Open Access Article
Open Access ArticleDesign, synthesis and insecticidal activity of novel analogues of flubendiamide containing alkoxyhexafluoroisopropyl groups†
Xianghu Zhao‡
,
Sixue Xu‡,
Chuan Liu,
Jingjing He,
Chunmei Li,
Yupian Deng and
Song Cao *
*
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology (ECUST), Shanghai 200237, China. E-mail: scao@ecust.edu.cn
First published on 17th September 2020
Abstract
Flubendiamide has received considerable attention in the agriculture field due to its novel mode of action and excellent insecticidal activity. However, the high cost and toxicity to aquatic invertebrates associated with flubendiamide limit its agronomic utility. On the basis of the structure of the lead compound, flubendiamide, we designed and synthesized a series of novel analogues of flubendiamide bearing a alkoxyhexafluoroisopropyl moiety using 2-methyl-4-(2-alkoxyhexafluoroisopropyl) anilines as the key intermediates. Their insecticidal activities against the oriental armyworm (Mythimna separata Walker) were evaluated. The results indicated that most of the target compounds exhibited high insecticidal activities. Specifically, compound 8h showed the best insecticidal activity against the armyworm and its insecticidal activity reached 70% at 0.156 mg L−1. The LC50 value of compound 8h (0.0512 mg L−1) is nearly the same as the corresponding commercial product flubendiamide (0.0412 mg L−1). Furthermore, the acute toxicity test showed that the 48 h LC50 values of compound 8h and flubendiamide against Daphnia magna Straus were 0.0066 and 0.0021 mg L−1, respectively. The toxicity of compound 8h is obviously lower than flubendiamide.
Introduction
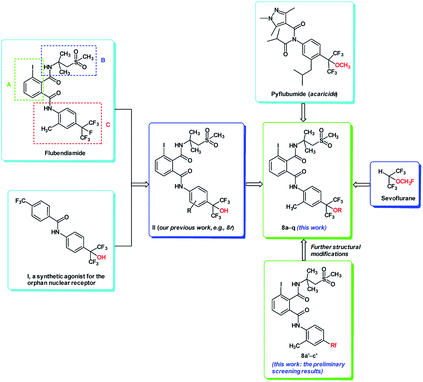
Flubendiamide, a new generation of synthetic insecticide, has attracted continuing interest in recent years because of its unique insecticide mode of action, high activity against a broad spectrum of lepidopterous insects and low acute toxicity to mammals.1,2 It directly activates the insect ryanodine receptors (RyRs), which causes permanent muscle contraction, paralysis and eventually results in the death of the insect.3,4 Since the first commercial Ca2+ channel modulator, flubendiamide, was launched in 2007 by Nihon Nohyaku and Bayer CropScience, there have been many reports on the synthesis and bioassay of flubendiamide.5–7Flubendiamide often serves as an ideal lead compound for developing a new and selective ryanodine receptor activator.8,9 Generally, the structure of flubendiamide is composed of three parts as shown in Fig. 1: (A) the phthaloyl moiety, (B) the aliphatic amine moiety and (C) the aromatic amine moiety.10–12 Therefore, extensive efforts have been focused on the modification of these three moieties and a variety of structurally diverse novel flubendiamide analogues have been discovered.13–16 It has been demonstrated that the heptafluoroisopropyl group in the aromatic amine moiety (part C) is essential for high insecticidal activity and remarkably broadens the insecticidal spectrum.17 However, the use of expensive starting material (heptafluoroisopropyl iodide) and the poor stability and operational inconvenience of this reagent greatly restrict the widespread applications of flubendiamide in crop protection.18 The high cost of heptafluoroisopropyl iodide likely drives researchers to search other alternative polyfluorinated substrates. For example, in 2010, Zhu et al. synthesized a series of phthalic acid diamides bearing the CF3 group at meta position on the aniline ring.19 In 2014, the group of Zheng-ming Li modified the structure of flubendiamide by replacing heptafluoroisopropyl group with different fluorinated functionalities in the aromatic amine moiety.20 Some of these novel flubendiamide derivatives exhibited high insecticidal activities. Tohnishi et al. indicated that incorporation of fluoroalkoxy group at the 4-position of the aromatic amine moiety could improve insecticidal activity.21 In addition, like heptafluoroisopropyl group, the hexafluorocarbinol moiety (–C(CF3)2OH) is also an attractive pharmacophore that is often included in medicines or bioactive compounds (e.g. Fig. 1, compound I).22–25
 | ||
| Fig. 1 Design strategy for novel flubendiamide derivatives containing alkoxyhexafluoroisopropyl group 8a–q. | ||
