Open Access Article
Open Access ArticleSynthesis of benzonaphthofuroquinones and benzoylnaphthindolizinediones by reactions of flavonoids with dichlone under basylous, oxygenous and aqueous conditions: their cytotoxic and apoptotic activities†
Peng Luoac,
Wanxing Weia,
Saqlain Haiderb,
Shabana I. Khanb,
Mei Wangb,
Weigao Pan *ac and
Amar G. Chittiboyina*b
*ac and
Amar G. Chittiboyina*b
aCollege of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China. E-mail: agao81706672@163.com
bNational Center for Natural Products Research, School of Pharmacy, University of Mississippi, University Park, Mississippi 38677, USA. E-mail: amar@olemiss.edu
cGuangxi University of Chinese Medicine, Nanning 530001, China
First published on 4th August 2020
Abstract
Using flavonoids and dichlone as substrates, benzonaphthofuroquinones (1, 2, 3, 5, 6, novel; 4 new) and benzoylnaphthindolizinediones (7, 8, known; 9, new) were synthesized through common base-catalyzed method and a new method of combining base-catalyzed with O2/H2O exposing. The possible reaction mechanisms may involve the process like isomerization, hydration, oxidation, decomposition and intermolecular condensation. Benzonaphthofuroquinones (2, 3, 4, 5) were found to exhibit potent cytotoxicity against carcinoma cell lines and low toxicity to normal cell lines. The compounds 4 and 5 not only expressed a significant late-stage-apoptosis against human leukemia and melanoma, but also promoted the cleavage of caspase-3 and PARP in human leukemia, which suggested that the late-stage-apoptosis and caspase-3 pathway may be responsible for the cytotoxicities of these benzonaphthofuroquinones. The replacement of the furan ring with pyrrole system in benzoylnaphthindolizinediones (7, 8, 9) resulted in the loss of anticancer activity.
1. Introduction
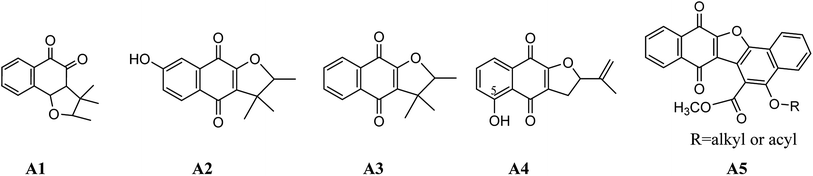
Quinones, an important class of compounds, are found in many biochemical systems. Anticancer drugs like daunorubicin and mitomycin C are known to contain the quinone scaffold in their structures. Their cytotoxic activity is attributed to different mechanisms including redox cycling, intercalation of DNA strands, free radical generation and alkylation via quinone methide formation.1,2Dunnione analogs, members of naphthoquinones family, were found to exhibit potent cytotoxicity against breast and pancreatic cancer cell lines (Scheme 1, A1–A3).3 Presence of a phenolic hydroxy group at C-5 position in 2,3-dihydro-5-hydroxy-2-(prop-1-en-2-yl)naphtho[2,3-b]furan-4,9-dione (Scheme 1, A4) results in an increase in the antiproliferative effects.1 α-Dunnione (CS-3, Scheme 1, A3) inhibited the respiration of the tumor cells by interfering with the electron transport at some point between NADH and ubiquinone which mechanism give poor selectivity for cytotoxicities between carcinoma and normal cells, and its furan ring attached to the naphthoquinone scaffold along with the lipophilic character of the alkyl substituents is a key feature required for the enhancement of anticancer activity.4 While, dinaphtho[1,2-b:2′,3′-d]furan-7,12-dione derivatives (Scheme 1, A5), with additional naphtha-moiety on α-dunnione, had anticancer mechanisms of topoisomerase-I mediated DNA cleavage, epidermal and vascular endothelial growth factor receptors inhibitory actions, which mechanisms owned high selectivity for cytotoxicities.5
So modifying dunniones with aromatic ring-group may be a good way to achieve anticancer mechanisms correlated with cancer-related-biomacromolecules, which may result in high selectivity for cytotoxicity. Flavonoids were potential phenol source for modifying uses, in which the two phenol moieties and a variable central-three-carbons-chain may provide various substituent groups. Meanwhile, constructing other phenol-modified α-dunnione-like derivatives (such as using pyrrol system instead of furan system) may be useful to investigate the changes of cytotoxicities or anticancer mechanisms.
