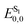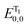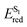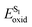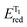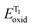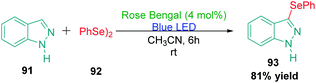Open Access Article
Open Access ArticleRecent applications of Rose Bengal catalysis in N-heterocycles: a short review
Arjita Srivastava
 a,
Pravin K. Singh
a,
Akram Ali
a,
Pravin K. Singh
a,
Akram Ali
 a,
Praveen P. Singh
a,
Praveen P. Singh
 b and
Vishal Srivastava
*a
b and
Vishal Srivastava
*a
aDepartment of Chemistry, CMP Degree College, Prayagraj, India. E-mail: vishalgreenchem@gmail.com
bDepartment of Chemistry, United College of Engineering and Research, Prayagraj, India
First published on 28th October 2020
Abstract
The visible light harnessing ability of Rose Bengal, an organic dye, has been extensively employed in organic chemistry over the last few years. In visible light mediated reactions, this photoredox catalyst operates through multiple pathways and has the ability to provide distinctly different and valuable results. The most significant of these results are bond creation, bond functionalization, particularly for C–H and C–heteroatom bonds, and cross couplings. It is crucial to study these cases whenever these bond formations and couplings lead to the formation of heterocyclic compounds or their functionalization. The diverse biological activity and medicinal applications of heterocyclic compounds is an extensively explored area. This review primarily attempts to demonstrate the synthetic potential of Rose Bengal for synthesis and site selective functionalization of nitrogen containing heterocycles.
1. Introduction
The applications of heterocyclic compounds are widely spread across medicinal chemistry, industry and applied chemistry. Most of the commercially available drugs use some derivative of or a functionalized heterocyclic moiety. Nitrogen containing heterocycles such as indoles, imidazoles, pyrrolidines, indolizine, quinolines etc. constitute a myriad of such biologically active compounds. This makes their functionalization strategies as significant as their synthesis. There has been extensive research regarding the applications of nitrogen containing heterocyclic compound libraries.1–5 The development of sustainable chemical strategies for synthesizing organic compound libraries is currently the field evoking the most interest in organic chemistry research. Visible light, the most sustainable reaction inducer, has been increasingly used to promote many synthetic transformations in organic chemistry.6–15 There have been numerous efforts to mimic the ease with which plants use visible light to carry out photosynthesis. Scientists have faced multiple challenges to devise practical and diverse applications of visible light in synthesis, the biggest limitation in this progress being the incapability of most organic molecules to absorb light in visible spectrum. Ever since Ciamician documented the application of visible light as a sustainable energy source for chemical transformations in laboratory,16 this research field has grown tremendously. With the combination of wavelength specific LEDs and light absorbing photocatalysts, it has become possible to apply visible light as a “green energy source” for many reactions.17–19 “Photocatalysts”, which basically act as intermediaries between visible light and organic substrates, have made possible for scientists to harness the energy of visible light to achieve chemical transformations.The pioneers of photocatalysis were Honda–Fujishima who applied visible light for splitting of water using a titania semiconductor.20 Ever since, a plethora of transition metal complexes21–33 and organic dyes34–42 have been used as photocatalysts. The most commonly used catalysts for such reactions are photoredox catalysts which induce SET pathways – single electron transfer to or from substrates and EnT pathways (light induced energy transfer pathways).43–45 Most of the photoredox catalysts, however, are metallophotocatalysts. The application of transition metal complexes as photocatalysts somehow contradicts the purpose of sustainability. This is why, organocatalysts are gaining a lot of popularity as they are excellent photoredox catalysts, are environmentally friendly and are inexpensive. Photocatalysed synthesis, particularly organic dye catalyzed synthesis, is a relatively novel area which is still being explored. Rose Bengal (RB) is one such versatile photocatalyst which is being extensively employed in a variety of organic transformations (Fig. 1). This versatile photocatalytic nature of Rose Bengal is primarily the result of its redox potential values (Table 1).46 It has emerged as an excellent photocatalyst for both synthesis and directed functionalization of heterocycles.47–59
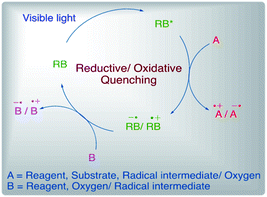
RB is generally supplied and used as its disodium salt RB-Na2 (RB2−), yet most of the reports depict the disodium salt itself as RB only. Rose Bengal excited by a light source is depicted generally as RB*. This excited photocatalyst RB* can influence a reaction either by energy transfer (EnT) pathway or single electron transfer (SET) pathway, the latter being the most common mode of action. The excited state photocatalyst RB* is capable of acting both as an oxidant and a reductant and hence it can undergo either a reductive quenching or an oxidative quenching photoredox cycle via a single electron transfer (SET) pathway. The type of cycle RB will follow depends upon the redox potentials of RB* and the reagents/substrates/intermediates being used/formed in the reaction. In the oxidative quenching cycle, excited state RB* first gets converted to RB˙+ by a reagent or molecular oxygen from air and later on gets quenched to ground state RB by another reagent or reaction intermediate. In the reductive quenching cycle, RB* first gets converted to its radical anion RB˙− by a substrate or intermediate of the reaction. In most of the reactions, radical anion RB˙− later gets quenched by molecular oxygen from air to produce ground state RB and superoxide radical anion O2˙−, which often takes part in further reaction mechanism (Fig. 2). However, in some cases, reaction may occur through energy transfer (EnT) pathway, either by energy transfer from excited state RB* to a substrate or intersystem crossing (ISC) of singlet excited state 1RB* to triplet excited state 3RB* and subsequent energy transfer from 3RB* to a substrate. There might be variations to the general mechanism in different reactions, especially in cases where a different terminal oxidant is employed.60,61
A detailed comprehensive insight into the mechanistic pathways and recent manipulations in the mode of action of Rose Bengal was reported by Sharma et al.62 in 2019. This review, however, aims to present the synthetic applications of RB, in particular for N-heterocyclic rings, with a basic discussion of its mechanism in such syntheses. In continuation of our work on development of photocatalysed synthesis63a–n this review might be particularly useful for those who are already involved in the area of heterocycles and interested in the use of visible light and photocatalysts for heterocyclic synthesis. Since this is a short review, only a selected number of published reports on this topic have been covered.
