 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Temperature effects on the C–H symmetric stretching vibrational frequencies of guest hydrocarbon molecules in 512, 51262 and 51264 cages of sI and sII clathrate hydrates†
Go Fuseya a,
Satoshi Takeya
a,
Satoshi Takeya b and
Akihiro Hachikubo
b and
Akihiro Hachikubo *a
*a
aKitami Institute of Technology, 165, Koen-cho, Kitami 090-8507, Japan. E-mail: hachi@mail.kitami-it.ac.jp
bNational Institute of Advanced Industrial Science and Technology (AIST), Central 5, 1-1-1, Higashi, Tsukuba 305-8565, Japan
First published on 12th October 2020
Abstract
C–H symmetric stretching vibrational frequencies of CH4, C2H4 and C2H6 molecules encapsulated in 512, 51262 and 51264 cages of structures I (sI) and II (sII) clathrate hydrates measured by Raman spectroscopy in the temperature range of 93–183 K was analysed. The slopes of the symmetric stretch vibrational frequencies under changing temperatures (Δv/ΔT) for CH4, C2H4 and C2H6 molecules encapsulated in sII 51264 cages were smaller than those for molecules in sI 51262 cages, although sI 51262 cages are smaller than sII 51264 cages. We compared the results of Δv/ΔT in this study with the geometrical properties of each host water cage, and these comparisons suggest that the geometry of host water cages affects Δv/ΔT.
Introduction
Clathrate hydrates, commonly known as gas hydrates, are crystalline inclusion compounds consisting of guest molecules of suitable sizes and shapes encapsulated in well-defined cages formed by water molecules. Gas hydrates with encapsulated hydrocarbon gases, which exist in sea/lake bottom sediments, have attracted considerable interest as a potential source of natural gas.1–3 There are three common crystallographic structures of hydrates, structure I (sI), structure II (sII) and structure H (sH).4–6 The unit cell of sI hydrates comprises two pentagonal dodecahedral (512) and six tetrakaidecahedral (51262) water cages.4 For sII hydrates, the unit cell is formed by sixteen 512 cages and eight hexakaidecahedral (51264) water cages.5 Small guest molecules such as methane (CH4), ethylene (C2H4) and ethane (C2H6) form sI hydrates, whereas larger molecules such as propane (C3H8) or 2-methylpropane (C4H10) form sII hydrates. C2H4 and C2H6 are encapsulated in sI 51262 cages, while sI 512 cages remain almost empty at equilibrium pressure conditions except at high-pressure conditions (∼100 MPa).7–10 A mixture of CH4 + C2H6 hydrates can form both sI and sII hydrates, depending on the gas composition of CH4 and C2H6.11 Coexisting states of both sI and sII CH4 + C2H6 hydrates have also been observed in natural settings.12 Moreover, in environments where larger hydrocarbons, e.g., C3H8 or C4H10 are present, smaller hydrocarbon molecules can also be encapsulated in the sII hydrate.13 Thus, accumulating knowledge about hydrocarbon hydrates in sI and sII structures is important.Raman spectroscopy has been frequently used for gas hydrates to identify the type of crystal structure11,14,15 or to analyse cage occupancy14 and type of guest molecule.15 The Raman spectra of the C–H symmetric stretch region of encapsulated CH4 and C2H6 have been used to identify the types of crystal structures and guest molecules.16,17 Raman spectra of hydrocarbon hydrates have shown that the C–H stretching frequencies of hydrocarbon molecules in large cages are generally lower than those of molecules in small cages.11,14,15 Subramanian and Sloan rationalised this observation in terms of the guest–host intermolecular interactions using the loose cage–tight cage (LCTC) model as an explanation for matrix-isolation IR experiments.15,18 The variation in the C–H stretching frequencies of various hydrocarbons with varying types of water cages has been computed by quantum chemical computations.19 Investigating the C–H stretching frequencies of guest hydrocarbons in water cages is important for understanding the fundamental properties of gas hydrates. In addition, the guest–host interactions in gas hydrates play a role in the thermal expansion ratio of different types of guests and crystallographic structures20 and in the expression of a unique phenomenon known as self-preservation.21
