Open Access Article
Open Access ArticleLigand exchange processes between molybdenum and zinc additives in lubricants: evidence from NMR (1H, 13C, 31P) and HPLC-MS analysis†
Yu Min Kiwab,
Philippe Schaeffera,
Pierre Adam*a,
Benoît Thiébautb,
Chantal Boyerb and
Géraldine Papinb
aUniversity of Strasbourg, CNRS, Institut de chimie de Strasbourg, UMR 7177, F-67000 Strasbourg, France. E-mail: padam@unistra.fr
bTOTAL Solaize Research Center, BP22-69360 Cedex, France
First published on 14th October 2020
Abstract
The tribological performances of engine oils have been shown to be enhanced by the synergistic interactions between Mo dithiocarbamates (Mo(DTC)2) with other additives, and notably Zn dithiophosphates (Zn(DTP)2). Being two key components in formulated lubricants, a detailed understanding of the mechanisms involved between these two types of additives is needed to develop engine oils with enhanced friction reduction performances, and improved fuel economy. In this context, we report here the investigation at the molecular level of the interactions between Mo and Zn complexes with DTC and DTP ligands using laboratory experiments. Our analytical approach comprised NMR spectroscopy (1H, 13C, 31P) allowing direct investigation of both homoleptic and heteroleptic Mo and Zn complexes as well as a specifically-developed HPLC-MS method for the investigation of the different DTC species formed during lubricant ageing experiments. The results showed that ligand exchange reactions between Mo(DTP)2 and Zn(DTC)2 complexes strongly favor the migration of the DTC ligands from Zn to Mo, illustrating the higher affinity of Mo for DTC ligands. In the case of binary mixtures involving Mo(DTC)2 and Zn(DTP)2 – a combination of additives frequently used in formulated lubricants – the formation of mixed complexes (Mo(DTC)(DTP)) resulting from ligand exchange reactions could be directly evidenced for the first time by the analytical methods used. These species could account, at least to some extent, for the synergistic effect of Mo(DTC)2 and Zn(DTP)2 on the friction reducing properties of engine oils. However, they were formed in significantly lower proportions than those previously reported in the literature using indirect methods.
1. Introduction
With the latest introduction of new motor oil specifications, automotive manufacturers, transportation companies and lubricant industries are constantly searching for new lubricant formulations or additional engine oil characteristics to meet the needs of the new engine tests. Two general methods used to improve fuel economy are the reduction of lubricant viscosity and the incorporation of appropriate friction modifiers. Among the latter,1 organomolybdenum compounds such as molybdenum dialkyldithiocarbamates (Mo(DTC)2) are able to generate a thin film of molybdenum disulfide (MoS2) with low shear strength between the rubbing surfaces as a result of chemical transformations,2–4 and have also proved to be substantially effective in lowering boundary frictions.5–7However, upon oil ageing, molybdenum additives undergo oxidative decomposition that leads to a significant drop in their tribological performances in the lubrication systems.8–13 According to Jensen et al.12 and Arai et al.,13 the loss of Mo(DTC)2 additives could be attributed to their thermo-oxidative degradation during oil oxidation upon engine functioning. Igarashi et al.11 also suggested that the accumulation of oil oxidation products could inhibit the formation of the MoS2 tribofilm, thus reducing the tribological performances of the oil. Given the presence of metallic parts in the engines which can act as effective oxidation catalysts, internal combustion engines can be considered as chemical reactors that are able to catalyze the oxidation processes. Therefore, engine oils are more prone to oxidation than lubricants used in other applications.
Since Mo(DTC)2 complexes decompose easily and hence loose their functionality during engine oil oxidation over time, investigation of the mechanisms of action of Mo(DTC)2 in the presence of other additives in oil, as well as the study of factors that can potentially increase their functional lifespan during engine operation are necessary in order to achieve substantial improvements of friction reduction and fuel economy of engine oils. Therefore, it is of importance to understand the nature of the interactions between molybdenum compounds and other additives, which can have either synergistic or antagonistic effects. Previous investigations have indeed shown the importance of adding other additives to the oil blend in order to reduce the loss rate of molybdenum compounds during ageing, thereby extending their useful life as efficient friction modifiers.14,15 In this respect, Minami et al.16 and Greene et al.17 reported that the addition of sulfur-containing additives improved the friction reducing properties of molybdenum compounds, and interactions between molybdenum and zinc derivatives were notably found to be extremely important with regard to fuel efficiency improvement15 and to lifespan extension of Mo(DTC)2 additives.3 Particularly, the combination of Mo(DTC)2 and zinc dithiophosphates (Zn(DTP)2) was proved to show synergistic effects on the friction reducing properties of engine oils.18–25
According to the literature, ligand exchange reactions occur when Mo(DTC)2 and Zn(DTP)2 are mixed, resulting in the formation of mixed molybdenum and zinc complexes with all possible combinations of dithiophosphate and dithiocarbamate ligands.12,26–29 These reactions are believed to activate the transformation of Mo(DTC)2 to form the MoS2 tribofilm on the rubbing surfaces.2,30,31 Other authors have, however, reported that Mo(DTC)2 complexes demonstrate a better capacity than Mo(DTP)2 complexes to generate MoS2 at the tribocontact and thus showed a better friction reducing ability,32 suggesting that enhanced capacity to form MoS2 films using mixtures of Mo(DTC)2 and Zn(DTP)2 does not exclusively result from ligand exchange reactions between Mo and Zn complexes. Yagashita et al.29 have investigated ligand exchange reactions between Mo(DTC)2 and Zn(DTP)2 and concluded that the equilibrium was strongly in favor of Mo(DTC)2 and Zn(DTP)2 and that Mo(DTP)2, Zn(DTC)2 and related complexes with mixed ligands were formed only to a very limited extent. Nevertheless, the method used to evaluate these ligand exchange reactions was not described with further details. Jensen et al.12 have documented that the ligand exchange reactions between Mo(DTC)2 and Zn(DTP)2 lead to the formation of Mo(DTP)2 and Zn(DTC)2, the formation of mixed derivatives being favored at high temperatures (160 °C) and enhanced in lubricants which have undergone thermooxidative alteration. However, the methods used by these authors to evaluate these exchange reactions were circumstantial (see Discussion section). Important ligand exchanges between Mo(DTC)2 and Zn(DTP)2 were also reported to occur at lower temperatures (80 °C) and appeared to be very sensitive to the polarity of the solvent used according to Shea et al.27
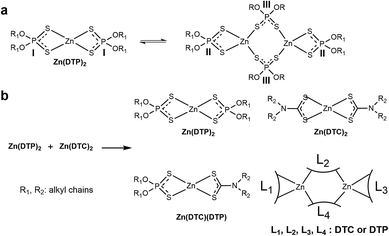
In this context, we have re-investigated the possibility and extent of ligand exchange reactions between molybdenum and zinc complexes of dialkyldithiocarbamates and dialkyldithiophosphates (Fig. 1) using an analytical approach involving NMR spectroscopy (1H, 13C, 31P) and HPLC-MS analysis. Since molybdenum and zinc derivatives are both key components used in engine oils, investigation at the molecular level of the underlying mechanisms of interactions between these compounds will be beneficial for the future improvement of energy-conserving lubricant formulations.
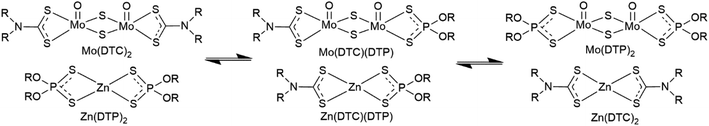 | ||
| Fig. 1 Ligand exchange reactions between Mo(DTC)2 and Zn(DTP)2 (adapted from Jensen et al.12). | ||
2. Experimental section
2.1. Materials (base oil and additives)
The commercial mineral base oil used in this work was provided by Total Marketing & Services. The chemical structures of the Mo and Zn complexes used and/or discussed in this study are shown in Fig. 2. The additives Mo(DTC)2 2a–2c (Sakuralube 525), Mo(DTC)2 2d (Sakuralube 600) and Mo(DTP)2 1 (Sakuralube 400) were purchased from Adeka, whereas Zn(DTP)2 4a–4d were purchased from Infineum. Sakuralube 525 comprises a mixture of Mo(DTC)2 2a–2c whereas Sakuralube 600 is a pure compound corresponding to Mo(DTC)2 2d (Fig. 2). Mo(DTP)2 1 (Sakuralube 400) and Zn(DTP)2 4a correspond to primary dithiophosphate complexes with C8 (ethylhexyl) alkyl chains. Secondary Zn(DTP)2 additives were purchased as a mixture of compounds 4b–4d with C3 and C6 alkyl chains. Purchased compounds were used without any further purification except for the compounds 1 and 4a which were purified by chromatography on a silica gel column prior NMR measurements in order to remove the hydrocarbon base oil and polar constituents (see Section 2.2.). Zn bis(di-n-decyldithiocarbamate) 3a was synthesized according to a published procedure (see Section 2.3.).332.2. Purification of compounds 1 and 4a
An aliquot of the commercial additive containing compound 1 (respectively compound 4a) was fractionated on a silica gel column using CH2Cl2 as eluent yielding, respectively, fraction F1 (1.2 dead volumes) containing the hydrocarbon base oil and a more polar fraction F2 (2 dead volumes) containing compound 1 (respectively compound 4a).2.3. Synthesis of Zn bis(di-n-decyldithiocarbamate) 3a
An heterogeneous mixture containing didecylamine (4.00 g, 13.44 mmol) and sodium hydroxide (811 mg, 14.45 mmol) in 11 mL of water was stirred with a magnetic stirrer at 10 °C for 10 min. Carbon disulfide (1.30 mL, 1.64 g, 21.50 mmol) was then added dropwise at 30 °C during 1 h followed by addition of 16 mL of water. Stirring was continued for 2 h, after which a solution of zinc chloride (1.39 g, 10.20 mmol) in water (6 mL) was slowly added at 40 °C in the course of 1 h. After stirring for a further 3.5 h, the reaction mixture was cooled down to room temperature and filtered using a Büchner funnel to recover the solid material formed. The yellowish solid obtained was dried under vacuum for a few hours at room temperature and subsequently filtered through silica gel using dichloromethane as eluent to get rid of the salts formed during the reaction. After removal of the solvent under reduced pressure, Zn bis(di-n-decyldithiocarbamate) 3a was obtained as a white solid in 58% yield (purity > 95%; see 1H- and 13C-NMR in ESI Fig. 5S†).1H-NMR (500 MHz; CDCl3): 3.75 (t, J = 7.8 Hz, 8H), 1.80–1.70 (m, 8H), 1.34–1.22 (m, 56H), 0.88 (t, J = 6.9 Hz, 12H). 13C-NMR (125 MHz; CDCl3): 202.6, 55.1, 32.0, 29.7, 29.6, 29.4, 29.4, 27.0, 26.93, 22.8, 14.3. Probe-MS (EI, 70 eV): m/z (relative intensity) 812 (M+, 9%), 810 (15), 808 (16), 775 (12), 436 (14), 372 (7), 340 (57), 308 (100).