2. C(sp2)–H functionalization of imidazoles
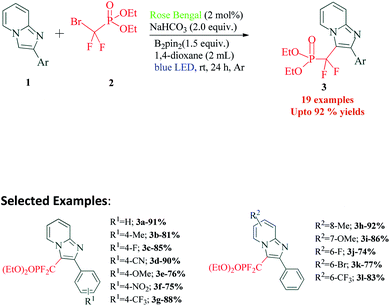
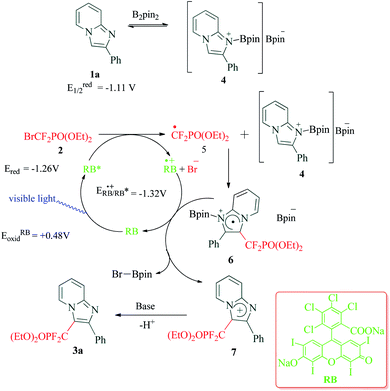
Functionalized imidazoles are versatile in their applications and are utilized in various areas of chemistry and industry. Besides being key pharmacophores in a wide range of commercial drugs, they find their applications in co-ordination chemistry, as catalysts, in material sciences and for natural product synthesis.64–67 Singsardar et al.68 recently reported C(sp2)–H difluoromethylenephosphonation of imidazoheterocycles catalyzed by RB via a single electron transfer pathway (Scheme 1). The reaction required bis(pinacolato)diboron (B2pin2) as an additive, for the activation and stabilization of the heterocyclic system. In the absence of additive B2pin2, the authors observed decomposition of imidazopyridine and no desired coupling product. The reaction was carried out under argon atmosphere in 34 W blue LED for 24 h. Their optimizations revealed 1,4-dioxane as solvent, 2 mol% of Rose Bengal, 2.0 equiv. of NaHCO3 and 1.5 equiv. of B2pin2 at room temperature as the optimum conditions. In their control experiments, they found out that the reaction did not yield any products in the presence of radical scavengers like TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl), BHT (2,6-di-tert-butyl-4-methyl phenol) etc. The reaction was found to be completely suppressed on addition of 1,3-diphenylethylene. Both these observations suggest a radical pathway for the mechanism. In their cyclic voltammetry measurements, authors probably obtained reversible response for 1a (Ered1/2 = −1.11 V) and irreversible response for RB* (Eoxid = +0.48 V) and compound 2 (Ered = −1.26 V). Their cyclic voltammetry results clearly showed that BrCF2PO(OEt)2 (2) has the potential to oxidize the excited state of photocatalyst RB* to generate RB˙+ and radical intermediate 5. This radical intermediate 5 reacts with B2pin2 activated 2-phenylimidazo[1,2-a]pyridine 4 to produce radical intermediate 6. This intermediate 6 gets converted to intermediate 7 in the photoredox cycle completion process simultaneously generating ground state RB. The final product 3a is eventually obtained by a proton abstraction from intermediate 7 by the base (Scheme 2). To demonstrate the substrate scope and generality of the reaction, the authors also explored the reaction with other heterocycles such as indoles, imidazo[2,1-b]thiazoles and benzo[d]-imidazo[2,1-b]thiazoles, and obtained good to excellent yields.3. Synthesis of pyrrolo[2,1,5-cd]indolizines
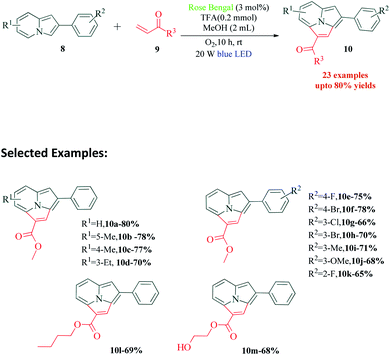
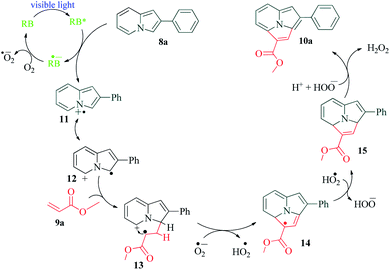
The synthesis of fused heterocyclic rings has always been an attractive area for synthetic chemists. The combination and modification of activities of two heterocyclic rings opens up new arenas for drug discovery. Fused indolizines have found applications in pharmaceutical industry, agrochemical industry and cell functional studies.69–73 In a recent report published by Liang et al.,74 Rose Bengal was used as a photosensitiser for an intermolecular [3 + 2] alkenylation–cyclization strategy for the synthesis of pyrrolo[2,1,5-cd]indolizine derivatives (Scheme 3). The authors report that other photocatalysts also provided product of the reaction, but with lower yields. Their fluorescence quenching experiments revealed that the fluorescence intensity of Rose Bengal decreased on interaction with 8. Based on this fact, the postulated mechanism shows energy transfer via activated Rose Bengal RB* to 8a, resulting in the formation of radical intermediate 11 and RB˙−. The completion of photoredox cycle by O2 results in the formation of ground state photocatalyst RB and superoxide radical anion O2˙−. Intermediate 11 transforms to its resonance structure 12, which then undergoes addition with 9a to produce radical intermediate 13. A sequential dehydrogenation oxidation of 13 with the superoxide radical anion O2˙−, then produces intermediate 15 via radical intermediate 14. This finally undergoes an electron transfer to produce product 10a (Scheme 4). The authors were able to synthesize 23 different variants of fused indolizine derivatives.4. C3-Functionalization of indoles
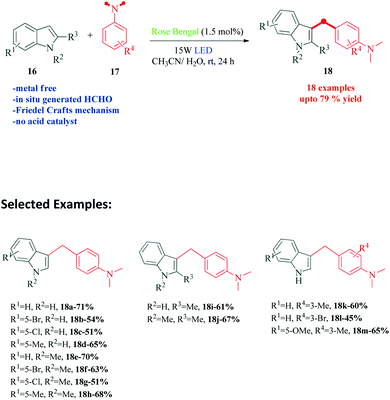
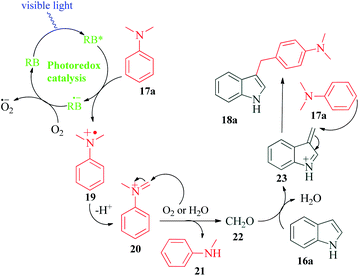
The indole skeleton and its functionalized derivatives are one of the most extensively studied heterocyclic skeletons because of their presence in a large number of drugs and its capability to be utilized as a versatile building block. The modifications of this ring, has therefore warranted a lot of attention.75–80 In 2018, Dai et al.81 reported an efficient visible light promoted, Rose Bengal catalysed synthesis of C3-alkylated indoles by Friedel–Crafts alkylation using N,N-dimethylanilines as the carbon source (Scheme 5). Although the authors obtained considerable yields in CH3CN as solvent, mixed CH3CN/H2O solvents were employed to obtain fast demethylation of tertiary amines. According to the mechanism postulated by the authors, visible light activated Rose Bengal undergoes reductive quenching by N,N-dimethylaniline 17a to give radical cation 19 and RB˙−. The oxidation of RB˙− with O2 produces ground state RB and superoxide radical anion O2˙−. The radical cation 19 looses a proton to superoxide radical anion O2˙− to produce iminium ion 20, which gets oxidized or hydrolyzed and gets decomposed in the process to give compounds 21 and 22. The decomposition product 21, obtained as a by-product of the reaction, was isolated from the reaction mixture. The other decomposition product 22, undergoes condensation with indole 16a to produce intermediate cation 23. This intermediate finally reacts with N,N-dimethylaniline 17a to produce the desired product 18a (Scheme 6). To support the mechanism, the authors carried out a Rose Bengal catalyzed three component reaction of indole 16a (1 eq.), N,N-dimethylaniline 17a (2 eq.) and formaldehyde 22 (3 eq.) under their standard reaction conditions and obtained the desired product 18a.5. Rose Bengal as photocatalyst for Friedel–Crafts alkylation of indoles with nitroalkenes in water
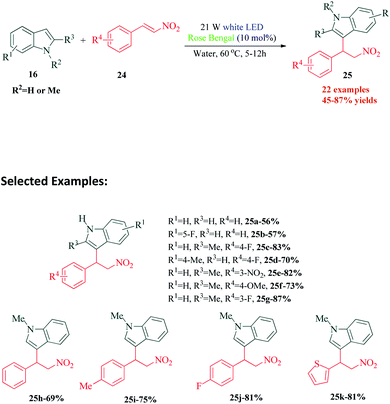