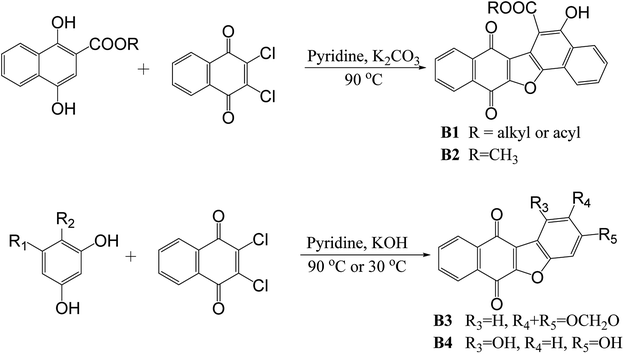
In reported methods, benzo- or naphthanaphthofuroquinones (α-dunniones analogs) was synthesized mainly through base-catalyzed condensation reaction. Condensation of dichlone with methyl 1,4-dihydroxy-2-naphthoate or 2-acetyl-1,4-dihydronaphthalene with presence of K2CO3 under reflux in pyridine at 90 °C resulted in dinaphtho[1,2-b:20,30-d]furan-7,12-dione (Scheme 2, B1)6 or 6-acetyl-5-hydroxydinaphtho[2,3-b:2′,1′-d]furan-7,12-dione (Scheme 2, B2),7 respectively. [1,3]Dioxolo[4,5-f]naphtho[2,3-b]benzofuran-6,11-dione (Scheme 2, B3) or 1,3-dihydroxybenzo[b]naphtho[2,3-d]furan-6,11-dione (Scheme 2, B4) was synthesized from the reaction of dichlone with sesamol (or phloroglucinol) in presence of KOH under reflux in pyridine at 90 °C (or 30 °C).8
Intermolecular C–C-bond formation and intermolecular O-alkylation were the two condensation processes for the synthesis of naphthofuroquinones scaffold. Other methods of preparation included light irradiation and oxidation were reported. 2-Hydroxynaphtho-1,4-dione was converted to negative ion by pyrex-filtered light, and reacted with 2-naphthol exciplex to produce a C–C bond firstly, and then to form a C–O bond by dehydration.9 Quinone-arenols were prepared from hydroquinone monomethylethers by oxidants to form C–C bond in the first step, and by oxidative cyclization of quinone-arenols to give C–O bond in the second step to produce dibenzofuranquinones.10
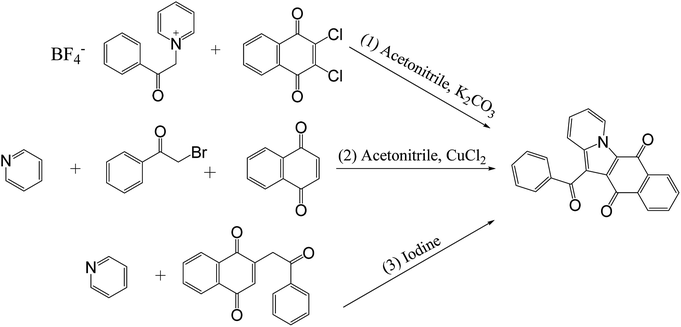
In reported methods, benzoylnaphthindolizinediones (α-dunnione-like compounds) were synthesized by potassium carbonate-catalyzed one-pot tandem reactions of the N-ylides with 2,3-dichloro-1,4-naphthoquinone (Scheme 3, (1)),11 or by copper(II)-catalyzed three-component reactions of 2-bromoacetophenone, 1,4-naphthoquinone, and pyridine via sp2-C–H difunctionalization of naphthoquinone followed by intramolecular cyclization and oxidative aromatization (Scheme 3, (2)),12 or by iodine oxidation of 2-alkyl-1,4-naphthoquinones in the presence of substituted pyridines (Scheme 3, (3)).13
In our initial plan, in order to get naphthanaphthofuroquinones, dihydroflavone and chalcone were respectively used to react with dichlone in pyridine under anoxic and anhydrous conditions, however, no targeted products were obtained. Unintentionally, the anoxic and anhydrous conditions being removed and reaction system being exposed to the moist air, unexpected benzoylnaphthindolizinediones were found. So this O2/H2O exposing combining with base-catalyzed method were developed.
With regard to recent works about the utilization of water to promote reaction, ecofriendly and efficient synthesis of sulfonylated N-heteroaromatics14 and 2-sulfonyl quinolines/pyridines15 were conducted in water, and in the iodine-catalyzed odorless synthesis of S-thiocarbamates, water played a dual role, viz. co-solvent and H/O source.16
2. Results and discussion
2.1 Chemistry
Structures of 1–10 were elucidated and characterized by NMR (1H, 13C, DEPT, 1H–1H COSY, HSQC, HMBC), MS (ESI-MS, APCI-MS and HR ESI-MS) and IR spectra (see the ESI† file).
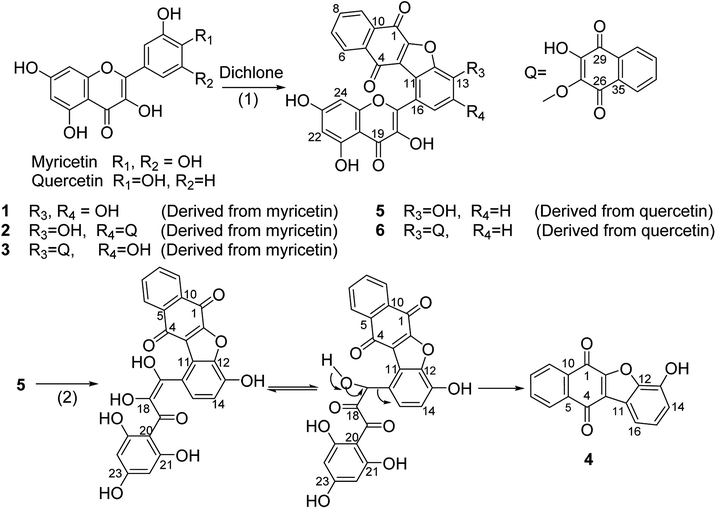
In NMR spectra of 1, total of seven aromatic methines signals were observed. As concerns resonances changes occurring on the B-ring of flavonoid moiety, one methane signal at δH/δC 7.36(s)/118.6 (position 15) was remained while the other one disappeared, which suggested that intermolecular C–C condensation reaction occurred in position 11. Furthermore, a new aromatic oxygenated carbon signal at δC 154.1 (C-2) was found, so an additional C–O covalent bond was formed, which contributed to produce the furan ring (Scheme 4). As compared with H-15/C-15 resonances of 1 at δH-15/δC-15 7.36/118.6, the corresponding resonances of 2 moved to up-field shift at δH-15/δC-15 7.29/113.3, and as to 3, δH-15 move to down-field shift at 7.56 while δC-15 almost unchanged at 117.8, which meaned that the second dichlone moiety, through intermolecular O-alkylation condensation, was located at 14-O (ortho effect) in 2, and at 13-O in 3 (Scheme 4).
