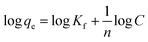Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gold nanoparticle decorated titania for sustainable environmental remediation: green synthesis, enhanced surface adsorption and synergistic photocatalysis†
Maheshika Perera‡
a,
Lahiru A. Wijenayaka *ab,
Kumudu Siriwardanaa,
Damayanthi Dahanayake
*ab,
Kumudu Siriwardanaa,
Damayanthi Dahanayake a and
K. M. Nalin de Silva
a and
K. M. Nalin de Silva ac
ac
aSri Lanka Institute of Nanotechnology (SLINTEC), Mahenwatte, Pitipana, Homagama, 10200, Sri Lanka. E-mail: lawij@ou.ac.lk
bDepartment of Chemistry, The Open University of Sri Lanka, Nawala, 11222, Sri Lanka
cCentre for Advanced Materials and Devices (CAMD), Department of Chemistry, University of Colombo, Colombo, 00300, Sri Lanka
First published on 11th August 2020
Abstract
Developing materials for efficient environmental remediation via cheap, nontoxic and environmentally benign routes remains a challenge for the scientific community. Here, a novel, facile, and green synthetic approach to prepare gold nanoparticle decorated TiO2 (Au/TiO2) nanocomposites for sustainable environmental remediation is reported. The synthesis involved only TiO2, metal precursor and green tea, obviating the need for any solvents and/or harsh chemical reducing or stabilizing agents, and was efficiently conducted at 50 °C, indicating the prominent sustainability of the novel synthetic approach. The synthesis indicated notable atom economy, akin to that observed in a typical chemical mediated synthesis while high-resolution transmission electron microscopy (HRTEM) findings suggest the presence of a pertinent decoration of spherical and homogeneous gold nanoparticles on the titania surface. Notably, the Au/TiO2 nanocomposite demonstrated appreciable stability during preparation, subsequent processing and prolonged storage. Further, the nanocomposite was found to have a superior adsorption capacity of 8185 mg g−1 towards methylene blue (MB) in solution using the Freundlich isotherm model, while the rate constants for the photocatalytic degradation of MB on the nanocomposite under UV irradiation indicated a 4.2-fold improvement compared to that of bare TiO2. Hence, this novel green synthesized Au/TiO2 nanocomposite shows promising potential for sustainable environmental remediation via efficient contaminant capture and subsequent synergistic photocatalysis.
Introduction
Among the many metal oxide semiconductor based photocatalysts, titanium dioxide (TiO2) is the most widely used, owing to its excellent optical transmittance, high refractive index and chemical stability, while concurrently being stable, nontoxic, and inexpensive.1 However, the wide band gap (∼3.2 eV) of TiO2 allows absorption of energy in the UV region of the solar spectrum, corresponding to only ∼5% of the solar spectrum.2,3 Additionally, TiO2 inherently suffers from the fast recombination of photogenerated excitons.4 Typically, for TiO2, the charge recombination time has been determined to be in the order of 10−9 s, while the chemical reaction time between photogenerated charges and any adsorbed species is 10−8 to 10−3 s.4 Hence, the fast recombination of charges hinder the photocatalytic reactivity of a species adsorbed on a TiO2 nanoparticle catalyst. TiO2, therefore, is far from being a perfect photocatalyst regardless of its widespread applications.Nevertheless, there is ample room to further improve the photocatalytic activity of TiO2-based photocatalysts. According to recent findings, the localized surface plasmon resonance (LSPR) photosensitization or the electromagnetic field enhancement of catalytic material via the deposition of noble metal nanoparticles has been reported as an effective strategy in enhancing visible light absorption,5–7 thus leading to significantly improved photocatalysis. Notably, here the chemically inert metal is in a separate phase in interfacial contact with semiconducting titania, in contrast to what would result from doping.8 This strategy has been reported to be very effective in enhancing photocatalysis as the Fermi levels of noble metals are lower than that of TiO2. This allows the photo-excited electrons to be transferred from the conduction band (CB) to metal particles deposited on the TiO2 surface, while photo-generated valence band (VB) holes remain on the latter, thereby diminishing the possibility of electron–hole recombination.4
Noble metal nanoparticles such as gold (Au) and silver (Ag) have tremendous interest in photo reactions since their optical and electronic properties are highly tunable by changing the size, shape, and surface charge. As per recent literature, there are several reports on gold nanoparticles (AuNPs) loaded onto TiO2, where the immobilization of AuNPs on TiO2 produces visible light induced photocatalysis for the oxidation of organic substances in water.9 In their recent work, Guo et al. has demonstrated the development of Au@TiO2 plasmonic films with enhanced photocatalysis resulting from the surface plasmonic resonance of isolated AuNPs in TiO2 nanocavities and suppressed electron recombination.10
Li et al. have reported the synthesis of highly active mesoporous titania photocatalyst by embedding gold nanoparticles homogenously within the framework, where significantly improved photocatalytic activity is observed due to enhanced light absorption and improved quantum efficiency.11 Additionally, Bian et al. have demonstrated that the modification of TiO2 mesocrystals with AuNPs allows a strong photoelectrochemical response in the visible electromagnetic region. Diffuse reflectance spectroscopy measurements have demonstrated that a substantial portion of electrons injected from the AuNPs to TiO2 through the plasmonic excitation, anisotropically migrate through the TiO2 nanocrystal significantly hindering potential charge recombination.7
However, coupling of metal nanomaterials to a catalytic surface would typically utilize non-sustainable methods incorporating harsh or even hazardous chemicals and strong reaction conditions to allow efficient preparation of nanoparticles. Additionally, although TiO2 has been used as a white pigment from ancient times, while its safety to humans and environment is well established,12 reagents and conditions used for coupling of AuNPs onto it may hinder the intrinsic environmental and biological compatibility of titania. Hence, the development of composite materials for the efficient environmental remediation via cheap, nontoxic and environmentally benign routes remains a challenge to the scientific community.