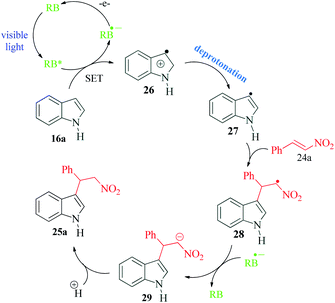
In another interesting methodology for functionalization of indoles, Yu et al.82 reported a metal free photocatalyzed Friedel–Crafts alkylation of indole ring with nitroalkenes using water as the reaction medium (Scheme 7). The strategy is highly efficient and it is noteworthy that good to excellent yields of the desired product were obtained without the use of any external oxidant. The authors performed control experiments under oxygen and nitrogen atmosphere to establish that external oxidant was unnecessary in this protocol. Their cyclic voltammetry experiments further proved that RB* and indole 16 could react spontaneously with each other. Based on their data, the proposed mechanism by the authors involves SET between RB* and indole 16a to produce RB˙− and radical cation 26. This radical cation 26 undergoes deprotonation to radical 27, which undergoes Michael addition with nitroethenyl benzene 24a to produce intermediate 28. The intermediate 28, after undergoing another SET cycle with RB˙− and protonation, yields the desired product 25a (Scheme 8).6. Visible light mediated dearomatisation of indoles and pyrroles
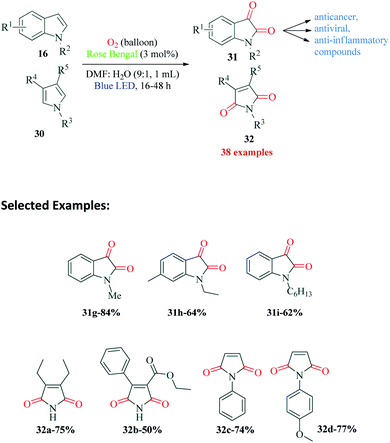
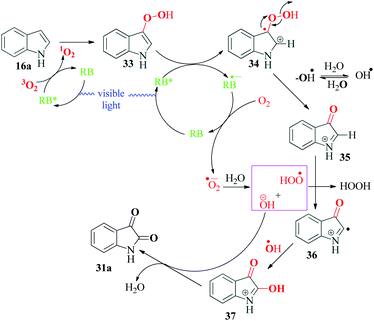
The dearomatisation of indoles to isatin derivatives and pyrroles to imides was reported by Schilling et al.83 in 2019 (Scheme 9). The structural modification of any heterocyclic ring is a highly promising area and continues to be explored for synthesis of potential pharmacologically active compounds. Isatin is one of the privileged building blocks of heterocyclic chemistry and numerous structural modifications are possible. In a considerable improvement over the already reported methods, the authors devised an atom efficient, mild, metal free single step protocol for dearomatisation of indoles and pyrroles. Their optimization studies revealed Rose Bengal to be the best catalyst for the reaction. The reaction could tolerate a wide range of substitutions in the aromatic ring, even C2- and C3-substituted indole carboxylic acids provided good to excellent yields. From their dearomatised product, the authors also successfully synthesized different pharmaceuticals exhibiting anticancer, anticonvulsant, antitumor, antiviral and anti-inflammatory activities. The radical pathway of the reaction was confirmed by the authors by addition of CuCl2, benzoquinone, sodium azide and Stern–Volmer quenching experiments. In the mechanism postulated by the authors, the excited state of Rose Bengal RB* is quenched by oxygen to form singlet oxygen, which then reacts with indole 16a to form peroxo species 33. This peroxo species 33 gets oxidized by RB* to form peroxoindole intermediate 34 and RB˙−. While the intermediate 34 cleaves to form cation 35 and hydroxyl radical, RB˙− reduces molecular oxygen to produce superoxide radical anion (O2˙−), which then reacts with water to form hydroxide ion and peroxide radical. The peroxide radical formed probably abstracts a proton from cation 35 to form intermediate 36. The reaction between intermediate 36 and hydroxyl radical produces 37, which looses a proton to hydroxide ion formed earlier to finally give the product 31a (Scheme 10). Apart from the various differently substituted indole derivatives, the reaction conditions were also optimal for dearomatisation via decarboxylation of C-2 and C-3 carboxylated indoles. The authors also synthesized significant pharmaceuticals from specific substituted indoles.7. Oxidative radical cyclization for synthesis of phenanthridines
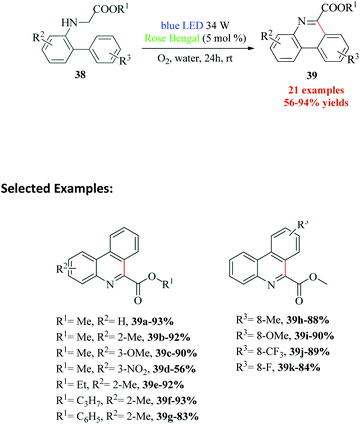
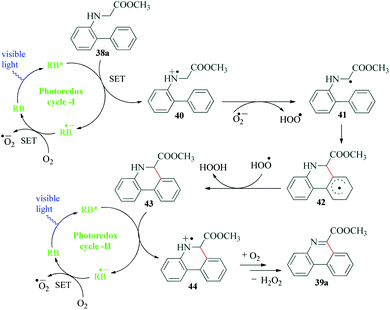
Phenanthridine are core heterocycles in many anticancer, antibacterial, antiprotozoal, antifungal, antiviral and other biologically active compounds. There are also a number of natural products constituted of phenanthridine ring.84–88 In a first ever attempt for visible light photocatalysed conversion of biocompatible N-biarylglycine esters to phenanthridines, Natarajan et al.89 recently published a report which involves a Rose Bengal catalyzed intramolecular cyclization–dehydrogenation to provide excellent yields of the desired products (Scheme 11). The authors demonstrated the requirement of oxygen in the reaction, since the replacement of oxygen with nitrogen, inferior yields of the product was obtained. Based on the mechanism postulated by the authors (Scheme 12), the reductive quenching of RB by glycine ester 38a leads to the formation of RB˙−, which further produces superoxide radical anion O2˙− from molecular oxygen. This superoxide anion radical O2˙− and the amine radical cation 40 react to afford biarylglycine ester radical 41 and peroxide radical. The biarylglycine ester 41 undergoes C–H aromatic coupling to yield another radical 42, which on reaction with the peroxide radical, produces the phenanthridine derivative 43. Another photoredox cycle between excited state photocatalyst RB*, molecular oxygen and phenanthridine derivative 43 results in the formation of radical cation 44, which loses a H+ and a H˙ to yield the final product 39a. An additional experiment was conducted by the authors to confirm the mechanism where effective conversion of compound 43 to methyl phenanthridine-6-carboxylate 39a was achieved under optimized reaction conditions.8. Functionalization of quinoxalin-2(H)-ones
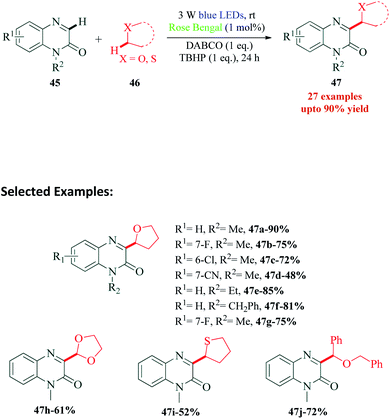
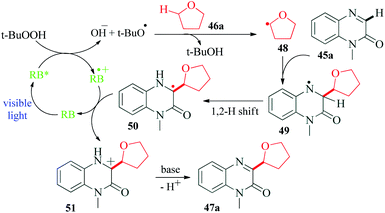
