Open Access Article
Open Access ArticleEnvironmental valorisation of bagasse fly ash: a review†
Himanshu Patel *
*
Department of Applied Science and Humanities, Pacific School of Engineering, Kadodara Palsana Road (NH-8), V: Sanki, Ta. Palsana, Surat-394305, Gujarat, India. E-mail: hjpatel123@yahoo.co.in; Tel: + 91-261-2772233
First published on 26th August 2020
Abstract
Worldwide, each year the sugar industry generates bagasse fly ash (BFA) in the process of producing sugar including ethanol and jaggery. With the increasing average value of 2% consumption of sugar per annum, the quantity of solid waste BFA continuously increases and creates environmental problems. The valorisation of BFA is a convenient and sustainable means for decreasing the environmental burden. This paper aims to review the various important analyses and valorisation of BFA. BFA is a porous material and has oxides of several elements, such as silicon, aluminium, iron, sodium and other metals. Based on some of its excellent properties, untreated and modified BFA can represent an important source in different fields. Metals, dyes, petrochemicals, insecticides and other contaminants can be adsorbed using BFA, where the maximum adsorption capacities can be described using different adsorption variables and isotherms. It is convenient and a sustainable resolution to use traditional adsorbents for water treatment. This also decreases the environmental solid burden, eventually reducing greenhouse gas emissions. This ash has been incorporated into different construction materials, including cement and brick in different percentages to enhance their mechanical characteristics and durability, thus maintaining economic and environmental sustainability. Also, several products such as zeolites, silica, briquette catalyst and other important materials, which are promising energy sources, have been prepared using the BFA.
1. Introduction
Rapid industrialization, haphazard use of non-renewable resources, vast pollution growth, and improper legislative pollution management are the main drawbacks for sustainable development. As earth's resources, such as petroleum, coal and ores are being used up, it is feared that these might soon get exhausted. Consequently, there is always a need for the newly developed materials in various fields. Scientists are continuously working on applications of industrial sludge, food processing waste, agriculture by-products, animal waste, and marine extracts to minimize the solid waste burden associated with environmental pollution. These materials are inexpensive, easily available, locally accessible, safe to use and environmentally sustainable renewable resources and are alternatives to traditional materials.1 It is estimated that 2 billion tonnes were collected world-wide in 2016 and it may increase to 3.4 billion tons in 2050, with Asian and African countries being the main sources. This solid waste also causes global greenhouse gas release due to the decaying organic sections of solid waste. About 34% of total solid waste is recycled, 13% is composted or burnt to yield energy and the remaining waste is dumped or not properly managed. Only 3.0–3.5% of solid waste is municipal solid water and the remaining 76.5–77.0% of waste is industrial solid waste. Solid waste, especially the industrial solid waste, therefore, has become one of the environmental issues worldwide.2,3Sugar, used as a sweetener in food and beverages, is a sweet crystalline substance acquired from various plants, such as sugar cane and sugar beet, but approximately 80% of the world's sugar is produced from sugarcane. Sugarcane is grown in tropical and subtropical climates having temperatures of 21–27 °C and annual rainfall between 75 and 100 cm. Sugarcane is an important commercial crop and is one of the principal sources of sugar, ethanol, and jaggery (a semi-refined sugar product used in the Indian subcontinent) globally. The by-product, jaggery has various health benefits and ethanol as well has industrial importance. Bagasse (fibrous residue behind from sugarcane after extraction juice) has a net calorific value of around 8000 kJ kg−1 and is therefore utilized as a fuel in boilers in the sugar mills to generate steam and electricity. The efficiency of boilers used in sugar mills is typically 60–70%. The burning of bagasse could lead to the generation of around 3 million tonnes of fly ash annually, i.e. 4% of the weight of bagasse, called bagasse fly ash (BFA).4,5 For the production of 1.0 metric tonne (MT) of sugar and 0.07 MT of ethanol, it is estimated that up to 9.05 MT sugar cane is required, which would generate up to 2.61 MT bagasse, from which 2.15 MT bagasse is required to generate electricity and yield 0.045 MT bagasse fly ash. According to the International Sugar Organization, world sugar consumption has increased from 123.454 to 172.441 MT from 2001 to 2018, having an average annual growth of 2.01%. Fig. 1 represents the sugar production by continent in the year 2018, where the continent with the highest sugar production was Asia, having 75 million tonnes, followed by South America (37 MT) and Europe (30 MT). A bar chart presentation of the sugar production of Asian countries is given in Fig. 2 for the year 2018, which indicates that India has the largest production of sugar (50.92%), followed by China (14.69%) and Thailand (14.10%). In that year, India produced 29.5 million metric tons of sugar. From Fig. 1 and 2, it was concluded that India has the highest sugar production from sugarcane and generated BFA.6
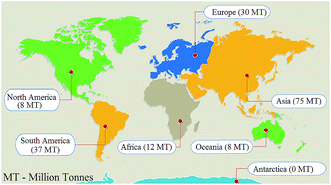 | ||
| Fig. 1 World sugar production by continents.6 | ||
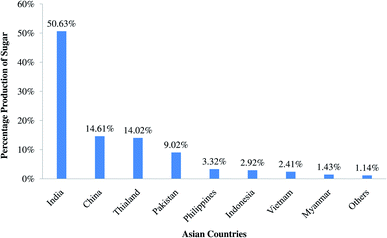 | ||
| Fig. 2 Asian sugar production by major countries.6 | ||
Bagasse fly ash contains a high amount of unburned carbon and can have several potential effects on humans, plants and animals through the air, water and soil. (1) Since BFA has very small particle sizes ranging from 0.5 to 300 microns and being lightweight, it can be easily airborne and tends to cause air pollution and respiratory problems. (2) It contains traces of heavy metals, which pollute the air, soil and groundwater. (3) It can be used as fertilizer but it hardens underground. (4) Only land-filling (dumping) techniques are used to dispose of it, which requires large areas. This fly ash is not disposed of in open fields or aquatic bodies such as seas, rivers or ponds.7,8 In this review, we focus on the valorisation of bagasse fly ash (the solid waste of the sugar industry) in different areas to reduce the solid waste generation and environmental pollution load, thereby eliminating the environmental load.