Inspired by the structure of flubendiamide and compound I, in 2012, we synthesized a series of novel analogues of flubendiamide containing a hexafluoro-2-hydroxypropan-2-yl moiety (Fig. 1, compound II).26 Compound II has some significant advantages over its leading compound, flubendiamide, due to the use of cheap, stable and commercially available starting material, CF3COCF3·H2O. However, compound II such as 8r (R = 2-CH3) (Fig. 1) exhibited worse activity than flubendiamide. Therefore, the requirements to develop novel analogues of flubendiamide with low cost, low aquatic species toxicity and excellent insecticidal activity are highly desirable. In our initial experiments, three polyfluorinated groups (Rf) were introduced into the aromatic amine moiety of flubendiamide to replace heptafluoroisopropyl group (Fig. 1, 8a′–c′). Preliminary results indicated that analogue of flubendiamide bearing fluoroalkoxy group 8c′ possessed high insecticidal activity, implying that the introduction of fluoroalkoxy group might be favorable for retaining insecticidal activity. In addition, pyflubumide is a novel acaricide with remarkable activity against spider mites. The structural feature of pyflubumide is that it contains a methoxy-substituted hexafluoroisopropyl group on the aromatic amine moiety (Fig. 1, pyflubumide).27 Another bioactive compound bearing 2-fluoroalkoxy-hexafluoroisopropyl group is Sevoflurane, a widely used inhalational anesthetic agent (Fig. 1, Sevoflurane).28
In 2016, Cruciani et al. replaced the tert-butyl group of bosentan with heptafluoroisopropyl, hexafluoro-2- hydroxyprop-2-yl and hexafluoro-2-methoxyprop-2-yl group, respectively (Fig. 2a).29 These fluorinated analogues of bosentan exhibited an improved metabolic stability towards certain specific cytochromes. More recently, after carefully analysis of three X-ray crystal structures of polyfluorinated isopropyl benzenes (Fig. 2b, compounds X1, X2 and X3), Maienfisch et al. found that the dihedral angles of X1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–F), X2 (C
C/C–F), X2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–OH), and X3 (C
C/C–OH), and X3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–OCH3) were slightly different. The subtle differences in the conformation of polyfluorinated substituents on the benzene ring were observed.30
C/C–OCH3) were slightly different. The subtle differences in the conformation of polyfluorinated substituents on the benzene ring were observed.30
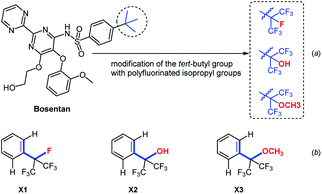 | ||
| Fig. 2 (a) Modification of the tert-butyl group of Bosentan with the polyfluorinated isopropyl groups. (b) The dihedral angles of different polyfluorinated isopropyl benzenes. | ||
On the basis of the above consideration and preliminary results of bioassay, we envisioned that the introduction of hexafluoro-2-alkoxyprop-2-yl group to the aromatic amine moiety of flubendiamide might retain or improve the activity of parent compound. In this paper, we designed and synthesized a series of novel analogues of flubendiamide bearing alkoxyhexafluoroisopropyl moiety (Fig. 1, 8a–q). Their insecticidal activities of the target compounds against oriental armyworm and the acute toxicity of 8h against Daphnia magna Straus were also evaluated.
Results and discussion
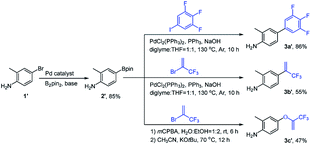
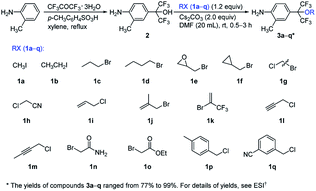
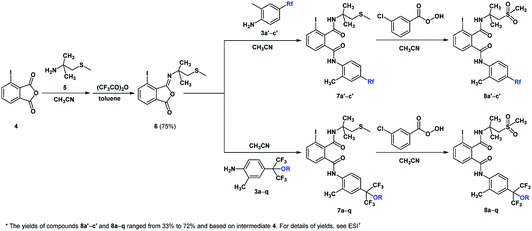
The synthetic routes of the intermediates 2′, 3a′–c′ and 3a–q and compounds 8a′–c′ and 8a–q are outlined in Schemes 1–4, respectively. These intermediates and title compounds were readily synthesized according to the reported methods.26,31–33 In some case, slight modifications of these reaction conditions were necessary in order to obtain satisfying yields (see ESI† for details).The highly efficient synthesis of the key intermediates 3a–q was one of the key steps in the total synthesis of these novel analogues of flubendiamide (Scheme 2). A survey of the literature revealed that only a few methods were used to synthesize the analogues of intermediates 3a–q (Scheme 3, methods A and B).34–36 However, these methods suffer from the use of expensive starting material (heptafluoroisopropyl iodide) and/or the lengthy protection and deprotection of amido group.