Quinoxalines and their derivatives, particularly C-3 functionalized, are one of the most extensively studied moieties of heterocyclic chemistry. They exhibit a remarkable range of biological activities such as anticancer, antimicrobial, anti-inflammatory, benzodiazepine receptor agonist, DNA cleaving agents and protein kinase inhibitory activities.90–95 Wei et al.96 reported a visible light promoted synthesis of 3-oxyalkylated quinoxalin-2(1H)-ones at room temperature (Scheme 13). The C3-functionalization of quinoxalin-2-ones is one of the significant merits of this method. Their control experiment with TEMPO completely suppressed the reaction and TEMPO–THF complex was also detected, suggesting a radical pathway for the mechanism. The reaction proceeded through Rose Bengal catalyzed C–H/C–H cross-dehydrogenative-coupling (CDC) of quinoxalin-2(H)-ones with simple ethers. TBHP (tert-butyl hydroperoxide) used in the reaction causes oxidative quenching of RB to produce hydroxide ion and t-butyloxy radical. The next step is α-C–H bond cleavage of furan 46a in the presence of t-butyloxy radical, to generate radical intermediate 48, which on reaction with 45a produces radical intermediate 49. A 1,2-hydrogen shift from 49 generates radical intermediate 50. The photoredox cycle is completed by a SET between this radical intermediate 50 and RB˙+ providing cation intermediate 51. Finally, a β-H abstraction from 51 yields the desired product 47a (Scheme 14).9. Rose Bengal catalysed synthesis of quinoxalines
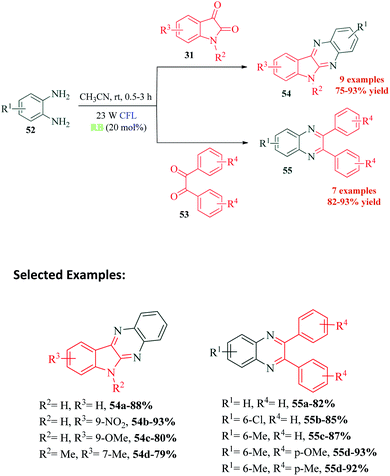
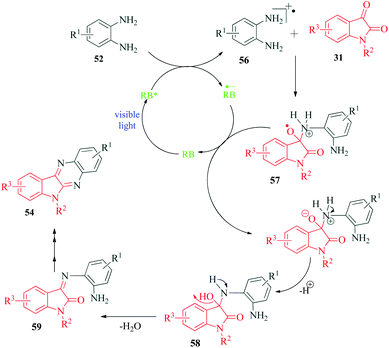
Jaiswal et al.97 reported a novel protocol for photocatalyzed coupling of phenylene 1,2-diamines and 1,2-dicarbonyls for the synthesis of quinoxaline derivatives (Scheme 15). The protocol is an improvement over the already reported conventional methods, is sustainable and exhibits excellent atom economy. Rose Bengal is reported to have good catalytic performance in many organic solvents, yet CH3CN seems to be the optimum choice for most reactions. The authors propose that photoexcited singlet state photocatalyst RB* (S1) undergoes ISC to more stable triplet state RB* (T1), which then acts as the photocatalyst to produce radical cation 56 from 1,2-phenylenediamine 52 (Scheme 16). This radical cation 56, on reaction with isatin 31, produces 57, which through a series of single electron transfer, deprotonation and dehydration produces intermediate 59. This intermediate undergoes cyclization to finally yield the target compound 54.10. C–H oxidation of tryptoline and tetrahydroisoquinoline substrates to corresponding δ-lactams
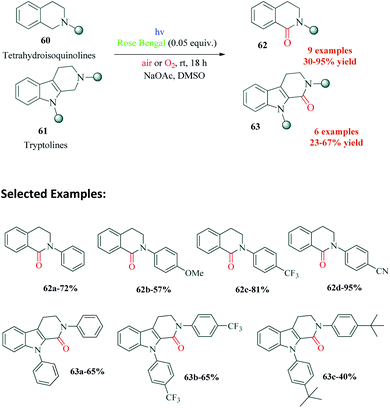
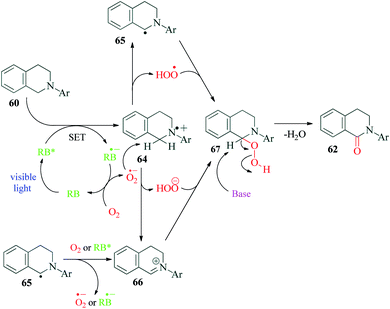
Guryev et al.98 reported a highly efficient Rose Bengal catalyzed protocol for the synthesis of amide derivatives of tetrahydroisoquinoline and tryptoline (Scheme 17). The lactams of these heterocyclic rings are frameworks of many biologically active compounds. The authors were able to synthesize antiviral activity exhibiting derivatives a plant alkaloid strychnocarpine99 (a dihydrocarbolinone compound), which is otherwise used as a muscle relaxant and 5-hydroxytryptamine receptor stimulant.100–103 This is the first ever report on facile access of such selective derivatives of Strychnos alkaloid. The biological activity investigation of all synthesized δ-lactams is underway according to the authors. This environmentally friendly protocol represents an excellent example of metal free C–H photooxidation of cyclic amines. NaOAc was required as a base in the reaction in an attempt to accelerate the formation of α-amine radical. The mechanism proposed by the authors successfully explains the requirement of oxygen as the terminal oxidant (Scheme 18). The usual photoredox cycle between RB, substrate 60 and molecular oxygen results in the formation of superoxide radical anion and radical cation 64. The superoxide radical anion can react with 64 to form either α-aminoradical 65 or iminium ion 66, both of which pathways ultimately result in the final product 62, via intermediate 67.11. Visible light promoted synthesis of tetrahydroquinoline derivatives
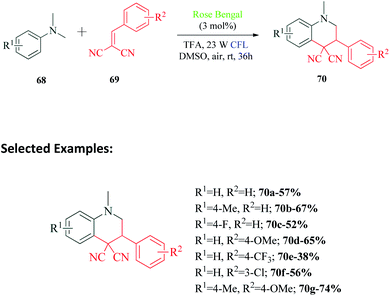
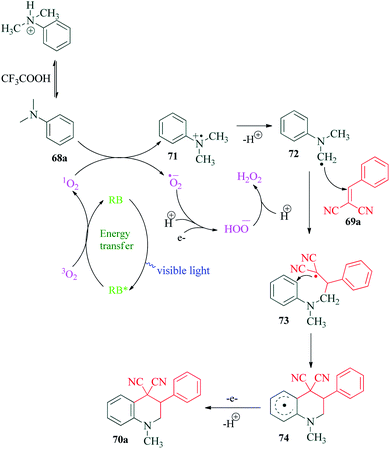
Quinoline and its derivatives have a ubiquitous presence in medicinal agents and natural products. The quinoline ring is one of the most researched heterocycles with regards to drug discovery. There is a long list of therapeutic agents that are constituted of this compound.104–108 In 2017, Xin et al.109 reported the synthesis of tetrahydroquinoline derivatives via visible light promoted reaction of N,N-dimethylanilines and 2-benzylidenemalononitriles using Rose Bengal as the photosensitizer (Scheme 19). The authors performed different control experiments to establish the reaction mechanism (Scheme 20). They observed complete inhibition of the reaction on addition of TEMPO and addition of DABCO provided only trace amounts of product. The collective interpretation was that the reaction involved a radical pathway and singlet oxygen participation. TFA was added to the reaction in an attempt to regulate the balance between free amine 68a and its unreactive ammonium salt. The 1O2 generated by interaction of excited Rose Bengal RB* and 3O2, reacts with free amine 68a to produce amine radical cation 71. This radical 71 undergoes deprotonation to produce radical 72, which on reaction with 2-benzylidenepropanedinitrile 69a, provides radical intermediate 73. This radical intermediate undergoes a series of intramolecular cyclization (through intermediate 74) and proton elimination to finally produce desired product 70a.12. Rose Bengal catalyzed oxidative dehydrogenation of N-heterocycles
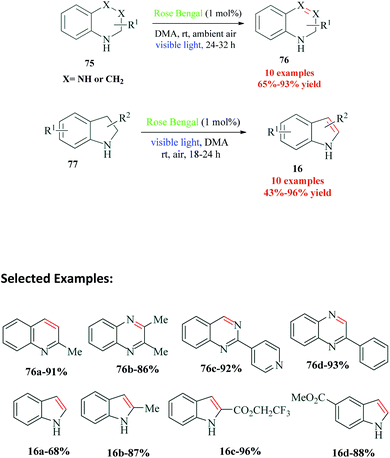
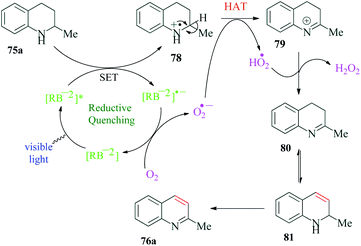
Sahoo et al.110 reported Rose Bengal catalyzed synthesis of different medicinally significant N-heteroarenes such as quinoline, quinoxaline, quinazoline and acridine (Scheme 21). This strategy was further utilized for oxidative dehydrogenation of indolines. They obtained excellent yields of desired dehydrogenated heterocycles using a catalytic amount of Rose Bengal. The authors emphasize the fact that this organic dye catalyzed process provided good yields of dehydrogenated products even from halogen-substituted tetrahydroisoquinolines, which is generally difficult to achieve by conventional transition metal catalysts because of unwanted side reactions. It is significant to note that their control experiments proved that N–H motif in cycloalkane was a prime requirement for the reaction. The mechanism postulated by the authors shows reaction initiation by ISC from singlet state excited photocatalyst RB−2* (S1) to triplet state photoexcited catalyst RB−2* (T1). This triplet state photocatalyst oxidizes the amine 75a to radical cation 78, and the reduced photocatalyst [RB−2]˙− thus generated, reduces O2 to superoxide radical anion O2˙−. This superoxide radical anion helps in the oxidation of radical cation 78 to imine 80, through a hydrogen atom transfer (HAT) step. Under the reaction conditions, imine 80 isomerizes to provide imine 81, which then undergoes a similar dehydrogenation step to finally provide the desired product 76a (Scheme 22).13. Ether functionalization of 2H-indazoles