2. Properties of BFA
For a better understanding of its application, the physical, chemical and other properties of BFA play significant roles. Various properties such as the chemical constitution, proximate analysis, physicochemical properties, surface areas of pores, Barrett–Joyner–Halenda (BJH) cumulative pore volume, average pore diameter, BJH – pore size distribution and elemental analysis were determined and are depicted in Table 1. The chemical composition of BFA was determined using standard methods. BFA mainly consists of calcareous materials, viz., silica (63.1%), alumina (16.9%), calcium oxide (3.50%) and magnesia (0.80%), determined using XRF (the X-ray fluorescence method). It is also comprised of several oxides of metal such as iron, manganese, titanium, sodium and potassium. Moisture, loss of drying, ash, volatile matter and fixed carbon were determined in proximate analysis using standard methods,9 and the values obtained were 2.51, 12.3, 30.95, 23.48 and 43.03%, respectively. The total carbon content was 60.04%, as derived by elemental analysis; thus, BFA has a high unburnt carbon content. The specific density, bulk density, dry density, void ratio, fractional porosity and point of zero charge (pHpzc) of BFA are 1.882, 1.725 g cm−3, 1.081 g cm−3, 0.747, 0.428 and 8.18, respectively. The Barrett–Joyner–Halenda (BJH) cumulative pore volume, average pore diameter, and BJH – pore size distribution were determined and presented in Table 1. It contains amorphous calcareous materials, high unburnt carbon content, high surface area, low moisture content, high porosity percent, high pore size distribution and characterization, and therefore, can be utilized for different purposes.| Type of analysis | Particular | Reference |
|---|---|---|
| a XRF – X-ray fluorescence, pHpzc – the point of zero charge, BET – Brunauer–Emmett–Teller, BJH – Barrett–Joyner–Halenda. | ||
| Chemical constituents (%) by XRF | ||
| MgO | 0.80 | 9 |
| Al2O3 | 16.9 | |
| SiO2 | 63.1 | |
| Cl | 3.00 | |
| K2O | 3.27 | |
| CaO | 3.50 | |
| TiO2 | 0.34 | |
| MnO | 0.12 | |
| Fe2O3 | 4.40 | |
| Na2O | 4.57 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Physiochemical properties | ||
| Specific density | 1.882 | |
| Bulk density (g cm−3) | 1.725 | |
| Dry density (g cm−3) | 1.081 | |
| Void ratio | 0.747 | |
| Fractional porosity | 0.428 | |
| pHpzc | 8.18 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Proximate analysis (%) | ||
| Moisture content | 2.51 | 10 |
| Loss on drying | 12.3 | |
| Ash content | 30.95 | |
| Volatile matter | 23.48 | |
| Fixed carbon | 43.03 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Surface area of pores (m2 g−1) | ||
| BET | 168.39 | 11 |
| BJH – adsorption cumulative | 70.90 | |
| BJH – desorption cumulative | 45.30 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Average pore diameter (Å) | ||
| BET | 25.54 | |
| BJH – adsorption cumulative | 49.85 | |
| BJH – desorption cumulative | 58.44 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| BJH cumulative pore volume (cm2 g−1) | ||
| Single point total | 0.1067 | 12 |
| BJH adsorption | 0.0844 | |
| BJH desorption | 0.0622 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| BJH – pore size distribution | ||
| Average adsorption pore diameter (nm) | 6.33 | 13 |
| Average desorption pore diameter (nm) | 5.23 | |
| Total pore volume (cm3 g−1) | 0.0669 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Elemental analysis (%) | ||
| Carbon | 60.04 | 14 |
| Hydrogen | 0.62 | |
| Nitrogen | 0.28 | |
| Sulphur | 0.00 | |
| Oxygen | 6.77 | |
| Others | 32.29 | |
Fourier transform infrared (FT-IR) spectroscopy of BFA was performed to identify its functional groups. Fig. 3 shows the FT-IR spectrum of BFA, where the medium and sharp peaks near 3774 and 3100 cm−1 indicate the presence of the O–H stretching groups of silanol and N–H stretching respectively. Two medium peaks at 3000–2840 cm−1 are due to the C–H stretching of alkanes. The peak due to –OH stretching in carboxylic acid was seen near 2200 cm−1. Three medium peaks in the range of 1750–1650 cm−1 are due to different groups in BFA like the carbonyl stretching of the aromatic groups and alkene groups, and the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the alkene and aromatic groups. The 1385–1123 cm−1 bands can be attributed to the –CO and –C–OH stretching peaks of carboxylic acids, lactones, and alcohols. The weak peaks in the lower IR region at 870 and 476 cm−1 are assigned to the bending vibrations of alkenes and C–OH. Further, XRD was used to identify the crystalline structure and the pattern is presented in Fig. 4, which indicates the presence of minerals with respect to d-spacing. Table 2 shows the d-spacings obtained from the diffraction pattern, and the respective minerals.16
O stretching of the alkene and aromatic groups. The 1385–1123 cm−1 bands can be attributed to the –CO and –C–OH stretching peaks of carboxylic acids, lactones, and alcohols. The weak peaks in the lower IR region at 870 and 476 cm−1 are assigned to the bending vibrations of alkenes and C–OH. Further, XRD was used to identify the crystalline structure and the pattern is presented in Fig. 4, which indicates the presence of minerals with respect to d-spacing. Table 2 shows the d-spacings obtained from the diffraction pattern, and the respective minerals.16
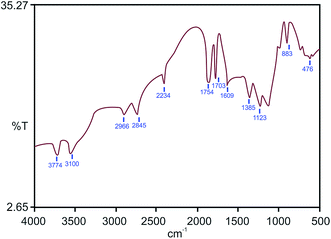 | ||
| Fig. 3 FT-IR spectrum of BFA.15 | ||
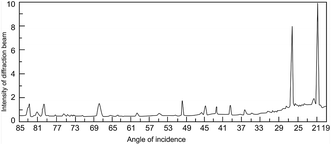 | ||
| Fig. 4 X-ray diffraction pattern of BFA.16 | ||
| S. no. | D (Å) value | Preferable mineral |
|---|---|---|
| 1 | 4.690 | Geothite |
| 2 | 3.740 | Mullite |
| 3 | 2.730 | Hematite |
| 4 | 2.528 | Kaolinite |
| 5 | 2.234 | α-Quartz |
| 6 | 2.012 | γ-Aumina |
| 7 | 1.496 | Hematite |
| 8 | 1.314 | Hematite |
Thermal analyses of BFA were accomplished using TG (Thermogravimetry), DTG (Derivative Thermogravimetry) and DTA (Differential Thermal Analysis) techniques, which demonstrated the decomposition temperature of the various oxides and functional groups. Available surface carbon and the various forms were constant at 150 °C but above this temperature, the surface carbon decomposed to yield CO (200–600 °C), CO2 (450–1000 °C), water vapour and free hydrogen (500–1000 °C). Thermal decomposition of BFA was performed using an oxidizing environment like oxygen and also, an inert atmosphere (nitrogen) at a heating rate of 10 K min−1. TG and DTG thermographs were divided into three zones: the first (25–400 °C), second (400–523 °C) and third (523–1000 °C) temperature zone with respect to mass loss. In oxygen and nitrogen atmospheres, maximum mass losses were found to be 22.8 and 19.2% of the mass respectively, due to active pyrolysis and oxidation. Further, 6.4 and 1.1% mass losses were observed in the first and third zones, respectively in an oxygen atmosphere, while 4.8 and 0.0% mass losses were observed in the first and third zone respectively in a nitrogen atmosphere. Moisture and the light volatiles were removed in the first zone, while hydrogen, carbon monoxide and carbon dioxide were removed in the third zone. After the third zone, i.e., above 1000 °C, the residue remaining was ash, i.e., 70 and 76% of the total weight in air and nitrogen atmospheres.17 In the DTA thermogram, a strong exothermic peak was observed in the range of 450–480 °C, due to the oxidative degradation of BFA.
