Open Access Article
Open Access ArticlePreparation and modification of cellulose sponge and application of oil/water separation†
Xu Mengabc,
Yanyan Donga,
Yajun Zhaoa and
Liping Liang *a
*a
aCollege of Textile and Garment, College of Life Science, Shaoxing University, Shaoxing, China. E-mail: liangliping0702@163.com; Fax: +86-575-88341506; Tel: +86-575-88341506
bKey Laboratory of Clean Dyeing and Finishing Technology of Zhejiang Province, Shaoxing University, Shaoxing, 312000, China
cZhejiang Sub-center of National Carbon Fiber Engineering Technology Research Center, Shaoxing, 312000, China
First published on 16th November 2020
Abstract
This work presents a facile preparation and modification of cellulose sponge with hydrophobic/oleophilic surface wetting properties. The modification method included several steps: sodium hydroxide (NaOH) and urea were introduced to dissolve cellulose, after which calcium carbonate (CaCO3) and epichlorohydrin were added into the solution for formation of a hydrogel. Finally, the cellulose sponge was obtained through hydrochloric acid (HCl) etching of CaCO3, and octadecyl trichlorosilane (OTS) self-assembly modification. The prepared cellulose sponge exhibited hydrophobicity with a water contact angle of 153.5°, and oleophilicity with an oil contact angle of 0°. The prepared cellulose sponge demonstrated a separation efficiency as high as 92% for various types of oil/water mixtures. The prepared cellulose sponge could achieve continuous separation with the assistance of a peristaltic pump. The material will be a promising candidate to be used in oil/water separation.
Introduction
Increasing industrial oily wastewater and frequent oil leakage accidents have caused serious harm to the ecological environment.1–4 Therefore, the research and development of effective, convenient, and low-cost oil/water separation materials has attracted extensive attention worldwide.So far, there have been various kinds of methods for oil/water separation, such as gravity separation, degradation separation, absorption methods, etc.5–7 Adsorption is one of the simple and convenient methods for oil/water separation. However, most of the traditional oil/water separation materials have weak absorption capacity, poor selectivity, low separation efficiency, and the disadvantage of not being easy to recycle which is not in line with the theme of green development. Therefore, it is extremely urgent to develop an environmentally friendly material with good performances. Sponges which can be manufactured with the materials such as melamine, polyurethane, carbon, polyvinyl alcohol, and polydimethylsiloxane have attracted attention due to their high absorbency.8–12 Among them, cellulose sponge is considered as a green and environmentally friendly material due to its abundant resources, renewability, high porosity, biodegradability and low cost. In addition, it is characterized by low density, which reduces the barrier layer formed by the direct contact of water (greater density than oil) and the surface of the high-density material to promote oil penetration.13–15 In order to make the materials have unique wetting properties, various substances with different properties have been introduced on the sponge matrix to change their surface properties and surface structures.16–19
In recent years, it has become a hot spot to prepare oil/water separation materials with environmental-friendly substances as the matrix.20,21 Thus, to date, a lot of researches on super hydrophobic and super lipophilic cellulose sponges have been conducted. In this respect, Halim et al.22 prepared a super lipophilic and underwater super oleophobic cellulose sponge by modifying with bamboo-based counter collision cellulose nanofiber and wood-free fiber-based 2,2,6,6-tetramethylpiperidine-1-oxyl oxidized cellulose nanofiber, respectively, whose separation efficiency could both come up to 99% with gravity alone. Chen et al.23 fabricated a porous cellulose sponge with steady super hydrophilicity and oleophobicity even under harsh environment by grafting amphiphilic molecular brushes of polyethylene glycol with short perfluorinated end caps on the surface of isocyanate-functionalized cellulose sponge, which had excellent separation efficiency and antifouling property. However, the complex preparation processes and high cost limited their applications in practice. Hence, it is significant to search after a convenient and low-cost method for preparing oil/water separation cellulose sponge.
