Open Access Article
Open Access ArticleNear-infrared-IIb probe affords ultrahigh contrast inflammation imaging†
Cong Hua*a,
Biao Huangb,
Yingying Jiangc,
Shoujun Zhucd and
Ran Cui *b
*b
aDepartment of Surgical Neuro-oncology, The First Hospital of Jilin University, Changchun, 130061, PR China. E-mail: huacong@jlu.edu.cn
bCollege of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430070, PR China. E-mail: cuiran@whu.edu.cn
cState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China
dKey Laboratory of Organ Regeneration & Transplantation of the Ministry of Education, The First Hospital of Jilin University, Changchun, 130061, PR China
First published on 11th September 2020
Abstract
Deep tissue imaging in the near-infrared II (NIR-II) window with significantly reduced tissue autofluorescence and scattering provides an important modality to visualize various biological events. Current commercially used contrast agents in the near-infrared spectrum suffer from severe photobleaching, high tissue scattering, and background signals, hampering high-quality in vivo bioimaging, particularly in small animals. Here, we applied a NIR-IIb quantum dot (QD) probe with greatly suppressed photon scattering and zero autofluorescence to map inflammatory processes. Two-layer surface modification by a combination of amphiphilic polymer and mixed linear and multi-armed polyethylene glycol chains prolonged probe circulation in vivo and improved its accumulation in the inflammation sites. Compared to indocyanine green, a widely applied dye in the clinic, our QD probe showed greater photostability and capacity for deeper tissue imaging with superior contrast. The longer circulation of QDs also improved vessel imaging, which is vital for better understanding of biological mechanisms of the inflammation microenvironment. Our proposed NIR-IIb in vivo imaging modality proved effective for the visualization of inflammation in small animals, and its use may be extended in future to studies of immunity and cancer.
Introduction
Inflammation is a very important physiological process that represents an adaptive response to noxious events following infection and tissue injury.1 Although the types of inflammatory processes and their interrelations have been described in detail, the physiological mechanisms of inflammation, particularly during chronic infections, are not completely understood. Such mechanisms are especially complicated for systemic chronic inflammation, which is associated with diseases such as metabolic disorder and cardiovascular diseases.1–7 Inflammation in response to infection is mediated by the components of the innate immune system, such as local macrophages and mast cells.8 Mapping in detail the processes and microenvironment of inflammation by an in vivo imaging modality9,10 enables to characterize the key events in the induction of inflammatory response, activation of inflammatory pathways, parainflammation processes and other related phenomena.Modern biomedical techniques employ a variety of imaging modalities to visualize biological events and disease states.11–13 Compared to magnetic resonance imaging, computed tomography, positron emission tomography, or single-photon emission computed tomography, optical imaging, especially fluorescence-based imaging, has several advantages with regard to the real-time imaging mode, multicolor labeling, and high resolution.14,15 Fluorescence imaging has been used with several advanced techniques, such as small animal imaging platform, confocal laser scanning microscopy, and light sheet microscopy, to investigate various biological processes. Fluorescence imaging navigation system has been also applied in the clinical setting, e.g., indocyanine green is successfully used for near-infrared imaging-guided surgery.16,17 Given that imaging penetration depth is greatly limited by tissue autofluorescence and light scattering, current fluorescence imaging probes for small laboratory animals mainly focus on the red to near-infrared I (NIR-I) range (∼600–1000 nm).18 Imaging at longer wavelengths, particularly in the NIR-II window, enhances visualization of inflammation in vivo, affording greater tissue penetration and imaging contrast.19,20
In several recent studies, donor–acceptor–donor dyes with NIR-II peak emission21–30 and cyanine dyes with both NIR-II peak31,32 and tail emission33–39 have been successfully applied for NIR-II imaging. However, increasing amount of evidence suggests that the true advantage of NIR-II imaging is achieved in the NIR-IIb sub-window (>1500 nm),40–43 in which the emission light penetrates different types of tissues with little energy loss and scattering, and with nearly zero autofluorescence. Due to water absorbance at ∼1400 nm, the NIR-IIb window can also avoid this usually unavoidable disturbance although it has been claimed that imaging at the peak absorbance of water (near 1450 nm) may provide the highest image contrast in the shortwave infrared.44 Thus, NIR-IIb imaging has been used to achieve high resolution of mouse vasculature, tumors, lymph nodes and visualize immune therapy process.41,42,45–48 More importantly, broadening the NIR window from 700 to over 1500 nm may potentially integrate multiple channels for imaging complex biological events.