C. W. Purnomo et al. analyzed the surface morphology of BFA using Scanning Electron Microscopy (SEM).18 Three types of BFA was separated according to the size fractions, viz., large (>1.4 mm), medium (0.7–1.4 mm) and small (<0.7 mm) using mechanical sieving and SEM images. SEM images of the small fraction showed small-sized particles adjacent to the fibrous particles and also, some non-fibrous particles. Non-fibrous particles had some identical shapes like cenospheres, spheres and prisms. This shape was due to the melting and re-solidification of the silica, alumina and metal content. A large fraction of SEM contains the cell walls of fibrous particles. The SEM of silicon elemental mapping for the small fraction was examined, which showed that silica was conserved during combustion and was arranged systemically within the fibrous particles while producing the configuration of cell walls.
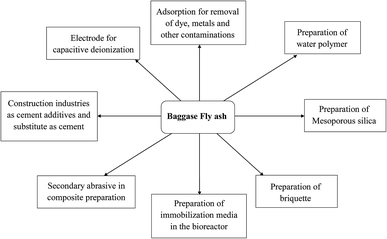
3. Valorisation of BFA
BFA has some extraordinary properties, and an outline of the valorisation of BFA is presented in Fig. 5, as per author knowledge.3.1 Adsorption
Adsorption has shown extraordinary properties for the removal of various contaminants including dyes, metals, pharmaceutical products, herbicides, insecticides, and also chemicals contributing to COD, BOD, colour and other contaminants from their synthetic solutions as well as real (industrial) wastewater. Two materials interact for adsorption: (1) the adsorbent, the material adsorbed on the surface and available in the bulk; (2) the adsorbate, the materials being adsorbed on the surface of the adsorbent material.19 Lots of varieties in adsorption-like treatments were investigated: [batch, continuous moving bed, continuous fixed bed (up-flow or down-flow), continuous fluidized bed and pulsed bed], different parameters [adsorbent dose, pH, contact time, initial concentration, temperature, flow rate, bed height, particle size], adsorption isotherms [Langmuir, Freundlich, Dubinin–Radushkevich, Temkin, Redlich–Peterson, Sips, Thomas, Bed Depth Service Time, Adam & Bohart, Yoon–Nelson, Clark model and Wolborska model], adsorptive kinetic models [pseudo-first-order, pseudo-second-order, Elovich, intra-particle diffusion, Boyd and surface mass transfer model], desorption/regeneration/elution of adsorbent, thermodynamic studies (change in free energy, enthalpy and entropy), statistical analysis [RSM (Response Surface Methodology), ANOVA (Analysis Of Variance), t-test, IAST (Ideal Adsorbed Solution Theory) model and other analyses], error analysis [R2 (coefficient of determination), ERRSQ (the sum of the squares of the errors), HYBRID (a composite fractional error function), EABS (sum of the absolute errors), χ2 (nonlinear chi-square test), ARE (Average Relative Error), RMSE (Root Mean Square Error), MPSD (Marquardt's Percent Standard Deviation), SNE (Sum of Normalized Errors) and SD (Standard Deviation)].20–22The surface of the adsorbent plays an important role in the adsorption phenomena. The adsorbent surface was intentionally made more porous, to increase the adsorption and hence, the percentage removal of adsorbate was higher.23 For confirmation of the adsorbent surface, various characterizations like BET surface area, pore volume, pore diameter, porosity percentage, FT-IR, SEM, TEM (Transmission Electron Microscopy) and XRD were performed. For example, Vidya S. Batra found that BFA contained unburned carbon, which was characterized by surface analysis (BET surface area, pore volume and pore size), thermal analysis (DTA, TGA and TG), electron microscopy [SEM, TEM and EDS (Energy-Dispersive Detector)] and nitrogen adsorption. These analyses indicated that this carbon has a combination of meso- and micro-pores and is suitable for the adsorption of several contaminants.24
Adsorption studies were carried out on two basic dyes, i.e., Rhodamine B (RB) and Methylene Blue (MB), using BFA and various experimental parameters such as adsorbent dose, pH, contact time and initial concentration. The data were analyzed using adsorption isotherms, thermodynamics, and kinetics studies. The highest removal amounts of 14.3 and 0.202 × 103 mol g−1 for these two dyes were achieved using Langmuir isotherms for RB and MB, respectively.30 BFA (collected from bagasse fired boilers) along with activated char (commercial-grade (ACC) and laboratory-grade (ACL)) were utilized for the adsorption of Congo red, in which various process parameters (adsorbent dose, pH, contact time, initial concentration) were used. The data were incorporated with adsorption isotherms, thermodynamics, error analysis and kinetics studies. Maximum removal capacities for BFA, ACC and ACL were found to be 11.885, 0.638 and 1.875 mg g−1, respectively.30 Comparative studies of these three carbons for the removal of malachite green dye were conducted and their adsorption capacities were calculated, in which BFA (170.33 mg g−1) was a better adsorbent than ACC (8.27 mg g−1) and ACL (42.18 mg g−1).31 Surface analyses such as bulk density, particle size, and proximate analysis, SEM, XRD, TGA, DTA and dTG of BFA were conducted by I. D. Mall and team members. Also, experiments were conducted for the removal of orange-G and methyl violet dyes. The adsorption data for the maximum adsorption capacities suggested that carbon-rich BFA has the potential for use as an adsorbent.32 Batch experiments were carried out for the adsorption of brilliant green (BG) using BFA without any pre-treatment. Different process parameters (adsorbent dose, pH, contact time, initial concentration and temperature), isotherms (Freundlich, Langmuir, Redlich–Peterson, Dubinin–Radushkevich and Temkin model), kinetics (pseudo-first-order, pseudo-second-order, Bangham's equation and intra-particle diffusion model), thermodynamic parameters and error analysis were evaluated. The maximum adsorption capacity was found to be 133.33 mg g−1 for the Langmuir model.17
BFA was converted to synthesized zeolite (ZFA) by alkaline hydrothermal treatment. Surface analysis of ZFA and raw BFA was conducted using surface area, density, proximate analysis, XRD, FT-IR and chemical composition and it was found that ZFA surface was more porous than that of raw BFA. Batch studies for the adsorption of methylene blue dye were conducted using different experimental parameters such as adsorbent dose, pH, contact time, initial concentration and temperature. Also, adsorption isotherms, thermodynamics, kinetic studies, economic evaluation were carried out to analyze the feasibility for industrial use.33,34 Comparative studies of two adsorbents, ZFA and raw BFA, for the removal of turquoise blue (TB) and brilliant magenta (BM) dyes were accomplished by B. A. Shah et al. The maximum adsorption capacities of TB and BM of ZFA (21.74 and 100 mg g−1 respectively) achieved were approximately double that of BFA (12.66 and 45.45 mg g−1 respectively).35