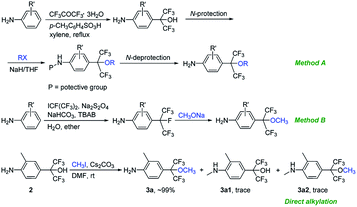 | ||
| Scheme 3 Two reported and our methods for the synthesis of 4-(hexafluoro-2-alkoxypropan-2-yl)anilines. | ||
To access the above-mentioned intermediates in a convenient and efficient manner, we tried to alkylate intermediates 2 with various alkyl halides (RX) directly. However, the chemoselective alkylation of intermediates 2 remains a challenge due to the presence of two possible highly reactive sites (NH2 and OH groups),37 which leads to the formation of different products (Scheme 3, 3a, 3a1 and 3a2, RX![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3I). After careful screening of bases, solvents, and reaction temperatures, we found that the chemoselective alkylation of hydroxyl group in intermediate 2 could proceed smoothly in the presence of 1.2 equiv. of CH3I and 1.5 equiv. of Cs2CO3 using DMF as the solvent at 25 °C for 0.5 h and afforded the desired O-alkylated product 3a in excellent yields. Only trace amounts of N-alkylated product 3a1 and double alkylated product 3a2 were detected. Subsequently, compound 2 was alkylated with a variety of alkyl halides RX under the optimized experimental conditions to afford O-alkylated products 3a–q in high yields and high purity (Scheme 2). Some crude O-alkylation products 3a–q could be used for the next step without additional purification (see ESI† for details). The structures of 3a, 3a1 and 3a2 were determined by the 1H NMR spectra and GC-MS, or 1H NMR spectra of their corresponding pure compounds reported in the literature.34–36
CH3I). After careful screening of bases, solvents, and reaction temperatures, we found that the chemoselective alkylation of hydroxyl group in intermediate 2 could proceed smoothly in the presence of 1.2 equiv. of CH3I and 1.5 equiv. of Cs2CO3 using DMF as the solvent at 25 °C for 0.5 h and afforded the desired O-alkylated product 3a in excellent yields. Only trace amounts of N-alkylated product 3a1 and double alkylated product 3a2 were detected. Subsequently, compound 2 was alkylated with a variety of alkyl halides RX under the optimized experimental conditions to afford O-alkylated products 3a–q in high yields and high purity (Scheme 2). Some crude O-alkylation products 3a–q could be used for the next step without additional purification (see ESI† for details). The structures of 3a, 3a1 and 3a2 were determined by the 1H NMR spectra and GC-MS, or 1H NMR spectra of their corresponding pure compounds reported in the literature.34–36
The insecticidal activities of compounds 8a′–c′, 8a–r and flubendiamide (as a control) against oriental armyworm were listed in Tables 1 and 2.
| Compds | Insecticidal activity (%) at concentration (mg L−1) | |||
|---|---|---|---|---|
| 400 | 200 | 100 | 10 | |
| 8a' | 47 | 7 | 3 | 0 |
| 8b' | 100 | 90 | 20 | 0 |
| 8c' | 100 | 100 | 70 | 43 |
| Flu | 100 | 100 | 100 | 97 |
| Compds | Insecticidal activity (%) at different concentration (mg L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 50 | 10 | 5 | 2.5 | 1.25 | 0.625 | 0.156 | |
| a Note that blank cells mean not tested. | ||||||||
| 8a | 100 | 100 | 100 | 80 | 67 | 57 | 50 | 0 |
| 8b | 100 | 100 | 100 | 87 | 77 | 73 | 40 | 0 |
| 8c | 70 | 57 | 40 | 0 | ||||
| 8d | 20 | |||||||
| 8e | 73 | 60 | 50 | 10 | ||||
| 8f | 100 | 100 | 100 | 90 | 87 | 83 | 73 | 40 |
| 8g | 100 | 100 | 90 | 83 | 80 | 77 | 70 | 23 |
| 8h | 100 | 100 | 100 | 100 | 90 | 87 | 85 | 70 |
| 8i | 100 | 100 | 100 | 97 | 90 | 83 | 80 | 60 |
| 8j | 100 | 100 | 70 | 50 | 13 | |||
| 8k | 100 | 100 | 100 | 90 | 90 | 87 | 83 | 60 |
| 8l | 100 | 100 | 100 | 90 | 90 | 87 | 60 | 17 |
| 8m | 100 | 100 | 87 | 83 | 80 | 77 | 73 | 30 |
| 8n | 100 | 90 | 67 | 47 | 20 | |||
| 8o | 10 | |||||||
| 8p | 10 | |||||||
| 8q | 30 | |||||||
| 8r | 100 | 100 | 100 | 80 | 80 | 67 | 30 | 0 |
| Flu | 100 | 100 | 100 | 97 | 93 | 90 | 85 | 70 |
As shown in Table 1, analogues of flubendiamide having fluoroalkoxy group 8c′ showed higher activity than other compounds (8a′ and 8b′). These preliminary bioassay results suggested that the incorporation of fluoroalkoxy group into the aromatic amine moiety (part C) could provide useful clue for further structural optimization for the discovery of novel analogues of flubendiamide.