 | ||
| Scheme 4 Synthesis of benzonaphthofuroquinones and the possible reaction mechanism of 4. Reagents and conditions: (1) pyridine, 85 °C, 24 h; (2) pyridine, moist air (O2/H2O) injection, 85 °C, 24 h. | ||
In case of 5, eight aromatic methines signals were found. As for resonances changes occurring on the B-ring of flavonoid moiety, two methine-protons signals at δH 7.22–7.24 (1H, d, J = 8.3 Hz)] and δH 7.61–7.63 (1H, d, J = 8.3 Hz) with their coupling in 1H–1H COSY were observed while the third one vanished, which meant that C–C condensation reaction occurred in position 11 (Scheme 4). Furthermore, a new aromatic oxygenated carbon signal at δC 154.7 (C-2) was found, so an additional C–O covalent bond was formed to produce the furan ring (Scheme 4). 6 manifested obvious proton/carbon resonances changes on the B-ring comparing to those of 5, so the second naphthoquinone moiety was located at 13-O (Scheme 4). 4 was produced from 5 (Scheme 4), so the hydroxyl group was located at position 13, which had been confirmed by its NMR data and single crystal structure in our previous work.17
7 had been confirmed by its NMR data and single crystal structure in our previous work.18
By the coupling relationships in 1H–1H COSY of 8, proton signals of δH 7.91–7.93 (2H, d, J = 7.5 Hz), 7.46–7.49 (2H, t, J = 7.5 Hz), 7.60–7.63 (1H, t, J = 7.5 Hz) were assigned to a five-consecutive-methines-chain of phenyl group. Considering the facts that the HR ESI-MS spectra gave a molecular formula of C23H13NO3 and pyridine was used as solvent in the reaction, proton signals of δH 9.83–9.85 (1H, d, J = 7.0 Hz), 7.21–7.24 (1H, t, J = 7.3 Hz), 7.41–7.44 (1H, m), 7.97–7.99 (1H, d, J = 9.0 Hz) were assigned to a four-consecutive-methines-chain of pyridine moiety by the 1H–1H COSY coupling relationships, which meant that pyridine was involved in the intermolecular condensation reaction (Scheme 5).
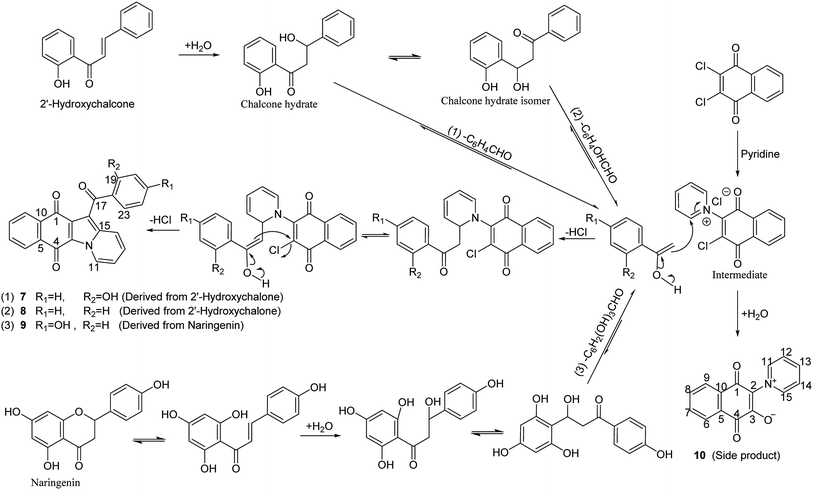 | ||
| Scheme 5 Synthesis of benzoylnaphthindolizinediones and the possible reaction mechanisms. Reagents and conditions: pyridine, moist air (O2/H2O) injection, 85 °C, 24 h. | ||
Instead of five consecutive coupling protons in 8, four protons with coupling relationships between signals of δH 6.80–6.82 (2H, d, J = 8.5 Hz) and those of δH 7.73–7.77 (2H, m) were observed in 9. Furthermore, one more aromatic oxygenated carbon at δC 162.8 was found in 9 than in 8 (Scheme 5).
The structure of 4 was confirmed by both single crystal X-ray diffraction and NMR-spectrum in our previous work.17 In the condensation reaction of quercetin with dichlone under basylous, anoxic and anhydrous conditions, 4 was not found, while providing additional condition of O2/H2O exposing, 4 was obtained (Scheme 4). It may indicate that C-ring of flavonol moiety in 5 was cleaved by hydration, and then a “2,3-dioxo-3-(2,4,6-trihydroxyphenyl)propanal” group was eliminated to give a benzonaphthofuroquinone core (4, Scheme 4). However, this “2,3-dioxo-3-propanal” elimination mechanism was not found in the literature, so further work should be done to confirm it.
In Scheme 5, occurrence of side product (10) provided evidence for the existence of intermediate of dichlone reacted with pyridine, and the structure of 10 and its synthetic method (under similar conditions and using the same substrates) were reported by Andreas Schmidt et al.19 Dihydroflavone (naringenin) isomerized into trans-chalcone. Acetophenone blocks, which were generated from trans-chalcone via retro-aldol decomposition after hydration and isomerization, reacted with above intermediate to build a benzoylnaphthindolizinedione skeleton (7, 8, 9, Scheme 5). The fact that 7 and 8 were derived from the different part of chalcone provided evidence for the isomerization reaction of chalcone hydrate.
Two phases of the hydration and dealdolization reactions of chalcone (1,3-diphenyl-2-propen-1-one) and their reaction rate constants were reported by J. Peter Guthrie et al. Hydration of chalcone was catalyzed by aqueous sodium hydroxide to give the hydrate (1,3-diphenyl-3-hydroxy-1-propanone) firstly, and then retro-aldol reaction of this hydrate was also catalyzed by aqueous sodium hydroxide to produce benzaldehyde and acetophenone.20
2.2 Biology
| Treatments | Cancer cell linesc (IC50, μM) | Non-cancer cell linesd (IC50, μM) | |||||
|---|---|---|---|---|---|---|---|
| SK-MEL | KB | BT-549 | SK-OV-3 | HL-60 | LLC-PK1 | Vero | |
| a NA or NC mean not active up to 40 μM.b Not tested.c SK-MEL: malignant melanoma, KB: oral epidermal carcinoma, BT-549: ductal breast carcinoma, SK-OV-3: ovary carcinoma, HL-60: human leukemia.d LLC-PK1: kidney epithelial, VERO: African green monkey kidney fibroblast. | |||||||
| 1 | 14.83 | 29.66 | 23.31 | NAa | NAa | NCa | NCa |
| 2 | 3.88 | 10.87 | 10.87 | 27.95 | 18.17 | 24.84 | 38.82 |
| 3 | 2.02 | 5.90 | 3.57 | 6.21 | 17.32 | 27.95 | 15.53 |
| 4 | 12.88 | 37.88 | 37.88 | 22.73 | 2.57 | 18.18 | 15.91 |
| 5 | 5.26 | 10.96 | 28.51 | 21.93 | 4.29 | 21.93 | NCa |
| 6 | 39.8 | NAa | NAa | NAa | NAa | 39.8 | NCa |
| 7 | NAa | NAa | NAa | NAa | NAa | NCa | NCa |
| 8 | NAa | NAa | NAa | NAa | NAa | NCa | NCa |
| 9 | NAa | NAa | NAa | NAa | NAa | NCa | NCa |
| Doxorubicin | 1.29 | 0.92 | 1.84 | 4.05 | —b | 0.92 | 9.21 |
| Cisplatin | —b | —b | —b | —b | 1.46 | —b | —b |
Doxorubicin manifested strong cytotoxicities against both carcinoma and normal cell lines, while 1, 2, 3, 4, 5 possessed good anticancer activities and low toxicity to normal cells. 3 owed high antagonistic effect against almost all test solid carcinoma cell lines, while 2, 5 just showed good inhibition against SK-MEL (solid carcinoma), and 4, 5 had good inhibition against HL-60 (non-solid carcinoma) (Table 1). The potent cytotoxic derivatives (2, 3, 4, 5) were selected for further apoptotic test and western blotting analysis.