2.4. Oil thermal ageing experiments
Thermal ageing experiments were carried out on lubricants using 50 g of hydrocarbon base oil samples containing molybdenum or/and zinc additives (1 weight%) at 135 °C under a stream of argon (100 mL min−1) in a multi-necked round-bottomed flask for the following studies:– Thermal stability of Mo(DTC)2 2a–2c at 135 °C.
– Ligand exchange reactions between Mo(DTC)2 2a–2c and Zn(DTC)2 3a.
Oil samples (1 mL aliquots) were collected over a period of 16–18 hours at 1 h intervals through a septum using a syringe for HPLC-MS qualitative and quantitative analysis. The first sample (defined as T = 0 h) was collected when the oil-additive mixture reached 135 °C.
2.5. Investigation of ligand exchange reactions using HPLC-MS
Each sample from oil thermal ageing experiments was prepared as follows: 20 μL of internal standard (2,3-bis(n-octadecyloxy)propan-1-ol; 1.7 mg mL−1) were added to an aliquot (20 mg) of each collected sample (1 mL) diluted in 1 mL of solvent (n-heptane/isopropanol: 95/5). The sample was then diluted by 10-fold in the same solvent before being analysed by HPLC-MS. Quantification of a given Mo(DTC)2 homologue was performed by integrating the area of the peak on the sum of the mass chromatograms corresponding to the pseudo-molecular ions [M + H]+ of the various Mo isotopologues of this compound (between 92Mo and 100Mo) (cf. ESI Fig. 1S† for the mass spectra and extracted ion chromatogram of 2a–2c) and comparison with the area of the internal standard. Absolute response factors have not been determined for the different Mo(DTC)2 complexes. Concentration calibration curves have been established for compounds 2a–2c and were shown to be linear (R2 > 0.97) in the range 0–2000 ng per injection (cf. ESI Fig. 2S†). Complexes with DTP ligands including Mo(DTP)2 1 and Zn(DTP)2 4a–4d could not be detected with our HPLC-MS method.2.6. Investigation of ligand exchange reactions using NMR
Ligand exchange reactions were performed in NMR glass tubes using CDCl3 as solvent for the experiments at 25 °C, and D8-toluene for the experiments at 105 °C. The full 1H- and 13C-NMR spectra at 25 °C of the 4 substrates 1, 2d, 3a and 4a used for these experiments are presented in ESI Fig. 3S–6S.† The partial 1H-NMR spectra (3.0–5.0 ppm) at 105 °C of the 4 substrates 1, 2d, 3a and 4a are presented in ESI Fig. 7S† and the 31P-NMR spectra at 25 °C of 1 and 4a are shown in ESI Fig. 8S.† The conditions related to the different ligand exchange experiments performed are listed in Table 1.| Substrates | Molar ratio | Temperature |
|---|---|---|
| Zn(DTC)2 3a/Zn(DTP)2 4a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−80 °C, −40 °C, −10 °C, 25 °C, 105 °C |
| Mo(DTP)2 1/Zn(DTC)2 3a | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 °C, 105 °C |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 °C, 105 °C | |
| Mo(DTC)2 2d/Zn(DTP)2 4a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
25 °C, 105 °C |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 °C, 105 °C |
2.7. High pressure liquid chromatography-mass spectrometry (HPLC-MS)
Samples were analysed by HPLC-MS using an Agilent HP 1100 series instrument equipped with an auto-injector, and connected to a Bruker Esquire 3000+ ion trap mass spectrometer. A Chemstation chromatography manager software (HPLC) and a Bruker Data Analysis software (MS) were used. Separations were achieved on a Zorbax SIL 5 μm column (Agilent, 4.6 × 250 mm) at 30 °C. The sample injection volume was set at 10 μL. Compounds were eluted with a flow rate of 0.4 mL min−1 in isocratic mode using n-heptane/isopropanol: 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 v/v as the mobile phase. The mass spectrometer was equipped with an atmospheric pressure photoionization source (APPI) used in the positive ion mode. Conditions for MS analyses were: nebulizer pressure 50 psi, APPI temperature 420 °C, drying temperature 350 °C, drying gas (N2) flow 5 L min−1, capillary voltage −2 kV, corona 4 μA, scan range m/z 400–2000. HPLC-MS analyses were performed in triplicate for each sample collected except for the experiment dealing with the investigation of the thermal stability of Mo(DTC)2 additives 2a–2c at 135 °C under argon (see Section 3.2.1.).
1.5 v/v as the mobile phase. The mass spectrometer was equipped with an atmospheric pressure photoionization source (APPI) used in the positive ion mode. Conditions for MS analyses were: nebulizer pressure 50 psi, APPI temperature 420 °C, drying temperature 350 °C, drying gas (N2) flow 5 L min−1, capillary voltage −2 kV, corona 4 μA, scan range m/z 400–2000. HPLC-MS analyses were performed in triplicate for each sample collected except for the experiment dealing with the investigation of the thermal stability of Mo(DTC)2 additives 2a–2c at 135 °C under argon (see Section 3.2.1.).
2.8. Nuclear magnetic resonance (NMR) spectroscopy
NMR spectra were recorded on a Bruker Avance I 500 MHz spectrometer (500 MHz for 1H; 125 MHz for 13C; 202 MHz for 31P) or on a Bruker Avance I 300 MHz spectrometer (121 MHz for 31P) for NMR measurements at 25 °C and on a Bruker Avance III 400 MHz spectrometer (400 MHz for 1H; 100 MHz for 13C; 162 MHz for 31P) for NMR measurements at 105 °C. The 1H- and 13C-chemical shifts are reported in ppm relative to tetramethylsilane with the residual protons and carbon atoms of the solvent used as internal standards (CDCl3: δ1H 7.26 ppm; δ13C 77.2 ppm; D8-toluene: δ1H 7.09, 7.00, 6.98, 2.09 ppm; δ13C 129.2, 128.3, 125.5, 20.4 ppm). The coupling constants (J) are expressed in Hertz (Hz). CDCl3 and D8-toluene were used for measuring NMR spectra at 25 °C and 105 °C, respectively, on 5–10 mg of molybdenum or zinc complexes diluted in ca. 0.8 mL solvent.3. Results
3.1. 1H, 13C and 31P NMR investigation of ligand exchange reactions between Mo and Zn dithiocarbamate and dithiophosphate complexes
DTC/DTP ligand exchange reactions between molybdenum and zinc complexes were investigated using 1H, 13C and 31P NMR spectroscopy at 25 °C and 105 °C (Table 1). The authentic additives Mo(DTP)2 1, Mo(DTC)2 2d, Zn(DTC)2 3a and Zn(DTP)2 4a (Fig. 2) were used as substrates. Three types of experiments were performed, involving either a mixture containing Zn(DTC)2 3a and Zn(DTP)2 4a, a mixture of Mo(DTP)2 1 and Zn(DTC)2 3a or a mixture of Mo(DTC)2 2d and Zn(DTP)2 4a at different relative molar ratios (Table 1). In the experiments with 1 + 3a or 2d + 4a, symmetrical complexes as well as related complexes with mixed dithiocarbamate and dithiophosphate ligands were expected to be formed (cf. Fig. 1).The 1H chemical shifts at 25 °C of the protons on methylene groups adjacent to the functional groups (α-OP or α-N) of the substrates are all in the 3.5–4.5 ppm range (Fig. 3) and clearly allow the various types of homoleptic complexes likely to occur during the ligand exchange reactions (i.e. Mo(DTP)2, Mo(DTC)2, Zn(DTC)2 and Zn(DTP)2) to be distinguished. For symmetry reasons, the 4 methylene groups adjacent to the functional groups of Zn(DTC)2 3a (resp. Zn(DTP)2 4a) have the same chemical shift and only 1 signal is observed for these protons at 3.75 ppm for 3a (resp. 4.07 ppm for 4a). In the case of Mo(DTC)2 2d, a complex signal at 3.90 ppm is observed for the protons of the 4 methylene groups α to the functional groups due to the fact that the 2 protons at each methylene group are diastereotopic (related to the axial chirality of Mo(DTC)2 complexes). In the case of Mo(DTP)2 1, the two ligand alkyl chains are located below and above the “median plane” of the Mo2O2S6 core of the molecule and are therefore not equivalent. Consequently, 2 signals at 3.78 and 4.49 ppm are observed for the methylene groups α to the functional groups.