Based on above background, in the present study we applied a NIR-IIb probe with for ultrahigh contrast imaging of inflammation, enabling future expansion for studies of inflammation microenvironment. The NIR-IIb quantum dot (QD) probe with the core–shell structure was synthesized by using a protocol similar to that described in our previous work.45–47 The amphiphilic polymer oleylamine-poly(acrylic acid) (OPA) was used to deliver the oil-soluble QDs to a water-soluble phase and a combination of m-polyethyleneglycol (PEG)-amine and 8-arm PEG-amine was used to improve the biocompatibility and in vivo circulation time of the QD probe. The as-prepared QDs enabled high-quality vessel imaging, whereas the prolonged circulation also improved probe accumulation in the sites of inflammation. We demonstrated that our NIR-IIb QD probe provided high imaging quality in a small laboratory animal (inflammation imaging in this work). We also outlined the key challenges that will shape the future application of the probe, particularly with regard to clarifying the origin and physiological roles of the inflammation.
Results and discussion
NIR-IIb probes with extremely low tissue scattering and autofluorescence have afforded ultrahigh imaging contrast and penetration depth.45–47,49 We initially tested the optimal surface modification and identified a two-layer-surface coating that improved biosafety of QDs (the dynamic light scattering (DLS) size of QDs is about 35 nm and the zeta potential of the QDs is about −1.4 eV). The coated QDs possessed superbright NIR-IIb emission with peak emission at 1600 nm.45–47 The QDs also exhibited outstanding photostability during the exposure to a high-power 808 nm laser, eliminating quantification problems that could be caused by signal decay. The large Stokes shift, widely recognized to be critical for background signal reduction, improved imaging quality (Fig. 1a). We used a 1% intralipid solution to simulate the tissue to test the penetration depth of the QDs with that of indocyanine green (ICG), a probe used in the clinic.50 QDs imaging signals were collected in the >1500 nm region. From the fluorescence images of capillaries filled with QDs or ICG solutions, we observed that the former penetrated deeper and significantly improved contrast in the NIR-IIb window (Fig. 1b). Multilayer surface modification with both OPA and PEGylation layers can significantly improve in vivo stability and in vivo circulation time of QDs. We further optimized the surface coating of PbS/CdS core–shell QDs and proved that the two-layer surface coating by OPA and PEGylation dramatically improved the stability and biosafety of QDs (Fig. 1c). As shown in Fig. S1,† OPA-coated QDs had short vessel circulation time, whereas further PEGylation greatly enhanced the circulation time and in vivo stability of the NIR-IIb QD probe. The rotated whole body imaging of mice after tail vein administration of the NIR-IIb probe provided high-quality 360° omnidirectional visualization of mouse vessels (Fig. 1d, S2, S3 and Video S1–S3†). The extremely low background signal enabled super-high imaging contrast.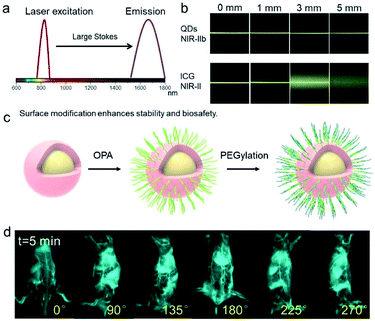 | ||
| Fig. 1 Imaging with a NIR-IIb probe with extremely low tissue scattering and autofluorescence affords ultrahigh imaging contrast and penetration depth. (a) Scheme of “the large Stokes shift accounts for low background signal and superior imaging quality”. (b) Fluorescence images of capillaries filled with aqueous solutions of quantum dots (QDs) and indocyanine green (ICG), covered by a 1% intralipid solution at varying depths. QDs imaging signals were collected in the >1500 nm region. Data was plotted from previous report.47 (c) Scheme of the PbS/CdS core–shell QDs after coating by OPA and PEGylation. The two-layer surface coating significantly improved the stability and biosafety of QDs. (d) Whole-body rotating images after administration of the NIR-IIb probe into the tail-vein (100–150 μL of 3 μM solution of coated QDs).47 | ||
