieved above 800 °C calcination temperature.
1, respectively. The mixing of the snail shell and kaoline together provides the catalyst with extra stability compared to their pure form.227 In addition, Laskar et al.228 developed a solid basic catalyst CaO derived from a calcined snail shell for the conversion of soybean oil to biodiesel. Under the ideal reaction states, 98% biodiesel yield was achieved. It is reported that at 400–600 °C calcination temperature, CaCO3 of the snail shell was transformed to calcite. When the calcination temperature was further increased to 700 and 800 °C, a minor and major component of CaO was achieved, which was later completely transformed into CaO at 900 °C calcination temperature. Fig. 18 reveals that 100% transformation of CaCO3 into CaO can be achieved above 800 °C calcination temperature.
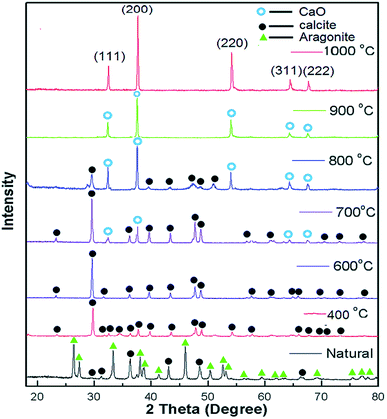 | ||
| Fig. 18 XRD spectra of normal and calcined (400–1000 °C) snail shells. Reproduced from ref. 228. | ||
In another work, El-Gendy et al.229 reported on a CaO catalyst originated from snail shell calcined at 800 °C, and utilized it in the transesterification reaction. RSM was utilized to investigate the influence of the reaction parameters on biodiesel production, and it was reported that 96.76% yield was observed under the optimized reaction conditions. Similarly, there are various studies in the literature available for the transesterification of WCO to FAME using CaO derived from snail shell collected from different sources.230,231 Very recently, Krishnamurthy et al.232 developed a solid catalyst, CaO nanoparticles derived from snail shell via the hydrothermal method, and investigated its application in the transesterification of H. wightiana oil to produce FAME. However, a high FFA content (7.57%) in the oil led the authors to follow a two-step process: (1) pre-esterification and (2) transesterification for the production of FAME. RSM was utilized to examine the impact of reaction parameters on FAME synthesis, which resulted in 96.92% yield under the optimal reaction conditions. In a similar vein, CaO derived from snail shell was also investigated for the transformation of A. africana seed oil233 and showed 85% FAME yield.
A calcined mussel/cockle/scallop shell-derived CaO was developed for the transformation of palm oil for FAME production. The authors reported on the high catalyst reactivity catalytic activity with great stability towards the transesterification of palm oil with 95% conversion.234 In the meantime, Hadiyanto et al.235 developed a solid catalyst, modified CaO (derived from green mussel shell) with activated carbon (C), followed by impregnation of NaOH, and utilized the catalyst in the transformation of palm oil. The wt% C/CaO ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 showed the maximum yield of 95.12% under the optimal reaction conditions. Similarly, KOH impregnated mussel shell derived CaO was examined for castor oil transformation to biodiesel. The authors made a comparison between the non-impregnated and KOH impregnated catalysts, and revealed that the KOH impregnated catalyst displayed higher reactivity, as well as basicity, and they reported 91.7% FAME yield using the KOH impregnated catalyst.236 Moreover, the calcined mussel shell-derived catalysts were widely examined for the transformation of vegetable oils, for example, soybean oil,237,238 chinese tallow oil,239 and Camelina sativa oil240 for biodiesel production.
3 showed the maximum yield of 95.12% under the optimal reaction conditions. Similarly, KOH impregnated mussel shell derived CaO was examined for castor oil transformation to biodiesel. The authors made a comparison between the non-impregnated and KOH impregnated catalysts, and revealed that the KOH impregnated catalyst displayed higher reactivity, as well as basicity, and they reported 91.7% FAME yield using the KOH impregnated catalyst.236 Moreover, the calcined mussel shell-derived catalysts were widely examined for the transformation of vegetable oils, for example, soybean oil,237,238 chinese tallow oil,239 and Camelina sativa oil240 for biodiesel production.
Syazwani et al.241 examined CaO, which originated from angel wing shell (AWS) and was calcined at 900 °C for 2 h, for the conversion of N. oculata micro-algae oil to FAME. The catalyst possessed high reactivity with great stability, and could be reused for 3 consecutive cycles. Furthermore, a bifunctional catalyst was developed for the conversion of palm fatty acid distillate (PFAD) to FAME. The angel wing shell was calcined to form CaO, followed by sulfonation to afford the catalyst. The authors reported that the catalyst surface area increased by two-fold after the modification. As a result, the catalyst showed excellent activity towards the esterification of PFAD. Unfortunately, the catalyst was reusable only for two cycles as blocking of active sites occurred in each reaction cycles. Therefore to enhance the reusability of the catalyst, pretreatment of the catalyst such as washing and re-calcination are necessary before each reaction cycles.242 In 2015, Asikin-Mijan et al.243 developed a waste clam shell-derived CaO using hydration–dehydration treatment, and investigated its catalytic application in the conversion of palm oil to FAME. They also examined the effect of the hydration–dehydration time on biodiesel conversion. The authors found that the catalytic activity increased with increasing hydration time. This was because the extended hydration enhanced the formation of Ca(OH)2, increased the basicity, reduced the crystallinity, and enhanced the surface area. They reported that the rehydration for 12 h showed the maximum 98% FAME yield under optimized reaction conditions. Similarly, an investigation of the naked CaO catalyst, derived from a calcined short-necked clamshell, recorded 93% biodiesel yield under the optimal reaction conditions.244 In addition, CaO derived from various calcined clamshell was utilized for the transformation of diverse edible/non-edible oils, for example, palm oil245,246 and WFO,247 to produce biodiesel.
A solid ethanol-treated catalyst CaO, derived from calcined abalone shell, was examined for the production of FAME from palm oil. The authors investigated the impact of ethanol treatment at different temperatures (RT, 100 °C and 160 °C). They found that the catalyst treated with ethanol at 100 °C showed the maximum yield of 96.2%, as the ethanol treatment provides high basicity, high surface area and lowered the catalyst crystallinity. Moreover, a comparison of the modified CaO with naked CaO showed that the modified CaO has higher reusability and provided higher biodiesel yield.248 In addition, there are several reports available in the literature regarding the transesterification of palm oil to FAME utilizing the CaO-based solid catalyst originating from various waste shells, such as T. jourdani shell,249A. cristatum shell,250 cockle shell251 and obtuse horn shell.252
In 2009, Xie et al.253 synthesized a solid catalyst via three-step process: (i) incomplete carbonization of a biont shell at 500 °C, (ii) KF impregnation and (iii) catalyst activation at 300 °C. The developed catalyst was utilized for the conversion of rapeseed oil to FAME. They reported that the catalyst displayed excellent reactivity due to the formation of a higher amount of active sites during the reaction between the incomplete carbonized shell and KF. The effect of KF loading was also examined, and it was found that 25% KF loading is optimal and showed 97% FAME yield under the optimized reaction conditions. Correspondingly, Boro et al.254 demonstrated the synthesis of the CaO catalyst by calcination of Turbonilla striatula shell, and utilized it for the transformation of mustard oil to FAME. The effect of the calcination temperature was examined, and it was observed that the catalyst calcined at 900 °C displayed the maximum 93.3% FAME yield. In addition, CaO derived from calcined Turbonilla striatula was modified with Ba in the range of 0.5–1.5 wt%. It was utilized for the transformation of WCO to biodiesel. Due to the high acid value of 22 mg KOH per g, the oil was pretreated with sulfuric acid to reduce the acid value to <1. Then, the pretreated oil was transesterified with Ba/CaO catalyst. The authors also examined the effect of Ba loading and found that 1% of Ba doping showed >98% biodiesel yield.255 In addition, Chicoreus brunneus shell was calcined above 800 °C to convert CaCO3 to CaO, followed by hydration/dehydration to form a solid base catalyst. It was then examined for the transformation of rice bran oil. Calcination and hydration provided the catalyst with high porosity, enhancing the basicity, catalytic activity and reusability.256 In addition, shrimp shell originated catalysts have been utilized for the transformation of various edible/non-edible oils to FAME. Yang et al.257 synthesized a catalyst via a three-step process; (i) inadequate carbonization of shrimp shell, (ii) reaction with KF, and (iii) activation of the catalyst under the heating condition for the rapeseed oil transformation. The authors examined the impact of the carbonization temperature, KF amount and activation temperature. They found that 89.1% biodiesel was achieved under the reaction states: carbonization temperature of 450 °C, KF amount of 25 wt%, and an activation temperature of 250 °C. The excellent catalyst reactivity is attributable to the formation of active sites during the reaction between the incomplete carbonized shrimp shell and KF. Moreover, a solid catalyst, CaO nanoparticles with a diameter of 66 nm derived from Polymedosa erosa shell via calcination–hydration–dehydration process was developed for the transformation of JCO to FAME in a two-step procedure: (1) pre-esterification and (2) transesterification. The influence of the reaction parameters on the oil conversion was examined by RSM technique, and displayed 98.54% FAME yield.258
In the recent past, Sivakumar et al.259 developed a solid catalyst derived from Scylla tranquebarica crab shell calcined at 750 °C for sunflower oil transformation to FAME. The developed catalyst displayed similar reactivity to that of commercial CaO, and reported a very high conversion of 94.2% under the optimal reaction conditions. Similarly, Shankar et al.260 prepared a solid catalyst, where CaO (derived from crab shell calcined at 900 °C) was impregnated on Na-ZSM-5 followed by activation at 550 °C for 10 h. It was utilized for the production of FAME from neem oil. The impact of CaO loading was examined, and it was found that 15 wt% CaO impregnation showed a maximum 95% biodiesel formation. Moreover, various reports are available for the transesterification of edible/non-edible oils, such as palm oil261 and karanja oil,262 utilizing CaO originated from calcined crab shells.
7.1.7.2 Ashes of biomass. In recent years, the application of waste plant ashes as a highly active heterogeneous catalyst has drawn increasing attention in the realm of biodiesel production. A huge amount of alkali or alkaline earth elements, mostly K, Ca and Mg present in the ashes of waste plant biomass, acted as a highly basic catalyst in the transesterification reaction to produce biodiesel from vegetable oil with low FFA. In the case of vegetable oil with high FFA, a reduction of FFA to <1% (by acid-catalyzed esterification) before the transesterification reaction is mandatory to elude catalyst consumption in soap formation, which otherwise leads to low biodiesel yield. Usually, the biomass is collected, washed and dried either in oven or sunlight, burnt in the open air or burnt in the air. This is followed by calcination to produce a highly basic ash catalyst, as shown in Fig. 19. Different basic ash catalysts were utilized, and their efficacy in the synthesis of biodiesel is presented in Table 14. In a pioneering work, Chouhan et al.263 reported the use of amphibian plant L. perpusilla Torrey ash as a solid catalyst in biodiesel synthesis from JCO. The plant biomass was subjected to calcination at 550 ± 5 °C for 2 h to obtain the ash catalyst. The crystallinity of the catalyst was affirmed by XRD patterns. The impact of catalyst loading revealed that 5 wt% (w.r.t. oil) is enough to obtain a high 89.43% biodiesel yield under the optimal reaction conditions. Nevertheless, the reusability study demonstrated that the catalyst lost its reactivity in each progressive reaction cycle, owing to leaching of the reactive elements in the catalyst. Thereby, the catalyst was recycled up to 3 cycles only.
| No. | Catalyst source | Feedstock | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. c mL g−1. d v/v. | |||||
| 1 | L. perpusilla Torrey | JCO | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65 ± 5, 300 1, 5, 65 ± 5, 300 |
89.43 | 263 |
| 2 | Oil palm ash | WCO | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 5.35, 60, 30 0, 5.35, 60, 30 |
71.74 | 264 |
| 3 | Oil palm ash/boiler ash (BA) | Palm olein | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 30 1, 3, 60, 30 |
90 | 265 |
| 4 | Musa paradisiaca L. (plantain) peels | Thevetia peruviana oil | 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 60 1, 3, 60, 60 |
95.2 | 266 |
| 5 | Ripe plantain fruit peel | Azadirachta indica oil | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.73, 0.65, 65, 57 0.73, 0.65, 65, 57 |
99.2 | 267 |
| 6 | Coconut husk | JCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, 45, 30 min 1, 7, 45, 30 min |
99.86 | 268 |
| 7 | Cocoa pod husks | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 60, 60/120 1, 1, 60, 60/120 |
98.7/91.4 | 269 |
| 8 | Musa balbisiana Colla peel | Thevetia peruviana seed oil | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 20, RT, 180 1c, 20, RT, 180 |
96b | 270 |
| 9 | Musa balbisiana Colla underground stem | JCO | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 275, 60 1, 5, 275, 60 |
98 | 271 |
| 10 | Musa ‘Gross Michel’ peel | Napoleon's plume seed oil | 7.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.75, 65, 69.02 1, 2.75, 65, 69.02 |
98.5 | 272 |
| 11 | Rubber seed shell | Rubber seed oil | 0.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d, 2.2, 60, 60 1d, 2.2, 60, 60 |
83.06 | 273 |
| 12 | Musa balbisiana Colla peel | WCO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 60, 180 1, 2, 60, 180 |
100b | 274 |
| 13 | M. acuminata peel | Soybean | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, RT, 240 1, 7, RT, 240 |
98.95 | 275 |
| 14 | Wood (Acacia nilotica) stem | JCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 180 1, 5, 65, 180 |
98.7b | 276 |
| 15 | Birch bark/fly ash | Palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 180 1, 3, 60, 180 |
88.06 ± 0.72/99.92 ± 0.01 | 277 |
| 16 | Musa spp “Pisang Awak” peduncle | Ceiba pentandra oil | 9.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.978, 65, 60 1, 1.978, 65, 60 |
98.69 ± 0.18 | 278 |
| 17 | Musa acuminata peduncle | Ceiba pentandra oil | 11.46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.68, 65, 106 1, 2.68, 65, 106 |
98.73 ± 0.50b | 279 |
| 18 | Theobroma grandiflorum seeds | Soybean oil | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 80, 480 1, 10, 80, 480 |
98.36b | 280 |
| 19 | Brassica nigra plant | Soybean oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, 65, 25 1, 7, 65, 25 |
98.79 | 281 |
| 20 | Kola nut pod husk | Kariya seed oil (KSO) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 65, 75 1, 3, 65, 75 |
98.67 ± 0.01 | 156 |
| 21 | Orange peel | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, RT, 420 1, 7, RT, 420 |
98b | 282 |
| 22 | Sesamum indicum plant | Sunflower oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, 65, 40 1, 7, 65, 40 |
98.9 | 283 |
| 23 | Tucumã peels | Soybean oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 80, 240 1, 1, 80, 240 |
97.3b | 284 |
| 24 | Tectona grandis leaves | WCO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5, RT, 180 1, 2.5, RT, 180 |
100b | 285 |
| 25 | Cocoa pod husk | Azadirachta indica oil | 0.73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d, 0.65, 65, 57 1d, 0.65, 65, 57 |
99.3 | 286 |
| 26 | Walnut shell | Soybean oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 10 1, 5, 60, 10 |
98 | 287 |
| 27 | Sugar beet waste | Sunflower oil | 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 75, 60 1, 1, 75, 60 |
93b | 288 |
| 28 | M. acuminata trunk | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 14, RT, 360 1, 14, RT, 360 |
98.39b | 289 |
| 29 | Banana peel/cocoa pod husk | Palm kernel oil | 0.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d, 4, 65, 65 1d, 4, 65, 65 |
99.5/99.3 | 290 |
| 30 | Carica papaya stem | Scenedesmus obliquus | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 60, 180 1, 2, 60, 180 |
93.33b | 291 |
| 31 | Musa balbisiana underground stem | Mesua ferrea oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 275 1, 5, 60, 275 |
95b | 292 |
In another work, oil palm ash was seen as an active catalyst for biodiesel synthesis from WCO by Chin et al.264Fig. 20 depicts the SEM micrograph of the palm ash, which displayed the porous nature of the ash catalyst, while Table 15 lists the elements existing in the palm ash determined from the EDX analysis. It was observed that the palm ash consisted of a large amount of potassium, while a relatively low quantity of aluminum, zinc, and magnesium was also found. Besides, it was seen that K2O was the primary driver for the high basicity and catalytic activity of the catalyst towards biodiesel synthesis. CCD was utilized to investigate the impact of the optimized reaction conditions in biodiesel synthesis, such as M/O ratio, reaction time, temperature and catalyst loading. Accordingly, the predicted and experimental biodiesel yields were found to be 60.07% and 71.74%, respectively.
 | ||
| Fig. 20 SEM micrograph of palm ash. Reproduced from ref. 264. | ||
| Elements | Atomic wt% |
|---|---|
| Potassium (K) | 40.59 |
| Magnesium (Mg) | 0.76 |
| Silicone (Si) | 2.63 |
| Aluminum (Al) | 0.50 |
| Zinc (Zn) | 0.33 |
| Oxygen (O) | 29.36 |
| Carbon (C) | 14.56 |
| Chlorine (Cl) | 7.07 |
In the meantime, Boey et al.265 reported on a solid base, derived from boiler ash (BA) via calcination, that catalyzed biodiesel synthesis from palm oil. BA effectively transformed palm oil to FAME at moderate reaction conditions and delivered 90% FAME yield. Ironically, the ash is intolerant of the presence of moisture and FFA at 1 wt% in the feedstock. Betiku et al.266 reported a process for biodiesel synthesis from Thevetia peruviana oil by utilizing calcined Musa paradisiaca L. (plantain) peel ash catalyst. The dried powdered plantain peels were calcined at 500 °C for 3.5 h to produce plantain peels ash. A biodiesel yield of 95.2% was acquired using the optimized reaction conditions. In addition, Etim et al.267 utilized ripe plantain fruit peel as a solid catalyst in biodiesel synthesis from Azadirachta indica oil. At the onset, pre-esterification of the oil was performed to diminish the FFA contents from 5.81 wt% to 0.90 wt%, utilizing a M/O molar ratio of 2.19 v/v and 6 wt% of Fe2(SO4)3. Finally, the pre-esterified oil was transformed to FAME via transesterification reaction catalyzed by plantain fruit peel ash. Coconut husk ash catalyst was also reported for biodiesel synthesis from JCO.268 The husks were subjected to calcination at various temperatures ranging from 250–500 °C. It was identified that the catalyst produced at 350 °C calcination temperature was found to be the most reactive one for biodiesel synthesis, giving 99.86% yield within 30 min at the moderate reaction temperature. XRD patterns of the catalysts are presented in Fig. 21, which revealed the presence of several components of ash, such as KCl, K2Si2O5, K2SO4, K2S3, KAlO2, K4CaSi3O9, and FeCa2Al2BSi4O15OH.
 | ||
| Fig. 21 XRD patterns of calcined coconut husk calcined at different temperatures. Reproduced from ref. 268. | ||
Cocoa pod husks (CPHs) were used as a solid catalyst for biodiesel synthesis from soybean oil by Ofori-Boateng et al.269 The authors examined the reactivity of MgO impregnated CPH (MgO@CPH) and bare CPH in biodiesel synthesis under the optimal reaction states, and achieved 98.7% and 91.4% biodiesel yields, respectively. Moreover, the synthesized fuel satisfies the European biodiesel quality norm (EN 14112). In another study, the production of biodiesel from yellow oleander (Thevetia peruviana) seed oil using banana (Musa balbisiana Colla) peel ash was reported.270 The K, Na, CO3, and Cl present in the ash are responsible for the high basicity, and thus the reactivity of the catalyst. Oil transformation of 96% was demonstrated in just 3 h time under room temperature. The produced biodiesel conforms to standards set for ASTM D6751, EN 14214 and others. The BET surface area measurement of the catalyst revealed that the surface area is 1.487 m2 g−1. The biodiesel was free from sulfur, and has displayed a high cetane number. Meanwhile, Musa balbisiana Colla underground stem (MBCUS) ash was examined as a solid base catalyst for biodiesel synthesis from high FFA containing JCO by Sarma et al.271 Characterization of the ash catalyst revealed that it is composed of oxides and carbonates of various alkali and alkaline earth metals, which leads to the high basicity of the catalyst, and the surface area is 39 m2 g−1. It was reported that the catalyst is very effective during the biodiesel synthesis process at 275 °C and internal pressure (4.2 MPa), and resulted in 98.0% biodiesel yield.
Betiku et al.272 led an investigation on the application of banana (Musa ‘Gross Michel’) peel waste as a catalyst for biodiesel synthesis from Bauhinia monandra (Napoleon's plume) seed oil (BMSO), with a motive to develop a low-cost fuel. The burnt ash of the banana peel was further calcined at 700 °C for 4 h to produce a highly active catalyst. They utilized the RSM model to determine the optimal reaction conditions for biodiesel synthesis using the ash catalyst. The RSM plot of the M/O molar ratio and catalyst loading on Bauhinia monandra (Napoleon's plume) methyl ester (BMME) yield is shown in Fig. 22a. It was observed that the BMME yield improved from 0 to >90 wt% as the M/O molar ratio expanded from 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 14
1 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the catalyst loading increased from 1.5 to 3.5 wt%. This might be ascribed to the increase in the active site number as a result of the increased catalyst loading. Besides, the BMME yield diminished marginally when the catalyst loading was above 3.5 wt% (Fig. 22a). In addition, the plot revealed a direct connection between the M/O molar ratio and catalyst loading on the biodiesel yield. As the two parameters increased, the biodiesel yield also increased (Fig. 22a). The transformation of the pre-esterified oil to biodiesel was done inside the time span of 33.79–76.21 min. The extended reaction time, somewhere in the range of 33.79 and 55 min, favoured biodiesel yield. After 55 min, the yield diminished. Fig. 22b displays the impact of the reaction time and catalyst loading on the biodiesel yield. It was observed from the surface plot that the rise in catalyst loading and reaction time led to an increase in the biodiesel yield. Moreover, the plot displayed that 90 wt% biodiesel yield was reached using 4.5 wt% catalyst loading within 80 min reaction time. In addition, Fig. 22c illustrates the surface plot to examine the impact of the M/O molar ratio and reaction time on the biodiesel yield. It was observed from the plot that the increases in two parameters, such as the M/O molar ratio and reaction time, led to a rise in the biodiesel yield. It can be seen from the figure that the increases in M/O molar ratio from 7
1, and the catalyst loading increased from 1.5 to 3.5 wt%. This might be ascribed to the increase in the active site number as a result of the increased catalyst loading. Besides, the BMME yield diminished marginally when the catalyst loading was above 3.5 wt% (Fig. 22a). In addition, the plot revealed a direct connection between the M/O molar ratio and catalyst loading on the biodiesel yield. As the two parameters increased, the biodiesel yield also increased (Fig. 22a). The transformation of the pre-esterified oil to biodiesel was done inside the time span of 33.79–76.21 min. The extended reaction time, somewhere in the range of 33.79 and 55 min, favoured biodiesel yield. After 55 min, the yield diminished. Fig. 22b displays the impact of the reaction time and catalyst loading on the biodiesel yield. It was observed from the surface plot that the rise in catalyst loading and reaction time led to an increase in the biodiesel yield. Moreover, the plot displayed that 90 wt% biodiesel yield was reached using 4.5 wt% catalyst loading within 80 min reaction time. In addition, Fig. 22c illustrates the surface plot to examine the impact of the M/O molar ratio and reaction time on the biodiesel yield. It was observed from the plot that the increases in two parameters, such as the M/O molar ratio and reaction time, led to a rise in the biodiesel yield. It can be seen from the figure that the increases in M/O molar ratio from 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 14
1 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 improved the biodiesel yield from 33% to 100%. Therefore, the highest biodiesel yield was recorded at 14
1 improved the biodiesel yield from 33% to 100%. Therefore, the highest biodiesel yield was recorded at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M/O molar ratio and 80 min reaction time.
