Open Access Article
Open Access ArticleAuNPs@PMo12 nanozyme: highly oxidase mimetic activity for sensitive and specific colorimetric detection of acetaminophen†
Tahereh Rohani Bastami *a and
Zeynab Dabirifarb
*a and
Zeynab Dabirifarb
aDepartment of Chemical Engineering, Quchan University of Technology, Quchan 94771-67335, Iran. E-mail: t.rohani@qiet.ac.ir; tahereh.rohani@gmail.com
bDepartment of Chemical Engineering, Quchan University of Technology, Quchan 94771-67335, Iran
First published on 30th September 2020
Abstract
The design of a highly specific and sensitive approach for the quantitative and qualitative determination of acetaminophen (AP) is crucial from a human health point of view. In this study, AuNPs@PMo12, as a nanozyme, has been developed for the highly sensitive and selective detection of AP with 3,3′,5,5′-tetramethylbenzidine (TMB) within a few seconds without adding oxidizing reagents (e.g. H2O2). Synthesized nanosensors are able to oxidize TMB to yellow-brown oxidized TMB (oxTMB). The maximum peak wavelength of oxTMB was observed at 450 nm. The addition of AP and then increasing its concentration led to the production of different products in blue color. In experimental measurements, the limit of detection was obtained as 14.52 mg L−1. The quantitative determination of AP concentrations can be carried out using UV-vis spectroscopy. The design of nanosensors is cost-effective and application of them in H2O2-free and enzyme-free conditions provides a rapid sensing approach for practical use in disease monitoring and diagnosis.
Introduction
Colorimetric sensing using nanomaterials provides a competitive approach in point-of-care sensing owing to not using enzymes.1,2 One of the best candidates for colorimetric detection is gold nanoparticles (AuNPs), due to their surface plasmon resonance which depends on distance.3,4 Recently, the development of enzyme mimetic nanostructured materials has been a matter of great concern.5,6 Using nanomaterials as enzymes (i.e. nanozymes) in comparison with natural enzymes shows many advantages such as cost-effective, easy and green production, high stability, and robustness. However, metal-based nanomaterials exhibit enzyme-like activities and have been used in immunoassay applications and biosensing.7,8 It is important to notice that the enzyme-like activities of nanozymes depend on the manipulation of some properties, including size, shape, composition, and surface modification.9 It is reported that surface modifications can be more prevailing due to catalytic reactions that occur on the surfaces of nanozymes. 3,3′,5,5′-Tetramethylbenzidine (TMB) is a green noncarcinogenic chromogenic material for colorimetric sensing,19,20 normally applied as a noncarcinogenic colour reagent in enzyme-linked immunosorbent assays (ELISA).10According to the literature, AuNPs show high activities in catalytic oxidation of TMB in the presence of hydrogen peroxide.10 Ni et al.11 developed a technique to detect melamine according to the peroxidase-like activity of gold nanoparticles.11 Wang et al.12 proposed the peroxidase-like activity of AuNPs and the effect of surface modification. They showed that bare AuNPs exhibit higher peroxidase activity in comparison to amino or citrate modified gold nanoparticles. It was also found that the surface charge of nanoparticles and substrates could be an important parameter in the catalytic activity.12 Jv et al. reported that cysteamine-modified positively charged gold nanoparticles and citrate-capped negatively charged gold nanoparticles have different peroxidase-like activities.13 They found that the surface charge of gold nanoparticles plays a crucial role in their catalytic activity.
Polyoxometalates (POMs) have received great interest owing to their wide range of applications in biochemistry, catalyst,14 and sensing.15 POMs can be used as reducing and stabilizing agents in aqueous solutions in ambient conditions.16,17 Also, POMs can be used as a multi-electron reducing agent which is useful for the preparation of noble metal nanoparticles.18,19
According to the literature, POMs can be used as a catalyst for H2O2-based oxidation of organic materials in the presence of O2 and H2O2 via multistep electron-transfer processes.20–22 Thus, POMs can be applied as enzyme mimics to catalyse H2O2-based oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB).23 Li et al. prepared three tetra-nuclear ZrIV-substituted POMs, which exhibit peroxidase-like activities.24 It was found that most of the POMs are stable and have more enzyme activities at low pH values (about 3.0 or 4.0).23,24 However, it is interesting to develop intrinsic oxidase nanozymes since they do not use harmful H2O2 as a co-substrate.