Subsequently, seventeen novel analogues of flubendiamide containing alkoxyhexafluoroisopropyl group 8a–q were designed and synthesized. Their bioassay results of seventeen novel analogues of flubendiamide containing alkoxyhexafluoroisopropyl group 8a–q and 8r are summarized in Table 2. Most of the alkoxyhexafluoroisopropyl-containing compounds displayed good to excellent larvicidal activities. The bioactivities of compounds 8a–q were significantly affected by the electronic nature and the steric properties of the alkyl group (R) attached to the oxygen atom. Generally, the larvicidal activities decreased with an increase in the size of the substituent R in the C(CF3)2OR moiety. The steric bulk of substituents was detrimental for activity (for example, 8a versus 8p and 8q). The target compounds bearing short-chain alkyl group such as methyl (8a) showed better larvicidal activities than that of compound bearing long-chain alkyl group (8c and 8d). It was observed that the introduction of appropriate small substituents with terminal halide atoms or unsaturated groups into the (2-hydroxyhexafluoroisopropyl) aniline moiety often had a positive effect on insecticidal activity. For example, compound 8g (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2Cl) and compound 8h (R
CH2Cl) and compound 8h (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2CN) exhibited 23% and 70% larvicidal activity against oriental armyworm at 0.156 mg L−1, respectively. Especially, compound 8h showed nearly the same larvicidal activity as flubendiamide on armyworm. Furthermore, compound 8i (R
CH2CN) exhibited 23% and 70% larvicidal activity against oriental armyworm at 0.156 mg L−1, respectively. Especially, compound 8h showed nearly the same larvicidal activity as flubendiamide on armyworm. Furthermore, compound 8i (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2CH
CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), compound 8j (R
CH2), compound 8j (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2C(CH3)
CH2C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), compound 8k (R
CH2), compound 8k (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF3C
CF3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), compound 8l (R
CH2), compound 8l (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2C
CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) and compound 8m (R
CH) and compound 8m (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2C
CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) also had good insecticidal activities due to the presence of unsaturated carbon–carbon bonds in alkyl group. Interestingly, replacement of a cyclopropylmethyl group (8f) by an oxiran-2-ylmethyl group (8e) or an acetamide group (8n) by an acetate group (8o) resulted in a remarkable decrease in activity. These two compounds (8e and 8o) exhibited weak insecticidal activities against oriental armyworm at a test concentration of 5 mg L−1 (10% or 47%, respectively). Delightfully, the insecticidal activities of compounds 8f–i and 8k–m exhibited apparently higher larvicidal activities than compound 8r, previously reported by our group (Scheme 1), suggesting that the introduction of alkyl group into the (2-hydroxyhexafluoroisopropyl) aniline moiety might have a beneficial effect on the insecticidal activity of the title compounds.
CH) also had good insecticidal activities due to the presence of unsaturated carbon–carbon bonds in alkyl group. Interestingly, replacement of a cyclopropylmethyl group (8f) by an oxiran-2-ylmethyl group (8e) or an acetamide group (8n) by an acetate group (8o) resulted in a remarkable decrease in activity. These two compounds (8e and 8o) exhibited weak insecticidal activities against oriental armyworm at a test concentration of 5 mg L−1 (10% or 47%, respectively). Delightfully, the insecticidal activities of compounds 8f–i and 8k–m exhibited apparently higher larvicidal activities than compound 8r, previously reported by our group (Scheme 1), suggesting that the introduction of alkyl group into the (2-hydroxyhexafluoroisopropyl) aniline moiety might have a beneficial effect on the insecticidal activity of the title compounds.
Furthermore, the LC50 values of compounds 8a–c, 8e–n, 8r as well as flubendiamide against oriental armyworm were calculated and summarized in Table 3. Compounds 8d and 8o–q were excluded from the regression analysis because these compounds did not give acceptable LC50 values. As shown in Table 3, compounds 8f, 8i and 8k exhibited excellent insecticidal activity against armyworm, with the LC50 values of 0.2158, 0.0868, and 0.0722 mg L−1, respectively. In particular, the LC50 value of compound 8h was 0.0512 mg L−1, which was near that of flubendiamide (0.0412 mg L−1).
