Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Z-scheme BiVO4/Ag/Ag2S composites with enhanced photocatalytic efficiency under visible light†
Yi Liu *abd,
Jiajia Chenc,
Jinfeng Zhangabd,
Zhongliang Tanga,
Haibin Lia and
Jian Yuana
*abd,
Jiajia Chenc,
Jinfeng Zhangabd,
Zhongliang Tanga,
Haibin Lia and
Jian Yuana
aCollege of Physics and Electronic Information, Huaibei Normal University, Huaibei, Anhui 235000, P. R. China. E-mail: yiliu@chnu.edu.cn
bKey Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education, Huaibei Normal University, Huaibei, Anhui 235000, P. R. China
cCollege of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui 235000, P. R. China
dAnhui Province Key Laboratory of Pollutant Sensitive Materials and Environmental Remediation, Huaibei Normal University, Huaibei, Anhui 235000, P. R. China
First published on 18th August 2020
Abstract
The Z-scheme BiVO4/Ag/Ag2S photocatalyst was fabricated via a two-step route. The as-prepared samples were characterized by XRD, FE-SEM, HRTEM, XPS and UV-vis diffuse reflectance spectroscopy. The results of PL and photocurrent response tests demonstrate that the ternary BiVO4/Ag/Ag2S composites had a high separation and migration efficiency of photoexcited carriers. As a result, the ternary photocatalyst exhibits enhanced photocatalytic activity for decomposing Rhodamine B (RhB) under LED light (420 nm) irradiation. The results of trapping experiments demonstrate both h+ and ˙OH play crucial roles in decomposing RhB molecules. Additionally, the energy band structures and density of states (DOS) of BiVO4 and Ag2S were investigated via the density functional theory (DFT) method. Finally, a Z-scheme electron migration mechanism of BiVO4 → Ag → Ag2S was proposed based on the experimental and calculated results.
1 Introduction
Nowadays, photocatalysis technology is regarded as one of the best ways to alleviate energy and environmental issues. TiO2 is a widely known photocatalyst for water splitting and decomposing organic pollutants. However, TiO2 is only sensitive to UV radiation due to its large bandgap (3.2 eV). Accordingly, a maximum of 6% of solar energy can be harvested using this material, which limits its practical application.1 This obvious disadvantage is the main motivation for the search for new and more efficient visible-light-driven photocatalysts.Among the developed photocatalysts, monoclinic structured BiVO4 has emerged as a potentially suitable visible-light-driven photocatalyst because its exceptional properties, such as narrow bandgap, good photocatalytic activity, and nontoxicity. However, the practical applications of BiVO4 is significantly limited owing to the weak charge-transfer rate and rapid recombination of photoinduced charge carriers.2,3 It has been reported that constructing semiconductor heterojunction structures could promote the photoexcited charge carrier separation, and hence improve the performance of photocatalyst. Cao et al. prepared Au/BiVO4 nanocomposites with excellent activity for water splitting owing to the improved charge separation and surface plasmon resonance of Au nanoparticles.4 Regmi et al. reported that Ag-modified BiVO4 sample exhibits better photocatalytic activity than BiVO4, which can effectively degrade bisphenol A and deactivate the Escherichia coli.5 Li et al. synthesized Ag3PO4/BiVO4 composites with type-II heterojunction, which can effectively degrade methylene blue (MB).6 Li et al. reported that the MoS2/BiVO4 composites also show excellent photoactivity for decoloring MB.7 Similarly, a number of studies of BiVO4 coupled with metal nanoparticles or other oxides were reported for the purpose of improving photocatalytic activity as well, such as Bi/BiVO4,8 Pd/BiVO4,9 CdS/BiVO4,10 g-C3N4/BiVO4,11,12 Bi2S3/BiVO4,13 WO3/BiVO4,14 Ag2O/BiVO4,15 Cu2O/BiVO4,16 and BiVO4/RGO.17