In earlier studies, the temperature dependence of the C–H symmetric stretching frequencies of encapsulated CH4 as a guest molecule were investigated using Raman spectroscopy.22–24 In the case of sI CH4 hydrate, the thermal variation in the frequencies of CH4 in larger cages (sI 51262 cages) is greater than that of frequencies of CH4 in smaller cages (sI 512 cages).24 In another earlier study, the neutron diffraction experiments for sI deuterated CD4 hydrate showed that CD4 in the sI 51262 cage distributes longitudinally within the cage at temperatures higher than 80 K, whereas CD4 in the 512 cage distributes spherically around the center of the cage even at higher temperatures.25 These results suggest that the distance between the guest and host molecules in the sI 51262 cage is smaller than that in the sI 512 cage. We proposed the idea that the difference in the thermal variations of the C–H symmetric stretching frequencies of CH4 in sI 512 and 51262 cages is caused by the difference in distribution changes of guest CH4 under changing temperature.24 The trend of the variations of the C–H vibrational frequencies of the encapsulated hydrocarbon molecules in the gas hydrate under changing temperature is useful for better understanding the trends of the temperature change of the distribution of guest molecules. However, these trends for hydrocarbons encapsulated in sII hydrate have not been investigated. It has also been reported that the distribution of guest molecules differs depending on the geometry of guest molecules.9,10,26,27 Hence, additional investigation for the C–H vibrational frequencies of encapsulated hydrocarbon molecules in various combinations of host cages and guest molecules are expected.
In this study, we observed the variations in the Raman shift of C–H symmetric stretching vibrations for various guest hydrocarbon molecules in sI 512 and 51262 cages and in sII 512 and 51264 cages under changing temperatures. Gas hydrates of sI C2H4 hydrate, sII Kr + C2H4 hydrate, sI C2H6 hydrate, sI CH4 + C2H6 hydrate and sII CH4 + C2H6 hydrate were investigated. From these results, we discuss the variations in the Raman shift of the C–H symmetric stretching vibrations and the geometric dependence of the intermolecular interaction energies within the water cages of sI and sII hydrates with varying temperature.
Experimental section
Sample preparation
To synthesise gas hydrate samples, research-grade CH4, C2H4 and C2H6 (purities of 99.99, 99.9 and 99.99%, respectively; Takachiho Chemical Industry, Japan) and Kr (99.9% purity; Air Liquide Japan Ltd.) were used as the guest gases. These gas hydrate samples were formed from fine ice powder at 273.2 K and high-pressure conditions. For C2H4 hydrate and C2H6 hydrate, 1.2 MPa of C2H4 and C2H6 gas pressure was applied. The Kr + C2H4 hydrate was prepared from a gas mixture containing 9 mol% C2H4 and 91 mol% Kr at 1.6 MPa. sI CH4 + C2H6 hydrates were prepared from a gas mixture containing 70 mol% C2H6 and 30 mol% CH4 at 2.0 MPa. sII CH4 + C2H6 hydrates were prepared from a gas mixture containing 15 mol% C2H6 and 85 mol% CH4 at 2.0 MPa.Fine ice powder (1.0 g) was prepared for preparing high-purity hydrate sample and was loaded into a high-pressure cell (internal volume: ∼30 mL), which was precooled in a freezer at 253 K. After loading at 253 K, the high-pressure cell was cooled to below 90 K, and pure CH4, C2H4, C2H6 or Kr gas was slowly introduced into the cell. The high-pressure cell was then transferred into a water bath kept at 273.2 K for hydrate formation. As the hydrates formed, the pressure decreased. When the pressure stabilised more than 12 hours later, the cell was cooled below 90 K, and the sample was retrieved from the cell.