| Compds | Regression equation | LC50 (mg L−1) | 95% confidence interval of LC50 (mg L−1) | r |
|---|---|---|---|---|
| 8a | y = 0.91177x + 5.1361 | 0.7092 | 0.4376–1.1492 | 0.9839 |
| 8b | y = 1.4626x + 5.1970 | 0.7334 | 0.5419–0.9925 | 0.9457 |
| 8c | y = 0.7456x + 3.9798 | 23.3582 | 14.2972–38.1618 | 0.9864 |
| 8e | y = 0.5605x + 4.4081 | 11.3793 | 4.5377–28.5361 | 0.9475 |
| 8f | y = 1.0690x + 5.7120 | 0.2158 | 0.1426–0.3265 | 0.9752 |
| 8g | y = 1.7053x + 5.7005 | 0.3884 | 0.3126–0.4825 | 0.9804 |
| 8h | y = 1.0188x + 6.3151 | 0.0512 | 0.0168–0.1560 | 0.9760 |
| 8i | y = 0.9241x + 5.9809 | 0.0868 | 0.0428–0.1760 | 0.9743 |
| 8j | y = 2.667x + 2.9565 | 5.8372 | 5.1008–6.6797 | 0.9785 |
| 8k | y = 0.8938x + 6.0201 | 0.0722 | 0.0315–0.1657 | 0.9802 |
| 8l | y = 1.9854x + 5.6844 | 0.4521 | 0.3770–0.5423 | 0.9839 |
| 8m | y = 1.1781x + 5.5729 | 0.3264 | 0.2357–0.4520 | 0.9392 |
| 8n | y = 1.604x + 3.7044 | 6.4223 | 5.2645–7.8353 | 0.9832 |
| 8r | y = 2.2959x + 5.0371 | 0.9635 | 0.8172–1.1359 | 0.9729 |
| Flu | y = 0.8931x + 6.2370 | 0.0412 | 0.0146–0.1160 | 0.9996 |
To get a better understanding of the insecticidal activity of synthesized compounds, the best bioactive compound 8h and commercial insecticide chlorantraniliprole (CAP) were selected to further evaluate the activities against oriental armyworm, tea geometrid, cabbage butterfly and diamondback moths (Table 4). The results of the preliminary bioassays indicated that compound 8h displayed good to high insecticidal activities against these four insects. The larvicidal activity of compound 8h was comparable to that of chlorantraniliprole.
| Concentration (mg L−1) | Insecticidal activity (%) at different concentration (mg L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Oriental armyworm | Tea geometrid | Cabbage butterfly | Diamondback moth | |||||
| 8h | CAP | 8h | CAP | 8h | CAP | 8h | CAP | |
| a Note that blank cells mean not tested. | ||||||||
| 100 | 100 | 100 | 100 | 100 | 100 | 100 | 87 | 100 |
| 20 | 100 | 100 | 90 | 90 | 90 | 90 | 60 | 90 |
| 4 | 100 | 100 | 50 | 60 | 50 | 70 | 50 | 70 |
| 0.8 | 90 | 100 | 30 | 30 | 30 | 60 | 40 | 50 |
| 0.16 | 70 | 80 | 0 | 0 | 0 | 0 | ||
Recently, it was reported that flubendiamide was restricted or banned in certain countries due to its toxicity to aquatic invertebrates.38,39 Consequently, the acute toxicity tests of compound 8h and flubendiamide to Daphnia magna were carried out. Daphnia magna were exposed to different concentrations of compound 8h and flubendiamide after 48 h. The LC50 values of compound 8h and flubendiamide against Daphnia magna were 0.0066 and 0.0021 mg L−1, respectively (Tables 5 and 6). The toxicity of compound 8h is lower than that of flubendiamide. It implied that the replacement of secondary C–F bond in flubendiamide by C–OR moiety might lead to a decrease in the acute toxicity for Daphnia magna, whereas this modification in the aromatic amine moiety could retain or improve insecticidal activity.