Two types of modified bagasse, namely, acrylic acid-grafted bagasse fly ash (BFAG) and hydrochloric acid-treated bagasse fly ash (BFA/HCl) were prepared and various physicochemical characteristics were determined such as BET surface area, total pore volume (VT), primary mesopore volume (Vme), micropore volume (Vmi), and zero-point of charge (pHzpc). Comparative studies of BFA, BFAG, BFA/HCl and AC (commercially available activated charcoal) were performed for the adsorption of antimony using contact duration and system pH and it was found that BFA/HCl was more preferable than other investigated adsorbents based on the value of the maximum adsorption capacity (Qmax). Sorption-desorption studies were also conducted using various concentrations of sodium hydroxide solution and it was revealed that a small amount of Sb molecules from the adsorbent was eluted from the adsorbent. This may be due to chemisorptions and strong bonds formed between the adsorbent and adsorbate.40 Batch and column studies were conducted on the adsorption of arsenic species (arsenate and arsenite) onto BFA by Imran Ali. BFA was characterized by surface area, density, porosity, moisture content, elemental analysis, FT-IR, scanning electron microscopy (SEM), and point of zero charge, and it was concluded that BFA is a preferable adsorbent. Various parameters such as initial concentration, pH, process time, adsorbent dose and temperature for the adsorption of arsenic species were determined. Thermodynamic, chemical kinetics and isotherms were also studied for batch treatment. Breakthrough curves were plotted for different flow rates (0.5–2.50 mL min−1) and the optimum flow rate was found to be 0.5 mL min−1. The column data were analyzed by the bed depth service time and Yoon and Nelson models. Retention of BFA was studied using sulphuric acid, hydrochloric acid, nitric acid, sodium hydroxide, and potassium hydroxide and it was revealed that 1.0 M NaOH was the more preferable desorption solvent.41
Two heavy metals, cadmium [Cd(II)] and zinc [Zn(II)] ions, were adsorbed onto BFA in individual form and a binary system. The effects of different metal initial concentrations, temperature, time and BFA were examined. Individual metal data were analyzed using Redlich–Peterson (R–P) and Freundlich isotherms, which showed that the Langmuir model was the best-fitted model, and zinc was better adsorbed than cadmium. Also, non-modified Langmuir, modified Langmuir, extended-Langmuir, Sheindorf–Rebuhn–Sheintuch (SRS), non-modified R–P and modified R–P adsorption models were utilized for the binary adsorbent system.42 A fixed-bed column study of the adsorption of Cr(VI), Cu(II) and Ni(II) was conducted and breakthrough curves were prepared using tubes with different lengths.43 Iron-coated bagasse fly ash (BFA-IC) and sponge iron char (SIC) were prepared and the surface areas were determined by BET surface area, ZPC and PZC techniques. Using these newly prepared adsorbents, batch studies for the removal of trivalent arsenic [As(III), or arsenite] and pentavalent arsenic [As(V), or arsenate] were carried out. Different parameters, i.e. adsorbent dose, pH, contact time, temperature and initial concentration, and various isotherms and kinetic models were performed. From the correlation coefficient values (R2), the Temkin and pseudo-second-order models were found to be the best fitting among the investigated models. The maximum adsorption capacities for BFA-IC [25.82 mg g−1 for As(V) and 39.83 mg g−1 314 for As(III)] and SIC [28.58 mg g−1 for As(V); 27.85 mg g−1 for As(III)] were achieved, which showed that the modified BFA had excellent potential for use as an adsorbent.44
Ferric chloride-coated BFA was characterized using the BET surface area, particle size, proximate analyses, SEM, FT-IR, EDAX and TGA, and utilized for the adsorption of selenium(IV). Apart from the process parameters (adsorbent dose, pH, contact time and temperature), isotherms (Freundlich, Langmuir and Temkin), kinetics (pseudo-first-order, intra-particle diffusion and pseudo-second-order), thermodynamics and error analysis, the thermal degradation of the spent adsorbent was determined to analyze the feasibility of re-use/recycling.45 Table 3 depicts the details of the metal adsorption study using BFA and its activated forms, in which the value of maximum adsorption capacity is from the Langmuir isotherm.
| S. no. | Adsorbate | Variable | Details of adsorption study | Maximum adsorption capacitya (mg of metal per g of BFA) | Reference |
|---|---|---|---|---|---|
| a The value of the maximum adsorption capacity is corresponds to the Langmuir isotherm. | |||||
| 1 | Cr(VI) | pH, temperature, time duration, adsorbent dose, initial concentration | Adsorption isotherms (batch and column); thermodynamic study; regeneration of adsorbent; cost estimation | 5.0 × 103 mol g−1 | 46 |
| 2 | Pb(II) | pH, adsorbent dose, initial concentration | Adsorption isotherms (batch); thermodynamic study; regeneration of adsorbent | 2.60 × 103 mol g−1 | 47 |
| 3 | Cu(II) & Zn(II) | pH, adsorbent dose, initial concentrations, temperature, particle size | Adsorption isotherms (batch and column); thermodynamic study; regeneration of adsorbent | 2.36 & 2.54 | 48 |
| 4 | Zn(II) | pH, time duration, adsorbent dose, initial concentration | Adsorption isotherms (batch); thermodynamic study; kinetic analysis; cost estimation | 2.02 × 104 mol g−1 | 49 |
| 5 | Cd(II) & Ni(II) | pH, adsorbent dose, initial concentrations, temperature, particle size | Adsorption isotherms (batch); thermodynamic study; kinetic analysis | 2.00 & 1.70 | 50 |
| 6 | Pb(II) & Cr(III) | pH, adsorbent dose, initial concentrations, contact duration, temperature, particle size | Adsorption isotherms (batch and column); thermodynamic study; regeneration of adsorbent; cost estimation | 2.50 & 4.35 | 51 |
| 7 | Cr(VI) & Ni(II) | Hydrogen ion concentration, contact time, adsorbent dose, initial concentration, particle size | Adsorption isotherms (batch) including Bhattacharya and Venkobachar model | 0.001 & 0.001 | 52 |
| 8 | Pb(II) & Cd(II) | pH, agitation time, adsorbent dose, initial concentration, temperature | Adsorption isotherms (batch and column); thermodynamic study; regeneration of adsorbent | 93.20 & 77.10 | 9 |
| 9 | Cd(II) & Ni(II) | pH, contact duration | Adsorption isotherms (single & multi-components); regeneration of adsorbent | 6.1942 & 6.4887 | 53 |
| 10 | Cd(II), Ni(II) & Zn(II) | Temperature, initial concentration | Adsorption isotherms (batch); thermodynamic study | 0.225, 0.432 & 0.399 mmol g−1 | 54 |
| 11 | Cd(II), Ni(II) & Zn(II) | Adsorbent dose, temperature, initial concentration, contact duration | Determination of statistic parameters | — | 55 |
| 12 | Cd(II), Ni(II) & Zn(II) | Initial concentration | Adsorption isotherms (single & multi-components) | 6.13, 6.49 & 7.03 | 56 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4% wt/wt). Maximum adsorption capacities were achieved up to 258 and 263 mg g−1 using batch and column treatment, respectively.60