Raman spectroscopy
A Raman spectrometer (Jasco Corporation, RMP-210) equipped with a 532 nm excitation source (100 mW), a single holographic diffraction grating (1800 grooves per mm) and a CCD detector were used. The spectrum pixel resolution, which is the sampling interval of the spectrum, was 0.9 cm−1 per pixel in the range of 2500–3100 cm−1. The wave number was calibrated using atomic emission lines from a neon lamp. The Raman spectra for the C–H symmetric stretch region (2500–3100 cm−1) of the encapsulated hydrocarbon molecules in the gas hydrate water cages were obtained at ambient pressure within a temperature range of 93–183 K at 15 K intervals. The measured temperature was confirmed by using a thermocouple (Type T, 01-T, Ninomiya Electric Wire Co. Ltd., Japan). The calibrated thermocouple had an accuracy within 0.1 K. The peak positions could be rigorously analysed by fitting the data to a mixed Gaussian–Lorentzian function, which allowed us to obtain a high positional accuracy. We measured the C–H symmetric stretch of the sI C2H6 hydrate 18 times at 123 K at the same sample position. From these measurements, the standard deviation of the peak positions was found to be approximately 0.1 cm−1.Powder X-ray diffraction (PXRD)
Temperature-dependent PXRD measurements were performed using an X-ray diffractometer (40 kV, 40 mA; Rigaku model Ultima-III) with parallel beam optics and a low-temperature chamber. Finely powdered hydrate samples were mounted on a PXRD sample holder made of 2.5 mm thick Cu at a temperature of around 100 K. Each measurement was performed in a θ/2θ step scan mode with a step width of 0.02° using Cu Kα radiation (λ = 1.541 Å).Gas chromatography
Molecular compositions of CH4 and C2H6 in gas hydrate samples were determined using a gas chromatograph (Shimadzu Corporation, GC-2014) equipped with a packed column (Shimadzu Corporation, Sunpak-S), along with a thermal conductivity detector and fame ionisation detector.Results and discussion
Fig. 1 depicts the Raman spectra of the C–H stretching region of sI C2H4 hydrate, sII Kr + C2H4 hydrate, sI C2H6 hydrate, sI CH4 + C2H6 hydrate and sII CH4 + C2H6 hydrate at a temperature range of 93–183 K. We confirmed the crystal structures of sI C2H4 hydrate and sII Kr + C2H4 hydrate and their lattice constants by the PXRD method (Fig. S1 and S2†).The Raman spectra of the C–H symmetric stretch of encapsulated CH4 in sI CH4 + C2H6 hydrate and sII CH4 + C2H6 hydrate were observed at 2912.7 cm−1 (in sI 512 cages) and 2901.6 cm−1 (in sI 51262 cages); 2913.5 cm−1 (in sII 512 cages) and 2902.2 cm−1 (in sII 51264 cages) at 93 K. Here, each yC2H6 (bulk guest composition of C2H6) of sI and sII CH4 + C2H6 hydrates were 79.7% and 35.3%, respectively.
For sI C2H4 hydrate, the Raman spectrum of the C–H symmetric stretch of encapsulated C2H4 in sI 51262 cages were observed at 3011.3 cm−1 at 93 K. This result was consistent with an earlier study.7 The Raman shift of the C–H symmetric stretch of encapsulated C2H4 in larger sII 51264 cages (3007.0 cm−1 at 93 K) was red-shifted relative to that of the encapsulated C2H4 in smaller sI 51262 cages. Furthermore, the Raman shift of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C symmetric stretch of the encapsulated C2H4 in sII 51264 cages of sII Kr + C2H4 hydrate (1340.7 ± 0.4 cm−1 at 93 K) was red-shifted relative to that of sI 51262 cages of sI C2H4 hydrate (1342.6 ± 0.4 cm−1 at 93 K) (Fig. 2). These results agree with the LCTC model. This is also the first report of the Raman spectrum of C2H4 encapsulated in sII hydrate.