| Compds | Acute toxicity (%) at different concentration (mg L−1) | ||||||
|---|---|---|---|---|---|---|---|
| 0.175 | 0.0875 | 0.04375 | 0.02188 | 0.01092 | 0.00546 | 0.00273 | |
| 8h | 97 | 95 | 79 | 66 | 61 | 44 | 39 |
| Flu | 97 | 95 | 81 | 76 | 70 | 67 | 58 |
Conclusions
In summary, we designed and synthesized a variety of structurally diverse analogues of flubendiamide containing alkoxyhexafluoroisopropyl group. The key intermediates, 2-methyl-4-(2-alkoxyhexafluoroisopropyl) anilines (3a–q), were synthesized in good to excellent yields by the straightforward chemoselective alkylation of 2-methyl-4-hexafluoroisopropanol aniline. The bioassay results indicated that some compounds displayed high insecticidal activities against oriental armyworm (Mythimna separata Walker) in comparison with flubendiamide. Particularly, the insecticidal activity of compound 8h against armyworm was as high as 70% at 0.156 mg L−1, the same as that of control flubendiamide (0.156 mg L−1, 70%). The LC50 values of 8h and flubendiamide were 0.0512 and 0.0412 mg L−1, respectively. Furthermore, compound 8h also exhibited strong insecticidal activities against tea geometrid, cabbage butterfly and diamondback moths, which is comparable to commercial chlorantraniliprole. In addition, the acute toxicity test showed that the 48 h LC50 value of compound 8h against Daphnia magna Straus was 0.0066 mg L−1, whereas the LC50 value of flubendiamide was 0.0021 mg L−1. The modification resulted in an obvious decrease in toxicity. The preliminary structure–activity relationship of the title compounds revealed that the introduction of small, electron-poor substituent or unsaturated group into the (2-hydroxyhexafluoroisopropyl) aniline moiety may be favorable for retaining high insecticidal activity. The findings obtained in these studies provided useful guidance for the design of novel analogues of flubendiamide bearing polyfluorinated group. Further modification of flubendiamide and field trials of compound 8h are currently underway.Experimental
General information
All reagents were of analytic grade and obtained from commercial suppliers and used without further purification. Reactions were monitored by GC-MS or thin-layer chromatography (TLC) on silica gel 60 GF254 with ultraviolet detection. Melting points were measured in an open capillary using Büchi melting point B-540 apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Bruker AM-400 spectrometer (400 MHz and 100 MHz, respectively) using TMS as the internal standard. CDCl3 and DMSO-d6 were used as NMR solvent. The 19F NMR spectra were recorded on a Bruker AM-400 spectrometer (376 MHz) using CF3CO2H as external standard. Gas chromatography-mass spectra (GC-MS) were recorded on HP 5973 MSD with 6890 GC. High resolution mass spectra (HRMS) were recorded under electron impact conditions using a MicroMass GCT CA 055 instrument.Procedures for the synthesis of compounds in Scheme 4
Intermediates 4, 5 and 6 were prepared according to the literature procedures with some modification.26,33 General procedure for the synthesis of target compounds 8a′–c′ and 8a–q. To a solution of 6 (1.87 g, 5 mmol) in acetonitrile (25 mL) was added substituted anilines 3a′–c′ or 3a–q (5.5 mmol) and trifluoroacetic acid (28.5 g, 0.25 mmol). The mixture was then stirred for 3 h. The progress of the reaction was monitored by TLC. When the reaction was complete, without further purification, the intermediate 7a′–c′ or 7a–q (5 mmol) was allowed to react with the solution of m-chloroperoxybenzoic acid (1.90 g, 11 mmol) in acetonitrile (25 mL). The solution was stirred for another 3 h at room temperature until the reaction was complete. Then the solution was concentrated under reduced pressure and the residue was dissolved in dichloromethane (10 mL). The organic layer was washed by water and dried over anhydrous MgSO4. The CH2Cl2 was removed under reduced pressure, and the residue was purified by silica gel column chromatograph (eluent: petroleum ether (60–90 °C)/ethyl acetate = 3/2, v/v) to afford compounds 8a′–c′ and 8a–q. The physical characteristics, yields, 1H NMR, 13CNMR, 19F NMR and HRMS (ESI) data of the target compounds can be found in ESI.†Biological assays
All bioassays were performed on representative test organisms reared in the laboratory. The bioassay was repeated at 25 ± 1 °C according to statistical requirements. Assessments were made on a dead/alive basis, and mortality rates were corrected using Abbott's formula. Evaluations are based on a percentage scale of 0–100 in which 0 = no activity and 100 = total kill.Larvicidal activity against oriental armyworm (Mythimna separata Walker). The larvicidal activity of compounds 8a′–c′ and 8a–r (8r, Fig. 1, R = 2-CH3) against oriental armyworm was tested according to the leaf-dip method using the literature procedures.40,41 In leaf-dip bioassay, leaf disks (about 5 cm) were cut from fresh corn leaves and dipped in insecticide solutions for 5 s, and then air-dried on filter paper. Leaf disks dipped in water were used as controls. After drying, the treated leaf disks were placed on a bed of agar in a small Petri dish (7 cm in diameter). Each dried treated leaf disk was infested with 10 third-instar oriental armyworm larvae. Percentage mortalities were assessed 3 days later. Each treatment was performed three times. To compare their activities, the commercial flubendiamide was tested under the same conditions. The larvicidal activities of 8a′–c′, 8a–r and flubendiamide against oriental armyworm are listed in Tables 1 and 2. In addition, the LC50 values of compounds 8a–c, 8e–n, 8r and flubendiamide (Flu) against oriental armyworm as shown in Table 3.
