Open Access Article
Open Access ArticleMulticomponent crystals of anti-tuberculosis drugs: a mini-review†
Eustina Batisai

Department of Chemistry, University of Venda, P. Bag X5050, Thohoyandou, 0920, South Africa. E-mail: Eustina.Batisai@univen.ac.za
First published on 7th October 2020
Abstract
Tuberculosis (TB) is the leading cause of death from a single infectious agent globally. Some of the early research on TB treatment indicated drug resistance as one of the key challenges in fighting this disease. The discovery that administering two or more drugs simultaneously could lead to much more effective treatment, with reduced drug resistance and shorter periods of chemotherapy, was, therefore, a very significant breakthrough in TB drug research. Pursuant to this discovery, the World Health Organisation (WHO) recommended TB treatment employing fixed-dose combinations (FDCs) containing first line anti-TB drugs; rifampicin, isoniazid, pyrazinamide, streptomycin and ethambutol. Regardless, certain challenges associated with FDCs remain and these include chemical instability and reduced bioavailability of rifampicin. Therefore, some research effort has been directed towards finding ways to deal with these challenges. One such effort involves the use of pharmaceutical co-crystals of the active pharmaceutical ingredients. Consequently, several pharmaceutical co-crystals of isoniazid and pyrazinamide have been reported. This paper aims at reviewing the multicomponent crystal structures of two first-line anti-TB drugs; isoniazid and pyrazinamide. The review will first set out a brief history of the disease, milestones in TB chemotherapy and the challenges associated with current treatment regimens. This will then be followed by a brief introduction to pharmaceutical co-crystals and how they can improve the physical and chemical properties of the active pharmaceutical ingredients. Secondly, multicomponent crystals of the two active pharmaceutical ingredients will be analysed by manual inspection for common supramolecular synthons between the drug molecules as well as between drug molecules and co-formers. Lastly; stability, solubility and dissolution experiments carried out on the pharmaceutical co-crystals of pyrazinamide and isoniazid will be analysed to gain insights into progress made with regards to improving stability and solubility of the active pharmaceutical ingredients.
1. Introduction
Tuberculosis (TB) is an infectious disease caused by the bacillus Mycobacterium tuberculosis. Even though the bacterium was discovered in March 1882 by Robert Koch, studies have only recently revealed that the bacterium may, in fact, have infected humans for thousands of years before its discovery.1 TB is currently one of the leading causes of death globally, and the World Health Organisation (WHO) estimates 10.0 million infections and 1.3 million deaths worldwide in 2018.2 It is, therefore, a global epidemic of significant concern. The prevention of new infections and their progression to TB disease is at the core of the fight against TB. This is currently only possible through the vaccination of children using the Bacille Calmette–Guérin (BCG) vaccine. Fully progressed TB, on the other hand, can be treated using a fixed-dose combination (FDC) containing four first line TB drugs: rifampicin, isoniazid, pyrazinamide and ethambutol.3 Such treatment entails a six-to eight-month-long FDC course at an approximate cost of USD40 per person and has an 85% cure rate. Regardless, the history of TB treatment is plagued with various challenges, in particular, emergent drug resistance towards the first line drugs rifampicin and isoniazid.4 Treatment of multi-drug resistant TB is much more expensive (1000 USD per person) and it involves the use of toxic drugs and a longer treatment period. Even then, such treatment is successful in only 55% of reported cases.3A survey of the history of the development of TB treatment drugs reveals that, despite many drug candidates showing initial promise, the emergence of drug resistance in the bacteria has posed one of the biggest challenges. The earliest TB treatment attempts employed the antibiotic streptomycin, which was discovered by Schatz, Bugie and Waksman in 1944.5 In monotherapy trials conducted by the British Medical Research Council (BMRC),6 for the treatment of pulmonary TB from 1946 onwards, a streptomycin-resistant strain was isolated in 85% of the patients under treatment within two months of commencement.6 It quickly became evident that single-drug therapy would most likely result in the emergence of drug resistance. A breakthrough in TB drug research came in 1948 when it was shown that combined therapy of streptomycin and para-aminosalicylic acid (PAS) led to improved protection against the emergence of resistance.7 Para-aminosalicylic acid's antibacterial activity had first been reported in 1946.8 Another milestone followed in 19529 when isoniazid, a drug first synthesized in 1912,10 showed anti-TB activity. Subsequent studies showed that even though isoniazid monotherapy is effective in pulmonary TB treatment, it is not more effective than combined therapy of streptomycin plus PAS.11 It was now becoming clear that mono-therapeutic drugs were much more susceptible to the emergence of drug resistance, TB drug development increasingly focused on combination therapy and the development of FDCs. The challenge lay in finding both the suitable candidate drugs and the correct combination. During the 1952 to 1955 period, combination therapy utilizing PAS plus isoniazid and, streptomycin plus isoniazid were explored.12 These studies led to the triple therapy lasting up to twenty-four months which involved the use of all three drugs for TB treatment for almost fifteen (15) years.13,14 A very important breakthrough came in the 1970s when it was discovered that the addition of rifampicin and pyrazinamide to the regimens shortened chemotherapy to six months.15,16 The potential efficacy of pyrazinamide17 and rifampicin18 had been reported over the course of TB drug research. A fourth drug usually included in combination therapy in case there is unknown resistance to isoniazid is ethambutol whose anti TB activity was first realized in 1961.19