C symmetric stretch of the encapsulated C2H4 in sII 51264 cages of sII Kr + C2H4 hydrate (1340.7 ± 0.4 cm−1 at 93 K) was red-shifted relative to that of sI 51262 cages of sI C2H4 hydrate (1342.6 ± 0.4 cm−1 at 93 K) (Fig. 2). These results agree with the LCTC model. This is also the first report of the Raman spectrum of C2H4 encapsulated in sII hydrate.
 | ||
| Fig. 2 Raman spectra of C–C stretching region of sI C2H4 hydrate, sII Kr + C2H4 hydrate, sI C2H6 hydrate, sI CH4 + C2H6 hydrate (yC2H6: 79.7%) and sII CH4 + C2H6 hydrate (yC2H6: 35.3%) at 93 K. | ||
In the case of sI C2H6 hydrate, sI CH4 + C2H6 hydrate and sII CH4 + C2H6 hydrate, the Raman spectrum of the C–H symmetric stretch of encapsulated C2H6 in the water cages of gas hydrate were observed at 2942.1 cm−1, 2941.9 cm−1 and 2940.1 cm−1 at 93 K, respectively. The attribution of this vibrational mode was based on the previous literature.28 Furthermore, structures of sI C2H6 hydrate, sI CH4 + C2H6 hydrate and sII CH4 + C2H6 hydrate samples were confirmed from the Raman spectra of the C–C symmetric stretch of encapsulated C2H6 in each gas hydrate (1002.2 ± 0.4 cm−1, 1001.9 ± 0.4 cm−1 and 992.4 ± 0.4 cm−1, respectively, at 93 K; see Fig. 2).11
Here, the notation Δv/ΔT refers to the temperature-dependent slope of the Raman shifts of the C–H symmetric stretching vibrations in guest hydrocarbon molecules encapsulated in the gas hydrate water cages. Fig. 3 shows Δv/ΔT of the encapsulated CH4, C2H4 and C2H6 in water cages of various gas hydrates in a temperature range of 93–183 K. In the case of CH4, Δv/ΔT of encapsulated CH4 in sI 51262 cages (sI CH4 + C2H6 hydrate) were greater than that of encapsulated CH4 in sII 512 and 51264 cages. We observed that Δv/ΔT of CH4 in the larger sI 51262 cages was greater than that of CH4 in 512 cages of sI hydrates.24 Our measurements for the encapsulated CH4 in the sI CH4 + C2H6 hydrate agree with this trend. In the case of C2H4, Δv/ΔT of C2H4 in sI 51262 cages (sI C2H4 hydrate) was greater than that of C2H4 in sII 51264 cages (sII Kr + C2H4 hydrate). Furthermore, in the case of C2H6, Δv/ΔT of C2H6 in sI 51262 cages (sI C2H6 hydrate and sI CH4 + C2H6 hydrate) was greater than that of C2H6 in sII 51264 cages (sII CH4 + C2H6 hydrate). For CH4, C2H4 and C2H6 as guest molecules, Δv/ΔT for molecules in the sI 51262 cages were greater than that for molecules in sII 51264 cages. Moreover, we found that Δv/ΔT of CH4 in sII 512 cages was almost the same as that of CH4 in sII 51264 cages. Specific values of Δv/ΔT from Fig. 3 are summarised in Table 1.