With a well-established NIR-IIb probe in hand, we first tested whether it was suitable for high-quality and high-contrast vessel imaging. To this end, a shaved mouse was immobilized in the supine position with the left leg exposed under the imaging field of view for video-rate hind limb vessel imaging (Fig. 2a and Video S4†). Following an injection of 100–150 μL of 3 μM two-layer coated QDs, the vessel structure was visualized clearly and with extremely low background signal by video-rate imaging under the 1500 nm long pass filter. The hind limb vein was visualized first, and the NIR-IIb signal spread in both the veins and arteries, highlighting the potential of the probe for real-time monitoring of inflammatory processes. Additionally, we performed high-quality vessel imaging in other parts of the body, including leg, backside, abdomen, tail, and others (Fig. 2b). In all cases, our probe allowed for high-quality vessel imaging under surgical light exposure with maximal vessel signal to background (muscle) ratio. Multiplexed NIR-II visualization of metastatic tumors and sentinel lymph node resection with full light exposure surgery has been achieved previously.47 The current study also proves that NIR-IIb imaging modality enables universal navigation under surgical light exposure for both preclinical and clinical procedures.
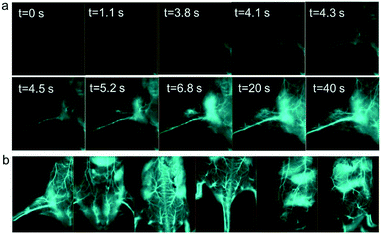 | ||
| Fig. 2 NIR-IIb probe enables high contrast vessel imaging. (a) The mouse was fixed on the operation platform with the left leg exposed under the imaging field of view. Video-rate images were recorded under 1500 nm long pass filter after tail vein injection of coated NIR-IIb probe. (b) High-quality vessel images visualized at various body parts: leg, abdomen, backside, tail, and lateral side. NIR-II imaging was conducted at the excitation power density of 150 mW cm−2 and 808 nm wavelength.35,47,51,58–60 Due to the energy loss after passing through the 850/1000 nm SP excitation-filter set, the actual exposure power received by the mice was weaker. All 2.5× high-magnification data were simultaneously collected using the same cohort of mice when conducting 1× whole-body imaging during the previous and current projects.47 | ||
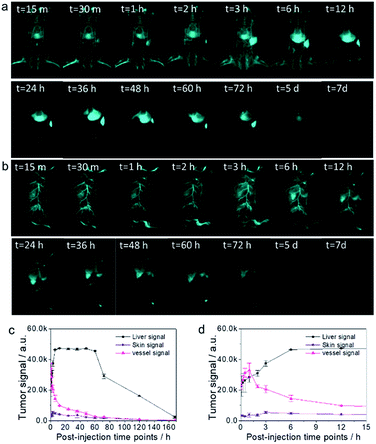
We next carried out whole body NIR-IIb imaging and traced signal accumulation locations of the coated QDs probes. Because two-layer coated QDs showed improved in vivo pharmacokinetic properties compared to those of previous probes with suboptimal surface modifications, we assumed that our probe would remain in blood circulation for longer and would have lower skin uptake in the whole-body imaging mode. Fig. 3a illustrates NIR-IIb imaging of mice in the supine position after administration of QDs under 1500 nm long pass filter and 808 nm laser excitation. Vessels were clearly observed even at >12 h post-injection. NIR-IIb imaging of mice in the lateral position provided even better vessel visualization with extremely low background signals and allowed to map small vessels (Fig. 3b). Statistical analysis of the long-term and short-term liver, skin, and vessel signals at different post-injection time points (Fig. 3c and d) indicated that coated QDs had very low skin uptake due to the rational surface coating with favorable in vivo pharmacokinetics. In contrast, low amount of PEGylation precluded high-quality NIR-IIb imaging, particularly during later post-injection time points, and the skin signal appeared at 4 to 20 h post-injection time points (Fig. S4†). It's very hard to rule out the possibility of the QDs being degraded within the body, which needs further investigation. However, previous study indicated that “∼76% of injected QDs were excreted from the body with feces after 4 weeks”,45 which was roughly consistent with current study.