Despite the progress, TB drug resistance remains a global problem. Therefore, current approaches in TB drug development that seek to address this problem include the development of new drugs, as well as re-formulations of existing drugs. According to a recent WHO report, there are twenty (20) new TB drugs as well as combination regimens currently being tested in clinical trials.3 The development of FDCs represents significant progress in combating drug resistance. However, FDCs are also plagued by their challenges which include chemical instability as well as the reduced bioavailability of rifampicin. The reduction in the bioavailability of rifampicin has been attributed to drug–drug interactions between rifampicin and isoniazid. Singh et al. postulated that under acidic conditions rifampicin is first hydrolysed to 3-formylrifamycin which then reacts with isoniazid to yield isonicotinyl hydrazone (HYD). The isonicotinyl hydrazone, which is unstable under acidic condition is then converted back to isoniazid and 3-formylrifamycin. This results in the recovery of isoniazid but not rifampicin.20–22 The chemical instability of the FDCs arises due to the direct interaction of the imine group on the rifampicin and the amine group of the isoniazid. This results in the formation of isonicotinyl hydrazone. The other reason is due to the moisture gain by ethambutol hydrochloride which creates a hydrolytic environment. This accelerates the reaction between isoniazid and rifampicin.20
There is a lot of ongoing research focused on combating the low-bioavailability of rifampicin and the chemical instability of FDCs. Crystal engineering, defined as the study and utilization of intermolecular interactions in designing new solids with specific desired properties,23 is one of the most promising techniques employed to solve these problems faced with FDCs. Co-crystallization is a subfield of crystal engineering which involves crystallizing an active pharmaceutical ingredient with a pharmaceutically acceptable compound known as a co-former. The resulting compound, known as a pharmaceutical co-crystal or salt, usually exhibits improved physical and chemical properties compared to those of the active pharmaceutical ingredient.24–26
Hydrogen bonding, ionic interactions, π–π stacking interactions, van der Waals interactions and halogen bonding are some of the interactions utilized in co-crystal formation.
Different methods are employed in the synthesis of co-crystals. These may be broadly classified based on whether a solvent is involved in the crystallization process. Solution-based methods include solvent evaporation, reaction co-crystallization, cooling co-crystallization, anti-solvent addition co-crystallization, slurry co-crystallization and ultrasound assisted co-crystallization. Solvent-free methods, on the other hand, include neat grinding, liquid assisted grinding, polymer assisted grinding, hot melt extrusion and matrix-assisted co-crystallization.27 A more thorough discussion of these as well as other related techniques; their advantages and disadvantages can be found in the review by Rodrigues and co-workers.27 Studies have shown that the solvent polarity plays a very important role in the self-assembly process during co-crystal formation in both solvent-based techniques as well as solvent-less techniques.28,29 Co-crystals can be characterised using crystallographic techniques (single crystal X-ray diffraction and powder X-ray diffraction), spectroscopy (solid state nuclear magnetic resonance and NMR crystallography and, vibrational spectroscopy) and thermal analysis (differential scanning calorimetry, thermogravimetric analysis, hot stage microscopy).30 For a more comprehensive discussion of these techniques the reader is referred to a review by Pindelska and co-workers.30
A significant amount of work that addresses how co-crystallization has improved some properties of anti-TB drugs has been published. This paper reviews the pharmaceutical co-crystals of pyrazinamide and isoniazid, published to date which comprise the one hundred and ten (110) multicomponent crystals of isoniazid and forty-nine (49) co-crystals of pyrazinamide deposited in the Cambridge Structural Database (CSD) (Version 5.5, March 2020).31 Data on the solubility, stability and dissolution rates for these co-crystals will be analysed to evaluate the progress made in improving the stability and solubility of the drugs. In addition, the presence of common supramolecular synthons between drug molecules, as well as between drug molecules and co-formers will be examined. Understanding the nature of these interactions is crucial for the further development of pharmaceutical co-crystals of these drugs.
2. Common synthons in multicomponent crystals of isoniazid and pyrazinamide
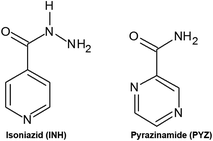
Isoniazid (INH) and pyrazinamide (PYZ) (Fig. 1) are excellent supramolecular reagents possessing both hydrogen bond acceptors and donors. The pyridyl functionality in INH can act as a hydrogen bond acceptor, whilst the –CO–NH–NH2 functionality possesses both hydrogen bond acceptors and donors. PYZ contains the pyrazyl functionality which can act as a hydrogen bond acceptor whilst the –CO–NH2– group can act as both a hydrogen bond acceptor and a hydrogen bond donor. This presents opportunities in understanding intermolecular interactions formed with the different co-formers and, therefore, both drugs have been successfully co-crystallized with a variety of organic and inorganic compounds. This section thus analyses structures of multicomponent crystals of both drugs deposited in the CSD for common supramolecular synthons.2.1. Isoniazid
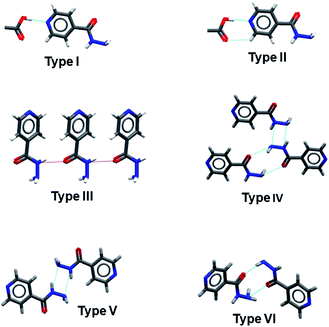
