Open Access Article
Open Access ArticleSequestration of pyridinium herbicides in plants by carboxylated pillararenes possessing different alkyl chains†
Mian Tang‡
a,
Qiang Bian‡b,
Ying-Ming Zhang*a,
Muhammad Arifc,
Qiong Luoc,
Shuzhen Menc and
Yu Liu *a
*a
aCollege of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China. E-mail: ymzhang@nankai.edu.cn; yuliu@nankai.edu.cn
bNational Pesticide Engineering Research Center, College of Chemistry, Nankai University, Tianjin 300071, China
cDepartment of Plant Biology and Ecology, College of Life Sciences, Nankai University and Tianjin Key Laboratory of Protein Sciences, Tianjin 300071, China
First published on 22nd September 2020
Abstract
We report that the sequestration of pyridinium-containing herbicides can be achieved on plant foliage through the strong supramolecular complexation with water-soluble pillararenes. The host–guest interaction appears to exert a protective effect on the plant growth, thus holding great promise in agricultural application.
Indiscriminate and inefficient use of agrochemicals, such as herbicides, pesticides, and fertilizers, may cause their loss, decomposition, and bioaccumulation in natural ecological circulation, thus leading to many adverse environmental concerns and social impacts.1 Therefore, it is absolutely imperative to reduce the hazards of surplus agrochemicals from crop plants and other sites of action to ensure the sustainable agricultural development.2 To this end, the present acceptable decontamination and sequestration procedures of agrochemicals generally involve incineration, photo-degradation, soil disposal, and chemical redox treatment, but they always suffer from problems, including the release of unclean flue gases and toxic byproducts, as well as the installation and operation of costly equipment.3
To date, supramolecular encapsulation by synthetic cavity-bearing macrocycles (for example, cyclodextrin, calixarene, cucurbituril, pillararene, and others), have emerged as a feasible approach to remove many toxic substances from external environment and animal models without changing the chemical structures, which can greatly alleviate their undesired side effects on biological and ecological systems and thus exhibit appealing practical prospects. A plethora of fundamental research have shown the power of supramolecular scavengers based on the host–guest molecular recognition, such as clearance of organic pollutants by cross-linked cyclodextrins and amphiphilic calixarene,4 removal of cytotoxic bile acids in vivo by amino acid-modified cyclodextrins,5 and treatment of herbicide poisoning by cucurbituril, sulfonated calixarene, and carboxylated pillararene,6 as well as many cases of macrocycle-based antidotes with great clinic potentials.7
Paraquat (PQ) and diquat (DQ) possessing 4,4′-bipyridinium core are two of the globally used herbicidal agents for weed and grass control but they also have irreversible toxicity/damage to the human body. The IC50 (half maximal inhibitory concentration) value of PQ is 117 μM and LC50 (median lethal concentration) value of DQ is 14 μM, respectively.8 Moreover, they are a typical class of positively charged molecules that can form a variety of extraordinarily strong host–guest complexes with many different macrocycles, which is regarded as the structural basis in the treatment for PQ poisoning (Scheme 1). Encouragingly, it is worth appreciating that in parallel with the rapid evolution of stimuli-responsive nano-based agrochemicals, the role of pyridinium-derived compounds in supramolecular chemistry rotates back toward the area of herbicides and agricultural application whence they started.9 Meanwhile, in the realm of supramolecular chemistry, carboxylated pillararenes with excellent water solubility and electron-rich cavities have been widely explored as the promising macrocyclic receptors in achieving strong and selective molecular binding processes, particularly with bispyridinium salts to form fairly stable inclusion complexes.10 In this work, by virtue of the high binding affinity between the pyridinium-containing herbicides and the water-soluble pillararenes with different alkyl chains, supramolecular methodology has been successfully implemented in the sequestration of PQ and DQ from plant foliage, with the final goal of extending the supramolecular therapeutics from disease treatment to plant protection and crop production.2,6,11 Significantly, it is also demonstrated that the complexation-assisted decontamination efficiency is highly dependent on their host–guest complexation between pillararenes and herbicides, thereby providing a valuable strategy in attaining more advanced herbicidal nanoformulations.