With regard to apoptosis against melanoma (Fig. S2,† see the ESI†), 2 (30 μM) and 3 (30 μM) had no apoptotic activities; while 4 (30 μM) and 5 (30 μM) induced obvious Annexin V-positive apoptosis (45.8% and 17.0%, respectively), in which, the major apoptotic proportions (41.7% and 15.1%, respectively) were observed in the upper right quadrant (Annexin V+/7-AAD+, late-apoptotic-stage).
3. Experimental
3.1 General information and characterization data
Flash column chromatographic separations were accomplished on Biotage Isolera Four system (Biotage, Uppsala, Sweden). NMR was recorded on DRX-400 and DRX-500 NMR spectrometers (Bruker, Rheinstetten, Germany). ESI-, APCI- and HR ESI-MS were recorded on Agilent 1290 LC-MS or Agilent G6530 TOF MS spectrometer (Agilent Technologies Inc., California, USA). Western blot results were visualized on Mini-HD9-Auto Biomolecular imager (Leader Oriental Technology. LTD, Beijing, China). Dichlone and flavonoids were purchased from Fisher Scientific (Fair Lawn, NJ, USA) and Ark Pharm, Inc. (Arlington Heights, IL, USA). Cell lines were gotten from the American Type Culture Collection (Manassas, VA). Caspase-3 (3G2) mouse mAb, caspase-3 antibody, cleaved caspase-3 (Asp175) (5A1E) rabbit mAb, PARP (46D11) rabbit mAb and horseradish peroxidase (HRP) were purchased from Cell Signaling Technology, Inc (Danvers, MA, USA).2: red powder; 126 mg, yield 18% (final product/myricetin × 100%); mp 319–320 °C. ESI-MS: 659.0 [M + CH3OH–H2O + H]+, 657.0 [M + CH3OH–H2O–H]−; ACPI-MS: 659.0 [M + CH3OH–H2O + H]+, 657.0 [M + CH3OH–H2O–H]−. HRESI-MS found [M − H]+ 643.0463; C35H16O13 [M − H]+ requires 643.0518. IR (solid, cm−1): 3530, 3078, 1671, 1655, 1627, 1606, 1588, 1498, 1383, 1363, 1322, 1286, 1246, 1186, 1161, 1112, 1051, 975, 897, 841, 794, 711, 655. 1H NMR (400 MHz, DMSO-d6) δ: 8.10–8.12(1H, m, H-9), 7.99–8.01 (2H, m, H-31, 34), 7.94–7.96 (2H, m, H-32, 33), 7.92–7.94 (1H, m, H-6), 7.84–7.86 (2H, m, H-7, 8), 7.29 (1H, s, H-15), 6.40 (1H, s, H-24), 6.20 (1H, s, H-22). 13C NMR and DEPT (101 MHz, DMSO-d6) δ: 182.3 (C-4), 177.4 (C-29), 176.9 (C-19), 176.6 (C-26), 174.9 (C-1), 165.0 (C-23), 162.0 (C-21), 160.1 (C-27), 156.9 (C-25), 153.5 (C-14), 148.3 (C-17), 140.6 (C-2), 140.2 (C-12), 139.5 (C-28), 138.5 (C-18), 134.9 (C-7, 32, 33), 134.7 (C-8), 132.7 (C-10), 132.2 (C-13), 131.1 (C-5), 130.6 (C-16), 130.2 (C-35), 130.0 (C-30), 127.0 (C-3), 126.9 (C-31), 126.5 (C-9), 126.3 (C-34), 126.2 (C-6), 113.3 (C-15), 110.7 (C-11), 105.1 (C-20), 99.5 (C-22), 94.4 (C-24). 1H was assigned to 13C by HSQC correlations. In the 1H–1H COSY spectrum, the couplings of H-6 with H-7, H-7 with H-8, H-8 with H-9, H-31 with H-32, H-32 with H-33, H-33 with H-34 were determined. In the HMBC spectrum, the correlations of H-6 with C-7, 8; H-9 with C-7, 8; H-15 with C-11, 13, 14, 17; H-22 with C-20, 24; H-31 with C-33; H-34 with C-32 were observed.