A Cambridge Structural Database (CSD) (Version 5.5, March 2020) search produced one hundred and ten (110) multicomponent crystals of isoniazid. Of these, one hundred and one (101) contain organic compounds as co-formers, whilst the remaining are prepared from various inorganic acids. The multicomponent crystals were analysed by manual inspection and the most common synthons observed in the structures are shown in Fig. 2. The co-crystal formers, CCDC reference codes, common synthons and physical properties addressed for multicomponent crystals of isoniazid are given in Table S1.†Carboxylic acid is the most commonly utilized functionality in the preparation of co-crystals of isoniazid, accounting for 86% (87 hits) of the multicomponent crystals that contain organic co-formers. The remaining contain macrocyclic compounds (five (5) hits) and other organic compounds (nine (9) hits). The eighty-seven (87) multicomponent crystals containing carboxylic acids comprise seventeen (17) salts and seventy (70) co-crystals. Fifty-nine (59) of the co-crystal structures display the expected pyridyl⋯acid heterosynthon (synthon type I) (Fig. 2). Of these fifty-nine (59), twenty-seven (27) display synthon type II. Synthon types III, IV, V and VI are observed in ten (10), eleven (11), six (6) and twenty-four (24) structures respectively (Fig. 2). Analysis of the seventeen (17) salts indicated that the pyridyl-H+⋯−OOC heterosynthon is utilized in 69% of the salts. The co-crystal formers, CCDC reference codes, common synthons and physical properties addressed for multicomponent crystals of isoniazid are given in Table S2.†
2.2. Pyrazinamide
The first co-crystal of pyrazinamide reported by Aakeröy and co-workers in 2004![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 was prepared from the slow evaporation of an ethanol solution of a 1
32 was prepared from the slow evaporation of an ethanol solution of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
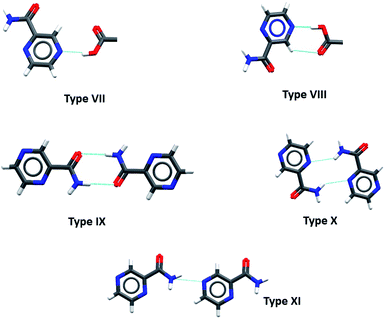
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of pyrazinamide and 4-nitrobenzamide. The interactions in this co-crystal were described by the authors as unpredictable because of the absence of primary interactions between pyrazinamide and the 4-nitrobenzamide molecules. Since 2004, a lot of research has been conducted in the study of co-crystals of pyrazinamide, and to date, there are forty-nine (49) multicomponent crystal structures of pyrazinamide deposited in the CSD. The most common synthons between the pyrazinamide molecules in the multicomponent crystals are presented in Fig. 3. Of the reported forty-nine (49) structures, thirty-four (34) contain carboxylic acid co-formers, thirteen (13) contain other organic compounds including drug co-formers (hydrochlorothiazide, temozolomide, theophylline and quercetin), one (1) contains an inorganic compound and one (1) is a clathrate prepared from cyclodextrin. Of the thirty-four (34) containing carboxylic acid co-formers, fifteen (15) contain the pyrazyl-N⋯HOOC heterosynthon (synthon VII) with eleven (11) of these featuring synthon VIII. Synthon IX appears in twenty-two (22) structures, synthon X appears in eight (8) structures and synthon XI appears in six (6) structures (Fig. 3).
1 mixture of pyrazinamide and 4-nitrobenzamide. The interactions in this co-crystal were described by the authors as unpredictable because of the absence of primary interactions between pyrazinamide and the 4-nitrobenzamide molecules. Since 2004, a lot of research has been conducted in the study of co-crystals of pyrazinamide, and to date, there are forty-nine (49) multicomponent crystal structures of pyrazinamide deposited in the CSD. The most common synthons between the pyrazinamide molecules in the multicomponent crystals are presented in Fig. 3. Of the reported forty-nine (49) structures, thirty-four (34) contain carboxylic acid co-formers, thirteen (13) contain other organic compounds including drug co-formers (hydrochlorothiazide, temozolomide, theophylline and quercetin), one (1) contains an inorganic compound and one (1) is a clathrate prepared from cyclodextrin. Of the thirty-four (34) containing carboxylic acid co-formers, fifteen (15) contain the pyrazyl-N⋯HOOC heterosynthon (synthon VII) with eleven (11) of these featuring synthon VIII. Synthon IX appears in twenty-two (22) structures, synthon X appears in eight (8) structures and synthon XI appears in six (6) structures (Fig. 3).
 | ||
| Fig. 3 Common synthons formed by PYZ molecules in the multicomponent crystal structures of pyrazinamide deposited in the CSD. | ||
3. Multicomponent crystals of isoniazid that address the issue of stability and solubility
Isoniazid, on its own, is stable at ambient conditions (30 °C and 40% relative humidity) as well as at accelerated conditions (40 °C and 75% relative humidity). However, in FDCs, its stability is reduced due to drug–drug interactions. This section reviews the pharmaceutical co-crystals of isoniazid that sought to address the issues of stability and solubility. The equilibrium solubility values of the multicomponent crystals reviewed in this section are given in Table 1.| Dissolution medium | Co-crystal | Solubility (mg mL−1) | Reference |
|---|---|---|---|
| Water | INH | 137.96 | 33 |
| INH·OXA | 5.43 | ||
| INH·MAL | 403.95 | ||
| INH·MES | 485.91 | ||
| pH 6.8 buffer | INH | 137.96 | 33 |
| INH·OXA | 8.13 | ||
| INH·MAL | 307.25 | ||
| INH·MES | 322.67 | ||
| pH 4.5 buffer | INH | 108.75 | 33 |
| INH·OXA | 8.64 | ||
| INH·MAL | 411.7 | ||
| INH·MES | 372.14 | ||
| pH 1.2 buffer | INH | 148.41 | 33 |
| INH·OXA | 8.96 | ||
| INH·MAL | 422.79 | ||
| INH·MES | 410.82 | ||
| pH 7.5 buffer | INH | 183 | 34 |
| INH·HBA hydrate (form II) | 17.9 | ||
| INH·HBA anhydrous | Converts to hydrate | ||
| INH·FA (form I) | 66.5 | ||
| INH·NA·FA | 131 | ||
| INH·NA·SA | 285 | ||
| Water | INH·CIN | 6.9 ± 0.5 | 37 |
| INH·BA | 137 ± 9 | ||
| INH·MALO | 375 ± 23 | ||
| INH·SUC | 128 ± 7 | ||
| INH·GLT | 302 ± 9 | ||
| INH·ADI | 73 ± 2 | ||
| INH·PIM | 83 ± 1 | ||
| INH SUB | 164 ± 4 | ||
| INH·SEB | 86 ± 4 |
Diniz et al. crystallized isoniazid (INH) with oxalic acid (OXA), maleic acid (MAL) and methanesulfonic acid (MES).33 The solubility of the resulting salts, as well as INH, were determined in water, and in pH 6.8, 4.5 and 1.2 buffers (Table 1). Except for the INH·OXA salt, the other salts generally have higher solubility in all dissolution media compared to INH. The solubility of the salts in water and pH 6.8 buffer follows the order INH·MES > INH·MAL > INH > INH·OXA while the solubility in pH 4.5 and 1.2 buffers follow the order INH·MAL > INH·MES > INH > INH·OXA.
