Open Access Article
Open Access ArticleSynthesis of 2D cobalt oxide nanosheets using a room temperature liquid metal†
Jessica Crawfordab,
Aidan Cowmanb and
Anthony P. O'Mullane *ab
*ab
aSchool of Chemistry and Physics, Queensland University of Technology (QUT), Brisbane, QLD 4001, Australia. E-mail: anthony.omullane@qut.edu.au
bCentre for Materials Science, Queensland University of Technology (QUT), Brisbane, QLD 4001, Australia
First published on 11th August 2020
Abstract
Room temperature liquid metals based on Ga can be used as a synthesis medium for the creation of metal oxide nanomaterials, however one thermodynamic limitation is that metals that are more easily oxidised than Ga are required to ensure their preferential formation. In this work we demonstrate a proof of principle approach whereby exposing the liquid metal alloyed with the required metal to acidic conditions circumvents preferential formation of Ga2O3 and allows for the formation of the required 2D transition metal oxide nanosheets. The synthesis procedure is straightforward in that it only requires bubbling oxygen gas through the liquid metal alloy into a solution of 10 mM HCl. We show that the formation of thin nanosheets of ca. 1 nm in thickness of CoO is possible. The material is characterised using transmission electron microscopy, atomic force microscopy, X-ray photoelectron and Raman spectroscopy. The electrocatalytic activity of the CoO nanosheets was investigated for the oxygen evolution reaction where the nanosheet thickness was found to be a factor influencing the activity. This proof of principle offers a route to the possible formation of many other 2D transition metal oxides from metals that are less readily oxidised than Ga by taking advantage of the interesting properties of room temperature liquid metals.
Introduction
Galinstan is a eutectic alloy made from gallium, indium and tin with the ratio (Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68.5%, In
68.5%, In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21.5%, Sn
21.5%, Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%) that is a liquid at room temperature. Recently it has received significant attention due its physical and chemical properties and has been identified as an emerging material in nanotechnology1–3 while being studied for many applications including use as a conductive ink,4 stretchable and self-healing wires,5 soft matter circuitry,6 actuators,7–11 and energy storage.12 Recent work has shown that Ga based liquid metals can also be used as a solvent for the synthesis of a wide range of nanomaterials via chemical reactions that occur at the surface of the liquid metal under a variety of conditions including galvanic exchange at the surface of large liquid metal droplets or under sonication conditions which leads to composites containing gold that are catalytically active,13 metal oxides such as MnO2 that have photocatalytic properties,14 Prussian blue nanomaterials,15 as well as the ability to polymerise materials around the surface of liquid metal droplets.16–18 One aspect that always needs to be considered when using galinstan, EGaIn or Ga in the liquid form is that when these materials are exposed to an oxygen containing atmosphere they spontaneously form a self limiting ∼0.7 nm layer of Ga2O3 around the outside of the droplet, even when containing additional In or Sn components.19 This phenomenon can be explained by calculation of the Gibbs free energy of formation (ΔGf) of gallium oxide which is much lower than In2O3 or SnO2 which therefore results in preferential formation of Ga2O3 at the surface. This phenomenon is also evident when other transition metals are incorporated in liquid metal galinstan such as Gd, Al or Hf. The Gibbs free energy of formation for Gd2O3 (−1732.3 kJ mol−1), Al2O3 (−1582.3 kJ mol−1) and HfO2 (−1088.2 kJ mol−1) are lower than Ga2O3 (−998.3 kJ mol−1)20 which implies that these oxides would preferentially form over Ga2O3 to ensure the maximum reduction in the Gibb's free energy of the system. This was exploited in previous work where Gd2O3, Al2O3 and HfO2 were expelled from the surface of galinstan into water via purging oxygen gas through a reservoir of liquid metal that contained the relevant metal to form atomically thin nanosheets.21 With this approach many 2D transition metal oxide materials can in principle be produced as long as the incorporated metal is more readily oxidised than Ga.