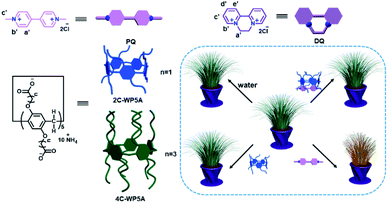 | ||
| Scheme 1 Schematic illustration of inclusion complexes consisting of PQ, DQ, and carboxylated pillararenes for herbicide sequestration from plant foliage. | ||
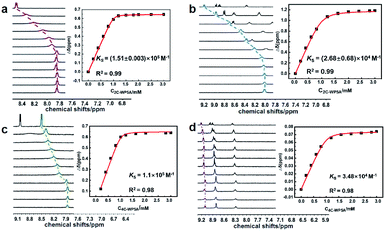
The schematic illustration of sequestration effect by the formation of binary inclusion complexes is depicted in Scheme 1. The molecular binding behaviors of PQ and DQ with the carboxylated pillararenes possessing different alkyl chains in length (2C-WP5A and 4C-WP5A) were preliminarily investigated by 1H NMR titration method. As a class of macrocyclic receptors with inherent electron-rich cavity, pillararenes can readily form stable host–guest complexes with electron-deficient molecules via the cooperative π-stacking and electrostatic interactions.12 In our case, as discerned from Fig. 1, the resonance peaks of aromatic (Ha′,b′) and methyl sites (Hc′) in PQ underwent pronounced complexation-induced upfield shifts in the presence 2C-WP5A, proton attributions were described in ESI (Fig. S1†). Meanwhile, similar tendency was also observed in the case of PQ⊂4C-WP5A complexation, implying that the whole PQ molecule can keep close contact with the macrocyclic cavity and adopt the same inclusion modes with 2C-WP5A and 4C-WP5A (Fig. 1a and c). In comparison, the proton signals of pyridinium core in DQ gave slight chemical shift changes, accompanied by the downfield shift in the methylene group upon complexation with 2C-WP5A and 4C-WP5A (Fig. 1b and d). These phenomena are mainly contributed to the more flexible binding geometry in the DQ⊂4C-WP5A complexation and may be indicative of a relatively weaker affinity upon association with DQ, which can be further confirmed by their stability constants (KS) in solution as described below.
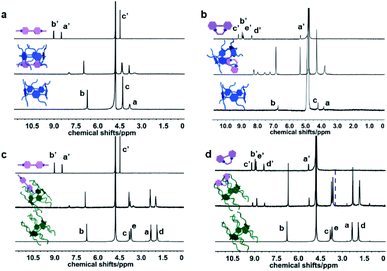 | ||
| Fig. 1 Partial 1H NMR spectra (D2O, 400 MHz, 25 °C) of (a) PQ⊂2C-WP5A, (b) DQ⊂2C-WP5A, (c) PQ⊂4C-WP5A, and (d) DQ⊂4C-WP5A complexes, respectively ([host] = [guest] = 1 mM). | ||
Furthermore, after validating the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry by Job's plots, the KS values were accordingly calculated as 1.51 × 106, 2.68 × 104, 1.10 × 106, 3.48 × 104 M−1 for PQ⊂2C-WP5A, DQ⊂2C-WP5A, PQ⊂4C-WP5A, DQ⊂4C-WP5A complexes, respectively, by using the nonlinear least-squares curve-fitting method in the 1H NMR titration experiments (Fig. 2 and S2, ESI†). Benefitting from the preferable size/shape matching effect that makes the accommodated PQ more immovable around the macrocyclic cavity, the guest selectivity for PQ is found to be 30–50 times higher than the one for DQ, regardless of the different spacer length in the WP5A hosts.