Aitipamula and co-workers studied the stability and solubility of co-crystals of isoniazid with fumaric acid (FA), succinic acid (SA), nicotinamide (NA) and 4-hydroxybenzoic acid (HBA).34 The authors also report two concomitant polymorphs of the INH·HBA hydrate, a new polymorph of INH·FA co-crystal and two ternary co-crystals; INH·NA·FA and INH·NA·SA. Form I of the INH·HBA hydrate crystallizes in the monoclinic space group P21/n while form II crystallizes in the monoclinic space group P21. Form I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 and form II of INH·FA both crystallize in the monoclinic space group P21/n. The stability of the co-crystals was determined by performing slurry experiments at 37 °C for 24 hours, storing the co-crystals at 40 °C and 75% RH and dynamic vapour sorption (DVS) experiments. The ternary co-crystals were found to be stable while the form I of INH·HBA hydrate, as well as the anhydrous INH·HBA co-crystal,36 were converted to form II INH·HBA hydrate, whereas form II of INH·FA converts to form I INH·FA upon slurrying. Stability experiments performed at accelerated conditions showed that the two ternary co-crystals, INH·FA form I and INH·HBA hydrate form II are stable up to thirteen (13) weeks while the anhydrous INH·HBA converts to form II INH·HBA hydrate within two days of storage. The solubility studies carried out on the co-crystals in the pH 7.5 buffer showed that, except in the case of the ternary co-crystal INH·NA·SA which has solubility 1.5 times higher than the INH, the solubility of the co-crystals were lower than the solubility of INH (Table 1). The high solubility of INH·NA·SA was attributed to the high solubility of the SA and NA.
35 and form II of INH·FA both crystallize in the monoclinic space group P21/n. The stability of the co-crystals was determined by performing slurry experiments at 37 °C for 24 hours, storing the co-crystals at 40 °C and 75% RH and dynamic vapour sorption (DVS) experiments. The ternary co-crystals were found to be stable while the form I of INH·HBA hydrate, as well as the anhydrous INH·HBA co-crystal,36 were converted to form II INH·HBA hydrate, whereas form II of INH·FA converts to form I INH·FA upon slurrying. Stability experiments performed at accelerated conditions showed that the two ternary co-crystals, INH·FA form I and INH·HBA hydrate form II are stable up to thirteen (13) weeks while the anhydrous INH·HBA converts to form II INH·HBA hydrate within two days of storage. The solubility studies carried out on the co-crystals in the pH 7.5 buffer showed that, except in the case of the ternary co-crystal INH·NA·SA which has solubility 1.5 times higher than the INH, the solubility of the co-crystals were lower than the solubility of INH (Table 1). The high solubility of INH·NA·SA was attributed to the high solubility of the SA and NA.
Sarcevica et al.37 prepared INH co-crystals of suberic acid (SUB), sebacic acid (SEB) benzoic acid (BA) and cinnamic acid (CIN). The stability and solubility of the resulting co-crystals as well as the previously reported co-crystals of INH and malonic (INH·MALO), succinic (INH·SUC), glutaric (INH·GLT), adipic (INH·ADI) and pimelic (INH·PIM) were investigated. Co-crystallization of isoniazid with suberic acid yielded two polymorphs crystallizing in the triclinic space group P1 and monoclinic space group P21/c. Isoniazid and cinnamic acid also yielded two polymorphs crystallizing in the triclinic space group P1 and monoclinic space group P21/c. The stability of INH·MALO, INH·SUC, INH·GLT, INH·ADI and INH·PIM were investigated by storing the compounds at 30 °C and 75% relative humidity for eight (8) weeks. The stability experiments indicated that the INH·MALO decomposed after four (4) weeks while the rest of the co-crystals remained intact for the entire duration of the testing period. To determine the stability of pure INH and the triclinic form of INH·CIN, the compounds were stored at 30 °C and 75% relative humidity for eleven (11) weeks. The compounds were stable over the experimental period. Stability experiments for INH·BA, INH·SEB and INH SUB (monoclinic form) conducted at 30 °C and 75% relative humidity for 22 weeks showed that the INH·BA co-crystal decomposes after 2 weeks while the INH·SEB remained stable over the experimental period. The poor stability of the INH·BA co-crystal was attributed to the fewer hydrogen bonds between the benzoic acid and the isoniazid molecules. When determined using deionized water at 22 ± 1 °C, the solubility of INH·MALO, INH·SUC, INH·GLT, INH·SUB and INH·BA were higher than the solubility of INH while the remaining were lower than that of INH (Table 1). The higher solubility values of the INH·MALO, INH·SUC, INH·GLT co-crystals were attributed to the high solubility values of the dicarboxylic acids, while the high solubility of INH·BA was attributed to its instability.