10%) that is a liquid at room temperature. Recently it has received significant attention due its physical and chemical properties and has been identified as an emerging material in nanotechnology1–3 while being studied for many applications including use as a conductive ink,4 stretchable and self-healing wires,5 soft matter circuitry,6 actuators,7–11 and energy storage.12 Recent work has shown that Ga based liquid metals can also be used as a solvent for the synthesis of a wide range of nanomaterials via chemical reactions that occur at the surface of the liquid metal under a variety of conditions including galvanic exchange at the surface of large liquid metal droplets or under sonication conditions which leads to composites containing gold that are catalytically active,13 metal oxides such as MnO2 that have photocatalytic properties,14 Prussian blue nanomaterials,15 as well as the ability to polymerise materials around the surface of liquid metal droplets.16–18 One aspect that always needs to be considered when using galinstan, EGaIn or Ga in the liquid form is that when these materials are exposed to an oxygen containing atmosphere they spontaneously form a self limiting ∼0.7 nm layer of Ga2O3 around the outside of the droplet, even when containing additional In or Sn components.19 This phenomenon can be explained by calculation of the Gibbs free energy of formation (ΔGf) of gallium oxide which is much lower than In2O3 or SnO2 which therefore results in preferential formation of Ga2O3 at the surface. This phenomenon is also evident when other transition metals are incorporated in liquid metal galinstan such as Gd, Al or Hf. The Gibbs free energy of formation for Gd2O3 (−1732.3 kJ mol−1), Al2O3 (−1582.3 kJ mol−1) and HfO2 (−1088.2 kJ mol−1) are lower than Ga2O3 (−998.3 kJ mol−1)20 which implies that these oxides would preferentially form over Ga2O3 to ensure the maximum reduction in the Gibb's free energy of the system. This was exploited in previous work where Gd2O3, Al2O3 and HfO2 were expelled from the surface of galinstan into water via purging oxygen gas through a reservoir of liquid metal that contained the relevant metal to form atomically thin nanosheets.21 With this approach many 2D transition metal oxide materials can in principle be produced as long as the incorporated metal is more readily oxidised than Ga.
Nanostructured transition metal oxides have shown excellent activity for many electrocatalytic reactions including those associated with water splitting, i.e. the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). The OER has received particular attention given the sluggish kinetics of the process which limits overall performance for electrochemical water splitting. Many transition metal oxides based on Fe, Co, Ni and Mn have been studied for alkaline electrolysis and several comprehensive reviews are available in this area.22–26 The realisation of ultrathin nanosheets of such oxides is attractive for exposing edge sites and increasing the amount of active sites available for the reaction which is highly important in the development of new electrolysers for the generation of green hydrogen. At present there is an urgent need to replace currently used expensive precious metal oxide electrocatalysts and promisingly previous work has shown that cobalt oxide/hydroxide materials in particular show activity for the sluggish OER.27–32
In this work we extend the applicability of nanosheet formation from Ga based liquid metals by producing 2D nanosheets of a material that is unfavoured, namely oxidised Co (ΔGf for CoO is −214.2 kJ mol−1 which is significantly higher than that for Ga2O3), via control of the solution pH into which the material is ejected. This material is then investigated for the OER under alkaline conditions.
Experimental
Materials
Cobalt powder 2 μm particle size (99.8%), Galinstan (gallium (68.5%) indium (21.5%) and tin (10%)) eutectic alloy and hydrochloric acid 37% were purchased from Sigma Aldrich. NaOH was purchased from Chem Supply. Ultra High Purity Oxygen was purchased from Supagas. Lacey Carbon copper coated TEM grids and silicon wafers were purchased from Ted Pella.Methods
| ERHE = EAg/AgCl + 0.059 × pH + E0Ag/AgCl V. |
Samples were imaged using a Zeiss Sigma Scanning Electron Microscope. A JEOL 2100 transmission electron microscope was also used where the material was drop cast onto a lacey carbon copper TEM grid. Images were taken at 200 kV using diffraction mode with a selected area aperture to take diffraction patterns. A Bruker Atomic Force Microscope (AFM) using ScanAsyst in air method in contact mode was used to measure the thickness of samples. Gwyddion AFM analysis software was used to treat images and analyse all thicknesses. For every thickness measurement the mean from the low area and high area was taken and the difference calculated and rounded to 1 decimal point.