1 M/O molar ratio and 80 min reaction time.
 | ||
| Fig. 22 3-D plots of biodiesel yield. (a) Impact of M/O molar ratio and catalyst loading, (b) reaction time and catalyst loading, and (c) M/O molar ratio and reaction time on the biodiesel yield. Reproduced from ref. 272. | ||
Meanwhile, Onoji et al.273 built up a novel technique to utilize rubber seed shell (RSS) ash calcined at 800 °C as a solid base catalyst for the transformation of rubber seed oil to biodiesel. The high FFA content of the RSS (9.01 ± 0.07%) was pre-esterified using H2SO4 to >1% FFA. The reusability study of the catalyst revealed that >80% biodiesel yield was noticed after 4 successive reaction cycles. The surface area and pore size of the calcined RSS was found to be 2.29 nm and 352.51 m2 g−1, respectively. Similarly, Gohain et al.274 studied the application of the Musa balbisiana Colla peel ash catalyst to produce biodiesel from WCO. It was observed that the calcination procedure improved the mesoporous and microporous morphology of the catalyst, and upgraded its surface area, bringing about the higher catalytic activity. The external morphology of the catalyst examined by SEM analysis revealed aggregation of the particles, and porosity in the range of micro and meso. Moreover, 100% conversion of WCO to biodiesel was confirmed by 1H NMR spectra (Fig. 23b), utilizing the Knothe and Kenar eqn (1). The 1H NMR spectrum of WCO (Fig. 23a) displays two peaks at 4.1 and 5.3 ppm because of the glyceridic protons (Fig. 23a). The presence of a peak of the methoxy protons at ∼3.6 ppm and the vanishing of the signs of the glyceridic peak close to 4–4.2 ppm (Fig. 23b) confirmed the formation of biodiesel.
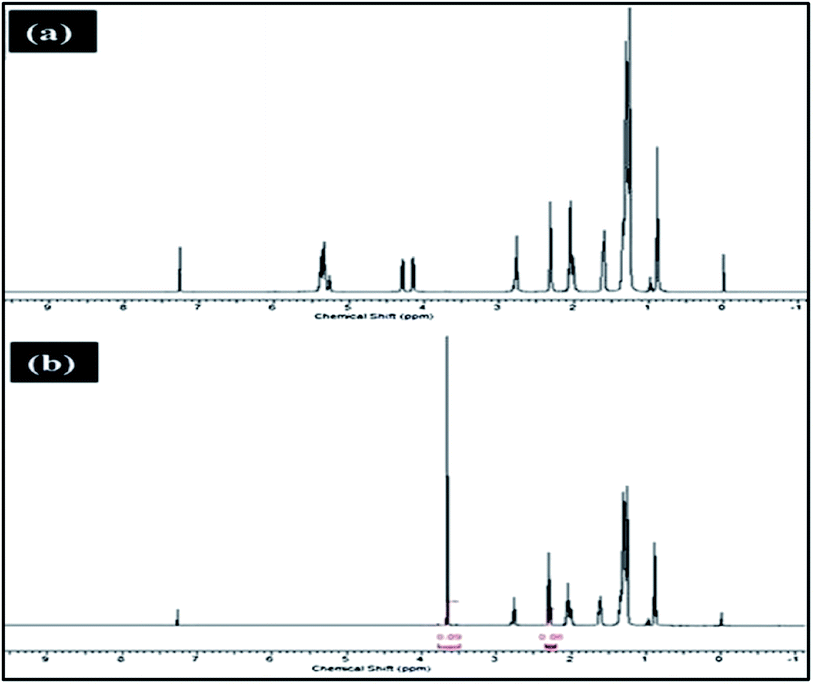 | ||
| Fig. 23 1H NMR spectrum of (a) WCO and (b) Biodiesel. Reproduced from ref. 274. | ||
In recent year, Pathak et al.275 utilized the Musa acuminata peel ash (MAPA) catalyst for biodiesel synthesis from soybean oil at room temperature. The catalyst characterization reported the existence of various alkali and alkaline earth metals that enhance the catalyst basicity and reactivity of the ash catalyst. K (14.27%), C (47.51%) and O (30.27%) are the primary/main elements that exist in MAPA, as revealed by the XPS data (Fig. 24). The authors reported 98.95% biodiesel yield under the optimized reaction conditions.
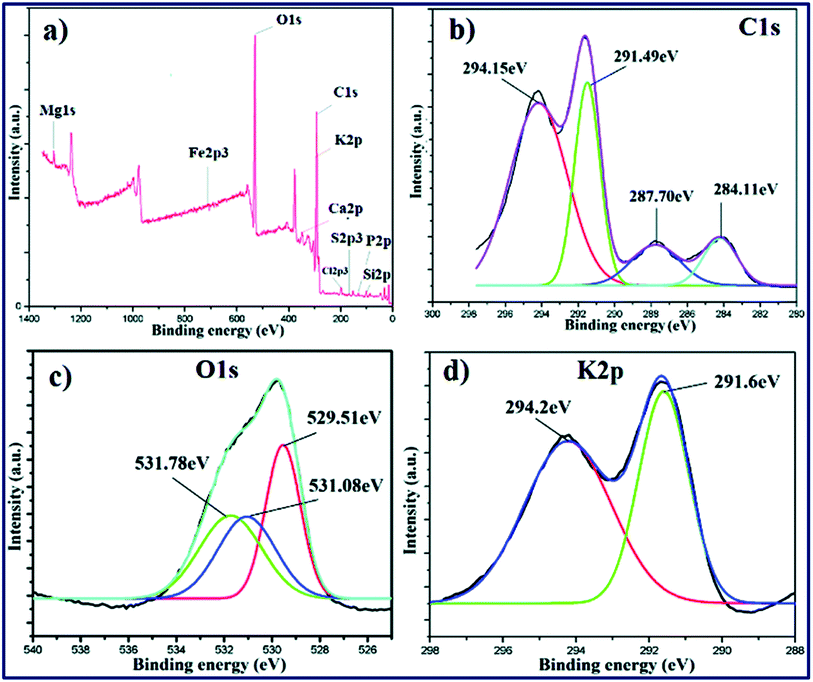 | ||
| Fig. 24 XPS survey (a), C 1s (b), O 1s (c), and K 2p (d) spectra of MAPA. Reproduced from ref. 275. | ||
Sharma et al.276 investigated the reactivity of wood ash catalyst calcined at different temperatures for biodiesel synthesis from JCO. Ester conversion in the range of 97–99% could be achieved with wood ash catalysts. Wood ash calcined at 800 °C afforded 98.7% oil conversion under the ideal reaction conditions. Uprety et al.277 studied the application of wood ash derived from birch bark and fly ash blazed at 800 °C for 4 h synthesis of biodiesel from palm oil. Birch bark ash gave a FAME yield of 88.06 ± 0.72, whereas fly ash from wood pellet afforded 99.92 ± 0.01% yield. Recently, the application of banana peduncle ash as an efficient solid base catalyst for the synthesis of biodiesel from Ceiba pentandra oil (CPO) was investigated.278 Based on the response surface methodology (RSM) study, the ideal reaction conditions for the transformation of CPO into FAME was found to be 1.978 wt% catalyst loading, 60 min response time, 9.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M/O molar ratio with a maximum predicted FAME yield of 99.36%, which was assessed experimentally as 98.69 ± 0.18%. The same research team also investigated the utilization of Musa acuminata peduncle for biodiesel preparation from CPO.279 The authors calculated the surface area and pore diameter of the calcined ash catalyst from BET analysis data, and reported 45.99 m2 g−1 and 9.77 nm, respectively. Moreover, the catalyst consists of diverse minerals (along with potassium) as primary components, which leads to the higher reactivity of the catalyst (Fig. 25). A high conversion of 98.73 ± 0.50% FAME was observed under the optimum reaction conditions.
1 M/O molar ratio with a maximum predicted FAME yield of 99.36%, which was assessed experimentally as 98.69 ± 0.18%. The same research team also investigated the utilization of Musa acuminata peduncle for biodiesel preparation from CPO.279 The authors calculated the surface area and pore diameter of the calcined ash catalyst from BET analysis data, and reported 45.99 m2 g−1 and 9.77 nm, respectively. Moreover, the catalyst consists of diverse minerals (along with potassium) as primary components, which leads to the higher reactivity of the catalyst (Fig. 25). A high conversion of 98.73 ± 0.50% FAME was observed under the optimum reaction conditions.
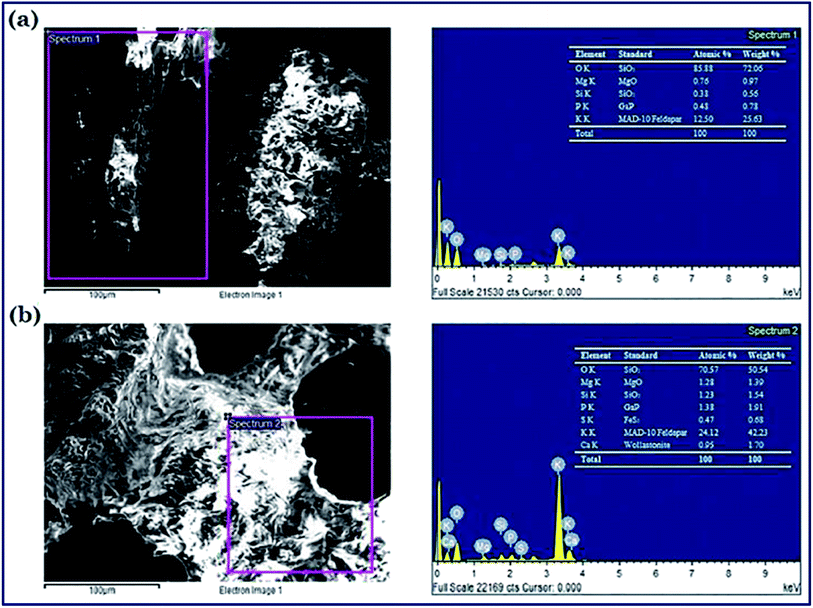 | ||
| Fig. 25 EDS images of (a) uncalcined and (b) calcined banana peduncle. Reproduced from ref. 279. | ||
In 2019, Mendonça et al.280 reported the utilization of calcined (800 °C for 4 h) waste cupuaçu seeds as a solid base catalyst in the synthesis of biodiesel from soybean oil and ethanol. Similarly, Nath et al.281 utilized a solid base catalyst derived from waste Brassica nigra plant for the efficient preparation of biodiesel. The SEM-EDX analysis of the catalyst revealed the existence of potassium (56.13%) and calcium (26.04%) in a huge amount, which may be considered as key ingredients for the high basicity of the catalyst. The authors also measured the surface area pore volume of the catalyst via BET analysis, and came about 7.308 m2 g−1 and 0.011 cm3 g−1, respectively. The catalyst possessed excellent reactivity in transforming the soybean oil to FAME and displayed 98.79% FAME yield in a short time frame of 25 min under the optimum states. Betiku et al.156 prepared an ash catalyst from kola nut pod husk and used it to convert Kariya seed oil (KSO) to biodiesel, namely Kariya oil methyl esters (KOME), via transesterification process. A maximum of 98.67 ± 0.01 wt% of FAME yield was observed. Moreover, the reusability examination of the catalyst suggests that it can be reused for 4 progressive cycles. Recently, Changmai et al.282 converted soybean oil to biodiesel using orange peel ash in 98% yield. XRF analysis showed the presence of potassium oxide (51.64%) and calcium. The Hammet indicator strategy was employed to examine the catalyst basicity, and it was seen as 9.8 < H_ < 12.2. The authors measured the catalyst pore volume and surface area from BET analysis, and found 0.428 cm3 g−1 and 605.60 m2 g−1, respectively. Moreover, GC-MS analysis (Fig. 26) revealed the existence of six components in the synthesized FAME; methyl palmitate (11.63%), methyl oleate (25.32%) and methyl linoleate (54.34%) were found to be the major components.
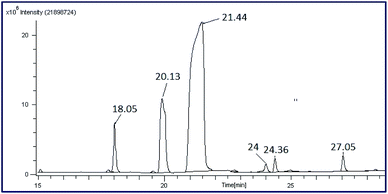 | ||
| Fig. 26 GC-MS spectrum of biodiesel from soybean oil. Reproduced from ref. 282. | ||
The waste Sesamum indicum plant ash catalyst was also successfully utilized for the transformation of sunflower oil to biodiesel.283 The measured surface area of the catalyst was 3.66 m2 g−1, as obtained from the BET analysis data. A high 98.9% biodiesel yield was accomplished. They reused the catalyst up to the 3rd cycle, which yielded 94.2% biodiesel. In addition, Mendonça et al.284 utilized waste tucumã peels ash catalysts for biodiesel synthesis from soybean oil. The catalyst characterization by XRF showed that it was mostly composed of potassium oxides, calcium and magnesium. Because of its heterogeneous and non-leachable nature, the catalyst derived from tucumã peels could be reused at least 5 times. In another study, an ash catalyst from Tectona grandis leaves was developed and utilized for the transformation of WCO to FAME by Gohain et al.285 The measured surface area and pore size of the catalyst were 116.833 m2 g−1 and 112.210 Å, respectively, as calculated from BET data. 100% oil transformation to FAME was accomplished at room temperature using the optimized reaction conditions. Furthermore, cocoa pod husk-derived solid base catalyst was employed in the transformation of neem seed oil to FAME.286 A two-step process was employed for the conversion of neem seed oil to FAME: (i) pretreatment of the oil was performed using the Fe2(SO4)3 catalyst to reduce the FFA content from 28.76% to 0.39%, and (ii) the transesterification of the pretreated oil using the calcined bio waste-derived catalyst. The authors also studied the effect of the reaction parameters using the Box–Behnken design (BBD), and the CCD of RSM was utilized to determine the optimized reaction conditions. Similarly, a walnut shell derived catalyst was developed for the transformation of sunflower oil to biodiesel.287 The catalyst was prepared from walnut shells via air combustion, thereby bringing down the cost involved in the calcination process to afford ash. The authors reported a 98% FAME yield within a brief time frame of 10 min. Recently, the transformation of sunflower oil to synthesize FAME using calcined sugar beet generated from agro-industry waste was reported.288 The catalyst has a high amount of highly basic CaO, and showed very high reactivity towards the transesterification process to afford about 93% FAME yield. 98.39% soybean oil transformation to FAME under room temperature was recently reported using M. acuminata trunk ash catalyst.289
Most biomass ash catalysts are usually applied for the transesterification reactions of different biodiesel feedstocks and different reaction conditions. These make a comparison of the effectiveness of such catalysts under the same reaction condition impossible. Hence, to have a better insight into the activities of the catalysts under the same reaction conditions and feedstock, Odude et al.290 examined the transformation of the pre-esterified palm kernel oil (PKO) to FAME utilizing two diverse catalysts, viz., calcined banana peel ash (CBPA) and calcined cocoa pod husk ash (CCPHA) under the same reaction conditions. The RSM technique was utilized for the optimization of both CBPA and CCPHA catalyzed transformation processes of PKO to FAME. CCD was utilized to acquire the best possible combination of the M/O ratio, catalyst loading and reaction time for the highest conversion of oil to FAME, as portrayed in Fig. 27. The observed FAME yields under the optimized conditions utilizing the catalysts CBPA and CCPHA were 99.5 and 99.3 wt%, respectively. The created models, when exposed to statistical assessment, demonstrated that the CBPA-catalyzed transformation model was better than the CCPHA-catalyzed transformation model. In the meantime, the Carica papaya stem291 and Musa balbisiana underground stem292 were also reported as a solid catalyst to convert Scenedesmus obliquus and Mesua ferrea oil, respectively, to FAME.
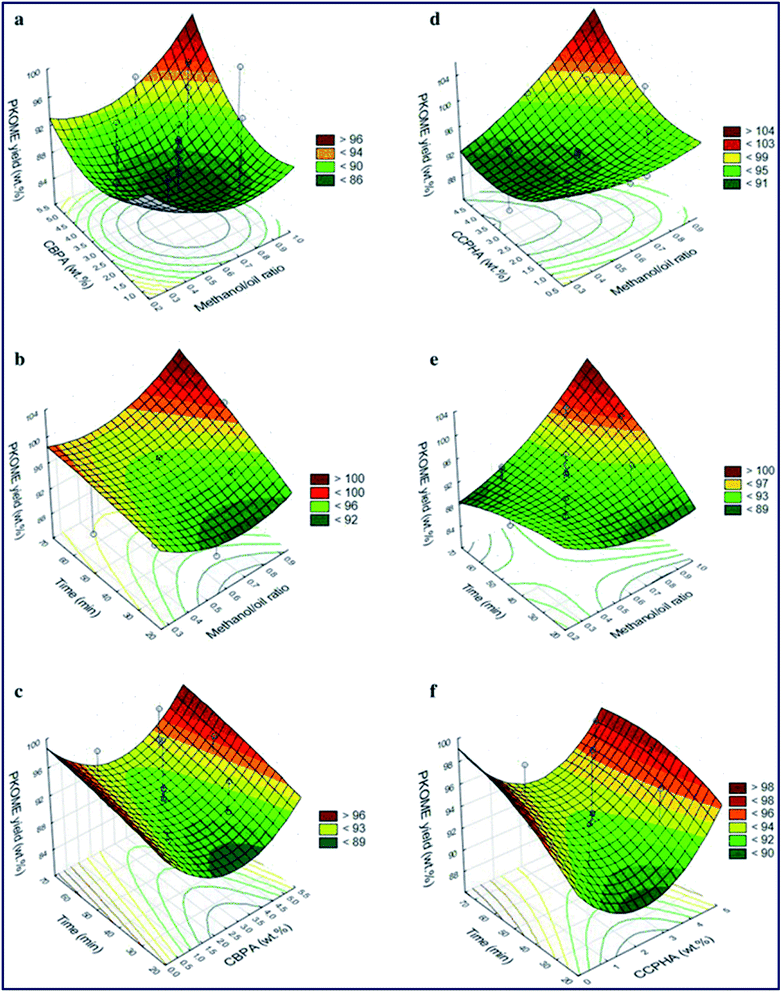 | ||
| Fig. 27 Contour and surface plots for PKOME synthesis. Reproduced from ref. 290. | ||
7.2 Acid catalysts
Acids can catalyze both transesterification and esterification reactions without soap formation.293 Hence, unlike base catalysts, an acid catalyst has the potential to afford biodiesel from poor quality oil with high FFA and high water content. In the transesterification reaction, alkaline catalysts are superior in promoting methoxide anion formation from methanol. In contrast, acidic catalysts are less active in methoxide anion formation, but could activate the carbonyl bonds via H+ addition (Brønsted acidic sites) or via coordination of the carbonyl oxygen with the coordinatively unsaturated metal ion sites (Lewis acidic sites), and thereby promote transesterification. Hence, an increase in the number of either Brønsted or Lewis acidic sites promotes faster FAME formation via transesterification. Delightfully, heterogeneous acid catalysts are endorsed as a potential alternative to homogeneous acid catalysts as they possess certain advantages. These include their ease of separation and reuse, lower corrosiveness and lower toxicity.294 In recent years, several research groups have studied the feasibility of solid acid catalysts for esterification/transesterification processes, and proposed economical and environment-friendly approaches for biodiesel production.295–297Since the last few years, the cationic resins have gained considerable attention due to their advantages, such as functioning at soft reaction conditions, non-corrosive nature, more numbers of active sites and lower residual water production.302,303 The cationic resin catalysts possess numerous active acid sites that play a crucial role in FAME production via esterification/transesterification reactions.304,305 Various ion exchange resin catalysts utilized for FAME production, together with ideal reaction conditions, are listed in Table 16. In 2007, Shibasaki-Kitakawa et al.302 reported in a comparative study that cation exchange resins showed less efficacy than anion exchange resins towards the conversion of triacylglycerols to biodiesel. Moreover, while evaluating the conversion rates of various commercial resins, such as Diaion PA308, PA306, PA306S and Diaion HPA25, it was observed that highly porous resin-like Diaion HPA25 showed a low conversion rate. It is believed that this might be due to resistance of the resin towards water. According to Ren et al.,303 transformation of soybean oil to FAME was reduced from 95.2% to 87.7% in the presence of D261 anion-exchange resin when the water content was enhanced from 0.0% to 1.0% by the mass of oil. Similarly, in another study, Deboni et al.304 also reported a lowering of the reaction rate due to the presence of water inside the resins.
| No. | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. c NR: not reported, PFAD: palm fatty acid distillate. | |||||
| 1 | D261 anion-exchange resin | Soybean oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 50.15, 56 1, 50.15, 56 |
95.2b | 303 |
| 2 | Amberlyst A26 OH anion exchange resin | Acid soybean oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 50, NR 1, 2, 50, NR |
78 | 304 |
| 3 | Amberlyst-15 | Hydrolyzed sea mango oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 30, 30, NR 1, 30, 30, NR |
>90 | 305 |
| 4 | Basic anion exchange resin. | Pongamia oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 75, 60 1, 75, 60 |
85 | 306 |
| 5 | Amberlyst 15 ion exchange resin | Lagenaria vulgaris seed oil | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 40 1, 5, 60, 40 |
93.2 | 307 |
| 6 | Amberlyst | Hydrolyzed sea mango oil | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 100, 60 12, 100, 60 |
>80 | 308 |
| 7 | Amberlyst-26 | Canola oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 45, 90 1, 3, 45, 90 |
67 | 309 |
| 8 | Amberlyst-A26 OH | Tallow fat | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mol L−1, 65, 360 1, 2 mol L−1, 65, 360 |
95 | 310 |
| 9 | Amberlite gel resin | WCO | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 60, 120 1, 60, 120 |
85.94 | 311 |
| 10 | Cation-exchange resin | Rice bran oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20, 63.83, 120 1, 20, 63.83, 120 |
79.7 | 313 |
| 11 | Purolite-PD206 | Corn oil | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 65, 2880 1, 65, 2880 |
79.45 | 315 |
Generally, ion exchange resins are utilized for the purification and softening of water at room temperature. Recently, Kansedo et al.305 compared the catalytic efficiencies of different ion exchange resins like Amberlyst 15, Dowex DR-2030 and DR-G8 for the transformation of FFA into FAME via esterification of the sea mango oil (hydrolyzed) at RT. The results revealed that Amberlyst 15 showed maximum efficacy with the highest FAME production compared to Dowex DR-2030 and Dowex DR-G8. However, Jaya et al.306 utilized ion exchange resin catalysts at a moderately lower temperature (50 °C to 80 °C) for biodiesel production, which is analogous to those of the homogeneous catalytic process. Furthermore, Umer and co-worker investigated the transformation of Lagenaria vulgaris seed oil to biodiesel, exploiting the Amberlyst 15 resin and calcium oxide (egg cell) catalyst. The authors reported 93.2% yield of biodiesel when the Amberlyst 15 ion exchange resin was used as a catalyst with the loading of 5% w/w and M/O ratio of 40% w/w for 40 min reaction time at 60 °C.307 Similarly, Kansedo and Lee308 investigated the esterification of hydrolyzed sea mango oil utilizing different cationic ion exchange resins, and over 80% yield of FAME was recorded using the Amberlyst 15 catalyst at a comparatively lower temperature within 1 h of reaction time and with catalyst loading of less than 5% w/w.