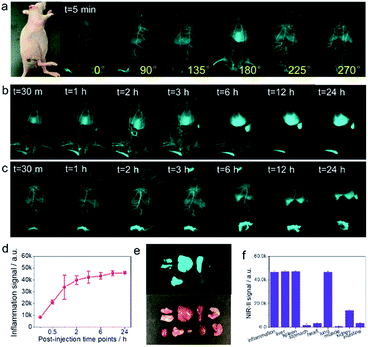
Inflammation is an adaptive response during which chemical and biological substances are released into the blood to protect specific sites of the body, causing increased blood flow in the inflamed areas. The increased number of cells and inflammatory substances within the sites of inflammation augments accumulation of foreign substances.1 Because large numbers of immune cells accumulate in the sites of inflammation, we hypothesized that they could take up coated QDs probes, enabling targeted high-quality NIR-IIb inflammation imaging. To verify this assumption, a mouse with on-going inflammation was injected with the QD probe through the tail vein (Fig. 4a). NIR-IIb imaging of this mouse at both the supine and lateral positions after the administration of the QD probe under 1500 nm long pass filter and 808 nm laser excitation clearly visualized relatively fast QD probe accumulation with the signal in the inflammation site reaching the peak at around 4–6 h (Fig. 4b and c). Organ distribution of QDs at 24 h post-injection indicated that besides the accumulation in site of inflammation, the QD probe also accumulated in high amounts in the liver, spleen, and lung (Fig. 4e and f).
Next, we examined the accumulation of the QD probe in tumors. SKOV3 tumor cells were inoculated subcutaneously into the armpit of mice, which were monitored until the tumor size reached 0.5 × 0.5 cm. The QD probe was administered to mice with tumors (Fig. S5†) and was found to provide high-quality NIR-IIb tumor imaging in room light conditions. The 2.5× magnification of the tumor site at early time points after QD probe injection visualized tumor structure in detail (Fig. S5b†). Although a previous study indicated much higher accumulation of the QD probe in the U87 tumor model,45 it had relatively low accumulation in the SKOV3 tumor model in our present experiments. Whole body NIR-IIb imaging of SKOV3 tumor-bearing mouse (1× magnification) with surrounding inflammation sites at different post-injection time points indicated that the accumulated signal was stronger in the sites of inflammation than in the tumor site. We speculated that inflammation site was looser than the solid-tumor site. In the same conditions of injection dose and imaging set-up, the abundant accumulation of NIR-IIb QDs in the inflammation sites will possibly result in bright NIR-II signal. This point needs further investigation.
Conclusions
Inflammation comprises many physiological and pathological processes.1 Mechanisms of the acute inflammatory response to the infection are becoming increasingly elucidated. Contemporary experimental and clinical evidence suggests that inflammation has close relationship with many normal and pathological processes, such as metabolism, immunity,2 metabolic disorders,3 cancer progression,6,8 atherosclerosis,5 autoimmune disease,4 Alzheimer's disease,7 and others. However, the exact details of the interrelationships between inflammation and these processes are often not known. Therefore, it is essential to be able to reveal important constituents in the local microenvironment that mediate inflammatory responses and to develop efficient therapeutic approaches on the basis of this knowledge. The present study describes an important imaging probe for clear visualization of the inflammation process. Application of the proposed NIR-II imaging in combination with confocal51–53 and light sheet microscopy42,48 in future will enable detailed studies of the inflammation microenvironment and mechanisms of cancer-related inflammation.Our NIR-IIb imaging modality provides a potential solution to investigate complex biological events in the real-time manner. A