Ag2S has aroused much research interest owing to its narrow bandgap, which means that it can utilize more energy from the solar light spectrum. Therefore, Ag2S can act as photocatalyst or suitable sensitizer for photocatalysts under visible light irradiation. For example, Ghafoor et al. reported that Ag2S nanoparticles photosensitized TiO2 nanofibers display enhanced simulated solar light driven photocatalytic performance.18 Kumar et al. reported that a p–n heterojunction was fabricated by modifying NaNbO3 nanorods with Ag2S particle, which possesses excellent photoelectrochemical and photocatalytic activity.19 Jiang et al. found that compared with g-C3N4, the Ag2S/g-C3N4 photocatalyst exhibits improved activity for H2-evolution. Interestingly, Ag was formed in the process of H2-production. It was suggested that both Ag and Ag2S played a synergistic role in hydrogen production.20 Guan et al. prepared a p–n junction of Ag2S/BiVO4 with significantly improved photoelectrochemical water splitting ability.21 Zhao et al. reported that Ag2S/BiVO4 junction displays a better photocatalytic efficiency with the aid of plasmon resonance of Ag nanoparticles.22
However, it is known that the redox ability of photoexcited electrons and holes on reaction sites deteriorated during the charge transfer process in p–n or type-II heterojunction. As a result, the traditional semiconductor heterojunction could not exhibits both excellent charge-separation efficiency and powerful redox ability simultaneously.23 To overcome this drawback, the Z-scheme photocatalytic system has been developed. It was believed that the Z-scheme photocatalytic system not only promotes the separation of the photo-induced carriers, but also can optimizes carriers redox ability.24–26 For example, Tada et al. reported a Z-scheme CdS–Au–TiO2 composites with high photocatalytic activity, which exhibit an electron migration of TiO2 → Au → CdS.27 This unique electron migration is different with previously reported CdS/TiO2,28 which was attributed to the Au nanoparticles acting as the electron mediator. Yin et al. reported that C3N4/Cu2O photocatalyst with a p–n heterojunction can be changed to a Z-scheme system C3N4/Pd/Cu2O by inserting metal Pd into the C3N4/Cu2O interface, which has enhanced photocatalytic activity and stability.29 The inserted Pd nanocubes in the stack structure and the unique design are the critical roles in the formation of Z-scheme system. Deng et al. also synthesized a Z-scheme system BiVO4/Ag/Cu2O by deposing metallic Ag to the p–n heterojunction BiVO4/Cu2O.30 Lin et al. reported that a Z-scheme electron migration was confirmed in Ag3PO4/Ag/Bi2MoO6 composites, which is different with typical Ag3PO4/Bi2MoO6 type-II heterojunction.31,32 Li et al. found that the Ag/Ag3PO4/WO3 nanocomposites show better photocatalytic performance than traditional Ag3PO4/WO3 heterojunction, which can be attributed to the formation of Z-scheme structure.33 Zhao et al. prepared Z-scheme g-C3N4/Au/P25 composites with excellent photoactivity by introducing metal Au to the g-C3N4/P25 heterojunction.34 Liu et al. fabricated Z-scheme Cu2O/Au@CeO2 composites with strong redox ability by embedding Au nanoparticles in the yolk–shell Cu2O@CeO2 structure, which can effectively oxidize amines into imines.35 Bao et al. prepared efficient Z-scheme Cu2O/Cu/g-C3N4 photocatalyst for decomposing phenol via reduction route, in which partial metal Cu was preserved as a bridge for the transfer of photoexcited charge.36 Shen et al. reported that when reduced graphene oxide was introduced to a Cu2O/Fe2O3 type-II junction, the electron migration of Fe2O3 → RGO → Cu2O was confirmed in the Z-scheme RGO-Cu2O/Fe2O3 composites.37 Similarly, some Z-scheme photocatalysts were prepared by introducing Ag or Au to a type-II heterojunction, such as Ag2CO3/Ag/AgBr,38 g-C3N4/Ag/Ag3VO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 and Au/TiO2-gC3N4.40 Therefore, it was possible that a Z-scheme photocatalytic system could be fabricated by properly adding electron mediator to a p–n or type-II heterojunction.
39 and Au/TiO2-gC3N4.40 Therefore, it was possible that a Z-scheme photocatalytic system could be fabricated by properly adding electron mediator to a p–n or type-II heterojunction.
Herein, the Z-scheme system BiVO4/Ag/Ag2S photocatalyst was prepared via two-step route in this work. The photocatalytic activity of the synthesized Z-scheme BiVO4/Ag/Ag2S photocatalyst was investigated in this study. Additionally, the possible mechanism of the improved photocatalytic performance of BiVO4/Ag/Ag2S was discussed.
2 Experimental
2.1 Synthesis of photocatalysts
