Open Access Article
Open Access ArticleChinese chive and Mongolian leek suppress heterocyclic amine formation and enhance nutritional profile of roasted cod†
Qi Wangabc,
Yifeng Zhanga,
Yuanyuan Renabc,
Weiwei Cheng bc,
Yuge Bia,
Feng Chen
bc,
Yuge Bia,
Feng Chen bc and
Ka-Wing Cheng
bc and
Ka-Wing Cheng *bc
*bc
aInstitute for Food and Bioresource Engineering, College of Engineering, Peking University, Beijing, 100871, China
bShenzhen Key Laboratory of Marine Microbiome Engineering, Institute for Advanced Study, Shenzhen University, Shenzhen 518060, China. E-mail: kwcheng@szu.edu.cn
cInstitute for Innovative Development of Food Industry, Shenzhen University, Shenzhen 518060, China
First published on 22nd September 2020
Abstract
Heterocyclic amines (HAs) are potent mutagens, which can form DNA adducts in various human tissues. There is increasing evidence that mutagenic HA formation and nutrition loss can occur concurrently in fish during vigorous heat treatment. Our study investigated the effects of five Allium spp. (garlic, onion, welsh onion, Chinese chive, and Mongolian leek) on reducing HA formation and improving nutritional quality of roasted cod (Gadus morhua). The results showed that cod patties pretreated with powders of the selected Allium spp. had significantly (P < 0.05) lower levels of HAs (82–92%, except garlic, 49%) than the control. The contents of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the patties exhibited strong negative correlations with total antioxidant activity (−0.937), phenolic (−0.948), and lipophilic flavonoid (−0.933) contents, whereas the 2-amino-3,8 dimethylimidazo [4,5-f] quinoxaline (MeIQx) (made up only ∼0.7–3% of total HAs) contents exhibited significant positive correlations with these antioxidant parameters. In terms of nutrient composition change, Chinese chive and Mongolian leek were the most effective in preventing oxidative degradation of proteins and unsaturated fatty acids in roasted cod patties, which was translated into significantly higher contents of soluble proteins, essential amino acids, and polyunsaturated fatty acids. This has been the first report on the strong HA-formation inhibitory effect of Chinese chive and Mongolian leek. The dual beneficial functionality of these two Allium spp. may be utilized to reduce the intake of hazardous by-products while enhancing the nutritional and antioxidant properties of roasted cod and probably other protein-rich heat-processed foods.
1. Introduction
Cod (Gadus morhua) is one of the most popular seafood dishes worldwide. It has dense, flaky and white flesh with low fat content and a balanced protein profile. Some of its proteins could be a source of bioactive peptides that possess functional properties such as antioxidation and inhibition of angiotensin converting enzyme.1,2 Meanwhile, these nutritional and potentially health promoting attributes could be lost during the process of heat treatment, which causes oxidative degradation of proteins, fatty acids (especially polyunsaturated fatty acids, PUFAs) and other nutrients.3–7 Concurrently, heat-induced reactions among food components can also give rise to harmful compounds.8 Heterocyclic amines (HAs) are one of the most important categories of these hazardous by-products in muscle-based foods including meat and fish.9 More than two dozen HAs have been identified in thermally processed foods. Some of them, such as 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,8 dimethylimidazo [4,5-f] quinoxaline (MeIQx) and 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxalin (4,8-DiMeIQx), are classified as possible human carcinogens by the International Agency for Research on Cancer.10 Many reports indicate a dose–dependent relationship between HA intake and DNA adduct formation that may increase the risk of various cancers, especially liver, colon and prostate cancers.11,12 Considering their wide occurrence in major dietary components, HAs represent a significant health risk in the long term.12 Hence, it is of high priority to inhibit HA formation in order to attenuate the health risk due to dietary exposure, while preserving or improving the nutritional quality of the associated food products. HA contents in foods can be reduced by lowering heating temperature, reducing concentrations of HA precursors and/or using alternative heating methods known to generate less HAs.13,14 The use of plant-based extracts and phytochemicals as additives or seasoning ingredients have also gained increasing attention and ample evidences suggest it to be one of the most promising approaches to attenuate HA-associated health risk.15–17Members of the Allium genus are common culinary ingredients in many parts of the world. Garlic (Allium sativum) and onion (Allium cepa) have been the most extensively studied Allium species both as seasoning ingredients and as modulators of undesirable heat-induced reactions in foods. For example, they were reported to effectively lower the content of HAs in pan-fried pork patties and in deep-fried meatballs.18,19 Garlic powders were also reported to inhibit acrylamide formation in a low-moisture chemical model and in baked bread.20 Organosulfur compounds have been proposed to be major bioactive compounds in Allium plants.21 Some organosulfur compounds, such as γ-glutamyl-S-allyl-cysteine peptide isolated from garlic, showed antiglycative activity in a bovine serum albumin-glucose model.22 Meanwhile, accumulating evidence indicates that polyphenols, in particular flavonoids are another important class of bioactive constituents in Allium spp.23–25 Lu and coworkers recently reported a significant negative correlation (coefficient (r) = −0.712 to − 0.853) between antioxidant capacity of spices (including garlic, onion, red chilli, paprika, ginger and black pepper powder) and total HA contents, which indicated that antioxidants in these spices played a significant role in inhibiting the formation of HAs during heating.19 However, no further data was provided regarding the type of phytochemicals which were likely the principal inhibitors.
The distinctive flavor and aroma and rich phytochemical contents have been the major impetus for the increasing research interest in Allium spp. both as functional food ingredients and as modulators of sensory attributes in foods. Nonetheless, this genus remains underexplored, especially with regard to their potential for simultaneous improvement of nutrition profiles and mitigation of the formation of heat-induced toxicants in foods. The hypothesis of the present study was that pretreatment with powders of certain Allium spp. could decrease the content of mutagenic HAs and enhance the nutritional and antioxidant properties of roasted cod. Herein, the effects of powders of five Allium spp., garlic, onion, welsh onion (Allium fistulosum), Chinese chive (Allium tuberosum), and Mongolian leek (Allium mongolicum Regel) on the formation of major HAs (PhIP, MeIQx, and DiMeIQx), and on the changes in the profiles of major nutrients (proteins/amino acids, and fatty acids) in roasted cod patties were evaluated. Antioxidant activity, phenolic and flavonoid contents (total, lipophilic and hydrophilic) were also evaluated to better understand their relationships with the modulating effects of the selected Allium spp. on HA formation and nutrient composition changes.