In this work, the cellulose sponge was fabricated with a cost-efficient and simple operation and ended with vacuum freeze-drying technology. And then, the obtained sponge was grafted with OTS, which endowed the sponge with a certain hydrophobic/oleophilic property. Fig. 1 showed the design scheme of the modification of the cellulose sponge and the oil/water separation process of the as-prepared sponge. The modified cellulose sponge with the above properties could be an environmentally friendly and efficient oil/water separation material in practical application.
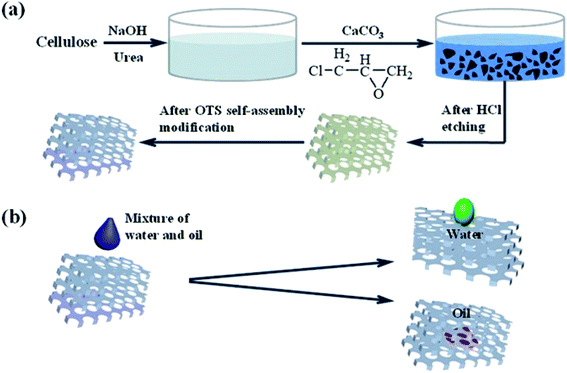 | ||
| Fig. 1 (a) Schematic diagram of the modification of cellulose sponge, (b) the oil/water separation process of prepared cellulose sponge. | ||
Experimental
Materials
Sodium hydroxide (NaOH), carbamide (H2NCONH2), cellulose (C6H10O5), epichlorohydrin (ECH), calcium carbonate (CaCO3), hydrochloric acid (HCl), and octadecyl trichlorosilane (OTS) were supplied by Aladdin Industrial Inc., Shanghai, China. The reagents used for oil/water separation, such as toluene, chloroform, hexane, and cyclohexane were also obtained from Aladdin Industrial Inc., Shanghai, China. Vegetable oil was purchased from Kerry Oils & Grains (China) Co., Ltd, China.Preparation of cellulose sponge
Firstly, 7 g of NaOH and 12 g of carbamide were mixed and dissolved in a beaker which contained 60 mL deionized (DI) water. Secondly, 3.5 g of cellulose was dissolved in 17.5 g of deionized water under intense stirring until spreading evenly before adding into the NaOH/carbamide solution. After that, the mixture was stirred at 0 °C until completely dissolved. Thirdly, the different weights (1 g, 1.5 g, 3 g, 5 g, 8 g and 10 g) of CaCO3 and 3 mL ECH were added into the above transparent solution (30 g) under stirring until there was no bubbles. Then, placed it in a 60 °C water bath and kept stirring until it became slightly pasty before pouring into a Petri dish where the thickness of the mixture was 0.5 cm. After all that, sealed the Petri dish with plastic wrap and placed it back in the 60 °C water bath for two hours. The solid product obtained by the reaction was washed with DI water, and then immersed in hydrochloric acid (1%) until the product became transparent. Lastly, the transparent product was freeze-dried for 24 hours in the lyophilizer which was precooled for 2 hours.Modification of OTS grafting on the cellulose sponge
The prepared cellulose sponge was immersed in the toluene (50 mL), which was added with OTS (500 μL), for 2 hours. And then, it was taken out and freeze-dried for 8 hours to obtain the as-prepared sponge.Characterizations
The chemical states of pristine sponge and modified sponge were analyzed by the infrared spectrogram which was conducted with an IRPrestige-21 Fourier transform infrared spectrometer (FTIR, Shimadzu Corporation, Japan). The surface topography investigation was achieved by placing the gold-sprayed samples in a scanning electron microscope (SEM, SNE-3000, SEC, Korea). Analysis of thermal stability was accomplished by an apparatus (TG, TG/DTA 6300, Japan) at a heating rate of 15 °C min−1 from 30 °C to 600 °C and a cooling rate of 25 °C min−1 from 600 °C back to 30 °C. The wettability of sponge was characterized with water contact angle (WCA) and oil contact angle (OCA), which were measured by an OCA50 Micro machine (Data-Physics, Germany) with the 2 μL droplet. The oil/water separation