Results and discussion
The method used to produce 2D nanosheets from a liquid metal precursor is simple to set up and consists of an inlet tube allowing oxygen gas to flow through a reservoir of liquid Galinstan that is mixed beforehand with Co metal (0.5 to 1.5 wt%) for 30 min. The oxygen gas flows through the liquid metal causing disruption of the surface and expulsion of the outermost layers into an aqueous solution as shown in Fig. 1.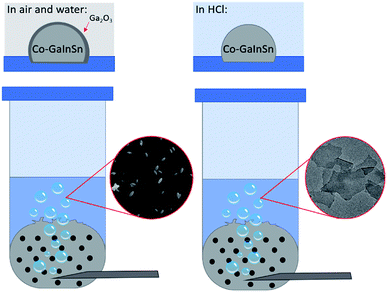 | ||
| Fig. 1 The gas injection method showing Co–GaInSn in water forming gallium oxide and Co–GaInSn in hydrochloric acid forming cobalt oxide nanosheets. | ||
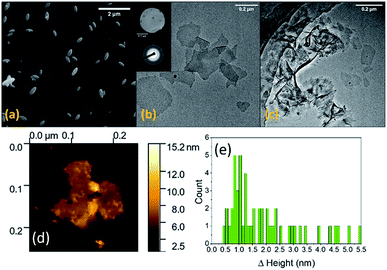
Our initial experiments were conducted with MilliQ water as the solvent using 0.5 wt% Co in Galinstan. As anticipated we did not observe the formation of any Co containing species but saw an abundance of ellipsoid shaped particles generated in water when imaged by SEM (Fig. 2a) which are consistent with the formation of Ga oxides.33–35 It is known that gallium oxides are soluble in acidic solution36 and therefore the pH of the solution was controlled via the addition of HCl at various concentrations to minimise its formation. When the solution was acidified using 10 mM HCl there was a clear change in the morphology of the material ejected from the liquid metal (Fig. 2b and c). The formation of thin nanosheets can be observed (Fig. 2b) with some areas showing multiple sheets on top of each other or folded sheets (Fig. 2c). It should be noted that the sample was allowed to settle prior to analysis to remove any residual droplets that may have formed in the supernatant.
Electron diffraction measurements indicated that the material is amorphous (Fig. 2b inset). The individual sheets can be up to 500 nm in length whereas the folded sheets are larger. The thickness of the sheets was measured by atomic force microscopy (AFM) where an individual sheet is shown in Fig. 2d. The thickness of 50 different sheets was then measured and the histogram showing the distribution in thickness values (Fig. 2e) indicates that the sheets show that a high proportion are ca. 1 nm thick, indicating 2D nanosheet formation, with very few sheets as thick as 4.5 nm. The latter observation is due to the presence of sheets on top of each other or folded sheets as seen in the TEM images (Fig. 2c).
Raman spectroscopy was then undertaken and a typical Raman spectrum of the 2D nanosheets is shown in Fig. 3a. The peaks at (195.7, 619.7), 484.3 and 691.8 cm−1 can be assigned to the F2g, Eg and A1g active modes of CoO respectively.37 The intense sharp peaks at 516.1 and 526.6 cm−1 are due to the underlying Si substrate that was used for AFM imaging purposes. X-ray photoelectron spectroscopy (XPS) analysis confirmed the formation of CoO. The Co 2p spectrum (Fig. 3b) can be fitted to the Co2+ oxidation state while the spin orbit splitting of 15.5 eV is also indicative of Co2+.38 The O 1s spectrum shows a peak at 531.8 eV which is generally consistent with the presence of hydroxide groups,39 however there have been many reports which show that CoO nanomaterials exhibit a O 1s peak at a higher binding energy of 531.2 eV.40,41 In particular it has been reported that this higher binding energy peak for CoO compared to that normally seen for lattice oxygen is due to a large number of defects which is encountered in very thin films.40 It should also be noted that there was no evidence of gallium in the sample when analysed using XPS.