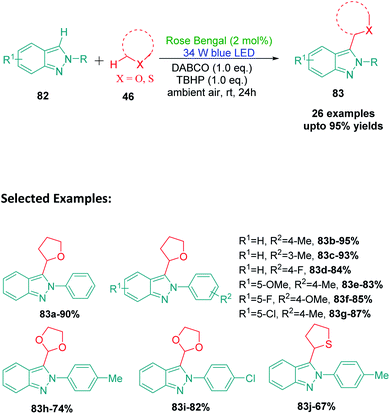
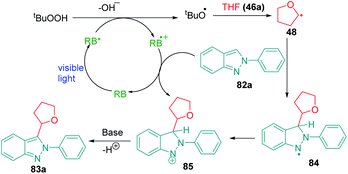
The bicyclic fused indazole ring is one of the most significant N-heterocycle and has widespread applications in medicinal chemistry. Indazole and its derivatives are well known to be exhibit multiple biological activities, particularly against different types of cancers, including inducer of tumor regression, selective CRAF inhibitor activity against melanoma cell lines, inhibition of hepatocellular carcinoma proliferation, multikinase inhibitor activity, and many more.111–117 The spectrum of biological activities that can be obtained by targeted functionalization of the ring is very interesting and has great potential for drug discovery. In 2019, Singsardar et al.118 developed a method for Rose Bengal catalyzed regioselective C(sp2)–H/C(sp3)–H cross-dehydrogenative coupling of indazoles with ethers (Scheme 23). The authors used THF as the oxyalkylating agent in this Rose Bengal catalyzed protocol and obtained excellent yields with TBHP as an additive and DABCO (1,4-diazabicyclo[2.2.2]octane) as a base. The reaction conditions were thoroughly optimized by the authors and they performed a series of control experiments using radical scavengers like TEMPO and BHT to establish the radical pathway of the reaction mechanism. According to the mechanism proposed by the authors, a single electron transfer between excited Rose Bengal RB* and TBHP produces RB˙+, hydroxide ion and t-butyloxy radical. The t-butyloxy radical deprotonates the α-C(sp3)–H of THF 46a to produce alkoxyalkyl radical 48 which then reacts with indazole 82a to provide radical intermediate 84. This radical intermediate 84, on reaction with RB˙+, furnishes a cationic intermediate 85 which finally undergoes deprotonation to produce the desired 3-oxyalkylated indazole 83a (Scheme 24).14. Phosphonylation of indazoles using diphenylphosphine oxide
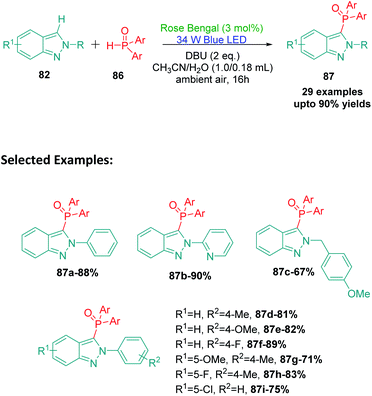
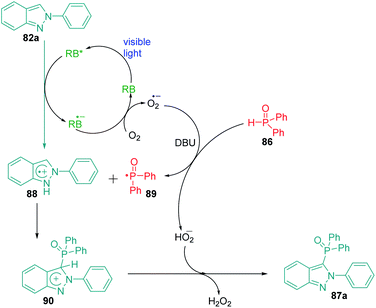
Phosphines containing organic compounds have found various applications in organic synthesis and material sciences. The functionalization of indazole ring with phosphines has the potential to generate derivatives with interesting applications. The introduction of phosphine to indazole has the potential to cause interesting modifications in the properties and activities of the ring owing to the formation of N–C–P bond.119,120 In 2018, Singsardar et al.121 reported an excellent method for Rose Bengal catalysed C(sp2)–H functionalization of indazoles using diphenylphosphine oxide at room temperature (Scheme 25). The authors were able to achieve excellent substrate scope with this protocol. Based on the mechanism proposed by the authors, excited state RB* undergoes a single electron transfer process (SET) with indazole 82a to produce radical cation intermediate 88 and RB˙−. The photoredox cycle then gets completed by reduction of molecular oxygen by RB˙− to produce ground state RB and superoxide radical anion O2˙−. A hydrogen atom transfer process (HAT) then occurs between superoxide radical anion O2˙− and diphenylphosphine oxide 86 in the presence of DBU (1,8-diazabicyclo[5.4.0]undec-7-ene), to produce diphenylphosphinoyl radical 89 and HO2−. This radical 89 now undergoes coupling with radical cation intermediate 88 to produce cation intermediate 90, which undergoes deprotonation by HO2− to finally produce H2O2 and the desired product 87a (Scheme 26). The authors also report that no phosphonylation of DBU was observed during the reaction suggesting that it only acts as a base in this protocol.15. Rose Bengal catalysed selenylation of indazoles
Organoselenium compounds are becoming more prominent in the area of medicinal chemistry because of their pharmacological activities and their applications in material science.122,123 The selenylation of indazole ring to produce a potentially bioactive compound is a highly promising area. One such highly efficient protocol was developed by Saba et al.124 in 2018. The authors achieved effective Rose Bengal catalysed selenylation of various heterocyclic rings, including indazole, using diorganoyl diselenides (Scheme 27).16. Conclusion
Nitrogen containing heterocycles are ubiquitous in pharmaceutical industry and their synthesis and site selective targeted functionalization has always held a significant place in the area of drug discovery. Synthetic chemists continue to explore and develop novel, sustainable methods for synthesis of potentially bioactive N-heterocycles. With the boom of visible light promoted synthesis using organic dyes as photoredox catalysts, the area of sustainable synthesis is being utilized like never before and holds vast potential. Rose Bengal has emerged as an excellent photoredox catalyst for a variety of organic syntheses and has been the catalyst of choice for many protocols involving synthesis and functionalization of nitrogen containing heterocycles. Further developments in the site selective functionalization of heterocycles would prove extremely beneficial for synthetic and medicinal chemistry. Rose Bengal catalysed synthetic strategies would prove as viable tools for construction of potentially bioactive N-heterocycles and other heterocyclic moieties. Rose Bengal could also be employed as a better alternative to metal based catalysts in a variety of organic transformations. In conclusion, the harnessing of visible light using Rose Bengal is a highly promising area for pharmaceutical industry and needs to be explored to a much greater extent in the future.Conflicts of interest
There are no conflicts to declare.References
- Y. Wang, B. Xu, R. Sun, Y. J. Xu and J. F. Ge, J. Mater. Chem. B, 2020, 8, 7466–7474 RSC.