tests were accomplished by placing the modified sponge in the Petri dish whose upper layer was toluene and lower layer was DI water. The wettability and adsorption capacity were investigated by putting the blank sponge and as-prepared sponge in the DI water and vegetable oil, respectively. The modified cellulose sponge was fastened to the inlet of the constant flow pump with fine copper wire and placed in a beaker filled with the toluene/DI water mixture to study the continuous oil/water separation capacity. Every picture was obtained via a Canon camera. The absorption capacity was investigated by measuring the weight of the sponge before and after adsorption, which was based on the equation: C (%) = [(M2 − M1)/M1] × 100% where C (%)represents the adsorption capacity of the modified sponge, M1 and M2 are the weights of the grafted sponge before and after oil adsorption, respectively.24Results and discussion
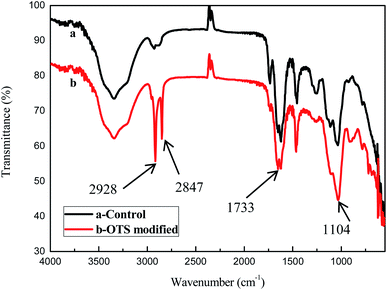
Fig. 2 showed the FTIR spectra of the untreated sponge and the OTS-grafted sponge. The bands at about 2928 cm−1 and 2847 cm−1 were ascribed to the stretching vibration of –CH2 and the area of them increased significantly.25 The peak at 1104 cm−1 corresponded to the stretching vibration of Si–O–Si groups indicated OTS was grafted on the surface of the cellulose sponge.26 Besides the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appeared at about 1733 cm−1.27 These consequences confirmed that OTS was successfully grafted on the sponge.
O appeared at about 1733 cm−1.27 These consequences confirmed that OTS was successfully grafted on the sponge.
The morphological structures of the original and OTS-grafted sponge were investigated by SEM. From Fig. 3a and a′, it could be clearly seen that the untreated sponge exhibited a highly porous structure and a smooth skeleton surface. As shown in Fig. 3b and b′, the modification with OTS led to the significant change on the sponge surface. The grafted sponge covered uniformly by OTS exhibited its evident rough surface, which might be the reason of the reduction of pore structure. And this difference also proved that grafting reaction had taken place on the surface of the sponge. It is worth noting that one of the prerequisites for hydrophobic/oleophilic property is the rough surface mentioned above.28
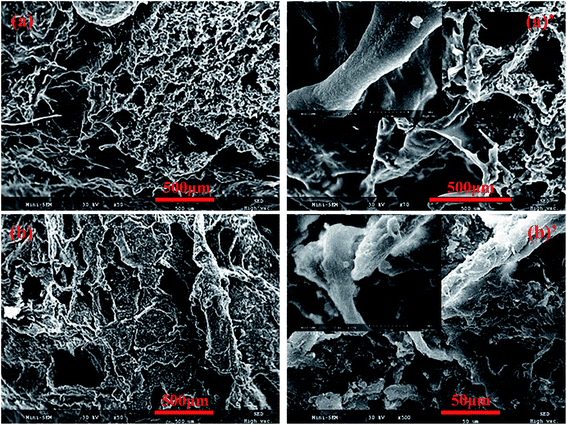 | ||
| Fig. 3 SEM images of prepared cellulose sponge: (a) control, (a′) a control one with high magnification, (b) cellulose sponge after modifying with OTS, (b′) a modifying one with high magnification. | ||
The maximum operating temperature is determined by the thermal performance of the sponge. TG was conducted for the original sponge and the modified one, and the results were shown in Fig. 4. From the curve, it could be found that the mass loss of each sponge was recorded three stages. For the blank sponge, the maximum decomposition temperature of the first stage was between about 40 °C and 170 °C, which was the result of the loss of water and cellulose. Compared to the blank one, the modified sponge's maximum decomposition temperature was increased to about 180 °C and the weightlessness rate was lower, which indicated its better thermal stability due to the addition of OTS and less water adsorbed from the air. In the second stage, both the blank and modified sponges lost their weight rapidly, which could be owing to the degradation of the cellulose sponge backbone.