Recently, Deboni et al.304 reported 99% yield of methyl and ethyl esters from soybean oil with methanol and ethanol, respectively, using optimal reaction conditions. Conversely, Ilgen et al.309 recorded 63% yield of FAME from canola oil using Amberlyst-26 under the optimized reaction conditions. Moreover, in another study, a yield of about 67% was observed for canola oil and methanol with almost similar reaction conditions.301,309 The conversion of tallow fat with methanol showed the yield of methyl and ethyl esters around 95% using Amberlyst-A26 OH with reaction conditions, like a tallow fat-to-methanol molar ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a resin loading of 2 mol L−1 at 65 °C temperature for about 8.5 hours.310
1, and a resin loading of 2 mol L−1 at 65 °C temperature for about 8.5 hours.310
Hartono et al.311 investigated the catalytic efficacy of a heterogeneous catalyst obtained from a different source, like Lewatit macroporous resin, Amberlite gel resin and natural zeolite from Bayah, to transform WCO to biodiesel. Authors reported the 85.94% yield of biodiesel production by Lewatit macroporous anion exchanger with 6 M NaOH. Whereas, Amberlite gel with 6 M HCl displayed 65.22% biodiesel generation. Previously, Shibasaki-Kitakawa et al.312 reported the usefulness of the anion-exchange resin from their catalytic and adsorption abilities for the transformation of WCO to FAME. In their other study, Shibasaki-Kitakawa et al.313 also developed an ion-exchange resin catalyst-based continuous process for the production of biodiesel. The FFA conversion rate was estimated for different catalysts with reactions conditions, like the mole ratio of M/O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), temperature (63.83 °C), reaction time (2 h) and catalyst load (20 wt%). The maximum FFA conversions of 79.7% were recorded for NKC-9. For 001 × 7 and D61 catalysts, it was found to be only 32.2% and 10.3%, respectively.314 Jalilnejad-Falizi et al.315 achieved the highest FFA conversions by ion exchange resins (PD206-Na+ and PD206-H+) under the optimal reaction conditions. All of the above-mentioned reports are enough to summarize that ion exchange resins can be employed as one the potential heterogeneous catalysts in biodiesel production.
1), temperature (63.83 °C), reaction time (2 h) and catalyst load (20 wt%). The maximum FFA conversions of 79.7% were recorded for NKC-9. For 001 × 7 and D61 catalysts, it was found to be only 32.2% and 10.3%, respectively.314 Jalilnejad-Falizi et al.315 achieved the highest FFA conversions by ion exchange resins (PD206-Na+ and PD206-H+) under the optimal reaction conditions. All of the above-mentioned reports are enough to summarize that ion exchange resins can be employed as one the potential heterogeneous catalysts in biodiesel production.
| No. | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b NR: not reported. | |||||
| 1 | SO42−/ZrO2 | Neem oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1, 65, 120 1, 1, 65, 120 |
95 | 321 |
| 2 | SO42−/SnO2–SiO2 | WCO | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 150, 180 1, 3, 150, 180 |
92.3 | 322 |
| 3 | SnSO4 | Soybean oil | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 100 1, 5, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
92 | 323 |
| 4 | SO42−/SnO2–SiO2 | Jatropha oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 180, 120 1, 3, 180, 120 |
97 | 324 |
| 5 | SO42−/TiO2 | Rapeseed oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NR. 80, 720 1, NR. 80, 720 |
51 | 325 |
| 6 | Ti(SO4)O | WCO | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 75, 180 1, 1.5, 75, 180 |
97.1 | 328 |
| 7 | TiO2/PrSO3H | WCO | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4.5, 60, 540 1, 4.5, 60, 540 |
98.3 | 329 |
Xia et al.317 demonstrated the synthesis of mesoporous materials, which has the potential to improve the activity of the sulfated zirconia catalyst owing to their promising and outstanding properties, like high surface area, uniform and controllable pore size. According to Alexander et al.,318 the modification of the sulfated zirconia catalyst enhanced the total acidity, which basically increased the catalyst active sites. In another study, Guoliang et al.319 proposed that a change in the phase structure of sulfated zirconia can also increase its catalytic activity. Therefore, they developed tetragonal sulfated zirconia, which showed enhanced catalytic activity in the FAME synthesis procedure. Moreover, some of the studies proposed the modification of sulfated zirconia on a MCM-41 (Mobil Composition of Matter No. 41) support for the generation of methyl tert-butyl ether to improve its catalytic performance. The results obtained revealed that the catalytic performance of the prepared supported sulfated zirconia catalyst was 2.5–3.0 times greater than neat sulfated zirconia.317,320 Similarly, Muthu et al.321 reported the preparation of FAME from neem (Azadirochta indica) oil using sulfated zirconia catalyst. It was revealed that the catalyst is highly stable to oils with high FFA concentration. The strong acid sites of this catalyst showed a considerable impact on its reactivity in the transformation of neem oil.
Recently, Lam et al.322 developed a SO42−/SnO2 catalyst by impregnation method, and exploited it for the conversion of WCO to biodiesel. Furthermore, the authors studied the bi-metallic impact of the catalyst, in which SnO2 was blended in with SiO2 and Al2O3, at various weight ratios to increase the activity of SnO2. The finding confirmed that the SO42−/SnO2–SiO2 weight ratio of 3 showed exceptionally high reactivity with 92.3% biodiesel yield using optimal reaction conditions. Similarly, Pereira et al.323 demonstrated the application of the SnSO4 catalyst for the esterification of oleic acid (as model feedstock) and acid soybean oil having high contents of FFA. It was found that the model feedstock containing 70 wt% of FFA showed 92% FAME yield using excess ethanol, 5 wt% SnSO4 at 100 °C for 3 h. Moreover, it was also reported that the catalyst is stable up to ten cycles without any significant decrease in the biodiesel yield. Moreover, one of the studies involved the application of sulfated tin oxide modified with the SiO2 (SO42−/SnO2–SiO2) catalyst to produce FAME from JCO.324 The sulfated titania-based solid superacid catalysts are another kind of sulfated catalysts. Li et al.325 prepared three different titania-based solid superacid catalysts, and these were exploited for the transformation of rapeseed oil to FAME at 353 K with a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of M/O under atmospheric pressure. It was found that all three prepared catalysts showed a significant yield of biodiesel due to their stronger surface acidities. Moreover, Alaba et al.316 reviewed that apart from these, there are various other sulfated metal oxides, such as titania and silica, and a combination of both also showed remarkable performance. It was also proved thorough the investigation led by several researchers, who applied sulfated silica as catalysts for esterification and transesterification.326,327 In this context, Gardy and co-workers demonstrated a facile preparation of the sulfated doped TiO2 catalyst that was utilized efficiently in the petroleum refinery. The authors reported that the synthesized catalyst has better reactivity than other sulfated metal oxides, primarily because of the acidic properties of the TiO2 particles, which was subjected to sulfonation to enhance its acidity. The catalyst displayed great efficiency in the synthesis of FAME from WCO.328,329
1 molar ratio of M/O under atmospheric pressure. It was found that all three prepared catalysts showed a significant yield of biodiesel due to their stronger surface acidities. Moreover, Alaba et al.316 reviewed that apart from these, there are various other sulfated metal oxides, such as titania and silica, and a combination of both also showed remarkable performance. It was also proved thorough the investigation led by several researchers, who applied sulfated silica as catalysts for esterification and transesterification.326,327 In this context, Gardy and co-workers demonstrated a facile preparation of the sulfated doped TiO2 catalyst that was utilized efficiently in the petroleum refinery. The authors reported that the synthesized catalyst has better reactivity than other sulfated metal oxides, primarily because of the acidic properties of the TiO2 particles, which was subjected to sulfonation to enhance its acidity. The catalyst displayed great efficiency in the synthesis of FAME from WCO.328,329
| No. | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. | |||||
| 1 | Fe2O3–SiO2 | Jatropha oil | 218![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15, 220, 180 1, 15, 220, 180 |
95.6 | 330 |
| 2 | ZnAl2O4/ZnFe2O4 | Sunflower oil, WCO, Jatropha oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 180, 600 1, 5, 180, 600 |
>90 | 331 |
| 3 | SnO2–SiO2 | Soybean oil | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 180, 300 1, 5, 180, 300 |
81.7 | 332 |
| 4 | Fe–Mn–MoO3/ZrO2 | WCO | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 200, 300 1, 4, 200, 300 |
95.6 ± 0.15 | 333 |
| 5 | WO3–SnO2 | Soybean oil | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 110, 300 1, 5, 110, 300 |
79.2 | 334 |
| 6 | WO3 (30 wt%)/AlPO4 | Soybean oil | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 180, 300 1, 5, 180, 300 |
72.5 | 335 |
| 7 | Mn1.4Zr0.35Al0.6O3 | WCPO | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5, 150, 300 1, 2.5, 150, 300 |
>93 | 336 |
| 8 | Zr–Mo | Oleic acid | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 180, 120 1, 4, 180, 120 |
94.2b | 337 |
| 9 | FMWMo | WCO | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 200, 480 1, 6, 200, 480 |
92.3 ± 1.12 | 338 |
Impregnation followed by calcination (600 °C) was used to synthesize the Fe–Mn–MoO3/ZrO2 catalyst, which could provide a high 95.6 ± 0.15% yield of FAME.333 It is interesting to observe that ZrO2 and MoO3/ZrO2 gave a lower FAME yield of 48.6 ± 1.14 and 73.0 ± 0.25%, respectively. The high activity of the Fe–Mn–MoO3/ZrO2 catalyst is attributed to the high surface area (49.5 m2 g−1) and availability of huge active sites (2411 μmol g−1) in the catalyst. Moreover, the catalyst reusability examination revealed that it is stable up to 6 progressive reaction cycles of transesterification of WCO without a loss in its efficiency. On the other hand, the enhanced catalytic activity was observed in a mixed metal oxide of WO3/SnO2 in the soybean oil transformation in comparison with the individual WO3 and SnO2 species.334 The bonding of WO3 with SnO2 was believed to upgrade the WO3/SnO2 acidity. The catalyst is highly stable and was reused up to 4 times without much depreciation in the biodiesel yield.
Further, Xie et al.335 studied 30 wt% WO3 loading on the AlPO4 catalyst and recorded a good 72.5% conversion to biodiesel under the optimized reaction condition. The high catalyst reactivity was attributed to the existence of WO3 that enhanced the surface acid sites. Similarly, Amani et al.336 reported a series of Mn3.5xZr0.5yAlxO3 catalysts for the transformation of WCO to FAME. The Mn1.4Zr0.35Al0.6O3 catalyst demonstrates better catalyst reactivity, as far as the FAME yield (>93%), than the Mn1.4Zr0.35O3 catalyst (52.8%). The bonding between metals in the crystal structure efficiently influenced the catalyst reactivity. It was observed that the amphoteric component of the Al developed the surface region of the catalyst and framed a complex structure with other metal oxides, although Mn alternated the morphology and catalyst basic site density. In the meantime, Zhang et al.337 reported the Zr–Mo mixed metal oxide functionalized with various carboxylic acids, for example, lauric acid, stearic acid, palmitic acid and myristic acid for the biodiesel production from oleic acid. The modification of the Zr–Mo metal oxide using such monofunctional carboxylic acids enhances the catalyst acidity and surface area, and thus upgraded the rate of the reaction. They also reported that among all catalysts, the stearic acid-functionalized Zr–Mo metal oxide showed the best result with the maximum oleic acid conversion of 94.2%. The catalyst reusability test revealed that the catalyst is stable for up to 6 progressive cycles. Similarly, WCO was utilized for the FAME production using ferric-manganese doped tungstate molybdena nanoparticles (FMWMo).338 The Fe–Mn dopants enhance the surface area, density of acidic sites, and the stability towards the esterification of WCO. A maximum yield of 92.3 ± 1.12% methyl ester was achieved under the optimized reaction conditions.
The acid-catalyzed chemical reactions, such as saccharification, esterification, transesterification and acetylation, are vital operations commonly used for the valorization of biomass or their components to useful products in various food, fuel and chemical industries.350 The functionalized acidic carbons from inexpensive sources, including natural organic carbon matter (such as sugars, carbohydrates, cellulosic materials, and lignin), have been achieved by several researchers.341,351–353 Besides this, agro waste such as husk, straw, seed cover, cow manure, corn cob,342,343,354,355 carbonaceous waste from industries (char, oil pitch, coke, glycerol)346,348,356,357 and polymer resins349,358,359 were also used. Various carbon supports (e.g., zeolite-templated carbons, mesoporous carbons, active carbon)352,353,360,361 and more recently nanostructured carbons (such as graphene, graphene oxide, carbon nanotubes, and carbon dots)362–367 have been exploited for the same purpose.
Over the last few years, there is growing interest from researchers towards the application of sulfonated carbon-based catalysts due to their noteworthy efficacies mentioned earlier. Many reports are available, which demonstrated the efficient nature of the sulphonic acid-functionalized catalyst in biodiesel production using various feedstocks.356,362,367 One of the reports presented the synthesis of organosulfonic acid (i.e., propylsulfonic and arenesulfonic groups) functionalized mesoporous silicas through a simple one-step process. The synthesized novel catalysts that possessed propylsulfonic groups and arenesulfonic groups were further evaluated for their catalytic efficacy in the esterification of fatty acids with methanol to produce methyl esters, and the authors also compared the efficacy of these heterogeneous catalysts with a variety of commercially available catalysts (such as sulfuric acid, p-toluene sulfonic acid, Nafion NR50, and Amberlyst-15). The obtained results indicated that the organosulfonic acid-functionalized mesoporous silica catalysts showed the highest reactivity compared to all of the above-mentioned commercial solid acid catalysts in the fatty acid esterification process. Moreover, it was also recorded that the efficiency of these catalysts largely depended on important factors, such as the median pore diameter of the catalyst and the acidic strength of the organosulfonic acid group present over this catalyst. Considering these findings, it can be proposed that there is a huge potential to develop catalysts using organic–inorganic mesoporous materials.363 In general, the activity of the carbon-based catalysts upon fatty acid (C16–C18) esterification to produce biodiesel primarily depends on three primary factors: (i) –SO3H group density, (ii) total acid density, and (iii) porosity. Different sulfonated carbon-based acid catalysts utilized for FAME production are listed in Table 19. Numerous reported catalysts demonstrated promising outcomes in the (trans)esterification of biodiesel feedstocks with high FFA and afforded >85% FAME yield. In the meantime, several investigations had been conducted using model acids (e.g., palmitic acid, oleic acid, which are the major components of vegetable oil as a reactant) that mainly focused on the esterification reaction.
| No. | Catalyst | Feedstock | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. c Ethanol to oil molar ratio. d NR: not reported. | |||||
| 1 | Sulfonated sugar | Oleic acid | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 7.4, 80, 240 1c, 7.4, 80, 240 |
NR | 364 |
| 2 | Sulfonated carbon | Oleic acid | 2.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 17.2, 95, 240 1c, 17.2, 95, 240 |
99.9 | 365 |
| 3 | ACPhSO3H | Rapeseed oil | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 65, 420 1, 10, 65, 420 |
95 | 366 |
| 4 | Sulfonated AC | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20, 75, 20 1, 20, 75, 20 |
88.7 | 355 |
| 5 | H2SO4/C | Castor oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 60 1, 5, 65, 60 |
94 | 369 |
| 6 | SAM | Vegetable oil | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 180, 120 1, 6, 180, 120 |
95 | 370 |
| 7 | SO3H/SBA-15 | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 190, 30 1, 5, 190, 30 |
90 | 371 |
| 8 | SiO2–Pr–SO3H | Acid oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 100, 480 1, 4, 100, 480 |
96.78b | 372 |
| 9 | OPPSO3H | Soybean oil | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 10, 70, 600 1c, 10, 70, 600 |
93b | 373 |
| 10 | Coal based solid acid | Oleic acid | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8, 240, 67 1, 8, 240, 67 |
97.6b | 375 |
| 11 | Sulfonated carbon-based solid acid | Oleic acid | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 65, 120 1, 10, 65, 120 |
97.3 | 376 |
| 12 | Sulfonated activated carbon | Oleic acid | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 12, 180, 85 1c, 12, 180, 85 |
96b | 377 |
| 13 | C–SO3H | Waste cooking oil | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 60, 180 1, 10, 60, 180 |
93.6 | 378 |
| 14 | Sulfonated multiwalled carbon nanotube | Triglycerides | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 3.7, 60, 150 1c, 3.7, 60, 150 |
97.8b | 379 |
| 15 | ICS-SO3H | Palm fatty acid distillate | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 180, 75 1, 2, 180, 75 |
90.4 | 380 |
| 16 | CMR-DS-SO3H | Waste palm oil | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 65, 72 1, 5, 65, 72 |
92.7 | 381 |
| 17 | HS/C–SO3H | Oleic acid | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3.5, 80, 300 1, 3.5, 80, 300 |
96.9b | 382 |
| 18 | SOMC | Oleic acid | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3.5, 80, 600 1, 3.5, 80, 600 |
73.59b | 383 |
| 19 | SO42−/corncob | Oleic acid | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 60, 480 1, 5, 60, 480 |
>80 | 384 |
| 20 | C–SO3H | Oleic acid | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 67, 120 1, 1.5, 67, 120 |
93.04 | 385 |
| 21 | C–SO3H | Oleic acid | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 17, 95, 240 1, 17, 95, 240 |
99.9 | 386 |
| 22 | C–SO3H | WCO | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 110, 240 1, 10, 110, 240 |
89.6 | 387 |
| 23 | C–SO3H | PFAD | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5, 80, 240 1, 2.5, 80, 240 |
95.3b | 388 |
| 24 | C–SO3H | Mesua ferrea Linn oil | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 120, 1440 1, 5, 120, 1440 |
97.79 | 389 |
| 25 | Coconut shell-SO3H | Palm oil | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 60, 360 1, 6, 60, 360 |
88.03 | 390 |
| 26 | Oil palm trunk/sugarcane bagasse-SO3H | Waste oil | 1.17 mL min−1, 12, 130, 240 | 80.6/83.2 | 391 |
| 27 | Corn straw-SO3H | Oleic acid | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 60, 240 1, 3, 60, 240 |
92 | 392 |
| 28 | Bamboo-SO3H | Oleic acid | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 2, 90, 360 1c, 2, 90, 360 |
98.4 | 393 |
| 29 | Jatropha curcas Seed-SO3H | JCO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7.5, 60, 60 99.13 1, 7.5, 60, 60 99.13 |
99.13b | 394 |
| 30 | Bio-glycerol | Karanja oil | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20, 160, 240 1, 20, 160, 240 |
99.5 | 395 |
| 31 | Glycerol | Palmitic acid | 9.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 10, 65, 240 1c, 10, 65, 240 |
99b | 396 |
| 32 | Microalgae residue | Oleic acid | NR, 5, 80, 720 | 98b | 397 |
| 33 | Oil cake waste-SO3H | JCO/M. ferrea L. oil | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 80, 480 1, 5, 80, 480 |
99 | 398 |
| 34 | Oil cake waste-SO3H | Oleic acid | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20, 60, 120 1, 20, 60, 120 |
94b | 399 |
| 35 | De-oiled waste cake | Oleic acid | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 64, 600 1, 3, 64, 600 |
97b | 400 |
| 36 | De-oiled canola meal-SO3H | Oleic acid | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7.5, 65, 1440 1, 7.5, 65, 1440 |
93.8b | 401 |
| 37 | Pine chip char | Palmitic acid | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 55–60, 300 1, 5, 55–60, 300 |
97 | 402 |
| 38 | Biochar | Canola oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 5, 65, 1440 1c, 5, 65, 1440 |
92 | 403 |
| 39 | Biochar | Canola oil, oleic acid | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 315, 180 1, 5, 315, 180 |
48 | 404 |
In a pioneering work towards the preparation of the biomass-based sulfonated carbon catalyst, Toda et al.364 synthesized the sulfonated carbon catalyst by partial carbonization of sugar, followed by sulfonation in fuming H2SO4. The prepared catalyst consists of sheets of indistinctive carbon having a high amount of sulfonic groups, along with hydroxyl and carboxyl as a minor group (Fig. 28). The highly active bio-based carbon catalyst was utilized for the transformation of oleic and stearic acid to FAME via esterification. Apart from the –SO3H group, the presence of the –OH and –COOH groups in the catalyst greatly enhance the catalytic activity and make it highly water tolerant. The successful incorporation of the –SO3H group and the formation of carbonized materials can be easily confirmed by using FT-IR and 13C MAS NMR analysis, respectively, as depicted in Fig. 33.368 The FT-IR spectra (Fig. 29a) displayed two bands at 1040 and 1377 cm−1 (in SO3H), ascribed to the SO3 and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, respectively, suggesting the existence of the –SO3H groups. 13C MAS NMR (Fig. 29b) depicted three major peaks at 130, 155, and 180 ppm, ascribed to the polycyclic aromatic carbon atoms, phenolic OH, and COOH groups, respectively.
O stretching vibrations, respectively, suggesting the existence of the –SO3H groups. 13C MAS NMR (Fig. 29b) depicted three major peaks at 130, 155, and 180 ppm, ascribed to the polycyclic aromatic carbon atoms, phenolic OH, and COOH groups, respectively.
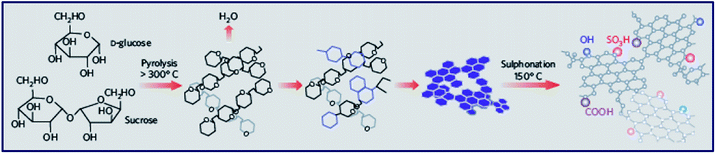 | ||
| Fig. 28 Synthesis of sulfonated carbon catalyst from sucrose and D-glucose. Reproduced from ref. 364. | ||
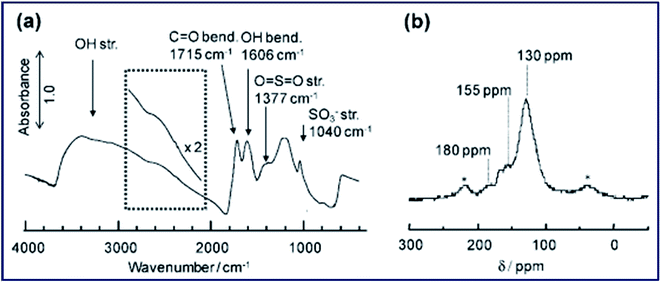 | ||
| Fig. 29 FT-IR (a) and 13C MAS NMR (b) spectra for the sulfonated carbon catalyst originated from cellulose. Reproduced from ref. 368. | ||
In another work, Hara et al.356 examined the sulfonated carbon catalyst in biodiesel synthesis. The findings showed that the amorphous carbon material-containing sulfonic acid groups enhances the catalytic performance, and thus displayed extraordinary reactivity in the esterification/transesterification reactions in comparison with the ordinary solid acid catalyst.