3: black powder; 112 mg, yield 16% (final product/myricetin × 100%); mp 425–426 °C. ESI-MS: 659.0 [M + CH3OH–H2O + H]+, 656.8 [M + CH3OH–H2O–H]−; ACPI-MS: 657.0 [M + CH3OH–H2O–H]−. HRESI-MS found [M − H]+ 643.0463; C35H16O13 [M − H]+ requires 643.0518. IR (solid, cm−1): 3647, 3317, 3071, 1673, 1645, 1630, 1603, 1591, 1571, 1508, 1474, 1434, 1369, 1320, 1277, 1246, 1226, 1211, 1178, 1156, 1104, 1060, 1010, 974, 948, 916, 900, 887, 833, 789, 750, 710, 695. 1H NMR (400 MHz, DMSO-d6) δ: 8.09–8.11 (1H, m, H-9), 8.04–8.05 (1H, m, H-34), 7.99–8.01 (1H, m, H-31), 7.89–7.90 (1H, m, H-6), 7.87–7.88 (2H, m, H-32, 33), 7.82–7.86 (2H, m, H-7, 8), 7.56 (1H, s, H-15), 6.22 (2H, s, H-22, 24). 13C NMR and DEPT (101 MHz, DMSO-d6) δ: 179.4 (C-4), 177.0 (C-29), 177.0 (C-19), 176.8 (C-26), 174.9 (C-1), 164.5 (C-23), 161.5 (C-21), 157.3 (C-25), 156.2 (C-27), 146.2 (C-17), 143.3 (C-2), 140.6 (C-14), 139.6 (C-28), 138.9 (C-12), 137.7 (C-18), 135.1–135.2 (C-7, 32, 33), 134.6 (C-8), 133.5 (C-10), 131.8 (C-5), 130.0 (C-35), 129.9 (C-30), 128.5 (C-13), 127.2 (C-6), 126.6 (C-9), 126.4 (C-31, 34), 124.0 (C-3), 123.0 (C-16), 121.1 (C-11), 117.8 (C-15), 104.6 (C-20), 98.8 (C-22), 94.0 (C-24). 1H was assigned to 13C by HSQC correlations. In the 1H–1H COSY spectrum, the couplings of H-6 with H-7, H-7 with H-8, H-8 with H-9, H-31 with H-32, H-32 with H-33, H-33 with H-34 were determined. In the HMBC spectrum, the correlations of H-6 with C-5; H-7 with C-9; H-8 with C-10; H-9 with C-7; H-15 with C-11, 13, 14, 17; H-22 with C-20, 21, 23, 24; H-24 with C-20, 22, 23, 25; H-31 with C-33; H-32 with C-33, 34; H-33 with C-31, 32; H-34 with C-32 were observed.
4: red columnar crystals. 133 mg, yield 20% (final product/quercetin × 100%); mp 263 °C. ESI-MS: 263.0 [M − H]−; ACPI-MS: 265.0 [M + H]+, 263.0 [M − 1]−. HRESI-MS found [M + H]+ 265.0492; C16H9O4 [M + H]+ requires 265.0495. Crystal structure, IR and NMR data were reported in our previous work.17
5: orange powder; 319 mg, yield 48% (final product/quercetin × 100%); mp 387–388 °C. ESI-MS: 457.0 [M + H]+, 455.0 [M − H]−; ACPI-MS: 457.0 [M + H]+, 455.0 [M − H]−. HRESI-MS found [M + H]+ 457.0554; C25H13O9 [M + H]+ requires 457.0554. IR (solid, cm−1): 3545, 3531, 3288, 3095, 1672, 1646, 1625, 1600, 1577, 1559, 1497, 1438, 1358, 1305, 1278, 1218, 1193, 1166, 1090, 1015, 971, 943, 900, 842, 825, 798, 751, 709, 695. 1H NMR (400 MHz, DMSO-d6) δ: 8.12–8.14 (1H, m, H-9), 7.91–7.93 (1H, m, H-6), 7.83–7.87 (1H, m, H-8), 7.76–7.80 (1H, m, H-7), 7.61–7.63 (1H, d, J = 8.3 Hz, H-15), 7.22–7.24 (1H, d, J = 8.3 Hz, H-14), 6.25 (1H, d, J = 2.0 Hz, H-22), 6.22 (1H, d, J = 2.0 Hz, H-24). 13C NMR and DEPT (101 MHz, DMSO-d6) δ: 179.9 (C-4), 177.1 (C-19), 175.5 (C-1), 164.3 (C-23), 161.5 (C-21), 157.3 (C-25), 154.7 (C-2), 149.1 (C-17), 145.9 (C-13), 145.4 (C-12), 137.0 (C-18), 135.0 (C-7), 134.4 (C-8), 133.7 (C-10), 132.1 (C-5), 129.8 (C-15), 127.1 (C-6), 126.5 (C-9), 124.5 (C-3), 123.3 (C-11), 116.9 (C-16), 115.0 (C-14), 104.5 (C-20), 98.6 (C-22), 93.9 (C-24). 1H was assigned to 13C by HSQC correlations. In the 1H–1H COSY spectrum, the couplings of H-6 with H-7, H-7 with H-8, H-8 with H-9 were determined. In the HMBC spectrum, the correlations of H-6 with C-8; H-8 with C-5, 6, 9, 10; H-9 with C-7; H-14 with C-12, 16; H-15 with C-11, 13, 17; H-22 with C-20, 21, 24; H-24 with C-20, 22, 25 were observed.
6: brownish red powder; 98 mg, yield 15% (final product/quercetin × 100%); mp 390–391 °C. ESI-MS: 629.0 [M + H]+, 627.0 [M − H]−; ACPI-MS: 629.0 [M + H]+. HRESI-MS found [M + H]+ 629.0711; C35H17O12 [M + H]+ requires 629.0715. IR (solid, cm−1): 3651, 3292, 3125, 2925, 2851, 1654, 1636, 1590, 1560, 1508, 1474, 1368, 1306, 1264, 1215, 1192, 1165, 1090, 1073, 1011, 981, 941, 899, 796, 711, 696. 1H NMR (400 MHz, DMSO-d6) δ: 8.15–8.18 (1H, m, H-9), 8.09–8.11 (1H, m, H-34), 7.99–8.02 (1H, m, H-31), 7.95–7.97 (1H, m, H-6), 7.89–7.91 (1H, m, H-8), 7.88–7.89 (2H, m, H-32, 33), 7.85–7.87 (1H, m, H-7), 7.62–7.64 (1H, d, J = 8.4 Hz, H-15), 7.48–7.50 (1H, d, J = 8.4 Hz, H-14), 6.26–6.27 (1H, d, J = 2.0 Hz, H-22), 6.23–6.24 (1H, d, J = 2.0 Hz, H-24). 13C NMR and DEPT (101 MHz, DMSO-d6) δ: 182.0 (C-29), 181.5 (C-26), 179.8 (C-4), 177.1 (C-19), 175.4 (C-1), 164.4 (C-23), 161.5 (C-21), 157.4 (C-25), 155.2 (C-2), 148.4 (C-17), 144.8 (C-13), 144.8 (C-12), 137.3 (C-18), 135.8 (C-28), 135.1 (C-7), 135.1 (C-33), 134.7 (C-27), 134.5 (C-8), 134.1 (C-32), 133.7 (C-10), 132.2 (C-5), 131.3 (C-30), 130.9 (C-35), 129.4 (C-15), 127.2 (C-6), 126.6 (C-9), 126.5 (C-34), 126.2 (C-31), 124.2 (C-3), 123.7 (C-11), 120.3 (C-16), 114.3 (C-14), 104.5 (C-20), 98.7(C-22), 94.0 (C-24). 1H was assigned to 13C by HSQC correlations. In the 1H–1H COSY spectrum, the couplings of H-6 with H-7, H-7 with H-8, H-8 with H-9, H-14 with H-15, H-31 with H-32, H-32 with H-33, H-33 with H-34 were determined.