 | ||
| Fig. 3 Effect of temperature on Raman shifts of C–H symmetric stretch of encapsulated CH4, C2H4 and C2H6 in various water cages of sI and sII hydrates.29 | ||
| Guest molecule | Cage | Structure | Hydrate | Raman shift at 93 K [cm−1] | Slope of Raman shift between 93 K and 183 K (Δv/ΔT) [10−2 cm−1/K] |
|---|---|---|---|---|---|
| CH4 | 512 | sI | CH4 + C2H6 (yC2H6: 79.7%) | 2912.7 ± 0.1 | +0.9 ± 0.1 |
| 51262 | sI | CH4 + C2H6 (yC2H6: 79.7%) | 2901.6 ± 0.1 | +2.0 ± 0.3 | |
| 512 | sII | CH4 + C2H6 (yC2H6: 35.3%) | 2913.5 ± 0.1 | +1.3 ± 0.2 | |
| 51264 | sII | CH4 + C2H6 (yC2H6: 35.3%) | 2902.2 ± 0.1 | +1.2 ± 0.1 | |
| C2H4 | 51262 | sI | C2H4 | 3011.3 ± 0.1 | +2.4 ± 0.1 |
| 51264 | sII | Kr + C2H4 | 3007.0 ± 0.1 | +1.0 ± 0.2 | |
| C2H6 | 51262 | sI | C2H6 | 2942.1 ± 0.2 | +3.1 ± 0.1 |
| 51262 | sI | CH4 + C2H6 (yC2H6: 79.7%) | 2941.9 ± 0.1 | +3.2 ± 0.1 | |
| 51264 | sII | CH4 + C2H6 (yC2H6: 35.3%) | 2940.1 ± 0.1 | +1.6 ± 0.1 |
The Raman spectra of sI and sII CH4 + C2H6 hydrates having different cage occupancies of large cages were obtained for verification of the effect of cage occupancies of large cages on Δv/ΔT. The cage occupancies of large cages of sI and sII CH4 + C2H6 hydrates were estimated from yC2H6 (see Table S1†).30 The Raman spectra of sI and sII CH4 + C2H6 hydrates which have different guest composition are shown in Fig. 1 and S3,† and these Δv/ΔT are compared in Fig. S4.† We observed the consistent trends of Δv/ΔT of encapsulated CH4 and C2H6 in sI and sII CH4 + C2H6 hydrates regardless of different cage occupancies of large cages: Δv/ΔT for guest molecules in the 51262 cages were greater than that for molecules in 512 and 51264 cages (see Fig. S4 and Table S1†). Although different distortion and thermal expansion in sII hydrates dependent on the guest species encapsulated in 51264 cages were reported,31 it turned out to that the effect of cage occupancies of large cages have very little impact on Δv/ΔT.
We compared the geometrical properties of 512, 51262 and 51264 cages. sI 512, sII 512 and 51264 cages are known to be almost spherical, although they are slightly distorted, depending on the type of guest molecule. sI 51262 cages, however, are known to be spheroidal and extend along the equatorial plane (Fig. 4). Δv/ΔT values of CH4 in both sII 512 and 51264 cages were equivalent, although these two host water cages were the smallest and largest cages in this study, respectively (see Table 1). Δv/ΔT of guests in the sI 51262 cages was greater compared with the 512 cages and sII 51264 cages. The earlier study indicated that distributions and reorientations of guest molecules were affected by the distortion of the shapes of the host cages. For instance, C2H6 and carbon dioxide (CO2) molecules within the sI 51262 cage lie near the equatorial plane of the cages, with the long axis of the guest molecules lying in the plane.9,10 By contrast, the spherically extended distribution of C3H8 and C4H10 molecules in the sII 51264 cages obtained by the X-ray diffraction structure analysis was apparent.26,27 In addition, in the case of sI CD4 hydrate, the CD4 in sI 512 cages showed a spherical density distribution at the centre of the 512 cage at temperatures of 7.7–185 K, while CD4 in sI 51262 cages showed only a longitudinal density distribution between the two hexagonal faces of the sI 51262 cages.25 These differences in the distributions of guest molecules encapsulated in the different host water cages of gas hydrates may cause thermal vibrations in the guest molecules and variations in guest–host and guest–guest interactions due to varying temperatures.