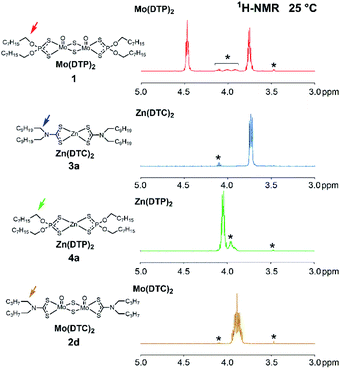 | ||
| Fig. 3 Partial 1H-NMR spectra (3.0–5.0 ppm, 500 MHz, CDCl3, 25 °C) of the reference additives 1, 2d, 3a and 4a used to investigate DTC/DTP ligand exchange reactions. *: Impurities. | ||
The NMR spectra of Mo and Zn complexes bearing mixed ligands (i.e., Mo(DTC)(DTP) and Zn(DTC)(DTP)), which are potential intermediates formed in reactions involving Mo and Zn complexes, are however more difficult to obtain, since these compounds can probably not be isolated as pure compounds due to ligand exchange reactions (i.e., equilibrium between all possible homoleptic and heteroleptic complexes). The 1H, 13C and 31P chemical shifts of DTP ligands bound to Mo (resp. Zn) mixed complexes are expected to be very close to those of DTP ligands on Mo(DTP)2 (resp. Zn(DTP)2) complexes. However, small differences may affect, notably, the chemical shifts of the phosphorus atom, the methylene protons and the carbon atoms located α to the oxygen atom from DTP bound to a Mo complex, allowing DTP from homo- and heteroleptic Mo complexes to be distinguished. The same holds for the chemical shifts of 1H and 13C from DTC ligands from Mo and Zn complexes.
Therefore, in order to investigate specifically the 1H, 13C and 31P chemical shifts of ligands from mixed complexes and to test whether the occurrence of these complexes can be detected and eventually quantified using NMR spectroscopy during ligand exchange reactions, experiments postulated to favour the formation of such mixed complexes were performed. To this aim, the evolution over time of a mixture of Zn(DTP)2 4a and Zn(DTC)2 3a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio postulated to lead to the formation of mixed Zn(DTC)(DTP) complexes was investigated by 1H, 13C and 31P NMR in CDCl3 at 25 °C. Similarly, an experiment likely to yield Mo(DTC)(DTP) complexes, for which Mo(DTP)2 1 and Zn(DTC)2 3a were mixed in a 4
1 molar ratio postulated to lead to the formation of mixed Zn(DTC)(DTP) complexes was investigated by 1H, 13C and 31P NMR in CDCl3 at 25 °C. Similarly, an experiment likely to yield Mo(DTC)(DTP) complexes, for which Mo(DTP)2 1 and Zn(DTC)2 3a were mixed in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, was carried out in CDCl3 at 25 °C in order to detect the chemical shifts of the mixed complex formed. Since the affinity of DTC has been shown to be greater for Mo than for Zn (e.g. Yagashita and Igarashi29), transfer of the DTC ligands from Zn to Mo has been postulated to occur to a significant extent. However, in the latter experiment, the formation of the mixed Mo(DTP)(DTC) complex 5a was anticipated along with the formation of Mo(DTC)2 2e, since Mo(DTP)2 1 was present in a higher molar abundance.
1 molar ratio, was carried out in CDCl3 at 25 °C in order to detect the chemical shifts of the mixed complex formed. Since the affinity of DTC has been shown to be greater for Mo than for Zn (e.g. Yagashita and Igarashi29), transfer of the DTC ligands from Zn to Mo has been postulated to occur to a significant extent. However, in the latter experiment, the formation of the mixed Mo(DTP)(DTC) complex 5a was anticipated along with the formation of Mo(DTC)2 2e, since Mo(DTP)2 1 was present in a higher molar abundance.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio should result in the formation of the mixed Zn(DTC)(DTP) complex. The 1H-NMR spectrum of the mixture after 1 h at 25 °C displayed the typical signals of the two substrates and, notably, the signal at 3.75 ppm and 4.07 ppm corresponding to the protons from the methylene groups adjacent to the functional groups (α-OP or α-N), respectively (Fig. 4a). No additional signals likely to correspond to methylene groups of DTC or DTP ligands from the mixed complex could be detected even after 24 h of reaction. Similarly, only one signal at 96.0 ppm could be detected in the 31P-NMR spectrum of the mixture (Fig. 4b) whereas one would expect specific additional P signals related to the newly-formed mixed species to appear. The same experiment was also performed in D8-toluene at 105 °C and, again, only the signals corresponding to the substrates in the 1H- and 31P-NMR spectra could be detected. These results could be explained by an extremely slow kinetic of the ligand exchange reaction, by the fact that the exchange is not thermodynamically favoured, or because the chemical shifts of 1H and 31P of the DTC and DTP ligands in the mixed complex have exactly the same values as in the symmetrical Zn complexes. Alternatively, in the 31P- and 1H-NMR spectra of the mixture, it can be envisaged that only 1 signal was observed for P and 1 signal for the 1H from the methylene groups adjacent to N or O due to the fact that the ligand exchange reaction is a rapid process on the NMR time scale.
1 molar ratio should result in the formation of the mixed Zn(DTC)(DTP) complex. The 1H-NMR spectrum of the mixture after 1 h at 25 °C displayed the typical signals of the two substrates and, notably, the signal at 3.75 ppm and 4.07 ppm corresponding to the protons from the methylene groups adjacent to the functional groups (α-OP or α-N), respectively (Fig. 4a). No additional signals likely to correspond to methylene groups of DTC or DTP ligands from the mixed complex could be detected even after 24 h of reaction. Similarly, only one signal at 96.0 ppm could be detected in the 31P-NMR spectrum of the mixture (Fig. 4b) whereas one would expect specific additional P signals related to the newly-formed mixed species to appear. The same experiment was also performed in D8-toluene at 105 °C and, again, only the signals corresponding to the substrates in the 1H- and 31P-NMR spectra could be detected. These results could be explained by an extremely slow kinetic of the ligand exchange reaction, by the fact that the exchange is not thermodynamically favoured, or because the chemical shifts of 1H and 31P of the DTC and DTP ligands in the mixed complex have exactly the same values as in the symmetrical Zn complexes. Alternatively, in the 31P- and 1H-NMR spectra of the mixture, it can be envisaged that only 1 signal was observed for P and 1 signal for the 1H from the methylene groups adjacent to N or O due to the fact that the ligand exchange reaction is a rapid process on the NMR time scale.
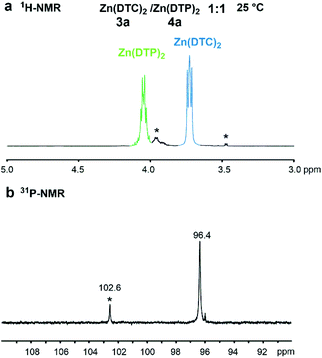 | ||
Fig. 4 Partial NMR spectra (CDCl3, 25 °C) of a mixture of Zn(DTC)2 3a and Zn(DTP)2 4a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio after 1 h reaction. (a) 1H-NMR spectrum (3.0–5.0 ppm, 500 MHz); (b) 31P-NMR spectrum (90–110 ppm, 121 MHz). Green colour: Zn(DTP)2, blue colour: Zn(DTC)2 (cf. Fig. 3). *: Impurities. 1 molar ratio after 1 h reaction. (a) 1H-NMR spectrum (3.0–5.0 ppm, 500 MHz); (b) 31P-NMR spectrum (90–110 ppm, 121 MHz). Green colour: Zn(DTP)2, blue colour: Zn(DTC)2 (cf. Fig. 3). *: Impurities. | ||
In this respect, it should be noted that Harrison et al.34 have shown that Zn(DTP)2 complexes in solution exist as a mixture of monomers and dimers in equilibrium (Fig. 5a), but these various types of DTP ligands cannot be resolved by NMR at room temperature because the exchange between the different forms of complexes is too rapid on the NMR time scale. These forms can, however, be revealed by 31P-NMR measurements at low temperatures. A similar situation might occur with the mixture of Zn(DTC)2 3a and Zn(DTP)2 4a in which several complexes, comprising the monomer and dimer complexes related to Zn(DTP)2 reported by Harrison et al.34 likely co-exist, but also analogous structures for which DTP ligands are replaced by DTC ligands.
 | ||
| Fig. 5 (a) Monomeric and dimeric forms of Zn(DTP)2 complexes in equilibrium in solution (adapted from Harrison et al.34); (b) various monomeric and dimeric complexes in equilibrium in solution formed by reaction of Zn(DTP)2 with Zn(DTC)2. For P atoms, at least 8 types of different chemical environments are potentially distinguishable by 31P NMR. I, II, and III (a) correspond to P atoms with 3 different chemical environments distinguishable by 31P-NMR at low temperature. | ||
We have, therefore, performed temperature dependant 1H- and 31P-NMR measurements at −10 °C, −40 °C and −80 °C in D8-toluene. At −80 °C, at least 6 31P-NMR signals (vs. 1 broad signal at ca. 97.4 ppm at 25 °C) were detected at 104.7, 104.4, 104.1, 103.9, 100.7, 100.3 ppm (Fig. 6), thus indicating that magnetically equivalent 31P from DTP ligands at 25 °C are resolved at the low temperature of −80 °C.