4% wt/wt). Maximum adsorption capacities were achieved up to 258 and 263 mg g−1 using batch and column treatment, respectively.60Adsorptive comparative studies of two types of biomass ash, namely rice husk ash (RHA) and bagasse fly ash (BFA) for the removal of diuron (herbicide) were carried out. The surface characteristics (BET surface area, particle size and silica-to-carbon ratio), chemical composition, the effects of different variables (adsorbent dose, initial diuron concentration, contact time, pH, particle size and temperature), thermodynamic properties, adsorption kinetic modelling (pseudo-first-order and pseudo-second-order), isotherm modelling (Langmuir, Freundlich and Temkin model) and also a comparison of the diuron adsorption of this work with previous studies were performed. The results revealed that BFA has a greater adsorption capacity than RHA.61 The adsorption behaviour of another herbicide 2,4-D (2,4-dichlorophenoxyacetic acid) was studied by Deokar et al.62 using the batch (initial concentration, contact time, temperature, pH and particle size) and continuous packed bed (influent concentration, flow rate, and bed height) techniques. Adsorption isotherms, i.e., Langmuir and Freundlich isotherms were obtained for the batch technique, and the BDST, Thomas, Bohart–Adams, Clark and Wolborska models were applied to the data. The application of the deactivation kinetic model was also analyzed. Maximum adsorption was achieved, up to 7.14 mg g−1, using the Langmuir isotherm. Studies of COD and colour removal from sugar industry effluent were performed.63 Highly polluted distillery spent wash of sugar industries was characterized by Jemal Fito and it was found that this wash was acidic, with a high content of organic pollutants, and elevated amounts of heavy metals and other pollutants. Batch experiments were conducted for the reduction of COD, in which various adsorption parameters, isotherms, kinetic and thermodynamic studies were performed. Maximum adsorption capacities and removal efficiency was achieved up to 92.40 mg g−1 and 61.6%, respectively, using untreated BFA.64
Phenolic inhibitors were adsorbed using untreated BFA65 and carbon dioxide-treated BFA66 for the improvement of biomass saccharification, enzymatic hydrolysis and alcoholic fermentation in bio-refineries. Hydrogen peroxide-treated BFA was characterized, and adsorptive batch treatment and column treatment were carried out for the removal of toxic and carcinogenic pesticides, DDD and DDE. Various parameters, isotherms, kinetics, thermodynamic desorption studies, and cost estimation were also determined. Maximum adsorption capacities were found to be 7.69 and 6.67 μg g−1 for DDD and DDE, respectively.67 The remaining details of adsorption studies for other adsorbates using BFA and its activated forms are mentioned in the ESI† table, in which the value of the maximum adsorption capacity is from the Langmuir isotherm.
3.2 Construction industries
For the preparation of cement and other construction materials, natural materials, i.e., calcareous materials, argillaceous materials, gypsum and pulverised coal are utilized. Further, calcareous and argillaceous materials contain oxides of calcium, silica, aluminium and iron, and BFA is also comprised of oxides of various materials as per XRF, so, BFA was utilized for construction purposes.68 Sieved BFA (SBFA) and fly ash (FA) were used to prepare pastes and mortars and analyze the rheological and mini-slump properties like viscosity, yield stress, Herschel–Bulkley yield stress, mini-slump cone test and flow properties. The results indicated that SBFA and FA (individual as well as mixtures) efficiently worked in pastes and mortars, as the viscosities of the pastes and mortars increased using SBFA, and yield stress was reduced in the presence of FA and SBFA.69 Various combinations using FA and BFA for manufacturing reinforced ternary concrete were prepared to evaluate unessential chloride-induced corrosion. Chloride-ion diffusion coefficients, long-term microstructure, mineralogical and microstructural analysis, percentage of voids and compressive strength, estimated time for depassivation, densities, and electrochemical testing were determined and it was found that a mixture of FA and BFA is capable of reducing redundant chloride-induced corrosion in concrete.70 Experiments on BFA for application as an additive in cement were conducted by A. K. Anupam and co-scientists. Analyses such as California bearing ratio, unconfined compressive strength, triaxial test and micro-structural investigation were performed, and it was concluded that BFA has the properties of cementitious materials.71 Mortar was prepared by mixing BFA and FA in various proportions and were characterized via chemical composition, particle size distribution, BET surface area, SEM and XRD. Mechanical and durability properties like the strength activity index, compressive strength, ultrasonic pulse velocity, electrical resistivity and rapid chloride permeability were also determined by J. C. Arenas-Piedrahita.72 Economical and eco-friendly fly ash and sugar cane bagasse fly ash were used as binders by blending them with activated alkaline solutions of SiO2/K2O in different ratios in the preparation of pastes and mortars. Different proportions of SiO2/K2O were also taken. The mechanical properties of these ratios were assessed by different analytical techniques such as XRD, FT-IR, DTG, SEM, mercury intrusion porosimetry, pH and electrical conductivity, and it was concluded that the molar ratio of 0.75 for SiO2/K2O and the 75/25 proportion for FA/SCBA provided the best results.73 Bagasse-rice husk-wood ash (BRWA) was used as an additive to improve the strength and durability of coarse fly ash (FA) concrete. Strength and corrosion resistance were increased in concrete containing 60% FA concrete with ground BRWA.74Bagasse fly ash (BFA) industrial waste was included with base materials of cement-like clinker, trash, gypsum and limestone having different percentage weights. The chemical composition determined by XRF, the fineness, setting time, expansion, and compressive strength of cement and concrete indicated that BFA (up to 15% total weight) can be utilized as a cement additive.68 Recycled aggregate concrete was crushed, and ground fly ash (GFA) and ground bagasse ash (GBA) were added in different proportions of 20%, 35%, and 50% by weight respectively. Concrete was prepared using these mixtures and various analyses were conducted. Of these proportions, favourable results, i.e., suitable compressive strength, reduced water permeability, elevated chloride penetration and sulphate resistance were obtained at 20% by weight of the binder.75 The utilization of sugarcane fly ash in the construction industries was also reviewed.76,77 A short review on the valorisation of BFA in the manufacture of stabilized/sintered earth blocks and tiles was published.78
3.3 Other uses