Acetaminophen (AP) is normally used as a medicine in the world.25 The toxic dose of AP leads to acute liver injuries and liver failures.26 The majority of acute liver failures are because of acetaminophen overdose, so it is vital to design a method with rapid quantitative detection of AP in emergency rooms. The most recent techniques for the detection of AP are a variety of analytical approaches like titrimetric methods,27 high-performance liquid chromatography (HPLC),28 spectrophotometry,29 chemiluminescence,30 and electrochemical analysis.31 Colorimetric sensing of analytes is one of the techniques which provide personalized and clinical point-of-care (POC) diagnosis.
In the present research, a nanozyme, polyoxometalate-stabilized gold nanoparticles (AuNPs@PMo12) was prepared under ultrasonic irradiation according to the previous literature.32 Sono-synthesized samples were kept at room temperature and remained stable for more than three months. The as-prepared nanosensor showed remarkable oxidase-like activity of colourless TMB into the coloured product in the free H2O2 medium. The synthesized nanosensor was used for the naked-eye detection and showed a highly selective and specific determination of AP. The obtained results confirmed that the colorimetric reaction between AuNPs–PMo12 as a nanoprobe takes place within a few seconds. The color of the AuNPs@PMo12 nanohybrid solution changed a few seconds after mixing with TMB solution which confirms the rapid sensing of synthesized nanozyme. Moreover, it was found that the oxidase-like activity of AuNPs@PMo12 was improved in the presence of ionic species like PMo12 and Au3+, [AuCl4]−. Synthesized nanosensors could oxidize TMB to (oxTMB) and induced a yellow-brown colour solution corresponding to an absorption peak centered at 450 nm. The addition of AP and increasing its concentration led to the production of blue products. In experimental measurements, the limit of detection was obtained 14.52 μg mL−1. The quantitative determination of AP concentrations can be carried out using UV-vis spectroscopy. To achieve the optimized value of AuNPs@PMo12 nano-hybrids for sensing application, reaction temperature, and sample pH were investigated. The design of nanosensor is cost-effective and is operated in H2O2-free and enzyme-free conditions, which provide a new avenue for rapid sensing in disease monitoring and diagnosis.
Experimental
Materials
All reagents were obtained and used without further purification. Phosphomolybdic acid (H3PMo12O40) (PMo12), sodium hydroxide, and hydrochloric acid (37%) were purchased from Merck Company. 1-Propanol (C3H7OH) and 3,3′,5,5′-tetramethylbenzidine (TMB) were commercially available from Sigma-Aldrich. HAuCl4 solution (0.1 M, 3.4% w/v) was freshly purchased from Kimia Next Company, Iran. Acetaminophen (C8H9NO2), methadone (C21H27NO), methylphenidate (C14H19NO2), aspirin (C9H8O4), and tramadol (C16H25NO2) were obtained from Temad Pharmaceutical Company in Iran. The physicochemical properties of AP are illustrated in Table S1.†33 Milli-Q water was used with minimum resistivity of 18.2 MΩ cm−1.Sonochemical preparation of AuNPs@PMo12
Sonochemical preparation of AuNPs@PMo12 carried out by high power sonicator (20 kHz) at three different temperatures (27 ± 1 °C, 37 ± 1 °C, and 47 ± 1 °C) for 30 minutes with Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POM ratio of 1
POM ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and acoustic amplitude of 50%. The as-prepared products were used for the characterization analysis and detection of AP. The synthesis pathway was according to the previous study.32 Briefly, the HAuCl4 solution (1 mM, 16.5 mL) and PMo12 (1 mM, 33 mL) were mixed and stirred at room temperature. After that, the resulting solution was transferred into 100 mL Dewar cell, and 1.2 mL of propanol was added into this solution. The final solution was exposed to ultrasonic irradiation through an ultrasonic horn (20 kHz, Branson Digital Sonifier-USA, and W-450 D). The acoustic amplitude of the sonicator was adjusted on 50%, which delivered acoustic power of 25 W. The obtained reaction medium turned into wine red, showing the production of gold nanoparticles. Finally, the as-prepared AuNPs@PMo12 solution was kept at room temperature and remained stable for more than three months.
2 and acoustic amplitude of 50%. The as-prepared products were used for the characterization analysis and detection of AP. The synthesis pathway was according to the previous study.32 Briefly, the HAuCl4 solution (1 mM, 16.5 mL) and PMo12 (1 mM, 33 mL) were mixed and stirred at room temperature. After that, the resulting solution was transferred into 100 mL Dewar cell, and 1.2 mL of propanol was added into this solution. The final solution was exposed to ultrasonic irradiation through an ultrasonic horn (20 kHz, Branson Digital Sonifier-USA, and W-450 D). The acoustic amplitude of the sonicator was adjusted on 50%, which delivered acoustic power of 25 W. The obtained reaction medium turned into wine red, showing the production of gold nanoparticles. Finally, the as-prepared AuNPs@PMo12 solution was kept at room temperature and remained stable for more than three months.