1 binding stoichiometry by Job's plots, the KS values were accordingly calculated as 1.51 × 106, 2.68 × 104, 1.10 × 106, 3.48 × 104 M−1 for PQ⊂2C-WP5A, DQ⊂2C-WP5A, PQ⊂4C-WP5A, DQ⊂4C-WP5A complexes, respectively, by using the nonlinear least-squares curve-fitting method in the 1H NMR titration experiments (Fig. 2 and S2, ESI†). Benefitting from the preferable size/shape matching effect that makes the accommodated PQ more immovable around the macrocyclic cavity, the guest selectivity for PQ is found to be 30–50 times higher than the one for DQ, regardless of the different spacer length in the WP5A hosts.
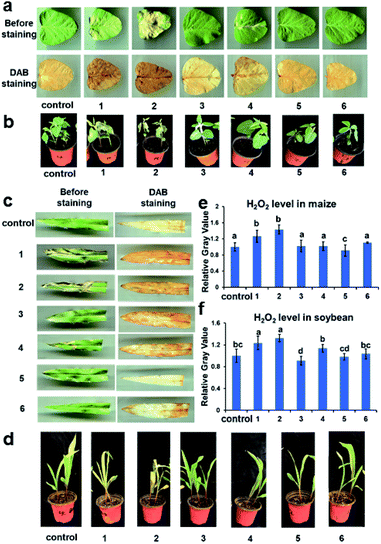
With a clear understanding of the binding modes and strengths, plant phenotypes were further compared to test the sequestration effect by supramolecular inclusion complexation. In this case, maize and soybean were employed as model plants and sprayed with 0.2 mg mL−1 of PQ or DQ, which is an equivalent dose treated in the field application. As shown in Fig. S5a,† the soybean leaves turned yellow after 6 h and were desiccated and withered within 1 day. In addition, no negative impact was found on the soybean growth in the presence of free WP5A. In sharp contrast, when sprayed with the binary inclusion complexes, the colour of soybean leaves still remained green and no leaf senescence was observed for quite a long time, which resembles the one in the control group. More photographic images in the maize group were presented in the ESI (Fig. S5†).
More evidence on the sequestration effect by supramolecular complexation comes from the concentration-variable and leaf-staining experiments. After 6 h treatment, compared to the free PQ groups with severe necrosis, the protection effect was clearly observed in the complex-treated groups cultivated with soybean and maize at both concentrations of PQ (0.1 and 0.2 mg mL−1 in Fig. 3b and d). Moreover, it is known that as the extremely poisonous, rapid-acting, and nonselective herbicides, PQ can interrupt the photosynthesis system and redox pathway by massive generation of the reactive oxygen species (ROS), such as superoxide (O2−), hydroxyl (˙OH) radicals, and hydrogen peroxide (H2O2).13 Thus, the ROS accumulation at elevated level is identified as the signalling factor in leaf senescence. To further explore the role of WP5A in achieving the decontamination effect, the H2O2 content in different plants was measured by the 3,3-diaminobenzidine (DAB) staining experiments. After 6 h treatment, the colour depth of the PQ-treated soybean and maize was significantly greater than that of the WP5A- and complex-treated plants, indicating that the concentrations of H2O2 and DAB oxidation products were largely decreased in the presence of WP5A (Fig. 3a and c). Meanwhile, the quantitative grey values for ROS production were accordingly measured, which is well consistent with those phenotypes (Fig. 3e and f). Collectively, these results jointly corroborate that PQ participates in leaf senescence by upregulating the ROS production and the WP5A-mediated sequestration can efficiently protect the plant from the attack of pyridinium herbicides mainly through the reduction of ROS content.