BFA is also used to produce several materials that may be utilized as alternative sources of energy, thus preserving natural products. Amorphous silica was obtained from BFA by treating it with an alkaline solution, followed by heating. Beta zeolite was prepared by treating purified silica with several chemicals such as sodium hydroxide, tetraethylammonium hydroxide, sodium chloride, sodium aluminate and water. This beta zeolite was characterized by XRD, BET surface area, SEM and particle size distribution. This zeolite was used as a catalyst for the dehydration of methanol to dimethyl ether.79 Another ion-exchange beta zeolite from BFA was prepared and employed as a catalyst for the dehydration of methanol to dimethyl ether.80 Due to the presence of alumina and silica, FA and BFA were used in secondary abrasives. BFA and FA having different concentrations up to 12% of total weight were used with primary abrasives for the preparation of phenolic-based composites. Various determinations such as hardness, compressive strength, friction and wear properties were conducted and it was observed that 4% of the total weight was optimum concentration.81 BFA and rice husk ash were explored for the preparation of water glass. First, both types of ash were added to the NaOH solution and heated to give the geopolymer. This geopolymer was further treated with NaOH, followed by a curing process at room temperature for 7 days to yield water glass. Various investigations such as XRD, SEM, FT-IR, setting time and mechanical strength of water glass were conducted and favourable results were obtained.82Comparisons were made of untreated sugarcane bagasse ash83 and activated BFA84 in electrodes for capacitive deionization (CDI) to improve the electrosorption of salts. The carbon remaining during the consumption of BFA was distinct and was activated using phosphoric acid, followed by loaded iron. This iron-loaded activated carbon having high surface area was employed for the oxidation of volatile organic compounds (VOCs).85 Pure Na–X and Na–A zeolites with very high porosity and ion exchange capacity were manufactured from BFA. Different zeolitization process parameters like curing time, hydrothermal temperature, anion addition and Si/Al molar ratio in the reaction mixture were assessed. The morphologies of these newly prepared zeolites were determined using SEM, XRD and FT-IR.86 Two mesoporous silicas, MCM-41 and SBA-15 were prepared from BFA, which was used as the precursor.87 Since BFA contains various inorganic fractions, viz., oxides of silicon, aluminum oxides, iron, calcium, magnesium, and potassium, it was exploited in red ceramic industries for the replacement of quartz.88 Also, it contains higher amounts of carbon for the preparation of briquette as an alternative fuel.89 Comparative studies of BFA and natural zeolite as the immobilization medium in the anaerobic digester were carried out to improve the digestion rate of a landfill leachate and it showed that the zeolite was more feasible than BFA.90
4. Challenges and perspectives
(1) Bagasse fly ash, the sugar industry waste, is discharged as solid waste. BFA is mostly used for the adsorption of metals, dyes and other contaminants from their synthetic solutions. Limited research has been conducted on the treatment of real wastewater from different industries. The adsorption process is a tertiary treatment, so, it is used after primary and secondary treatments. Most adsorptive experiments are performed on batch treatments, though column treatment is more feasible industrially. BFA can be used as an adsorbent in wastewater treatment by maintaining suitable operating conditions and requirements.(2) BFA has been used as an additive for cement and brick preparation in the construction industry. Only a small portion of BFA is used in cement. More theoretical and experimental works are needed in this area to increase its usability according to its huge production.
(3) Most experiments for the exploitation of BFA are at the laboratory scale. Pilot and large-scale experiments are required for proper industrial applications. Few researchers have tried to estimate the cost of BFA in terms of US dollars.47,50,52 The price of commercially available charcoal ($285 per tonne) was compared with that of BFA ($15 per tonne), considering all the expenses including transport, chemicals and electrical energy, to analyze the feasibility of BFA as an adsorbent in terms of cost, efficiency and efficacy.
(4) The production of sugarcane crops is much higher in tropical zone countries like India, Africa, China and some parts of the United States. In these countries, sugar is produced from sugarcane, and solid waste, i.e. BFA, is generated. Other countries like Russia, France, Italy, UK and Germany produce sugar from beet sugar, and its waste is used as cattle feed. Also, sugarcane is cut once a year, probably in two to three months. Hence, sugar industries are open for only four to five months in a year. The generation of BFA is time-limited, so sugarcane production depends on seasonality, geography and the agricultural productivity of crops to generate BFA. Also, the properties of BFA change according to the origin, variety, treatment method, activation process and geographic location.
(5) There have only been a few reports on its regeneration. Regeneration may reduce the solid waste quantity.
5. Conclusion
(1) Sugar industries utilize bagasse, sugarcane waste, to generate energy and its fly ash is called bagasse fly ash (BFA). This BFA is considered industrial solid waste. This solid waste is characterized using various sophisticated techniques, which show that BFA contains unburnt carbon and different metal oxides, including silicon and aluminum. The surface morphology of BFA shows that it is a porous material. BFA has outstanding usage in various fields due to versatile properties.(2) Untreated and/or modified BFA using various activated agents is extensively used as an adsorbent for the removal of metals, dyes, petrochemicals, insecticides and hazardous environmental pollutants. Various process parameters, adsorption isotherms, kinetic models, thermodynamics, error and statistical studies were executed in the adsorption exploration including the calculation of the maximum adsorption capacity. Well-known dyes were adsorbed using BFA. Most of the metal adsorption research was carried out on cadmium(II), and the maximum adsorption capacity was 6.13 mg g−1. Hence, BFA may replace commercially available adsorbents for environmental and economic benefits. Also, it was regenerated using different solvents to eliminate the solid industrial waste load.
(3) Since BFA contains oxides of various metals, it is also used as an additive in cement, bricks, pastes, blocks, tiles and mortar. The combination of fly ash, bagasse-rice husk-wood ash, and other ashes in different concentrations improved the compressive strength, reduced water permeability, chloride-induced corrosion and sulphate resistance. Therefore, it reduced the utilization of cement, ultimately reducing the natural calcareous and argillaceous materials.
(4) It is also used to prepare different zeolites, water polymers, briquettes, mesoporous silica, electrodes, immobilization media, catalysts and is a secondary abrasive. It also decreases the use of natural materials.
Conflicts of interest
There are no conflicts to declare.References
- H. I. Abdel-Shafy and M. S. M. Mansour, Egypt. J. Pet., 2018, 27, 1275–1290 CrossRef.
- D. Hoornweg and P. Bhada-Tata, What a Waste: A Global Review of Solid Waste Management. Urban development series, knowledge papers no. 15, World Bank, Washington, DC, 2012 Search PubMed.
- R. M. Yoada, D. Chirawurah and P. B. Adongo, BMC Public Health, 2014, 14, 697–706 CrossRef PubMed.
- S. Solomon, Sugar Tech, 2016, 18, 588–602 CrossRef.
- A. Singh, U. R. Lal, H. M. Mukhtar, P. S. Singh, G. Shah and R. K. Dhawan, Pharmacogn. Rev., 2015, 9, 45–54 CrossRef CAS PubMed.
- E. Martinez-Hernandez, M. A. Amezcua-Allieri, J. Sadhukhan and J. A. Anell, Sugarcane - Technology and Research, 2018, ch. 5, DOI:10.5772/intechopen.72237.
- M. H. Fulekar and J. M. Dave, Int. J. Environ. Stud., 1986, 26, 191–215 CrossRef CAS.