Detection of acetaminophen (AP) by UV-vis spectrum measurements
AuNPs@PMo12 nanohybrid was used for the colorimetric detection of AP. 0.9 mL of an AP aqueous solution with different concentrations (0–120 mg L−1) were mixed separately with 0.9 mL of AuNPs@PMo12 solution and 0.1 mL of TMB solution (0.7 mM in ethanol). After that, the color change of the solution was monitored by UV-visible spectroscopy. Control solutions containing 0.1 mL of TMB (0.7 mM in ethanol), 0.9 mL of AuNPs@PMo12 solution and 0.9 mL of Milli-Q-water were prepared.Characterization and apparatus
To obtain the crystal structure of nanohybrids, Bruker D8-Focus with Cu Kα radiation (λ = 0.154056 nm) was used in the range of 2θ = 10–80°. High-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) images were taken with TecnaiS-Twin 30, 300 keV, GIF-TRIDIEM. Energy-dispersive X-ray spectroscopy (EDS) data were measured using the same instrument. The UV-vis spectra were performed using an Evolution 201 Spectrophotometer (Thermo Fisher Scientific). IR spectra were recorded on a Thermo Nicolet Avatar-370-FTIR Spectrometer (Thermo Fisher Scientific, United States). Zeta-potential measurement was carried out using Zeta Compact (CAD Instrumentation, France). Dynamic Light Scattering (DLS) was conducted on Vasco Particle Size Analyzer (Cordouan Technologies). The electrochemical analysis was conducted using a μ-Autolab type III electrochemical workstation. The analysis was done at room temperature using a conventional three-electrode set-up. Platinum wire (Azar Electrode Co.) served as the counter electrode and Ag/AgCl as the reference electrode (Azar Electrode Co.). The sonochemical procedure was conducted using equipment operating at 20 kHz (Branson Digital Sonifier, Model W-450 D). It includes a double cylindrical jacket with a volume of 100 mL to control the temperature. The 20 kHz ultrasonic irradiations were emitted from the tip with a diameter of 1.1 cm which was located at the end of the horn.Determination of acoustic intensity
Acoustic power and intensity were determined by the colorimetric method.34 In this work, an amplitude of 50% was used to deliver acoustic power of 25 W and an intensity of 26 W cm−2.Results and discussions
After the reduction of Au3+ ions with PMo12 as a stabilizing and reducing agents, the AuNPs@PMo12 nano-hybrids were characterized by UV-visible, FT-IR, HAADF-STEM, EDS, zeta potential, cyclic voltammograms (CVs), and DLS, respectively. The details of the reaction mechanism are presented in ESI.† The characterization of AuNPs@PMo12 nano-hybrids is also discussed in ESI.†Design of the proposed colorimetric sensor
The colorimetric analytical technique has attracted considerable attention due to some advantages such as simplicity, cost-effectiveness, and no need of any complicated or expensive instruments. TMB is commonly used as a chromogenic substrate, especially in enzyme-linked immunosorbent assay (ELISA). TMB oxidation by catalyst or enzyme/enzyme-like in the presence of H2O2 generates a blue color, with absorbance peaks at 370 and 652 nm.35 There are some limitations, in terms of reaction conditions, on biological detection. Besides, the enzymatic reaction requires strict conditions, such as pH control, catalyst type, and temperature control of the reaction.As reported elsewhere, gold nanoparticles can oxidize 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of hydrogen peroxide to form a blue product.12 TMB includes two amino groups, which show affinity to the surface of nanoparticle with a negative charge.36 Besides, in acidic media (pH of 3.0 or 4.0), POMs show both higher enzyme activity and stability. But, the POM nanozymes become catalytically inactive in the physiological solutions (pH 7.0–7.5). The enzyme-like activities of POMs with Keggin type structures were reported.23 It was claimed that Mo and W as coordination atoms show more influence on the enzyme-like activity of POM.23 According to another literature, polyoxomolybdates (H3PMo12O40) can oxidize TMB as a substrate to form a blue color substance with maximum absorbance at 650 nm and without the presence of H2O2.23
So, we choose AuNPs decorated with PMo12 to oxidize TMB due to more stability of AuNPs than enzymes. Here, the catalytic activity of AuNPs@PMo12 on the colorimetric reaction of TMB in the absence of H2O2 was investigated. Also according to another literature, unreacted Au3+ ions and PMo12 which exist in the AuNPs@PMo12 solution could help to oxidize TMB to oxTMB.12
Mechanism investigation on colorimetric sensing of AP
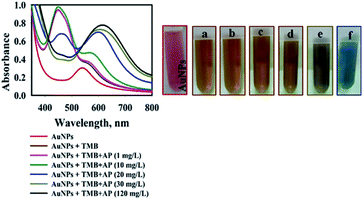
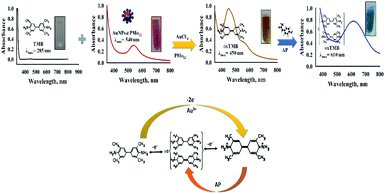
As stated in the literature, TMB can be oxidized into its free cationic radical form via single electron transfer.37 Furthermore, different TMB derivatives in the reaction medium can be generated like charge-transfer complex and di-imine (di-cation due to double electron transfer).37 The charge-transfer complex is blue, with absorbance peaks at 652 and 370 nm and two-electron oxidation, di-imine derivative (450 nm) with yellow color (Fig. S2†).37In our study, with the addition of as-prepared AuNPs@PMo12 solution, the colorless solution of TMB changed into yellow-brown, and a maximum absorbance at 450 nm was monitored (Fig. 1). These results indicate that TMB is oxidized directly into di-imine derivative by the AuNP@PMo12 solution in the absence of H2O2.