Likewise, Nakajima et al.365 synthesized an amorphous cellulose-originated carbon solid acid (CCSA) catalyst and exploited it in the transformation of oleic acid to FAME, and observed a 99.9% yield under the optimized conditions. The carbon material displayed much higher catalytic activity in the esterification reaction in comparison with the ordinary solid acid catalysts examined, such as niobic acid, Amberlyst-15 and Nafion NR50. Interestingly, those CCSA catalysts prepared at a lower carbonization temperature before being subjected to sulfonation gave a much better biodiesel yield, as compared to those prepared at higher carbonization temperature. This is attributed to the huge amount of –OH and –COOH groups in the former, which enhanced its acidic nature, and thereby its catalytic activities (Fig. 30). The catalyst reactivity remains intact after 10 progressive cycles.
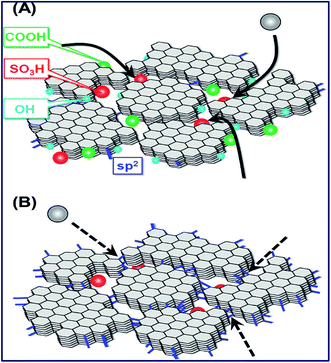 | ||
| Fig. 30 Schematic structures of the SO3H-bearing CCSA materials carbonized at below 723 K (A) and above 823 K (B). Reproduced from ref. 365. | ||
The simultaneous carbonization and sulfonation in a one-pot synthesis of solid acid catalyst directly from biomass have also been explored by various experts, as it is a straightforward, cost and time-efficient approach. Malins et al.366 synthesized C–SO3H via the simultaneous carbonization-sulfonation approach, and utilized it for FAME production. The C–SO3H catalysts with the highest density of SO3H groups (0.81 mmol Hβ per g) were prepared using optimal reaction conditions. It was noted that under these optimized reaction conditions, 96.5% of FAME was recorded. Interestingly, the catalyst has great stability, and can be easily recovered and reused for subsequent reaction cycles. Moreover, in the comparative study of the esterification reactions of rapeseed oil fatty acids, the prepared catalyst exhibited similar reactivity to Amberlyst-15.
Another recent report proposed a synthesis of the heterogeneous sulfonated catalyst using activated carbon to overcome several problems, like drastic reaction conditions (such as very high temperature, pressure, longer reaction time and expensive overall process cost). The above-mentioned activated carbon catalyst was prepared from corncobs as a precursor, and utilized in the microwave-assisted conversion of soybean oil with ethanol to FAME. In this study, about 88.7% yield of pure biodiesel was reported at 0–600 W of microwave power. Moreover, the catalyst was reused for up to 5 cycles.355Fig. 31 represents the schematic illustration of the application of the activated carbon-based catalyst in the transesterification of various oils using methanol.
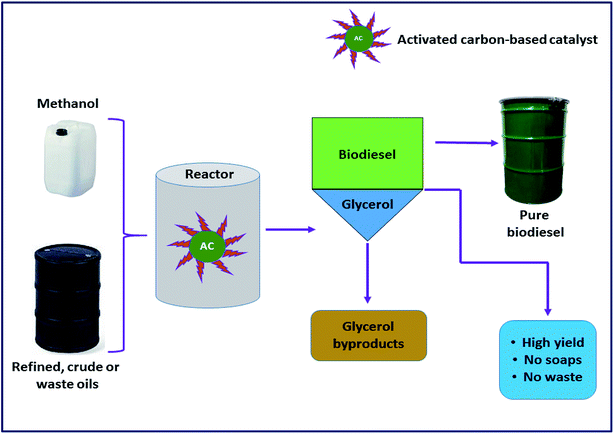 | ||
| Fig. 31 Schematic representation of transesterification of various oils using activated carbon-based catalysts. | ||
In 2009, Yuan et al.369 examined the application of a solid acid catalyst originated from sulfonated activated carbon (H2SO4/C) for catalyzing the transesterification of castor oil and methanol as feedstock. Melero et al.370 synthesized the sulfonic acid-modified mesostructured (SAM) catalysts and studied their efficacy in the transformation of crude vegetable oils to FAME. The results obtained noted that this catalyst has the ability to yield 95 wt% pure FAME and oil transformation close to 100%. Despite the presence of FFAs, this catalyst displayed significantly high activity toward the simultaneous esterification and transesterification reactions. Similarly, Zuo et al.371 developed various sulfonic acid-functionalized mesoporous SBA-15 catalysts, and tested their catalytic activity in the microwave-assisted conversion of soybean oil and 1-butanol to biodiesel. The authors observed that the catalytic efficacy of these catalysts mainly depends on the acid strength and not on the number of acid sites. Furthermore, propyl-SO3H and arene-SO3H functionalized SBA-15 catalysts were found to have comparatively better reactivity in the transesterification process. However, the perfluoro-SO3H functionalized SBA-15 catalyst displayed leaching of the active sites in each progressive cycle, and thus, the reactivity decreased. Shah et al.372 demonstrated esterification of FFAs in acid oil (which is a byproduct of oil refining) using a sulfonic acid-functionalized silica (SiO2–Pr–SO3H) catalyst to prepare the biodiesel. Furthermore, the authors optimized various reaction conditions, such as temperature, reaction time, catalyst concentration, and M/O molar ratio, which usually affect the conversion to FAME. A high conversion (i.e., 96.78% conversion after 8 h was reported at optimized conditions) can be achieved using these solid acid catalysts.
Moreover, in the recent past, Varyambath et al.373 developed different sulfonic acid-functionalized organic knitted porous polyaromatic microspheres (OPPSO3H) utilizing pyrene, anthracene, and naphthalene as monomers via Friedel–Crafts alkylation, followed by crosslinking reactions. Furthermore, these heterogeneous catalysts were utilized for the transformation of long-chain fatty acids and triglycerides to biodiesel. These solid acid catalysts were found to be very promising for biodiesel synthesis, as they showed excellent surface acidity. In addition, several other sulphonic acid-functionalized catalysts were successfully developed and exploited in the production of biodiesel. In this context, Shagufta et al.374 reviewed all such sulphonic acid-functionalized catalysts in esterification and transesterification reactions. This review can be consulted for more detailed information.
Yu et al.375 studied biodiesel production by exploiting coal-based acid catalysts, and reported an oleic acid conversion of 97.6% under the optimal reaction conditions. Similarly, Tang and Niu376 investigated the synthesis of carbon-based solid acid catalysts from bamboo through the partial carbonization and sulfonation approach. The microstructure of the catalyst was activated by phosphoric acid impregnation. The catalyst afforded a biodiesel yield of 97.3% at optimum conditions, which decreased to 83.7% in the fourth reaction cycles. In addition, biodiesel production from oleic acid was reported using sulfonated activated carbon from bamboo.377 A sulfonated carbonaceous material synthesized via the single-step hydrothermal sulfonation of glucose has also been used as a catalyst for the esterification of waste cooking oil to produce biodiesel.378 FESEM images of the carbonaceous material (C) (Fig. 32a) and the sulfonated carbonaceous material (C–SO3H) (Fig. 32b) showed the carbonaceous microsphere and the sulfonated carbonaceous microsphere with an attached sulfonic group on the surface, respectively. The catalyst showed great stability with 93.4% FAME yield under the optimized reaction conditions.
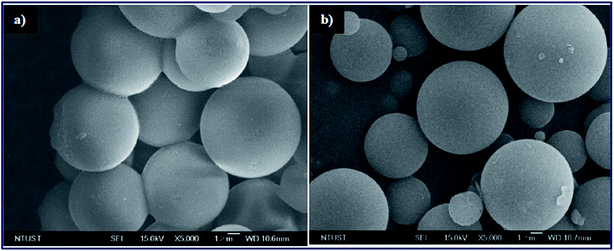 | ||
| Fig. 32 FESEM images of (a) C and (b) C–SO3H. Reproduced from ref. 378. | ||
Guan et al.379 synthesized the sulfonated multi-walled carbon nanotube (S-MWCNT) for the conversion of triglyceride to FAME in 97.8%. The high catalytic reactivity is because of the high surface area (198.9 m2 g−1), high porosity (10–15 nm) and high acid sites. Similarly, the sulfonated carbonaceous material from starch was utilized as a solid catalyst for the esterification of PFAD.380 A novel, efficient, inexpensive and environment-friendly acid catalyst was synthesized from coconut meal residue (CMR). The CMR-DS-SO3H catalyst was prepared by a one-step direct in situ carbonization in concentrated H2SO4, and reported for the transformation of the waste palm oil (WPO) to biodiesel. The prepared sulfonated catalyst has an acid density of 3.8 mmol g−1, surface area of 1.33 m2 g−1 and means pore volume of 0.31 cm3 g−1. The results obtained recorded a high yield of 92.7% biodiesel from WPO.381 Moreover, Wang et al.382 investigated the application of the monodispersed hollow carbon/silica solid acid catalyst HS/C–SO3H, which was prepared by chemical activation approach, in the esterification of oleic acid with methanol to produce the biodiesel.
Besides this, another kind of sulfonated functionalized carbon material, i.e., sulfonated ordered mesoporous carbon (SOMC) catalyst, showed promising biodiesel production (73.59% yield).383 Recently, the sulfonated acid catalyst obtained from corncob (SO42−/corncob) has been reported as an excellent catalyst for the conversion of oleic acid to obtain methyl oleate in good yield (>80% after 8 h at 60 °C).384 Mahdavi and Darab385 prepared a sulfonated carbon catalyst by treatment of sucrose and concentrated H2SO4 at high temperature (sulfonation and carbonization approach). The synthesized C–SO3H catalyst was further utilized for the conversion of oleic acid to FAME in 93.04% yield. Moreover, a solid acid catalyst generated from the sulfonation of microcrystalline cellulose powder was successfully applied for oleic acid esterification, showed 99.9% biodiesel yield under the optimized reaction conditions.386 In another investigation, waste cooking oil was transformed to produce biodiesel, utilizing an environmentally benign sulfonated carbon microsphere catalyst.387 The catalyst with surface area 86 m2 g−1 and acidity 1.38 mmol g−1 was developed by consecutive hydrothermal carbonization and sulfonation of xylose. Using this catalyst, a biodiesel yield of 89.6% was recorded at optimal reaction conditions. The catalyst reusability report revealed that in each cycle, the biodiesel yield was reduced by 9%. Furthermore, the sulfonated carbon-based solid acid catalyst was also utilized for the transformation of PFAD388 and Mesua ferrea Linn oil389 to biodiesel.
To bring down the cost of biodiesel production, several sulfonated raw biomasses have been prepared and investigated for their catalytic activities. In this line, a sulfonated solid-acid catalyst obtained from coconut shells (SO42−/coconut shell) reported 88.03% biodiesel yield.390 In the same vein, oil palm trunk/sugarcane bagasse,391 corn straw,392 bamboo,393Jatropha curcas seed,394 bio-glycerol,395 glycerol,396 microalgae residue,397 oil cake waste,398,399 de-oiled waste cake,400 de-oiled canola meal-SO3H,401 pine chip char402 and biochar403,404 are reported as a catalysts for FAME production.
7.3 Enzyme catalyst
In recent years, enzyme catalysts have been widely examined for the production of biodiesel, as they produce high-quality biodiesel, improve the product separation process, mild reaction conditions and most importantly, their ecological benignness (Table 20).405,406 Besides, they do not form soap with FFA, contrary to the alkaline catalyst. Hence, they can be utilized in biodiesel production on the industrial scale.| S. no. | Catalyst | Feedstock | Conditionsa | Yield | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). NR: not reported. b Miligram. c 2-Propanol/oil molar ratio. d Ethanol/oil molar ratio. e Methyl acetate/fat molar ratio. | |||||
| 1 | Lipase immobilized on biosupport beads | Hybrid non edible oils | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c, 10, 50, 1440 1c, 10, 50, 1440 |
∼78 | 407 |
| 2 | Lipase | WCO | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5, 65, 240 1, 1.5, 65, 240 |
88 | 423 |
| 3 | Thermolysis lanugonosus lipase | Rubber seed oil | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, NR, 65 1, 5, NR, 65 |
92.83 | 416 |
| 4 | CalleraTM Trans L lipase | Soybean oil | 4.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.45, 35, 1440 1, 1.45, 35, 1440 |
96.9 | 417 |
| 5 | Lipase@AC | Sardine oil | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 30, 600 1, 10, 30, 600 |
94.5 | 418 |
| 6 | Lipase@APTES-Fe3O4 | Aspergillus lipid | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 300b, 45, 240 1, 300b, 45, 240 |
84 | 419 |
| 7 | Lipase@ZIF-67 | Soybean oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 45, 3600 1, 10, 45, 3600 |
78 | 420 |
| 8 | Lipase@[bmim][PF6] | Food compost | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 40, 50, 840 1, 40, 50, 840 |
72 | 421 |
| 9 | Lipase@[bmim][NTf2] | Food compost | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 40, 50, 840 1, 40, 50, 840 |
48 | 421 |
| 10 | Lipase@Immobead | Blended non-edible oils | 7.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3.55, 36, 120 1, 3.55, 36, 120 |
94 | 422 |
| 11 | Novozym 435 lipase | Waste fish oil | 35.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d, 50, 35, 480 1d, 50, 35, 480 |
82.91 wt% | 424 |
| 12 | Novozym 435 lipase | BSFL fat | 14.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1e, 17.58, 39.5, 720 1e, 17.58, 39.5, 720 |
96.97 | 425 |
| 13 | Immobilized lipase (Epobond-Pseudomonas cepacia) | Waste vegetable oil | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d, 3, 37, 90 1d, 3, 37, 90 |
46.32 | 426 |
| 14 | Immobilized Candida cylindracea lipase | Jatropha curcas oil | HR, 8, 40, 1440 | 78 | 427 |
| 15 | Immobilised Rhizopus oryzae lipase | Sludge palm oil (SPO) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 40, 240 1, 5, 40, 240 |
91.30 | 428 |
| 16 | Lipase (from rice bran) | Rice bran oil | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NR, 40, 17 1, NR, 40, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
83.4 wt% | 429 |
In biocatalyst-mediated reactions, enzymes can usually be used in the free form or they can be immobilized on a matrix, i.e., immobilized lipase.407 The free enzymes are more sensitive towards the pH, temperature and impurities of the reactants, which may create an obstacle in the bioprocesses. However, these problems can be overcome by immobilizing the enzyme onto different types of support materials.408 The commonly adopted immobilization methods for biological processes include entrapment, adsorption and covalent bonding. Among these techniques, the entrapment method was found to be effective, offering greater advantages, such as ease of process scale-up, higher stability of the enzyme, and longer enzymatic activity retention.409,410 Mostly, the lipase enzymes obtained from microbial sources that have been used for biodiesel production411 proposed the entrapment method for the large scale production of bacterial or fungal lipases due to their extracellular nature. Moreover, lipases obtained from diverse plant sources are also considered as the potential substitute for catalysing the transesterification process.412 The advantages associated with the lipase catalyst over the other catalysts used in biodiesel production are its superior quality and higher yield of biodiesel, freedom from soap formation, lower reaction temperature and ability to work on a variety of feedstock.413
Compared to homogeneous and heterogeneous catalysts, enzymatic catalysts are less studied; hence, there is scant literature that is available when compared with reports on the above-mentioned two catalysts. However, the high cost of the free lipase catalyst along with the limited long-term use has led to the exploitation of the immobilized lipase catalyst to reduce the cost of the catalyst and its reusability. Apart from that, the immobilized lipase catalyst showed greater tolerance to pH variation, high thermal stability and high substrate selectivity.414,415 To date, a large number of studies in the literature are available in the field of biodiesel production using both free416–418 and immobilized419–422 enzyme catalysts.
Recently, Jayaraman et al.423 demonstrated the lipase enzyme-mediated transesterification of waste cooking oil (WCO), and reported 88% of biodiesel yield. Marín-Suárez et al.424 demonstrated the lipase-catalyzed transesterification of low quality fish oil through the process optimization. Moreover, the reusability of the enzyme was also studied. Authors evaluated the efficacy of the commercially available immobilized enzymes, such as Liposome RM IM, Lipozyme TL IM and Novozym 435 (ref. 425) for biodiesel production from waste fish oil. The results obtained revealed that Novozym 435 showed the maximum catalytic activity, resulting in the highest yield of FAME, i.e., 82.91 wt% and the enzyme can be reused for about ten successive cycles. In another study, it was reported that the immobilized lipase (Epobond P. cepacia) employed in the transesterification of waste vegetable oil was reported to achieve an ester yield of 46.32%.426 Similarly, the Candida cylindracea lipase immobilised on the functionalised activated carbon was tested as a catalyst in the transesterification of Jatropha curcas oil. It was found that a free fatty acid yield of 78% was achieved at the optimized reaction conditions. Furthermore, the biocatalyst was found to be stable for up to four consecutive cycles of transesterification.427 Besides, the lipase obtained from the plant source (like the rice bran lipase) produced 83.4 wt% FAME yield from rice bran oil under optimized conditions.428
Moreover, Muanruksa and Kaewkannetra429 examined the biodiesel production from sludge palm oil (SPO) via two steps of extraction and enzymatic esterification. The immobilised Rhizopus oryzae lipases on alginate-polyvinyl alcohol (PVA) beads were used for the conversion of FFAs from SPO to fatty acid methyl esters (biodiesel). It was found that at the optimum condition, the maximum biodiesel yield of 91.30% was achieved and the biocatalyst showed higher stability and catalytic efficiency for up to 15 cycles. It is reported that the enzymatic transesterification reaction for producing biodiesel is the slowest pathway among all of the known transformations. Taking this into account, the application of ultrasonication in the enzyme-catalyzed transesterification improves the reaction rate and hence, reduces the reaction time.414,422 Thus, it can be a promising technique for the industrial-scale production of biodiesel in a very short time.
7.4 Bifunctional solid catalysts
Despite the high reactivity of the basic solid catalyst towards biodiesel production, they are not an effective catalyst for the transesterification of oils having a high amount of FFA, as such catalysts are highly sensitive to the FFA, which leads to soap generation and thus interferes in the separation process of glycerol from biodiesel. On the other hand, solid acid catalysts are insensitive to the FFA content and esterify waste oils or low-cost oils without any requirement of pretreatment. However, water formed during the course of the reaction may lead to the decomposition of triglycerides to diglycerides, resulting in the formation of more FFA and catalyst leaching.430 Taking these difficulties into account, developing a new type of solid catalyst that possesses dual characteristics, such as solid acidic character, to tackle the FFA and solid basic character for easy transesterification of triglycerides to FAME has been a recent interest in the realm of biodiesel research. To date, numerous bifunctional catalysts are reported for the FAME production (Table 21), which will be discussed in this section. Farooq et al.78 developed a bifunctional Mo–Mn/γ-Al2O3–MgO catalyst and utilized it for the simultaneous esterification/transesterification of WCO, having FFA content of 3.27 mg KOH per g. The authors investigated the effect of MgO loading (5–20 wt%) on its catalytic activity, and found that 15 wt% MgO loading showed the highest catalytic activity with 91.4% biodiesel yield under the ideal reaction conditions. Moreover, the catalyst showed excellent stability towards the biodiesel production from WCO, as it is stable for up to 8 progressive reaction cycles without any major loss of its activity. In another study, the Cu/Zn/γ-Al2O3 catalyst was utilized for the simultaneous esterification/transesterification of WCO for the production of FAME via RSM.431 The effect of the Cu/Zn wt% ratio and calcination temperature on the catalytic reactivity was also examined, and it was found that the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 Cu/Zn wt% ratio and 800 °C calcination temperature showed 88.82% FAME yield. The authors also studied the structure and particle size of the synthesized catalyst via TEM micrographs (Fig. 33). Fig. 33a showed that the average diameter of the particles lies in between 4–6 nm. The lattice fringes measured from Fig. 33b, c and d are 0.201, 0.282 and 0.242 nm, and matched with the hkl planes (400), (220) and (311) of alumina, respectively. The lattice fringe in Fig. 33e is 0.240 nm fitted with the hkl plane (200) of CuO, and the lattice fringe 0.281 nm (Fig. 33f) fitted with the ZnO plane (100). Similarly, the biodiesel production from WCO was reported using diverse bifunctional solid catalysts, such as Mg/MCM-41,432 γ-Al2O3–CeO2,433 KAcZX434 and Sr/ZrO2.435
90 Cu/Zn wt% ratio and 800 °C calcination temperature showed 88.82% FAME yield. The authors also studied the structure and particle size of the synthesized catalyst via TEM micrographs (Fig. 33). Fig. 33a showed that the average diameter of the particles lies in between 4–6 nm. The lattice fringes measured from Fig. 33b, c and d are 0.201, 0.282 and 0.242 nm, and matched with the hkl planes (400), (220) and (311) of alumina, respectively. The lattice fringe in Fig. 33e is 0.240 nm fitted with the hkl plane (200) of CuO, and the lattice fringe 0.281 nm (Fig. 33f) fitted with the ZnO plane (100). Similarly, the biodiesel production from WCO was reported using diverse bifunctional solid catalysts, such as Mg/MCM-41,432 γ-Al2O3–CeO2,433 KAcZX434 and Sr/ZrO2.435
| No | Catalyst | Feedstocks | Conditionsa | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Methanol-to-oil molar ratio, catalyst loading (wt%), temperature (°C), reaction time (min). b Conversion. | |||||
| 1 | Mo–Mn/γ-Al2O3-15% MgO | WCO | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 100, 240 1, 3, 100, 240 |
91.4 | 78 |
| 2 | Cu/Zn(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90)/γ-Al2O3-800 °C 90)/γ-Al2O3-800 °C |
WCO | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 65 ± 5, 180 1, 6, 65 ± 5, 180 |
88.82 | 431 |
| 3 | Mg/MCM-41 | WCO | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10, 80, 180 1, 10, 80, 180 |
94 | 432 |
| 4 | γ-Al2O3–CeO2 | WCO | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7, 110, 270 1, 7, 110, 270 |
81.1 | 433 |
| 5 | KAcZX | WCO | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 120, 180 1, 6, 120, 180 |
80.8 | 434 |
| 6 | Sr/ZrO2 | WCO | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.7, 115.5, 169 1, 2.7, 115.5, 169 |
79.7 | 435 |
| 7 | Bi2O3–La2O3 | JCO | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2, 150, 240 1, 2, 150, 240 |
94 | 436 |
| 8 | CaO–La2O3 | JCO | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 160, 180 1, 3, 160, 180 |
98.76 | 437 |
| 9 | Mn@MgO–ZrO2 | Kernel oil | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3, 90, 240 1, 3, 90, 240 |
96.4 | 438 |
| 10 | HPA@ZIF-8 | Rapeseed oil | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4, 240, 300 1, 4, 240, 300 |
98.02b | 439 |
| 11 | AWS/SO42− | PFAD | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5, 80, 180 1, 5, 80, 180 |
98 | 441 |
| 12 | [Zn(4,4′-bipy)(OAc)2]n | Soybean oil | 3.2/5 (v/v), 2, 180, 120 | 98 | 442 |
| 13 | K/TiO2 | Canola oil | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, 70, 180 1, 6, 70, 180 |
100b | 443 |
 | ||
Fig. 33 TEM micrograph for Cu/Zn(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90)/γ-Al2O3-800 °C (a). The HRTEM images displayed the lattice fringes of (b) Al2O3 (400), (c) Al2O3 (220), (d) Al2O3 (311), (e) CuO (200) and (f) ZnO (100). Reproduced from ref. 431. 90)/γ-Al2O3-800 °C (a). The HRTEM images displayed the lattice fringes of (b) Al2O3 (400), (c) Al2O3 (220), (d) Al2O3 (311), (e) CuO (200) and (f) ZnO (100). Reproduced from ref. 431. | ||
Nizah et al.436 synthesized a bifunctional catalyst Bi2O3–La2O3via wet impregnation procedure, and employed it for the one-pot esterification/transesterification of JCO, having a FFA content of 6.1 mg KOH per g. The authors investigated the influence of Bi2O3 impregnation on La2O3 support by varying the wt% of Bi2O3 in the range of 1–7 wt%, and found that 5 wt% Bi2O3 impregnated on La2O3 showed the maximum biodiesel yield of 94%. The high catalyst reactivity is attributed to the good dispersion of Bi2O3 on the La2O3 support, which directly enhanced the surface area, and thus increases the selectivity and rate of the reaction. Similarly, the biodiesel production from JCO having a high amount of FFA was reported by using a bifunctional solid catalyst CaO–La2O3.437 The esterification/transesterification was performed in a high-temperature reactor (Fig. 34). The effect of the Ca/La atomic ratio on the catalytic activity was examined, and it was observed that a Ca/La atomic ratio of 0.8 showed the maximum biodiesel yield of 98.76% under the optimized reaction conditions. The high catalytic reactivity is because of the good dispersion of CaO on the surface of La2O3, which led to an increase in the catalyst surface area. Moreover, the synthesized catalyst is chemically stable and can be used for 4 consecutive cycles.