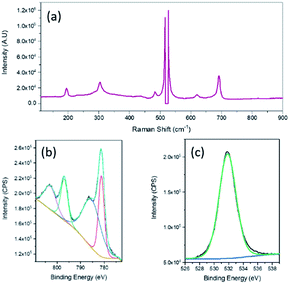 | ||
| Fig. 3 (a) Raman spectrum, XPS spectra of nanosheets (b) Co 2p and (c) O 1s fabricated from 0.5% Co–GaInSn exposed to 10 mM HCl. | ||
Therefore the use of 10 mM HCl was successful in minimising the formation of gallium oxide while allowing for the oxidation of metallic Co to occur which resulted in the generation of 2D nanosheets of CoO with a thickness of ca. 1 nm that contain a significant number of defects and is denoted as CoOx. This value is consistent with the ultrathin nature of nanosheets that can be produced using a similar approach from metals alloyed with galinstan reported previously that included the much more reactive metals of Gd, Hf and Al which all produced nanosheets less than 1.1 nm in thickness,21 and Ti with nanosheets of 2 nm thickness.42 Even though thermodynamically the oxidation of Ga is preferred, as ΔGf of CoO is much higher at −214.2 kJ mol−1, the presence of 10 mM HCl results in the dissolution of any gallium oxides that are formed which subsequently promotes CoOx formation. The conditions are also appropriate for the existence of CoOx in this solution as previous work has shown that CoO only begins to dissolve to a significant extent at concentration of 0.5 M HCl.43 Therefore the mechanism of formation is due to oxygen bubbles travelling rapidly through the liquid metal causing rapid interfacial oxidation at the air/liquid metal/electrolyte interface due to the Cabrera–Mott oxidation process which limits the growth of the oxide layer at the Co–GaInSn surface to the several Å to nm scale. The nanosheets are then ejected into solution where any Ga2O3 that would have formed dissolves allowing for the accumulation of CoOx nanosheets in solution. The concentration of HCl is enough to dissolve any Ga2O3 but allows for CoOx to exist stably in solution. This will result in some consumption of the Ga component, however the liberated Ga3+ ions that are generated can be in principle be recovered via electrochemical reduction to Ga.44 An advantage of this approach is that it can be done in a single step in a relatively straightforward manner. Other approaches to creating isolated nanosheets of 2D metal oxides require layered materials to be initially synthesised followed by various exfoliation methods which can produce polydispersity in sheet thickness. Another report has shown that indium tin oxide nanosheets can be printed from a liquid InSn alloy held at 200 °C via sandwiching the alloy between two substrates which when removed results in ITO nanosheets deposited on the top substrate.45 The method presented here produces a high proportion of very thin nanosheets in a relatively straightforward process at room temperature.
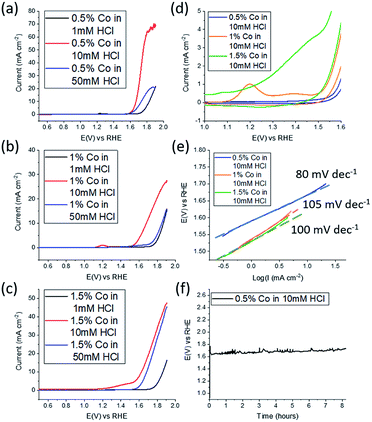
Electrochemical water splitting in alkaline conditions using various types of cobalt oxides/hydroxides, have been studied previously at a variety of morphologies, including nanosheets, however they have been significantly thicker than reported here at around 6 monolayers per sheet for Co(OH)2.31 Therefore the 2D CoOx nanosheets fabricated here were studied for the OER in 0.1 M NaOH. As mentioned previously the effect of the pH of the solution into which the material is ejected is expected to play a critical role. Therefore 1 mM, 10 mM and 50 mM HCl solutions were used to synthesise CoOx which was then investigated for the OER (Fig. 4a). From this data the material synthesised from 10 mM HCl shows the best performance in terms of current density where values as high as 70 mA cm−2 were reached. For the 1 mM HCl solution the formation of gallium oxide is still likely to occur inhibiting the extensive formation of CoOx while for the 50 mM HCl solution dissolution of CoOx will be a complicating factor, which results in poor performance due to a lack of material. The amount of Co in the Co–GaInSn mixture was then increased to 1 wt% (Fig. 4b) and 1.5 wt% (Fig. 4c) as a means to increase OER performance. However, in both cases inferior OER current densities were attained compared to 0.5 wt% Co although a slightly better onset potential was achieved for the 1.5 wt% sample. It was also found that the 10 mM HCl solution used in the synthesis was the optimum solution at all weights of Co that was used.