The BiVO4 sample was obtained via a previously reported route.41 0.3 g BiVO4 and 0.1 mmol AgNO3 were added into 50 mL of distilled (DI) water and stirred for 3 h. Then, the mixture was added with 5 mL of methanol and irradiated by a UV light (100 W) for 1 h. The resulting powders (BiVO4/Ag composites) were collected by centrifuge and washed with DI water and ethanol, then dried at 70 °C for 8 h. 0.3 g as-synthesized BiVO4/Ag composites was ultrasonically dispersed in 50 mL DI water. Then, AgNO3 was added and stirred for 1 h. Subsequently, 50 mL of Na2S solution was dropped into the mixture and stirred for 8 h under dark condition. Similarly, the obtained product was centrifuged, washed and dried, in which the weight ratio of BiVO4, Ag and Ag2S is 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
2.2 Characterization of the as-prepared samples
X-ray diffraction (XRD) was recorded by X-ray diffractometer (Bruker D8) with Cu Kα radiation. The samples' morphologies were characterized by field emission scanning electron microscopy (JSM 6701F) and high-resolution transmission electron microscopy (HRTEM, FEI Tecnai G2 F20 S-TWIN). X-ray photoelectron spectroscopy (XPS) was collected on ESCALAB 250Xi to identify the chemical compositions and the chemical states of the sample. UV-vis diffuse reflectance spectra (DRS) of the samples were acquired using BaSO4 as a reference material by a PerkinElmer Lambda 950 UV-vis spectrophotometer. Steady-state and time-resolved photoluminescence spectra (PL) were performed on FLS 920 fluorescence spectrometer.2.3 Photoelectrochemical measurements
The photocurrent responses of as-synthesized photocatalysts were investigated on a Zahner PP211 electrochemical workstation in a three-electrode cell. The FTO coated with photocatalysts served as the working electrode. A Pt wire and an Ag/AgCl (saturated KCl) electrode were applied as counter and reference electrode, respectively. The photocurrent measurements were carried out in 0.1 M Na2SO4 solution. A 30 W LED lamp with lighting wavelength of 420 nm was utilized as the light source. The light spectrum of the LED lamp is presented in Fig. S1.†2.4 Photocatalytic test
The photocatalytic activity of the samples was evaluated by photo-decomposing RhB. The obtained photocatalysts (50 mg) were dispersed into 50 mL RhB aqueous solution (10 mg L−1) and stirred in a dark condition for 30 min to ensure an adsorption–desorption equilibrium before irradiation. A LED lamp (30 W, 420 nm) was used as the visible light source. During the photodegradation experiment, the suspension was under continuous magnetic stirring. 5 mL of reaction solution was taken at 30 min intervals for analysis. The RhB concentration was monitored by Lambda 950 spectroscopy at a wavelength of 553 nm.2.5 Computational details
Energy band structures and DOS of BiVO4 and Ag2S were investigated by the DFT method, in which plane-wave pseudopotential with CASTEP code was adopted. The generalized gradient approximation with the Perdew–Burke–Ernzerhof was applied as the exchange and correlation terms. The cutoff energies of 450 eV were used for all calculations. Monkhorst–Pack K-points grids of 5 × 5 × 2 and 6 × 3 × 3 were applied for BiVO4 and Ag2S, respectively.3 Results and discussion
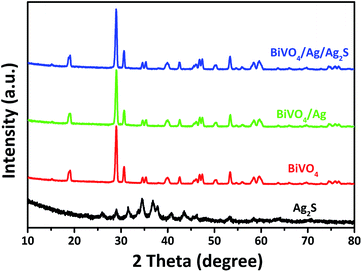
Fig. 1 presents the XRD patterns of the as-synthesized BiVO4, Ag2S, BiVO4/Ag and BiVO4/Ag/Ag2S samples. For the BiVO4 and Ag2S samples, all the peaks could be assigned to BiVO4 (JCPDS 14-0668) and Ag2S (JCPDS 14-0072) respectively. The XRD patterns of BiVO4, BiVO4/Ag and BiVO4/Ag/Ag2S samples were similar. No diffraction peaks from Ag are observed in the BiVO4/Ag sample. Similar phenomenon was observed in the XRD pattern of BiVO4/Ag/Ag2S sample and Ag2S was not detected as well. These results can be ascribed to the high crystallinity of BiVO4 powders and low contents of Ag and Ag2S. To further verify the existence of Ag and Ag2S, the as-obtained photocatalysts were further investigated by SEM, HRTEM and XPS.Fig. 2 shows the SEM, TEM and HRTEM images of the as-obtained products. As shown in Fig. 2a, plate-like BiVO4 powders were obtained, which have a thickness of ca. 350 nm. Furthermore, the particles surface is rather smooth and clean. Fig. 2b shows the SEM image of the BiVO4/Ag