2. Materials and methods
2.1 Chemicals and reagents
HA standards, PhIP, MeIQx, and 4,8-DiMeIQx were purchased from Toronto Research Chemicals (Toronto, Canada). Oasis MCX cartridges (500 mg) were from Waters Inc. (Milford, MA, USA). Amino acid standards, fatty acid methyl ester standards, Bond-Elut reservoirs, diatomaceous earth, Folin–Ciocalteu reagent, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,4,6-tripyridyl-s-triazine (TPTZ), and quercetin were from Sigma-Aldrich (St Louis, MO, USA). Mass spectrometry grade methanol, acetonitrile, ammonium acetate, and formic acid were purchased from Merck KGaA (Darmstadt, Germany). Amino acid test kit (MSLAB-45+AA) was from Beijing Mass Spectrometry Medical Research Co., Ltd. (Beijing, China). Aluminum chloride (AlCl3), sodium carbonate and other general reagents were from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).2.2 Preparation of Allium powders
Five fresh Allium spp. (garlic, red onion, green welsh onion, Chinese chive, and Mongolian leek) were purchased in four batches (one in each season) from a local supermarket (Beijing, China) to minimize seasonal variation of the samples. After cleaning and drying (∼4 h) to constant weight in an oven set at 95 °C, they were pulverized in a grinder (M 20, IKA, Germany). Powders of the four batches of each Allium spp. were mixed thoroughly and stored in a desiccator.2.3 Roasted cod sample preparation
Cod fillets were obtained from a local supermarket (Beijing, China). Fillet samples (∼120 g each; dimensions: 7.5 ± 0.5, 10.5 ± 1.0, and 2.0 ± 0.2 cm) were boned. For every batch of experiments, 100 g of minced cod was weighed for each treatment, which was mixed with 2 g (2%, w/w) of garlic, onion, welsh onion, Chinese chive, or Mongolian leek powders, respectively. The control group was not given any pretreatment. After marinating for an hour at 4 °C, the fillets were homogenized in a blender separately and four accurately weighed (30 ± 0.2 g) samples from each treatment were formed into patties with the aid of a Petri dish (diameter 6 cm, height 1.5 cm). The patties were roasted in a BAKLON-58M oven (Germany) at 225 °C for 10 min on each side. After roasting, each replicate was divided into two parts. One part was directly used for the analysis of HAs, and the other part was freeze-dried and stored at −80 °C until used for analysis of other endpoints.2.4 UPLC-MS analysis of HAs in roasted cod patties
HA content of roasted cod patties was evaluated according to the literature with slight modifications.26 Briefly, the patties from each treatment were homogenized in 150 mL of 1 M NaOH for 2 min to a dense paste. For each sample, 17.5 ± 0.1 g of the paste was mixed with 22.5 mL of 1 M NaOH in a beaker, and the mixture was sonicated for 20 min. The suspension was then mixed with diatomaceous earth and packed into empty reservoirs fitted with a bottom frit (20 μm). Eluate from the reservoir (200 mL ethyl acetate for each sample) was passed through an Oasis MCX cartridge, which was pre-conditioned sequentially with 6 mL of methanol, 6 mL of distilled water, and 6 mL of ethyl acetate. The cartridges were then dried under maximum vacuum for 5 min and washed with 6 mL of 0.1 N HCl, followed by 6 mL of methanol. The retained analytes were eluted with 6 mL of methanol–ammonia solution (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The eluate was evaporated to dryness under nitrogen, and the residue was re-dissolved in 300 μL of methanol for UPLC-MS analysis. Triplicate analyses were performed for each treatment.
1, v/v). The eluate was evaporated to dryness under nitrogen, and the residue was re-dissolved in 300 μL of methanol for UPLC-MS analysis. Triplicate analyses were performed for each treatment.
Identification and quantification of HAs were performed on a UPLC equipped with a triple quadrupole mass spectrometer (Waters, Milford, MA, USA) and an electrospray ionization interface. Separation of the analytes was carried out on an ACQUITY UPLC BEH C18 reversed-phase column (1.7 μm particle size, 2.1 mm × 50 mm, Waters Inc.; USA) at 35 °C. Gradient elution with a binary mobile phase (solvent A: 0.1% formic acid and 5 mM ammonium acetate in water; solvent B: acetonitrile) was used: 0–0.1 min, 95% A, 5% B; 0.1–10 min, 70% A, 30% B; 10–14 min, 40% A, 60% B; 14–16 min, 20% A, 80% B; 16–18 min, 95% A, 5% B; 18–23 min, 95%A, 5% B. The flow rate was set as 0.3 mL min−1, and the injection volume was 1 μL. Mass spectrometric detection was conducted in the multiple reaction monitoring (MRM) scan mode. The operating conditions were: capillary voltage 3.5 kV, ion source temperature 120 °C, and desolvation temperature 350 °C. Quantitative determination was performed using an external calibration curve. Correlation coefficients (r2) for HA standard curves were 0.9995 for PhIP, 0.9996 for MeIQx, and 0.9999 for 4,8-DiMeIQx.
2.5 Preparation of hydrophilic and lipophilic extracts from roasted cod patties
Hydrophilic and lipophilic extracts of the samples were prepared for the determination of phenolic and flavonoid contents, and total antioxidant activity according to the method of Manful et al. with minor modifications.5 Approximately 3 g of roasted cod samples were homogenized in 9 mL of sodium phosphate buffer (50 mM, pH 7.5). The homogenate was centrifuged (6000 rpm, 15 min) to obtain the supernatant as the hydrophilic extract. The residue was suspended in 9 mL of acetone/ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% v/v), vortexed, and incubated in darkness at room temperature for 30 min. The mixture was centrifuged to collect the supernatant as the lipophilic extract.
50% v/v), vortexed, and incubated in darkness at room temperature for 30 min. The mixture was centrifuged to collect the supernatant as the lipophilic extract.
2.6 Analysis of antioxidant activity
Antioxidant activity was analyzed spectrophotometrically using the ferric reducing antioxidant power (FRAP) assay.27 The FRAP reagent was freshly prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ in 40 mM HCl, and 20 mM ferric chloride hexahydrate in a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/v), and incubated at 37 °C for 60 min. Briefly, 100 μL of each standard or sample was reacted with 2.9 mL of the FRAP reagent in the dark for 30 min. Then absorbance of the samples was measured at 593 nm in a spectrophotometer (Tecan, Switzerland). Total antioxidant activity (TAA) values of the samples were the sum of their respective hydrophilic (HAA) and lipophilic (LAA) antioxidant activity values. Trolox (0–100 μM) was used as reference standard and antioxidant activities were expressed as μmol Trolox/g of cod.
1 (v/v/v), and incubated at 37 °C for 60 min. Briefly, 100 μL of each standard or sample was reacted with 2.9 mL of the FRAP reagent in the dark for 30 min. Then absorbance of the samples was measured at 593 nm in a spectrophotometer (Tecan, Switzerland). Total antioxidant activity (TAA) values of the samples were the sum of their respective hydrophilic (HAA) and lipophilic (LAA) antioxidant activity values. Trolox (0–100 μM) was used as reference standard and antioxidant activities were expressed as μmol Trolox/g of cod.
2.7 Analysis of total phenolic contents
Total phenolic content (TPC) was determined by the Folin–Ciocalteau assay as described by Aryal et al. with minor modifications.28 Diluted hydrophilic and lipophilic extract samples were each added with 1 mL of Folin–Ciocalteu reagent and 3 mL of 7.5% sodium carbonate solution. The resulting mixtures were incubated in darkness at room temperature for 2 h and absorbance was measured at 765 nm in a spectrophotometer (Tecan, Switzerland). TPC values were the sum of the respective hydrophilic (HPC) and lipophilic (LPC) values. Gallic acid (0–1 mg L−1) was used to construct the standard calibration curve and phenolic contents were expressed as ng gallic acid/g cod.2.8 Analysis of total flavonoid contents
Total flavonoid content (TFC) was measured by the method of Sharma et al.27 One mL of extract or quercetin standard (0–1 mg mL−1) solution was mixed with 1 mL of AlCl3 (0.1 M), 1 mL of potassium phosphate monobasic (0.1 M, pH 5.5), and 7 mL of ultrapure water. The mixture was incubated for 30 min at room temperature followed by measurement of the absorbance at 415 nm against the blank in a spectrophotometer (Tecan, Switzerland). The data obtained were expressed as mg quercetin/g cod.2.9 Total amino acid content analysis
Amino acid compositions of roasted cod patties were analyzed by LC-MS/MS (Shimadzu LC20AD, Kyoto, Japan and API 3200MD TRAP, Framingham, MA, USA). Approximately 0.1 g freeze-dried sample was hydrolyzed in 2 mL of 6 M hydrochloric acid solution at 110 °C for 24 h. The tube was pre-filled with nitrogen to prevent oxidation of amino acids. An aliquot (50 μL) of each sample was treated with sodium hydroxide solution to precipitate proteins. After centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm at 4 °C for 4 min, 8 μL of the supernatant were incubated with 42 μL of labeling buffer and 20 μL of derivatization reagent at 50 °C for 15 min. The derivatized samples were chilled, centrifuged, and the supernatants analyzed by LC-MS/MS according to the method of Xu et al.29
200 rpm at 4 °C for 4 min, 8 μL of the supernatant were incubated with 42 μL of labeling buffer and 20 μL of derivatization reagent at 50 °C for 15 min. The derivatized samples were chilled, centrifuged, and the supernatants analyzed by LC-MS/MS according to the method of Xu et al.29
Estimated protein efficiency ratio (ePER), an indicator of protein quality, was calculated using the following equation:
| ePER = 0.468 + 0.454 × Leu − 0.105 × Tyr | (1) |
Fischer ratio (FR) was also calculated to evaluate protein quality with the following equation:30
| FR = (Val + Leu + Ile)/(Phe + Tyr) | (2) |
2.10 Analysis of soluble proteins in roasted cod patties
Approximately 1 g of freeze-dried sample was homogenized in 10 mL of sodium phosphate buffer (50 mM, pH 7.5). The homogenate was mixed on a shaker at 4 °C for 4 h and then centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min) to obtain the supernatant. Soluble protein content of the supernatant was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher, USA). Briefly, 20 μL of each albumin standards or supernatant was added to a microplate well containing 200 μL of the BCA reagent mixture. The mixtures were incubated in darkness at room temperature for 1 h and the absorbance was measured at 595 nm in a spectrophotometer (Tecan, Switzerland). Soluble protein contents were expressed as mg protein/g cod.