previous study has demonstrated application of dual-channel NIR-II probes for simultaneous metastatic tumor imaging and sentinel lymph node resection with full surgery with light exposure and in vivo vessel imaging from both macro- and micromicroscopic dimensions.45,47 Our probe for imaging at >1500 nm NIR-IIb window with extremely low tissue autofluorescence and scattering affords great penetration depth, high spatial resolution, and real-time imaging speed.47 NIR-II molecular imaging at longer wavelengths is favorable for muscle, brain, skin and subcutaneous tissues.54 Our NIR-IIb probe yielded greater spatial resolution and penetration depth by tissues in the NIR-IIb window due to a combination of factors such as low-autofluorescence, minimized photon scattering, water absorption, and large Stokes shift (excitation at 808 nm and signal collection at >1500 nm).47
It is widely accepted that imaging at the NIR region has several advantages, particularly for studies in vivo and clinical imaging-guided surgery.14,15,55–57 Current commercial NIR probes mainly focus on the narrow NIR-I window between 700 and 1000 nm. It is very important to extend the imaging emission range for multicolor channels with non-overlapping emission spectra to label different biological processes and objects simultaneously without any crosstalk between channels.47 This increased wavelength range will provide sufficient space to integrate multiplexed channels and enable real-time visualization of complex biological events with unprecedented imaging contrast and penetration depth in the true NIR-II transparent imaging windows.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Institute of Translational Medicine, The First Hospital, Jilin University, and the National Natural Science Foundation of China (21535005). We thank Dr Xue Zhang of the Jilin Agricultural University for scheme designing.Notes and references
- R. Medzhitov, Nature, 2008, 454, 428–435 CrossRef CAS.
- S. I. Grivennikov, F. R. Greten and M. Karin, Cell, 2010, 140, 883–899 CrossRef CAS.
- G. S. Hotamisligil, Nature, 2006, 444, 860–867 CrossRef CAS.
- B. Levine, N. Mizushima and H. W. Virgin, Nature, 2011, 469, 323–335 CrossRef CAS.
- P. Libby, Nature, 2002, 420, 868–874 CrossRef CAS.
- A. Mantovani, P. Allavena, A. Sica and F. Balkwill, Nature, 2008, 454, 436–444 CrossRef CAS.
- H. Akiyama, Neurobiol. Aging, 2000, 21, 383–421 CrossRef CAS.
- L. M. Coussens and Z. Werb, Nature, 2002, 420, 860–867 CrossRef CAS.
- M. Zhao, R. Wang, B. Li, Y. Fan, Y. Wu, X. Zhu and F. Zhang, Angew. Chem., Int. Ed., 2019, 58, 2050–2054 CrossRef CAS.
- S. Wang, L. Liu, Y. Fan, A. M. El-Toni, M. S. Alhoshan, D. Li and F. Zhang, Nano Lett., 2019, 19, 2418–2427 CrossRef CAS.
- Z. Zhou, R. Bai, J. Munasinghe, Z. Shen, L. Nie and X. Chen, ACS Nano, 2017, 11, 5227–5232 CrossRef CAS.
- X. Sun, X. Huang, J. Guo, W. Zhu, Y. Ding, G. Niu, A. Wang, D. O. Kiesewetter, Z. L. Wang, S. Sun and X. Chen, J. Am. Chem. Soc., 2014, 136, 1706–1709 CrossRef CAS.
- Y. Wang, L. Lang, P. Huang, Z. Wang, O. Jacobson, D. O. Kiesewetter, I. U. Ali, G. Teng, G. Niu and X. Chen, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 208–213 CrossRef.
- A. L. Vahrmeijer, M. Hutteman, J. R. van der Vorst, C. J. van de Velde and J. V. Frangioni, Nat. Rev. Clin. Oncol., 2013, 10, 507–518 CrossRef CAS.
- R. R. Zhang, A. B. Schroeder, J. J. Grudzinski, E. L. Rosenthal, J. M. Warram, A. N. Pinchuk, K. W. Eliceiri, J. S. Kuo and J. P. Weichert, Nat. Rev. Clin. Oncol., 2017, 14, 347–364 CrossRef CAS.
- C. Vinegoni, I. Botnaru, E. Aikawa, M. A. Calfon, Y. Iwamoto, E. J. Folco, V. Ntziachristos, R. Weissleder, P. Libby and F. A. Jaffer, Sci. Transl. Med., 2011, 3, 84ra45 Search PubMed.