Swapna et al.38 reported stability studies of co-crystals of INH with ferulic acid (FRA), vanillic acid (VLA), caffeic acid (CFA) and resorcinol (RES) namely INH·FRA form I, INH·FRA form II, INH·VLA-form 1, INH·VLA form II, INH·CFA form I, INH·CFA form II, INH·CFA form III and INH·RES. Stability tests were carried out at ambient conditions (35 °C and 40% RH) as well as at accelerated conditions (40 °C, 75% RH). All the co-crystals were stable for more than twelve months at ambient conditions. At accelerated conditions, INH-RES dissociated after one month while the rest were stable for up to six months. In addition, no polymorphic transition or hydrate formation was observed for all the co-crystals. Rosa et al.39 prepared a co-crystal of INH and resveratrol; INH·RES. The solubility was determined in the pH range 1.2 to 7.4 and it was found that INH·RES has low solubility compared to that of INH.
During treatment, TB patients may experience oxidative stress due to tissue inflammation and free radical burst from activated macrophages. These free radicals are usually treated with anti-oxidants. Therefore, there has been a few reports on the pharmaceutical co-crystals of anti-TB drugs containing anti-oxidant co-formers, these include a co-crystal of INH and p-coumaric acid by Ravikumar et al.40 and co-crystals of INH and gallic acid, 3-hydroxybenzoic acid, 3,5-dihydroxybenzoic acid and 2,3-dihydroxybenzoic acid by Mashhadi et al.41 Mashhadi et al. also reported a hydrate co-crystal of INH with an antioxidant and anti-bacterial protocatechuic acid.42 The co-crystal was found to be less soluble in a pH 7.4 buffer but had greater stability at 80 °C compared to INH.
4. Drug–drug and drug-bridge-drug co-crystals of PYZ and INH
Drug–drug co-crystals present a new potential strategy for administering the fixed dose combinations (FDCs). This would be possible if the two drugs have complementary functional groups. Grobelny et al.43 prepared a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal of INH and 4-aminosalicylic acid. The molecules in the co-crystal are joined by the COOH⋯N-pyridyl heterosynthon. The authors also reported a 1
1 co-crystal of INH and 4-aminosalicylic acid. The molecules in the co-crystal are joined by the COOH⋯N-pyridyl heterosynthon. The authors also reported a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monohydrate of pyrazinamide and 4-aminosalicylic acid, in which the COOH⋯N-pyrazyl heterosynthon also joins the two drugs.43 Other drug–drug co-crystals of pyrazinamide reported in the literature include co-crystals of pyrazinamide and, temozolomide,44 entacapone,45 quercetin46 and hydrochlorothiazide.47 Drug–drug co-crystals containing isoniazid and, hydrochlorothiazide48 and 5-fluorocytosine49 have also been reported. In some cases, however, co-crystallizing two drugs may be challenging. One approach that has been developed for dealing with drugs that are difficult to co-crystallize has been through the formation of a drug-bridge-drug co-crystal in which two drug molecules are linked via a pharmaceutically acceptable molecule. Cherukuvada and co-workers35 attempted to prepare a 1
1 monohydrate of pyrazinamide and 4-aminosalicylic acid, in which the COOH⋯N-pyrazyl heterosynthon also joins the two drugs.43 Other drug–drug co-crystals of pyrazinamide reported in the literature include co-crystals of pyrazinamide and, temozolomide,44 entacapone,45 quercetin46 and hydrochlorothiazide.47 Drug–drug co-crystals containing isoniazid and, hydrochlorothiazide48 and 5-fluorocytosine49 have also been reported. In some cases, however, co-crystallizing two drugs may be challenging. One approach that has been developed for dealing with drugs that are difficult to co-crystallize has been through the formation of a drug-bridge-drug co-crystal in which two drug molecules are linked via a pharmaceutically acceptable molecule. Cherukuvada and co-workers35 attempted to prepare a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal of isoniazid and pyrazinamide, INH·PYZ via solution crystallization and grinding. Solution crystallization was unsuccessful, however, the grinding method yielded INH·PYZ eutectic. In addition, two ternary eutectics containing succinic acid (SA), and fumaric acid (FA) namely PYZ·SA·INH and PYZ·FA·INH were also prepared via grinding. Solubility studies on the three eutectics indicated that they display a superior intrinsic dissolution rate (IDR) compared to PYZ and follow the order PYZ·SA·INH > INH > PYZ·INH > PYZ·FA·INH > PYZ. Stability studies conducted at ambient conditions (15–40 °C and 30–70% RH) showed that the compounds are stable up to 1 year. Liu et al.50 prepared ternary co-crystal containing INH and pyrazinamide (PYZ) linked by a fumaric acid bridge (PYZ·FA·INH). The solubility tests were conducted in pH 1.2, 4.0 and 6.8 buffer solutions. The results indicated that the ternary co-crystal is most soluble in pH 6.8 buffer and that the formation of a ternary co-crystal with the two drugs managed to increase the poor solubility of PYZ and reduce the solubility of INH. It is postulated that the co-crystal may be more bioavailable compared to the two drugs.