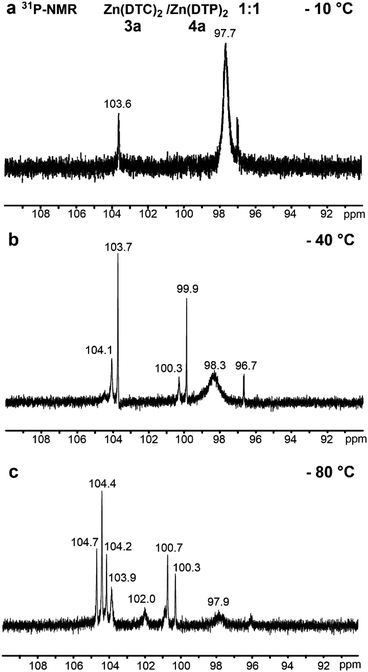 | ||
Fig. 6 Partial 31P-NMR spectra (90–110 ppm, 243 MHz, D8-toluene) of a mixture of Zn(DTC)2 3a and Zn(DTP)2 4a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio after 24 h at (a) −10 °C; (b) −40 °C; (c) −80 °C. 1 molar ratio after 24 h at (a) −10 °C; (b) −40 °C; (c) −80 °C. | ||
The number of signals detected clearly indicates that they are not only related to the monomer and dimer complexes reported by Harrison et al.,34 but most likely also comprise additional monomeric and dimeric structures for which DTP ligands are replaced by DTC ligands (Fig. 5b). Our 31P-NMR investigation of the mixture of 3a and 4a thus clearly showed that ligand exchange reactions occur between both types of complexes at 25 °C, that both monomeric and dimeric structures exist (Fig. 5b and c) and that this exchange is a fast process on the NMR time scale.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and the evolution of the two compound mixtures over time was followed by 1H-, 13C- and 31P-NMR at 25 °C (in CDCl3) and 105 °C (in D8-toluene). Since the affinity of DTC has been suggested to be greater for Mo than for Zn (e.g. Yagashita and Igarashi29), transfer of the DTC ligands from Zn to Mo complexes was expected to occur to a significant extent. In particular, in the experiment for which 1 and 3a were mixed in a 4
1 molar ratio and the evolution of the two compound mixtures over time was followed by 1H-, 13C- and 31P-NMR at 25 °C (in CDCl3) and 105 °C (in D8-toluene). Since the affinity of DTC has been suggested to be greater for Mo than for Zn (e.g. Yagashita and Igarashi29), transfer of the DTC ligands from Zn to Mo complexes was expected to occur to a significant extent. In particular, in the experiment for which 1 and 3a were mixed in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, the formation of both Mo(DTP)(DTC) 5a and Mo(DTC)2 2e complexes in significant proportions along with Zn(DTP)2 4a was anticipated.
1 molar ratio, the formation of both Mo(DTP)(DTC) 5a and Mo(DTC)2 2e complexes in significant proportions along with Zn(DTP)2 4a was anticipated.
Mixture of 1 and 3a in a 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 molar ratio at 25 °C and 105 °C. The results of the 1H-NMR analyses carried out on the mixture of 1 and 3a in a 4
1 molar ratio at 25 °C and 105 °C. The results of the 1H-NMR analyses carried out on the mixture of 1 and 3a in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio after 0.5 h, 18 h and 47 h at 25 °C, focusing on the methylene protons located α of the functional groups, are shown in Fig. 7. The formation of new compounds was already observed after 0.5 h of reaction (Fig. 7a), their relative amounts progressively increasing with time. The 1H-NMR spectra obtained after 18 h and 47 h were almost identical (Fig. 7b and c), indicating that an equilibrium between the various complexes in solution was already reached after 18 h.
1 molar ratio after 0.5 h, 18 h and 47 h at 25 °C, focusing on the methylene protons located α of the functional groups, are shown in Fig. 7. The formation of new compounds was already observed after 0.5 h of reaction (Fig. 7a), their relative amounts progressively increasing with time. The 1H-NMR spectra obtained after 18 h and 47 h were almost identical (Fig. 7b and c), indicating that an equilibrium between the various complexes in solution was already reached after 18 h.
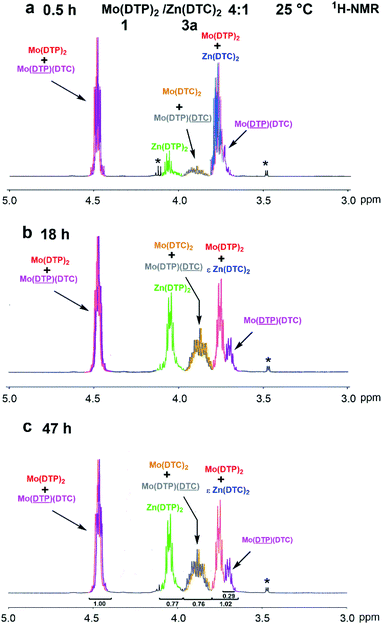 | ||
Fig. 7 Partial 1H-NMR spectra (3.0–5.0 ppm, 500 MHz, CDCl3, 25 °C) of a mixture of Mo(DTP)2 1 and Zn(DTC)2 3a in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio after (a) 0.5 h; (b) 18 h; (c) 47 h. *: Impurities. 1 molar ratio after (a) 0.5 h; (b) 18 h; (c) 47 h. *: Impurities. | ||
The formation of Mo complexes with DTC ligands comprising Mo(DTC)2 2e and Mo(DTC)(DTP) 5a was evidenced by the comparison between the chemical shifts of the 1H-NMR spectra of the reaction mixture and of the reference compounds (Fig. 3 and ESI Fig. 3Sa–6Sa†). Indeed, the signal at 3.90 ppm (Fig. 7; orange colour) presents strong similarities (i.e., comparable chemical shifts and coupling pattern) with that of the methylene groups adjacent to the nitrogen atoms of Mo(DTC)2 2d bearing butyl alkyl chains (ESI Fig. 4Sa†).
As already mentioned, the chemical shifts of methylene protons located α to N-CS2 bonds of DTC from homoleptic or heteroleptic Mo complexes are expected to be very close or even identical. In parallel, the formation of Zn complexes with DTP ligands was evidenced by the appearance of a signal at 4.07 ppm (Fig. 7; green colour) which is similar to that observed for protons of the methylenes adjacent to the O–P bond of the reference complex 4a (Fig. 3 and ESI Fig. 6Sa†). Interestingly, the signals at 3.90 and 4.07 ppm have, as expected, the same integral value since to each DTP ligand on a Zn complex must correspond one DTC ligand transferred to a Mo complex. This further supports the hypothesis that protons from DTC of the mixed complex 5a have chemical shifts almost identical to those of DTC in Mo(DTC)2 2e. As shown by the measured integrals, this signal must indeed comprise the signals of all DTC ligands from both Mo(DTC)2 2e and Mo(DTC)(DTP) 5a.
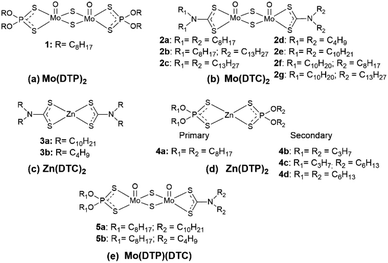
The remaining signals in the 1H-NMR spectrum of the mixture of Mo(DTP)2 1 and Zn(DTC)2 3a after 18 h and 47 h (Fig. 7b and c; red and purple colours) correspond most likely to protons located α to the O–P bonds in DTP from Mo complexes. The signals at 4.49 ppm and 3.78 ppm are identical to those detected for the Mo(DTP)2 reference compound 1 (Fig. 3) and correspond to the alkyl chains of DTP ligands on both sides of the MoO2S6 core. The signal at 3.78 ppm (red colour) is, however, accompanied by a slightly shifted signal centred around 3.70 ppm (Fig. 7b and c; purple colour). Remarkably, the integral value of the signal at 4.49 ppm is almost identical to that corresponding to the sum of the signals at 3.78 and 3.70 ppm. On this basis, it can be proposed that the signals at 3.78 and 3.70 ppm correspond, respectively, to the protons of the methylenes adjacent to the O–P bond from the alkyl chain located below or above the “median plane” of the Mo2O2S6 core from, respectively, Mo(DTP)2 and Mo(DTP)(DTC) complexes 1 and 5a, whereas the signal at 4.49 ppm corresponds to the superimposition of the signal from protons of the methylene groups located α to the O–P bonds from the alkyl chain located on the other side of the MoO2S6 core from both Mo(DTP)2 and Mo(DTP)(DTC) complexes 1 and 5a. This therefore indicates that mixed Mo complexes are indeed present in the reaction mixture and can be distinguished by 1H-NMR spectroscopy. This is further supported by the interpretation of the 31P-NMR spectrum which clearly showed the presence of two signals at 96.0 and 112.2 ppm corresponding to P from Zn(DTP)2 and Mo(DTP)2 complexes 4a and 1, respectively (cf. 31P-NMR of standards, ESI Fig. 8S†), as well as a new signal at 112.8 ppm which can be ascribed to P from the mixed complex 5a (Fig. 8a). The occurrence of the mixed Mo complex 5a also appeared in the 13C-NMR spectrum from the reaction mixture after 18 h and 47 h (Fig. 8b). Indeed, the signals corresponding to the C atoms from the methylene groups located α to the O–P bonds from Mo(DTP)2 1 (cf. ESI Fig. 3Sb†) on both side of the MoO2S6 core at 73.1 and 71.6 ppm are accompanied by two smaller signals at, respectively, 73.0 and 71.4 ppm which potentially correspond to the same carbon atoms of DTP ligands on mixed Mo(DTC)(DTP) complexes (Fig. 8b). Each signal appears as a doublet due to the presence of an asymmetric centre on the ethylhexyl alkyl chain. Our results thus clearly showed that mixed Mo(DTC)(DTP) complexes can be directly and unambiguously detected by 1H-, 13C- and 31P-NMR spectroscopy. Based on the values of the integrals measured for the methylene protons located α to the O–P bonds in Mo complexes, the relative proportion of Mo(DTP)2 and Mo(DTP)(DTC) complexes 1 and 5a corresponded to 55% and 45%, respectively, in the reaction mixture after 47 h.