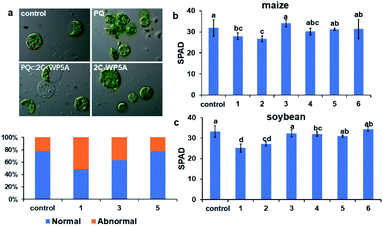
Furthermore, as can be seen from Fig. 4a, damaged plasma membrane and shrunken chloroplast occurred in Arabidopsis protoplasts when treated with PQ. In comparison, such PQ-induced morphological change in protoplasts was largely inhibited upon addition of 2C-WP5A, suggesting that WP5A is more effective in maintaining the integrity of cell structure in plant. Meanwhile, the quantitative percentage of chlorophyll retention was fully consistent with these phenotypes (Fig. 4b and c). These phenomena in the cellular environment strongly manifest that the inclusion complexation with pillararenes is of critical importance in reducing the PQ toxicity in plants.
Finally, potted miniature plants containing three main kinds of crop plants (maize, soybean, and rice) as models were cultivated together as a small-scale filed to simulate the natural growing environment. As expected, only after 12 h, severe leaf senescence symptoms appeared in all the plants when sprayed with PQ at 0.1 mg mL−1 (Fig. 5b). More gratifyingly, newly emerging leaves had been constantly observed during the following 15 days, which eventually reached the same growth stages as the control group (Fig. 5b). However, plants in the complex-treated groups were still alive under the same growth conditions, although visible wilting with some brown spots were sporadically found in the plant foliage on the first 7 days (Fig. 5a). These exciting results demonstrate the great potential of supramolecular complexation-assisted sequestration procedure for the large-scale agricultural application.
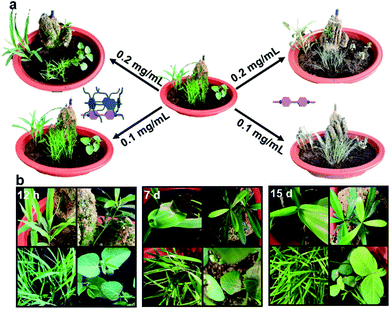 | ||
| Fig. 5 (a) Miniature plants after treatment with PQ and PQ⊂2C-WP5A complex for 7 days. (b) Miniature plants after treatment with PQ for 12 h, 7 days and 15 days. | ||
In conclusion, benefiting from the high binding affinities with WP5A, the herbicidal toxicity of PQ and DQ could be greatly inhibited in many species of living plants. Moreover, as investigated by the morphological characterization, pillararenes could efficiently prevent the chloroplasts and cell membranes from the irreversible damages on photosynthesis system and eventually promote the plant growth with a longer life span. Thus, the present work may offer a feasible and environmentally attractive alternative to substantially eliminate the intrinsic toxicity and diminish the indiscriminate use of conventional herbicides. Although there are tremendous challenges in environmental and cost issues, it is possible to envision that, once equipped with active targeting groups onto the backbone of macrocyclic receptors, our supramolecular sequestration approach featuring the complexation-enhanced sequestration behaviours may open up new opportunities for practical translation of nano-based smart herbicide formulations in the safe agricultural development.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grants 21871154, 21772099, and 21861132001), and the Fundamental Research Funds for the Central Universities, Nankai University.Notes and references
- T. R. Hawkes, Pest Manage. Sci., 2014, 70, 1316–1323 CrossRef CAS
.
-
(a) F. Gomollón-Bel, Chem. Int., 2019, 41, 12–17 Search PubMed
; (b) S. Kumar, M. Nehra, N. Dilbaghi, G. Marrazza, A. A. Hassan and K.-H. Kim, J. Controlled Release, 2019, 294, 131–153 CrossRef CAS
; (c) M. Kah, R. S. Kookana, A. Gogos and T. D. Bucheli, Nat. Nanotechnol., 2018, 13, 677–684 CrossRef CAS
; (d) G. W. Walker, R. S. Kookana, N. E. Smith, M. Kah, C. L. Doolette, P. T. Reeves, W. Lovell, D. J. Anderson, T. W. Turney and D. A. Navarro, J. Agric. Food Chem., 2018, 66, 6480–6486 CrossRef CAS
; (e) X. Zhao, H. Cui, Y. Wang, C. Sun, B. Cui and Z. Zeng, J. Agric. Food Chem., 2018, 66, 6504–6512 CrossRef CAS
.