000 rpm, 10 min) to obtain the supernatant. Soluble protein content of the supernatant was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher, USA). Briefly, 20 μL of each albumin standards or supernatant was added to a microplate well containing 200 μL of the BCA reagent mixture. The mixtures were incubated in darkness at room temperature for 1 h and the absorbance was measured at 595 nm in a spectrophotometer (Tecan, Switzerland). Soluble protein contents were expressed as mg protein/g cod.
2.11 Total fatty acid analysis
Lipids were extracted from the freeze-dried samples according to the literature with minor modifications.31 Approximately 0.1 g of sample was extracted with 2 mL of hexane at 50 °C on a shaker for 30 min, followed by derivatization with 3 mL of 0.4 M methanolic potassium hydroxide solution for 20 min. Fatty acid methyl esters (FAMEs) were extracted with 3 mL of hexane-water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and quantified with reference to calibration curves of FAMEs in a gas chromatography mass spectrometer system (7890B-5977A, Agilent Technologies Inc.; USA) equipped with a DB-23 column (0.25 μm film, 0.32 mm i.d. × 30 m length). GC-MS conditions were as follows: injection volume 1 μL; split injection; split ratio 5
1, v/v) and quantified with reference to calibration curves of FAMEs in a gas chromatography mass spectrometer system (7890B-5977A, Agilent Technologies Inc.; USA) equipped with a DB-23 column (0.25 μm film, 0.32 mm i.d. × 30 m length). GC-MS conditions were as follows: injection volume 1 μL; split injection; split ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; carrier gas (helium) 3.0 mL min−1; injection port temperature 250 °C; detector temperature 230 °C; oven temperature program: 50 °C held for 1 min, then increased at a rate of 25 °C min−1 to 175 °C and held for 1 min, followed by an increase to 230 °C at a rate of 4 °C min−1.
1; carrier gas (helium) 3.0 mL min−1; injection port temperature 250 °C; detector temperature 230 °C; oven temperature program: 50 °C held for 1 min, then increased at a rate of 25 °C min−1 to 175 °C and held for 1 min, followed by an increase to 230 °C at a rate of 4 °C min−1.
After determining individual fatty acids, the contents of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were calculated.
2.12 Statistical analysis
Statistical analysis was performed using the SPSS statistical package (SPSS 20). Significant differences in treatment means were identified at a level of P < 0.05 by one-way analysis of variance (ANOVA) and the Duncan test. Pearson's correlations were used for factor analyses. Principal component analysis (PCA) was performed by using OriginPro 2019b.3. Results and discussion
3.1 Effect of Allium powders on the formation of HAs in roasted cod patties
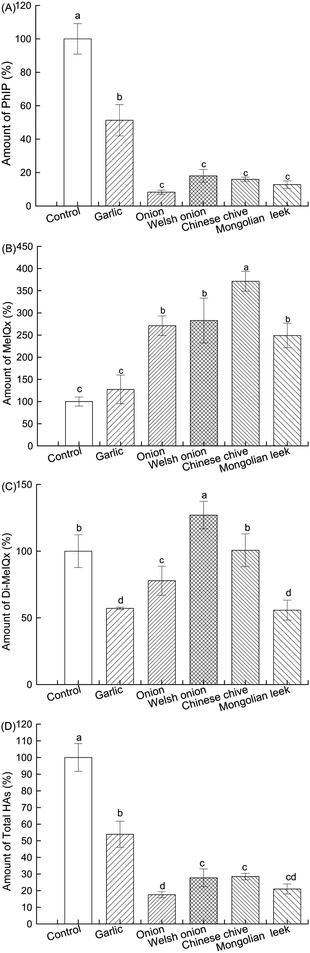
Heating of meat and fish can give rise to highly hazardous by-products, particularly HAs, through the Maillard reaction. Five Allium spp. in form of powders were used for pretreatment of cod samples respectively prior to heating, and the contents of three major HAs (PhIP, MeIQx and 4,8-DiMeIQx) which have been reported to account for most of the food-borne HA-associated mutagenic/carcinogenic activity were determined by LC-MS (ESI Fig. S1† and 1). In the control patties, the contents of PhIP, MeIQx and 4,8-DiMeIQx were 10.35 ± 0.95, 0.32 ± 0.07, and 0.07 ± 0.02 ng g−1, respectively. Consistent with previous reports, PhIP was the most abundant and its content was much higher than that of MeIQx and 4,8-DiMeIQx.32 As shown in Fig. 1A, all Allium plants significantly inhibited the formation of PhIP in cod patties, which was in agreement with previous reports on effective inhibition of PhIP formation in fried beef patties by onion and garlic.33,34 Except garlic, which reduced PhIP content by 49% relative to control, the inhibition rates of the other four ranged from 82% to 92%. In contrast, these four Allium plants resulted in higher MeIQx contents compared with control, while garlic did not significantly affect it (Fig. 1B). The totally different effects of onion on PhIP and on MeIQx formation was also reported in a previous study in fried chicken.35 Another study using fried beef observed a dose–dependent enhancement in MeIQx formation by onion, and the authors attributed it to the introduction of sugar from onion into the meat during the marination and/or heating process.36 For 4,8-DiMeIQx, similarly, no consistent inhibitory effect was observed (Fig. 1C); Mongolian leek, garlic and onion exhibited moderate inhibition (P < 0.05), while the others were either ineffective or mildly promoted its formation.Based on the following two considerations: (1) the levels of MeIQx and 4,8-DiMeIQx only accounted for ∼0.7–3% of total HAs in the roasted cod patties, and (2) the relative degrees of reduction in PhIP and in total HA contents by pretreatment with different Allium plants (Fig. 1A and D), Mongolian leek and onion represent the most promising candidates, followed by Chinese chive and welsh onion. This has been the first report on the potent HA-formation inhibitory effect of Chinese chive and Mongolian leek. In particular, 2% level of Mongolian leek-addition was able to reduce the content of total HAs by 80% relative to control.
3.2 Effect of Allium powders on antioxidant capacity, polyphenol and flavonoid contents, and their correlations with HA contents in roasted cod patties
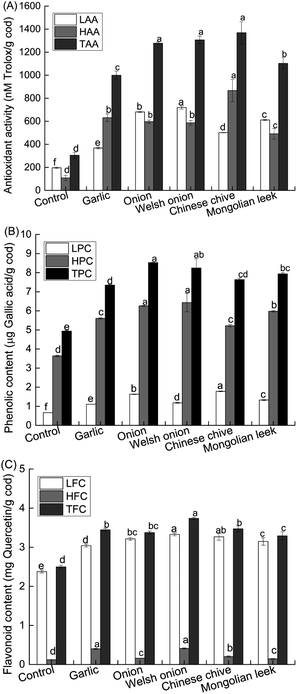
Allium spp. are well-known phytochemical-rich culinary ingredients in many cuisines worldwide. Our data thus far showed that four out of the five selected Allium spp. could strongly reduce the contents of HAs in roasted cod patties by >70% relative to control. Considering ample evidence suggesting that antioxidant activity is a critical functional property of Allium spp.,21,37–39 we next analyzed the antioxidant capacities and phenolic contents of the roasted cod samples pretreated with powders of the different Allium spp. As expected, Allium-pretreated roasted cod patties all had significantly (P < 0.05) higher TAA, LAA and HAA than the control (Fig. 2A). Consistent with the trend in their relative antioxidant activities, phenolic and flavonoid contents of the Allium-pretreated cod patties were all significantly (P < 0.05) higher than the control patties (Fig. 2B and C). Allium spp. are known to be good sources of phenolic and flavonoid compounds, such as quercetin, kaempferol, chlorogenic acid, gallic acid and others.21,25,38,39 Consistent with literature data, the phenolic and flavonoid contents of Allium-pretreated patties were significantly higher than the control patties.5 These data suggest that pretreatment of fish or meat with powders of certain Allium spp. prior to heating could indirectly improve our dietary intake of antioxidants.Next, we sought to understand the correlations between the antioxidant activity and phenolic and flavonoid contents of the roasted cod samples, as well as their correlations with the HA-formation inhibitory effects of the Allium spp. The phenolic and flavonoid contents (except for HFC) all had significant (P < 0.001) positive correlations with TAA of the roasted cod patties (Table 1), suggesting that Allium lipophilic flavonoids were key components of the antioxidant capacity of the roasted cod patties. In addition, it was found that the antioxidant activity, phenolic and flavonoid content parameters (except HFC) all had strong negative correlations with the contents of PhIP in the roasted cod samples (Table 2). On the other hand, these parameters were significantly and positively correlated with MeIQx contents in the roasted cod patties. No significant correlation was found with 4,8-DiMeIQx. These data indicate an inhibitory and a promoting effect of Allium antioxidants on PhIP and MeIQx formation in roasted cod patties, respectively.