In addition, the complex 3a has apparently almost completely disappeared after 47 h. Indeed, the signal corresponding to the methylene protons adjacent to N–CS2 bonds from DTC on Zn complexes (cf. 1H-NMR spectrum of 3a, Fig. 3) is normally superimposed with that of the signal at 3.78 ppm corresponding to the methylene protons located α to O–P bonds of DTP alkyl chains located on one side of Mo(DTP)2 complexes. Since the value of the integrated signals at 3.78 and 3.70 ppm corresponding to DTP alkyl chains on the same side of the MoO2S6 core from, respectively, Mo(DTP)2 and Mo(DTP)(DTC) complexes 1 and 5a ppm (Fig. 7b and c; red and purple colours) is identical to that of the DTP methylene protons at 4.49 ppm (Fig. 7b and c; red and purple colours) corresponding to DTP alkyl chains on the other side of the MoO2S6 core, it can be concluded that the contribution of protons from Zn(DTC)2 to this signal is extremely small.
An experiment using the same mixture of 1 and 3a in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was also performed in D8-toluene at 105 °C. Fig. 9 shows the chemical shifts of the 1H-NMR signals between 3.5–4.5 ppm from the reaction mixture. By comparison with the 1H-NMR spectra of the reference compounds 1, 2d, 3a and 4a measured under the same conditions (ESI Fig. 7S†), it appeared that the ligand exchange reactions between Mo(DTP)2 and Zn(DTC)2 took place at a much faster rate at 105 °C (as compared to 25 °C) and that a very similar composition of the reaction mixture as that obtained at 25 °C was observed after only 15 minutes (vs. 18 h; see above). In particular, the specific signals corresponding to the methylene protons located α to O–P bonds of DTP from the mixed complex 5a were observed for both types of DTP ligands alkyl chains (above and below the MoO2S6 core). The formation of the mixed complex 5a could also be evidenced by 31P-NMR spectroscopy (ESI Fig. 9S†). Interpretation of the integral values from the 1H-NMR spectrum showed that the relative proportions of the different complexes in D8-toluene at 105 °C were close to those observed in CDCl3 at 25 °C and consequently, neither the nature of the solvent nor the temperature seem to have an important effect on the composition of the reaction mixture. A higher temperature seemed, however, to slightly favour the formation of the mixed complex.
1 molar ratio was also performed in D8-toluene at 105 °C. Fig. 9 shows the chemical shifts of the 1H-NMR signals between 3.5–4.5 ppm from the reaction mixture. By comparison with the 1H-NMR spectra of the reference compounds 1, 2d, 3a and 4a measured under the same conditions (ESI Fig. 7S†), it appeared that the ligand exchange reactions between Mo(DTP)2 and Zn(DTC)2 took place at a much faster rate at 105 °C (as compared to 25 °C) and that a very similar composition of the reaction mixture as that obtained at 25 °C was observed after only 15 minutes (vs. 18 h; see above). In particular, the specific signals corresponding to the methylene protons located α to O–P bonds of DTP from the mixed complex 5a were observed for both types of DTP ligands alkyl chains (above and below the MoO2S6 core). The formation of the mixed complex 5a could also be evidenced by 31P-NMR spectroscopy (ESI Fig. 9S†). Interpretation of the integral values from the 1H-NMR spectrum showed that the relative proportions of the different complexes in D8-toluene at 105 °C were close to those observed in CDCl3 at 25 °C and consequently, neither the nature of the solvent nor the temperature seem to have an important effect on the composition of the reaction mixture. A higher temperature seemed, however, to slightly favour the formation of the mixed complex.
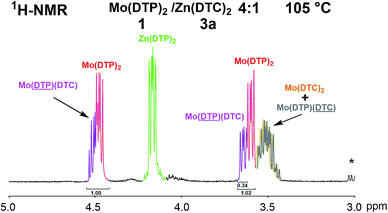 | ||
Fig. 9 Partial 1H-NMR spectrum (3.0–5.0 ppm, 500 MHz, D8-toluene, 105 °C) of a mixture of Mo(DTP)2 1 and Zn(DTC)2 3a in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio after 15 min. *: Impurities. 1 molar ratio after 15 min. *: Impurities. | ||
Mixture of 1 and 3a in a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 molar ratio at 25 °C and 105 °C. Based on the interpretation of the 1H-NMR spectra, the reaction between 1 and 3a in solution in a 1
1 molar ratio at 25 °C and 105 °C. Based on the interpretation of the 1H-NMR spectra, the reaction between 1 and 3a in solution in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio led predominantly to the stoichiometric formation of Mo(DTC)2 and Zn(DTP)2 complexes 2e and 4a at 25 °C (ESI Fig. 10S†). The ligand exchange reaction between the two substrates was relatively fast, and ligand exchange products were already observed 1 h after mixing 1 and 3a. The characteristic 1H-NMR signals from 1 and 3a decreased over time while those of Mo(DTC)2 2e and Zn(DTP)2 4a increased. After 95 h at 25 °C, 1 and 3a had almost totally disappeared, leaving large amounts of 2e and 4a in the solution. The small signals at, respectively, 4.49 ppm and 3.70 ppm (ESI Fig. 10Sc;† purple colour) could be ascribed to minor DTP from Mo complexes, mainly as the mixed Mo(DTP)(DTC) complex 5a (cf. Fig. 7). This was further confirmed by the presence of a signal at 112.8 ppm in the 31P-NMR spectrum of the reaction mixture after 95 h (ESI Fig. 11S†) as well as by the small signals at 73.0 and 71.4 ppm in the 13C-NMR spectrum (cf. Fig. 8b). Similar results were obtained when the reaction was performed in D8-toluene at 105 °C.
1 molar ratio led predominantly to the stoichiometric formation of Mo(DTC)2 and Zn(DTP)2 complexes 2e and 4a at 25 °C (ESI Fig. 10S†). The ligand exchange reaction between the two substrates was relatively fast, and ligand exchange products were already observed 1 h after mixing 1 and 3a. The characteristic 1H-NMR signals from 1 and 3a decreased over time while those of Mo(DTC)2 2e and Zn(DTP)2 4a increased. After 95 h at 25 °C, 1 and 3a had almost totally disappeared, leaving large amounts of 2e and 4a in the solution. The small signals at, respectively, 4.49 ppm and 3.70 ppm (ESI Fig. 10Sc;† purple colour) could be ascribed to minor DTP from Mo complexes, mainly as the mixed Mo(DTP)(DTC) complex 5a (cf. Fig. 7). This was further confirmed by the presence of a signal at 112.8 ppm in the 31P-NMR spectrum of the reaction mixture after 95 h (ESI Fig. 11S†) as well as by the small signals at 73.0 and 71.4 ppm in the 13C-NMR spectrum (cf. Fig. 8b). Similar results were obtained when the reaction was performed in D8-toluene at 105 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 2
4 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios were performed at 25 °C (CDCl3) and at 105 °C (D8-toluene) and their evolution over time was followed by 1H, 13C and 31P NMR spectroscopy.
1 molar ratios were performed at 25 °C (CDCl3) and at 105 °C (D8-toluene) and their evolution over time was followed by 1H, 13C and 31P NMR spectroscopy.