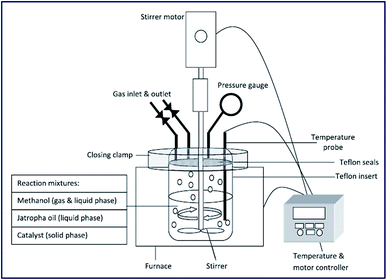 | ||
| Fig. 34 Schematic diagram of a high-temperature reactor. Reproduced from ref. 437. | ||
Another study revealed the synthesis of the mixed metal oxide Mn@MgO–ZrO2via co-precipitation and impregnation method, and the utilization of the catalyst in the FAME production from kernel oil.438 The efficiency of the catalyst in the FAME production was tested by changing the Mg/Zr ratio from 0.2 to 0.5, and it was found that 0.4 Mg/Zr has the optimal active sites, followed by impregnation of 4 wt% Mn to the MgO–ZrO2 composite to enhance its reactivity and displayed 96.4% biodiesel yield. The high catalyst reactivity is due to a large number of active sites and the mesoporous nature of the catalyst. Jeon et al.439 synthesized heteropolyacid (HPA) functionalized ZIF-8 (zeolite imidazole framework-8) to form a bifunctional catalyst for the production of biodiesel from rapeseed oil in a batch reactor. The catalyst possesses a core–shell nanostructure as displayed by the TEM micrograph (Fig. 35), where the rhombic dodecahedral ZIF-8 core was surrounded by thin-wrinkled HPA shell, and thus enhances the surface area and catalyst reactivity. Moreover, the effect of the concentration of HPA for the functionalization was also tested by varying the amount of HPA, such as 0.05, 0.1, 0.3 and 0.5. It was found that 0.1 g HPA functionalized ZIF-8 showed a maximum FAME conversion of 98.02% under the optimized reaction conditions. Similarly, another bifunctional catalyst organotriphosphonic acid-functionalized ferric alginate (ATMP-FA) was developed for the oleic acid esterification to produce biodiesel.440 The reaction conditions were optimized by using the Box–Behnken model of RSM. Moreover, the catalyst is very stable towards the esterification reaction, and can be reused for 5 consecutive cycles.
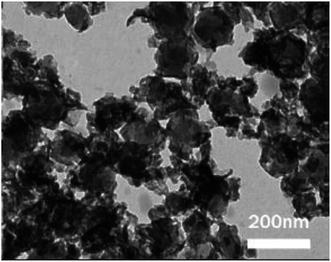 | ||
| Fig. 35 TEM image of HPA-ZIF-8. Reproduced from ref. 439. | ||
Recently, a solid bifunctional catalyst originating from the bio-waste angel wing shell (AWS) via two-step processes: (i) calcination of angel wing shell, and (ii) sulfonation of the calcined angel wing shell to produce sulfonated angel wing shell (AWS/SO42−), was reported for the esterification of PFAD to produce biodiesel.441 The sulfonation procedure increases the surface area of bare AWS from 3.88 to 6.53 m2 g−1, and thus enhanced the catalytic reactivity. The authors tested the influence of the sulfuric acid concentration by varying the sulfuric acid amount from 3 to 11 M, and found that the sulfonation with 7 M sulfuric acid showed 98% FAME yield. The authors also checked the reusability of the catalyst, and observed a blockage of the active sites of the catalyst after the 2nd consecutive cycles, which necessitated pretreatment of the spent catalyst to increase its reusability. In addition, a coordinated polymer of Zn, [Zn(4,4′-bipy)(OAc)2]n, was tested for the soybean oil transformation to FAME.442 The catalyst showed excellent reactivity and showed 98% FAME yield under the optimized reaction conditions. The authors reported that the high reactivity of the catalyst is attributed to the bipyridine present in the catalyst. In another study, the conversion of canola oil to FAME was reported using potassium-impregnated titania (K/TiO2).443 The addition of K on the surface of titania increases the surface energy from 86 to 102 m2 g−1, and thus enhanced the catalytic activity. The authors investigated the effect of K loading on the catalytic activity, and found that 20 wt% K-loaded titania was optimal and showed 100% conversion of canola oil to biodiesel.
8. Biodiesel production process
Biodiesel can be produced by (trans)esterification, thermal cracking and pyrolysis.444–447 Among all these methods, transesterification is generally utilized for the synthesis of biodiesel.447 The generalized diagram for the biodiesel production process is presented in Fig. 36, which consists of the synthesis and purification steps.447 Alkali, acid and enzyme are routinely exploited as a catalyst for the transesterification reactions. These catalysts had their own merits and demerits, as compiled in Table 22.448 Until now, the homogeneous base catalysts (such as NaOH, KOH) are normally utilized for biodiesel synthesis in the industrial scale. In the meantime, owing to their capacity to catalyze both esterification/transesterification reactions, a homogeneous acid catalyst (such as H2SO4) and HCl are generally picked for feedstock having high FFA, such as non-edible vegetable oil, WCO and animal fats. Recently, the heterogeneous catalyst has attracted interest to a great extent for biodiesel synthesis because of their easy recyclability and reusability for successive reaction cycles.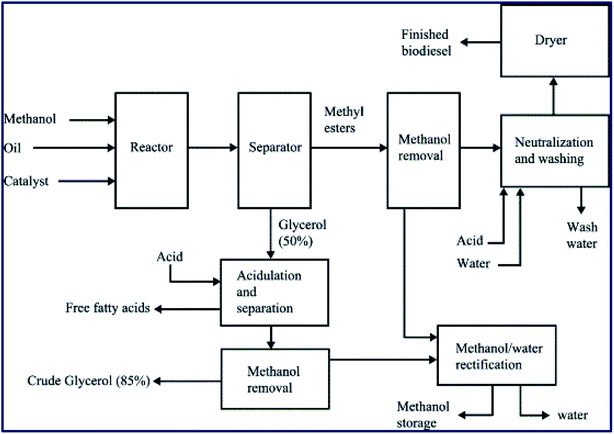 | ||
| Fig. 36 Representative diagram for biodiesel production. Reproduced from ref. 447. | ||
| Catalyst types | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Homogeneous | |||
| Alkali | NaOH, KOH | • High reactivity | • Inappropriate for high FFA in feedstocks |
| • Faster reaction rate | • Deactivates in the presence of moisture and FFA. | ||
| • Minimum cost | • Requirement of high amount of waste water | ||
| • Encouraging kinetics | • Saponification occurs as a side reaction. | ||
| • Moderate working conditions | • Non-recyclable | ||
| • Corrosive in nature | |||
| Acid | H2SO4, HCl, HF. | – Non-reactive to moisture and FFA content in oil. | – Slow reaction rate |
| – Catalyzed simultaneous esterification/transesterification reactions. | – Long reaction time | ||
| – Avoids formation of soap. | – Equipment corrosion | ||
| – Higher reaction temperature and pressure | |||
| – High alcohol/oil requirement | |||
| – Weak catalytic activity | |||
| – Catalyst is difficult to recycle | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Heterogeneous | |||
| Alkali | CaO, SrO, MgO, mixed oxide and hydrotalcite | • Non-corrosive | • Slow reaction rate compared to homogeneous one |
| • Environmentally benign | • Low FFA requirement in the feedstock (<1 wt%) | ||
| • Recyclable | • Highly sensitive to water and FFA | ||
| • Fewer disposal problems | • Saponification as a side reaction | ||
| • Easy separation | • Soap formation | ||
| • Higher selectivity | • High volume of wastewater | ||
| • Longer catalyst life | • Leaching of active catalyst sites | ||
| • Diffusion limitations | |||
| • Complex and expensive synthesis route | |||
| • High cost of catalyst synthesis | |||
| Acid | ZrO, TiO, ZnO, ion-exchange resin, sulfonic modified | – Insensitive to FFA and water content in the oil | – Moderate reaction rate |
| Mesostructured silica | – Catalyzed simultaneous esterification and transesterification reactions | – Long reaction time | |
| – Recyclable, eco-friendly | – Higher reaction temperature and pressure | ||
| – Non-corrosive to reactor and reactor parts | – High alcohol/oil requirement | ||
| – Weak catalytic activity | |||
| – Low acidic site | |||
| – Low micro porosity | |||
| – Leaching of active catalyst sites | |||
| – Diffusion limitations | |||
| – Complex and expensive synthesis route | |||
| – High cost of catalyst synthesis | |||
9. Catalyst comparison
It can be seen from the literature that the reactivity of both homogeneous base and acid catalysts are very high compared to heterogeneous catalysts.61,70 Despite the high reactivity, homogeneous catalysts have some serious shortfalls. These include the low quality of glycerol produced, the inability to regenerate the catalyst, and the lengthy process involved in the purification of biodiesel. Thus, the whole process becomes labour-intensive and uneconomical.76 To overcome these shortfalls, solid catalysts have been widely investigated. Alkaline earth, basic metal oxides and supported solid base catalysts show excellent activity towards biodiesel production. However, their low stability and high sensitivity against the FFA limit its industrial application.143 In contrast, their acid counterparts are not efficient towards the transesterification reactions. Recently, mixed metal oxides are gaining immense attention in the field of biodiesel production due to their generally high surface area, excellent thermal and chemical stability, and tailored acid–base properties. Hence, they can be predominantly used for the (trans)esterification of vegetable oil having high FFA.145Prior studies from literature revealed that the enzyme-based catalysts have various advantages over other catalysts, such as being environmentally benign, operating at mild reaction conditions and displaying high specificity.432 Unfortunately, due to their sensitivity towards heat, poor operational stability and narrow pH range, the use of such catalysts for the industrial scale production of biodiesel is not a wise choice.433 However, the immobilized lipase has various advantages compared to free lipase, such as cost-effectiveness, high thermal stability and greater tolerance to pH changes.435 Thus, it has a scope for utilization in biodiesel production on the industrial scale. Besides, the present study suggests that the bio-waste derived catalyst can potentially be used in the industrial scale production of biodiesel as they are easily available, cost-effective and most importantly, environmentally benign.161 The main limitation is their reusability due to the leaching of the active sites.165 Apart from that, the metal-free carbon based solid acid catalyst is also a promising candidate for the industrial scale production of biodiesel as these materials possess promising features, such as being biogenic and environment-friendly, and having lower production costs, distinctive surface chemistry, high chemical and thermal stability.383 The bifunctional catalyst has been of recent interest in the realm of biodiesel research, as it possesses dual characteristics such as solid acidic character to tackle the FFA and solid basic character for the easy transesterification of triglycerides to FAME. Hence, it can be utilized for the (trans)esterification of diverse oil systems. Apart from that, the bifunctional catalysts are highly reusable, thermostable and insensitive to the moisture.438 Thus, the bifunctional solid catalyst can be utilized in the successful production of industrial scale biodiesel.
10. Conclusion and outlook
The exponential growth in the human population around the globe and industrial globalization tremendously increases the demand for petroleum fuels like diesel for various purposes. However, considering the limited resources of fossil fuels, searching for a novel, renewable and sustainable alternative fuel was required. In this context, researchers focused on the FAME production from different renewable sources as an effective way. A variety of methods have been proposed for biodiesel production. However, among all the existing methods, transesterification is considered as the foremost choice.The transesterification reaction involves the use of a basic catalyst, such as homogeneous and heterogeneous catalysts. The use of homogeneous catalysts is found to be promising as far as the rate of biodiesel production is concerned. However, it is associated with certain limitations. The homogeneous catalyst-based transesterification reaction involves the consumption of high energy. Moreover, the treatment of wastewater generated is essential due to the presence of unreacted chemicals. These limitations created the need for the development of efficient catalysts, which was completed in terms of the heterogeneous catalysts. These catalysts attracted a great deal of attention from the scientific community all over the world because of its several advantages over homogeneous catalysts, such as the simple realization of continuous reactors, production of cleaner glycerol, and the absence of both the alkaline catalyst neutralization step and the necessity to replace the consumed catalyst. Due to these advantages, heterogeneous catalysts have opened up the chance for another powerful pathway for FAME production. However, the reactivity of the solid catalyst is dependent on several variables, which mainly involve the oil type, alcohol to oil molar ratio, temperature, and type of reactor. Therefore, the selection of these variables at an optimum level is a crucial step. The heterogeneous catalysts are considered comparatively promising because only the external-surface active species of the porous solid support is involved in the reaction, and these catalysts can be recovered in some cases. However, in the case of certain catalysts like CaO, leaching was reported, which adversely influences the reaction. Hence, researchers are looking at nanotechnology as a new hope.
Nanotechnology is the most emerging branch of science, having promising applications in catalysis. Moreover, it is reported to have the ability to fabricate the catalyst surface in order to meet the prerequisites of explicit applications, and beat the different issues related to both homogeneous and heterogeneous catalysts. Nanocatalysts can act as an interface between the homogeneous and heterogeneous catalysts having the possibility to develop promising solid-acid or solid-base catalysts, which can be easily recovered using conventional filtration and centrifugation techniques. The development and use of magnetic nanoparticle-supported catalysts is a path-breaking research because such catalysts can be easily recovered by using a simple magnetic field and reused for progressive reaction cycles, which helps to reduce the overall process cost involved in biodiesel production, which is the ultimate aim.
It is well proven that the application of a biological catalyst (enzyme) is more effective over all kinds of chemical catalysts, but the involvement of an expensive enzyme increases the overall cost of the FAME production process. In this context, immobilization of such enzymes on the surface of various magnetic nanoparticles was found to be a novel concept because of the easy recovery of the immobilized enzyme, along with magnetic nanoparticles and its reusability. Moreover, it also solves the problem of leaching the enzymes during the reaction due to immobilization. Although nanocatalysts were reported to have promising applications, the toxicological concerns associated with nanoparticles are a topic of debate because there are mixed opinions from the scientific community.
The present study revealed that the properties of the catalyst (such as basicity and acidity) play a pivotal role in the biodiesel production. Several literature studies suggest that the basicity of the catalyst is directly proportional to the transesterification activity.171,195 Similarly, the acidity of the catalyst decides the esterification activity of the catalyst.383,390 The esterification activity increases with increasing acidity of the catalyst. Apart from the basicity and acidity, the catalytic activity of the solid catalyst depends on its surface area and porosity. Literature studies revealed that the high surface area of the catalyst enhances the rate of biodiesel production.184,225
It is believed that several newly introduced catalysts will take a central position in the near future, and help produce biodiesel through eco-friendly and economically viable processes. The development of a novel heterogeneous catalyst having both acid and basic sites on its surface will have a promising future in biodiesel production technologies because it will have the ability to overcome the issues usually caused because of the utilization of homogeneous catalysts. The application of bifunctional solids can be a novel way in heterogeneous catalyst-mediated biodiesel production because they showed the capability to accomplish the simultaneous esterification and transesterification reactions in a one-pot process. In addition, the development and application of the nanocatalysts will be a milestone in biodiesel production. These nanocatalysts will be the next-generation catalysts, which will help to develop the most effective, sensitive, sustainable and economically viable technology for the FAME production in the near future. Although recent advances in the developments of various homogeneous, heterogeneous and nanocatalysts showed a promising future for biodiesel industries or biorefineries, more efforts are required to develop even more effective and cheap catalysts, which will help overcome the present issues with all of the above-mentioned catalysts and increase the efficiency of sustainable biodiesel production.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Science and Engineering Research Board (SERB), India is thankfully acknowledged for the research fund (Grant No. SB/FT/CS-103/2013 and SB/EMEQ-076/2014).References
- G. Ciarrocchi, A. Montecucco, G. Pedrali-Noy and S. Spadari, Biochem. Pharmacol., 1988, 37, 1803–1804 CrossRef CAS PubMed
.
- X. Yin, X. Duan, Q. You, C. Dai, Z. Tan and X. Zhu, Energy Convers. Manage., 2016, 112, 199–207 CrossRef CAS
.
-
International Renewable Energy Agency (IRENA), Global Energy Transformation: A Roadmap to 2050, 2018 Search PubMed
.
-
IEA, Int. Energy Agency, Peris, 2016, pp. 1–77 Search PubMed
.
- M. G. Kulkarni and A. K. Dalai, Ind. Eng. Chem. Res., 2006, 45, 2901–2913 CrossRef CAS
.
- S. Chatterjee, Dhanurdhar and L. Rokhum, Renewable Sustainable Energy Rev., 2017, 72, 560–564 CrossRef CAS
.
- A. da Silva César, M. A. Conejero, E. C. Barros Ribeiro and M. O. Batalha, Renewable Energy, 2019, 133, 1147–1157 CrossRef
.
- M. T. Lund, T. K. Berntsen and J. S. Fuglestvedt, Environ. Sci. Technol., 2014, 48, 14445–14454 CrossRef CAS PubMed
.
- F. C. De Oliveira and S. T. Coelho, Renewable Sustainable Energy Rev., 2017, 75, 168–179 CrossRef CAS
.
- J. Ling, S. Nip, W. L. Cheok, R. A. de Toledo and H. Shim, Bioresour. Technol., 2014, 173, 132–139 CrossRef CAS PubMed
.
-
L. E. Singer and D. Peterson, International energy outlook, 2010, p. 0484 Search PubMed
.
- D. Y. C. Leung, X. Wu and M. K. H. Leung, Appl. Energy, 2010, 87, 1083–1095 CrossRef CAS
.
- G. Pathak, D. Das and L. Rokhum, RSC Adv., 2016, 6, 93729–93740 RSC
.
- G. Pathak and L. Rokhum, ACS Comb. Sci., 2015, 17, 483–487 CrossRef CAS PubMed
.
- B. Mallesham, P. Sudarsanam and B. M. Reddy, Ind. Eng. Chem. Res., 2014, 53, 18775–18785 CrossRef CAS
.
- B. H. Hameed, L. F. Lai and L. H. Chin, Fuel Process. Technol., 2009, 90, 606–610 CrossRef CAS
.
- D. R. Lathiya, D. V. Bhatt and K. C. Maheria, Bioresour. Technol. Rep., 2018, 2, 69–76 CrossRef
.
- J. M. Encinar, N. Sánchez, G. Martínez and L. García, Bioresour. Technol., 2011, 102, 10907–10914 CrossRef CAS PubMed
.
- L. Li, W. Du, D. Liu, L. Wang and Z. Li, J. Mol. Catal. B: Enzym., 2006, 43, 58–62 CrossRef CAS
.
- J. Kansedo, K. T. Lee and S. Bhatia, Biomass Bioenergy, 2009, 33, 271–276 CrossRef CAS
.
- M. N. Nabi, M. M. Rahman and M. S. Akhter, Appl. Therm. Eng., 2009, 29, 2265–2270 CrossRef CAS
.
- S. V. Ghadge and H. Raheman, Biomass Bioenergy, 2005, 28, 601–605 CrossRef CAS
.
- X. Meng, G. Chen and Y. Wang, Fuel Process. Technol., 2008, 89, 851–857 CrossRef CAS
.
- S. A. Shaban, Egypt. J. Chem., 2012, 55, 437–452 CrossRef
.
- H. N. Bhatti, M. A. Hanif, M. Qasim and A.-u. Rehman, Fuel, 2008, 87, 2961–2966 CrossRef CAS
.
- P. Cao, M. A. Dubé and A. Y. Tremblay, Biomass Bioenergy, 2008, 32, 1028–1036 CrossRef CAS
.
- H. Y. Shin, S. H. Lee, J. H. Ryu and S. Y. Bae, J. Supercrit. Fluids, 2012, 61, 134–138 CAS
.
- M. Gürü, A. Koca, Ö. Can, C. Çinar and F. Şahin, Renewable Energy, 2010, 35, 637–643 CrossRef
.
- E. Alptekin and M. Canakci, Fuel, 2010, 89, 4035–4039 CrossRef CAS
.
- C. Y. Lin and R. J. Li, Fuel Process. Technol., 2009, 90, 130–136 CrossRef CAS
.
- J. F. Costa, M. F. Almeida, M. C. M. Alvim-Ferraz and J. M. Dias, Energy Convers. Manage., 2013, 74, 17–23 CrossRef CAS
.
- B. M. S. Hossain and S. Aishah, Am. J. Biochem. Biotechnol., 2008, 4, 250–254 CrossRef
.
- G. Najafi, B. Ghobadian and T. F. Yusaf, Renewable Sustainable Energy Rev., 2011, 15, 3870–3876 CrossRef CAS
.
- L. Chen, T. Liu, W. Zhang, X. Chen and J. Wang, Bioresour. Technol., 2012, 111, 208–214 CrossRef CAS PubMed
.
-
U. Zur and R. V. O. N. Oel-proteinpflanzen, Union Zur Förderung Von Oel- Und Proteinpflanzen E.V., 2017, p. 51 Search PubMed
.
- I. M. Atadashi, M. K. Aroua, A. R. Abdul Aziz and N. M. N. Sulaiman, J. Ind. Eng. Chem., 2013, 19, 14–26 CrossRef CAS
.
- S. P. Singh and D. Singh, Renewable Sustainable Energy Rev., 2010, 14, 200–216 CrossRef CAS
.
- S. D. A. P. Apptanaidu, A. M. Ali and M. H. Alias, J. Ekon. Malaysia, 2014, 48, 29–40 CrossRef
.
-
B. Flach, S. Lieberz, M. Rondon, B. Williams and C. Teiken, GAIN Report: EU-28 Biofuels Annual, 2015, pp. 14–21 Search PubMed
.