| Variable | TAA | LAA | HAA |
|---|---|---|---|
| a TAA = HAA + LAA, TPC = HPC + LPC, TFC = HFC + LFC. TAA: total antioxidant activity, HAA: hydrophilic antioxidant activity, LAA: lipophilic antioxidant activity; TPC: total phenolic content, HPC: hydrophilic phenolic content, LPC: lipophilic phenolic content; TFC: total flavonoid content, HFC: hydrophilic flavonoid content, LFC: lipophilic flavonoid content. Significant at *P < 0.05; **P < 0.01; ***P < 0.001. | |||
| TPC | 0.929*** | 0.917*** | 0.733** |
| LPC | 0.860*** | 0.641** | 0.844*** |
| HPC | 0.841*** | 0.909*** | 0.599** |
| TFC | 0.910*** | 0.779*** | 0.812*** |
| LFC | 0.965*** | 0.860*** | 0.834*** |
| HFC | 0.346 | 0.206 | 0.381 |
| TAA | — | — | — |
| LAA | — | — | — |
| HAA | — | — | — |
| Variable | PhIP | MeIQx | Di-MeIQx | Total HAs |
|---|---|---|---|---|
| a TAA = HAA + LAA, TPC = HPC + LPC, TFC = HFC + LFC. TAA: total antioxidant activity, HAA: hydrophilic antioxidant activity, LAA: lipophilic antioxidant activity; TPC: total phenolic content, HPC: hydrophilic phenolic content, LPC: lipophilic phenolic content; TFC: total flavonoid content, HPC: hydrophilic flavonoid content, LFC: lipophilic flavonoid content. Significant at *P < 0.05; **P < 0.01; ***P < 0.001. | ||||
| TAA | −0.937*** | 0.818*** | 0.054 | −0.927*** |
| LAA | −0.910*** | 0.695** | 0.128 | −0.910*** |
| HAA | −0.750*** | 0.735** | −0.017 | −0.733** |
| TPC | −0.948*** | 0.642** | −0.111 | −0.959*** |
| LPC | −0.850*** | 0.847*** | −0.078 | −0.830*** |
| HPC | −0.867*** | 0.484* | −0.11 | −0.889*** |
| TFC | −0.816*** | 0.605** | 0.079 | −0.819*** |
| LFC | −0.933*** | 0.737*** | 0.021 | −0.932*** |
| HFC | −0.128 | −0.026 | 0.200 | −0.140 |
Thus far, no unified conclusion is available in the literature regarding the effect of antioxidants on PhIP and MeIQx/Di-MeIQx formation. For instance, β-carotene and γ-tocopherol were reported to counteract the enhancing effect of some pro-oxidants such as iron compounds and hydroquinone on MeIQx/DiMeIQx formation in chemical models.40 In another recent study, capsaicin from chilli pepper was found to be capable of inhibiting the formation of both PhIP and MeIQx-type HAs, whereas other chili pepper antioxidants promoted their formation in roasted beef patties.41 Our previous study and other groups also found a lack of significant positive correlation between antioxidant activity and HA-formation inhibitory effect.16,42 In particular, dietary polyphenols naringenin, quercetin-3-O-glucoside and quercetin significantly inhibited PhIP formation, hesperidin and rutin had no significant effect, while carnosic acid and rosmarinic acid enhanced it. These inconsistent findings could be attributed to the following factors: (1) heat-induced degradation fragments of some polyphenols may take part in the formation pathways of HAs;42,43 (2) some polyphenols may function as prooxidants under the experimental conditions;40 and (3) mechanism other than antioxidant/radical scavenging plays a dominant role in the effect of some polyphenols on HA formation.44–46 Polyphenols are important dietary antioxidants that have been amply demonstrated to help attenuate the risk of many chronic diseases.47 Apparently, the incorporation of plant extracts or purified polyphenols to our daily cuisine could be a convenient and economic way of obtaining the purported health benefits. However, literature data together with the data from the present study highlight a need to use these phytochemical-rich natural agents in heat-processed muscle-based foods with caution since they could potentially increase the contents of some hazardous chemicals including HAs.
3.3 Effect of Allium powders on total amino acid and soluble protein profiles of roasted cod patties
During roasting, proteins/amino acids may be degraded or consumed in different chemical reactions, including the formation of HAs.5,9,48 Having established that phenolic antioxidants from Allium powders could effectively reduce the contents of HAs in roasted cod patties, we then proceeded to examine if this beneficial effect could be accompanied by a protective effect on deterioration of protein/amino acid quality. Table 3 shows the amino acid content, FR, ePER and soluble protein content of roasted cod patties pretreated respectively with powder of the five Allium spp. versus control. The content of essential amino acids (EAAs) and non-essential amino acids (NEAAs) ranged from 142.67 to 217.34 μg mg−1 and 203.54 to 260.53 μg mg−1 dry weight, respectively. The ratios of EAA to AA and EAA to NEAA in dietary proteins have been used to evaluate protein quality because of their important effects on protein utilization.49 Roasted cod patties pretreated with Chinese chive powder exhibited the highest EAA content and EAA to AA and EAA to NEAA ratios. Notably, the content of lysine in the Chinese chive-pretreated samples was 2-3-fold higher than the other samples, suggesting a strong protective effect against degradation of this EAA. Proteins with higher FR values are likely to have better health benefits owing to their potential antioxidant property, especially free radical scavenging and anti-peroxidation activity of the associated peptides.50 Mongolian leek-pretreated cod patties had significantly higher FR and ePER values than the control and other Allium-pretreated cod samples likely due to its high intrinsic content of branched chain AAs.51 The soluble protein contents of the Allium-pretreated cod samples were 40–75% higher than control. These data together provide strong evidence that pretreatment with powder of Allium spp. could effectively preserve protein/amino acid quality in roasted cod likely by interrupting their participation in heat-induced reactions via the action of the Allium phenolic antioxidants.| Control | Garlic | Onion | Welsh onion | Chinese chive | Mongolian leek | |
|---|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation (SD) (n = 3). A−E Values in the same row with different letters are significantly different (P < 0.05). ND: not detected. FR: Fischer ratio, ePER: estimated protein efficiency ratio. | ||||||
| Essential amino acids | ||||||
| Leucine | 30.00 ± 0.68BC | 31.16 ± 0.56B | 28.97 ± 0.38BC | 28.85 ± 0.74C | 30.83 ± 0.97BC | 34.95 ± 2.43A |