7: red needle crystals; 326 mg, yield 66% (final product/chalcone × 100%); mp 264–265 °C. Its crystal structure, MS, IR and NMR data were reported in our previous work.18
8: red needle crystals; 336 mg, yield 68% (final product/chalcone × 100%); mp 258–259 °C. ESI-MS: 352.2 [M + H]+; ACPI-MS: 352.2 [M + H]+. HRESI-MS found [M + Na]+ 374.0792, [2M + Na]+ 725.1689; C23H13NO3Na [M + Na]+ requires 374.0788, C46H26N2O6Na [2M + Na]+ requires 725.1683. IR (solid, cm−1): 3690, 3650, 3110, 3098, 3079, 3056, 2956, 2919, 1851, 1667, 1640, 1623, 1588, 1570, 1490, 1466, 1447, 1392, 1365, 1307, 1276, 1227, 1209, 1159, 1134, 1062, 1037, 1018, 998, 905, 877, 834, 808, 758, 748, 706, 693, 662. 1H NMR (500 MHz, CDCl3) δ: 9.83–9.85 (1H, d, J = 7.0 Hz, H-11), 8.27–8.29 (1H, d, J = 7.5 Hz, H-6), 8.03–8.05 (1H, d, J = 7.5 Hz, H-9), 7.97–7.99 (1H, d, J = 9.0 Hz, H-14), 7.91–7.93 (2H, d, J = 7.5 Hz, H-19, 23), 7.74–7.77 (1H, td, J = 7.5, 1.1 Hz, H-7), 7.65–7.68 (1H, td, J = 7.5, 1.1 Hz, H-8), 7.60–7.63 (1H, t, J = 7.5 Hz, H-21), 7.46–7.49 (2H, t, J = 7.5 Hz, H-20, 22), 7.41–7.44 (1H, m, H-13), 7.21–7.24 (1H, t, J = 7.3 Hz, H-12). 13C NMR and DEPT (126 MHz, CDCl3) δ: 191.9 (C-17), 180.9 (C-1), 175.0 (C-4), 139.2(C-15), 138.9(C-18), 134.3(C-5), 133.8(C-7), 133.6 (C-10), 133.1 (C-8), 133.1 (C-21), 129.3 (C-19, 23), 128.4 (C-20, 22), 128.4 (C-3), 128.3 (C-13), 127.8 (C-11), 127.2 (C-9), 126.4 (C-6), 121.4 (C-2), 120.4 (C-14), 117.8 (C-12), 113.7 (C-16). 1H was assigned to 13C by HSQC correlations. In the 1H–1H COSY spectrum, the couplings of H-6 with H-7, H-7 with H-8, H-8 with H-9, H-11 with H-12, H-12 with H-13, H-13 with H-14, H-19 with H-20, H-20 with H-21, H-21 with H-22, H-22 with H-23 were determined. In the HMBC spectrum, the correlations of H-6 with C-4, 8, 10; H-7 with C-5, 9; H-8 with C-6, 7, 10; H-9 with C-1, 5, 7; H-11 with C-3, 12, 13, 15; H-12 with C-13, 14; H-13 with C-11, 12, 15; H-14 with C-12, 15; H-19 with C-17, 21, 23; H-20 with C-18, 19, 21, 22; H-21 with C-19, 23; H-22 with C-18, 20, 21, 23; H-23 with C-17, 19, 21 were observed.
9: red needle crystals; 389 mg, yield 65% (final product/naringenin × 100%); mp 318–319 °C. ESI-MS: 368.0 [M + H]+, 366.0 [M − H]−; ACPI-MS: 368.0 [M + H]+, 366.0 [M − H]−. HRESI-MS found [M + H]+ 368.0916; C23H14NO4 [M + H]+ requires 368.0917. IR (solid, cm−1): 3650, 3358, 3071, 1670, 1629, 1614, 1599, 1566, 1560, 1491, 1474, 1436, 1394, 1368, 1306, 1276, 1226, 1211, 1162, 1137, 1063, 1037, 906, 878, 845, 777, 755, 713, 704, 688. 1H NMR (400 MHz, DMSO-d6) δ: 10.41 (1H, s, 21-OH), 9.63–9.66 (1H, m, H-11), 8.12–8.15 (1H, m, H-6), 7.91–7.93 (1H, m, H-9), 7.80–7.85 (1H, m, H-7), 7.73–7.77 (4H, m, H-8,14, 19, 23), 7.47–7.51 (1H, m, H-13), 7.36–7.39 (1H, m, H-12), 6.80–6.82 (2H, d, J = 8.5 Hz, H-20, 22). 13C NMR and DEPT (101 MHz, DMSO-d6) δ: 189.5 (C-17), 181.1 (C-1), 174.1 (C-4), 162.8 (C-21), 138.1(C-15), 134.6 (C-7), 134.6 (C-5), 133.7 (C-8), 133.5 (C-10), 132.4 (C-19, 23), 130.1 (C-18), 128.3 (C-13), 128.1 (C-11), 127.7 (C-3), 126.9 (C-9), 126.4 (C-6), 120.9 (C-2), 120.1 (C-14), 118.7 (C-12), 115.7 (C-20, 22), 114.3 (C-16). 1H was assigned to 13C by HSQC correlations. In the 1H–1H COSY spectrum, the couplings of H-6 with H-7, H-7 with H-8, H-8 with H-9, H-11 with H-12, H-12 with H-13, H-13 with H-14, H-19 with H-20, H-22 with H-23 were determined. In the HMBC spectrum, the correlations of H-6 with C-4, 10; H-7 with C-5, 9; H-8 with C-6, 10; H-9 with C-1, 5, 7; H-11 with C-3, 12, 13, 15; H-12 with C-11, 13, 14; H-13 with C-11, 15; H-14 with C-12, 15; H-19 with C-17, 21; H-20 with C-18, 21, 22; H-22 with C-18, 20, 21; H-23 with C-17, 21; 21-OH with 20, 21, 22 were observed.