composites. Clearly, Ag nanoparticles with diameter of 50–100 nm are attached on the surface of BiVO4. It was suggested that metal Ag was deposited on the plate-like BiVO4 particles. Fig. 2c and d present the SEM images of BiVO4/Ag/Ag2S composites with different magnification. In contrast to Fig. 2b, fine nanoparticles with a size of ca. 5 nm are observed on the BiVO4 particles, which could be referred as Ag2S nanoparticles formed on BiVO4/Ag composites. In addition, the TEM and HRTEM images of the BiVO4/Ag/Ag2S composites were recorded to further reveal its composition (Fig. 2e–g). In Fig. 2g, the lattice spacing of 0.24 nm and 0.34 nm are corresponded to the crystal plane of Ag (111) and Ag2S (110), respectively. This result indicates that some Ag2S nanoparticles were formed on the Ag particles.
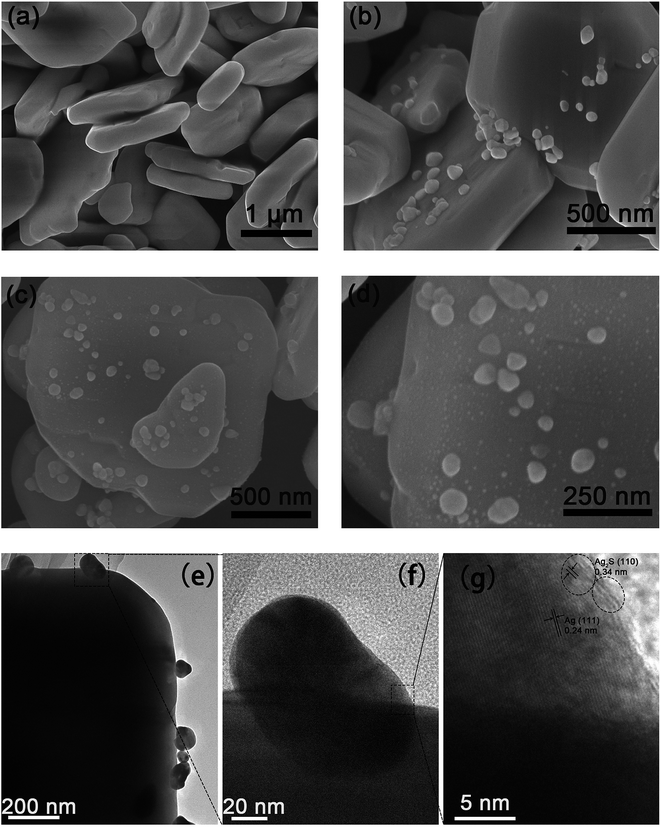 | ||
| Fig. 2 FESEM images of (a) BiVO4, (b) BiVO4/Ag and (c and d) BiVO4/Ag/Ag2S, (e and f) TEM and (g) HRTEM images of BiVO4/Ag/Ag2S composites. | ||
X-ray photoelectron spectroscopy (XPS) provided further evidence as to the existence of Ag and Ag2S in the ternary BiVO4/Ag/Ag2S composites. Ag, S, Bi, V and O elements were detected by the XPS survey spectrum of the ternary composites, as presented in Fig. 3a. From high resolution XPS analysis as depicted in Fig. 3b, two strong peaks at 373.5 and 374.2 eV were observed, which are indexed to Ag 3d5/2 and Ag 3d3/2, respectively. These two peaks could be fitted by four bands. Two peaks at 368.5 and 374.5 eV correspond to Ag0 3d5/2 and 3d3/2 respectively, which demonstrated the presence of metal Ag. Another two peaks at 368.0 and 374.0 eV correspond to Ag+ 3d5/2 and 3d3/2 respectively. Furthermore, the peak located at 160.8 eV can be assigned to S 2p binding energy for Ag2S (Fig. 3c). It demonstrates that both Ag and Ag2S were contained in the as-prepared BiVO4/Ag/Ag2S sample. The Fig. 3d exhibits two peaks at 159.2 and 164.5 eV, which are ascribed to Bi 4f7/2 and Bi 4f5/2 of Bi3+ in the BiVO4, respectively. Two peaks at 516.9 and 524.3 eV (Fig. 3e) are attributed to V 2p3/2 and V 2p1/2 of V5+ in the BiVO4, respectively. In Fig. 3f, the O 1s binding energy was observed at about 529.9 eV. The other O 1s peak can be attributed to –OH group or chemisorbed water molecule on BiVO4/Ag/Ag2S composites surface. Basing on the XPS result, the SEM and HRTEM images, it was suggested that the ternary BiVO4/Ag/Ag2S photocatalyst was successfully fabricated.
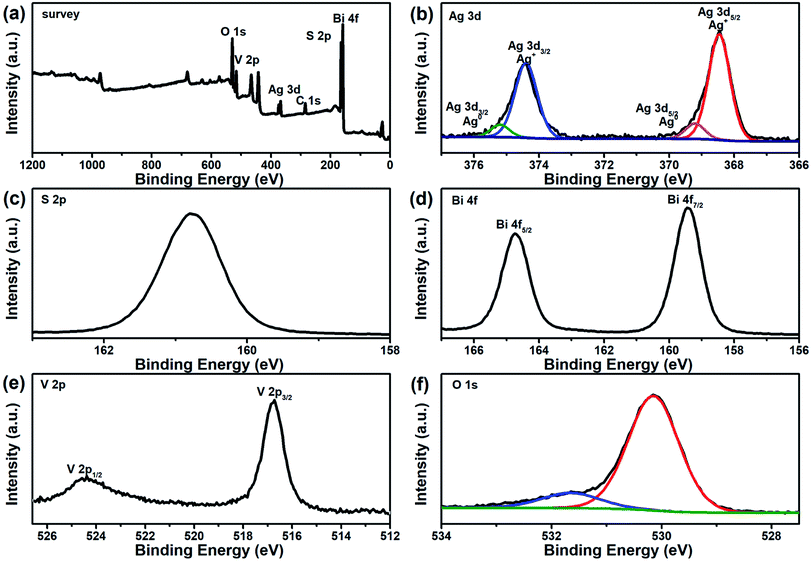 | ||
| Fig. 3 XPS spectra of BiVO4/Ag/Ag2S composites: (a) survey, (b) Ag 3d, (c) S 2p, (d) Bi 4f, (e) V 2p and (f) O 1s. | ||
Fig. 4a depicts the optical property of the as-prepared samples. It can be found that all BiVO4 based samples showed a strong absorption band in the 420–530 nm wavelength range, which suggested that they can absorb considerable amounts of visible light in this range. Therefore, the as-prepared BiVO4 based samples could act as visible-light-driven photocatalysts. Additionally, the bandgap of photocatalyst can be calculated by the following equation:42
| (αhν)n = A(hν − Eg) |
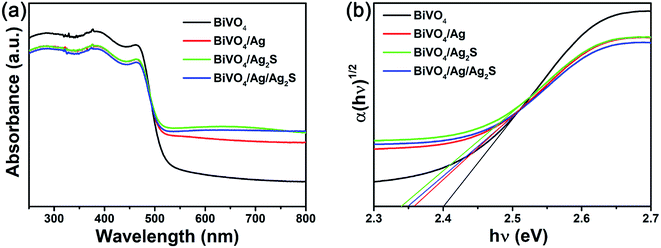 | ||
| Fig. 4 (a) UV-vis DRS absorption and (b) the plot of (αhν)1/2 vs. hν of the as-obtained BiVO4, BiVO4/Ag, BiVO4/Ag2S and BiVO4/Ag/Ag2S photocatalysts. | ||
Fig. 5a displays the PL spectra of BiVO4, BiVO4/Ag, BiVO4/Ag2S and BiVO4/Ag/Ag2S samples. It is widely accepted that high PL intensity suggests rapid recombination of excited pairs, and results in poor photocatalytic activity. The PL peak located at about 545 nm was recorded for all samples, which was result from the photogenerated carrier recombination in BiVO4 particles. Compared with BiVO4, both the BiVO4/Ag and BiVO4/Ag2S display lower PL intensity, indicating that the recombination of carriers could be suppressed by modifying with Ag or Ag2S nanoparticles. The ternary BiVO4/Ag/Ag2S sample shows the lowest PL intensity, which implying that it has the lowest carrier recombination rate. This is also confirmed by the time-resolved PL spectra (Fig. S2 and Table S1†). It is suggested that the synergistic effect of the Ag and Ag2S nanoparticles play a key role to promote the separation of photoexcited carriers in the BiVO4/Ag/Ag2S composites.