1 co-crystal of isoniazid and pyrazinamide, INH·PYZ via solution crystallization and grinding. Solution crystallization was unsuccessful, however, the grinding method yielded INH·PYZ eutectic. In addition, two ternary eutectics containing succinic acid (SA), and fumaric acid (FA) namely PYZ·SA·INH and PYZ·FA·INH were also prepared via grinding. Solubility studies on the three eutectics indicated that they display a superior intrinsic dissolution rate (IDR) compared to PYZ and follow the order PYZ·SA·INH > INH > PYZ·INH > PYZ·FA·INH > PYZ. Stability studies conducted at ambient conditions (15–40 °C and 30–70% RH) showed that the compounds are stable up to 1 year. Liu et al.50 prepared ternary co-crystal containing INH and pyrazinamide (PYZ) linked by a fumaric acid bridge (PYZ·FA·INH). The solubility tests were conducted in pH 1.2, 4.0 and 6.8 buffer solutions. The results indicated that the ternary co-crystal is most soluble in pH 6.8 buffer and that the formation of a ternary co-crystal with the two drugs managed to increase the poor solubility of PYZ and reduce the solubility of INH. It is postulated that the co-crystal may be more bioavailable compared to the two drugs.
5. Pharmaceutical co-crystals of pyrazinamide that address the issue of solubility
The solubility values of multicomponent crystals described in this section are given in Table 2. Luo and co-workers51 reported co-crystals of PYZ and malonic acid (PYZ·MALO) and PYZ and glutaric acid (PYZ·GLT) as well as a new polymorph of PYZ and succinic acid (PYZ·SUC). Solubility measurements were conducted using in situ ATR-FTIR spectroscopy. With malonic acid being the most soluble of the three acids and glutaric acid being the least soluble, the solubility of the three co-crystals obtained correlate with those of the acid co-formers.24 The most soluble acid co-former resulted in a most soluble co-crystal i.e. the solubility order was as follows; PYZ·MALO > PYZ·GLT > PYZ·SUC (Table 2). The dissolution rate measured by the rotating disk intrinsic dissolution rate (DIDR) in pH 1.2 aqueous HCl solution on in situ ATR-FTIR spectroscopy follows the order PYZ·SUC < PYZ·MALO < PYZ·GLT.| Dissolution medium | Co-crystal | Solubility (mg mL−1) | Reference |
|---|---|---|---|
| pH 1.2 HCl | PYZ | 22 | 51 |
| PYZ·MALO | 66.5 | ||
| PYZ·GLT | 49.7 | ||
| PYZ·SUC | 37.2 | ||
| pH 1.2 HCl | PYZ | 18.64 | 52 |
| PYZ·ADA | 12.57 | ||
| PYZ·SEB | 12.54 | ||
| PYZ·ACA | 30.60 | ||
| PYZ·CIA | 21.05 | ||
| Water | PYZ | 20.71 | 53 |
| PYZ·24DHBA | 7.07 | ||
| PYZ·26DHBA | 12.18 | ||
| PYZ·35DHBA | 24.26 | ||
| PYZ·FRA | 3.33 | ||
| PYZ+·pTSA− | 39.99 | ||
| Water | PYZ | 15.28(75) | 54 |
| PYZ·pNBA | 3.88(16) | ||
| (PYZ)2·pNBA | 4.15(11) |
Wang et al.52 prepared four PYZ co-crystals with carboxylic acids namely; adipic acid (ADA), sebacic acid (SEB), trans-aconitic acid (ACA) and citric acid (CIA). PYZ·ADA and PYZ·SEB co-crystals were found to have lower solubility and IDR than PYZ while PYZ·ACA and PYZ·CIA co-crystals were found to have greater solubility and IDR than PYZ (Table 2). The greater solubility of PYZ·ACA and PYZ·CIA was attributed to the high solubility of ACA and CIA.
Sarmah et al.53 reported five co-crystals of PYZ with 2,4 dihydroxybenzoic acid (24DHBA), 2,6 dihydroxybenzoic acid (26DHBA), 3,5 dihydroxybenzoic acid (35DHBA) and ferulic acid (FRA). The authors also report a salt of PYZ and p-toluenesulfonic acid (pTSA). The solubility of these compounds, measured using the shake flask method in distilled water, was found to correlate with the equilibrium solubility of the co-former and they follow the order PYZ+·pTSA− > PYZ·35DHBA > PYZ > PYZ·26DHBA > PYZ·24DHBA > PYZ·FRA (Table 2).
Abourahma et al.54 reported two stoichiometric variations of co-crystals of PYZ and p-nitrobenzoic acid (pNBA); (PYZ)2·pNBA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal) and PYZ·pNBA (1
1 co-crystal) and PYZ·pNBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal). The stability of the co-crystals was measured by suspending the compounds in methanol or acetonitrile for seven days. Analysis of the residual solid in the (PYZ)2·pNBA experiment confirmed the presence of only the 2
1 co-crystal). The stability of the co-crystals was measured by suspending the compounds in methanol or acetonitrile for seven days. Analysis of the residual solid in the (PYZ)2·pNBA experiment confirmed the presence of only the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal. On the other hand, analysis of the residual solid in the PYZ·pNBA experiment showed the presence of the 1
1 co-crystal. On the other hand, analysis of the residual solid in the PYZ·pNBA experiment showed the presence of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal as well as the 2
1 co-crystal as well as the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal indicating that the 1
1 co-crystal indicating that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal converts to the more stable 2
1 co-crystal converts to the more stable 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal. Equilibrium solubility measurements conducted on the two compounds as well as PYZ using the shake flask method follow the order PYZ > PYZ·pNBA > (PYZ)2·pNBA (Table 2).