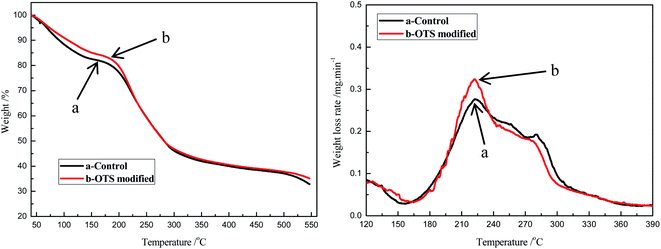 | ||
| Fig. 4 The TG curves of cellulose sponge before and after modifying: (a) control, (b) the modified one. | ||
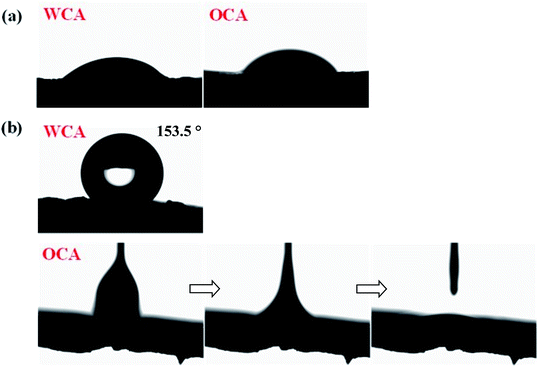
The contact angle measurements were implemented to evaluate the surface wettability of the original and grafted sponge, and the consequences were presented in Fig. 5. The pristine sponge was superhydrophilicity and superoleophilicity with very low WCA and OCA, which indicated that both water and oil could easily wet this sponge. Whereas, from the figure, it could be clearly seen that the water droplet exhibited spherical shape on the surface and the water contact angle increased rapidly to 153.5° with OTS grafted on the surface of the sponge. In the meantime, when an oil droplet dropped to the surface of OTS-grafted sponge, it spread and completely wetted the sample with the oil contact angle of about 0°. These consequences demonstrated the super-hydrophobicity/oleophilicity of the modified sponge.
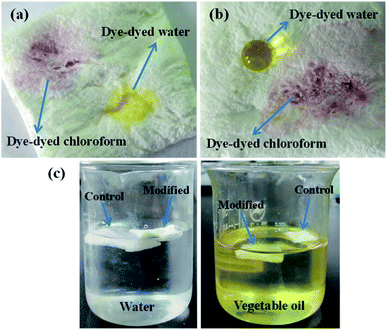
As mentioned above, the modified sponge showed superhydrophobic and superoleophilic. The phenomenon of water droplets and chloroform droplets on the sponge, and the sponge in the water and vegetable oil was looked into to further prove the unique wetting properties of the treated sponge. As shown in Fig. 6a, when a dye-dyed water droplet and a dye-dyed chloroform were placed on the surface, the blank sponge could be penetrated and wetted rapidly by both water and chloroform due to its superhydrophilicity and superoleophilicity. It could be seen from Fig. 6b that the OTS-grafted sponge still possessed the superlipophilicity and were only permeated by dye-dyed chloroform. Whereas, it was worth noting that the treated sponge showed a different wettability than before which might have something to do with their low surface energy and repelled the dye-dyed water which took on an almost perfect sphere on the surface.29 Fig. 6c showed the phenomena of the original sponge and modified sponge being placed on the surface of water and vegetable oil. It could be found that the blank sponge swelled rapidly caused by the water adsorption and suspended under the surface of water. In contrast, the OTS-grafted sponge floated on the surface of water without any visible changes, indicating the superhydrophobicity and certain structural strength of it. When immersed in vegetable oil, nevertheless, the treated sponge was soaked rapidly and suspended beneath the oil without swelling because of its super-oleophilicity.