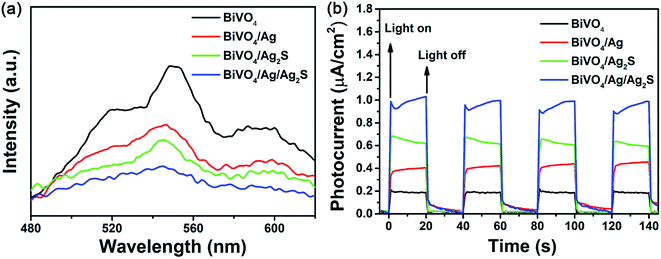 | ||
| Fig. 5 (a) PL spectrum and (b) photocurrent responses of the as-obtained BiVO4, BiVO4/Ag, BiVO4/Ag2S and BiVO4/Ag/Ag2S photocatalysts. | ||
To further understand the separation and migration efficiency of photoinduced electron–hole pairs, the photocurrent responses under dark and LED light irradiation were measured. As shown in Fig. 5b, the photocurrents of the prepared samples were stable and reversible at light-on and light-off. It is well known that larger photocurrent indicated a higher separation efficiency of photoexcited carriers. In comparison with BiVO4, BiVO4/Ag and BiVO4/Ag2S, BiVO4/Ag/Ag2S composites exhibited the highest photocurrent response density, about 4.5, 2.2 and 1.5 times that of BiVO4, BiVO4/Ag and BiVO4/Ag2S, respectively. It indicates that the BiVO4/Ag/Ag2S composites exhibit efficient charge separation, which is in agreement with the PL spectroscopy results.
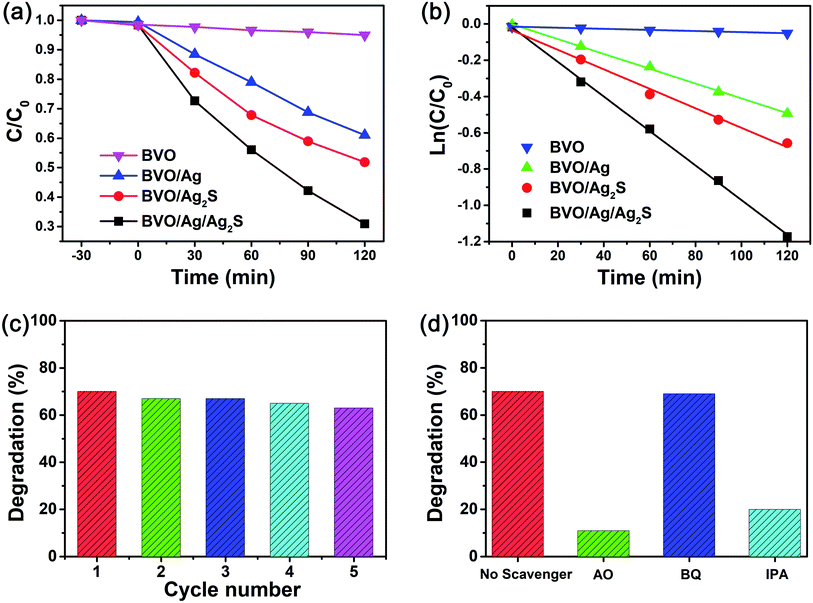
Fig. 6a depicts the photoactivity of the as-synthesized catalysts, which was estimated by degrading RhB. When the pure BiVO4 sample was used as photocatalyst, only about 5% of RhB was degraded after 120 min irradiation. For the binary catalysts of BiVO4/Ag and BiVO4/Ag2S, about 39% and 42% of RhB were degraded, respectively. It indicates that the photocatalytic activity of BiVO4 could be simply enhanced by decorating with Ag or Ag2S nanoparticles. The BiVO4/Ag/Ag2S photocatalyst shows excellent photocatalytic performance compared with other as-prepared samples. About 69% of RhB were degraded by the ternary composites. To directly show the improved photocatalytic activity of BiVO4/Ag/Ag2S sample, the degradation kinetics for the removal of RhB were calculated by the pseudo-first-order reaction:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C/C0 = −k × t C/C0 = −k × t |
The stability of photocatalyst is a crucial issue in its practice application. Fig. 6c shows the recycled experiments of the photodegradation of RhB by using the BiVO4/Ag/Ag2S composites for five cycles (120 min LED light irradiation for each cycle). It can be seen that only slight deterioration of the BiVO4/Ag/Ag2S sample was observed after five cycles, which implies the good recyclability of the prepared photocatalyst.
As is known, the superoxide radical (˙O2−), holes (h+) and hydroxyl radical (˙OH) as active species play crucial roles in photocatalytic reaction.43,44 In order to evaluate the main active species, the trapping experiments were carried out. The reactive species can be removed by adding corresponding scavengers into reaction solutions. The function of different reactive species could be made clear based on the change of photocatalytic performance. In this study, p-benzoquinone (BQ), ammonium oxalate (AO) and isopropanol (IPA) were added to act as ˙O2−, h+ and ˙OH scavenger, respectively. As presented in Fig. 6d, the ability of the BiVO4/Ag/Ag2S sample to remove RhB was not affected by adding BQ, which suggesting that ˙O2− radical does not participate in the photocatalytic reaction. However, when BQ and AO were added, the photocatalytic activity of the BiVO4/Ag/Ag2S composites decreased significantly. It demonstrates that h+ and ˙OH play important roles in the photocatalytic process.
To clarify the mechanism of the improved photocatalytic activity of the BiVO4/Ag/Ag2S composites, the band structure of the ternary system was studied. The band edge potentials of valence band (VB) and conduction band (CB) of BiVO4 and Ag2S can be estimated according the following equations:
| EVB = χ − Ee + 0.5Eg |
| ECB = EVB − Eg |
Fig. 7 presents the band structures and DOS of BiVO4 and Ag2S. The valence band maximum (VBM) and conduction band minimum (CBM) are located at Z and P point, respectively (Fig. 7a), which imply that BiVO4 is an indirect bandgap semiconductor. Ag2S belongs to the direct bandgap semiconductor because the position of VBM is same as that of CBM, as demonstrated in Fig. 7b. The bandgaps of BiVO4 and Ag2S are calculated to be 2.16 eV and 0.97 eV, respectively. However, these calculated bandgaps are smaller than experimental values (2.40 eV for BiVO4 and 1.46 eV for Ag2S), which are resulted from the well-known drawback of DFT calculations.46 As shown in Fig. 7c, the upper VB of BiVO4 consists largely of O 2p, V 3d and a small amount of Bi 6s and Bi 6p states. Meanwhile, the CB of BiVO4 mainly consist of O 2p, V 3d and Bi 6p states. For Ag2S (Fig. 7d), the upper VB is mainly composed by Ag 4d states and a few of S 3p states, and the CB is mainly occupied by Ag 4p, Ag 5s and S 3p states. It is reasonable that the S 3p states result in the narrow bandgap of Ag2S.47
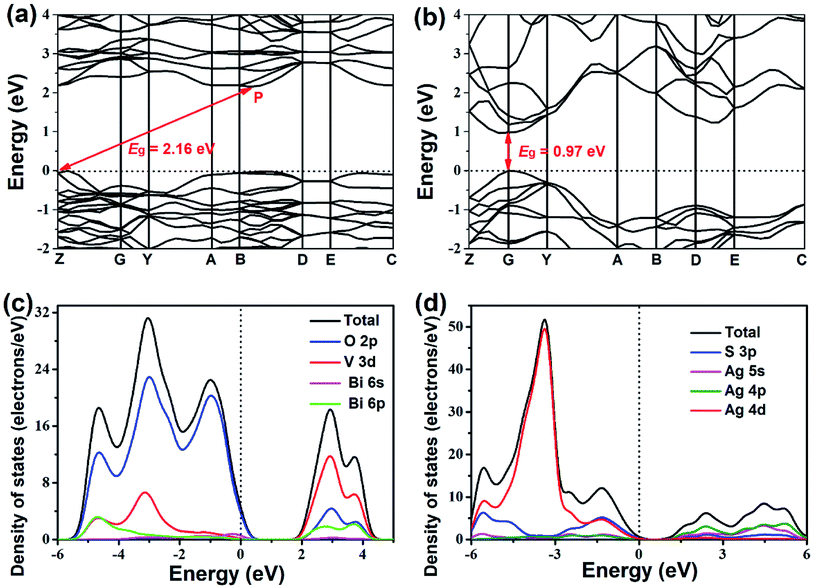 | ||
| Fig. 7 Electronic band structures of (a) BiVO4 and (b) Ag2S and density of states of (c) BiVO4 and (d) Ag2S. | ||
Based on the above results and discussion, the mechanism of the photodecomposition of RhB with the BiVO4/Ag/Ag2S composites is proposed, as shown in Fig. 8. When the ternary BiVO4/Ag/Ag2S composites were irradiated by LED light (420 nm), electrons and holes were generated in the CB and VB of BiVO4 and Ag2S. It is important to notice that the VB potential of Ag2S (1.195 V vs. NHE) is above the ˙OH/OH− potential (2.27 V vs. NHE at pH = 7),48 which demonstrating that the holes photogenerated in the VB of Ag2S cannot reacted with OH− to produce ˙OH active species. Therefore, it is not supposed that the photoinduced holes transfer from BiVO4 to Ag2S according to the traditional p–n heterojunction. It is suggested that a Z-scheme electron migration emerges in the ternary photocatalyst. The photoexcited electrons in the CB of BiVO4 transferred to Ag and then recombined with the photoinduced holes in the VB of Ag2S, which resulting in a better charge separation. The EVB of BiVO4 (3.37 V vs. NHE) is below the ˙OH/OH− potential. Hence, the photoexcited holes in the VB of BiVO4 could reacted with OH− to generate ˙OH.49 Additionally, the holes of BiVO4 can directly oxidized the RhB dye.8 Consequently, both h+ and ˙OH play crucial roles in decomposing RhB molecule, which is consist with the trapping experimental results.
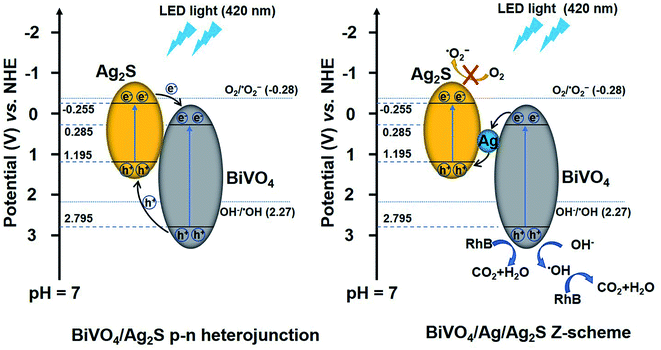 | ||
| Fig. 8 Schematic illustrating the proposed degradation mechanism of RhB over the ternary BiVO4/Ag/Ag2S composites. | ||
4 Conclusions
The Z-scheme system BiVO4/Ag/Ag2S photocatalyst was successfully prepared via two-step method. The as-synthesized ternary BiVO4/Ag/Ag2S composites exhibit higher separation efficiency and reduction of carrier recombination rate, which result in enhanced photocatalytic efficiency for decomposing RhB under LED light irradiation. The photocatalytic mechanism over the ternary composites was investigated in detail. It is suggested that Z-scheme electron migration of BiVO4 → Ag → Ag2S exists in the ternary system.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Anhui Provincial Natural Science Foundation (1908085QE222, 1708085QE119, 2008085QF319), the Natural Science Foundation of the Anhui Higher Education Institutions of China (KJ2019A0594, KJ2019A0595, KJ2019A0596) and the National Natural Science Foundation of China (51973078).References
- H. Xu, S. Ou Yang, L. Q. Liu, P. Reunchan, N. Umezawa and J. H. Ye, J. Mater. Chem. A, 2014, 2, 12642–12661 RSC.
- A. Malathi, J. Madhavan, M. Ashokkumar and P. Arunachalam, Appl. Catal., A, 2018, 555, 47–74 CrossRef CAS.
- H. L. Tan, R. Amal and Y. H. Ng, J. Mater. Chem. A, 2017, 5, 16498–16521 RSC.
- S. W. Cao, Z. Yin, J. Barber, F. Y. C. Boey, S. C. J. Loo and C. Xue, ACS Appl. Mater. Interfaces, 2012, 4, 418–423 CrossRef CAS PubMed.
- R. Chhabilal, D. Dipesh and L. Soo Wohn, Nanotechnology, 2018, 29, 064001 CrossRef PubMed.
- C. J. Li, P. Zhang, R. Lv, J. W. Lu, T. Wang, S. P. Wang, H. F. Wang and J. L. Gong, Small, 2013, 9, 3951–3956 CrossRef CAS PubMed.
- H. L. Li, K. Yu, X. Lei, B. J. Guo, H. Fu and Z. Q. Zhu, J. Phys. Chem. C, 2015, 119, 22681–22689 CrossRef CAS.
- Q. F. Jing, X. Y. Feng, J. L. Pan, L. M. Chen and Y. N. Liu, Dalton Trans., 2018, 47, 2602–2609 RSC.
- L. Y. Wang and Z. Y. Bian, Chemosphere, 2020, 239, 124815 CrossRef CAS PubMed.
- R. Guo, A. G. Yan, J. J. Xu, B. T. Xu, T. T. Li, X. W. Liu, T. F. Yi and S. H. Luo, J. Alloys Compd., 2020, 817, 153246 CrossRef CAS.
- J. Safaei, H. Ullah, N. A. Mohamed, M. F. Mohamad Noh, M. F. Soh, A. A. Tahir, N. Ahmad Ludin, M. A. Ibrahim, W. N. R. Wan Isahak and M. A. Mat Teridi, Appl. Catal., B, 2018, 234, 296–310 CrossRef CAS.
- Z. S. Zhang, M. Wang, W. Q. Cui and H. Sui, RSC Adv., 2017, 7, 8167–8177 RSC.
- W. Wang, X. W. Wang, C. X. Zhou, B. Du, J. X. Cai, G. Feng and R. B. Zhang, J. Phys. Chem. C, 2017, 121, 19104–19111 CrossRef CAS.
- C. Yin, S. M. Zhu and D. Zhang, RSC Adv., 2017, 7, 27354–27360 RSC.
- J. P. Ren and Y. Y. Zhu, RSC Adv., 2020, 10, 6114–6120 RSC.
- W. Wang, X. Huang, S. Wu, Y. Zhou, L. Wang, H. Shi, Y. Liang and B. Zou, Appl. Catal., B, 2013, 134, 293–301 CrossRef.
- Y. Wang, W. Wang, H. Mao, Y. Lu, J. Lu, J. Huang, Z. Ye and B. Lu, ACS Appl. Mater. Interfaces, 2014, 6, 12698–12706 CrossRef CAS PubMed.
- S. Ghafoor, S. Ata, N. Mahmood and S. N. Arshad, Sci. Rep., 2017, 7, 255 CrossRef PubMed.
- S. Kumar, A. P. Singh, C. Bera, M. Thirumal, B. R. Mehta and A. K. Ganguli, ChemSusChem, 2016, 9, 1850–1858 CrossRef CAS PubMed.
- D. L. Jiang, L. L. Chen, J. M. Xie and M. Chen, Dalton Trans., 2014, 43, 4878–4885 RSC.
- P. Guan, H. Y. Bai, F. G. Wang, H. Yu, D. B. Xu, B. Y. Chen, T. Xia, W. Q. Fan and W. D. Shi, ChemCatChem, 2018, 10, 4941–4947 CrossRef CAS.
- Z. Wei, D. Ben Lin, Z. Feng Xia, T. Xin Yue, X. Ji Ming, Z. Li Li, L. Shi Yin, D. Y. C. Leung and C. Sun, Appl. Catal., B, 2018, 229, 171–180 CrossRef CAS.
- P. Zhou, J. G. Yu and M. Jaroniec, Adv. Mater., 2014, 26, 4920–4935 CrossRef CAS PubMed.
- H. Li, W. G. Tu, Y. Zhou and Z. G. Zou, Adv. Sci., 2016, 3, 1500389 CrossRef PubMed.
- M. Liu, L. Z. Qiao, B. B. Dong, S. Guo, S. Yao, C. Li, Z. M. Zhang and T. B. Lu, Appl. Catal., B, 2020, 273, 119066 CrossRef CAS.
- L. X. Zhong, B. D. Mao, M. Liu, M. Y. Liu, Y. Q. Sun, Y. T. Song, Z. M. Zhang and T. B. Lu, J. Energy Chem., 2021, 54, 386–394 CrossRef.
- H. Tada, T. Mitsui, T. Kiyonaga, T. Akita and K. Tanaka, Nat. Mater., 2006, 5, 782–786 CrossRef CAS PubMed.
- N. Qin, Y. H. Liu, W. M. Wu, L. J. Shen, X. Chen, Z. H. Li and L. Wu, Langmuir, 2015, 31, 1203–1209 CrossRef CAS PubMed.
- W. J. Yin, L. J. Bai, Y. Z. Zhu, S. X. Zhong, L. H. Zhao, Z. Q. Li and S. Bai, ACS Appl. Mater. Interfaces, 2016, 8, 23133–23142 CrossRef CAS PubMed.
- Y. C. Deng, L. Tang, G. M. Zeng, C. Y. Feng, H. R. Dong, J. J. Wang, H. P. Feng, Y. Liu, Y. Y. Zhou and Y. Pang, Environ. Sci.: Nano, 2017, 4, 1494–1511 RSC.
- X. Lin, J. Hou, S. S. Jiang, Z. Lin, M. Wang and G. B. Che, RSC Adv., 2015, 5, 104815–104821 RSC.
- Y. S. Xu and W. D. Zhang, Dalton Trans., 2013, 42, 1094–1101 RSC.
- Q. Y. Li, F. L. Wang, Y. X. Hua, Y. T. Luo, X. H. Liu, G. R. Duan and X. J. Yang, J. Colloid Interface Sci., 2017, 506, 207–216 CrossRef CAS PubMed.
- W. R. Zhao, L. H. Xie, M. Zhang, Z. Y. Ai, H. P. Xi, Y. J. Li, Q. M. Shi and J. S. Chen, Int. J. Hydrogen Energy, 2016, 41, 6277–6287 CrossRef CAS.
- Y. Y. Liu, Y. J. Chen, W. Zhou, B. J. Jiang, X. Zhang and G. H. Tian, Catal. Sci. Technol., 2018, 8, 5535–5543 RSC.
- Y. C. Bao and K. Z. Chen, Mol. Catal., 2017, 432, 187–195 CrossRef CAS.
- H. Q. Shen, G. W. Liu, X. Yan, J. H. Jiang, Y. Z. Hong, M. Yan, B. D. Mao, D. Li, W. Q. Fan and W. D. Shi, Mater. Today Energy, 2017, 5, 312–319 CrossRef.
- J. J. Li, Y. L. Xie, Y. J. Zhong and Y. Hu, J. Mater. Chem. A, 2015, 3, 5474–5481 RSC.
- J. J. Wu, X. P. Shen, X. L. Miao, Z. Y. Ji, J. H. Wang, T. Wang and M. M. Liu, Eur. J. Inorg. Chem., 2017, 2017, 2845–2853 CrossRef CAS.
- C. Marchal, T. Cottineau, M. G. Méndez-Medrano, C. Colbeau-Justin, V. Caps and V. Keller, Adv. Energy Mater., 2018, 8, 1702142 CrossRef.
- W. Z. Wang, X. W. Huang, S. Wu, Y. X. Zhou, L. J. Wang, H. L. Shi, Y. J. Liang and B. Zou, Appl. Catal., B, 2013, 134, 293–301 CrossRef.
- M. A. Butler, J. Appl. Phys., 1977, 48, 1914–1920 CAS.
- H. B. Fu, C. S. Pan, W. Q. Yao and Y. F. Zhu, J. Phys. Chem. B, 2005, 109, 22432–22439 CAS.
- G. Sivalingam, K. Nagaveni, M. S. Hegde and G. Madras, Appl. Catal., B, 2003, 45, 23–38 CrossRef CAS.
- S. I. Sadovnikov, Y. V. Kuznetsova and A. A. Rempel, Nano-Struct. Nano-Objects, 2016, 7, 81–91 CrossRef CAS.
- J. P. Perdew and M. Levy, Phys. Rev. Lett., 1983, 51, 1884–1887 CrossRef CAS.
- K. Zhang and L. j. Guo, Catal. Sci. Technol., 2013, 3, 1672–1690 RSC.
- M. Kaneko and I. Okura, Photocatalysis: science and technology, Springer, 2002 Search PubMed.
- C. S. Turchi and D. F. Ollis, J. Catal., 1990, 122, 178–192 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05712f |
| This journal is © The Royal Society of Chemistry 2020 |