-
R. Delzeit, T. Heimann, F. Schuenemann and M. Soeder. GTAP, 2019 Search PubMed
.
-
U. Zur and R. V. O. N. Oel-proteinpflanzen, Union Zur Förderung Von Oel- Und Proteinpflanzen E.V. 2015 Search PubMed
.
- J. L. Shumaker, C. Crofcheck, S. A. Tackett, E. Santillan-Jimenez and M. Crocker, Catal. Lett., 2007, 115, 56–61 CrossRef CAS
.
- K. Bélafi-Bakó, F. Kovács, L. Gubicza and J. Hancsók, Biocatal. Biotransform., 2002, 20, 437–439 CrossRef
.
- S. Yan, H. Lu and B. Liang, Energy Fuels, 2008, 22, 646–651 CrossRef CAS
.
- D. A. G. Aranda, R. T. P. Santos, N. C. O. Tapanes, A. L. D. Ramos and O. A. C. Antunes, Catal. Lett., 2008, 122, 20–25 CrossRef CAS
.
- M. R. Avhad and J. M. Marchetti, Renewable Sustainable Energy Rev., 2015, 50, 696–718 CrossRef CAS
.
- A. Karmakar, S. Karmakar and S. Mukherjee, Bioresour. Technol., 2010, 101, 7201–7210 CrossRef CAS PubMed
.
- M. M. Gui, K. T. Lee and S. Bhatia, Energy, 2008, 33, 1646–1653 CrossRef CAS
.
- K. Shikha and C. Y. Rita, J. Chem. Pharm. Res., 2012, 4, 4219–4230 Search PubMed
.
- A. L. Ahmad, N. H. M. Yasin, C. J. C. Derek and J. K. Lim, Renewable Sustainable Energy Rev., 2011, 15, 584–593 CrossRef CAS
.
- S. L. Dmytryshyn, A. K. Dalai, S. T. Chaudhari, H. K. Mishra and M. J. Reaney, Bioresour. Technol., 2004, 92, 55–64 CrossRef CAS PubMed
.
- S. Yusup and M. A. Khan, Biomass Bioenergy, 2010, 34, 1500–1504 CrossRef CAS
.
- J. M. Dias, M. C. M. Alvim-Ferraz and M. F. Almeida, Fuel, 2008, 87, 3572–3578 CrossRef CAS
.
- U. Rashid and F. Anwar, Fuel, 2008, 87, 265–273 CrossRef CAS
.
- J. M. Encinar, J. F. González and A. Rodríguez-Reinares, Fuel Process. Technol., 2007, 88, 513–522 CrossRef CAS
.
- A. A. Refaat, N. K. Attia, H. A. Sibak, S. T. El Sheltawy and G. I. ElDiwani, Int. J. Environ. Sci. Technol., 2008, 5, 75–82 CrossRef CAS
.
- M. P. Dorado, E. Ballesteros, M. Mittelbach and F. J. López, Energy Fuels, 2004, 18, 1457–1462 CrossRef CAS
.
- O. J. Alamu, S. O. Jekayinfa and T. A. Akintola, Agric. Eng., 2007, 9, 1–11 Search PubMed
.
- K. H. Chung, J. Kim and K. Y. Lee, Biomass Bioenergy, 2009, 33, 155–158 CrossRef CAS
.
- S. K. Karmee and A. Chadha, Bioresour. Technol., 2005, 96, 1425–1429 CrossRef CAS PubMed
.
- P. Felizardo, M. J. Neiva Correia, I. Raposo, J. F. Mendes, R. Berkemeier and J. M. Bordado, Waste Manage., 2006, 26, 487–494 CrossRef CAS PubMed
.
- B. B. Uzun, M. Kiliç, N. Özbay, A. E. Pütün and E. Pütün, Energy, 2012, 44, 347–351 CrossRef CAS
.
- D. Y. C. Leung and Y. Guo, Fuel Process. Technol., 2006, 87, 883–890 CrossRef CAS
.
- U. Rashid, F. Anwar, B. R. Moser and S. Ashraf, Biomass Bioenergy, 2008, 32, 1202–1205 CrossRef CAS
.
- Z. Ilham, Malays. J. Biochem. Mol. Biol., 2009, 17, 5–9 Search PubMed
.
- S. T. Keera, S. M. El Sabagh and A. R. Taman, Fuel, 2011, 90, 42–47 CrossRef CAS
.
- U. Rashid, F. Anwar, T. M. Ansari, M. Arif and M. Ahmad, J. Chem. Technol. Biotechnol., 2009, 84, 1364–1370 CrossRef CAS
.
- K. S. Chen, Y. C. Lin, K. H. Hsu and H. K. Wang, Energy, 2012, 38, 151–156 CrossRef CAS
.
- K. Jacobson, R. Gopinath, L. C. Meher and A. K. Dalai, Appl. Catal., B, 2008, 85, 86–91 CrossRef CAS
.
- Y. Wang, S. Ou, P. Liu, F. Xue and S. Tang, J. Mol. Catal. A: Chem., 2006, 252, 107–112 CrossRef CAS
.
- M. Canakci and J. Van Gerpen, Trans. ASAE, 1999, 42, 1203–1210 CAS
.
- X. Miao, R. Li and H. Yao, Energy Convers. Manage., 2009, 50, 2680–2684 CrossRef CAS
.
- M. J. Nye, T. W. Williamson, W. Deshpande, J. H. Schrader, W. H. Snively, T. P. Yurkewich and C. L. French, J. Am. Oil Chem. Soc., 1983, 60, 1598–1601 CrossRef CAS
.
- J. Zhang and L. Jiang, Bioresour. Technol., 2008, 99, 8995–8998 CrossRef CAS PubMed
.
- V. B. Veljković, S. H. Lakićević, O. S. Stamenković, Z. B. Todorović and M. L. Lazić, Fuel, 2006, 85, 2671–2675 CrossRef
.
- P. Dalvand and L. Mahdavian, Biofuels, 2018, 9, 705–710 CrossRef CAS
.
- Y. Ma, Q. Wang, X. Sun, C. Wu and Z. Gao, Renewable Energy, 2017, 107, 522–530 CrossRef CAS
.
- M. Farooq, A. Ramli and D. Subbarao, J. Cleaner Prod., 2013, 59, 131–140 CrossRef CAS
.
- M. Zabeti, W. M. A. Wan Daud and M. K. Aroua, Fuel Process. Technol., 2009, 90, 770–777 CrossRef CAS
.
- T. F. Dossin, M. F. Reyniers, R. J. Berger and G. B. Marin, Appl. Catal., B, 2006, 67, 136–148 CrossRef CAS
.
- M. C. Math, S. P. Kumar and S. V. Chetty, Energy Sustainable Dev., 2010, 14, 339–345 CrossRef CAS
.
- M. Kouzu, S.-y. Yamanaka, J.-s. Hidaka and M. Tsunomori, Appl. Catal., A, 2009, 355, 94–99 CrossRef CAS
.
- M. L. Granados, M. D. Z. Poves, D. M. Alonso, R. Mariscal, F. C. Galisteo, R. Moreno-Tost, J. Santamaría and J. L. G. Fierro, Appl. Catal., B, 2007, 73, 317–326 CrossRef CAS
.
- A. Kawashima, K. Matsubara and K. Honda, Bioresour. Technol., 2009, 100, 696–700 CrossRef CAS PubMed
.
- X. Liu, H. He, Y. Wang and S. Zhu, Catal. Commun., 2007, 8, 1107–1111 CrossRef CAS
.
- H. Mootabadi, B. Salamatinia, S. Bhatia and A. Z. Abdullah, Fuel, 2010, 89, 1818–1825 CrossRef CAS
.
- J. Jitputti, B. Kitiyanan, P. Rangsunvigit, K. Bunyakiat, L. Attanatho and P. Jenvanitpanjakul, Chem. Eng. J., 2006, 116, 61–66 CrossRef CAS
.
- M. Stöcker, J. Mol. Catal., 1985, 29, 371–377 CrossRef
.
- S. J. Yoo, H.-s. Lee, B. Veriansyah, J. Kim, J. D. Kim and Y. W. Lee, Bioresour. Technol., 2010, 101, 8686–8689 CrossRef CAS PubMed
.
- R. B. da Silva, A. F. Lima Neto, L. S. Soares dos Santos, J. R. de Oliveira Lima, M. H. Chaves, J. R. dos Santos, G. M. de Lima, E. M. de Moura and C. V. R. de Moura, Bioresour. Technol., 2008, 99, 6793–6798 CrossRef PubMed
.
- G. Baskar, A. Gurugulladevi, T. Nishanthini, R. Aiswarya and K. Tamilarasan, Renewable Energy, 2017, 103, 641–646 CrossRef CAS
.
- S. Nakagaki, A. Bail, V. C. dos Santos, V. H. R. de Souza, H. Vrubel, F. S. Nunes and L. P. Ramos, Appl. Catal., A, 2008, 351, 267–274 CrossRef CAS
.
- M. Di Serio, M. Cozzolino, R. Tesser, P. Patrono, F. Pinzari, B. Bonelli and E. Santacesaria, Appl. Catal., A, 2007, 320, 1–7 CrossRef CAS
.
- B. Rongxian, T. Yisheng and H. Yizhuo, Fuel Process. Technol., 2004, 86, 293–301 CrossRef
.
- A. P. S. Chouhan and A. K. Sarma, Renewable Sustainable Energy Rev., 2011, 15, 4378–4399 CrossRef CAS
.
- W. Xie, X. Huang and H. Li, Bioresour. Technol., 2007, 98, 936–939 CrossRef CAS PubMed
.
- Q. Shu, B. Yang, H. Yuan, S. Qing and G. Zhu, Catal. Commun., 2007, 8, 2159–2165 CrossRef CAS
.
- M. J. Ramos, A. Casas, L. Rodríguez, R. Romero and Á. Pérez, Appl. Catal., A, 2008, 346, 79–85 CrossRef CAS
.
- H. Wu, J. Zhang, Q. Wei, J. Zheng and J. Zhang, Fuel Process. Technol., 2013, 109, 13–18 CrossRef CAS
.
- M. Feyzi and G. Khajavi, Ind. Crops Prod., 2014, 58, 298–304 CrossRef CAS
.
- N. Narkhede and A. Patel, Ind. Eng. Chem. Res., 2013, 52, 13637–13644 CrossRef CAS
.
- O. Babajide, N. Musyoka, L. Petrik and F. Ameer, Catal. Today, 2012, 190, 54–60 CrossRef CAS
.
- M. C. Manique, L. V. Lacerda, A. K. Alves and C. P. Bergmann, Fuel, 2017, 190, 268–273 CrossRef CAS
.
- N. Al-Jammal, Z. Al-Hamamre and M. Alnaief, Renewable Energy, 2016, 93, 449–459 CrossRef CAS
.
- L. Du, S. Ding, Z. Li, E. Lv, J. Lu and J. Ding, Energy Convers. Manage., 2018, 173, 728–734 CrossRef CAS
.
- S. Semwal, A. K. Arora, R. P. Badoni and D. K. Tuli, Bioresour. Technol., 2011, 102, 2151–2161 CrossRef CAS PubMed
.
- A. Bohlouli and L. Mahdavian, Biofuels, 2019, 1–14 CrossRef
.
- W. Xie and H. Li, J. Mol. Catal. A: Chem., 2006, 255, 1–9 CrossRef CAS
.
- J. Paulo, A. Duarte, L. Di and A. Souza, Renewable Sustainable Energy Rev., 2016, 59, 887–894 CrossRef
.
- H. Ma, S. Li, B. Wang, R. Wang and S. Tian, J. Am. Oil Chem. Soc., 2008, 263–270 CrossRef CAS
.
- Y. Chen, Y. Huang, R. Lin, N. Shang and C. Chang, J. Taiwan Inst. Chem. Eng., 2011, 42, 937–944 CrossRef CAS
.
- X. Zhang, Q. Ma, B. Cheng, J. Wang, J. Li and F. Nie, J. Nat. Gas Chem., 2012, 21, 774–779 CrossRef CAS
.
- E. S. Umdu, M. Tuncer and E. Seker, Bioresour. Technol., 2009, 100, 2828–2831 CrossRef CAS PubMed
.
- M. Zabeti, W. Mohd, A. Wan and M. K. Aroua, Fuel Process. Technol., 2010, 91, 243–248 CrossRef CAS
.
- C. Samart, C. Chaiya and P. Reubroycharoen, Energy Convers. Manage., 2010, 51, 1428–1431 CrossRef CAS
.
- T. Witoon, S. Bumrungsalee, P. Vathavanichkul and S. Palitsakun, Bioresour. Technol., 2014, 156, 329–334 CrossRef CAS PubMed
.
- H. Wu, J. Zhang, Y. Liu, J. Zheng and Q. Wei, Fuel Process. Technol., 2014, 119, 114–120 CrossRef CAS
.
- B. Narowska, M. Kułażyński, M. Łukaszewicz and E. Burchacka, Renewable Energy, 2019, 135, 176–185 CrossRef CAS
.
- A. Buasri, B. Ksapabutr, M. Panapoy and N. Chaiyut, Korean J. Chem. Eng., 2012, 29, 1708–1712 CrossRef CAS
.
- L. J. Konwar, J. Boro and D. Deka, Energy Sources, Part A, 2018, 40, 601–607 CrossRef CAS
.
- B. H. Hameed, C. S. Goh and L. H. Chin, Fuel Process. Technol., 2009, 90, 1532–1537 CrossRef CAS
.
- S. Baroutian, M. K. Aroua, A. Aziz, A. Raman, N. Meriam and N. Sulaiman, Fuel Process. Technol., 2010, 91, 1378–1385 CrossRef CAS
.
- X. Li, Y. Zuo, Y. Zhang, Y. Fu and Q. Guo, Fuel, 2013, 113, 435–442 CrossRef CAS
.
- A. Buasri, N. Chaiyut, V. Loryuenyong and C. Rodklum, ScienceAsia, 2012, 38, 283–288 CAS
.
- Z. Wan and B. H. Hameed, Bioresour. Technol., 2011, 102, 2659–2664 CrossRef CAS PubMed
.
- A. B. Fadhil, A. M. Aziz and M. H. Altamer, Fuel, 2016, 170, 130–140 CrossRef CAS
.
- H. Liu, L. Su, Y. Shao and L. Zou, Fuel, 2012, 97, 651–657 CrossRef CAS
.
- I. B. Laskar, L. Rokhum, R. Gupta and S. Chatterjee, Environ. Prog. Sustainable Energy, 2019, 39, 1–11 Search PubMed
.
- Taslim, O. Bani, Iriany, N. Aryani and G. S. Kaban, Key Eng. Mater., 2018, 777, 262–267 Search PubMed
.
- S. Abelló, F. Medina, D. Tichit, J. Pérez-Ramírez, J. C. Groen, J. E. Sueiras, P. Salagre and Y. Cesteros, Chem.–Eur. J., 2005, 11, 728–739 CrossRef PubMed
.
- D. P. Debecker, E. M. Gaigneaux and G. Busca, Chem.–Eur. J., 2009, 15, 3920–3935 CrossRef CAS PubMed
.
- A. Navajas, I. Campo, A. Moral, J. Echave, O. Sanz, M. Montes, J. A. Odriozola, G. Arzamendi and L. M. Gandía, Fuel, 2018, 211, 173–181 CrossRef CAS
.
- H.-y. Zeng, Z. Feng, X. Deng and Y.-q. Li, Fuel, 2008, 87, 3071–3076 CrossRef CAS
.
- Y. Ma, Q. Wang, L. Zheng, Z. Gao, Q. Wang and Y. Ma, Energy, 2016, 107, 523–531 CrossRef CAS
.
- H. Y. Zeng, S. Xu, M. C. Liao, Z. Q. Zhang and C. Zhao, Appl. Clay Sci., 2014, 91–92, 16–24 CrossRef CAS
.
- W. Trakarnpruk and S. Porntangjitlikit, Renewable Energy, 2008, 33, 1558–1563 CrossRef CAS
.
- Q. Liu, B. Wang, C. Wang, Z. Tian, W. Qu, H. Ma and R. Xu, Green Chem., 2014, 16, 2604–2613 RSC
.
- L. Gao, G. Teng, G. Xiao and R. Wei, Biomass Bioenergy, 2010, 34, 1283–1288 CrossRef CAS
.
- Y. Liu, E. Lotero, J. G. Goodwin and X. Mo, Appl. Catal., A, 2007, 331, 138–148 CrossRef CAS
.
- Z. Helwani, N. Aziz, M. Z. A. Bakar, H. Mukhtar, J. Kim and M. R. Othman, Energy Convers. Manage., 2013, 73, 128–134 CrossRef CAS
.
- C. S. Cordeiro, G. G. C. Arizaga, L. P. Ramos and F. Wypych, Catal. Commun., 2008, 9, 2140–2143 CrossRef CAS
.
- J. Tantirungrotechai, P. Chotmongkolsap and M. Pohmakotr, Microporous Mesoporous Mater., 2010, 128, 41–47 CrossRef CAS
.
- H. Hattori, Chem. Rev., 1995, 95, 537–558 CrossRef CAS
.
- A. Kawashima, K. Matsubara and K. Honda, Bioresour. Technol., 2008, 99, 3439–3443 CrossRef CAS PubMed
.
- H. Sun, Y. Ding, J. Duan, Q. Zhang, Z. Wang, H. Lou and X. Zheng, Bioresour. Technol., 2010, 101, 953–958 CrossRef CAS PubMed
.
- Z. Wen, X. Yu, S. T. Tu, J. Yan and E. Dahlquist, Bioresour. Technol., 2010, 101, 9570–9576 CrossRef CAS PubMed
.
- C. L. Chen, C. C. Huang, D. T. Tran and J. S. Chang, Bioresour. Technol., 2012, 113, 8–13 CrossRef CAS PubMed
.
- R. Madhuvilakku and S. Piraman, Bioresour. Technol., 2013, 150, 55–59 CrossRef CAS PubMed
.
- S. Yan, S. O. Salley and K. Y. Simon Ng, Appl. Catal., A, 2009, 353, 203–212 CrossRef CAS
.
- C. Ngamcharussrivichai, P. Totarat and K. Bunyakiat, Appl. Catal., A, 2008, 341, 77–85 CrossRef CAS
.
- J. Su, Y. Li, H. Wang, X. Yan and D. Pan, Chem. Phys. Lett., 2016, 663, 61–65 CrossRef CAS
.
- M. M. Ibrahim, H. R. Mahmoud and S. A. El-molla, Catal. Commun., 2019, 122, 10–15 CrossRef CAS
.
- E. A. Faria, I. M. Dias, P. A. Z. Suarez and A. G. S. Prado, J. Braz. Chem. Soc., 2009, 20, 1732–1737 CrossRef CAS
.
- M. C. G. Albuquerque, J. Santamaría-González, J. M. Mérida-Robles, R. Moreno-Tost, E. Rodríguez-Castellón, A. Jiménez-López, D. C. S. Azevedo, C. L. Cavalcante and P. Maireles-Torres, Appl. Catal., A, 2008, 347, 162–168 CrossRef CAS
.
- K. Rajkumari, D. Das, G. Pathak and L. Rokhum, New J. Chem., 2019, 43, 2134–2140 RSC
.
- E. Betiku, A. A. Okeleye, N. B. Ishola, A. S. Osunleke and T. V. Ojumu, Catal. Lett., 2019, 149, 1772–1787 CrossRef CAS
.
- R. Shan, L. Lu, Y. Shi, H. Yuan and J. Shi, Energy Convers. Manage., 2018, 178, 277–289 CrossRef CAS
.
- G. Pathak, K. Rajkumari and L. Rokhum, Nanoscale Adv., 2019, 1, 1013–1020 RSC
.
- B. Changmai, I. B. Laskar and L. Rokhum, J. Taiwan Inst. Chem. Eng., 2019, 102, 276–282 CrossRef CAS
.
- C. Xu, M. Nasrollahzadeh, M. Sajjadi, M. Maham, R. Luque and A. R. Puente-Santiago, Renewable Sustainable Energy Rev., 2019, 112, 195–252 CrossRef CAS
.
- Z. Wei, C. Xu and B. Li, Bioresour. Technol., 2009, 100, 2883–2885 CrossRef CAS PubMed
.
- J. Goli and O. Sahu, Renewable Energy, 2018, 128, 142–154 CrossRef CAS
.
- A. A. Ayodeji, M. E. Ojewumi, B. Rasheed and J. M. Ayodele, Data Brief, 2018, 19, 1466–1473 CrossRef PubMed
.
- G. Joshi, D. S. Rawat, B. Y. Lamba, K. K. Bisht, P. Kumar, N. Kumar and S. Kumar, Energy Convers. Manage., 2015, 96, 258–267 CrossRef CAS
.
- Y. C. Sharma, B. Singh and J. Korstad, Energy Fuels, 2010, 24, 3223–3231 CrossRef CAS
.
- N. Tshizanga, E. F. Aransiola and O. Oyekola, S. Afr. J. Chem. Eng., 2017, 23, 145–156 Search PubMed
.
- Y. H. Tan, M. O. Abdullah, C. Nolasco-Hipolito and N. S. Ahmad Zauzi, Renewable Energy, 2017, 114, 437–447 CrossRef CAS
.
- Y. C. Wong and R. X. Ang, Open Chem., 2018, 16, 1166–1175 CAS
.
- P. Suwannasom, R. Sriraksa, P. Tansupo and C. Ruangviriyachai, Energy Sources, Part A, 2016, 38, 3221–3228 CrossRef CAS
.
- G. Santya, T. Maheswaran and K. F. Yee, SN Appl. Sci., 2019, 1, 152–160 CrossRef CAS
.
- P. Parthasarathy and S. K. Narayanan, Environ. Prog. Sustainable Energy, 2014, 33, 676–680 CrossRef
.
- S. Niju, M. M. M. S. Begum and N. Anantharaman, J. Saudi Chem. Soc., 2014, 18, 702–706 CrossRef
.
- A. R. Gupta and V. K. Rathod, Waste Manage., 2018, 79, 169–178 CrossRef CAS PubMed
.
- N. S. El-Gendy, S. F. Deriase, A. Hamdy and R. I. Abdallah, Egypt. J. Pet., 2015, 24, 37–48 CrossRef
.
- Y. P. Peng, K. T. T. Amesho, C. E. Chen, S. R. Jhang, F. C. Chou and Y. C. Lin, Catalysts, 2018, 8, 81–91 CrossRef
.
- N. Viriya-Empikul, P. Krasae, W. Nualpaeng, B. Yoosuk and K. Faungnawakij, Fuel, 2012, 92, 239–244 CrossRef CAS
.
- P. Khemthong, C. Luadthong, W. Nualpaeng, P. Changsuwan, P. Tongprem, N. Viriya-Empikul and K. Faungnawakij, Catal. Today, 2012, 190, 112–116 CrossRef CAS
.
- N. Viriya-empikul, P. Krasae, B. Puttasawat, B. Yoosuk, N. Chollacoop and K. Faungnawakij, Bioresour. Technol., 2010, 101, 3765–3767 CrossRef CAS PubMed
.