-
(a) C. K. Remucal, Environ. Sci.: Processes Impacts, 2014, 16, 628–653 RSC
; (b) T.-T. Zhang, J. Huang, S.-B. Deng and G. Yu, Environ. Pollut., 2011, 159, 1744–1748 CrossRef CAS
; (c) T. Castelo-Grande, P. A. Augusto, P. Monteiro, A. M. Estevez and D. Barbosa, Int. J. Environ. Anal. Chem., 2010, 90, 438–467 CrossRef CAS
.
-
(a) M. J. Klemes, Y. Ling, C. Ching, C. Wu, L. Xiao, D. E. Helbling and W. R. Dichtel, Angew. Chem., Int. Ed., 2019, 58, 12049–12053 CrossRef CAS
; (b) Z. Zheng, H. Yu, W.-C. Geng, X.-Y. Hu, Y.-Y. Wang, Z. Li, Y. Wang and D.-S. Guo, Nat. Commun., 2019, 10, 5762 CrossRef CAS
.
-
(a) Y.-M. Zhang, X. Xu, Q. Yu, H.-J. Yu and Y. Liu, iScience, 2019, 15, 223–233 CrossRef CAS
; (b) Y.-M. Zhang, X. Xu, Q. Yu, Y.-H. Liu, Y.-H. Zhang, L.-X. Chen and Y. Liu, J. Med. Chem., 2017, 60, 3266–3274 CrossRef CAS
.
-
(a) X. Zhang, X. Xu, S. Li, L. Li, J. Zhang and R. Wang, Theranostics, 2019, 9, 633–645 CrossRef CAS
; (b) G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489–19497 CrossRef CAS
; (c) G.-F. Wang, X.-L. Ren, M. Zhao, X.-L. Qiu and A.-D. Qi, J. Agric. Food Chem., 2011, 59, 4294–4299 CrossRef CAS
; (d) K. Wang, D.-S. Guo, H.-Q. Zhang, D. Li, X.-L. Zheng and Yu Liu, J. Med. Chem., 2009, 52, 6402–6412 CrossRef CAS
.
-
(a) J. Chen, H. Ni, Z. Meng, J. Wang, X. Huang, Y. Dong, C. Sun, Y. Zhang, L. Cui, J. Li, X. Jia, Q. Meng and C. Li, Nat. Commun., 2019, 10, 3546 CrossRef
; (b) X. Zhang, Q. Cheng, L. Li, L. Shangguan, C. Li, S. Li, F. Huang, J. Zhang and R. Wang, Theranostics, 2019, 9, 3107–3121 CrossRef CAS
; (c) S. Zimmer, A. Grebe, S. S. Bakke, N. Bode, B. Halvorsen, T. Ulas, M. Skjelland, D. De Nardo, L. I. Labzin, A. Kerksiek, C. Hempel, M. T. Heneka, V. Hawxhurst, M. L. Fitzgerald, J. Trebicka, I. Björkhem, J.-Å. Gustafsson, M. Westerterp, A. R. Tall, S. D. Wright, T. Espevik, J. L. Schultze, G. Nickenig, D. Lütjohann and E. Latz, Sci. Transl. Med., 2016, 8, 333ra50 CrossRef
; (d) M. M. Nociari, G. L. Lehmann, A. E. Perez Bay, R. A. Radu, Z. Jiang, S. Goicochea, R. Schreiner, J. D. Warren, J. Shan, S. Adam de Beaumais, M. Menand, M. Sollogoub, F. R. Maxfield and E. Rodriguez-Boulan, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E1402–E1408 CrossRef CAS
; (e) A. I. Rosenbaum, G. Zhang, J. D. Warren and F. R. Maxfield, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5477–5482 CrossRef CAS
; (f) A. Bom, M. Bradley, K. Cameron, J. K. Clark, J. van Egmond, H. Feilden, E. J. Maclean, A. W. Muir, R. Palin, D. C. Rees and M.-Q. Zhang, Angew. Chem., Int. Ed., 2002, 41, 265–270 CrossRef CAS
.