Fig. 7 illustrated the adsorption capacities of the OTS-grafted sponge which could be estimated by several kinds of oils/organics, including vegetable oil, hexane, cyclohexane and chloroform. The results showed that the adsorption capacities of the as-prepared sponge for oils/organics were above 200% to around 700% of its weight, exhibiting a great oil adsorption performance. The multi-aperture structure and low surface energy of it might be the reason of this great property.30 Therefore, this modified sponge had a broad prospect as a sort of oil adsorbing material with great oil adsorption performance. However, there was such a huge difference in the adsorption capacities of vegetable oil and chloroform. The reason of this might be that the molecules of chloroform were smaller and more likely to enter the pores of the sponge.
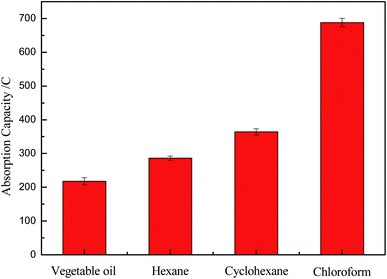 | ||
| Fig. 7 The absorption capacities of modified cellulose sponge for various types of oil or organic solvents. | ||
As shown in Fig. 8, the OTS-grafted sponge could be reused to absorb oil on the surface of oil/water mixture with high absorption capacity and great adsorption selectivity. In addition, the absorbed oil could be restored. These characteristics mentioned above were very crucial in practical applications. The separation process of collecting toluene from the surface of oil/water mixture by grafted sponge was demonstrated in Fig. 8a. The toluene in the mixture was rapidly absorbed by the OTS-grafted sponge when they came into contact with each other, and the sponge still remained the water-repellent at the same time. Obviously, it could be seen that the modified sponge still floated on the surface of water even after absorbing the toluene due to its light weight and superhydrophobicity. What's more, the as-prepared sponge could absorb toluene completely without absorbing water and the absorbed toluene could achieve recycling with mechanical extrusion. Fig. 8b showed the durability of the modified sponge after 5 adsorption–desorption cycles, taking vegetable oil as the sample. The results demonstrated that the absorption capacity of the sponge only reduced a little bit after 5 times of cyclic extrusion and still sustained about 200% of its weight, which indicated a great recyclability.
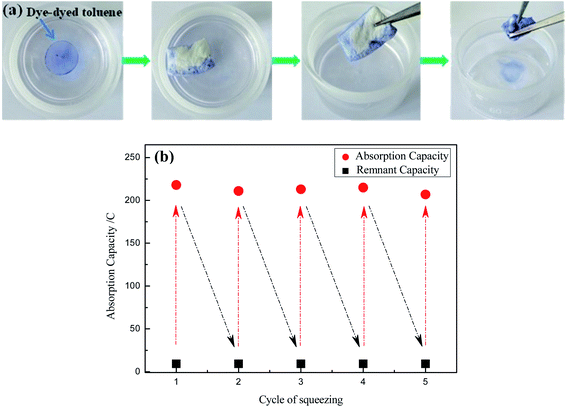 | ||
| Fig. 8 (a) The separation process of collecting toluene (dyed by dyestuff) from water surface, (b) the changes of absorption capacity for vegetable oil after 5 adsorption–desorption cycles. | ||
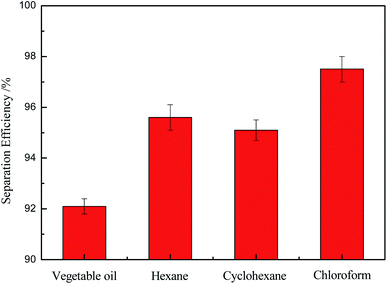
Fig. 9 showed the investigation results on the separation efficiency of the OTS-grafted sponge which was an important indicator for evaluating the separation property. It was worth noting that the modified sponge exhibited outstanding separation efficiencies of all above 92% towards these experimental mixtures (vegetable oil, hexane, cyclohexane, chloroform). Among them, the as-prepared sponge had the most excellent separation ability to chloroform, which could come up to about 98%. These consequences demonstrated that the modified sponge was a material worth choosing for oil/water separation.