| Lysine | 41.25 ± 1.829C | 49.71 ± 0.68B | 31.59 ± 1.82D | 33.00 ± 1.14D | 96.29 ± 9.35A | 52.86 ± 0.71B |
| Isoleucine | 18.05 ± 4.54B | 18.17 ± 0.58B | 16.02 ± 0.97D | 17.58 ± 0.97BC | 16.42 ± 0.85CD | 22.51 ± 0.45A |
| Valine | 19.43 ± 1.52B | 16.70 ± 0.44C | 18.17 ± 1.33BC | 18.17 ± 0.91BC | 17.87 ± 1.29BC | 24.77 ± 0.51A |
| Threonine | 18.64 ± 0.69C | 20.16 ± 0.17B | 17.92 ± 0.62C | 20.10 ± 0.59B | 20.07 ± 0.57B | 24.30 ± 1.04A |
| Phenylalanine | 18.86 ± 0.94B | 16.98 ± 0.88C | 16.61 ± 0.86C | 16.68 ± 0.17C | 19.20 ± 0.33B | 21.84 ± 0.32A |
| Methionine | 14.44 ± 1.41BC | 14.73 ± 0.64B | 13.12 ± 0.64CD | 12.39 ± 0.21D | 16.67 ± 0.57A | 15.32 ± 0.65AB |
| Tryptophan | ND | ND | ND | ND | ND | ND |
| Total essential amino acids (EAA) | 160.67 ± 3.19C | 167.62 ± 2.88C | 142.67 ± 3.99D | 146.78 ± 2.88D | 217.34 ± 11.81A | 196.55 ± 3.24B |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Non-essential amino acids | ||||||
| Tyrosine | 10.18 ± 0.61D | 10.41 ± 0.60CD | 13.17 ± 0.80A | 11.80 ± 0.55B | 11.58 ± 0.83BC | 9.14 ± 0.86D |
| Cysteine | 4.62 ± 0.07A | 4.62 ± 0.16A | 3.07 ± 0.21D | 3.56 ± 0.01C | 2.96 ± 0.04D | 3.95 ± 0.01B |
| Histidine | 8.92 ± 0.02BC | 9.60 ± 0.85B | 7.63 ± 0.26D | 8.30 ± 0.20CD | 8.47 ± 0.10C | 10.75 ± 0.17A |
| Glutamic acid | 57.92 ± 1.51CD | 60.79 ± 0.75B | 56.79 ± 0.18D | 62.15 ± 0.32B | 58.87 ± 1.57C | 79.45 ± 0.21A |
| Aspartic acid | 30.74 ± 1.02CD | 31.74 ± 0.64BC | 28.31 ± 0.66E | 33.28 ± 0.69B | 28.96 ± 1.65DE | 39.40 ± 2.01A |
| Arginine | 33.12 ± 0.45A | 14.57 ± 0.33E | 25.77 ± 0.43B | 19.51 ± 1.06D | 22.93 ± 0.85C | 24.95 ± 0.64B |
| Alanine | 24.06 ± 0.51CD | 26.89 ± 0.58B | 22.80 ± 1.16CD | 25.11 ± 1.40C | 24.19 ± 1.10D | 31.22 ± 0.65A |
| Glycine | 22.56 ± 1.21A | 18.35 ± 0.33C | 14.91 ± 0.87D | 18.84 ± 0.65C | 16.03 ± 0.14D | 20.89 ± 1.48B |
| Serine | 19.68 ± 1.04BC | 21.34 ± 0.49B | 18.46 ± 1.76C | 20.38 ± 0.86B | 21.22 ± 0.44B | 24.04 ± 0.85A |
| Proline | 13.48 ± 0.28BC | 14.59 ± 1.06B | 12.63 ± 0.85C | 13.43 ± 0.57BC | 14.17 ± 0.43BC | 16.75 ± 1.23A |
| Total non-essential amino acids (NEAA) | 225.41 ± 3.24B | 212.90 ± 4.14C | 203.54 ± 5.01D | 216.34 ± 3.73C | 209.38 ± 3.00CD | 260.53 ± 4.85A |
| Total amino acids (AA) | 386.08 ± 2.78C | 380.51 ± 3.78C | 346.21 ± 8.91E | 363.12 ± 5.99D | 426.72 ± 10.34B | 457.08 ± 7.89A |
| EAA/AA ratio | 0.42 ± 0.01CD | 0.44 ± 0.008B | 0.41 ± 0.001D | 0.40 ± 0.0009D | 0.51 ± 0.02A | 0.43 ± 0.001BC |
| EAA/NEAA ratio | 0.71 ± 0.02CD | 0.79 ± 0.02B | 0.70 ± 0.01CD | 0.68 ± 0.002D | 1.04 ± 0.07A | 0.75 ± 0.01BC |
| FR | 2.33 ± 0.14B | 2.41 ± 0.02B | 2.12 ± 0.06C | 2.27 ± 0.05BC | 2.12 ± 0.09C | 2.66 ± 0.16A |
| eFER | 13.02 ± 0.24BC | 13.52 ± 0.30B | 12.24 ± 0.14C | 12.33 ± 0.35C | 13.25 ± 0.40BC | 15.37 ± 1.15A |
| Soluble protein | 166.05 ± 3.25D | 260.63 ± 2.60B | 258.17 ± 5.12B | 233.96 ± 2.90C | 263.01 ± 1.36B | 292.53 ± 3.63A |
3.4 Effect of Allium powders on fatty acid profiles of roasted cod patties
The profiles of fatty acids (FAs) in roasted cod patties were evaluated by GC-MS (Table 4). Significant differences (P < 0.05) in SFA, MUFA and PUFA contents were observed among samples pretreated with Allium powders and control. In agreement with the literature, unsaturated FAs (MUFAs and PUFAs) represented 65–73% of the total FA contents with oleicacid (C18:1n9c) and docosahexaenoic acid (DHA C22:6n3) being the dominant MUFA and PUFA, respectively. Although PUFAs are highly valued health promoting nutrients in cod,52 they are susceptible to oxidative damage during thermal processes. Some of their peroxidation products, especially radicals may promote the formation of IQ- and MeIQx-type HAs.9,53 A corollary to this proposition is that the antioxidants introduced from the Allium powder into the cod patties could function to prevent these heat-induced lipid peroxidation reactions. Surprisingly, PUFA contents in garlic- and onion-pretreated samples were significantly lower than that in control patties. This might be due to the prooxidative effect of some of the constituents in garlic and onion under the experimental conditions,54,55 which caused oxidative degradation of PUFAs in the patties. The significantly higher contents of PUFAs in Mongolian leek, Chinese chive and Welsh onion pretreated samples were likely due to their much higher (∼10-fold) contents of α-linolenic acid (C18:3n3),56 an intrinsic property of these three Allium spp. that may be conveniently harnessed to increase dietary intake of PUFAs. These data tend to suggest that the phenolic antioxidants in the Allium spp. did not effectively prevent lipid peroxidation in the roasted cod patties at this condition, which could partly explain the lack of inhibition of MeIQx and 4,8-DiMeIQx formation. Meanwhile, the significantly higher contents of soluble proteins and amino acids in the Allium pretreated roasted cod patties (Table 3) together with their significant correlations with inhibitory effects on PhIP formation (Fig. 1) indicate the consumption of antioxidant capacity in the protection against protein/amino acid degradation and/or their involvement in the formation pathway of PhIP. Nonetheless, considering the fact that MeIQx and 4,8-DiMeIQx only accounted for <3% of the total HAs in the roasted cod patties, it could be concluded that phenolic antioxidants are principal constituents in the tested Allium spp. that may help mitigate dietary exposure to HAs.| Control | Garlic | Onion | Welsh onion | Chinese chive | Mongolian leek | |
|---|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation (SD) (n = 3). A−FValues in the same row with different letters are significantly different (P < 0.05). MUFA: monounsaturated fatty acid, PUFA: polyunsaturated fatty acid, SFA: saturated fatty acid, FA: fatty acid. | ||||||
| C8:0 | 0.09 ± 0.03B | 0.11 ± 0.01B | 0.12 ± 0.02B | 0.16 ± 0.03A | 0.18 ± 0.01A | 0.17 ± 0.02A |
| C10:0 | 0.17 ± 0.05BC | 0.14 ± 0.02C | 0.12 ± 0.01C | 0.20 ± 0.03AB | 0.23 ± 0.01A | 0.21 ± 0.02AB |
| C12:0 | 1.05 ± 0.03CD | 1.20 ± 0.02BC | 0.88 ± 0.06D | 1.58 ± 0.24A | 1.44 ± 0.86AB | 1.55 ± 0.23A |
| C14:0 | 55.92 ± 0.84BC | 53.22 ± 1.98D | 54.33 ± 0.80CD | 60.97 ± 1.85A | 58.01 ± 0.16B | 60.49 ± 0.21A |
| C15:0 | 8.31 ± 0.01B | 8.08 ± 0.47B | 8.56 ± 0.19B | 9.91 ± 0.37A | 9.51 ± 0.45A | 9.66 ± 0.19A |