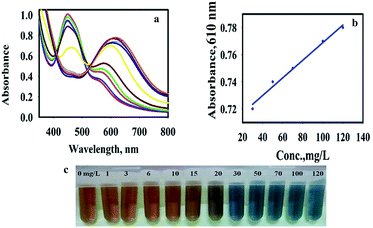
When the AP solution was added and its concentration increased, the yellow-brown color oxTMB transformed into a dark orange-brown and finally into blue. As shown in Fig. 1, by the addition of AP solution with a concentration of 20 mg L−1, two peaks were observed (460 and 600 nm) due to the presence of two forms of oxTMB in the solution (di-imine and charge-transfer complex). Increasing the concentration of AP to 30 mg L−1 led to the appearance of a peak at 610 nm and the absorbance peak of 460 nm diminished, which was due to the presence of one form of oxTMB (di-imine structures).
To prove the mechanism of the reaction, the effects of reagents, including HAuCl4, PMo12, precursor solution, and pure AuNPs@PMo12 on oxidation of TMB were separately investigated. In all cases, the color change was monitored a few seconds after mixing with TMB solution.
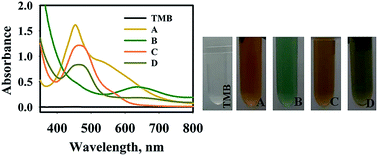
In the case of HAuCl4 solution, a few seconds after mixing TMB and HAuCl4 solution, the color of the solution changed into yellow (Fig. 2). The absorption peak at 450 nm appeared due to the formation of oxidized tetramethylbenzidine, diimine derivative (450 nm), which showed that HAuCl4 can directly oxidize TMB into the di-imine derivative. Generally, different specious of gold ions can be produced in an aqueous solution such as Au+, Au3+, and anionic-chloro complex [HAuCl4]−.38 It is proposed that Au3+ ions tend to withdraw electrons from TMB structure which leads to double electron transfer and production of the di-imine derivative.
As shown in Fig. 2, the absorption spectrum of TMB–PMo12 solution with an absorption peak at 640 nm was observed. Light green is assigned to the charge-transfer complex, which confirms the oxidation of TMB in presence of PMo12 in acidic medium. This observation confirms other literature results about the oxidization of TMB into blue color using polyoxomolybdates in the absence of H2O2.23
The addition of TMB into precursor solution in the absence and presence of AP (100 mg L−1) was conducted, appearing a yellow and olivaceous color with an absorption peak at 470 nm. These results suggest the direct oxidation of TMB into the di-imine derivative. It was observed that the intensity of the absorption peak of the TMB solution in the presence of AP drastically decreased compared to the absence of AP. It is proposed that the Au(III) ions which interacted with the –OH group of AP (C8H9NO2) molecules via donor–acceptor electron complex32 can reduce the catalytic activity of Au(III) on oxidation of TMB molecules. Besides, the electrostatic attraction of negatively charged anionic species including [AuCl4]− and PMo12 to the amine group of TMB could help to oxidize the TMB. By adding AP solution, anionic species interacted with the amine group of AP and reduced the interaction with TMB molecules.