As the 10 mM HCl solution provided the best results, the different weights of Co that were used were compared under these conditions (Fig. 4d). Here the redox processes for CoOx prior to the onset of the OER are shown. Over the potential range of 1.0 to 1.5 V vs. RHE the magnitude of the redox process increases upon increasing the Co content used for the synthesis. The peak that is evident at 1.2 V is due to the oxidation of CoO into CoOOH which is followed by a broader peak at 1.38 V attributed to the oxidation of CoOOH into CoO2 where the Co4+ species is regarded as the active component for the OER.46,47 From this data it is seen that upon increasing the Co content the magnitude of these redox processes increases prior to the OER, which indicates the formation of more CoOx, however this does not translate into improved activity.
This is also reflected in the Tafel slope values for the 0.5, 1.0 and 1.5 wt% Co samples which were determined to be 80, 105 and 100 mV dec−1 respectively. Therefore, the optimised conditions are gas injection into a Galinstan droplet containing 0.5 wt% Co immersed in a 10 mM HCl solution. It was also found that after 30 min of oxygen gas bubbling that the expulsion of CoOx nanosheets ceased. The stability of these 2D nanosheets was then investigated at a constant current of 10 mA cm−2 where a consistent potential of 1.62 V was maintained for 8 h. This overpotential value of 390 mV is comparable to liquid exfoliated layered Co(OH)2 that was ultrasonicated in aqueous surfactant solution for 4 h which gave an overpotential value of 440 mV.31 It should be noted that the inherent activity of Co(OH)2 is lower than many other OER electrocatalysts but can be improved by modifying the surface chemistry to carboxylate groups,48 incorporating S atoms,49 doping with Fe,50 adding graphene51 or gold nanoparticles32 which provides better activity than reported here. However in terms of the less studied CoOx material, the performance is slightly better than CoO nanofibers52 in terms of overpotential at 10 mA cm−2 and comparable to previous reports of CoO nanomaterials which were investigated in a more concentrated alkaline electrolyte of 1 M KOH, compared to 0.1 M used here, which gave values of 400 mV for both nanoparticles53 and nanoplates.54 Again the activity of CoO can be improved via doping with a second metal such as Fe52 and Zn54 but this was not the goal of the current study.