Larvicidal activity against armyworm and other three insects. The larvicidal activity of the typical compound 8h and chlorantraniliprole against oriental armyworm (Mythimna separata Walker), tea geometrid (Ectropis oblique hypulina Wehrli), cabbage butterfly (Pieris rapae L.) and diamondback moths (Plutella xylostella Linnaeus) were evaluated according to the leaf-dip method using the literature procedures (Table 4).13,41–43 The acute toxicity test of compound 8h and flubendiamide to Daphnia magna Straus were also performed according the reported method with some modifications.44–46 The results were summarized in Tables 5 and 6.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Key Research and Development Program of China (No. 2017YFD0200505).Notes and references
- H. Hamaguchi and T. Hirooka, in Modern Crop Protection Compounds, ed. W. K
![[r with combining umlaut]](https://www.rsc.org/images/entities/char_0072_0308.gif) amer and U. Schirmer, Wiley-VCH Verlag, Weinheim, Germany, 2nd edn, 2007, vol. 3, p. 1121 Search PubMed.
amer and U. Schirmer, Wiley-VCH Verlag, Weinheim, Germany, 2nd edn, 2007, vol. 3, p. 1121 Search PubMed. - P. L. George, C. Daniel and D. B. James, Bioorg. Med. Chem., 2009, 17, 4127 CrossRef.
- K. Kato, S. Kiyonaka, Y. Sawaguchi, M. Tohnishi, H. Takeshima and Y. Mori, Biochemistry, 2009, 48, 10342 CrossRef CAS.
- S. Z. Qi and J. E. Casida, Pestic. Biochem. Physiol., 2013, 107, 321 CrossRef CAS.
- U. Ebbinghaus-Kintscher, P. Lümmen, K. Raming, T. Masaki and N. Yasokawa, Pflanzenschutz-Nachr. Bayer, 2007, 60, 117 CAS.
- K. Tsubata, M. Tohnishi, H. Kodama and A. Seo, Pflanzenschutz-Nachr. Bayer, 2007, 60, 105 CAS.
- M. L. Feng, Y. F. Li, H. J. Zhu, J. P. Ni, B. B. Xi and L. Zhao, Pest Manage. Sci., 2012, 68, 986 CrossRef CAS.
- Y. T. Xie, S. Zhou, Y. X. Li, M. Chen, B. Wang, L. Xiong, N. Yang and Z. M. Li, Chin. J. Chem., 2018, 36, 129 CrossRef CAS.
- Y. B. Chen, Y. X. Xiao, X. S. Shao, X. Y. Xu and Z. Li, Chin. J. Chem., 2014, 32, 592 CrossRef CAS.
- S. Zhou, T. Yan, S. Zhou, X. W. Hua, L. X. Xiong and Z. M. Li, Chin. J. Chem., 2014, 32, 567 CrossRef CAS.
- S. Zhou, Z. H. Jia, L. X. Xiong, T. Yan and Z. M. Li, J. Agric. Food Chem., 2014, 62, 6269 CrossRef CAS.
- H. Hamaguchi and T. Hirooka, in Modern Crop Protection Compounds, ed. W. K
![[r with combining umlaut]](https://www.rsc.org/images/entities/char_0072_0308.gif) amer, U. Schirmer, P. Jeschke and M. Witschel, Wiley, Weinheim, Germany, 2nd edn, 2012; vol. 3, p. 1396 Search PubMed.
amer, U. Schirmer, P. Jeschke and M. Witschel, Wiley, Weinheim, Germany, 2nd edn, 2012; vol. 3, p. 1396 Search PubMed. - M. Liu, Y. Wang, Y. L. Cui, S. Z. Liu and C. H. Rui, J. Agric. Food Chem., 2010, 58, 6858 CrossRef CAS.
- C. Gnamm, A. Jeanguenat, A. C. Dutton, C. Grimm, D. P. Kloer and A. J. Crossthwaite, Bioorg. Med. Chem. Lett., 2012, 22, 3800 CrossRef CAS.
- S. Zhou, X. Meng, R. Jin, Y. Ma, Y. Zhao and Z. M. Li, Mol. Diversity, 2017, 21, 915 CrossRef CAS.
- S. Zhou, Y. C. Gu, M. Liu, B. L. Wang, L. X. Xiong, N. Yang and Z. M. Li, J. Agric. Food Chem., 2014, 62, 11054 CrossRef CAS.
- M. Tohnishi, T. Nishimatsu, K. Motoba, T. Hirooka and A. Seo, J. Pestic. Sci., 2010, 35, 490 CrossRef CAS.
- M. Onishi, A. Yoshiura, E. Kohno and K. Tsubata, EP 1006102 B1, 2000.
- M. L. Feng, Y. F. Li, H. J. Zhu, L. Zhao, B. B. Xi and J. P. Ni, J. Agric. Food Chem., 2010, 58, 10999 CrossRef CAS.
- Y. W. Chen, Y. X. Li, L. Pan, J. B. Liu, N. Yang, H. B. Song and Z. M. Li, Bioorg. Med. Chem., 2014, 22, 6366 CrossRef CAS.