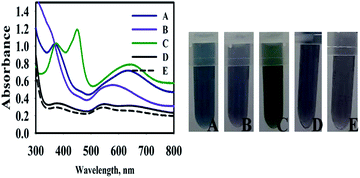
To investigate the mechanism of TMB oxidation in the presence of nanosensors, the final product was centrifuged and washed many times, in order to purify and separate it from the synthesis media. The purified AuNPs@PMo12 was dispersed in deionized water and tested with TMB solution in the presence and absence of an AP solution. According to the results (Fig. 3), no oxidase-like activity of the pure Au NPs was observed in the presence and absence of AP molecules. While, after the addition of HAuCl4 and PMo12 into the AuNPs solution in the presence of AP (100 mg L−1), the color of TMB changed to blue with observed peaks at 640 nm and 370 nm due to oxidation of TMB into charge-transfer complex. To investigate the effect of PMo12 and HAuCl4 separately, each of them was added in purified AuNPs in the presence of AP (100 mg L−1). As shown in Fig. 3, the addition of PMo12 led to a change of color into dark purple and a shift of absorption peak to 570 nm. The addition of HAuCl4 led to the appearance of three absorption peaks at 370, 450, and 652 nm on the absorption spectrum of TMB–Au(III) solution. These wavelengths (370 nm and 652 nm) are assigned to charge-transfer complex of oxidized tetramethylbenzidine and di-imine derivative (450 nm). It is concluded that the presence of HAuCl4 shows a predominant role in the oxidation of TMB. The significant performance of HAuCl4 is due to its ionic species including Au3+ and [AuCl4]−. Therefore, the synergic effect of HAuCl4, PMo12, and AuNPs on oxidation of TMB in the presence of AP molecules was confirmed.
It this research, the synergic effect of PMo12, AuCl4−, and AuNPs@PMo12 under acidic conditions (pH of nanosensor) led to direct oxidation of TMB into a di-imine derivative (λ = 450 nm) with yellow-brown color. With the addition of AP solution and consequently increasing its concentration, oxidized tetramethylbenzidine, di-imine derivative changed to a charge-transfer complex product and caused a color change.37
The brown solution turned into blue and the absorbance at 450 nm was slowly decreased, exhibiting a typical reaction product absorption at 610 nm. According to previous research, AuNPs@PMo12 nanohybrid is aggregated in the presence of Au(III) and AP.32 As seen in Fig. 4, by adding AP into AuNPs@PMo12 solution some islands of AuNPs aggregates were seen. As mentioned in the previous study, Au(III) ions interacted with the –OH group of AP (C8H9NO2) molecules via donor–acceptor electron complex.32 Besides, AP molecules with a functional group (–NH) form a hydrogen bonding and donor–acceptor electron complexes into the AuNPs@PMo12 surface. Therefore, AP molecules in the presence of Au(III) can induce the AuNPs@PMo12 aggregation.32 Thus, fewer amounts of active sites for the oxidation of TMB were accessible and the complete oxidation of TMB was prevented.
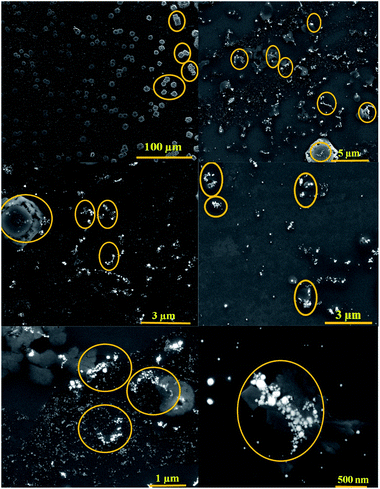 | ||
| Fig. 4 SEM images of AuNPs@PMo12 nano-hybrids after the addition of AP (70 mg L−1) (marked area shows aggregated particles). | ||
To prove the aggregation of AuNPs@PMo12 nanohybrids after the addition of AP (150 mg L−1) and TMB, DLS analysis was performed to measure the size distribution and hydrodynamic diameter of AuNPs@PMo12. The average size of AuNPs@PMo12 increased from 19.8 ± 1.7 nm to 42.17 ± 3.92 nm after the addition of AP (150 mg L−1) which confirmed the proposed mechanism about the aggregation AuNPs in presence of AP molecules. After adding TMB solution the size of AuNPs more increased to 72.41 ± 1.5 nm (Fig. S3†). It is suggested that Au+ ions reduced to Au in presence of TMB molecules according to the following equation:
| Au+ + TMB → Au + TMB+ | (1) |
Scheme 1, illustrates the oxidation pathway of 3,3′,5,5′-tetramethylbenzidine (TMB) using AuNPs@PMo12 in the presence of Au ionic species and unreacted PMo12.