Finally, the better performance of the 0.5 wt% Co sample is attributed to the thin nature of the nanosheets that in principle exposes more active sites for the OER. Therefore, to investigate this phenomenon the effect of sheet thickness was investigated. It was found that if the 2D CoOx nanosheet suspension was allowed to age for 1 week then the thickness of the nanosheets increased to a range of 2.5 to 4 nm as seen from AFM images and the corresponding histogram for sheet thickness (Fig. S1 and S2†). The electrochemically active surface area (ECSA) was determined using background capacitive measurements (Fig. S3†) where the value decreased by over a factor of 4 from 0.33 mF cm−2 to 0.073 mF cm−2 which is a result of the increased sheet thickness. This is reflected in a decrease in the OER activity where the OER current density reduced to a value of 25 mA cm−2 at 1.70 V (Fig. S4†). This observation is consistent with previous reports showing that increasing the thickness of 2D transition metal oxides decreases electrocatalytic activity.31
Conclusions
This study has demonstrated that 2D metal oxide expulsion from the room temperature liquid metal Galinstan is possible even if thermodynamically the formation of gallium oxide is preferred. By controlling the solution pH into which the material is ejected any gallium oxide formed is dissolved allowing for the formation of oxidised Co. Under these conditions the speciation of Co was found to be CoOx which was ejected into a 10 mM HCl solution in the form of thin 2D nanosheets of ca. 1 nm thickness with a length of ca. 200 nm. The 2D CoOx nanosheets were found to be electrocatalytically active for the OER under alkaline conditions to give a current density of 10 mA cm−2 at an overpotential of 390 mV with no loss in activity for an electrolysis period of 8 h. It is emphasised here that the goal of this study was not to produce the most active OER electrocatalyst, but to demonstrate a proof of principle that 2D metal oxide nanosheets can be produced that are thermodynamically unfavoured using this simple approach and could be useful for applications other than electrocatalysis. In essence this could be a route to open up many other elements in the periodic table that are difficult to fabricate into nanosheets by taking advantage of the self-limiting oxide formation process that is accessible in room temperature liquid metals.Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Kalantar-Zadeh, J. Tang, T. Daeneke, A. P. O'Mullane, L. A. Stewart, J. Liu, C. Majidi, R. S. Ruoff, P. S. Weiss and M. D. Dickey, ACS Nano, 2019, 13, 7388–7395 CrossRef CAS PubMed.
- Q. Wang, Y. Yu and J. Liu, Adv. Eng. Mater., 2017, 20, 1700781 CrossRef.
- T. Daeneke, K. Khoshmanesh, N. Mahmood, I. A. de Castro, D. Esrafilzadeh, S. J. Barrow, M. D. Dickey and K. Kalantar-zadeh, Chem. Soc. Rev., 2018, 47, 4073–4111 RSC.
- Y. Gao, H. Li and J. Liu, PLoS One, 2012, 7, e45485 CrossRef CAS PubMed.
- M. D. Dickey, Adv. Mater., 2017, 29, 1606425 CrossRef PubMed.
- H.-J. Koo, J.-H. So, M. D. Dickey and O. D. Velev, Adv. Mater., 2011, 23, 3559–3564 CrossRef CAS PubMed.
- M. F. Wang, M. J. Jin, X. J. Jin and S. G. Zuo, Phys. Chem. Chem. Phys., 2017, 19, 18505–18513 RSC.
- K. Khoshmanesh, S.-Y. Tang, J. Y. Zhu, S. Schaefer, A. Mitchell, K. Kalantar-zadeh and M. D. Dickey, Lab Chip, 2017, 17, 974–993 RSC.
- J. Zhang, Y. Yao, L. Sheng and J. Liu, Adv. Mater., 2015, 27, 2648–2655 CrossRef CAS PubMed.
- S.-Y. Tang, K. Khoshmanesh, V. Sivan, P. Petersen, A. P. O'Mullane, D. Abbott, A. Mitchell and K. Kalantar-zadeh, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3304–3309 CrossRef CAS PubMed.
- S. Holcomb, M. Brothers, A. Diebold, W. Thatcher, D. Mast, C. Tabor and J. Heikenfeld, Langmuir, 2016, 32, 12656–12663 CrossRef CAS PubMed.
- X. Guo, L. Zhang, Y. Ding, J. B. Goodenough and G. Yu, Energy Environ. Sci., 2019, 12, 2605–2619 RSC.
- F. Hoshyargar, J. Crawford and A. P. O'Mullane, J. Am. Chem. Soc., 2017, 139, 1464–1471 CrossRef CAS PubMed.
- M. B. Ghasemian, M. Mayyas, S. A. Idrus-Saidi, M. A. Jamal, J. Yang, S. S. Mofarah, E. Adabifiroozjaei, J. Tang, N. Syed, A. P. O'Mullane, T. Daeneke and K. Kalantar-Zadeh, Adv. Funct. Mater., 2019, 29, 1901649 CrossRef.
- B. Lertanantawong, P. Lertsathitphong and A. P. O'Mullane, Electrochem. Commun., 2018, 93, 15–19 CrossRef CAS.