- A. Annam Renita, P. P. Chowdhury, P. Sultana, P. Phukan and A. Hannan, Int. J. Pharm. Pharm. Sci., 2016, 8, 143–146 Search PubMed
.
- A. A. Jazie, H. Pramanik and A. S. K. Sinha, Spec. Issue Int. J. Sustain. Dev. Green Econ., 2013, 2, 2315–4721 Search PubMed
.
- F. Yasar, Fuel, 2019, 255, 115828 CrossRef CAS
.
- K. Kara, F. Ouanji, M. El Mahi, E. M. Lotfi, M. Kacimi and Z. Mahfoud, Biofuels, 2019, 24, 1–7 CrossRef
.
- E. Fayyazi, B. Ghobadian, H. H. Van De Bovenkamp, G. Najafi, B. Hosseinzadehsamani, H. J. Heeres and J. Yue, Ind. Eng. Chem. Res., 2018, 38, 12742–12755 CrossRef PubMed
.
- L. M. Correia, R. M. A. Saboya, N. de Sousa Campelo, J. A. Cecilia, E. Rodríguez-Castellón, C. L. Cavalcante and R. S. Vieira, Bioresour. Technol., 2014, 151, 207–213 CrossRef CAS PubMed
.
- I. Reyero, F. Bimbela, A. Navajas, G. Arzamendi and L. M. Gandía, Fuel, 2015, 158, 558–564 CrossRef CAS
.
- S. B. Chavan, R. R. Kumbhar, D. Madhu, B. Singh and Y. C. Sharma, RSC Adv., 2015, 5, 63596–63604 RSC
.
- P. R. Pandit and M. H. Fulekar, J. Environ. Manage., 2017, 198, 319–329 CrossRef CAS PubMed
.
- P. R. Pandit and M. H. Fulekar, Renewable Energy, 2019, 136, 837–845 CrossRef CAS
.
- P. R. Pandit and M. H. Fulekar, Mater. Today: Proc., 2019, 10, 75–86 CAS
.
- K. Kirubakaran and V. Arul Mozhi Selvan, J. Environ. Chem. Eng., 2018, 6, 4490–4503 CrossRef
.
- G. Santya, T. Maheswaran and K. F. Yee, SN Appl. Sci., 2019, 1, 152–160 CrossRef CAS
.
- M. L. Savaliya, M. S. Bhakhar and B. Z. Dholakiya, Catal. Lett., 2016, 146, 2313–2323 CrossRef CAS
.
- L. Da Silva Castro, A. G. Barañano, C. J. G. Pinheiro, L. Menini and P. F. Pinheiro, Green Process. Synth., 2019, 8, 235–244 Search PubMed
.
- Y. Hangun-Balkir, J. Chem., 2016, 1–10 Search PubMed
.
- A. Ansori, S. A. Wibowo, H. S. Kusuma, D. S. Bhuana and M. Mahfud, Open Chem., 2019, 17, 1185–1197 CAS
.
- N. Mansir, S. Hwa Teo, M. Lokman Ibrahim and T. Y. Yun Hin, Energy Convers. Manage., 2017, 151, 216–226 CrossRef CAS
.
- A. S. Yusuff, O. D. Adeniyi, M. A. Olutoye and U. G. Akpan, Int. J. Technol., 2018, 1, 1–11 Search PubMed
.
- M. J. Borah, A. Das, V. Das, N. Bhuyan and D. Deka, Fuel, 2019, 242, 345–354 CrossRef CAS
.
- A. S. Oladipo, O. A. Ajayi, A. A. Oladipo, S. L. Azarmi, Y. Nurudeen, A. Y. Atta and S. S. Ogunyemi, C. R. Chim., 2018, 21, 684–695 CrossRef CAS
.
- M. D. Putra, Y. Ristianingsih, R. Jelita, C. Irawan and I. F. Nata, RSC Adv., 2017, 7, 55547–55554 RSC
.
- N. Mansir, S. H. Teo, U. Rashid and Y. H. Taufiq-Yap, Fuel, 2018, 211, 67–75 CrossRef CAS
.
- S. H. Teo, A. Islam, H. R. F. Masoumi, Y. H. Taufiq-Yap, J. Janaun, E. S. Chan and M. A. khaleque, Renewable Energy, 2017, 111, 892–905 CrossRef CAS
.
- M. A. Olutoye, S. C. Lee and B. H. Hameed, Bioresour. Technol., 2011, 102, 10777–10783 CrossRef CAS PubMed
.
- G. Chen, R. Shan, S. Li and J. Shi, Fuel, 2016, 143, 110–117 CAS
.
- N. S. Lani, N. Ngadi, N. Y. Yahya and R. A. Rahman, J. Cleaner Prod., 2018, 164, 210–218 Search PubMed
.
- G. Y. Chen, R. Shan, J. F. Shi and B. B. Yan, Fuel Process. Technol., 2015, 133, 8–13 CrossRef CAS
.
- S. Sulaiman and N. I. F. Ruslan, Energy Sources, Part A, 2017, 39, 154–159 CrossRef CAS
.
- J. Boro, L. J. Konwar and D. Deka, Fuel Process. Technol., 2014, 122, 72–78 CrossRef CAS
.
- W. U. Rahman, A. Fatima, A. H. Anwer, M. Athar, M. Z. Khan, N. A. Khan and G. Halder, Process Saf. Environ. Prot., 2019, 122, 313–319 CrossRef CAS
.
- R. Chakraborty, S. Bepari and A. Banerjee, Chem. Eng. J., 2010, 165, 798–805 CrossRef CAS
.
- D. Zeng, Q. Zhang, S. Chen, S. Liu, Y. Chen, Y. Tian and G. Wang, J. Environ. Chem. Eng., 2015, 3, 560–564 CrossRef CAS
.
- S. Chowdhury, S. H. Dhawane, B. Jha, S. Pal, R. Sagar, A. Hossain and G. Halder, Biomass Convers. Biorefin., 2019, 1–11 Search PubMed
.
- G. Chen, R. Shan, J. Shi and B. Yan, Bioresour. Technol., 2014, 171, 428–432 CrossRef CAS PubMed
.
- Y. B. Cho and G. Seo, Bioresour. Technol., 2010, 22, 8515–8519 CrossRef PubMed
.
- A. Buasri and V. Loryuenyong, Mater. Today: Proc., 2017, 4, 6051–6059 Search PubMed
.
- J. Goli and O. Sahu, Renewable Energy, 2018, 128, 142–154 CrossRef CAS
.
- S. Niju, K. M. M. S. Begum and N. Anantharaman, Environ. Prog. Sustainable Energy, 2015, 34, 248–254 CrossRef CAS
.
- N. P. Asri, B. Podjojono, R. Fujiani and Nuraini, IOP Conf. Ser. Earth Environ. Sci., 2017, 67, 1–7 Search PubMed
.
- L. M. Correia, R. M. A. Saboya, N. de Sousa Campelo, J. A. Cecilia, E. Rodríguez-Castellón, C. L. Cavalcante and R. S. Vieira, Bioresour. Technol., 2014, 151, 207–213 CrossRef CAS PubMed
.
- S. Jairam, P. Kolar, R. Sharma-Shivappa Ratna, J. A. Osborne and J. P. Davis, Bioresour. Technol., 2012, 104, 329–335 CrossRef CAS PubMed
.
- N. Nakatani, H. Takamori, K. Takeda and H. Sakugawa, Bioresour. Technol., 2009, 100, 1510–1513 CrossRef CAS PubMed
.
- A. Buasri, T. Rattanapan, C. Boonrin, C. Wechayan and V. Loryuenyong, J. Chem., 2015, 1–7 Search PubMed
.
- S. Kaewdaeng, P. Sintuya and R. Nirunsin, Energy Procedia, 2017, 138, 937–942 CrossRef CAS
.
- W. Roschat, T. Siritanon, T. Kaewpuang, B. Yoosuk and V. Promarak, Bioresour. Technol., 2016, 209, 343–350 CrossRef CAS PubMed
.
- X. Liu, H. Bai, D. Zhu and G. Cao, Adv. Mater. Res., 2011, 148, 794–798 Search PubMed
.
- A. Birla, B. Singh, S. N. Upadhyay and Y. C. Sharma, Bioresour. Technol., 2012, 106, 95–100 CrossRef CAS PubMed
.
- H. Liu, H. s. Guo, X. j. Wang, J. z. Jiang, H. Lin, S. Han and S. p. Pei, Renewable Energy, 2016, 93, 648–657 CrossRef CAS
.
- I. B. Laskar, K. Rajkumari, R. Gupta, S. Chatterjee, B. Paul and L. Rokhum, RSC Adv., 2018, 8, 20131–20142 RSC
.
- N. S. El-Gendy, S. F. Deriase and A. Hamdy, Energy Sources, Part A, 2014, 36, 623–637 CrossRef CAS
.
- J. Sani, S. Samir, I. I. Rikoto, A. D. Tambuwal, A. Sanda, S. M. Maishanu and M. M. Laden, Innov. Energy Res., 2017, 6, 1–4 CAS
.
- V. A. Fabiani, R. O. Asriza, A. R. Fabian and M. Kafillah, IOP Conf. Ser. Earth Environ. Sci., 2019, 353, 12012 CrossRef
.
- K. N. Krishnamurthy, S. N. Sridhara and C. S. Ananda Kumar, Renewable Energy, 2020, 146, 280–296 CrossRef CAS
.
- A. A. Otori, A. Mann, M. A. T. Suleiman and E. C. Egwimvol, Niger. J. Chem. Res., 2011, 23, 837–846 Search PubMed
.
- A. Buasri, N. Chaiyut, V. Loryuenyong, P. Worawanitchaphong and S. Trongyong, Sci. World J., 2013, 1–7 Search PubMed
.
- H. Hadiyanto, A. H. Afianti, U. I. Navi'A, N. P. Adetya, W. Widayat and H. Sutanto, J. Environ. Chem. Eng., 2017, 5, 4559–4563 CrossRef CAS
.
- S. Nurdin, N. A. Rosnan, N. S. Ghazali, J. Gimbun, A. H. Nour and S. F. Haron, Energy Procedia, 2015, 79, 576–583 CrossRef CAS
.
- R. Rezaei, M. Mohadesi and G. R. Moradi, Fuel, 2013, 109, 534–541 CrossRef CAS
.
-
Y. Zhang, X. Shen, H. Bai and S. Liu, World Automation Congress Proceedings, 2012, pp. 1–4 Search PubMed
.
- S. Hu, Y. Wang and H. Han, Biomass Bioenergy, 2011, 35, 3627–3635 CrossRef CAS
.
- A. Perea, T. Kelly and Y. Hangun-Balkir, Green Chem. Lett. Rev., 2016, 9, 27–32 CrossRef CAS
.
- O. Nur Syazwani, U. Rashid and Y. H. Taufiq Yap, Energy Convers. Manage., 2015, 101, 749–756 CrossRef CAS
.
- O. N. Syazwani, U. Rashid, M. S. Mastuli and Y. H. Taufiq-Yap, Renewable Energy, 2019, 131, 187–196 CrossRef CAS
.
- N. Asikin-Mijan, H. V. Lee and Y. H. Taufiq-Yap, Chem. Eng. Res. Des., 2015, 102, 368–377 CrossRef CAS
.
- Y. Taufiq-Yap, H. Lee and P. Lau, Energy Explor. Exploit., 2012, 30, 853–866 CrossRef CAS
.
- P. Nair, B. Singh, S. N. Upadhyay and Y. C. Sharma, J. Cleaner Prod., 2012, 29, 82–90 CrossRef
.
- N. Girish, S. P. Niju, K. M. Meera Sheriffa Begum and N. Anantharaman, Fuel, 2013, 111, 653–658 CrossRef CAS
.
- O. N. Syazwani, S. H. Teo, A. Islam and Y. H. Taufiq-Yap, Process Saf. Environ. Prot., 2017, 105, 303–315 CrossRef CAS
.
- G. Y. Chen, R. Shan, B. B. Yan, J. F. Shi, S. Y. Li and C. Y. Liu, Fuel Process. Technol., 2016, 143, 110–117 CrossRef CAS
.
- S. Boonyuen, S. M. Smith, M. Malaithong, A. Prokaew, B. Cherdhirunkorn and A. Luengnaruemitchai, J. Cleaner Prod., 2018, 177, 925–929 CrossRef CAS
.
- W. Suryaputra, I. Winata, N. Indraswati and S. Ismadji, Renewable Energy, 2013, 50, 795–799 CrossRef CAS
.
- P. L. Boey, G. P. Maniam, S. A. Hamid and D. M. H. Ali, Fuel, 2011, 88, 283–288 CAS
.
- S. L. Lee, Y. C. Wong, Y. P. Tan and S. Y. Yew, Energy Convers. Manage., 2015, 93, 282–288 CrossRef CAS
.
- J. Xie, X. Zheng, A. Dong, Z. Xiao and J. Zhang, Green Chem., 2009, 11, 355–364 RSC
.
- J. Boro, A. J. Thakur and D. Deka, Fuel Process. Technol., 2011, 92, 2061–2067 CrossRef CAS
.
- J. Boro, L. J. Konwar, A. J. Thakur and D. Deka, Fuel, 2014, 129, 182–187 CrossRef CAS
.
- H. Mazaheri, H. C. Ong, H. H. Masjuki, Z. Amini, M. D. Harrison, C. T. Wang, F. Kusumo and A. Alwi, Energy, 2018, 144, 10–19 CrossRef CAS
.
- L. Yang, A. Zhang and X. Zheng, Energy Fuels, 2009, 23, 3859–3865 CrossRef CAS
.
- R. Anr, A. A. Saleh, M. S. Islam, S. Hamdan and M. A. Maleque, Energy Fuels, 2016, 30, 334–343 CrossRef
.
- P. Sivakumar, P. Sivakumar, K. Anbarasu, R. Mathiarasi and S. Renganathan, Int. J. Green Energy, 2014, 11, 886–897 CrossRef CAS
.
- V. Shankar and R. Jambulingam, Sustainable Environ. Res., 2017, 27, 273–278 CrossRef CAS
.
- P. L. Boey, G. P. Maniam and S. A. Hamid, Bioresour. Technol., 2011, 168, 15–22 CAS
.
- D. Madhu, S. B. Chavan, V. Singh, B. Singh and Y. C. Sharma, Bioresour. Technol., 2016, 214, 210–217 CrossRef CAS PubMed
.
- A. P. S. Chouhan and A. K. Sarma, Biomass Bioenergy, 2013, 55, 386–389 CrossRef CAS
.
- L. H. Chin, B. H. Hameed and A. L. Ahmad, Energy Fuels, 2009, 23, 1040–1044 CrossRef CAS
.
- P. L. Boey, S. Ganesan, S. X. Lim, S. L. Lim, G. P. Maniam and M. Khairuddean, Energy, 2011, 36, 5791–5796 CrossRef
.
- E. Betiku and S. O. Ajala, Ind. Crops Prod., 2014, 53, 314–322 CrossRef CAS
.
- A. O. Etim, E. Betiku, S. O. Ajala, P. J. Olaniyi and T. V. Ojumu, Sustain, 2018, 10, 707–715 CrossRef
.
- V. Vadery, B. N. Narayanan, R. M. Ramakrishnan, S. K. Cherikkallinmel, S. Sugunan, D. P. Narayanan and S. Sasidharan, Energy, 2014, 70, 588–594 CrossRef CAS
.
- C. Ofori-Boateng and K. T. Lee, Chem. Eng. J., 2013, 220, 395–401 CrossRef CAS
.
- D. C. Deka and S. Basumatary, Biomass Bioenergy, 2011, 35, 1797–1803 CrossRef CAS
.
- A. K. Sarma, P. Kumar, M. Aslam and A. P. S. Chouhan, Catal. Lett., 2014, 144, 1344–1353 CrossRef CAS
.
- E. Betiku, A. M. Akintunde and T. V. Ojumu, Energy, 2016, 103, 797–806 CrossRef CAS
.
- S. E. Onoji, S. E. Iyuke, A. I. Igbafe and M. O. Daramola, Energy Fuels, 2017, 31, 6109–6119 CrossRef CAS
.
- M. Gohain, A. Devi and D. Deka, Ind. Crops Prod., 2017, 109, 8–18 CrossRef CAS
.
- G. Pathak, D. Das, K. Rajkumari and L. Rokhum, Green Chem., 2018, 20, 2365–2373 RSC
.
- M. Sharma, A. A. Khan, S. K. Puri and D. K. Tuli, Biomass Bioenergy, 2012, 41, 94–106 CrossRef CAS
.
- B. K. Uprety, W. Chaiwong, C. Ewelike and S. K. Rakshit, Energy Convers. Manage., 2016, 115, 191–199 CrossRef CAS
.
- M. Balajii and S. Niju, Renewable Energy, 2020, 146, 2255–2269 CrossRef CAS
.
- M. Balajii and S. Niju, Energy Convers. Manage., 2019, 189, 118–131 CrossRef CAS
.
- I. M. Mendonça, F. L. Machado, C. C. Silva, S. Duvoisin Junior, M. L. Takeno, P. J. de Sousa Maia, L. Manzato and F. A. de Freitas, Energy Convers. Manage., 2019, 200, 112095 CrossRef
.
- B. Nath, B. Das, P. Kalita and S. Basumatary, J. Cleaner Prod., 2019, 239, 118112 CrossRef CAS
.
- B. Changmai, P. Sudarsanam and L. Rokhum, Ind. Crops Prod., 2019, 145, 111911–111919 CrossRef
.
- B. Nath, P. Kalita, B. Das and S. Basumatary, Renewable Energy, 2020, 151, 295–310 CrossRef CAS
.
- I. M. Mendonça, O. A. R. L. Paes, P. J. S. Maia, M. P. Souza, R. A. Almeida, C. C. Silva, S. Duvoisin and F. A. de Freitas, Renewable Energy, 2019, 130, 103–110 CrossRef
.
- M. Gohain, K. Laskar, H. Phukon, U. Bora, D. Kalita and D. Deka, Waste Manage., 2020, 102, 212–221 CrossRef CAS PubMed
.
- E. Betiku, A. O. Etim, O. Pereao and T. V. Ojumu, Energy Fuels, 2017, 31, 6182–6193 CrossRef CAS
.
- M. R. Miladinović, M. V. Zdujić, D. N. Veljović, J. B. Krstić, I. B. Banković-Ilić, V. B. Veljković and O. S. Stamenković, Renewable Energy, 2020, 147, 1033–1043 CrossRef
.
- H. H. Abdelhady, H. A. Elazab, E. M. Ewais, M. Saber and M. S. El-Deab, Fuel, 2020, 261, 116481 CrossRef CAS
.
- K. Rajkumari and L. Rokhum, Biomass Convers. Biorefin., 2020, 1–10 Search PubMed
.
- V. O. Odude, A. J. Adesina, O. O. Oyetunde, O. O. Adeyemi, N. B. Ishola, A. O. Etim and E. Betiku, Waste Biomass Valorization, 2019, 10, 877–888 CrossRef CAS
.
- M. Gohain, K. Laskar, A. K. Paul, N. Daimary, M. Maharana, I. K. Goswami, A. Hazarika, U. Bora and D. Deka, Renewable Energy, 2020, 147, 541–555 CrossRef CAS
.
- M. Aslam, P. Saxena and A. K. Sarma, Energy Environ. Res., 2014, 4, 1927-0569 Search PubMed
.
- M. Di Serio, R. Tesser, L. Pengmei and E. Santacesaria, Energy Fuels, 2008, 22, 207–217 CrossRef CAS
.
- N. S. Talha and S. Sulaiman, ARPN J. Eng. Appl. Sci., 2016, 11, 439–442 CAS
.
- F. Allioux, B. J. Holland, L. Kong and L. F. Dumée, Front. Mater., 2017, 4, 1–10 Search PubMed
.
- K. L. T. Rodrigues, V. M. D. Pasa and É. C. Cren, J. Environ. Chem. Eng., 2018, 6, 4531–4537 CrossRef CAS
.
- L. Ma, Y. Han, K. Sun, J. Lu and J. Ding, J. Energy Chem., 2015, 1–7 Search PubMed
.
- S. Xia, X. Guo, D. Mao, Z. Shi, G. Wu and G. Lu, RSC Adv., 2014, 4, 51688–51695 RSC
.
- N. Shibasaki-kitakawa, K. Hiromori, T. Ihara, K. Nakashima and T. Yonemoto, Fuel, 2015, 139, 11–17 CrossRef CAS
.
- M. Banchero and G. Gojjelino, Energies, 2018, 11, 1843–1851 CrossRef
.
-
D. R. Radu and G. A. Kraus, Heterogeneous Catalysis for Today's Challenges, 2015, pp. 117–130 Search PubMed
.
- N. Shibasaki-kitakawa, H. Honda, H. Kuribayashi and T. Toda, Bioresour. Technol., 2007, 98, 416–421 CrossRef CAS PubMed
.
- Y. Ren, B. He, F. Yan, H. Wang, Y. Cheng, L. Lin, Y. Feng and J. Li, Bioresour. Technol., 2012, 113, 19–22 CrossRef CAS PubMed
.
- T. M. Deboni, G. A. M. Hirata, G. G. Shimamoto, M. Tubino and A. J. A. Meirelles, Chem. Eng. J., 2018, 333, 686–696 CrossRef CAS
.
- J. Kansedo, Y. X. Sim and K. T. Lee, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 495, 012050–012060 CAS
.
- N. Jaya, B. K. Selvan and S. J. Vennison, Ecotoxicol. Environ. Saf., 2015, 121, 3–9 CrossRef CAS PubMed
.
- A. Umar, A. Uba, M. L. Mohammed, M. N. Almustapha, C. Muhammad and J. Sani, Nig. J. Basic Appl. Sci., 2019, 26, 88 CrossRef
.
- J. Kansedo and K. T. Lee, Energy Sci. Eng., 2014, 2, 31–38 CrossRef
.
- O. Ilgen, A. N. Akin and N. Boz, Turk. J. Chem., 2009, 33, 289–294 CAS
.
- B. Vafakish and M. Barari, Kem. Ind., 2017, 66, 47–52 CrossRef CAS
.
- R. Hartono, B. Mulia, M. Sahlan, T. S. Utami, A. Wijanarko and H. Hermansyah, AIP Conf. Proc., 2017, 1826, 020020 CrossRef
.
- N. Shibasaki-Kitakawa, T. Tsuji, M. Kubo and T. Yonemoto, BioEnergy Res., 2011, 4, 287–293 CrossRef
.
- N. Shibasaki-Kitakawa, T. Tsuji, K. Chida, M. Kubo and T. Yonemoto, Energy Fuels, 2010, 24, 3634–3638 CrossRef CAS
.
- Y. Feng, B. He, Y. Cao, J. Li, M. Liu, F. Yan and X. Liang, Bioresour. Technol., 2010, 101, 1518–1521 CrossRef CAS PubMed
.
- N. Jalilnejad Falizi, T. Güngören Madenoğlu, M. Yüksel and N. Kabay, Int. J. Energy Res., 2019, 43, 2188–2199 CrossRef CAS
.
- P. A. Alaba, Y. M. Sani, W. Mohd and A. Wan, RSC Adv., 2016, 6, 78351–78368 RSC
.
- Q. H. Xia, K. Hidajat and S. Kawi, Chem. Commun., 2000, 22, 2229–2230 RSC
.