Optimization studies for acetaminophen detection
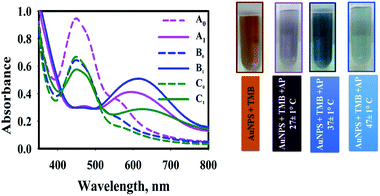
The sensing conditions such as reaction temperature and sample pH on AP sensing were further optimized.The effect of temperature was investigated at 27 ± 1 °C, 37 ± 1 °C, and 47 ± 1 °C (Fig. 5). To investigate the effect of reaction temperature, AP aqueous solution (0.9 mL) was added to the AuNPs@PMo12 solution (0.9 mL) and TMB solution (0.1 mL + 0.7 mM). After a few seconds, the color of the solution changed from yellow-brown (AP, 0 mg L−1) to blue (AP, 30 mg L−1).
As Fig. 5, the addition of AP into nanosensor solution, which was prepared in 37 ± 1 °C in the presence of TMB, led to a more significant SPR peak at 610 nm and a dark blue color. In the case of nanosensor which was synthesized at 27 ± 1 °C and 47 ± 1 °C, two peaks were observed (460 and 610 nm) due to the presence of two forms of oxTMB in solution. At higher temperatures, the UV-vis spectrum shows a significant sharp peak at 450 nm, which confirms the presence of di-imine structure as a predominant form of oxTMB in the solution.
It is assumed that in higher temperatures, fewer amounts of unreacted Au(III) ions leave in the solution due to the higher rate of reaction. So, fewer amounts of AuNPs were aggregated32 and AuNPs could completely oxidize TMB molecules to di-imine structures. In our case, as the monitoring of the blue color (λmax= 620 nm) was evident for quantitatively detection of AP, the temperature of 37 ± 1 °C was selected as the optimum temperature.
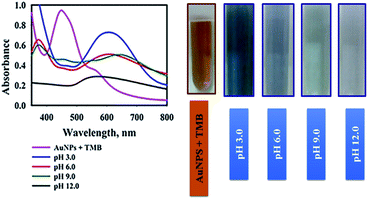
The second parameter which was optimized was the influence of sample pH on oxidation of TMB solution in the presence of AuNPs@PMo12 nanosensor. The pH of the AuNPs@PMo12 solution was 3.0 ± 0.5, and the other pH values were adjusted using NaOH solution. According to Fig. 6, the strong oxidation of TMB and the formation of dark blue color occurred at lower pH and acidic media. Also, increasing the pH of the solution led to fewer amounts of oxTMB forms.
According to the literature, in acidic media (about pH value 3 or 4), POMs are more stable and show more enzyme activities. Furthermore, at higher pH (pH ≥ 7.0–7.5), the POM nanozymes are catalytically inactive.23
Also in the basic media, PMo12 is not stable; therefore, it may be degraded to some new Mo-based compounds and change the structure of the nano-hybrid. Consequently, pH 3.0 ± 0.5 (natural pH of nanosensor) was selected for AuNPs@PMo12-based colorimetric assays of AP.32
Determination of AP on the proposed colorimetric sensor
The UV-vis absorption spectra of AP with different concentrations in the presence of AuNPs@PMo12 and TMB solution were recorded (Fig. 7). According to Fig. 7, the color turned from yellow-brown to orange-brown and then into blue by increasing the AP solution.As shown in Fig. 7, by increasing AP concentration, the absorbance of the TMB media at 450 nm significantly decreased. The absorption values also showed a good linear correlation with the concentration of AP in the range of 30–120 mg L−1 with a regression equation of y = 0.0007x + 0.704 (0.99) shown in Fig. 6b. Based on 3σ/s, where σ is the standard deviation of the blank measurements and s is the sensitivity of the calibration graph, the detection limit of AP was calculated to be 14.52 μg mL−1. It is important to notice that the toxic level of AP in human serum is more than 100 mg L−1.32
Specificity for AP
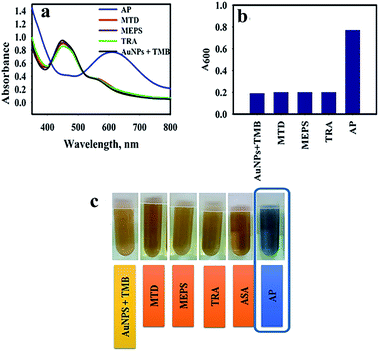
The specificity of the AuNPs@PMo12 nano-hybrid was performed by comparing the color change of solution, including seven drugs (acetaminophen, methadone, methylphenidate, tramadol, ibuprofen, aspirin, and diclofenac) separately. In this test, acetaminophen,AP (C8H9NO2), methadone, MTD (C21H27NO), methylphenidate, MEPS (C14H19NO2), tramadol, TRA (C16H25NO2), ibuprofen, IBP (C13H18O2), aspirin, ASA (C9H8O4), diclofenac, DIC (C14H11Cl2NO2) with a concentration of 0.4 mmol L−1 were added into AuNPs@PMo12 solution.As seen in Fig. 8, an obvious change in color, from yellow-brown to blue, was immediately observed after adding AP, whereas the others were still yellow-brown. The characteristic color changes were further confirmed by UV-Vis spectrum and only the target of AP leads to an obvious red-shift effect into 610 nm. According to the previous result, only AP molecules can react onto the surface of AuNPs@PMo12.32 So, the color change into the blue was seen in the presence of AP molecules.