10: yellow needle crystals; 300–599 mg, yield 20–40% (final product/dichlone × 100%); mp 296–297 °C. ESI-MS: 252.2 [M + H]+; ACPI-MS: 252.2 [M + H]+. HRESI-MS found [M + H]+ 252.0662; C15H10NO3 [M + H]+ requires 252.0655. 1H NMR (400 MHz, DMSO-d6) δ: 8.89–8.91 (2H, d, J = 5.7 Hz, H-11, 15), 8.57–8.61 (1H, t, J = 7.6 Hz, H-13), 8.15–8.19 (2H, t, J = 6.7 Hz, H-12, 14), 8.05–8.07 (1H, d, J = 7.5 Hz, H-6), 7.96–7.98 (1H, d, J = 7.5 Hz, H-9), 7.81–7.85 (1H, t, J = 7.4 Hz, H-7), 7.70–7.73 (1H, t, J = 7.3 Hz, H-8). 13C NMR and DEPT (101 MHz, DMSO-d6) δ: 184.4 (C-1), 172.4 (C-4), 165.4 (C-3), 148.1 (C-11,15), 145.1 (C-13), 134.9 (C-7), 134.1 (C-5), 132.1 (C-8), 131.6 (C-10), 127.4 (C-12, 14), 126.5 (C-9), 126.3 (C-6), 125.4 (C-2). 1H was assigned to 13C by HSQC correlations. In the 1H–1H COSY spectrum, the couplings of H-6 with H-7; H-7 with H-8; H-8 with H-9; H-13 with H-12, 14; H-11 with H-12; H-14 with H-15 were determined. In the HMBC spectrum, the correlations of H-6 with C-4, 10; H-7 with C-5, 9; H-8 with C-6, 10; H-9 with C-1, 5; H-11, 15 with C-13; H-13 with C-11, 15; H-12 with C-11; H-14 with C-15; H-12 with C-14; H-14 with C-12 were observed.
3.2 Synthesis and isolation
One of the selected flavonoids (0.002 mol) was mixed with dichlone (0.006 mol) uniformly, and then 50 mL of pyridine was added to dissolve the mixed solid in a 100-mL double mouth round bottom flask. The reacting system was stirred at 85 °C for 24 h (in order to synthesize 4, 7, 8, 9, additional conditions, air being pumped through a water-filled absorption bottle and then injected into this mixture solution with flow velocity of 1 bubble/2 s, must be provided), and was finally concentrated to give a semi-solid mass. The mass was chromatographed on Biotage Isolera Four flash column chromatography system with a SNAP Cartridge KP-Sil 10 g column eluted by hexane/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 90
0 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v), and then by chloroform/methanol (100
10, v/v), and then by chloroform/methanol (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 90
0 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) to give fractions. TLC was used to distinguish the eluted fractions. The fractions were further isolated by preparative TLC (1000 microns; 20 cm × 20 cm) using chloroform/methanol or hexane/ethyl acetate as developing agent to yield derivatives.
10, v/v) to give fractions. TLC was used to distinguish the eluted fractions. The fractions were further isolated by preparative TLC (1000 microns; 20 cm × 20 cm) using chloroform/methanol or hexane/ethyl acetate as developing agent to yield derivatives.
3.3 Assay for anticancer activity
Cytotoxicity against non-solid tumor and solid tumor were tested by Trypan blue dye exclusion method and Neutral red uptake method, respectively.21 IC50 was calculated from the dose curves generated by plotting percent growth vs. the test concentration on a logarithmic scale using Microsoft Excel. All assays were performed in triplicate and the mean values are given in Table 1.3.4 Apoptosis analysis
The sensitive cancer cell lines were separately incubated with derivatives, DMSO and doxorubicin (positive control) at different concentrations for 24 h, harvested by centrifugation at 300g, washed twice with cold 1× PBS and resuspended in 100 μL of binding buffer at a density of 2 × 106 cells per mL, stained with 5 μL of PE Annexin-V and 5 μL of 7-AAD for 15 min in the dark at room temperature and finally subjected to analysis via Accuri™ C6 flow cytometry.22,233.5 Western blotting analysis
The sensitive cancer cell lines were separately treated with derivatives, doxorubicin (positive control) and DMSO at different concentrations for 24 h, washed with cold 1× PBS, harvested in RIPA lysis solution for 60 min on ice, and then heated to boiling point for 10 min after adding loading buffer. The cell lysate were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min at 4 °C. Equal amounts of the protein samples were subjected to SDS-PAGE electrophoresis and transferred to PVDF membranes. The membranes were blocked in 5% nonfat milk, incubated with diluted primary antibodies and secondary antibodies conjugated with HRP, treated with Western Lighting Chemiluminescence Reagent and finally visualized on biomolecular imager to determine the expression levels of apoptosis marker proteins including caspase-3 and PARP cleavage (using β-actin as internal reference).23,24
000 g for 10 min at 4 °C. Equal amounts of the protein samples were subjected to SDS-PAGE electrophoresis and transferred to PVDF membranes. The membranes were blocked in 5% nonfat milk, incubated with diluted primary antibodies and secondary antibodies conjugated with HRP, treated with Western Lighting Chemiluminescence Reagent and finally visualized on biomolecular imager to determine the expression levels of apoptosis marker proteins including caspase-3 and PARP cleavage (using β-actin as internal reference).23,24
4. Conclusion
By the common base-catalyzed method, not only mono condensation products (1, 5) but also bis-naphthoquinones compounds (2, 3, 6) were obtained.Through a new method of combining base-catalyzed with O2/H2O exposing, benzonaphthofuroquinone (4) and benzoylnaphthindolizinediones (7, 8, 9) were synthesized. To our knowledge this is the first report about the reaction of chalcone/dihydroflavone with dichlone under given conditions to produce benzoylnaphthindolizinediones, as well as the reaction of flavonols with dichlone to achieve benzonaphthofuroquinones. Benzoylnaphthindolizinediones were previously synthesized by methods of potassium carbonate-catalyzing,11 or copper(II)-catalyzing,12 or iodine oxidation.13
Some of the derivatives (2, 3, 4, 5) showed good anticancer activities and low toxicity to normal cells, which indicated that they possess some cytotoxic selectivity. The formed furan-ring in benzonaphthofuroquinones being replaced by pyrrol-ring in benzoylnaphthindolizinediones resulted in the loss of anticancer activity. Late-stage-apoptosis and caspase-3 pathway may contribute to the cytotoxicities of the synthesized benzonaphthofuroquinones.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by State Scholarship Fund awarded by China Scholarship Council, 201706660015; Guangxi Scholarship Fund of Guangxi Education Department, guijiaoshipei-2016-22; Promotion of Young and Middle-aged Teachers' Basic Scientific Research Ability in Guangxi Universities by Guangxi Education Department, 2019KY0329; Opening Project of First-class Discipline Construction in Guangxi, 2019XK047, 2019XK132 and Guangxi Key Laboratory of Zhuang and Yao Ethnic Medicine, guikejizi-2014-32, GXZYZZ2019-7, GXZYKF2019-6.Notes and references
- E.-L. Bonifazi, C. Rios-Luci, L.-G. Leon, G. Burton, J.-M. Padron and R.-I. Misico, Bioorg. Med. Chem., 2010, 18, 2621–2630 CrossRef CAS PubMed
.