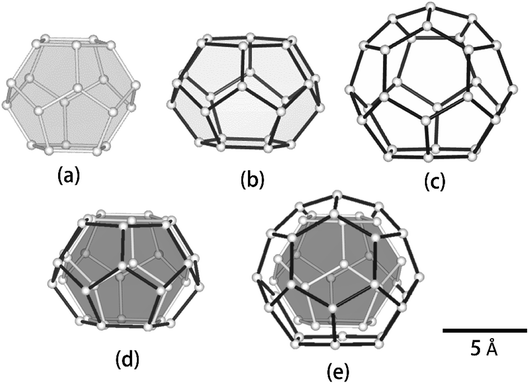 | ||
| Fig. 4 Geometry of water cages of sI and sII hydrates: (a) 512 cage, (b) 51262 cage, (c) 51264 cage, (d) 512 cage vs. 51262 cage and (e) 512 cage vs. 51264 cage. | ||
In our previous study, we considered the effect of the size of the host cages on Δv/ΔT and found that the slope increased with increasing host water cage volume.24 In this study, we revealed that Δv/ΔT depends on the geometry as well as the volume of host water cages. For a discussion about the effect of the conformation of guest molecules on Δv/ΔT, more studies of various combinations of guest molecules and host water cages are needed. These experimental trends of Δv/ΔT for the various water cages of gas hydrates may advance our understanding of the fundamental properties of sI and sII hydrocarbon gas hydrates.
Conclusions
In this work, we investigated the slopes of C–H symmetric stretching vibrational frequencies under changing temperatures (Δv/ΔT) for various hydrocarbon molecules in 512, 51262 and 51264 cages of sI and sII hydrates. Δv/ΔT values of CH4, C2H4 and C2H6 molecules encapsulated in sII 51264 cages were smaller than those for molecules in sI 51262 cages. In our previous study, we suggested that Δv/ΔT is greater with increasing volume of host water cages. In this study, we revealed that Δv/ΔT is dependent on the geometry, as well as the volume of host water cages and guest molecules.In the future, we intend to investigate the effects of the conformation of guest molecules on Δv/ΔT. For a discussion about the effect of the conformation of guest molecules on Δv/ΔT, more studies of various combinations of guest molecules and host water cages are required.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Grant-in-Aid for Scientific Research (B) 26303021 of the Japan Society for the Promotion of Science (JSPS).References
- E. D. Sloan, Nature, 2003, 426, 353–359 CrossRef CAS.
- Y. F. Makogon, S. A. Holditch and T. Y. Makogon, J. Pet. Sci. Eng., 2007, 56, 14–31 CrossRef CAS.
- R. Boswell and T. S. Collett, Energy Environ. Sci., 2011, 4, 1206–1215 RSC.
- H. R. Müller and M. V. Stackelberg, Naturwissenschaften, 1952, 39, 20 CrossRef.
- M. V. Stackelberg and H. R. Müller, Naturwissenschaften, 1951, 38, 456 CrossRef.
- J. A. Ripmeester, J. S. Tse, C. I. Ratcliffe and B. M. Powell, Nature, 1987, 325, 135–136 CrossRef CAS.
- T. Sugahara, K. Morita and K. Ohgaki, Chem. Eng. Sci., 2000, 55, 6015–6020 CrossRef CAS.
- K. Morita, S. Nakano and K. Ohgaki, Fluid Phase Equilib., 2000, 169, 167–175 CrossRef CAS.
- K. A. Udachin, C. I. Ratcliffe and J. A. Ripmeester, J. Supramol. Chem., 2002, 2, 405–408 CrossRef CAS.
- S. Takeya, K. A. Udachin, I. L. Moudrakovski, R. Susilo and J. A. Ripmeester, J. Am. Chem. Soc., 2010, 132, 524–531 CrossRef CAS.
- S. Subramanian, R. A. Kini, S. F. Dec and E. D. Sloan, Chem. Eng. Sci., 2000, 55, 1981–1999 CrossRef CAS.