Kinetic studies of AuNPs@PMo12 nanohybrid
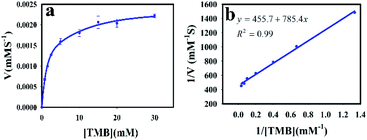
To understand the kinetic properties of AuNPs@PMo12 nanohybrid more precisely, steady-state kinetic assays were carried out in the presence of different concentrations of TMB. The experiments were conducted according to the enzyme kinetics theory. The kinetics data at the varying substrate (TMB) was plotted to fit the Michaelis–Menten equation (2). As seen in Fig. 9, the typical Michaelis–Menten (Fig. 9a) and Lineweaver–Burk plots (eqn (3)) (Fig. 9b) were plotted at a given concentration range of TMB.
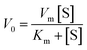 | (2) |
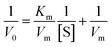 | (3) |
The enzymatic kinetic parameters like Michaelis–Menten constant (Km) and the maximum reaction velocity (Vmax) were determined by fitting the Lineweaver–Burk equation. Km is a kinetic parameter that identifies the enzyme affinity towards the substrates. The low value of Km is related to the higher enzyme affinity to a substrate and vice versa.39,40
The Km and Vm values of AuNPs@PMo12 for TMB have obtained 1.57 mM, and 2.2 × 10−3 mM s−1 which offer AuNPs@PMo12 as a suitable artificial enzyme.
Conclusions
In this research, for the first time, AuNPs@PMo12 as nanozyme was developed for the qualitative and quantitative determination AP based on TMB colorimetric nanosensor in H2O2-free conditions. The limit of detection reaches to 14.52 μg mL−1. The proposed mechanism of AP detection using AuNPs@PMo12 as nanozyme for the oxidation of TMB in H2O2 free media is as follows: the synergic effects of HAuCl4, PMo12, and AuNPs@PMo12 were effective for oxidation of TMB in the absence of H2O2. The role of Au(III) ionic species was predominant. Furthermore, in the presence of AP molecules due to aggregation of AuNPs via Au(III) ions, less active sites were available for the oxidation of TMB. Thus, di-imine derivative changed to charge-transfer complex product.The detection is quite simple in preparation and operation and can be completed within a few seconds. The developed strategy provides a cost-effective and efficient approach for potential applications in clinical diagnosis.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work has been financially supported by the Iranian National Science Foundation (INSF) (No. 97009286). The authors also wish to thank Dr Scott G. Mitchell from Zaragoza University for HAADF-STEM analysis.References
- H. H. Deng, X. L. Lin, Y. H. Liu, K. L. Li, Q. Q. Zhuang, H. P. Peng, A. L. Liu, X. H. Xia and W. Chen, Nanoscale, 2017, 9, 10292–10300 RSC.
- T. Wang, P. Su, F. Y. Lin and Y. Yang, Sens. Actuators, B, 2017, 254, 329–336 CrossRef.
- K. Hobbs, N. Cathcart and V. Kitaev, Chem. Commun., 2016, 52, 9785–9788 RSC.
- G. Sener, L. Uzun and A. Denizli, ACS Appl. Mater. Interfaces, 2014, 6, 18395–18400 CrossRef CAS.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- Q. Wang, H. Wei, Z. Zhang, E. Wang and S. Dong, TrAC, Trends Anal. Chem., 2018, 105, 218–224 CrossRef CAS.
- K. Korschelt, M. N. Tahir and W. Tremel, Chem.–Eur. J., 2018, 24, 9703–9713 CrossRef CAS.
- C. Liu, K. Chen, C. Su, P. Yu and P. Lee, Catalysts, 2019, 9, 517 CrossRef CAS.
- Z. Xue, L. Xiong, H. Rao, X. Liu and X. Lu, Dyes Pigm., 2019, 160, 151–158 CrossRef CAS.
- P. Ni, H. Dai, Y. Wang, Y. Sun, Y. Shi, J. Hu and Z. Li, Biosens. Bioelectron., 2014, 60, 286–291 CrossRef CAS.
- S. Wang, W. Chen, A. Liu, L. Hong, H. Deng and X. Lin, ChemPhysChem, 2012, 13, 1199–1204 CrossRef CAS.