- M. J. Whitley, D. M. Cardona, A. L. Lazarides, I. Spasojevic, J. M. Ferrer, J. Cahill, C. L. Lee, M. Snuderl, D. G. Blazer III, E. S. Hwang, R. A. Greenup, P. J. Mosca, J. K. Mito, K. C. Cuneo, N. A. Larrier, E. K. O'Reilly, R. F. Riedel, W. C. Eward, D. B. Strasfeld, D. Fukumura, R. K. Jain, W. D. Lee, L. G. Griffith, M. G. Bawendi, D. G. Kirsch and B. E. Brigman, Sci. Transl. Med., 2016, 8, 320ra324 Search PubMed.
- T. Ishizawa, N. Fukushima, J. Shibahara, K. Masuda, S. Tamura, T. Aoki, K. Hasegawa, Y. Beck, M. Fukayama and N. Kokudo, Cancer, 2009, 115, 2491–2504 CrossRef.
- K. Welsher, Z. Liu, S. P. Sherlock, J. T. Robinson, Z. Chen, D. Daranciang and H. Dai, Nat. Nanotechnol., 2009, 4, 773–780 CrossRef CAS.
- G. Hong, S. Diao, J. Chang, A. L. Antaris, C. Chen, B. Zhang, S. Zhao, D. N. Atochin, P. L. Huang, K. I. Andreasson, C. J. Kuo and H. Dai, Nat. Photonics, 2014, 8, 723–730 CrossRef CAS.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS.
- Y. Cai, Z. Wei, C. Song, C. Tang, W. Han and X. Dong, Chem. Soc. Rev., 2019, 48, 22–37 RSC.
- S. He, J. Song, J. Qu and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC.
- F. Ding, Y. Zhan, X. Lu and Y. Sun, Chem. Sci., 2018, 9, 4370–4380 RSC.
- S. J. Woo, S. Park, J. E. Jeong, Y. Hong, M. Ku, B. Y. Kim, I. H. Jang, S. C. Heo, T. Wang, K. H. Kim, J. Yang, J. H. Kim and H. Y. Woo, ACS Appl. Mater. Interfaces, 2016, 8, 15937–15947 CrossRef CAS.
- J. Qi, Y. Fang, R. T. K. Kwok, X. Zhang, X. Hu, J. W. Y. Lam, D. Ding and B. Z. Tang, ACS Nano, 2017, 11, 7177–7188 CrossRef CAS.
- X. D. Zhang, H. Wang, A. L. Antaris, L. Li, S. Diao, R. Ma, A. Nguyen, G. Hong, Z. Ma, J. Wang, S. Zhu, J. M. Castellano, T. Wyss-Coray, Y. Liang, J. Luo and H. Dai, Adv. Mater., 2016, 28, 6872–6879 CrossRef CAS.
- B. Guo, Z. Sheng, K. Kenry, D. Hu, X. Lin, S. Xu, C. Liu, H. Zheng and B. Liu, Mater. Horiz., 2017, 4, 1151–1156 RSC.
- S. Gao, G. Wei, S. Zhang, B. Zheng, J. Xu, G. Chen, M. Li, S. Song, W. Fu, Z. Xiao and W. Lu, Nat. Commun., 2019, 10, 2206 CrossRef.
- X. Lu, P. Yuan, W. Zhang, Q. Wu, X. Wang, M. Zhao, P. Sun, W. Huang and Q. Fan, Polym. Chem., 2018, 9, 3118–3126 RSC.
- E. D. Cosco, J. R. Caram, O. T. Bruns, D. Franke, R. A. Day, E. P. Farr, M. G. Bawendi and E. M. Sletten, Angew. Chem., 2017, 56, 13126–13129 CrossRef CAS.
- B. Li, L. Lu, M. Zhao, Z. Lei and F. Zhang, Angew. Chem., 2018, 57, 7483–7487 CrossRef CAS.
- A. L. Antaris, H. Chen, S. Diao, Z. Ma, Z. Zhang, S. Zhu, J. Wang, A. X. Lozano, Q. Fan, L. Chew, M. Zhu, K. Cheng, X. Hong, H. Dai and Z. Cheng, Nat. Commun., 2017, 8, 15269 CrossRef CAS.