1 co-crystal. Equilibrium solubility measurements conducted on the two compounds as well as PYZ using the shake flask method follow the order PYZ > PYZ·pNBA > (PYZ)2·pNBA (Table 2).
6. Conclusion
In summary, TB is a global health problem and a significant amount of research has been conducted since the discovery of Mycobacterium tuberculosis. The issue of drug resistance continues to be a major problem facing health systems in the treatment of TB. Even though WHO recommended the use of FDC regimens to combat resistance, they are still plagued by challenges which include chemical instability and reduced bioavailability of rifampicin. As this review shows, a significant amount of pharmaceutical co-crystal research is ongoing and is aimed at improving the stability of the active pharmaceutical ingredients in the FDCs. This review examined the multicomponent crystals of two anti-TB drugs; isoniazid and pyrazinamide. The research area itself is relatively young, with the first co-crystal of pyrazinamide having been reported less than two decades ago and a co-crystal of isoniazid first reported only eleven years ago.An analysis of the reported structures indicated that carboxylic acids were the most commonly utilized co-formers in the preparation of pharmaceutical co-crystals of both isoniazid and pyrazinamide. For the isoniazid co-crystals, the formation of the pyridyl-N⋯HOOC heterosynthon was observed in 84% of the co-crystals while in pyrazinamide the pyrazyl-N⋯HOOC heterosynthon was observed 45% of the co-crystals. The –COOH groups in the co-crystals in which the pyrazyl-N⋯HOOC heterosynthon is not utilized are involved in carboxylic acid dimer and/or acid⋯amide interactions.
The past fifteen years have seen an increase in research efforts dedicated to preparing multicomponent crystals of pyrazinamide and isoniazid. However, structure–property studies are still very limited considering that out of the one hundred and ten (110) co-crystals of isoniazid and the forty-nine (49) co-crystals of pyrazinamide solubility and/or stability studies were reported in approximately 28% and 51% of the isoniazid and pyrazinamide co-crystals respectively. Some of the studies reviewed here found a correlation between the solubility of co-crystal and solubility of co-former while other studies found no correlation.34,37,38,51,53 The nature of interactions in the crystal structure as well as the stability of the co-crystal were also shown to affect the solubility of some co-crystals.37
Co-crystallization offers the possibility of improving the physicochemical properties of isoniazid and pyrazinamide. There is still a lot that can be done given that there is a long list of compounds in the generally regarded as safe (GRAS) list which can be employed as co-formers. An increased focus on structure–property studies combined with computational studies may provide important information on how to design multicomponent crystals of isoniazid and pyrazinamide with the desired stability and solubility.
Conflicts of interest
There are no conflicts of interests to declare.Acknowledgements
E. B. thanks the National Research Foundation of South Africa for financial support.References
- I. Hershkovitz, H. D. Donoghue, D. E. Minnikin, H. May, O. Y. C. Lee, M. Feldman, E. Galili, M. Spigelman, B. M. Rothschild and G. K. Bar-Gal, Tuberculosis, 2015, 95, S122–S126 CrossRef.
- World Health Organisation, Global Tuberculosis Report, 2019 Search PubMed.
- World Health Organisation, Global Tuberculosis Report, 2018 Search PubMed.
- B. Blomberg and B. Fourie, Drugs, 2003, 63, 535–553 CrossRef CAS.
- A. Schatz, E. Bugie and S. A. Waksman, Proc. Soc. Exp. Biol. Med., 1944, 55, 66–69 CrossRef CAS.
- D. Weitzman, F. E. de Wend Cayley and A. L. Wingfield, Br. J. Tuberc. Dis. Chest, 1950, 44, 98–104 CrossRef CAS.
- The treatment of pulmonary tuberculosis with streptomycin and para-amino-salicylic acid, Br. Med. J. 1950, 2, 1073–1085 Search PubMed.
- J. Lehmann, Lancet, 1946, 1, 15–16 CrossRef CAS.
- E. Grunberg and R. J. Schnitzer, Q. Bull. Sea View Hosp., 1952, 13, 3–11 CAS.
- H. Meyer and J. Mally, Monatsh. Chem., 1912, 33, 393–414 CrossRef CAS.
- The treatment of pulmonary tuberculosis with isoniazid, Br. Med. J., 1952, 2, 735–746 Search PubMed.
- Various combinations of isoniazid with streptomycin or with P.A.S. in the treatment of pulmonary tuberculosis, Br. Med. J., 1955, 1, 441–445 Search PubMed.
- J. G. Scadding, Postgrad. Med. J., 1951, 27, 549–558 CrossRef CAS.
- J. F. Murray, D. E. Schraufnagel and P. C. Hopewell, Ann. Am. Thorac. Soc., 2015, 12, 1749–1759 CrossRef.
- Controlled clinical trial of four short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis, Lancet, 1974, 304, 237–240 Search PubMed.
- Clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis: the results up to 30 months, Am. Rev. Respir. Dis., 1979, 119, 579–589 Search PubMed.
- R. L. Yeagen, W. G. C. Munice and F. I. Dessai, Am. Rev. Tuberc., 1952, 65, 523–546 Search PubMed.
- N. Maggi, C. R. Pasqualucci, R. Ballotta and P. Sensi, Chemotherapy, 1966, 11, 285–292 CrossRef CAS.
- J. P. Thomas, C. O. Baughn, R. G. Wilkinson and R. G. Shepherd, Am. Rev. Respir. Dis., 1961, 83, 891–893 CAS.