Mixture of 2d and 4a in a 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 molar ratio at 25 °C. ESI Fig. 12Sa† shows the partial 1H NMR spectrum of the mixture of 2d and 4a in a 2
1 molar ratio at 25 °C. ESI Fig. 12Sa† shows the partial 1H NMR spectrum of the mixture of 2d and 4a in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio after 24 h at 25 °C. Comparison with the reference spectra (Fig. 3) clearly showed that Mo(DTP)2 1 and Zn(DTC)2 3b were not formed within this frame of time. Formation of the related mixed Mo complex 5b was not observed according to the 1H-NMR spectrum, but traces of the typical signal at 112.8 ppm from the mixed complex 5b was detected in the 31P-NMR spectrum of the reaction mixture (ESI Fig. 12Sb†). Our results thus indicate that ligand exchange reactions between these two types of complexes did not take place to a significant extent and that the amounts of 5b formed were extremely low under the conditions used. This likely reflects the stability of Mo complexes bearing dithiocarbamate ligands as already observed with the experiments involving Mo(DTP)2 and Zn(DTC)2. Our results might also be explained by the fact that the kinetic of ligand exchange reactions between 2d and 4a is extremely slow at 25 °C.
1 molar ratio after 24 h at 25 °C. Comparison with the reference spectra (Fig. 3) clearly showed that Mo(DTP)2 1 and Zn(DTC)2 3b were not formed within this frame of time. Formation of the related mixed Mo complex 5b was not observed according to the 1H-NMR spectrum, but traces of the typical signal at 112.8 ppm from the mixed complex 5b was detected in the 31P-NMR spectrum of the reaction mixture (ESI Fig. 12Sb†). Our results thus indicate that ligand exchange reactions between these two types of complexes did not take place to a significant extent and that the amounts of 5b formed were extremely low under the conditions used. This likely reflects the stability of Mo complexes bearing dithiocarbamate ligands as already observed with the experiments involving Mo(DTP)2 and Zn(DTC)2. Our results might also be explained by the fact that the kinetic of ligand exchange reactions between 2d and 4a is extremely slow at 25 °C.
Mixture of 2d and 4a in a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 4 molar ratio at 25 °C and 105 °C. The same experiment at 25 °C (CDCl3) using 2d and 4a in a 1
4 molar ratio at 25 °C and 105 °C. The same experiment at 25 °C (CDCl3) using 2d and 4a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
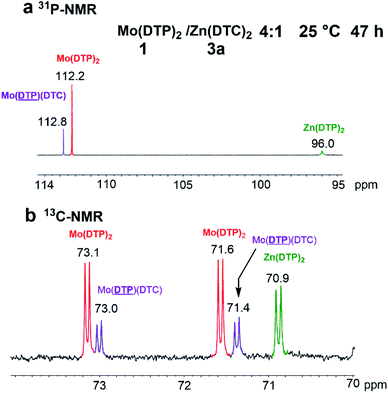
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio led to similar results. However, when this experiment was performed at 105 °C (D8-toluene), typical signals at 4.50 and 3.63 ppm corresponding to protons from methylenes adjacent to the oxygen atoms from DTP of Mo(DTP)(DTC) 5b as well as a signal at 3.57 ppm potentially corresponding to methylene protons adjacent to the functionality from DTC on Zn complexes were detected after 15 min of reaction (Fig. 10a).
4 molar ratio led to similar results. However, when this experiment was performed at 105 °C (D8-toluene), typical signals at 4.50 and 3.63 ppm corresponding to protons from methylenes adjacent to the oxygen atoms from DTP of Mo(DTP)(DTC) 5b as well as a signal at 3.57 ppm potentially corresponding to methylene protons adjacent to the functionality from DTC on Zn complexes were detected after 15 min of reaction (Fig. 10a).
It is worth noting that the value corresponding to the sum of the integral of the proton signals corresponding to DTP methylenes on mixed Mo complexes was identical to that measured for the signal at 3.57 ppm from DTC on Zn complexes, since each DTP migrated from Zn to Mo must correspond to one DTC which has migrated from Mo to Zn complexes. Calculation based on the values of the integrals of the signals corresponding to DTP and DTC from Mo complexes allowed to evaluate that Mo(DTP)(DTC) 5b represented ca. 16% and Mo(DTC)2 2d 84% of the Mo complexes in the solution.
The formation in rather low amounts of the mixed Mo(DTP)(DTC) complex 5b was further evidenced by 31P-NMR as shown by the occurrence of the typical signal at 112.8 ppm (Fig. 10b). After heating at 105 °C, the mixture was cooled down to 25 °C, redissolved in CDCl3 and the 1H- and 31P-NMR spectra re-measured. The typical signals of DTP from the mixed Mo(DTP)(DTC) complex 5b and of DTC from Zn complexes were detected. Furthermore, the relative contribution of 5b to the total Mo complexes was in the same range as that determined at 105 °C. It is therefore likely that the Mo(DTP)(DTC) 5b detected at 25 °C correspond in fact to the complex formed at 105 °C and that reequilibration at low temperature did not occur for kinetic reasons.
3.2. Study of the interactions between Mo(DTC)2 and zinc additives using HPLC-MS
A novel analytical method allowing detection and quantification of Mo(DTC)2 additives by normal phase HPLC-MS was developed (see Section 2.7.). Ionization was achieved using an APPI source. Under the HPLC conditions used, Mo(DTC)2 complexes with various alkyl chain lengths are partly separated and can be selectively detected based on their pseudo-molecular ions [M + H]+. On the contrary, dithiophosphate complexes (i.e., Mo(DTP)2, Zn(DTP)2) cannot be detected using this method.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
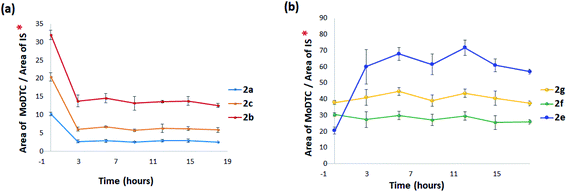
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) in a hydrocarbon base oil at 135 °C was carried out. The evolution of the composition of the reaction mixture was followed by HPLC-MS, all the substrates (2a–2c and 3a) and the postulated DTC complexes formed by ligand exchange reactions being detectable using our analytical method. It could thus be determined that new Mo complexes were already formed at T = 0 h (i.e., when the temperature of the reaction mixture just reached 135 °C). They comprised Mo(DTC)2 with two DTC ligands based on di n-decylamine 2e together with the asymmetric Mo complexes 2f and 2g.
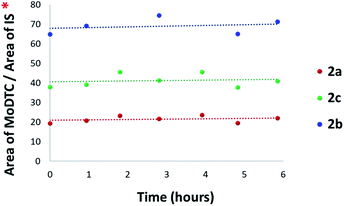
2 molar ratio) in a hydrocarbon base oil at 135 °C was carried out. The evolution of the composition of the reaction mixture was followed by HPLC-MS, all the substrates (2a–2c and 3a) and the postulated DTC complexes formed by ligand exchange reactions being detectable using our analytical method. It could thus be determined that new Mo complexes were already formed at T = 0 h (i.e., when the temperature of the reaction mixture just reached 135 °C). They comprised Mo(DTC)2 with two DTC ligands based on di n-decylamine 2e together with the asymmetric Mo complexes 2f and 2g.HPLC-MS relative quantification of the various Mo(DTC)2 homologues clearly showed a rapid decrease of the concentrations of the Mo(DTC)2 substrates 2a–2c (Fig. 12a) and the increase of the newly formed Mo(DTC)2 2e–2g over time (Fig. 12b) (cf. ESI Fig. 13S† for the mass spectra of 2e–2g). Since we have determined that Mo(DTC)2 are thermally stable under our experimental conditions (cf. Section 3.2.1.), the decrease of the concentrations of the initial Mo(DTC)2 most likely resulted from ligand exchange reactions. The rapid decrease of concentration of 2a–2c mainly occurred during the 3 first hours of the experiment whereas that of compound 2e progressively increased, clearly showing that this ligand exchange reaction is a rather fast process at 135 °C, though not instantaneous. After 3 h, the concentration of the various Mo(DTC)2 homologues remained stable, suggesting that an equilibrium between the various Mo(DTC)2 species has been reached.
The ligand exchange reactions between Mo(DTC)2 2a–2c and Zn(DTC)2 3a have also been evaluated at room temperature in cyclohexane. No ligand exchange products were formed during the first hour of the experiment. The mixture was then left at room temperature for another 6 h and was analysed again by HPLC-MS. This time, newly formed Mo(DTC)2 2e–2g were detected, but in very small amounts. Afterwards, the amounts of 2e–2g increased very slowly over a period of 18 h. Consequently, it appears that the ligand exchange reactions between Mo(DTC)2 2a–2c and Zn(DTC)2 3a also takes place, but the kinetic of this exchange is slow at room temperature.