- A. V. Ivanov, S. V. Lysenko, S. V. Baranova, A. V. Sungurov, T. N. Zangelov and E. A. Karakhanov, Microporous Mesoporous Mater., 2006, 91, 254–260 CrossRef CAS
.
- S. H. I. Guo-liang, Y. U. Feng, Y. A. N. Xiao-liang and L. I. Rui-feng, J. Fuel Chem. Technol., 2017, 45, 311–316 CrossRef
.
- Q. H. Xia, K. Hidajat and S. Kawi, J. Catal., 2002, 205, 318–331 CrossRef CAS
.
- H. Muthu, V. S. Selvabala, T. K. Varathachary, D. K. Selvaraj, J. Nandagopal and S. Subramanian, Braz. J. Chem. Eng., 2010, 27, 601–608 CrossRef CAS
.
- M. K. Lam, K. T. Lee and A. R. Mohamed, Appl. Catal., B, 2009, 93, 134–139 CrossRef CAS
.
- C. O. Pereira, M. F. Portilho, C. A. Henriques and F. M. Z. Zotin, J. Braz. Chem. Soc., 2014, 25, 2409–2416 CAS
.
- G. Kafuku, K. T. Lee and M. Mbarawa, Chem. Pap., 2010, 64, 734–740 CAS
.
- X. Li and W. Huang, Energy Sources, Part A, 2009, 31, 1666–1672 CrossRef CAS
.
- M. L. Testa, V. La Parola, L. F. Liotta and A. M. Venezia, J. Mol. Catal. A: Chem., 2013, 367, 69–76 CrossRef CAS
.
- M. L. Testa, V. La Parola and A. M. Venezia, Catal. Today, 2010, 158, 109–113 CrossRef CAS
.
- J. Gardy, A. Hassanpour, X. Lai and M. H. Ahmed, Appl. Catal., A, 2016, 527, 81–95 CrossRef CAS
.
- J. Gardy, A. Hassanpour, X. Lai, M. H. Ahmed and M. Rehan, Appl. Catal., B, 2017, 207, 297–310 CrossRef CAS
.
- T. Suzuta, M. Toba, Y. Abe and Y. Yoshimura, J. Am. Oil Chem. Soc., 2012, 89, 1981–1989 CrossRef CAS
.
- K. Thirunavukkarasu, T. M. Sankaranarayanan, A. Pandurangan, R. Vijaya Shanthi and S. Sivasanker, Catal. Sci. Technol., 2014, 4, 851–860 RSC
.
- W. Xie, H. Wang and H. Li, Ind. Eng. Chem. Res., 2012, 51, 225–231 CrossRef CAS
.
- F. H. Alhassan, U. Rashid and Y. H. Taufiq-Yap, J. Oleo Sci., 2015, 64, 505–514 CrossRef CAS PubMed
.
- W. Xie and T. Wang, Fuel Process. Technol., 2013, 109, 150–155 CrossRef CAS
.
- W. Xie and D. Yang, Bioresour. Technol., 2012, 119, 60–65 CrossRef CAS PubMed
.
- H. Amani, Z. Ahmad, M. Asif and B. H. Hameed, J. Ind. Eng. Chem., 2014, 20, 4437–4442 CrossRef CAS
.
- Q. Zhang, H. Li, X. Liu, W. Qin, Y. Zhang, W. Xue and S. Yang, Energy Technol., 2013, 1, 735–742 CrossRef CAS
.
- F. H. Alhassan, U. Rashid and Y. H. Taufq-Yap, J. Oleo Sci., 2015, 64, 91–99 CrossRef CAS PubMed
.
- A. Mahajan and P. Gupta, Environ. Chem. Lett., 2020, 18, 299–314 CrossRef CAS
.
- M. Hara, T. Yoshida, A. Takagaki, T. Takata, J. N. Kondo, S. Hayashi and K. Domen, Angew. Chem., Int. Ed., 2004, 43, 2955–2958 CrossRef CAS PubMed
.
- S. P. Adhikari, Z. D. Hood, S. Borchers and M. Wright, ChemistrySelect, 2020, 5, 1534–1538 CrossRef CAS
.
- R. A. Arancon, H. R. Barros, A. M. Balu, C. Vargas and R. Luque, Green Chem., 2011, 13, 3162–3167 RSC
.
- A. Sandouqa, Z. Al-Hamamre and J. Asfar, Renewable Energy, 2019, 132, 667–682 CrossRef CAS
.
- K. Malins, J. Brinks, V. Kampars and I. Malina, Appl. Catal., A, 2016, 519, 99–106 CrossRef CAS
.
- M. Kacem, G. Plantard, N. Wery and V. Goetz, Chin. J. Catal., 2014, 35, 1571–1577 CrossRef CAS
.
- Q. Shu, J. Gao, Z. Nawaz, Y. Liao, D. Wang and J. Wang, Appl. Energy, 2010, 87, 2589–2596 CrossRef CAS
.
- M. Goncìalves, V. C. Souza, T. S. Galhardo, M. Mantovani, F. C. A. Figueiredo, D. Mandelli and W. A. Carvalho, Ind. Eng. Chem. Res., 2013, 52, 2832–2839 CrossRef
.
- Y. Zhong, Q. Deng, P. Zhang, J. Wang, R. Wang, Z. Zeng and S. Deng, Fuel, 2019, 240, 270–277 CrossRef CAS
.
- V. Trombettoni, D. Lanari, P. Prinsen, R. Luque, A. Marrocchi and L. Vaccaro, Prog. Energy Combust. Sci., 2018, 65, 136–162 CrossRef
.
- M. Otadi, A. Shahraki, M. Goharrokhi and F. Bandarchian, Procedia Eng., 2011, 18, 168–174 CrossRef CAS
.
- K. Rajkumari, I. B. Laskar, A. Kumari, B. Kalita and L. Rokhum, React. Funct. Polym., 2020, 149, 104519 CrossRef CAS
.
- P. P. Upare, J. M. Lee, D. W. Hwang, S. B. Halligudi, Y. K. Hwang and J. S. Chang, J. Ind. Eng. Chem., 2011, 17, 287–292 CrossRef CAS
.
- K. Fukuhara, K. Nakajima, M. Kitano, S. Hayashic and M. Hara, Phys. Chem. Chem. Phys., 2013, 15, 9343 RSC
.
- T. S. Galhardo, N. Simone, M. Gonçalves, F. C. A. Figueiredo, D. Mandelli and W. A. Carvalho, ACS Sustainable Chem. Eng., 2013, 1, 1381–1389 CrossRef CAS
.
- P. D. Rocha, L. S. Oliveira and A. S. Franca, Renewable Energy, 2019, 143, 1710–1716 CrossRef CAS
.
- M. Hara, Energy Environ. Sci., 2010, 3, 601–607 RSC
.
- L. J. Konwar, P. Mäki-Arvela and J. P. Mikkola, Chem. Rev., 2019, 119, 11576–11630 CrossRef CAS PubMed
.
- I. B. Laskar, K. Rajkumari, R. Gupta and L. Rokhum, Energy Fuels, 2018, 32, 12567–12576 CrossRef CAS
.
- K. Rajkumari, I. B. Laskar, A. Kumari, B. Kalita and L. Rokhum, React. Funct. Polym., 2020, 149, 104519 CrossRef CAS
.
- M. M. Alam, M. A. Hossain, M. D. Hossain, M. A. H. Johir, J. Hossen, M. S. Rahman, J. L. Zhou, A. T. M. K. Hasan, A. K. Karmakar and M. B. Ahmed, Processes, 2020, 8, 203 CrossRef CAS
.
- X. J. Zhang, Y. Y. Wang, Z. C. Jiang, P. T. Wu, Y. M. Jin and Y. Q. Hu, New Carbon Mater., 2013, 28, 484–488 CAS
.
-
Q. Zhang, Y. Zhang, T. Deng, F. Wei, J. Jin and P. Ma, Sustainable production of biodiesel over heterogeneous acid catalysts, Elsevier B.V., 2020, pp. 407–432 Search PubMed
.
- I. K. Mbaraka, D. R. Radu, V. S. Y. Lin and B. H. Shanks, J. Catal., 2003, 219, 329–336 CrossRef CAS
.
- M. Toda, A. Takagaki, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Nature, 2005, 438, 178 CrossRef CAS PubMed
.
- K. Nakajima, M. Hara, B. Hu, Q. Lu, Y.-t. Wu, Z.-x. Zhang, M.-s. Cui, D.-j. Liu, C.-q. Dong, Y.-p. Yang, V. Aniya, A. Kumari, D. De, D. Vidya, V. Swapna, P. K. Thella, B. Satyavathi, H. Zhang, X. Meng, C. Liu, Y. Wang and R. Xiao, J. Anal. Appl. Pyrolysis, 2018, 2, 1296–1304 Search PubMed
.
- K. Malins, V. Kampars, J. Brinks, I. Neibolte and R. Murnieks, New Carbon Mater., 2015, 176, 553–558 Search PubMed
.
-
S. Pandian, A. Sakthi Saravanan, P. Sivanandi, M. Santra and V. K. Booramurthy, Refining Biomass Residues for Sustainable Energy and Bioproducts. Academic Press, 2020. pp. 87–109 Search PubMed
.
- M. Kitano, D. Yamaguchi, S. Suganuma, K. Nakajima, H. Kato, S. Hayashi and M. Hara, Langmuir, 2009, 25, 5068–5075 CrossRef CAS PubMed
.
- H. Yuan, B. L. Yang and G. L. Zhu, Energy Fuels, 2009, 23, 548–552 CrossRef CAS
.
- J. A. Melero, L. F. Bautista, G. Morales, J. Iglesias and D. Briones, Energy Fuels, 2009, 23, 539–547 CrossRef CAS
.
- D. Zuo, J. Lane, D. Culy, M. Schultz, A. Pullar and M. Waxman, Appl. Catal., B, 2013, 129, 342–350 CrossRef CAS
.
- K. A. Shah, J. K. Parikh and K. C. Maheria, Res. Chem. Intermed., 2015, 41, 1035–1051 CrossRef CAS
.
- A. Varyambath, M. R. Kim and I. Kim, New J. Chem., 2018, 42, 12745–12753 RSC
.
- Shagufta, I. Ahmad and R. Dhar, Catal. Surv. Asia, 2017, 21, 53–69 CrossRef CAS
.
- H. Yu, S. Niu, C. Lu, J. Li and Y. Yang, Fuel, 2017, 208, 101–110 CrossRef CAS
.
- X. Tang and S. Niu, J. Ind. Eng. Chem., 2019, 69, 187–195 CrossRef CAS
.
- S. Niu, Y. Ning, C. Lu, K. Han, H. Yu and Y. Zhou, Energy Convers. Manage., 2018, 163, 59–65 CrossRef CAS
.
- I. F. Nata, M. D. Putra, C. Irawan and C. K. Lee, J. Environ. Chem. Eng., 2017, 5, 2171–2175 CrossRef CAS
.
- Q. Guan, Y. Li, Y. Chen, Y. Shi, J. Gu, B. Li and R. Miao, RSC Adv., 2017, 7, 7250–7258 RSC
.
- I. M. Lokman, Arabian J. Chem., 2016, 9, 179–189 CrossRef CAS
.
- I. Thushari and S. Babel, Bioresour. Technol., 2018, 248, 199–203 CrossRef CAS PubMed
.
- Y. Wang, D. Wang, M. Tan, B. Jiang, J. Zheng, N. Tsubaki and M. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 26767–26775 CrossRef CAS PubMed
.
- R. Liu, X. Wang, X. Zhao and P. Feng, Carbon, 2008, 46, 1664–1669 CrossRef CAS
.
- S. Dechakhumwat, P. Hongmanorom, C. Thunyaratchatanon, S. M. Smith, S. Boonyuen and A. Luengnaruemitchai, Renewable Energy, 2020, 148, 897–906 CrossRef CAS
.
- M. Mahdavi and A.H. Darab, Preprints, 2019, 2019090110, DOI:10.20944/preprints201909.0110.v1
.
- M. Hara, Top. Catal., 2010, 53, 805–810 CrossRef CAS
.
- T. T. V. Tran, S. Kaiprommarat, S. Kongparakul, P. Reubroycharoen, G. Guan, M. H. Nguyen and C. Samart, Waste Manage., 2016, 52, 367–374 CrossRef CAS PubMed
.
- S. Hosseini, J. Janaun and T. S. Y. Choong, Process Saf. Environ. Prot., 2015, 98, 285–295 CrossRef CAS
.
- L. J. Konwar, J. Wärnå, P. Mäki-Arvela, N. Kumar and J. P. Mikkola, Fuel, 2016, 166, 1–11 CrossRef CAS
.
- A. Endut, S. Hanis, Y. Sayid, N. Hanis, M. Hanapi, S. Hajar, A. Hamid, F. Lananan, M. Khairul, A. Kamarudin, R. Umar and H. Khatoon, Int. Biodeterior. Biodegrad., 2017, 124, 250–257 CrossRef CAS
.
- F. Ezebor, M. Khairuddean, A. Z. Abdullah and P. L. Boey, Energy Convers. Manage., 2014, 88, 1143–1150 CrossRef CAS
.
- T. Liu, Z. Li, W. Li, C. Shi and Y. Wang, Bioresour. Technol., 2013, 133, 618–621 CrossRef CAS PubMed
.
- Y. Zhou, S. Niu and J. Li, Energy Convers. Manage., 2016, 114, 188–196 CrossRef CAS
.
- H. H. Mardhiah, H. C. Ong, H. H. Masjuki, S. Lim and Y. L. Pang, Energy Convers. Manage., 2017, 144, 10–17 CrossRef CAS
.
- B. L. A. Prabhavathi Devi, T. Vijai Kumar Reddy, K. Vijaya Lakshmi and R. B. N. Prasad, Bioresour. Technol., 2014, 153, 370–373 CrossRef CAS PubMed
.
- B. L. A. P. Devi, K. N. Gangadhar, P. S. S. Prasad, B. Jagannadh and R. B. N. Prasad, ChemSusChem, 2009, 2, 617–620 CrossRef PubMed
.
- X. Fu, D. Li, J. Chen, Y. Zhang, W. Huang, Y. Zhu, J. Yang and C. Zhang, Bioresour. Technol., 2013, 146, 767–770 CrossRef CAS PubMed
.
- L. J. Konwar, R. Das, A. J. Thakur, E. Salminen, P. Mäki-Arvela, N. Kumar, J. P. Mikkola and D. Deka, J. Mol. Catal. A: Chem., 2014, 388–389, 167–176 CrossRef CAS
.
- E. M. Santos, A. P. D. C. Teixeira, F. G. Da Silva, T. E. Cibaka, M. H. Araújo, W. X. C. Oliveira, F. Medeiros, A. N. Brasil, L. S. De Oliveira and R. M. Lago, Fuel, 2015, 150, 408–414 CrossRef CAS
.
- L. J. Konwar, P. Mäki-Arvela, E. Salminen, N. Kumar, A. J. Thakur, J. P. Mikkola and D. Deka, Appl. Catal., B, 2015, 176–177, 20–35 CrossRef CAS
.
- B. V. S. K. Rao, K. Chandra Mouli, N. Rambabu, A. K. Dalai and R. B. N. Prasad, Catal. Commun., 2011, 14, 20–26 CrossRef CAS
.
- J. R. Kastner, J. Miller, D. P. Geller, J. Locklin, L. H. Keith and T. Johnson, Catal. Today, 2012, 190, 122–132 CrossRef CAS
.
- A. M. Dehkhoda, A. H. West and N. Ellis, Appl. Catal., A, 2010, 382, 197–204 CrossRef CAS
.
- A. M. Dehkhoda and N. Ellis, Catal. Today, 2013, 207, 86–92 CrossRef CAS
.
- L. Fjerbaek, K. V. Christensen and B. Norddahl, Biotechnol. Bioeng., 2009, 102, 1298–1315 CrossRef CAS PubMed
.
- F. Moazeni, Y. C. Chen and G. Zhang, J. Cleaner Prod., 2019, 216, 117–128 CrossRef CAS
.
- D. Kumar, T. Das, B. S. Giri, E. R. Rene and B. Verma, Fuel, 2019, 255, 115801 CrossRef CAS
.
- D. Kumar, T. Das, B. S. Giri and B. Verma, New J. Chem., 2018, 42, 15593–15602 RSC
.
- K. C. Badgujar, K. P. Dhake and B. M. Bhanage, Process Biochem., 2013, 48, 1335–1347 CrossRef CAS
.
- N. R. Mohamad, N. H. C. Marzuki, N. A. Buang, F. Huyop and R. A. Wahab, Biotechnol. Biotechnol. Equip., 2015, 29, 205–220 CrossRef CAS PubMed
.
- Z. Amini, Z. Ilham, H. C. Ong, H. Mazaheri and W. H. Chen, Energy Convers. Manage., 2017, 141, 339–353 CrossRef CAS
.
- R. W. M. Mounguengui, C. Brunschwig, B. Baréa, P. Villeneuve and J. Blin, Prog. Energy Combust. Sci., 2013, 39, 441–456 CrossRef
.
- S. V. Ranganathan, S. L. Narasimhan and K. Muthukumar, Bioresour. Technol., 2008, 99, 3975–3981 CrossRef CAS PubMed
.
- A. Gusniah, H. Veny and F. Hamzah, Ind. Eng. Chem. Res., 2019, 58, 581–589 CrossRef CAS
.
- K. H. Kim, O. K. Lee and E. Y. Lee, Catalysts, 2018, 8, 68 CrossRef
.
- J. Sebastian, C. Muraleedharan and A. Santhiagu, Int. J. Green Energy, 2017, 14, 687–693 CrossRef CAS
.
- J. H. C. Wancura, D. V. Rosset, M. V. Tres, J. V. Oliveira, M. A. Mazutti and S. L. Jahn, Can. J. Chem. Eng., 2018, 96, 2361–2368 CrossRef CAS
.
- A. Arumugam and V. Ponnusami, Heliyon, 2017, 3, 486 CrossRef PubMed
.
- R. Jambulingam, M. Shalma and V. Shankar, J. Cleaner Prod., 2019, 215, 245–258 CrossRef CAS
.
-
S. Rafiei, S. Tangestaninejad, P. Horcajada, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork, R. Kardanpour and F. Zadehahmadi, Efficient biodiesel production using a lipase@ZIF-67 nanobioreactor, 2018, vol. 334 Search PubMed
.
- H. Taher, E. Nashef, N. Anvar and S. Al-Zuhair, Biofuels, 2019, 10, 463–472 CrossRef CAS
.
- R. S. Malani, S. B. Umriwad, K. Kumar, A. Goyal and V. S. Moholkar, Energy Convers. Manage., 2019, 188, 142–150 CrossRef CAS
.
- J. Jayaraman, K. Alagu, P. Appavu, N. Joy, P. Jayaram and A. Mariadoss, Renewable Energy, 2020, 145, 399–407 CrossRef CAS
.
- M. Marín-Suárez, D. Méndez-Mateos, A. Guadix and E. M. Guadix, Renewable Energy, 2019, 140, 1–8 CrossRef
.
- H. C. Nguyen, S. H. Liang, S. S. Chen, C. H. Su, J. H. Lin and C. C. Chien, Energy Convers. Manage., 2018, 158, 168–175 CrossRef CAS
.
- C. G. Lopresto, S. Naccarato, L. Albo, M. G. De Paola, S. Chakraborty, S. Curcio and V. Calabrò, Ecotoxicol. Environ. Saf., 2015, 121, 229–235 CrossRef CAS PubMed
.
- N. A. Kabbashi, N. I. Mohammed, M. Z. Alam and M. E. S. Mirghani, J. Mol. Catal. B: Enzym., 2015, 116, 95–100 CrossRef CAS
.
- N. Choi, Y. Kim, J. S. Lee, J. Kwak, J. Lee and I. H. Kim, J. Am. Oil Chem. Soc., 2016, 93, 311–318 CrossRef CAS
.
- P. Muanruksa and P. Kaewkannetra, Renewable Energy, 2020, 146, 901–906 CrossRef CAS
.
- N. Choi, D. S. No, H. Kim, B. H. Kim, J. Kwak, J. S. Lee and I. H. Kim, Ind. Crops Prod., 2018, 120, 140–146 CrossRef CAS
.
- N. F. Sulaiman, W. Azelee, W. Abu, S. Toemen, N. M. Kamal and R. Nadarajan, Renewable Energy, 2019, 135, 408–416 CrossRef CAS
.
- M. Pirouzmand, M. Mahdavi and Z. Ghasemi, Fuel, 2018, 216, 296–300 CrossRef CAS
.
- A. Ramli and M. Farooq, Malaysian J. Anal. Sci., 2015, 19, 8–19 Search PubMed
.
- M. E. Borges and A. Brito, Int. J. Chem. React. Eng., 2011, 9, 1–20 Search PubMed
.
- W. Nor, N. Wan, N. Aishah and S. Amin, Fuel Process. Technol., 2011, 92, 2397–2405 CrossRef
.
- M. F. R. Nizah, Y. H. Taufiq-yap, U. Rashid, S. Hwa and Z. A. S. Nur, Energy Convers. Manage., 2014, 88, 3–8 Search PubMed
.
- H. V. Lee, J. C. Juan and Y. H. Tau, Renewable Energy, 2015, 74, 124–132 CrossRef CAS
.
- F. Jamil, A. H. Al-muhatseb, M. Tay, Z. Myint, M. Al-hinai, L. Al-haj, M. Baawain, M. Al-abri, G. Kumar and A. E. Atabani, Energy Convers. Manage., 2018, 155, 128–137 CrossRef CAS
.
- Y. Jeon, W. S. Chi, J. Hwang, D. H. Kim, J. H. Kim and Y. Shul, Appl. Catal., B, 2019, 242, 51–59 CrossRef CAS
.
- W. Liu, P. Yin, X. Liu and R. Qu, Bioresour. Technol., 2014, 173, 266–271 CrossRef CAS PubMed
.
- O. Nur, U. Rashid and M. Sufri, Renewable Energy, 2019, 131, 187–196 CrossRef
.
- F. Farzaneh and F. Moghzi, React. Kinet., Mech. Catal., 2016, 118, 509–521 CrossRef CAS
.
- D. Salinas, S. Guerrero and P. Araya, Catal. Commun., 2010, 11, 773–777 CrossRef CAS
.
- K. Srilatha, N. Lingaiah, B. L. A. P. Devi, R. B. N. Prasad, S. Venkateswar and P. S. S. Prasad, Appl. Catal., A, 2009, 365, 28–33 CrossRef CAS
.
- C. M. R. Prado and N. R. Antoniosi Filho, J. Anal. Appl. Pyrolysis, 2009, 86, 338–347 CrossRef CAS
.
- A. Wisniewski, V. R. Wiggers, E. L. Simionatto, H. F. Meier, A. A. C. Barros and L. A. S. Madureira, Fuel, 2010, 89, 563–568 CrossRef CAS
.
- J. Van Gerpen, Fuel Process. Technol., 2005, 86, 1097–1107 CrossRef CAS
.
- S. H. Y. S. Abdullah, N. H. M. Hanapi, A. Azid, R. Umar, H. Juahir, H. Khatoon and A. Endut, Renewable Sustainable Energy Rev., 2017, 70, 1040–1051 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2020 |