Fig. 10 showed the continuous separation ability of the grafted sponge for toluene/water mixture, which was investigated through a constant flow pump. When the pump was taken on, toluene was drawn at a constant rate through a rubber tube to the beaker on the other side (Video S1 in ESI†). After a while, toluene was removed completely from the mixture, and no water was pumped away to the toluene collected beaker, which indicated that the modified sponge still maintained its superhydrophobicity and superoleophilicity even in the presence of pressure difference. These consequences indicated that the grafted sponge had a good application prospect in oil/water separation.
 | ||
| Fig. 10 The continuous oil/water separation process of modified cellulose sponge under being assisted by a peristaltic pump. | ||
Conclusions
In summary, a facile method to prepare cellulose sponge was demonstrated. And the OTS-grafted sponge with superhydrophobicity and superoleophilicity which were exhibited with a water contact angle of 153.5° and an oil contact angle of 0° was also successfully prepared. The modified sponge's absorption capacity could reach up to about 700% of its weight for chloroform, and still maintain around 200% of its weight for vegetable oil after 5 times of cyclic extrusion, indicating its excellent absorption performance and recyclability. In addition, the as-prepared sponge could realize ideal separation of water and oil with a high separation efficiency (98% for chloroform) and wonderful selectivity for various oil/water mixtures. In a word, the modified sponge with all the performances above will have a promising application in oil/water separation.Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Grant No. 51703130) and the International Science and Technology Cooperation Project of Shaoxing University (Grant No. 2019LGGH1004).References
- L. Zou, A. D. Phule, Y. Sun, T. Y. Zhu, S. Wen and Z. X. Zhang, Polym. Test., 2020, 85, 106451 CrossRef CAS.
- A. Parsaie, M. Mohammadi-Khanaposhtani, M. Riazi and Y. Tamsilian, Sep. Purif. Technol., 2020, 251, 117105 CrossRef CAS.
- Z. Lei, P. Zheng, L. Niu, Y. Yang, J. Shen, W. Zhang and C. Wang, Appl. Surf. Sci., 2019, 489, 922 CrossRef CAS.
- C. Cao, F. Wang and M. Lu, Colloids Surf., A, 2020, 601, 125033 CrossRef CAS.
- R. Zhang, Z. Zhou, W. Ge, Y. Lu, T. Liu, W. Yang and J. Dai, Chin. J. Chem. Eng., 2020 DOI:10.1016/j.cjche.2020.06.006.
- W. Qing, X. Li, Y. Wu, S. Shao, H. Guo, Z. Yao, Y. Chen, W. Zhang and C. Y. Tang, J. Membr. Sci., 2020, 612, 118476 CrossRef CAS.
- F. Li, Z. Yu, H. Shi, Q. Yang, Q. Chen, Y. Pan, G. Zeng and L. Yan, Chem. Eng. J., 2017, 322, 33 CrossRef CAS.
- Z. W. S. Zeng and S. E. Taylor, Sep. Purif. Technol., 2020, 247, 116996 CrossRef CAS.
- A. Jamsaz and E. K. Goharshadi, Process Saf. Environ. Prot., 2020, 139, 297 CrossRef CAS.
- N. Wang and Z. Deng, Mater. Res. Bull., 2019, 115, 19 CrossRef CAS.