- M. Kida, O. Khlystov, T. Zemskaya, N. Takahashi, H. Minami, H. Sakagami, A. Krylov, A. Hachikubo, S. Yamashita, H. Shoji, J. Poort and L. Naudts, Geophys. Res. Lett., 2006, 33, L24603 CrossRef.
- C. Bourry, B. Chazallon, J. L. Charlou, J. P. Donval, L. Ruffine, P. Henry, L. Geli, M. N. Çagatay, S. İnan and M. Moreau, Chem. Geol., 2009, 264, 197–206 CrossRef CAS.
- A. K. Sum, R. C. Burruss and E. D. Sloan, J. Phys. Chem. B, 1997, 101, 7371–7377 CrossRef CAS.
- S. Subramanian and E. D. Sloan, J. Phys. Chem. B, 2002, 106, 4348–4355 CrossRef CAS.
- S. Subramanian and E. D. Sloan, Ann. N. Y. Acad. Sci., 2000, 912, 583–592 CrossRef CAS.
- M. M. Murshed and W. F. Kuhs, J. Phys. Chem. B, 2009, 113, 5172–5180 CrossRef CAS.
- G. C. Pimentel and S. W. Charles, Pure Appl. Chem., 1963, 7, 111–124 CAS.
- Y. Liu and L. Ojamäe, J. Phys. Chem. C, 2015, 119, 17084–17091 CrossRef CAS.
- K. C. Hester, Z. Huo, A. L. Ballard, C. A. Koh, K. T. Miller and E. D. Sloan, J. Phys. Chem. B, 2007, 111, 8830–8835 CrossRef CAS.
- S. Takeya and J. A. Ripmeester, Angew. Chem., Int. Ed., 2008, 47, 1276–1279 CrossRef CAS.
- Y. Jin, M. Kida and J. Nagao, J. Phys. Chem. C, 2019, 123, 17170–17175 CrossRef CAS.
- J. Min, Y. H. Ahn, S. Baek, K. Shin, M. Cha and J. W. Lee, J. Phys. Chem. C, 2019, 123, 20705–20714 CrossRef CAS.
- G. Fuseya, S. Takeya and A. Hachikubo, RSC Adv., 2020, 10, 17473–17478 RSC.
- A. Hoshikawa, N. Igawa, H. Yamauchi and Y. Ishii, J. Chem. Phys., 2006, 125, 034505 CrossRef.
- K. A. Udachin, H. Lu, G. D. Enright, C. I. Ratcliffe, J. A. Ripmeester, N. R. Chapman, M. Riedel and G. Spence, Angew. Chem., Int. Ed., 2007, 46, 8220–8222 CrossRef CAS.
- S. Takeya, H. Fujihisa, A. Hachikubo, H. Sakagami and Y. Gotoh, Chem. – Eur. J., 2014, 20, 17207–17213 CrossRef CAS.
- G. Magnotti, U. KC, P. L. Varghese and R. S. Barlow, J. Quant. Spectrosc. Radiat. Transfer, 2015, 163, 80–101 CrossRef CAS.
- H. Ohno, M. Kida, T. Sakurai, Y. Iizuka, T. Hondoh, H. Narita and J. Nagao, ChemPhysChem, 2010, 11, 3070–3073 CrossRef CAS.
- M. Kida, H. Sakagami, N. Takahashi, A. Hachikubo, H. Shoji, Y. Kamata, T. Ebinuma, H. Narita and S. Takeya, J. Jpn. Pet. Inst., 2007, 50, 132–138 CrossRef CAS.
- S. Takeya, S. Alavi, S. Hashimoto, K. Yasuda, Y. Yamauchi and R. Ohmura, J. Phys. Chem. C, 2018, 122, 18134–18141 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06668k |
| This journal is © The Royal Society of Chemistry 2020 |