- C. Zhang, F.-M. Allioux, M. A. Rahim, J. Han, J. Tang, M. B. Ghasemian, S.-Y. Tang, M. Mayyas, T. Daeneke, P. Le-Clech, R. B. Kaner, D. Esrafilzadeh and K. Kalantar-Zadeh, Chem. Mater., 2020, 32, 4808–4819 CrossRef CAS.
- Y. Liu, J. Li and W. Zhang, Chem. Commun., 2020, 56, 6229–6232 RSC.
- J. Ma, Y. Lin, Y.-W. Kim, Y. Ko, J. Kim, K. H. Oh, J.-Y. Sun, C. B. Gorman, M. A. Voinov, A. I. Smirnov, J. Genzer and M. D. Dickey, ACS Macro Lett., 2019, 8, 1522–1527 CrossRef CAS.
- M. D. Dickey, ACS Appl. Mater. Interfaces, 2014, 6, 18369–18379 CrossRef CAS PubMed.
- R. A. Robie, B. S. Hemingway and J. R. Fisher, Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 pascals) pressure and at higher temperatures, United States Government Printing Office, Washington, 1978 Search PubMed.
- A. Zavabeti, J. Z. Ou, B. J. Carey, N. Syed, R. Orrell-Trigg, E. L. H. Mayes, C. Xu, O. Kavehei, A. P. O'Mullane, R. B. Kaner, K. Kalantar-zadeh and T. Daeneke, Science, 2017, 358, 332–335 CrossRef CAS PubMed.
- I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem., 2017, 1, 0003 CrossRef CAS.
- X. Li, X. Hao, A. Abudula and G. Guan, J. Mater. Chem. A, 2016, 4, 11973–12000 RSC.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- N. Cox, D. A. Pantazis, F. Neese and W. Lubitz, Interface Focus, 2015, 5, 20150009 CrossRef PubMed.
- O. M. Anthony Peter, J. Phys.: Energy, 2020 DOI:10.1088/2515-7655/ab8c5f.
- G. Liu, S. K. Karuturi, A. N. Simonov, M. Fekete, H. Chen, N. Nasiri, N. H. Le, P. Reddy Narangari, M. Lysevych, T. R. Gengenbach, A. Lowe, H. H. Tan, C. Jagadish, L. Spiccia and A. Tricoli, Adv. Energy Mater., 2016, 6, 1600697 CrossRef.
- A. Bergmann, E. Martinez-Moreno, D. Teschner, P. Chernev, M. Gliech, J. F. de Araujo, T. Reier, H. Dau and P. Strasser, Nat. Commun., 2015, 6, 8625 CrossRef CAS PubMed.
- Y.-C. Liu, J. A. Koza and J. A. Switzer, Electrochim. Acta, 2014, 140, 359–365 CrossRef CAS.
- B. H. R. Suryanto, X. Lu and C. Zhao, J. Mater. Chem. A, 2013, 1, 12726–12731 RSC.
- D. McAteer, I. J. Godwin, Z. Ling, A. Harvey, L. He, C. S. Boland, V. Vega-Mayoral, B. Szydłowska, A. A. Rovetta, C. Backes, J. B. Boland, X. Chen, M. E. G. Lyons and J. N. Coleman, Adv. Energy Mater., 2018, 8, 1702965 CrossRef.
- M. A. Sayeed and A. P. O'Mullane, J. Mater. Chem. A, 2017, 5, 23776–23784 RSC.
- B. Lertanantawong, J. D. Riches and A. P. O'Mullane, Langmuir, 2018, 34, 7604–7611 CrossRef CAS PubMed.
- C.-C. Huang and C.-S. Yeh, New J. Chem., 2010, 34, 103–107 RSC.
- L. S. Reddy, Y. H. Ko and J. S. Yu, Nanoscale Res. Lett., 2015, 10, 364 CrossRef PubMed.