| C16:0 | 493.96 ± 12.91B | 532.68 ± 38.95B | 505.29 ± 15.41B | 611.77 ± 17.90A | 646.34 ± 53.22A | 613.21 ± 15.65A |
| C17:0 | 40.04 ± 0.65A | 38.39 ± 0.46B | 29.62 ± 0.25E | 31.71 ± 1.46D | 38.52 ± 0.31B | 37.04 ± 0.20C |
| C18:0 | 581.84 ± 11.88A | 557.60 ± 7.60B | 497.62 ± 6.12D | 530.70 ± 23.13C | 544.88 ± 2.33BC | 534.50 ± 2.79C |
| C20:0 | 2.07 ± 0.15E | 5.33 ± 0.35C | 3.29 ± 0.37D | 10.78 ± 0.01A | 10.17 ± 0.48AB | 10.26 ± 0.02B |
| C22:0 | 35.25 ± 0.14D | 40.49 ± 0.41C | 39.65 ± 0.23C | 48.66 ± 1.57B | 50.73 ± 0.42A | 48.26 ± 1.43B |
| C23:0 | 0.61 ± 0.11D | 1.88 ± 0.13C | 1.00 ± 0.03D | 4.20 ± 0.58A | 3.30 ± 0.04B | 3.69 ± 0.40AB |
| C24:0 | 2.60 ± 0.14E | 5.48 ± 0.53D | 5.25 ± 0.48D | 19.72 ± 2.77A | 9.86 ± 0.38C | 17.11 ± 2.09B |
| SFA | 1227.86 ± 13.39B | 1244.60 ± 47.72B | 1145.74 ± 10.91C | 1330.36 ± 11.15A | 1373.17 ± 56.44A | 1335.47 ± 18.94A |
| C16:1 | 80.36 ± 1.65A | 69.09 ± 0.83C | 63.60 ± 0.82D | 63.60 ± 2.22D | 72.34 ± 0.38B | 68.76 ± 0.16C |
| C18:1n9c | 395.76 ± 8.25A | 377.80 ± 4.02A | 355.80 ± 3.33B | 350.66 ± 15.04B | 338.45 ± 18.10B | 347.57 ± 2.68B |
| C20:1 | 208.14 ± 4.60A | 180.88 ± 11.45B | 166.74 ± 1.76C | 165.78 ± 7.64C | 186.87 ± 0.86B | 180.40 ± 0.79B |
| C22:1n9 | 15.35 ± 1.58A | 13.06 ± 0.68A | 10.77 ± 1.38B | 10.50 ± 1.12B | 13.64 ± 0.71A | 13.18 ± 1.63A |
| C24:1 | 24.87 ± 2.16A | 16.53 ± 0.75C | 20.78 ± 1.99B | 20.45 ± 0.06B | 20.04 ± 1.47B | 22.19 ± 0.17B |
| MUFA | 724.48 ± 14.13A | 657.37 ± 14.71B | 617.68 ± 6.18C | 611.00 ± 25.37C | 631.35 ± 18.28BC | 632.11 ± 4.18BC |
| C18:3n3 | 25.12 ± 0.27E | 29.80 ± 0.54E | 68.49 ± 1.67D | 257.18 ± 8.00B | 352.50 ± 27.24A | 222.24 ± 5.11C |
| C20:3n3 | ND | ND | ND | ND | ND | ND |
| C20:5n3 (EPA) | 379.70 ± 6.47A | 345.17 ± 21.98B | 315.31 ± 3.17C | 320.95 ± 12.84BC | 333.60 ± 16.62BC | 331.38 ± 0.99BC |
| C22:6n3 (DHA) | 1593.94 ± 48.13B | 1442.57 ± 76.41C | 1533.38 ± 51.51BC | 1569.90 ± 51.68B | 1499.61 ± 64.85BC | 1753.92 ± 46.46A |
| PUFA n-3 | 1998.75 ± 52.16C | 1817.54 ± 67.54CD | 1917.18 ± 50.23CD | 2148.04 ± 47.54B | 2185.71 ± 105.27B | 2307.55 ± 52.48A |
| C18:2n6c | 220.53 ± 8.97B | 153.33 ± 9.16E | 110.51 ± 1.23F | 210.88 ± 4.68C | 250.66 ± 4.56A | 199.24 ± 0.38D |
| C18:3n6 | ND | ND | ND | ND | ND | ND |
| C20:3n6 | ND | ND | ND | ND | ND | ND |
| C20:4n6 | 100.77 ± 1.13B | 95.84 ± 0.47C | 105.52 ± 0.60A | 107.00 ± 3.14A | 95.51 ± 0.08C | 94.62 ± 0.20C |
| PUFA n-6 | 323.30 ± 10.10B | 249.18 ± 9.24D | 216.03 ± 1.82E | 317.88 ± 7.82B | 346.17 ± 4.55A | 293.86 ± 0.19C |
| C20:2 | 9.77 ± 0.17B | 9.06 ± 0.90B | 9.35 ± 0.04B | 10.22 ± 0.34A | 10.25 ± 0.39A | 9.79 ± 0.003AB |
| PUFA | 2331.82 ± 59.14C | 2075.78 ± 66.54D | 2142.56 ± 48.52D | 2476.14 ± 40.10B | 2542.13 ± 110.14AB | 2611.20 ± 52.66A |
| EPA + DHA | 1973.63 ± 51.93B | 1787.74 ± 67.07C | 1848.69 ± 48.59C | 1890.85 ± 39.69BC | 1833.21 ± 81.44C | 2085.31 ± 47.42A |
| FA | 4284.15 ± 81.01B | 3977.75 ± 120.26C | 3905.98 ± 55.00C | 4417.50 ± 13.95AB | 4546.65 ± 180.50A | 4578.79 ± 74.08A |
| PUFA n-3/FA | 0.467 ± 0.005C | 0.457 ± 0.007C | 0.491 ± 0.006B | 0.486 ± 0.010B | 0.481 ± 0.005B | 0.504 ± 0.004A |
3.5 Principal component analysis of the quality changes associated with different treatments
Principal component analysis (PCA) was applied to have a more comprehensive comparison of the cod patties subjected to different pretreatments. As shown in Fig. 3, the first two principal components accounted for 73.89% of the total changes of the samples (45.46% PC1 and 28.43% PC2). Fig. 3A shows the biplot of the scores and loadings located in the four quadrants of a confidence region (95%). Fig. 3B displays the score points in detail with PC1 and PC2 as loading factors. The control patties fall in the upper left quadrant and are distinctive from the other treatments in their higher HA and MUFA contents. Of note, HA content positively correlated with MUFAs (R = 0.910, P < 0.001), which suggests that higher MUFA levels might play a role in promoting the formation of HAs.57 Further investigation is warranted to clarify the potential correlation between the composition/content of MUFAs and HA formation in cod. Garlic-pretreated patties occupied a cluster in the lower left quadrant in the PCA plot near the control patties, which suggests that it may be a less preferable candidate to the other four Allium spp. in terms of potential to significantly improve the quality of roasted cod patties. Chinese chive- and Mongolian leek-pretreated patties occupied a cluster in the upper right quadrant which corresponds to their overall better nutrient profiles (i.e. n-3 PUFAs and soluble protein) than the other treatment groups. Thus, PCA indicates that powders of Chinese chive and Mongolian leek are promising culinary ingredients for improving both the safety and nutritional quality of roasted cod.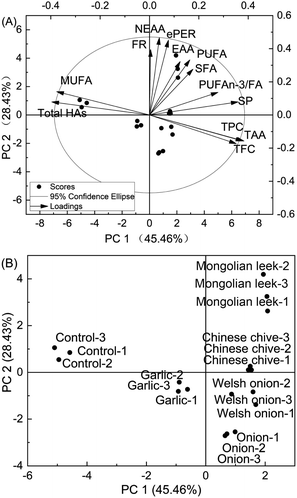 | ||
| Fig. 3 The bioplot (A) and score plot (B) of principal component analysis (PCA) of HA contents, nutrient compositions, and antioxidant attributes of roasted cod patties. | ||
4. Conclusion
In conclusion, the present study has demonstrated that pretreatment with powders of certain Allium spp. could lead to significantly lower HA contents and better nutritional profiles in roasted cod. This has been the first report on the strong HA-formation inhibitory effect of Chinese chive and Mongolian leek. It would be important to further characterize the principal phenolic component(s) and mechanism responsible for their differential effects on the formation of different types of HAs. The dual beneficial functionality of Chinese chive and Mongolian leek may be utilized to reduce the intake of hazardous by-products while enhance the nutritional and antioxidant properties of roasted cod and probably other protein-rich heat-processed foods.Conflicts of interest
The authors declare no conflicts of interestAcknowledgements
This work was supported by the Special National Key Research and Development Plan of China [grant number 2016YFD0400204], the Key Research and Development Program of Guangdong Province [grant number 2019B020212001], and the Postdoctoral Science Foundation of China [grant number 2018M630040].References
- P. A. Harnedy and R. J. FitzGerald, Bioactive peptides from marine processing waste and shellfish: A review, J. Funct. Foods, 2012, 4, 6–24 CrossRef CAS.