4. Discussion
By combining our 1H-, 13C- and 31P-NMR results, it clearly appears that the DTC/DTP ligand exchange reactions between molybdenum and zinc complexes goes strongly into favor of the migration of the DTC ligands from Zn complexes towards Mo complexes and of the formation of Mo(DTC)2 and Zn(DTP)2 complexes at 25 °C and 105 °C. Molybdenum showed indeed a greater affinity for dithiocarbamate vs. dithiophosphate ligands. It could also be determined that DTP and DTC ligands from Zn complexes are able to exchange rapidly at the NMR time scale. It is therefore not possible to detect specifically heteroleptic Zn complexes using NMR spectroscopy at room temperature.On the other hand, based on the ligand exchange reactions involving Mo(DTP)2 and Zn(DTC)2, it could be established that Mo complexes bearing mixed DTP and DTC ligands were formed and could be detected using 1H-, 13C- and 31P-NMR measurements. In the case of the reactions between Mo(DTP)2 and Zn(DTC)2, ligand exchange is a fast process, which is accelerated with increasing temperature (i.e., within 18 h at 25 °C vs. 15 min at 105 °C), and goes to completion until all the DTC ligands from Zn complexes have migrated to Mo complexes (depending on the relative concentration of the substrates). Mo(DTC)2 and Zn(DTP)2 complexes are formed together with mixed Mo(DTC)(DTP) complexes, the latter even becoming a predominant species depending on the relative proportions of each substrate. Consequently, in the case of experiments involving both Mo(DTC)2 and Zn(DTP)2 -an association of additives frequently used in formulated lubricants-, the formation of Mo(DTP)2 complexes was not observed regardless of the relative concentrations of the substrates. When a large excess of Zn(DTP)2 relative to Mo(DTC)2 species was used (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio), the formation of the mixed Mo(DTC)(DTP) complex in D8-toluene at 105 °C was unambiguously observed using 1H- and 31P-NMR measurements, the relative amounts of the mixed complex representing ca. 16% of all of the Mo complexes. This value is much lower than the proportions reported by Shea and Stipanovic27 with up to 80% conversion of Mo(DTC)2 obtained by reaction with Zn(DTP)2 at 80 °C in toluene. However, in our experiments, we did not detect the formation of this mixed complex at a lower temperature (25 °C, in CDCl3) even after 24 h of reaction, possibly due to the slow kinetics of the ligand exchange at this temperature, or because the equilibrium does not favor the formation of this compound at this temperature.
1 molar ratio), the formation of the mixed Mo(DTC)(DTP) complex in D8-toluene at 105 °C was unambiguously observed using 1H- and 31P-NMR measurements, the relative amounts of the mixed complex representing ca. 16% of all of the Mo complexes. This value is much lower than the proportions reported by Shea and Stipanovic27 with up to 80% conversion of Mo(DTC)2 obtained by reaction with Zn(DTP)2 at 80 °C in toluene. However, in our experiments, we did not detect the formation of this mixed complex at a lower temperature (25 °C, in CDCl3) even after 24 h of reaction, possibly due to the slow kinetics of the ligand exchange at this temperature, or because the equilibrium does not favor the formation of this compound at this temperature.
It should however be noted that the kinetics of the ligand exchange reactions might also depend on the nature of the solvent and could be significantly slower in a viscous medium such as a lubricant base oil. The kinetics of ligand exchange might also depend on the type of base oil or polarity of the solvent as proposed by Shea and Stipanovic27 or on the occurrence of impurities such as dithiocarbamates or dithiophosphates of monovalent metals which might catalyse the exchange of ligands.
The possibility of ligand exchange reactions between Mo(DTC)2 and Zn(DTP)2, which were postulated to play a role in the synergetic enhancement of the tribological properties of lubricant containing both types of additives, has been investigated previously by several authors.12,26–29,35 The present study, based on 1H, 13C and 31P NMR detection of the different Mo and Zn complexes, indicates that ligand exchange between Mo(DTC)2 and Zn(DTP)2 complexes occurs to a rather limited extent at 105 °C, provided that Zn(DTP)2 species are largely predominant. This contrasts with the findings from Jensen et al.12,35 and Shea and Stipanovic,27 in particular, which suggest that such exchanges might occur to an important rate, notably in lubricants which have undergone important oxidative alteration.
Jensen et al.12,35 have indeed documented ligand exchange between Mo(DTC)2 and Zn(DTP)2 complexes based on laboratory experiments involving both types of complexes at a high temperature (160 °C) either under an inert atmosphere or under oxidative conditions. In these experiments, the substrates, molybdenum di(2-ethylhexyl)dithiocarbamate [Mo(ehdtc)2] and zinc di(n-octyl)dithiophosphate [Zn(odtp)2], were used in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 molar ratio. Quantification of Mo complexes with odtp ligands resulting from the ligand exchange reaction was however shown to be challenging since Mo(odtp)2 and Mo(odtp)(ehdtc) could not be directly detected using the HPLC method developed by these authors. An indirect method was therefore developed based on the postulated efficient exchange between DTP and DTC ligands in the case of Mo complexes. To measure the concentrations of Mo(odtp)2 and Mo(odtp)(ehdtc), an excess of zinc di(n-butyl) dithiocarbamate [Zn(bdtc)2] was added into the reaction mixture at 160 °C, assuming a selective and quantitative exchange the odtp ligands from Mo complexes by di(n-butyl) dithiocarbamate ligands (bdtc) analyzable by HPLC. Thus, the resulting Mo(bdtc)2 and Mo(bdtc)(ehdtc) species could indeed be identified and quantified, and were shown to represent up to ca. 20% of the Mo complexes at 160 °C under inert atmosphere. When carried out under oxidative conditions, the Mo derivatives with bdtc ligands represented even more than 90% of the Mo complexes after 4 h. However, the approach used by Jensen et al.12 has two main flaws. Indeed, the ligand exchange step implies that this exchange must be selective and quantitative regarding the exchange between odtp ligands from Mo complexes by bdtc ligands. This assumption is of course questionable since ligand exchange reactions affecting Mo complexes and involving dithiocarbamates and dithiophosphates were precisely the processes investigated in the study. Furthermore, the addition of Zn(bdtc)2 in a large excess into the reaction mixture likely induces ligand exchange with the unreacted Mo(ehdtc)2 complex to form Mo(bdtc)2 and Mo(bdtc)(ehdtc) species as unambiguously demonstrated in our study. Therefore, it is more than likely that the amounts of Mo(odtp)2 and/or Mo(odtp)(ehdtc) complexes formed by ligand exchange reaction between Mo(DTC)2 and Zn(DTP)2 complexes and indirectly quantified by Jensen et al.12 were at least overestimated.
5.5 molar ratio. Quantification of Mo complexes with odtp ligands resulting from the ligand exchange reaction was however shown to be challenging since Mo(odtp)2 and Mo(odtp)(ehdtc) could not be directly detected using the HPLC method developed by these authors. An indirect method was therefore developed based on the postulated efficient exchange between DTP and DTC ligands in the case of Mo complexes. To measure the concentrations of Mo(odtp)2 and Mo(odtp)(ehdtc), an excess of zinc di(n-butyl) dithiocarbamate [Zn(bdtc)2] was added into the reaction mixture at 160 °C, assuming a selective and quantitative exchange the odtp ligands from Mo complexes by di(n-butyl) dithiocarbamate ligands (bdtc) analyzable by HPLC. Thus, the resulting Mo(bdtc)2 and Mo(bdtc)(ehdtc) species could indeed be identified and quantified, and were shown to represent up to ca. 20% of the Mo complexes at 160 °C under inert atmosphere. When carried out under oxidative conditions, the Mo derivatives with bdtc ligands represented even more than 90% of the Mo complexes after 4 h. However, the approach used by Jensen et al.12 has two main flaws. Indeed, the ligand exchange step implies that this exchange must be selective and quantitative regarding the exchange between odtp ligands from Mo complexes by bdtc ligands. This assumption is of course questionable since ligand exchange reactions affecting Mo complexes and involving dithiocarbamates and dithiophosphates were precisely the processes investigated in the study. Furthermore, the addition of Zn(bdtc)2 in a large excess into the reaction mixture likely induces ligand exchange with the unreacted Mo(ehdtc)2 complex to form Mo(bdtc)2 and Mo(bdtc)(ehdtc) species as unambiguously demonstrated in our study. Therefore, it is more than likely that the amounts of Mo(odtp)2 and/or Mo(odtp)(ehdtc) complexes formed by ligand exchange reaction between Mo(DTC)2 and Zn(DTP)2 complexes and indirectly quantified by Jensen et al.12 were at least overestimated.
5. Conclusions
Despite of being widely employed in commercial lubricants, a comprehensive description of Mo(DTC)2 interactions with other additives at the molecular level is still lacking. Moreover, the introduction of new engine tests has led to a strong interest in understanding the molecular transformations of the additives from engine oils. In this context, our study was dedicated to the investigation of the interaction between molybdenum and zinc derivatives using an analytical approach involving NMR spectroscopy (1H, 13C, 31P) and a specifically-developed HPLC-MS method permitting the analysis of Mo complexes bearing DTC ligands. This approach allowed, notably, direct investigation of both homoleptic and heteroleptic Mo complexes formed in experiments involving DTC and DTP complexes of Mo and Zn at 25 °C, 105 °C and 135 °C.Our results indicate that fast exchange of both DTC and DTP ligands between Zn complexes occur at room temperature and above, mixtures of DTP and DTC complexes of Zn most likely comprising homo- and heteroleptic complexes as well as dimeric structures. It could be shown also that ligand exchange reactions between Mo(DTP)2 and Zn(DTC)2 strongly favor the migration of DTC ligands from Zn to Mo, illustrating the higher affinity of Mo for DTC ligands at both 25 °C and 105 °C. The kinetic is, however, significantly faster at 105 °C. Mixed Mo(DTC)(DTP) complexes which can be specifically detected by 1H-, 13C- and 31P-NMR can become predominant species in experiments with a high Mo(DTP)2/Zn(DTC)2 ratio. Ligand exchange between Mo(DTC)2 and Zn(DTC)2 was shown to be a relatively fast process, but not immediate, at 135 °C.