- Y. Jv, B. Li and R. Cao, Chem. Commun., 2010, 46, 8017–8019 RSC.
- H. G. T. Ly, G. Absillis, R. Janssens, P. Proost and T. N. Parac-Vogt, Angew. Chem., Int. Ed., 2015, 54, 7391–7394 CrossRef CAS.
- A. González, N. Gálvez, M. Clemente-León and J. M. Dominguez-Vera, Chem. Commun., 2015, 51, 10119–10122 RSC.
- D.-F. Chai, Z. Ma, H. Yan, Y. Qiu, H. Liu, H.-D. Guo and G.-G. Gao, RSC Adv., 2015, 5, 78771–78779 RSC.
- L. Suo, W. Gao, Y. Du, R. Wang, L. Wu and L. Bi, New J. Chem., 2016, 40, 985–993 RSC.
- G. Zhang, B. Keita, R. N. Biboum, F. Miserque, P. Berthet, A. Dolbecq, P. Mialane, L. Catala and L. Nadjo, J. Mater. Chem., 2009, 19, 8639–8644 RSC.
- R. Liu, S. Li, G. Zhang, A. Dolbecq, P. Mialane and B. Keita, J. Cluster Sci., 2014, 25, 711–740 CrossRef CAS.
- D. L. Long, R. Tsunashima and L. Cronin, Angew. Chem., 2010, 122, 1780–1803 CrossRef.
- N. Mizuno, K. Yamaguchi and K. Kamata, Coord. Chem. Rev., 2005, 249, 1944–1956 CrossRef CAS.
- S. Liu, et al., ChemPlusChem, 2012, 77, 541–544 CrossRef CAS.
- B. Zhang, M. Zhao, Y. Qi, R. Tian, B. Carter, H. Zou, C. Zhang and C. Wang, Sci. Rep., 2019, 9, 14832 CrossRef.
- D. Li, et al., Chem.–Eur. J, 2013, 24, 1926–1934 CrossRef.
- E. Yoon, A. Babar, M. Choudhary, M. Kutner and N. Pyrsopoulos, J. Clin. Transl. Hepatol., 2016, 4, 131–142 Search PubMed.
- R. Clark, J. E. Fisher, I. S. Sketris and G. M. Johnston, BMC Clin. Pharmacol., 2012, 12, 11 CrossRef CAS.
- G. Burgot, F. Auffret and J.-L. Burgot, Anal. Chim. Acta, 1997, 343, 125–128 CrossRef CAS.
- S. Ravisankar, M. Vasudevan, M. Gandhimathi and B. Suresh, Talanta, 1998, 46, 1577–1581 CrossRef CAS.
- A. B. Moreira, H. P. Oliveira, T. D. Atvars, I. L. Dias, G. O. Neto, E. A. Zagatto and L. T. Kubota, Anal. Chim. Acta, 2005, 539, 257–261 CrossRef CAS.
- S. Zhao, W. Bai, H. Yuan and D. Xiao, Anal. Chim. Acta, 2006, 559, 195–199 CrossRef CAS.
- A. Cernat, M. Tertiş, R. Săndulescu, F. Bedioui, A. Cristea and C. Cristea, Anal. Chim. Acta, 2015, 886, 16–28 CrossRef CAS.
- T. Rohani Bastami, A. Ghaedi, S. Mitchell, A. Javadian-Sarafe and M. Karimi, RSC Adv., 2020, 10, 16805–16816 RSC.
- V. Bernal, A. Erto, L. Giraldo and J. C. Moreno-Piraján, Molecules, 2017, 22, 1032 CrossRef.
- T. Rohani Bastami and M. H. Entezari, Ultrason. Sonochem., 2012, 19, 560–569 CrossRef CAS.
- P. Ni, Y. Sun, H. Dai, J. Hu, S. Jiang and Y. Wang, et al., Biosens. Bioelectron., 2015, 63, 47–52 CrossRef CAS.
- F. Yu, Y. Huang, A. J. Cole and V. C. Yang, Biomaterials, 2009, 30, 4716–4722 CrossRef CAS.
- P. Josephygg, T. Elingg and R. Mason, J. Biol. Chem., 1982, 257, 3669–3675 Search PubMed.
- I. V. Mironov and E. V. Makotchenko, J. Solution Chem., 2009, 38, 725–737 CrossRef CAS.
- S. Mvango and P. Mashazi, Mater. Sci. Eng., C, 2019, 96, 814–823 CrossRef CAS.
- S. Rostamia, A. Mehdiniab and A. Jabbari, Mater. Sci. Eng., C, 2020, 114, 111034 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06545e |
| This journal is © The Royal Society of Chemistry 2020 |