- G. Ferraiolo, M. Zilli and A. Converti, J. Chem. Technol. Biotechnol., 1990, 41, 281–305 Search PubMed.
- B. Shah, C. Mistry and A. Shah, Environ. Sci. Pollut. Res. Int., 2013, 20, 2193–2209 CrossRef CAS PubMed.
- B. A. Shah, D. D. Pandya and H. A. Shah, Arabian J. Sci. Eng., 2016, 42, 241–260 CrossRef.
- V. C. Srivastava, M. M. Swamy, I. D. Mall, B. Prasad and I. M. Mishra, Colloids Surf., A, 2006, 272, 89–104 CrossRef CAS.
- V. C. Srivastava, I. D. Mall and I. M. Mishra, Chem. Eng. J., 2007, 132, 267–278 CrossRef CAS.
- B. Shah, D. Pandya, H. Patel and A. Shah, Int. J. Environ. Waste Manage., 2017, 20, 1–20 CrossRef CAS.
- J. F. Nure, N. T. Shibeshi, S. L. Asfaw, W. Audenaert and S. W. H. Van Hulle, Water SA, 2017, 43, 470 CrossRef CAS.
- B. A. Shah, A. A. Abebe and A. V. Shah, Arabian J. Sci. Eng., 2016, 42, 139–152 CrossRef.
- V. K. Gupta, D. Mohan and S. Sharma, Sep. Sci. Technol., 1998, 33, 1331–1343 CrossRef CAS.
- I. D. Mall, V. C. Srivastava and N. K. Agarwal, Dyes Pigm., 2006, 69, 210–223 CrossRef CAS.
- C. W. Purnomo, C. Salim and H. Hinode, Fuel Process. Technol., 2012, 102, 132–139 CrossRef CAS.
- H. Patel, Appl. Water Sci., 2019, 9(45), 1–17 Search PubMed.
- H. Patel and R. T. Vashi, Studies on Characterization and Treatment of Textile Wastewater, Elsevier Inc, 2015 Search PubMed.
- R. Pratibha, P. Malar, T. Rajapriya, S. Balapoornima and V. Ponnusami, Desalination, 2010, 264, 102–107 CrossRef CAS.
- G. W. Kajjumba, S. Emik, A. Ongen, H. K. Ozcan and S. Aydın, in Sorption, IntechOpen Limited, 2018 Search PubMed.
- H. Patel, Sep. Sci. Technol., 2018, 53, 2797–2812 CrossRef CAS.
- V. Batra, S. Urbonaite and G. Svensson, Fuel, 2008, 87, 2972–2976 CrossRef CAS.
- Z. Heinrich, Synthesis, Properties of Organic Dyes and Pigments, in Color Chemistry, VCH Publishers, New York, USA, 1987, pp. 92–102 Search PubMed.
- M. Berradi, R. Hsissou, M. Khudhair, M. Assouag, O. Cherkaoui, A. El Bachiri and A. El Harfi, Heliyon, 2019, 5, e02711 CrossRef PubMed.
- H. Patel and R. T. Vashi, E-J. Chem., 2010, 7, 975–984 CrossRef CAS.
- A. Pokharia and S. S. Ahluwalia, Curr. Trends Biotechnol. Chem. Res., 2015, 5, 1–17 Search PubMed.
- B. Lellis, C. Z. Favaro-Polonio, J. A. Pamphile and J. C. Polonio, Biotechnol. Res. Innov., 2019, 3, 275–290 CrossRef.
- V. K. Gupta, D. Mohan, S. Sharma and M. Sharma, Sep. Sci. Technol., 2000, 35, 2097–2113 CrossRef CAS.
- I. D. Mall, V. C. Srivastava, N. K. Agarwal and I. M. Mishra, Chemosphere, 2005, 61, 492–501 CrossRef CAS PubMed.
- I. D. Mall, V. C. Srivastava, N. K. Agarwal and I. M. Mishra, Colloids Surf., A, 2005, 264, 17–28 CrossRef CAS.
- B. A. Shah, H. D. Patel and A. V. Shah, Environ. Prog. Sustainable Energy, 2011, 30, 549–557 CrossRef CAS.
- B. A. Shah, A. V. Shah and H. D. Patel, Int. J. Environ. Waste Manage., 2011, 7, 192 CrossRef CAS.
- B. A. Shah, A. V. Shah, H. D. Patel and C. B. Mistry, Water Environ. Res., 2013, 85, 558–567 CrossRef CAS PubMed.
- J. Qin, Q. Chen, C. Yang and Y. Huang, J. Alloys Compd., 2016, 654, 39–44 CrossRef CAS.
- S. Jiwan and K. Ajay, Int. J. Res. Chem. Environ., 2011, 1, 15–21 Search PubMed.
- M. Jaishankar, T. Tseten, N. Anbalagan, B. B. Mathew and K. N. Beeregowda, Interdiscip. Toxicol., 2014, 7, 60–72 Search PubMed.
- M. Oves, M. Saghir Khan, A. Huda Qari, M. Nadeen Felemban and T. Almeelbi, J. Biorem. Biodegrad., 2016, 07(2), 1–15 Search PubMed.
- P. Leechart, D. Inthorn and P. Thiravetyan, Water Environ. Res., 2016, 88, 907–912 CrossRef CAS PubMed.
- I. Ali, Z. A. Al-Othman, A. Alwarthan, M. Asim and T. A. Khan, Environ. Sci. Pollut. Res. Int., 2014, 21, 3218–3229 CrossRef CAS PubMed.
- V. Chandra Srivastava, I. Deo Mall and I. Mani Mishra, Sep. Sci. Technol., 2006, 41, 2685–2710 CrossRef.
- C. W. Purnomo and A. Prasetya, AIP Conf. Proc., 2008, 1007, 24 CrossRef CAS.
- L. S. Yadav, B. K. Mishra, A. Kumar and K. K. Paul, J. Environ. Chem. Eng., 2014, 2, 1467–1473 CrossRef CAS.
- K. L. Wasewar, B. Prasad and S. Gulipalli, Clean: Soil, Air, Water, 2009, 37, 534–543 CAS.
- V. K. Gupta, D. Mohan, S. Sharma and K. T. Park, Environmentalist, 1999, 19, 129–136 CrossRef.
- V. K. Gupta, D. Mohan and S. Sharma, Sep. Sci. Technol., 1998, 33(9), 1331–1343 CrossRef CAS.
- V. K. Gupta and I. Ali, Sep. Purif. Technol., 2000, 18, 131–140 CrossRef CAS.
- V. K. Gupta and S. Sharma, Ind. Eng. Chem. Res., 2003, 42, 6619–6662 CrossRef CAS.
- V. K. Gupta, C. K. Jain, I. Ali, M. Sharma and V. K. Saini, Water Res., 2003, 37, 4038–4044 CrossRef CAS PubMed.