-
(a) E. Damiani, J. A. Solorio, A. P. Doyle and H. M. Wallace, Toxicol. Lett., 2019, 302, 28–34 CrossRef CAS
; (b) Q. Zhang, C. Wang, W.-P. Liu, X.-F. Zhang and S.-L. Zhuang, Environ. Chem. Lett., 2012, 10, 35–39 CrossRef CAS
.
-
(a) X.-S. Li, Y.-F. Li, J.-R. Wu, X.-Y. Lou, J. Han, J. Qin and Y.-W. Yang, J. Mater. Chem. A, 2020, 8, 3651–3657 RSC
; (b) L. Shangguan, B. Shi, Q. Chen, Y. Li, H. Zhu, Y. Liu, H. Yao and F. Huang, Tetrahedron Lett., 2019, 60, 150949 CrossRef CAS
; (c) X. Li, J. Han, X. Wang, Y. Zhang, C. Jia, J. Qin, C. Wang, J.-R. Wu, W. Fang and Y.-W. Yang, Mater. Chem. Front., 2019, 3, 103–110 RSC
; (d) X. Zhang, Q. Huang, Z.-Z. Zhao, X. Xu, S. Li, H. Yin, L. Li, J. Zhang and R. Wang, J. Agric. Food Chem., 2019, 67, 7783–7792 CrossRef CAS
; (e) C. Gao, Q. Huang, Q. Lan, Y. Feng, F. Tang, M. P. M. Hoi, J. Zhang, S. M. Y. Lee and R. Wang, Nat. Commun., 2018, 9, 2967 CrossRef
.
-
(a) T. Ogoshi, T. Kakuta and T.-a. Yamagishi, Angew. Chem., Int. Ed., 2019, 58, 2197–2206 CrossRef CAS
; (b) H. Zhang, Z. Liu and Y. Zhao, Chem. Soc. Rev., 2018, 47, 5491–5528 RSC
; (c) P. Li, Y. Chen and Y. Liu, Chin. Chem. Lett., 2019, 30, 1190–1197 CrossRef CAS
.
-
(a) X. Wang, Z.-J. Liu, E. H. Hill, Y. Zheng, G. Guo, Y. Wang, P. S. Weiss, J. Yu and Y.-W. Yang, Matter, 2019, 1, 848–861 CrossRef
; (b) H. Li, D.-X. Chen, Y.-L. Sun, Y. B. Zheng, L.-L. Tan, P. S. Weiss and Y.-W. Yang, J. Am. Chem. Soc., 2013, 135, 1570–1576 CrossRef CAS
.
-
(a) H.-C. Zhang and Y.-L. Zhao, Chem.–Eur. J., 2013, 19, 16862–16879 CrossRef CAS
; (b) M. Xue, Y. Yong, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS
; (c) T. Ogoshi and T.-a. Yamagishi, Eur. J. Org. Chem., 2013, 15, 2961–2975 CrossRef
; (d) L.-L. Tan and Y.-W. Yang, J. Inclusion Phenom. Macrocyclic Chem., 2015, 81, 13–33 CrossRef CAS
.
-
(a) Y. Gao, J. Ma, J.-C. Zheng, J. Chen, M. Chen, Y.-B. Zhou, J.-D. Fu, Z.-S. Xu and Y.-Z. Ma, Int. J. Mol. Sci., 2019, 20, 3001–3016 CrossRef CAS
; (b) L. Li, Y. Xing, D. Chang, S. Fang, B. Cui, Q. Li, X. Wang, S. Guo, X. Yang, S. Men and Y. Shen, Sci. Rep., 2016, 6, 31889 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06657e |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |