Open Access Article
Open Access ArticleEffective removal of aromatic pollutants via adsorption and photocatalysis of porous organic frameworks†
Congcong Wang‡
,
Wei Wang‡,
Jian Wang,
Peiping Zhang,
Shiding Miao ,
Bo Jin
,
Bo Jin and
Lina Li
and
Lina Li *
*
Key Laboratory of Automobile Materials of Ministry of Education, State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Solid Waste Recycling Engineering Research Center of Jilin, Jilin University, Changchun, 130022, Jilin Prov., China. E-mail: lilina@jlu.edu.cn
First published on 28th August 2020
Abstract
PAF-45 with a wholly aromatic framework, intrinsic microporosity and π–π conjugation system shows excellent performance in aromatic pollutant removal. It exhibits a high adsorption capacity for the benzene series and moderate photocatalytic performance. As an adsorbent, PAF-45 can adsorb 35 wt% benzene and 68 wt% chlorobenzene in static adsorption experiments at room temperature and pressure. In benzene simulation wastewater, PAF-45 also shows excellent adsorption capacity, without significant reduction after 10 cycles of the adsorption–desorption process. Moreover, PAF-45 exhibits an impressive photocatalytic degradability of aromatic compounds, like aniline and phenol, under visible light illumination.
Introduction
Volatile organic compounds (VOCs), including air and water pollutants, are widely considered as some of the most important components of man-made pollution in modern industrial society.1–7 Even though the utilization and emission of VOCs have caused great risks to the environment and human health, VOCs are still extensively employed, especially in the petroleum, chemical, medical and other industrial fields, as irreplaceable raw materials and inevitable by-products.8Aromatic compounds, including benzene, toluene, chlorobenzene and so on, are a series of the most commonly encountered VOCs, which are seriously toxic and carcinogenic.9 Detailed studies have been made on the harm caused by aromatic compounds, for example the potential relationship between the benzene series and the risk of hematologic cancers, like myeloid and lymphoid malignancies, in a large prospective cohort10; long term exposure to low concentration benzene series could predispose to the development of type 2 diabetes (T2D) and affect human metabolism11,12 while high concentration inhalation would result in unconsciousness, dizziness, and even death.13,14 Prenatal exposure to persistent organic pollutants may cause fetal malformations or even fetal death.15 Apart from this, aromatic organic compounds also contribute to serious environmental problems such as the water pollution, which may result in the demise of scarce species and biological genetic variation.16,17
The benzene series would enter the environment by the use and transportation of these raw materials and the impropriate emission of waste gases and water. As basic organic chemicals, benzene series commonly exist in oil/natural gas extraction/combustion, fuel combustion, traffic sources including gasoline exhaust, diesel exhaust, mixed fugitive emissions, and industrial coatings/solvents, which constitute main organic pollutants in the air. In the research of Md. Aynul Bari and Warren B. Kindzierski, aromatic compounds are the main reason why industrial coatings/solvents and fuel combustion are considered as severe pollutant sources, accounting for 12% and 20% respectively. The inadequately treated sewage discharge from industrial plants also leads to severe pollution in the water body.4 The pollution has already caused widespread attention from every corner of the society.
Currently, there are many ways to deal with the benzene series pollution in the environment. According to different technical solutions, the reported methods to effectively remove benzene series in the environment includes adsorption, chemisorption, thermal damage, membrane separation, microbial degradation, and photocatalytic degradation.18,19 Among those methods, adsorption and photo-catalysis receive more expectation. Therefore, to find a material as suitable adsorbent and photocatalyst becomes the key point of aromatic matter treatment. According to reports, PAF-45 is just one ideal option, but has never been tried in aromatic VOC removal.20 As a very stable microporous material, the surface area of PAF-45 is around 777 m2 g−1. The narrow irregular microporosity with diameter around 0.6 nm provides it with excellent adsorption ability towards gas adsorption. More importantly, PAF-45 was constituted with only benzene rings, and the large number of aromatic rings in the structure may lead to strong affinity with aromatic compounds.21,24–26 Moreover, this material has been proved with photocatalytic ability for RhB degradation,23 which makes it promising and valuable to explore its possibility in aromatic matter degradation. In addition, the preparation cost of PAF-45 is quite reasonable owing to its one-step synthesis and inexpensive raw materials, which becomes a great advantage when it comes to the pollutant removal in reality.
Based on all those facts, herein PAF-45 was chosen for adsorption and degradation of benzene series, taking advantage of its suitable microporosity, strong affinity with aromatic matter, and photocatalytic performance. In this protocol, PAF-45 shows high static adsorptions of benzene series under room temperature and pressure. The static adsorption rates of PAF-45 material can reach 30–68 wt% under room temperature and pressure, for example, 68 wt% for chlorobenzene. PAF-45 can effectively capture over 98% benzene from waste water while avoiding unnecessary water molecule capture. This process shows excellent repeatability, which also makes PAF-45 material promising in waste water treatment for benzene removal. In addition, PAF-45 exhibits possibility in aromatic compound photocatalytic degradation under visible light irradiation.
Results
Static adsorption of benzene series under room temperature and pressure
In order to test the VOCs adsorption ability of PAF-45, this material was measured with static adsorption of volatile benzene series. The excellent static adsorption performance of PAF-45 to benzene series can be well revealed with adsorption capacity curves, as shown in Fig. 1a. At room temperature and pressure, benzene, toluene, chlorobenzene, bromobenzene, nitrobenzene and aniline can be fast and efficiently adsorbed owing to its special structure. The adsorption curves rise rapidly in the first 5 hours and most of the curves reach its absorption equilibrium after 50 hour's adsorption. In general, PAF-45 shows great adsorption capacity towards these VOCs. To be exact, the adsorption amount of VOCs can reach 380.7 mg g−1 (benzene), 351.9 mg g−1 (toluene), 495.4 mg g−1 (bromobenzene), 675.3 mg g−1 (chlorobenzene), 466.5 mg g−1 (nitrobenzene) and 348 mg g−1 (aniline) respectively. The high adsorption performance of PAF-45 mainly comes from its typical skeleton structure, which is constructed completely by benzene rings. This special feature of PAF-45 makes it extremely hydrophobic, shown in Fig. 1b, whose water contact angle is 130°. Compared with the high adsorption performance of VOCs, PAF-45 can only adsorb race amount of water, around 0.251 mg g−1, which is less than 0.1‰. It is important to understand the possible adsorption mechanism between PAF-45 and aromatic organic compounds. The differences in affinity with organic compounds and water not only come from its molecule polarity, but also from phenyl group network (Fig. 1). The PAF-45 offers a great number of benzene rings and the adsorbed organic compounds all have aromatic rings. Therefore, PAF-45 would exert strong π–π interactions with the organic compounds.25 Moreover, pore-filling mechanism may be considered as a plausible mechanism.26 PAF-45 is a porous organic framework material with abundant micropore structures and possesses a satisfying surface area of 777 m2 g−1 (Table S1†), which provide adequate storage space for VOCs adsorption. Also, the molecular size of the adsorbed organic compounds (around 0.5 nm) is less than or similar the pore diameter of PAF-45. Those facts together guarantee PAF-45 excellent adsorption ability towards aromatic VOCs.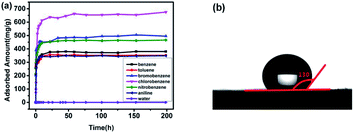 | ||
| Fig. 1 Static vapor adsorption capacity (benzene, toluene, chlorobenzene, bromobenzene, nitrobenzene, aniline and water) (a) and the contact angle (b) of PAF-45. | ||
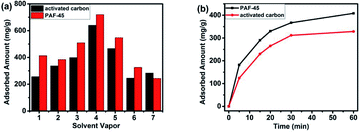
The excellent VOCs adsorption performance of PAF-45 is not inferior to classic absorbent materials. For example, activated carbon (AC) has been widely used in pollutant adsorption because of its overwhelming physical adsorption mechanisms, large surface area and rich pore structure. The comparison of AC and PAF-45 was listed in Table S1.† One of the advantages of AC is its large surface area (1270 m2 g−1), which greatly improves its VOC absorption ability. Compared with that, the specific surface area in Brunauer–Emmett–Teller (BET) theory of PAF-45 is only moderate (around 777 m2 g−1), which is only around 61% of AC. However, PAF-45 shows better performance than AC in benzene series adsorption (Fig. 2a), including benzene, toluene, chlorobenzene, bromobenzene, nitrobenzene and aniline. It is worth noting that PAF-45 is able to adsorb 60.87% more benzene than activated carbon. The great contrast between surface area and adsorption ability could again imply the effects of multiple-phenyl skeleton of PAF-45 towards aromatic VOCs. The affinity of aromatic organic compounds to absorbent can be well proved with the adsorption curve in the very early stage, e.g. in the first hour. As shown in Fig. 2b (take chlorobenzene as an example), PAF-45 could adsorb chlorobenzene faster than AC. Within first 5 minutes, the adsorption amount of PAF-45 is 1.5 times of AC. This again implies the stronger interactions between aromatic organic compounds with PAF-45 framework. In this protocol, the superior VOC adsorption performance of PAF-45 does not come only from its intrinsic microporosity but also from multiple inducing synergistic effects between adsorbate and adsorbent, which gives rise to a high driving force for the adsorption of VOCs in PAF-45.21 For cyclohexane, the result is just opposite. PAF-45 shows no advantage compared with activated carbon that it adsorbed 15% less cyclohexane than AC. This fact tells that surface area is still the most significant point for the adsorption of organic compounds without phenyl groups. It also confirms our point from the other side that whole phenyl group network of PAF-45 indeed promotes its aromatic organic compound adsorption ability.
Adsorption of benzene in simulation wastewater
With all those facts above, PAF-45 could be considered as a promising candidate material for wastewater treatment. Its high affinity for benzene series as well as strong hydrophobicity make it perfect for the removal of pollutant benzene in waste water, which is very dangerous in current industrial that urgently requires to be effectively solved.In the experiment of benzene removal in model wastewater, PAF-45 shows satisfying benzene removal performance. After 10 h stirring, the benzene removal rate can reach 98.36% with 50 mg PAF-45 in 100 mL 500 mg L−1 benzene simulation wastewater, shown in Fig. 3a (black line). The removal process is fast in the early period, that over 80% of benzene was adsorbed within 1 h. This adsorption process could be greatly accelerated, when more PAF-45 were added into the same experiment system, while the final removal rate keeps unchanged at around 98–99% (Fig. S2†). Moreover, the effect of pH on adsorption was also investigated. As shown in Fig. S6,† the adsorption rate of the PAF-45 increased as the pH decreased. The increase in the rate of PAF-45 to adsorb benzene in the wastewater as the pH decreased was probably related to the increase in the strength of the electrostatic attraction as the pH decreased.24 These facts indicate that PAF-45 can effectively remove benzene in waste water and the removal rate could be achieved adjusting the amount of PAF-45 the pH of wastewater.
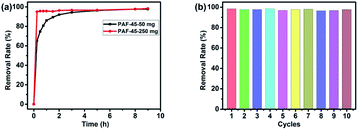 | ||
| Fig. 3 Adsorption of benzene simulation wastewater by PAF-45-50 mg (black) and PAF-45-250 mg (red) (a) and 10 cycles of adsorption–desorption of benzene by PAF-45-50 mg in simulation wastewater (b). | ||
Furthermore, in order to figure out the sustainability of our materials, PAF-45 was recycled after the first round of benzene removal in wastewater and repeatedly used. As a result, there is no significant reduction in the adsorption capacity of PAF-45 even after 10 cycles of adsorption–desorption experiments, that the removal rate is kept at about 97–98% (Fig. 3b). This demonstrates the strong stability and recyclability of PAF-45. Based on the above results presented, PAF-45 shows its potential to be utilized in real polluted effluent control.
Photodegradation of some liquid benzene series
Besides as excellent VOCs adsorbent, PAF-45 can also work as visible-light-responsive photocatalyst. By simple physical adsorption, usually aromatic organic compounds could not be completely eliminated, which may still lead to potential new round of pollution. Hence, further degradation seems to be a more effective method to solve the aromatic pollution. Under normal circumstances, most of aromatic organic compounds are fairly stable under visible light irradiation. This implies that a suitable photocatalyst is the key part to deal with the aromatic VOC pollution.In fact, PAF-45 has already been found with photocatalytic potentiality to some extent in the previous static adsorption experiment. Especially in the static adsorption experiment of aniline, the adsorption performance may change greatly in the dark or under natural light. As shown in Fig. 4a, in the dark, the adsorption of aniline could increase to its maximum within 10 hours. However, for the control group conducted under just natural daytime indoor light, the adsorption of aniline keeps increasing and could not reach its adsorption maximum yet after 816 h. In our assumption, the continuous increasement of weight of PAF-45 is just the result of its photoactivity. It is inferred that the already adsorbed aniline could be photodegraded by PAF-45 into others products, which may still attach to the PAF-45 but make room for further aniline adsorption. This information suggests that PAF-45 could be photoactive under visible light. In order to prove this assumption, further research on PAF-45 in aromatic organic compound photocatalysis have been carried out as below.
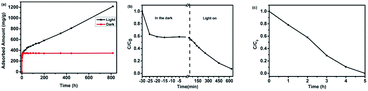 | ||
| Fig. 4 Aniline vapor adsorption capacity of PAF-45 (a); adsorption and degradation of aniline (b) and phenol (c) by PAF-45. | ||
The photocatalytic activities of PAF-45 were tested by estimating the removal efficiency of aniline in aqueous solution. For this experiment, about 5 mg of PAF-45 were added into a 20 mL glass bottle with 10 mL of aniline solution (1 mg L−1). The remaining aniline concentration could be tested by UV-spectra at the wavelength of 280 nm. Firstly, the concentration of aniline would decrease and reached equilibrium during stirring in the dark. After switch on the white light illumination (350 nm – infrared radiation), it could be seen from Fig. 4b that the concentration of aniline start to drop until there is almost no aniline left. The result demonstrates that aniline was degraded by PAF-45 under visible light illumination and confirms PAF-45 is with catalytic activity. In order to provide further proof, colorless phenol was also selected as a degraded matter. As a result, colorless phenol (1 mg L−1) could also be degraded by PAF-45 under white light illumination, only after four hours (Fig. 4c). This once again supports our point that PAF-45 possesses the photocatalytic activity.
According to previous literatures,22 conjugated microporous polymers constructed with benzene rings have been reported with outstanding photocatalytic activity, such as light-driven dye degradation and hydrogen evolution, because of its efficient electronic conjugation π–π packing in solid-state. The PAF-45, although prepared in a completely different method, also has similar π electronic conjugation packing skeleton with those CMPs, which provides evidence and theoretical basis for its photocatalytic performance. The key to PAFs degradation comes from photogenerated-hole oxidation. During the degradation process, O2 uptakes photogenerated electrons, and the produced ˙O2 would provide strong oxidation capability,23 this further confirms that PAF-45 has a moderate photocatalytic performance.
Conclusions
In summary, as a kind of wholly aromatic framework porous material, PAF-45 has excellent adsorption capacity on aromatic compounds and good photocatalytic degradation performance of benzene series. With strong hydrophobicity, PAF-45 also shows excellent aromatic adsorption capacity in benzene simulation wastewater, whose adsorption capacity of the regenerated agent exhibits no significant reduction after 10 cycles of adsorption–desorption experiments. In general, PAF-45 also has been proved to be an effective material in aromatic pollutant treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial supports from Jilin University Key Laboratory of Automobile Materials of Ministry of Education (45119031B028), National Natural Science Foundation of China (U1607122, 51874145), Qinghai Basic Program under Grant (2017-ZJ-705), the Open Foundation of State Key Laboratory of Mineral Processing, China Ocean Mineral Resources R&D Association (COMRA) Special Foundation (DY135-46, DY135-R2-1-01), and the China Ocean Mineral Resources University co-construction project – funds for new materials (SXGJSF2017-3).Notes and references
- M. Zang, C. Zhao, Y. Wang and S. Chen, J. Saudi Chem. Soc., 2019, 23, 645–654 CrossRef.
- F. Villanueva, A. Tapia, S. Lara and M. Amo-Salas, Sci. Total Environ., 2018, 622, 222–235 CrossRef PubMed.
- S. Yurdakul, M. Civan, O. Ozden, E. Gaga, T. Dogeroglu and G. Tuncel, Atmos. Pollut. Res., 2017, 8, 1–12 CrossRef.
- Md. Aynul Bari and W. B. Kindzierski, Sci. Total Environ., 2018, 631–632, 627–640 CrossRef PubMed.
- Md. Aynul Bari and W. B. Kindzierski, Environ. Pollut., 2018, 235, 602–614 CrossRef PubMed.
- D. Hsu, H. Chiang, R. Shie, C. Ku, T. Lin, M. Chen, N. Chen and Y. Chen, Environ. Pollut., 2018, 240, 95–104 CrossRef PubMed.
- Md. A. l. Bari, W. B. Kindzierski, A. J. Wheeler, M. Heroux and L. A. Wallace, Build. Environ., 2015, 90, 114–124 CrossRef.
- H. Wang, L. Nie, J. Li, Y. Wang, G. Wang, J. Wang and Z. Hao, Chin. Sci. Bull., 2013, 58, 724–730 CrossRef CAS.
- H. Grigoryan, W. M. B. Edmands, Q. Lan, H. Carlsson, R. Vermeulen, L. Zhang, S. Yin, G. Li, M. T. Smith, N. Rothman and S. M. Rappaport, Carcinogenesis, 2018, 39, 661–668 CrossRef CAS PubMed.
- L. R. Teras, W. R. Diver, E. L. Deubler, D. Krewski, C. R. Flowers, J. M. Switchenko and S. M. Gapstur, Int. J. Cancer, 2019, 145, 2647–2660 CrossRef CAS PubMed.
- G. Zong, D. Valvi, B. Coull, T. Göen, F. B. Hua, F. Nielsen, P. Grandjean and Q. Sun, Environ. Int., 2018, 114, 334–342 CrossRef CAS PubMed.
- V. Mustielesa, M. F. Fernández, P. Martin-Olmedo, B. González-Alzaga, A. Fontalba-Navas, R. Hauser, N. Olea and J. P. Arrebola, Environ. Int., 2017, 104, 48–57 CrossRef PubMed.
- S. L. Suib, New and Future Developments in Catalysis: Catalysis for Remediation and Environmental Concerns, Elsevier, Amsterdam, The Netherlands, 2013 Search PubMed.
- X. Y. Zhang, B. Gao, A. E. Creamer, C. C. Cao and Y. C. Li, J. Hazard. Mater., 2017, 338, 102–123 CrossRef CAS PubMed.
- M. Vrijheid, M. Casas, M. Gascon, D. Valvi and M. Nieuwenhuijsen, Int. J. Hyg. Environ. Health, 2016, 219, 331–342 CrossRef CAS PubMed.
- L. Z. Liu, J. X. Li, H. B. Zhang, L. Li, P. Zhou, X. L. Meng, M. M. Guo, J. P. Jia and T. H. Sun, J. Hazard. Mater., 2019, 362, 178–186 CrossRef CAS PubMed.
- H. Mosmeria, F. Gholamib, M. Shavandia, S. M. M. Dastgheibc and E. Alaied, J. Hazard. Mater., 2019, 371, 183–190 CrossRef PubMed.
- C. Yang, G. Miao, Y. Pi, Q. Xia, J. Wu, Z. Li and J. Xiao, Chem. Eng. J., 2019, 370, 1128–1153 CrossRef CAS.
- J. Liu, P. Wang, W. Qu, H. Li, L. Shi and D. Zhang, Appl. Catal., B, 2019, 257, 117880 CrossRef CAS.
- L. Li, K. Cai, P. Y. Wang, H. Ren and G. S. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 201–208 CrossRef CAS PubMed.
- L. Xie, X. Liu, T. He and J. Li, Chem, 2018, 4, 1911–1927 CAS.
- G. Q. Zhang, W. Ou, J. Wang, Y. S. Xu, D. Xu, T. Sun, S. N. Xiao, M. R. Wang, H. X. Li, W. Chen and C. L. Su, Appl. Catal., B, 2019, 245, 114–121 CrossRef CAS.
- J. Wang, P. P. Zhang, C. C. Wang, X. D. Zheng, Y. J. Zhao, L. N. Li and S. D. Miao, Mater. Des., 2020, 186, 108371 CrossRef.
- Q. Luo, C. W. Zhao, G. X. Liu and H. Ren, Sci. Rep., 2016, 6, 20311 CrossRef CAS PubMed.
- D. Y. Zhao, Y. Y. Tian, X. F. Jing, Y. Lu and G. S. Zhu, J. Mater. Chem. A, 2019, 7, 157 RSC.
- R. Zhao, T. T. Ma, S. Y. Li, Y. Y. Tian and G. S. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 16662–16673 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05724j |
| ‡ Congcong Wang and Wei Wang contribute equally in this article. |
| This journal is © The Royal Society of Chemistry 2020 |