- V. K. Gupta and I. Ali, J. Colloid Interface Sci., 2004, 271, 321–328 CrossRef CAS PubMed.
- M. Rao, A. V. Parwatea and A. G. Bholeb, Waste Manag., 2002, 22, 821–830 CrossRef CAS PubMed.
- V. C. Srivastava, I. D. Mall and I. M. Mishra, Chem. Eng. J., 2006, 117, 79–91 CrossRef CAS.
- V. C. Srivastava, I. D. Mall and I. M. Mishra, Chem. Eng. J., 2007, 132, 267–278 CrossRef CAS.
- V. C. Srivastava, I. D. Mall and I. M. Mishra, Ind. Eng. Chem. Res., 2007, 46, 5697–5706 CrossRef CAS.
- V. C. Srivastava, I. D. Mall and I. M. Mishra, Ind. Eng. Chem. Res., 2008, 47, 3129–3137 CrossRef CAS.
- World Health Organization (WHO), Guidelines for drinking-water quality, Geneva, 3rd edn, 2008, vol. 1, Recommendations Search PubMed.
- J. A. Sciortino and R. Ravikumar, Fishery Harbour Manual on the Prevention of Pollution - Bay of Bengal Programme, Bay of Bengal Programme, India, 1999 Search PubMed.
- Ashadi, M. Masykuri and Haryono, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 193, 012033 Search PubMed.
- K. E. Chingono, E. Sanganyado, E. Bere and B. Yalala, J. Environ. Manage., 2018, 224, 182–190 CrossRef CAS PubMed.
- S. K. Deokar, D. Singh, S. Modak, S. A. Mandavgane and B. D. Kulkarni, Desalin. Water Treat., 2016, 57, 22378–22391 CrossRef CAS.
- S. K. Deokar, S. A. Mandavgane and B. D. Kulkarni, Clean Technol. Environ. Policy, 2016, 18, 1971–1983 CrossRef CAS.
- L. D. Mall, Industrial Research, 1994, 39, 115–119 Search PubMed.
- J. Fito, N. Tefera and S. W. H. Van Hulle, J. Environ. Chem. Eng., 2017, 5, 5381–5388 CrossRef CAS.
- J. V. Freitas and C. S. Farinas, ACS Sustainable Chem. Eng., 2017, 5, 11727–11736 CrossRef CAS.
- J. V. Freitas, L. A. M. Ruotolo and C. S. Farinas, Fuel, 2019, 251, 1–9 CrossRef CAS.
- V. K. Gupta and I. Ali, Water Res., 2001, 35, 33–40 CrossRef CAS PubMed.
- N. Rauf, M. C. Damayanti and S. W. I. Pratama, AIP Conf. Proc., 2017, 1801, 1–3 CrossRef.
- V. G. Jiménez-Quero, F. M. León-Martínez, P. Montes-García, C. Gaona-Tiburcio and J. G. Chacón-Nava, Constr. Build. Mater., 2013, 40, 691–701 CrossRef.
- V. A. Franco-Luján, M. A. Maldonado-García, J. M. Mendoza-Rangel and P. Montes-García, Constr. Build. Mater., 2019, 198, 608–618 CrossRef.
- A. K. Anupam, P. Kumar, G. D. Ransinchung and Y. U. Shah, Presented in part at the International Conference on Highway Pavements and Airfield Technology, Philadelphia, Pennsylvania, August 27–30, 2017 Search PubMed.
- J. C. Arenas-Piedrahita, P. Montes-García, J. M. Mendoza-Rangel, H. Z. Lopez Calvo, P. L. Valdez-Tamez and J. Martínez-Reyes, Constr. Build. Mater., 2016, 105, 69–81 CrossRef CAS.
- V. N. Castaldelli, J. C. B. Moraes, J. L. Akasaki, J. L. P. Melges, J. Monzó, M. V. Borrachero, L. Soriano, J. Payá and M. M. Tashima, Fuel, 2016, 174, 307–316 CrossRef CAS.
- V. Horsakultulthia, Am. J. Appl. Sci., 2013, 10, 239–246 CrossRef.
- R. Somna, C. Jaturapitakkul and A. M. Amde, Cem. Concr. Compos., 2012, 34, 848–854 CrossRef CAS.
- M. Keramatikerman, A. Chegenizadeh and S. Terzaghi, Electron. J. Geotech. Eng., 2019, 2, 453–470 Search PubMed.
- M. Mohankumar, G. A. M. Tendulkar, P. G. Kumar and T. Arunkumar, Shanlax International Journal of Arts, Science & Humanities, 2017, 5, 89–93 Search PubMed.
- J. James and P. K. Pandian, Adv. Mater. Sci. Eng., 2017, 1–15 CAS.
- P. Assawasangrat, S. Neramittagapong, W. Pranee and P. Praserthdam, Energy Sources, Part A, 2016, 38, 3081–3088 CrossRef CAS.
- W. Pranee, P. Assawasaengrat, A. Neramittagapong, S. Intarachit and S. Neramittagapong, Adv. Mater. Res., 2014, 931–932, 3–6 Search PubMed.
- S. Choosri, N. Sombatsompop, E. Wimolmala and S. Thongsang, Proc. Inst. Mech. Eng., Part D, 2018, 233, 1296–1305 CrossRef.
- K. Gomonsirisuk and P. Thavorniti, Key Eng. Mater., 2019, 798, 364–369 Search PubMed.
- J. J. Lado, R. L. Zornitta, F. A. Calvi, M. I. Tejedor-Tejedor, M. A. Anderson and L. A. M. Ruotolo, J. Anal. Appl. Pyrolysis, 2016, 120, 389–398 CrossRef CAS.
- J. J. Lado, R. L. Zornitta, F. A. Calvi, M. Martins, M. A. Anderson, F. G. E. Nogueira and L. A. M. Ruotolo, J. Anal. Appl. Pyrolysis, 2017, 126, 143–153 CrossRef CAS.
- G. Pande, S. Selvakumar, V. S. Batra, O. Gardoll and J.-F. Lamonier, Catal. Today, 2012, 190, 47–53 CrossRef CAS.
- C. W. Purnomo, C. Salim and H. Hinode, Microporous Mesoporous Mater., 2012, 162, 6–13 CrossRef CAS.
- C. W. Purnomo, S. K. Wirawan and H. Hinode, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 543, 012040 CAS.
- S. R. Teixeira, A. E. de Souza, G. T. de Almeida Santos, A. F. Vilche Peña and Á. G. Miguel, J. Am. Ceram. Soc., 2008, 91, 1883–1887 CrossRef CAS.
- S. R. Teixeira, A. F. Pena and A. G. Miguel, Waste Manag., 2010, 30, 804–807 CrossRef CAS PubMed.
- H. Sudibyo, Z. L. Shabrina, H. R. Wondah, R. T. Hastuti, C. W. Purnomo and W. Budhijanto, Acta Polytech., 2018, 58, 57 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06422j |
| This journal is © The Royal Society of Chemistry 2020 |