- J. A. Carr, D. Franke, J. R. Caram, C. F. Perkinson, M. Saif, V. Askoxylakis, M. Datta, D. Fukumura, R. K. Jain, M. G. Bawendi and O. T. Bruns, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 4465–4470 CrossRef CAS.
- S. Zhu, Z. Hu, R. Tian, B. C. Yung, Q. Yang, S. Zhao, D. O. Kiesewetter, G. Niu, H. Sun, A. L. Antaris and X. Chen, Adv. Mater., 2018, 30, e1802546 CrossRef.
- S. Zhu, B. C. Yung, S. Chandra, G. Niu, A. L. Antaris and X. Chen, Theranostics, 2018, 8, 4141–4151 CrossRef.
- Z. Starosolski, R. Bhavane, K. B. Ghaghada, S. A. Vasudevan, A. Kaay and A. Annapragada, PLoS One, 2017, 12, e0187563 CrossRef.
- Z. Hu, C. Fang, B. Li, Z. Zhang, C. Cao, M. Cai, S. Su, X. Sun, X. Shi, C. Li, T. Zhou, Y. Zhang, C. Chi, P. He, X. Xia, Y. Chen, S. S. Gambhir, Z. Cheng and J. Tian, Nat. Biomed. Eng., 2019, 4, 259 CrossRef.
- Z. Feng, X. Yu, M. Jiang, L. Zhu, Y. Zhang, W. Yang, W. Xi, G. Li and J. Qian, Theranostics, 2019, 9, 5706–5719 CrossRef.
- C. N. Zhu, P. Jiang, Z. L. Zhang, D. L. Zhu, Z. Q. Tian and D. W. Pang, ACS Appl. Mater. Interfaces, 2013, 5, 1186–1189 CrossRef.
- Y. Fan, P. Wang, Y. Lu, R. Wang, L. Zhou, X. Zheng, X. Li, J. A. Piper and F. Zhang, Nat. Nanotechnol., 2018, 13, 941–946 CrossRef CAS.
- Y. Zhong, Z. Ma, F. Wang, X. Wang, Y. Yang, Y. Liu, X. Zhao, J. Li, H. Du, M. Zhang, Q. Cui, S. Zhu, Q. Sun, H. Wan, Y. Tian, Q. Liu, W. Wang, K. C. Garcia and H. Dai, Nat. Biotechnol., 2019, 37, 1322–1331 CrossRef CAS.
- O. T. Bruns, T. S. Bischof, D. K. Harris, D. Franke, Y. Shi, L. Riedemann, A. Bartelt, F. B. Jaworski, J. A. Carr, C. J. Rowlands, M. W. B. Wilson, O. Chen, H. Wei, G. W. Hwang, D. M. Montana, I. Coropceanu, O. B. Achorn, J. Kloepper, J. Heeren, P. T. C. So, D. Fukumura, K. F. Jensen, R. K. Jain and M. G. Bawendi, Nat. Biomed. Eng., 2017, 1, 0056 CrossRef CAS.
- J. A. Carr, M. Aellen, D. Franke, P. T. C. So, O. T. Bruns and M. G. Bawendi, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 201803210 Search PubMed.
- M. Zhang, J. Yue, R. Cui, Z. Ma, H. Wan, F. Wang, S. Zhu, Y. Zhou, Y. Kuang, Y. Zhong, D. W. Pang and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6590–6595 CrossRef CAS.
- Z. Ma, M. Zhang, J. Yue, C. Alcazar, Y. Zhong, T. C. Doyle, H. Dai and N. F. Huang, Adv. Funct. Mater., 2018, 28, 1803417 CrossRef.
- R. Tian, H. Ma, S. Zhu, J. Lau, R. Ma, Y. Liu, L. Lin, S. Chandra, S. Wang, X. Zhu, H. Deng, G. Niu, M. Zhang, A. L. Antaris, K. S. Hettie, B. Yang, Y. Liang and X. Chen, Adv. Mater., 2020, 32, 1907365 CrossRef CAS.