- D. Didier, A. N. Baumann and M. Eisold, Tetrahedron Lett., 2018, 59(45), 3975–638 CrossRef CAS.
- M. T. El-Sayed, N. A. Hamdy, D. A. Osman and K. M. Ahmed, Adv. Mod. Oncol. Res., 2015, 1, 20–638 CrossRef CAS.
- L. Wang, Y. Tian, W. Chen, H. Liu, P. Zhan, D. Li, H. Liu, E. D. Clercq, C. Pannecouque and X. Liu, Eur. J. Med. Chem., 2014, 85, 293–638 CrossRef CAS.
- N. Siddiqui, N. Andalip, S. Bawa, R. Ali, O. Afzal, M. J. Akhtar, B. Azad and R. Kumar, J. Pharm. BioAllied Sci., 2011, 3(2), 194–638 CrossRef CAS.
- Y. Yasu, T. Koike and M. Akita, Chem. Commun., 2013, 49, 2037–638 RSC.
- T. Chatterjee, G. Roh, M. A. Shoaib, C. H. Suhl, J. S. Kim, C. G. Cho and E. J. Cho, Org. Lett., 2017, 19(7), 1906–638 CrossRef CAS.
- Y. Z. Chen, D. H. Wang, B. Chen, J. J. Zhong, C. H. Tung and L. Z. Wu, J. Org. Chem., 2012, 77(16), 6773–638 CrossRef CAS.
- Q. Liu, F. Liu, H. Yue, X. Zhao, J. Li and W. Wei, Adv. Synth. Catal., 2019, 361(22), 5277–638 CrossRef CAS.
- Z. Li, H. Song, R. Guo, M. Zuo, C. Hou, S. Sun, X. He, Z. Sun and W. Chu, Green Chem., 2019, 21, 3602–638 RSC.
- A. N. Nadaf and K. Shivashankar, J. Heterocycl. Chem., 2018, 55(6), 1375–638 CrossRef CAS.
- X. F. Xia, G. W. Zhang and S. L. Zhu, Tetrahedron, 2017, 73(19), 2727–638 CrossRef CAS.
- J. Sun, Y. He, X. D. An, X. Zhang, L. Yu and S. Yu, Org. Chem. Front., 2018, 5, 977–638 RSC.
- M. Parasrama and V. Gevorgyan, Chem. Soc. Rev., 2017, 46, 6227–638 RSC.
- G. Zhao and T. Wang, Angew. Chem., 2018, 57(21), 6120–638 CrossRef CAS.
- G. Ciamician, Science, 1912, 36, 385–638 CrossRef CAS.
- J. Chen, J. Cen, X. Xu and X. Li, Catal. Sci. Technol., 2016, 6, 349–638 RSC.
- L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., 2018, 57(32), 10034–638 CrossRef CAS.
- B. König, Eur. J. Org. Chem., 2017, 15, 1979–638 CrossRef.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–638 CrossRef CAS.
- C. K. Prier, D. A. Rankic and D. W. C. Macmillan, Chem. Rev., 2013, 113(7), 5322–638 CrossRef CAS.
- J. Twilton, C. Le and P. Zhang, Nat. Rev. Chem., 2017, 1, 0052–638 CrossRef CAS.
- B. L. Toth, O. Tischler and Z. Novak, Tetrahedron Lett., 2016, 57(41), 4505–638 CrossRef CAS.
- Y. Wang, A. Liu, D. Ma, S. Li, C. Lu, T. Li and C. Chen, Catalysts, 2018, 8, 355–638 CrossRef.
- H. M. Yang, M. L. Liu, J. W. Tu, E. M. Stempel, M. G. Campbell and G. J. Chuang, J. Org. Chem., 2020, 85(4), 2040–638 CrossRef CAS.
- A. Graml, I. Ghosh and B. Konig, J. Org. Chem., 2017, 82(7), 3552–638 CrossRef CAS.
- X. Zhang, P. Zhu, R. Zhang, X. Li and T. Yao, J. Org. Chem., 2020, 85(15), 9503–9513 CrossRef CAS.
- W. M. Cheng and R. Shang, ACS Catal., 2020, 10(16), 9170–9196 CrossRef CAS.
- J. Tang, G. Grampp, Y. Liu, B. X. Wang, F. F. Tao, L. J. Wang, X. Z. Liang, H. Q. Xiao and Y. M. Shen, J. Org. Chem., 2015, 80(5), 2724–638 CrossRef CAS.
- R. Kancherla, K. Muralirajan, A. Sagadevan and M. Rueping, Trends Chem., 2019, 1(5), 510–638 CrossRef.
- Y. Z. Chen, D. H. Wang, B. Chen, J. J. Zhong, C. H. Tung and L. Z. Wu, J. Org. Chem., 2012, 77(16), 6773–638 CrossRef CAS.
- M. Parasram and V. Gevorgyan, Chem. Soc. Rev., 2017, 46, 6227–638 RSC.
- Y. Yasu, T. Koike and M. Akita, Chem. Commun., 2013, 49, 2037–638 RSC.
- X. Z. Fan, J. W. Rong, H. L. Wu, Q. Zhou, H. P. Deng, J. D. Tan, C. W. Xue, L. Z. Wu, H. R. Tao and J. Wu, Angew. Chem., 2018, 57(28), 8514–638 CrossRef CAS.
- V. Srivastava and P. P. Singh, RSC Adv., 2017, 50, 31377–638 RSC.
- X. Li, X. Gu, Y. Li and P. Li, ACS Catal., 2014, 4(6), 1897–638 CrossRef CAS.
- W. Fan, Q. Yang, F. Xu and P. Li, J. Org. Chem., 2014, 79(21), 10588–638 CrossRef CAS.
- S. Kaur, G. Zhao, E. Busch and T. Wang, Org. Biomol. Chem., 2019, 17, 1955–638 RSC.
- C. Ni, W. Chen, C. Jiang and H. Lu, New J. Chem., 2020, 44, 313–638 RSC.
- W. Zhang, X. X. Xiang, J. Chen, C. Yang, Y. L. Pan, J. P. Cheng, Q. Meng and X. Li, Nat. Commun., 2020, 11 Search PubMed.
- L. Teng, X. Liu, P. Guo, Y. Yu and H. Cao, Org. Lett., 2020, 22(10), 3841–638 CrossRef CAS.
- Y. Zhang, C. Ye, S. Li, A. Ding, G. Gu and H. Guo, RSC Adv., 2017, 7, 13240–638 RSC.
- F. S. Kalthoff, M. J. James, M. Teders, L. Pitzer and F. Glorius, Chem. Soc. Rev., 2018, 47, 7190–638 RSC.
- D. Staveness, I. Bosque and C. R. J. Stephenson, Acc. Chem. Res., 2016, 49(10), 2295–638 CrossRef CAS.
- Q. Q. Zhou, Y. Q. Zou, L. Q. Lu and W. J. Xiao, Angew. Chem., Int. Ed., 2019, 58(6), 1586–638 CrossRef CAS.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–638 CrossRef CAS.