- C. Zhang, Y. Li, S. Sun, M. Kalulu, Y. Wang, X. Zhou, X. Wang, Q. Du and Y. Jiang, Prog. Org. Coat., 2020, 139, 105369 CrossRef CAS.
- J. H. Shin, J. H. Heo, S. Jeon, J. H. Park, S. Kim and H. W. Kang, J. Hazard. Mater., 2019, 365, 494 CrossRef CAS.
- B. Peng, Z. Yao, X. Wang, M. Crombeen, D. G. Sweeney and K. C. Tam, Green Energy Environ., 2020, 5, 37 CrossRef.
- L. Li, L. Rong, Z. Xu, B. Wang, X. Feng, Z. Mao, H. Xu, J. Yuan, S. Liu and X. Sui, Carbohydr. Polym., 2020, 237, 116133 CrossRef CAS.
- C. Su, H. Yang, S. Song, B. Lu and R. Chen, Chem. Eng. J., 2017, 309, 366 CrossRef CAS.
- M. Zhao, Y. Tao, J. Wang and Y. He, J. Water Process. Eng., 2020, 36, 101279 CrossRef.
- S. Ye, B. Wang, Y. Shi, B. Wang, Y. Zhang, Y. Feng, W. Han, C. Liu and C. Shen, Compos. Commun., 2020, 21, 100378 CrossRef.
- Y. Yang, P. Peng, Q. Yang, D. Wang and J. Dong, Appl. Surf. Sci., 2020, 530, 147163 CrossRef CAS.
- H. Chen, Y. Shen, Z. He, Z. Wu and X. Xie, J. Mater. Sci. Technol., 2020, 51, 151 CrossRef.
- Q. Song, H. Wang, S. Han, J. Wang, B. Zhang and Y. Zhang, Prog. Org. Coat., 2020, 148, 105839 CrossRef CAS.
- Y. Deng, C. Peng, M. Dai, D. Lin, I. Ali, S. S. Alhewairini, X. Zheng, G. Chen, J. Li and I. Naz, J. Clean. Prod., 2020, 266, 121624 CrossRef.
- A. Halim, Y. Xu, K. H. Lin, M. Kobayashi, M. Kajiyama and T. Enomae, Sep. Purif. Technol., 2019, 224, 322 CrossRef CAS.
- J. Chen, Y. Zhang, C. Chen, M. Xu, G. Wang, Z. Zeng, L. Wang and Q. Xue, Macromol. Mater. Eng., 2017, 302, 1700086 CrossRef.
- X. Meng, H. Zhan, B. Xi, X. Li, X. Zhou, Q. Deng and L. Liang, J. Macromol. Sci., Part A: Pure Appl.Chem., 2017, 54, 877 CrossRef CAS.
- X. Chen, Y. He, Y. Fan, Q. Yang, X. Yang and G. Zeng, J. Mater. Sci., 2018, 53, 516 CrossRef CAS.
- L. Dashairya, D. D. Barik and P. Saha, J. Coat. Technol. Res., 2019, 16, 1021 CrossRef CAS.
- B. Jiang, K. Cheng, N. Zhang, N. Yang, L. Zhang and Y. Sun, Sep. Purif. Technol., 2021, 255, 117724 CrossRef CAS.
- K. Li, W. Chen, W. Wu, Z. Pan, Z. Liang and J. Gan, Prog. Org. Coat., 2021, 150, 105979 CrossRef CAS.
- Q. Cheng, C. Guan, Y. Li, J. Zhu and J. Zeng, Cellulose, 2019, 26, 2861 CrossRef CAS.
- L. Han, W. Wu, Z. Huang, W. Lei, S. Li, H. Zhang, Q. Jia and S. Zhang, Sep. Purif. Technol., 2021, 254, 117620 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07910c |
| This journal is © The Royal Society of Chemistry 2020 |