In the case of binary systems involving Mo(DTC)2 and Zn(DTP)2 additives frequently used in formulated lubricants, the formation of small amounts of Mo(DTC)(DTP) mixed complexes could be evidenced for the first time by direct analytical methods. The proportions observed were however significantly lower than those previously reported in the literature. These exchange reactions are operative at high temperature (105 °C) but seem to be inhibited at low temperatures (25 °C). It can be inferred from our results that these Mo(DTC)(DTP) mixed species can indeed be formed in engines and could account, at least to some extent, to synergistic effects of Mo(DTC)2 and Zn(DTP)2 on the friction reducing properties of engine oils.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Total Marketing Services for funding this research work that was carried out at the University of Strasbourg and Total Solaize Research Center (CRES), and for supplying the base oils and the additives used in this study. The authors also thank Maurice Coppe and Bruno Vincent from the NMR Department of the University of Strasbourg, and E. Motsch for her help in the MS analyses. Y. M. K. thanks Total Marketing Services and the French “Ministère de l'Enseignement Supérieur, de la Recherche et de l'Innovation” for a doctoral fellowship (CIFRE).Notes and references
- H. A. Spikes, Friction modifier additives, Tribol. Lett., 2015, 60, 5 CrossRef.
- M. I. De Barros Bouchet, J. M. Martina, Th. Le Mogne, P. Bilasa, B. Vachera and Y. Yamada, Mechanisms of MoS2 formation by MoDTC in presence of ZnDTP: effect of oxidative degradation, Wear, 2005, 258, 1643–1650 CrossRef CAS.
- J. Graham, H. Spikes and R. Jensen, The friction reducing properties of molybdenum dialkyldithiocarbamate additives: Part II - durability of friction reducing capability, Tribol. Lett., 2001, 44, 637–647 CAS.
- K. Inoue, E. Tominaga, K. Akiyama and T. Ashida, Effects of lubricant composition on fuel efficiency in modern engines, SAE Trans., 1995, 104, 728–736 Search PubMed.
- J. Graham, H. Spikes and S. Korcek, The friction reducing properties of molybdenum dialkyldithiocarbamate additives: Part I – factors influencing friction reduction, Tribol. Lett., 2001, 44, 626–636 CAS.
- G. Splengler and A. Webber, On the lubricating performance of organic molybdenum compounds, Chem. Ber., 1939, 92, 2163–2171 CrossRef.
- C. Grossiord, K. Varlot, J. M. Martin, T. L. Mogne, C. Esnouf and K. Inoue, MoS2 single sheet lubrication by molybdenum dithiocarbamate, Tribol. Int., 1998, 31, 737–743 CrossRef CAS.
- M. de Feo, C. Minfray, M. I. De Barros Bouchet, B. Thiébaut, T. L. Mogne, B. Vacher and J. M. Martin, Ageing impact on tribological properties of MoDTC-containing base oil, Tribol. Int., 2015, 92, 126–135 CrossRef CAS.
- M. De Feo, Impact of thermo-oxidative degradation of MoDTC additive on its tribological performances for steel-steel and DLC-steel contacts, PhD thesis, Université de Lyon, 2015.
- M. De Feo, C. Minfray, M. I. De Barros Bouchet, B. Thiébaut and J. M. Martin, MoDTC friction modifier additive degradation: Correlation between tribological performance and chemical changes, RSC Adv., 2015, 5, 93786–93796 RSC.
- J. Igarashi, Y. Yamada, M. Ishimaru and M. Kagaya, Degradation of friction modifiers, Proceedings of Japan International Tribology Conference, Nagoya, 1990, pp. 421–426 Search PubMed.
- R. K. Jensen, M. D. Johnson, S. Korcek and M. J. Rokosz, Friction reducing and antioxidant capabilities of engine oil additive systems under oxidative conditions. I. Effects of ligand exchange between molybdenum dialkyldithiocarbamate and zinc dialkyldithiophosphate in hexadecane, Lubr. Sci., 1998, 10, 99–120 CrossRef CAS.
- K. Arai, M. Yamada, S. Asano, S. Yoshizawa, H. Ohira, K. Hoshino, F. Ueda and K. Akiyama, Lubricant technology to enhance the durability of low friction performance of gasoline engine oils, SAE Trans., 1995, 104, 1964–1972 Search PubMed.
- S. Korcek, R. K. Jensen and M. D. Johnson, Interaction leading to formation of low friction films in system containing molybdenum dialkyldithiocarbamate and zinc dialkyldithiophosphate additives, Tribol. Ser., 2000, 38, 399–407 CrossRef CAS.
- M. D. Johnson, R. K. Jensen, E. M. Clausing, K. Schriewer and S. Korcek, Effects of ageing on frictional properties of fuel efficient engine oils, SAE Technical Paper No. 952532, 1995 Search PubMed.
- I. Minami, N. Kitayama and H. Okabe, Synthesis and evaluation of sulphurized MoDTP, Jpn. J. Tribol., 1992, 37, 113–119 Search PubMed.
- A. B. Greene and T. J. Risdon, Effects of Mo-containing oil soluble friction modifiers on engine fuel economy and gear oil efficiency, SAE Paper 811187, 1981 Search PubMed.
- M. Muraki and H. Wada, Frictional properties of organo molybdenum compounds in the presence of ZnDTP under sliding condition, Tribol. Ser., 1995, 30, 409–422 CrossRef CAS.
- M. Kasrai, J. N. Culter, K. Gore, G. Canning, G. M. Bancroft and K. H. Tan, The chemistry of antiwear films generated by the combination of ZDDP and MoDTC examined by X-ray absorption spectroscopy, Tribol. Lett., 1998, 41, 69–77 CAS.
- F. Rounds, Effects of organic molybdenum compounds on the friction and wear observed with ZDP-containing lubricant blends, Tribol. Lett., 1990, 3, 345–354 Search PubMed.
- J. M. Martin, C. Grossiord, K. Varlot, B. Vacher and J. Igarashi, Synergistic effects in binary systems of lubricant additives: A chemical hardness approach, Tribol. Lett., 2000, 8, 193–201 CrossRef CAS.
- A. Morina, A. Neville, M. Priest and J. H. Green, ZDDP and MoDTC interactions in boundary lubrication—The effect of temperature and ZDDP/MoDTC ratio, Tribol. Int., 2006, 39, 1545–1557 CrossRef CAS.
- A. Morina, A. Neville, M. Priest and J. H. Green, ZDDP and MoDTC interactions and their effect on tribological performance – tribofilm characteristics and its evolution, Tribol. Lett., 2006, 24, 243–256 CrossRef CAS.
- M. I. de Barros Bouchet, J. M. Martin, T. Le-Mogne and B. Vacher, Boundary lubrication mechanisms of carbon coatings by MoDTC and ZDDP additives, Tribol. Int., 2005, 38, 257–264 CrossRef CAS.
- M. Muraki and H. Wada, Influence of the alkyl group of zinc dialkyldithiophosphate on the frictional characteristics of molybdenum dialkyldithiocarbamate under sliding conditions, Tribol. Int., 2002, 35, 857–863 CrossRef CAS.
- A. S. Sarpal, J. Christopher, S. Mukherjee, M. B. Patel and G. S. Kapur, Study of additive-additive interactions in a lubricant system by NMR, ESCA, and thermal techniques, Lubr. Sci., 2005, 17, 319–345 CrossRef CAS.
- T. M. Shea and A. J. Stipanovic, Solution phase reactions of organomolybdenum friction modifier additives for energy conserving engine oils, Tribol. Lett., 2002, 12, 13–22 CrossRef CAS.
- S. Korcek, R. K. Jensen, M. D. Johnson and J. Sorab, Fuel efficient engine oils, additive interactions, boundary friction, and wear, Tribol. Ser., 1999, 36, 13–24 CAS.
- K. Yagashita and J. Igarashi, Analysis of ligand exchange reaction among sulphur containing complexes, Prepr. JAST Tribology Meeting, Fukuoka, 1991, pp. 673–676 Search PubMed.
- S. B. Arabyan, I. A. Homolomonov, A. K. Karaulov and A. B. Vipper, Investigation of the effectiveness of antifriction additives in motor oils by laboratory methods and engine tests, Lubr. Sci., 1994, 5, 241–256 CrossRef.
- E. R. Braithwaite and A. B. Greene, A critical analysis of the performance of molybdenum, Wear, 1978, 45, 405–433 CrossRef.
- Y. Yamamoto and S. Gondo, Friction and wear characteristics of molybdenum dithiocarbamate and molybdenum dithiophosphate, Tribol. Lett., 1989, 32, 251–257 CAS.
- R. Schubart and H. Engels, Process for the preparation of zinc dithiocarbamates, US Pat., 6534675B2, 2003.
- J. J. Harrison, C. Y. Chan, A. Onopchenko, A. R. Pradhan and M. Petersen, Neutral zinc(II) O,O-di-alkyldithiophosphates – variable temperature 31P NMR and quantum chemical study of the ZDDP monomer–dimer equilibrium, Magn. Reson. Chem., 2008, 45, 115–124 CrossRef.
- R. K. Jensen, S. Korcek and M. D. Johnson, Friction-reducing and antioxidant capabilities of engine oil additive systems under oxidative conditions. II. Understanding ligand exchange in a molybdenum dialkyldithiocarbamate zinc dialkyldithiophosphate additive system in various base oils, Lubr. Sci., 2001, 14, 25–42 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures. See DOI: 10.1039/d0ra07329f |
| This journal is © The Royal Society of Chemistry 2020 |