- G. Kibriya, S. Samanta, S. Jana, S. Mondal and A. Hajra, J. Org. Chem., 2017, 82, 13722–638 CrossRef CAS.
- Q. Yu, Y. Zhang and J. P. Wan, Green Chem., 2019, 21, 3436–638 RSC.
- W. Z. Weng, H. Liang and B. Zhang, Org. Lett., 2018, 20, 4979–638 CrossRef CAS.
- H. Cui, W. Wei, D. Yang, Y. Zhang, H. Zhao, L. Wang and H. Wang, Green Chem., 2017, 19, 3520–638 RSC.
- W. Guo, W. Tan, M. Zhao, K. Tao, L. Y. Zheng, Y. Wu, D. Chen and X. L. Fan, RSC Adv., 2017, 7, 37739–638 RSC.
- H. Cui, W. Wei, D. Yang, Y. Zhang, H. Zhao, L. Wang and H. Wang, Green Chem., 2018, 20, 141–638 RSC.
- W. Wei, P. Bao, H. Yue, S. Liu, L. Wang, Y. Li and D. Yang, Org. Lett., 2018, 20, 5291–638 CrossRef CAS.
- H. F. Qian, C. K. Li, Z. H. Zhou, Z. K. Tao, A. Shoberu and J. P. Zou, Org. Lett., 2018, 20, 5947–638 CrossRef CAS.
- L. Tang, X. M. Li, J. H. Matuska, Y. H. He and Z. Guan, Org. Lett., 2018, 20, 5618–638 CrossRef CAS.
- W. Wei, H. Cui, H. Yue and D. Yang, Green Chem., 2018, 20, 3197–638 RSC.
- J. Kovvuri, B. Nagaraju, A. Kamal and A. K. Srivastava, ACS Comb. Sci., 2016, 18, 644–638 CrossRef CAS.
- S. S. Shah and N. P. Singh, Tetrahedron Lett., 2018, 59, 247–638 CrossRef.
- J. G. Sun, H. Yang, P. Li and B. Zhang, Org. Lett., 2016, 18, 5114–638 CrossRef CAS.
- Z. C. Shen, P. Yang and Y. Tang, J. Org. Chem., 2016, 81(1), 309–638 CrossRef CAS.
- R. R. Mondal, S. Khamarui and D. K. Maiti, Org. Lett., 2017, 19, 5964–638 CrossRef CAS.
- S. Sharma and A. Sharma, Org. Biomol. Chem., 2019, 17, 4384–638 RSC.
- (a) V. Srivastava, P. K. Singh and P. P. Singh, Croat. Chem. Acta, 2014, 87(2), 91–638 CrossRef; (b) V. Srivastava, P. K. Singh and P. P. Singh, Croat. Chem. Acta, 2015, 88(1), 59–638 CrossRef CAS; (c) V. Srivastava, P. K. Singh and P. P. Singh, Croat. Chem. Acta, 2015, 88(3), 227–638 CrossRef CAS; (d) V. Srivastava, P. K. Singh and P. P. Singh, Asian J. Chem., 2016, 28(10), 2159–638 CrossRef CAS; (e) V. Srivastava, P. K. Singh and P. P. Singh, Rev. Roum. Chim., 2016, 61(10), 755–638 Search PubMed; (f) V. Srivastava, P. K. Singh and P. P. Singh, Croat. Chem. Acta, 2017, 90(3), 435–638 CrossRef CAS; (g) V. Srivastava, P. K. Singh, S. Kanaujia and P. P. Singh, New J. Chem., 2018, 42, 688–638 RSC; (h) P. K. Singh, P. P. Singh and V. Srivastava, Croat. Chem. Acta, 2018, 91(3), 383–638 CrossRef; (i) V. Srivastava, P. K. Singh and P. P. Singh, Tetrahedron Lett., 2019, 60, 40–638 CrossRef CAS; (j) V. Srivastava, P. K. Singh and P. P. Singh, Tetrahedron Lett., 2019, 60, 1333–638 CrossRef CAS; (k) V. Srivastava, P. K. Singh and P. P. Singh, Tetrahedron Lett., 2019, 60, 151041–638 CrossRef CAS; (l) V. Srivastava, P. K. Singh, A. Srivastava and P. P. Singh, RSC Adv., 2020, 10, 20046–638 RSC; (m) V. Srivastava, P. K. Singh and P. P. Singh, Rev. Roum. Chim., 2020, 65(3), 221–638, DOI:10.33224/rrch.2020.65.3.01; (n) A. Verma, A. Srivastava, S. K. Tiwari, N. Yadav, M. D. Ansari, V. B. Yadav, H. Sagir and I. R. Siddiqui, J. Heterocycl. Chem., 2020, 57(9), 3493–3498 CAS.
- B. Kuzu, M. Tan, P. Taslimi, I. Gülçin, M. Taşpınar and N. Menges, Bioorg. Chem., 2019, 86, 187–638 CrossRef CAS.
- M. S. Roy, X. Meg, K. Koda, S. Rasapalli, D. Gout and C. J. Lovely, Tetrahedron Lett., 2019, 60, 979–638 CrossRef.
- J. Muñoz and M. Köck, J. Nat. Prod., 2016, 79, 434–638 CrossRef.
- L. Zhang, X. M. Peng, G. L. V. Damu, R. X. Geng and C. H. Zhou, Med. Res. Rev., 2014, 34, 340–638 CrossRef CAS.
- M. Singsardar, S. Mondal, S. Laru and A. Hajra, Org. Lett., 2019, 21(14), 5606–638 CrossRef CAS.
- A. S. Jorgensen, P. Jacobsen, L. B. Christiansen, P. S. Bury, A. Kanstrup, S. M. Thorpe, S. Bain, L. Nerum and K. Wassermann, Bioorg. Med. Chem. Lett., 2000, 10, 399–638 CrossRef CAS.
- A. Shemet and E. M. Carreira, Org. Lett., 2017, 19, 5529–638 CrossRef CAS.
- K. C. Majumdar and S. K. Chattopadhyay, Heterocycles in Natural Product Synthesis, Wiley-VCH, United States, 2011 Search PubMed.
- B. Sadowski, J. Klajn and D. T. Gryko, Org. Biomol. Chem., 2016, 14, 7804–638 RSC.
- A. S. Jorgensen, P. Jacobsen, L. B. Christiansen, P. S. Bury, A. Kanstrup, S. M. Thorpe, S. Bain, L. Nerum and K. Wassermann, Bioorg. Med. Chem. Lett., 2000, 10, 2383–638 CrossRef CAS.
- Y. Liang, L. Teng, Y. Wang, H. Qiuxing and H. Cao, Green Chem., 2019, 21, 4025–638 RSC.
- G. Martinez-Ariza, B. T. Mehari, L. A. G. Pinho, C. Foley, K. Day, J. C. Jewett and C. Hulme, Org. Biomol. Chem., 2017, 15, 6076–638 RSC.
- V. Sharma, P. Kumar and D. Pathak, J. Heterocycl. Chem., 2010, 47, 491–638 CAS.
- G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875–638 CrossRef CAS.
- V. K. Outlaw, J. W. Zhou, A. E. Bragg and C. A. Townsend, RSC Adv., 2016, 6, 61249–638 RSC.
- J. Wang, J. Wang, Y. Zhu, P. Lu and Y. Wang, Chem. Commun., 2011, 47(11), 3275–638 RSC.
- D. A. Vargas, A. Tinoco, V. Tyagi and R. Fasan, Angew. Chem., Int. Ed., 2018, 57(31), 9911–638 CrossRef CAS.