- M.-G. Miller, A. Rodgers and G.-M. Cohen, Biochem. Pharmacol., 1986, 35, 1177–1184 CrossRef CAS PubMed
.
- H. Sheridan, C. Nestor, L. O'Driscoll and I. Hook, J. Nat. Prod., 2011, 74, 82–85 CrossRef CAS PubMed
.
- A. Morello, M. Pavani, J.-A. Garbarino, M.-C. Chamy, C. Frey, J. Mancilla, A. Guerrero, Y. Repetto and J. Ferreira, Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol., 1995, 112, 119–128 CAS
.
- H. Cho, S.-J. Park and K.-I. Lee, Bull. Korean Chem. Soc., 2005, 26, 1829–1832 CrossRef CAS
.
- K.-I. Lee, Y. Park, S.-J. Park, J.-H. Hwang, S.-J. Lee, G.-D. Kim, W.-K. Park, S. Lee, D. Jeong, J.-Y. Kong, H.-K. Kang and H. Cho, Bioorg. Med. Chem. Lett., 2006, 16, 737–742 CrossRef CAS PubMed
.
- J. Padwal, W. Lewis and C.-J. Moody, J. Org. Chem., 2011, 76, 8082–8087 CrossRef CAS PubMed
.
- C.-C. Cheng, Q. Dong, D.-F. Liu, Y.-L. Luo, L.-F. Liu, A.-Y. Chen, C. Yu, N. Savaraj and T.-C. Chou, J. Med. Chem., 1993, 36, 4108–4112 CrossRef CAS PubMed
.
- H. Suginome, A. Konishi, H. Sakurai, H. Minakawa, T. Takeda, H. Senboku, M. Tokuda and K. Kobayashi, Tetrahedron, 1995, 51, 1377–1386 CrossRef CAS
.
- T. Takeya, H. Kondo, T. Otsuka, K. Tomita, I. Okamoto and O. Tamura, Org. Lett., 2007, 9, 2807–2810 CrossRef CAS PubMed
.
- Y. Liu, H.-Y. Hu, Q.-J. Liu, H.-W. Hu and J.-H. Xu, Tetrahedron, 2007, 63, 2024–2033 CrossRef CAS
.
- Y. Liu and J.-W. Sun, J. Org. Chem., 2012, 77, 1191–1197 CrossRef CAS PubMed
.
- A. Citterio, M. Fochi, A. Marion, A. Mele, R. Sebastiano and M. Delcanale, Heterocycles, 1998, 48, 1993–2002 CrossRef CAS
.
- L.-Y. Xie, S. Peng, J.-X. Tan, R.-X. Sun, X.-Y. Yu, N.-N. Dai, Z.-L. Tang, X.-H. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2018, 6, 16976–16981 CrossRef CAS
.
- S. Peng, Y.-X. Song, J.-Y. He, S.-S. Tang, J.-X. Tan, Z. Cao, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019, 30, 2287–2290 CrossRef CAS
.
- W.-H. Bao, M. He, J.-T. Wang, X. Peng, M. Sung, Z.-L. Tang, S. Jiang, Z. Cao and W.-M. He, J. Org. Chem., 2019, 84, 6065–6071 CrossRef CAS PubMed
.
- P. Luo, A.-G. Chittiboyina, W.-G. Pan and W.-X. Wei, Z. Kristallogr. – New Cryst. Struct., 2020, 235, 565–567 CAS
.
- P. Luo, A.-G. Chittiboyina, M. Wang, I.-A. Khan, W.-G. Pan and W.-X. Wei, Z. Kristallogr. – New Cryst. Struct., 2020, 235, 105–107 CAS
.
- A. Schmidt and M. Albrecht, Z. Naturforsch., B: J. Chem. Sci., 2008, 63, 465–472 CAS
.
- J.-P. Guthrie, J. Cossar, P.-A. Cullimore, N.-M. Kamkar and K.-F. Taylor, Can. J. Chem., 1983, 61, 2621–2626 CrossRef CAS
.
- J. Mustafa, S.-I. Khan, G.-Y. Ma, L.-A. Walker and I.-A. Khan, Lipids, 2004, 39, 167–172 CrossRef CAS PubMed
.
- I. Vermes, C. Haanen, H. Steffens-Nakken and C. Reutelingsperger, J. Immunol. Methods, 1995, 184, 39–51 CrossRef CAS
.
- J. Wu, Y. Ding, C.-H.-Z. Chen, Z.-M. Zhou, C.-Y. Ding, H.-Y. Chen, J. Zhou and C.-S. Chen, Cancer Lett., 2016, 380, 393–402 CrossRef CAS PubMed
.
- Y.-J. Kong, F.-B. Li, Y. Nian, Z.-M. Zhou, R.-X. Yang, M.-H. Qiu and C.-S. Chen, Theranostics, 2016, 6, 875–886 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra, figures of apoptosis analysis and western blotting analysis. See DOI: 10.1039/d0ra06043g |
| This journal is © The Royal Society of Chemistry 2020 |