- S. Singh, H. Bhutani and T. T. Mariappan, Indian J. Tuberc., 2006, 53, 201–205 Search PubMed.
- R. Sankar, N. Sharda and S. Singh, Drug Dev. Ind. Pharm., 2003, 29, 733–738 CrossRef CAS.
- S. Singh, T. T. Mariappan, N. Sharda and B. Singh, Pharm. Pharmacol. Commun., 2000, 6, 491–494 CrossRef CAS.
- G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342–8356 CrossRef CAS.
- D. J. Good and R. H. Naír, Cryst. Growth Des., 2009, 9, 2252–2264 CrossRef CAS.
- N. Qiao, M. Li, W. Schlindwein, N. Malek, A. Davies and G. Trappitt, Int. J. Pharm., 2011, 419, 1–11 CrossRef CAS.
- L. Roy, M. P. Lipert, N. Rodríguez-Hornedo, W. Jones, R. Thakuria and A. Delori, Int. J. Pharm., 2012, 453, 101–125 Search PubMed.
- M. Rodrigues, B. Baptista, J. A. Lopes and M. C. Sarraguça, Int. J. Pharm., 2018, 547, 404–420 CrossRef CAS.
- D. Braga, S. L. Giaffreda, F. Grepioni, M. R. Chierotti, R. Gobetto, G. Palladino and M. Polito, CrystEngComm, 2007, 9, 879–881 RSC.
- C. C. Robertson, J. S. Wright, E. J. Carrington, R. N. Perutz, C. A. Hunter and L. Brammer, Chem. Sci., 2017, 8, 5392–5398 RSC.
- E. Pindelska, A. Sokal and W. Kolodziejski, Adv. Drug Delivery Rev., 2017, 117, 111–146 CrossRef CAS.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS.
- C. B. Aakeröy, J. Desper and B. A. Helfrich, CrystEngComm, 2004, 6, 19–24 RSC.
- L. F. Diniz, M. S. Souza, P. S. Carvalho, C. C. Correa and J. Ellena, J. Mol. Struct., 2018, 1171, 223–232 CrossRef CAS.
- S. Aitipamula, A. B. H. Wong, P. S. Chow and R. B. H. Tan, CrystEngComm, 2013, 15, 5877–5887 RSC.
- S. Cherukuvada and A. Nangia, CrystEngComm, 2012, 14, 2579–2588 RSC.
- A. Lemmerer, CrystEngComm, 2012, 14, 2465–2478 RSC.
- I. Sarcevica, L. Orola, M. V. Veidis, A. Podjava and S. Belyakov, Cryst. Growth Des., 2013, 13, 1082–1090 CrossRef CAS.
- B. Swapna, D. Maddileti and A. Nangia, Cryst. Growth Des., 2014, 14, 5991–6005 CrossRef CAS.
- J. Rosa, T. C. Machado, A. K. Da Silva, G. Kuminek, A. J. Bortolluzzi, T. Caon and S. G. Cardoso, Cryst. Growth Des., 2019, 19, 5029–5036 CrossRef CAS.
- N. Ravikumar, G. Gaddamanugu and K. Anand Solomon, J. Mol. Struct., 2013, 1033, 272–279 CrossRef CAS.
- S. M. A. Mashhadi, U. Yunus, M. H. Bhatti and M. N. Tahir, J. Mol. Struct., 2014, 1076, 446–452 CrossRef CAS.
- S. M. A. Mashhadi, U. Yunus, M. H. Bhatti, I. Ahmed and M. N. Tahir, J. Mol. Struct., 2016, 1117, 17–21 CrossRef CAS.
- P. Grobelny, A. Mukherjee and G. R. Desiraju, CrystEngComm, 2011, 13, 4358–4364 RSC.
- P. Sanphui, N. J. Babu and A. Nangia, Cryst. Growth Des., 2013, 13, 2208–2219 CrossRef CAS.
- M. K. Bommaka, M. K. Chaitanya Mannava, K. Suresh, A. Gunnam and A. Nangia, Cryst. Growth Des., 2018, 18, 6061–6069 CrossRef CAS.
- F. Liu, L. Y. Wang, Y. T. Li, Z. Y. Wu and C. W. Yan, Cryst. Growth Des., 2018, 18, 3729–3733 CrossRef CAS.
- J. R. Wang, C. Ye and X. Mei, CrystEngComm, 2014, 16, 6996–7003 RSC.
- S. P. Gopi, M. Banik and G. R. Desiraju, Cryst. Growth Des., 2017, 17, 308–316 CrossRef CAS.
- M. S. Souza, L. F. Diniz, L. Vogt, P. S. Carvalho, R. F. D'Vries and J. Ellena, Cryst. Growth Des., 2018, 18, 5202–5209 CrossRef CAS.
- F. Liu, Y. Song, Y. N. Liu, Y. T. Li, Z. Y. Wu and C. W. Yan, Cryst. Growth Des., 2018, 18, 1283–1286 CrossRef CAS.
- Y. H. Luo and B. W. Sun, Cryst. Growth Des., 2013, 13, 2098–2106 CrossRef CAS.
- J. R. Wang, C. Ye, B. Zhu, C. Zhou and X. Mei, CrystEngComm, 2015, 17, 747–752 RSC.
- K. K. Sarmah, T. Rajbongshi, S. Bhowmick and R. Thakuria, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2017, 73, 1007–1016 CrossRef CAS.
- H. Abourahma, D. D. Shah, J. Melendez, E. J. Johnson and K. T. Holman, Cryst. Growth Des., 2015, 15, 3101–3104 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06478e |
| This journal is © The Royal Society of Chemistry 2020 |