- X. Q. Dai, W. X. Xu, Y. L. Wen, X. H. Liu and J. Q. Weng, Tetrahedron Lett., 2018, 59, 2945–638 CrossRef CAS.
- Z. Y. Yu, J. N. Zhao, F. Yang, X. F. Tang, Y. F. Wu, C. F. Ma, B. Song, L. Yun and Q. W. Meng, RSC Adv., 2020, 10, 4825–638 RSC.
- W. Schilling, Y. Zhang, D. Reimer and S. Das, Chem.–Eur. J., 2020, 26(2), 390–638 CrossRef CAS.
- Y. Wu, S. M. Wong, F. Mao, T. L. Chan and F. Y. Kwong, Org. Lett., 2012, 14, 5306–638 CrossRef CAS.
- R. T. McBurney, A. M. Z. Slawin, L. A. Smart, Y. Yu and J. C. Walton, Chem. Commun., 2011, 47, 7974–638 RSC.
- T. Nakanishi and M. Suzuki, J. Nat. Prod., 1998, 61, 1263–638 CrossRef CAS.
- T. Ishikawa, Med. Res. Rev., 2001, 21, 61–638 CrossRef CAS.
- W. A. Denny, Curr. Med. Chem., 2002, 9, 1655–638 CrossRef CAS.
- P. Natarajan, D. Chuskit and Priya, Green Chem., 2019, 21, 4406–638 RSC.
- A. Carta, S. Piras, G. Loriga and G. Paglietti, Mini-Rev. Med. Chem., 2006, 6, 1179–638 CrossRef CAS.
- T. Kazunobu, T. Ryusuke, O. Tomohiro and S. Matsumura, Chem. Commun., 2002, 212–638 Search PubMed.
- H. M. Refaat, A. A. Moneer and O. M. Khalil, Arch. Pharmacal Res., 2004, 27, 1093–638 CrossRef CAS.
- S. L. Mangold, L. R. Prost and L. L. Kiessling, Chem. Sci., 2012, 3, 772–638 RSC.
- A. A. Hashem, M. A. Gouda and F. A. Badria, Eur. J. Med. Chem., 2010, 45, 1976–638 CrossRef.
- D. A. E. Issa, N. S. Habib and A. E. Abdel Wahab, Med. Chem. Commun., 2015, 6, 202–638 RSC.
- W. Wei, L. Wang, H. Yue, P. Bao, W. Liu, C. Hu, D. Yang and H. Wang, ACS Sustainable Chem. Eng., 2018, 6(12), 17252–638 CrossRef CAS.
- D. Jaiswal, J. Tiwari, S. Singh, A. K. Sharma, J. Singh and J. Singh, ChemistrySelect, 2019, 4, 8713–638 CrossRef CAS.
- A. A. Guryev, F. Hahn, M. Marschall and S. B. Tsogoeva, Chem.–Eur. J., 2019, 25(16), 4062–638 CrossRef CAS.
- R. Jokela and M. Lounasmaa, Tetrahedron, 1987, 43, 6001–638 CrossRef CAS.
- A. Putey, F. Popowycz, Q.-T. Do, P. Bernard, S. K. Talapatra, F. Kozielski, C. M. Galmarini and B. Joseph, J. Med. Chem., 2009, 52, 5916–638 CrossRef CAS.
- H. Rommelspacher, H. Kauffmann, C. H. Cohnitz and H. Coper, Naunyn-Schmiedeberg’s Arch. Pharmacol., 1977, 298, 83–638 CrossRef CAS.
- E. Lukevics, I. Segal, A. Zablotskaya and S. Germane, Molecules, 1997, 2, 180–638 CrossRef CAS.
- J. M. Harris and A. Padwa, Org. Lett., 2003, 5, 4195–638 CrossRef CAS.
- V. I. Nikulin, I. M. Rakov, J. E. De Los Angeles, R. C. Mehta, L. Y. Boyd, D. R. Feller and D. D. Miller, Bioorg. Med. Chem., 2006, 14, 1684–638 CrossRef CAS.
- T. Nagatsu, Neurosci. Res., 1997, 29, 99–638 CrossRef CAS.
- J. D. Scott and R. M. Williams, Chem. Rev., 2002, 102(5), 1669–638 CrossRef CAS.
- A. E. Wright, D. A. Forleo, G. P. Gunawardana, S. P. Gunasekera, F. E. Koehn and O. J. McConell, J. Org. Chem., 1990, 55(15), 4508–638 CrossRef CAS.
- A. C. Collins, J. L. Cashaw and V. E. Davis, Biochem. Pharmacol., 1973, 22(18), 2337–638 CrossRef CAS.
- J. R. Xin, J. T. Guo, D. Vigliaturo, Y. H. He and Z. Guan, Tetrahedron, 2017, 73, 4627–638 CrossRef CAS.
- M. K. Sahoo, G. Jaiswal, J. Rana and E. Balaraman, Chem.–Eur. J., 2017, 23(57), 14167–638 CrossRef CAS.
- S. P. Govek, J. Y. Nagasawa, K. L. Douglas, A. G. Lai, M. Kahraman, C. Bonnefous, A. M. Aparicio, B. D. Darimont, K. L. Grillot, J. D. Joseph, J. A. Kaufman, K.-J. Lee, N. Lu, M. J. Moon, R. Y. Prudente, J. Sensintaffar, P. J. Rix, J. H. Hager and N. D. Smith, Bioorg. Med. Chem. Lett., 2015, 25, 5163–638 CrossRef CAS.
- W. Aman, J. Lee, M. Kim, S. Yang, H. Jung and J.-M. Hah, Bioorg. Med. Chem. Lett., 2016, 26, 1188–638 CrossRef CAS.
- W. Yichao, H. Shengzhuo, L. Wei and T. Zilong, Anti-Cancer Agents Med. Chem., 2018, 18, 1228–638 Search PubMed.
- Y.-Y. Lu, J.-J. Wang, X.-K. Zhang, W.-B. Li and X.-L. Guo, J. Pharm. Pharmacol., 2015, 67, 1393–638 CrossRef CAS.
- A. Thangadurai, M. Minu, S. Wakode, S. Agrawal and B. Narasimhan, Med. Chem. Res., 2012, 21, 1509–638 CrossRef CAS.
- D. J. Dong, D. Q. Zhang, Z. Wang, D. G. Huang and P. S. Li, ChemMedChem, 2018, 13(15), 1490–638 CrossRef.
- T. Yakaiah, B. P. V. Lingaiah, B. Narsaiah, B. Shireesha, B. A. Kumar, S. Gururaj, T. Parthasarathy and B. Sridhar, Bioorg. Med. Chem. Lett., 2007, 17(12), 3445–638 CrossRef CAS.
- M. Singsardar, S. Laru, S. Mondal and A. Hajra, J. Org. Chem., 2019, 84, 4543–638 CrossRef CAS.
- A. Mucha, P. Kafarski and L. Berlicki, J. Med. Chem., 2011, 54, 5955–638 CrossRef CAS.
- A. George and A. Veis, Chem. Rev., 2008, 208, 1670–638 Search PubMed.
- M. Singsardar, A. Dey, R. Sarkar and A. Hajra, J. Org. Chem., 2018, 83(20), 12694–638 CrossRef CAS.
- H. J. Reich and R. J. Hondal, ACS Chem. Biol., 2016, 11, 821–638 CrossRef CAS.
- J. Trenner, C. Depken, T. Weber and A. Breder, Angew. Chem., Int. Ed., 2013, 125, 9121–638 CrossRef.
- S. Saba, J. Rafique, M. S. Franco, A. R. Schneider, L. Espindola, D. O. Silva and A. L. Braga, Org. Biomol. Chem., 2018, 16, 880–638 RSC.
| This journal is © The Royal Society of Chemistry 2020 |