- N. H. Ishak and N. M. Sarbon, A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing, Food Bioprocess Tech., 2018, 11, 2–16 CrossRef CAS.
- Q. X. Jiang, J. W. Han, P. Gao, L. X. Yu, Y. S. Xu and W. S. Xia, Effect of heating temperature and duration on the texture and protein composition of Bighead Carp (Aristichthys nobilis) muscle, Int. J. Food Prop., 2018, 21, 2110–2120 CrossRef CAS.
- B. Mitra, R. Lametsch, T. Akcan and J. Ruiz-Carrascal, Pork proteins oxidative modifications under the influence of varied time-temperature thermal treatments: A chemical and redox proteomics assessment, Meat Sci., 2018, 140, 134–144 CrossRef CAS.
- C. F. Manful, N. P. Vidal, T. H. Pham, M. Nadeem, E. Wheeler, M. C. Hamilton, K. M. Doody and R. H. J. F. C. Thomas, Unfiltered beer based marinades reduced exposure to carcinogens and suppressed conjugated fatty acid oxidation in grilled meats, Food Control, 2020, 111, 107040 CrossRef.
- S. Turhan, N. S. Ustun and H. Temiz, Lipid quality of anchovy (Engraulis encrasicholus) fillets affected by different cooking methods, Int. J. Food Prop., 2011, 14, 1358–1365 CrossRef.
- H. Hosseini, M. Mahmoudzadeh, M. Rezaei, L. Mahmoudzadeh, R. Khaksar, N. K. Khosroshahi and A. Babakhani, Effect of different cooking methods on minerals, vitamins and nutritional quality indices of kutum roach (Rutilus frisii kutum), Food Chem., 2014, 148, 86–91 CrossRef CAS.
- T. Yang, Z. He, F. Qin, M. Zeng and J. Chen, Research progress of the effects of Maillard reaction on flavor and quality of products as well as derivatized harmful substances, J. Food Saf. Food Qual., 2017, 8, 854–861 Search PubMed.
- K. W. Cheng, F. Chen and M. Wang, Heterocyclic amines: chemistry and health, Mol. Nutr. Food Res., 2006, 50, 1150–1170 CrossRef CAS.
- International Agency for Research on Cancer (IARC), Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins, IARC Monogr. Eval. Carcinog. Risks Hum., 1993, 56, 397–444 Search PubMed.
- C. L. Archer, P. Morse, R. F. Jones, T. Shirai, G. Haas and C. Wang, Carcinogenicity of the N-hydroxy derivative of 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine, 2-amino-3, 8-dimethyl-imidazo [4, 5-f] quinoxaline and 3, 2′-dimethyl-4-aminobiphenyl in the rat, Cancer lett., 2000, 155, 55–60 CrossRef CAS.
- T. Sugimura, K. Wakabayashi, H. Nakagama and M. Nagao, Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish, Cancer Sci., 2004, 95, 290–299 CrossRef CAS.
- A. Solyakov and K. Skog, Screening for heterocyclic amines in chicken cooked in various ways, Food Chem. Toxicol., 2002, 40, 1205–1211 CrossRef CAS.
- M. Gibis, M. Kruwinnus and J. Weiss, Impact of different pan-frying conditions on the formation of heterocyclic aromatic amines and sensory quality in fried bacon, Food Chem., 2015, 168, 383–389 CrossRef CAS.
- R. Salazar, G. Arambula-Villa, F. J. Hidalgo and R. Zamora, Structural characteristics that determine the inhibitory role of phenolic compounds on 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) formation, Food Chem., 2014, 151, 480–486 CrossRef CAS.
- Q. Zhu, S. Zhang, M. Wang, J. Chen and Z.-P. Zheng, Inhibitory effects of selected dietary flavonoids on the formation of total heterocyclic amines and 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) in roast beef patties and in chemical models, Food Funct., 2016, 7, 1057–1066 RSC.
- M. Gibis and J. Weiss, Inhibitory effect of cellulose fibers on the formation of heterocyclic aromatic amines in grilled beef patties, Food Chem., 2017, 229, 828–836 CrossRef CAS.
- B. Janoszka, Heterocyclic amines and azaarenes in pan-fried meat and its gravy fried without additives and in the presence of onion and garlic, Food Chem., 2010, 120, 463–473 CrossRef CAS.
- F. Lu, G. K. Kuhnle and Q. Cheng, The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs, Food Control, 2018, 92, 399–411 CrossRef CAS.
- J. Li, J. Zuo, X. Qiao, Y. Zhang and Z. Xu, Effect of garlic powder on acrylamide formation in a low-moisture model system and bread baking, J. Sci. Food Agr., 2016, 96, 893–899 CrossRef CAS.
- P. Putnik, D. Gabric, S. Roohinejad, F. J. Barba, D. Granato, K. Mallikarjunan, J. M. Lorenzo and D. B. Kovacevic, An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties, Food Chem., 2019, 276, 680–691 CrossRef CAS.
- D. Tan, Y. Zhang, L. Chen, L. Liu, X. Zhang, Z. Wu, B. Bai and S. Ji, Decreased glycation and structural protection properties of gamma-glutamyl-S-allyl- cysteine peptide isolated from fresh garlic scales (Allium sativum L.), Nat. Prod. Res., 2015, 29, 2219–2222 CrossRef CAS.
- I. S. Choi, H. S. Cha and Y. S. Lee, Physicochemical and Antioxidant Properties of Black Garlic, Molecules, 2014, 19, 16811–16823 CrossRef.
- J.-S. Kim, O.-J. Kang and O.-C. Gweon, Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps, J. Funct. Foods, 2013, 5, 80–86 CrossRef CAS.
- S. Aoyama and Y. Yamamoto, Antioxidant activity and flavonoid content of Welsh onion (Allium fistulosum) and the effect of thermal treatment, Food Sci. Technol. Res., 2007, 13, 67–72 CrossRef CAS.
- J. Chen, Z. He, F. Qin, J. Chen, D. Cao, F. Guo and M. Zeng, Inhibitory profiles of spices against free and protein-bound heterocyclic amines of roast beef patties as revealed by ultra-performance liquid chromatography–tandem mass spectrometry and principal component analysis, Food Funct., 2017, 8, 3938–3950 RSC.
- K. Sharma, E. Y. Ko, A. D. Assefa, S. Ha, S. H. Nile, E. T. Lee and S. W. Park, Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties, J. Food Drug Anal., 2015, 23, 243–252 CrossRef CAS.