- F. Wang, H. Wan, Z. Ma, Y. Zhong, Q. Sun, Y. Tian, L. Qu, H. Du, M. Zhang, L. Li, H. Ma, J. Luo, Y. Liang, W. J. Li, G. Hong, L. Liu and H. Dai, Nat. Methods, 2019, 16, 545–552 CrossRef CAS.
- Y. Zhong, Z. Ma, S. Zhu, J. Yue, M. Zhang, A. L. Antaris, J. Yuan, R. Cui, H. Wan, Y. Zhou, W. Wang, N. F. Huang, J. Luo, Z. Hu and H. Dai, Nat. Commun., 2017, 8, 737 CrossRef.
- S. Wang, Y. Fan, D. Li, C. Sun, Z. Lei, L. Lu, T. Wang and F. Zhang, Nat. Commun., 2019, 10, 1058 CrossRef.
- S. Zhu, S. Herraiz, J. Yue, M. Zhang, H. Wan, Q. Yang, Z. Ma, Y. Wang, J. He, A. L. Antaris, Y. Zhong, S. Diao, Y. Feng, Y. Zhou, K. Yu, G. Hong, Y. Liang, A. J. Hsueh and H. Dai, Adv. Mater., 2018, 30, e1705799 CrossRef.
- H. Wan, J. Yue, S. Zhu, T. Uno, X. Zhang, Q. Yang, K. Yu, G. Hong, J. Wang, L. Li, Z. Ma, H. Gao, Y. Zhong, J. Su, A. L. Antaris, Y. Xia, J. Luo, Y. Liang and H. Dai, Nat. Commun., 2018, 9, 1171 CrossRef.
- S. Zhu, Q. Yang, A. L. Antaris, J. Yue, Z. Ma, H. Wang, W. Huang, H. Wan, J. Wang, S. Diao, B. Zhang, X. Li, Y. Zhong, K. Yu, G. Hong, J. Luo, Y. Liang and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 962–967 CrossRef.
- G. Hong, A. L. Antaris and H. Dai, Nat. Biomed. Eng., 2017, 1, 0010 CrossRef.
- S. Daneshmand, A. K. Schuckman, B. H. Bochner, M. S. Cookson, T. M. Downs, L. G. Gomella, H. B. Grossman, A. M. Kamat, B. R. Konety, C. T. Lee, K. S. Pohar, R. S. Pruthi, M. J. Resnick, N. D. Smith, J. A. Witjes, M. P. Schoenberg and G. D. Steinberg, Nat. Rev. Urol., 2014, 11, 589–596 CrossRef.
- A. K. Parchur, Z. Fang, J. M. Jagtap, G. Sharma, C. Hansen, S. Shafiee, W. Hu, Q. R. Miao and A. Joshi, Biomater. Sci., 2020 10.1039/d0bm00873g.
- D. M. Montana, M. Nasilowski, W. R. Hess, M. Saif, J. A. Carr, L. Nienhaus and M. G. Bawendi, ACS Appl. Mater. Interfaces, 2020, 12, 35845–35855 CrossRef CAS.
- Y. Feng, S. Zhu, A. L. Antaris, H. Chen, Y. Xiao, X. Lu, L. Jiang, S. Diao, K. Yu, Y. Wang, S. Herraiz, J. Yue, X. Hong, G. Hong, Z. Cheng, H. Dai and A. J. Hsueh, Chem. Sci., 2017, 8, 3703–3711 RSC.
- R. Tian, H. Ma, Q. Yang, H. Wan, S. Zhu, S. Chandra, H. Sun, D. O. Kiesewetter, G. Niu, Y. Liang and X. Chen, Chem. Sci., 2019, 10, 326–332 RSC.
- R. Tian, Q. Zeng, S. Zhu, J. Lau, S. Chandra, R. Ertsey, K. Hetti, T. Teraphongphom, Z. Hu, G. Niu, D. O. Kiesewetter, H. Sun, X. Zhang, A. L. Antaris, B. R. Brooks and X. Chen, Sci. Adv., 2019, 5, eaaw0627 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, synthesis of quantum dots, NIR-II imaging. See DOI: 10.1039/d0ra06249a |
| This journal is © The Royal Society of Chemistry 2020 |