- P. Benézéth, I. I. Diakonov, G. S. Pokrovski, J.-L. Dandurand, J. Schott and I. L. Khodakovsky, Geochim. Cosmochim. Acta, 1997, 61, 1345–1357 CrossRef.
- J. S. Gwag and Y.-K. Sohn, Bull. Korean Chem. Soc., 2012, 33, 505–510 CrossRef CAS.
- M. M. Alsabban, X. Yang, W. Wahyudi, J.-H. Fu, M. N. Hedhili, J. Ming, C.-W. Yang, M. A. Nadeem, H. Idriss, Z. Lai, L.-J. Li, V. Tung and K.-W. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 20752–20761 CrossRef CAS PubMed.
- H. B. Li, M. H. Yu, X. H. Lu, P. Liu, Y. Liang, J. Xiao, Y. X. Tong and G. W. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 745–749 CrossRef CAS PubMed.
- S. C. Petitto, E. M. Marsh, G. A. Carson and M. A. Langell, J. Mol. Catal. A: Chem., 2008, 281, 49–58 CrossRef CAS.
- Y. Tang, L. Dong, S. Mao, H. Gu, T. Malkoske and B. Chen, ACS Appl. Energy Mater., 2018, 1, 2698–2708 CrossRef CAS.
- T. Alkathiri, N. Dhar, A. Jannat, N. Syed, M. Mohiuddin, M. M. Y. A. Alsaif, R. S. Datta, K. A. Messalea, B. Y. Zhang, M. W. Khan, A. Elbourne, N. Pillai, J. Z. Ou, A. Zavabeti and T. Daeneke, Chem. Commun., 2020, 56, 4914–4917 RSC.
- I. Boukerche, N. Habbache, N. Alane, S. Djerad and L. Tifouti, Ind. Eng. Chem. Res., 2010, 49, 6514–6520 CrossRef CAS.
- D. O. Flamini, S. B. Saidman and J. B. Bessone, J. Appl. Electrochem., 2007, 37, 467–471 CrossRef CAS.
- R. S. Datta, N. Syed, A. Zavabeti, A. Jannat, M. Mohiuddin, M. Rokunuzzaman, B. Yue Zhang, M. A. Rahman, P. Atkin, K. A. Messalea, M. B. Ghasemian, E. D. Gaspera, S. Bhattacharyya, M. S. Fuhrer, S. P. Russo, C. F. McConville, D. Esrafilzadeh, K. Kalantar-Zadeh and T. Daeneke, Nature Electronics, 2020, 3, 51–58 CrossRef CAS.
- M. A. Sayeed, T. Herd and A. P. O'Mullane, J. Mater. Chem. A, 2016, 4, 991–999 RSC.
- L. D. Burke, M. E. Lyons and O. J. Murphy, J. Electroanal. Chem. Interfacial Electrochem., 1982, 132, 247–261 CrossRef CAS.
- C. Qiao, S. Rafai, T. Cao, Z. Wang, H. Wang, Y. Zhu, X. Ma, P. Xu and C. Cao, ChemCatChem, 2020, 12, 2823–2832 CrossRef CAS.
- H. Zhang, B. Chen, H. Jiang, X. Duan, Y. Zhu and C. Li, Nanoscale, 2018, 10, 12991–12996 RSC.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed.
- R. Mehmood, N. Tariq, M. Zaheer, F. Bibi and Z. Iqbal, Sci. Rep., 2018, 8, 13772 CrossRef PubMed.
- W. Li, M. Li, C. Wang, Y. Wei and X. Lu, Appl. Surf. Sci., 2020, 506, 144680 CrossRef.
- Z.-J. Jiang and Z. Jiang, Sci. Rep., 2016, 6, 27081 CrossRef CAS PubMed.
- M. Huo, Z. Yang, C. Yang, Z. Gao, J. Qi, Z. Liang, K. Liu, H. Chen, H. Zheng and R. Cao, ChemCatChem, 2019, 11, 1480–1486 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: AFM images, OER activity of aged samples, electrochemical surface area measurements. See DOI: 10.1039/d0ra06010k |
| This journal is © The Royal Society of Chemistry 2020 |