- S. Aryal, M. K. Baniya, K. Danekhu, P. Kunwar, R. Gurung and N. Koirala, Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western nepal, Plants, 2019, 8, 96 CrossRef CAS.
- P. P. Xu, J. Xiong, S. Cheng, X. Zhao, C. F. Wang, G. Cai and W. L. Zhao, A phase II study of methotrexate, etoposide, dexamethasone and pegaspargase sandwiched with radiotherapy in the treatment of newly diagnosed, stage IE to IIE extranodal natural-killer/T-cell lymphoma, nasal-type, EBioMedicine, 2017, 25, 41–49 CrossRef.
- Y. Zhang, Q. Wang, Y. Bi, K. W. Cheng and F. Chen, Nutritional and functional activities of protein from steamed, baked, and high hydrostatic pressure treated cod (Gadus morhua), Food Control, 2018, 96, 9–15 CrossRef.
- S. A. Lagerstedt, D. R. Hinrichs, S. M. Batt, M. J. Magera, P. Rinaldo and J. P. McConnell, Quantitative determination of plasma C8-C26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders, Mol. Genet. Metab., 2001, 73, 38–45 CrossRef CAS.
- M. R. Khan, R. Busquets, J. Saurina, S. Hernández and L. Puignou, Identification of seafood as an important dietary source of heterocyclic amines by chemometry and chromatography–mass spectrometry, Chem. Res. Toxicol., 2013, 26, 1014–1022 Search PubMed.
- L. Rounds, C. M. Havens, Y. Feinstein, M. Friedman and S. Ravishankar, Concentration-dependent inhibition of Escherichia coli O157: H7 and heterocyclic amines in heated ground beef patties by apple and olive extracts, onion powder and clove bud oil, Meat Sci., 2013, 94, 461–467 CrossRef CAS.
- H. S. Shin, W. J. Rodgers, G. M. Strasburg and J. I. Gray, Reduction of heterocyclic aromatic amine formation and overall mutagenicity in fried ground beef patties by organosulfur compounds, J. Food Sci., 2002, 67, 3304–3308 CrossRef CAS.
- C. P. Salmon, M. G. Knize, J. S. Felton, B. Zhao and A. Seow, Heterocyclic aromatic amines in domestically prepared chicken and fish from Singapore Chinese households, Food Chem. Toxico., 2006, 44, 484–492 CrossRef CAS.
- M. Gibis, Effect of oil marinades with garlic, onion, and lemon juice on the formation of heterocyclic aromatic amines in fried beef patties, J. Agric. Food Chem., 2007, 55, 10240–10247 CrossRef CAS.
- M. Abdel-Gawad, M. Abdel-Aziz, M. El-Sayed, E. El-Wakil and E. Abdel-Lateef, In vitro antioxidant, total phenolic and flavonoid contents of six Allium species growing in Egypt, J. Microbiol., Biotechnol. Food Sci., 2014, 3, 343 Search PubMed.
- W. Wang, J. Li, H. Zhang, X. Wang, J. Fan and X. Zhang, Phenolic compounds and bioactivity evaluation of aqueous and methanol extracts of Allium mongolicum Regel, Food Sci. Nutr., 2019, 7, 779–787 CrossRef CAS.
- S. Dziri, I. Hassen, S. Fatnassi, Y. Mrabet, H. Casabianca, B. Hanchi and K. Hosni, Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum), J. Funct. Foods, 2012, 4, 423–432 CrossRef CAS.
- M. A. E. Johansson and M. Jägerstad, Influence of pro-and antioxidants on the formation of mutagenic-carcinogenic heterocyclic amines in a model system, Food Chem., 1996, 56, 69–75 CrossRef CAS.
- M. Zeng, M. Zhang, Z. He, F. Qin, G. Tao, S. Zhang and J. Chen, Inhibitory profiles of chilli pepper and capsaicin on heterocyclic amine formation in roast beef patties, Food Chem., 2017, 221, 404–411 CrossRef CAS.
- K.-W. Cheng, F. Chen and M. Wang, Inhibitory activities of dietary phenolic compounds on heterocyclic amine formation in both chemical model system and beef patties, Mol. Nutr. Food Res., 2007, 51, 969–976 CrossRef CAS.
- A. Oguri, M. Suda, Y. Totsuka, T. Sugimura and K. Wakabayashi, Inhibitory effects of antioxidants on formation of heterocyclic amines, Mutat. Res. Fund Mol. Mech. Mutagen, 1998, 402, 237–245 CrossRef CAS.
- Y. Zhao, D. Fan, Z. P. Zheng, E. T. Li, F. Chen, K. W. Cheng and M. Wang, 8-C-(E-phenylethenyl) quercetin from onion/beef soup induces autophagic cell death in colon cancer cells through ERK activation, Mol. Nutr. Food Res., 2017, 61, 1600437 CrossRef.
- K.-W. Cheng, C. C. Wong, J. Chao, C. Lo, F. Chen, I. K. Chu, C.-M. Che, C.-T. Ho and M. Wang, Inhibition of mutagenic PhIP formation by epigallocatechin gallate via scavenging of phenylacetaldehyde, Mol. Nutr. Food Res., 2009, 53, 716–725 CrossRef CAS.
- K.-W. Cheng, C. C. Wong, C. K. Cho, I. K. Chu, K. H. Sze, C. Lo, F. Chen and M. F. Wang, Trapping of Phenylacetaldehyde as a Key Mechanism Responsible for Naringenin's Inhibitory Activity in Mutagenic 2-Amino-1-methyl-6-phenylimidazo [4,5-b] Pyridine Formation, Chem. Res. Toxicol., 2008, 21, 2026–2034 Search PubMed.
- K. Rani, Role of antioxidants in prevention of diseases, J. Appl. Biotechnol. Bioeng., 2017, 4, 00091 Search PubMed.
- M. N. Lund, M. Heinonen, C. P. Baron and M. Estevez, Protein oxidation in muscle foods: A review, Mol. Nutr. Food Res., 2011, 55, 83–95 CrossRef CAS.
- J. A. Green, R. W. Hardy and E. L. Brannon, The optimum dietary essential: nonessential amino acid ratio for rainbow trout (Oncorhynchus mykiss), which maximizes nitrogen retention and minimizes nitrogen excretion, Fish Physiol. Biochem., 2002, 27, 109–115 CrossRef CAS.
- C. C. Udenigwe and R. E. Aluko, Antioxidant and angiotensin converting enzyme-inhibitory properties of a flaxseed protein-derived high Fischer ratio peptide mixture, J. Agric. Food Chem., 2010, 58, 4762–4768 CrossRef CAS.
- X. Liu and Sechenbater, Nutritional compositions and ethnobotany of Allium mongolicum regel, Grassland of China, 2002, 24, 52–54 Search PubMed.
- A. Ticinesi, T. Meschi, F. Lauretani, G. Felis, F. Franchi, C. Pedrolli and M. Maggio, Nutrition and inflammation in older individuals: focus on vitamin D, n-3 polyunsaturated fatty acids and whey proteins, Nutrients, 2016, 8, 186 CrossRef.
- A. M. Pearson, C. Chen, J. I. Gray and S. D. Aust, Mechanism(s) involved in meat mutagen formation and inhibition, Free Radical. Bio. Med., 1992, 13, 161–167 CrossRef CAS.
- D. K. Maurya and T. P. A. Devasagayam, Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids, Food Chem. Toxicol., 2010, 48, 3369–3373 CrossRef CAS.
- M. Nuray and F. Oz, The effect of using different types and rates of onion-water extract in meatball production on the formation of heterocyclic aromatic amines, J Sci. Food Agr., 2019, 99, 3538–3547 CrossRef CAS.
- M. C. Tsiaganis, K. Laskari and E. Melissari, Fatty acid composition of Allium species lipids, J. Food Compos. Anal., 2006, 19, 620–627 CrossRef CAS.
- M. Johansson, K. Skog and M. Jägerstad, Effects of edible oils and fatty acids on the formation of mutagenic heterocyclic amines in a model system, Carcinogenesis, 1993, 14, 89–94 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05758d |
| This journal is © The Royal Society of Chemistry 2020 |