- T. Masaki, N. Yasokawa, S. Fujioka, M. Tohnishi and T. Hirooka, J. Pestic. Sci., 2009, 34, 37 CrossRef CAS.
- Y. J. Wang, N. Kumar, P. Nuhant, M. D. Cameron and P. R. Griffin, ACS Chem. Biol., 2010, 5, 1029 CrossRef CAS.
- L. Li, J. W. Liu, L. S. Zhu, S. Cutler, H. Hasegawa, B. Shan and J. C. Medina, Bioorg. Med. Chem. Lett., 2006, 16, 1638 CrossRef CAS.
- R. Narlawar, K. Baumann, C. Czech and B. Schmidt, Bioorg. Med. Chem. Lett., 2007, 17, 5428 CrossRef CAS.
- L. T. Kong, J. Wang, X. C. Fu, Y. Zhong, T. Luo and J. H. Liu, Carbon, 2010, 48, 1262 CrossRef CAS.
- M. X. Wu, W. W. Zhao, G. Y. Jin, Q. C. Huang and S. Cao, Chin. J. Chem., 2012, 30, 1310 CrossRef CAS.
- T. Furuya, K. Machiya, S. Fujioka, M. Nakano and I. K. nagaki, J. Pestic. Sci., 2017, 42, 132 CrossRef CAS.
- K. Ramakrishna, C. Behme, R. M. Schure and C. Bieniarz, Org. Process Res. Dev., 2000, 4, 581 CrossRef CAS.
- S. Lepri, L. Goracci, A. Valeri and G. Cruciani, Eur. J. Med. Chem., 2016, 121, 658 CrossRef CAS.
- M. E. Qacemi, S. Rendine, P. Maienfisch, G. Haufe and F. R. Leroux, Recent applications of fluorine in crop protection—new discoveries originating from the unique heptafluoroisopropyl group, in Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals, Elsevier Academic Press, 2019, ch. 17, pp. 607–629, DOI:10.1016/B978-0-12-812733-9.00017-9.
- B. H. Lipshutz, R. Moser and K. R. Voigtritter, Isr. J. Chem., 2010, 50, 691 CrossRef CAS.
- R. Masciadri, M. Kamer and N. Nock, Eur. J. Org. Chem., 2003, 4286 CrossRef CAS.
- M. Tohnishi, H. Nakao, T. Furuya, T. Hirooka and T. Nishimatsu, J. Pestic. Sci., 2005, 30, 354 CrossRef CAS.
- R. Bakthavatchalam, WO 2010138879 A1, 2010.
- S. M. Masoud, A. K. Mailyan, V. Dorcet, T. Roisnel, P. H. Dixneuf, C. Bruneau and S. N. Osipov, Organometallics, 2015, 34, 2305 CrossRef CAS.
- T. Furuya, A. Suwa, M. Nakano, S. Fujioka, N. Yasokawa and K. Machiya, J. Pestic. Sci., 2015, 40, 38 CrossRef CAS.
- B. Grimm, C. Risko, J. D. Azoulay, J. Brédas and G. C. Bazan, Chem. Sci., 2013, 4, 1807 RSC.
- B. Erickson, Chem. Eng. News, 2016, 94(10), 18 Search PubMed.
- Bee, Chem. Eng. News, 2016, 94(7), 20 Search PubMed.
- S. C. Chen, Y. Zhang, Y. X. Liu and Q. M. Wang, J. Agric. Food Chem., 2019, 67, 13544 CrossRef CAS.
- J. Zhang, X. H. Tang, I. Ishaaya, S. Cao, J. J. Wu, J. L. Yu, H. Li and X. H. Qian, J. Agric. Food Chem., 2010, 58, 2736 CrossRef CAS.
- C. J. Cui, Y. Q. Yang, T. Y. Zhao, H. M. Cai, X. C. Wan and R. Y. Hou, Molecules, 2019, 24, 4518 CrossRef CAS.
- E. E. Mansour, G. Zhang, F. Y. Mi, Y. M. Wang and A. Kargbo, Pestic. Biochem. Physiol., 2012, 102, 237 CrossRef CAS.
- F. Cui, T. Chai, L. Qian and C. J. Wang, Chemosphere, 2017, 169, 107 CrossRef CAS.
- Y. H. Liu, R. X. Guo, S. K. Tang, F. Y. Zhu, Z. Y. Yan and J. Q. Chen, Chemosphere, 2018, 195, 542 CrossRef CAS.
- V. Lavtizar, R. Helmus, S. A. E. Kools, D. Dolenc, S. L. Waaijers and M. H. S. Kraak, Environ. Sci. Technol., 2015, 49, 3922 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07121h |
| ‡ These authors have equally contributed to this article. |
| This journal is © The Royal Society of Chemistry 2020 |