Notably, biocompatibility of nanoparticles may be significantly enhanced via the use of biogenic synthetic pathways such as the use of microorganisms or plant-based extracts in the synthesis of nanoparticles.13–15 Although the incorporation of AuNPs to TiO2 thus enhancing the photocatalysis of the latter is well demonstrated, there are no previous accounts of sustainable green approaches to develop such eco-friendly and innocuous composite nanomaterials. Additionally, although many recent scientific efforts have focused on the preparation of novel nanomaterials that are proficient in environmental remediation, the focus has continued to be on the light mediated degradation of contaminants; or photocatalysis. Thus, other mechanisms by which efficient contaminant capture and removal can be conducted, such as surface adsorption which is facile and spontaneous, are relatively less exploited.16–18
Here, the preparation of a AuNP decorated TiO2 nanocomposite using an entirely green chemical synthesis approach is reported. AuNPs were synthesized by reducing HAuCl4 onto TiO2 particles using a green tea extract. Green tea here acts both as a reducing and stabilizing agent, thus obviating the need to utilize any auxiliary chemicals during the preparation and/or the application of the catalyst. To the best of our knowledge, there is no previous report on the synthesis of AuNP decorated TiO2 using an entirely green chemical approach for efficient environmental remediation through contaminant capture and photocatalysis.
The environmental remediation efficiency of the AuNP decorated TiO2 nanocomposite was investigated via (1) adsorption and (2) photodegradation of methylene blue (MB). As per the findings, the decoration of the TiO2 surface with AuNPs significantly increases the MB adsorption capacity of the catalyst while demonstrating an improved photocatalytic degradation rate constant for the same. Overall, this indicates the significant aptitude of the green synthesized Au/TiO2 nanocomposite towards proficient environmental remediation.
Methods
Materials and instruments
Titanium dioxide (TiO2) (anatase) and tetrachloroauric(III) acid trihydrate (HAuCl4·3H2O) were purchased from Sigma Aldrich (USA) and Sisco Research Laboratories Pvt. Ltd. (India) respectively. Green tea extract was obtained from Fragrance Oils (International) Limited, UK. Methylene blue (MB) was obtained from HiMedia Laboratories (India). A Shimadzu UV-3600 UV-Vis-NIR spectrophotometer was used for all UV-visible spectroscopic and Localized Surface Plasmon Resonance (LSPR) measurements and centrifugation was performed using a Vision Scientific VS-15000N centrifuge. The hydrodynamic radii of the Au@Ag nanoparticles were determined via dynamic light scattering using a Malvern Nano-ZS zetasizer. Ultrapure water (conductivity < 0.05 μS cm−1) obtained from an Evoqua Water Technologies ultrapure water system was used in all synthesis, preparation, and experimental procedures.Synthesis of the Au/TiO2 nanocomposite
TiO2 powder was ground into fine particles and was suspended in ultrapure water to prepare a 250 ppm suspension. This was sonicated for 30 minutes to ensure the efficient dispersion of TiO2 particles resulting in a homogeneous milky suspension. Then, 25.0 mL of the as-prepared TiO2 suspension was heated to 50 °C in a water bath under continuous stirring. HAuCl4·3H2O (5 mM, 10.0 mL) and a solution containing the diluted green tea extract (25% v/v, 10.0 mL) were simultaneously added dropwise into the TiO2 suspension at a rate of 0.5 mL min−1 using a burette and a programmable syringe pump respectively. Here, the reduction of Au3+ ions was evidenced by a pink coloration of the originally milky suspension within few minutes after initiating the addition. The mixture was stirred for an additional 30 minutes after the complete addition of reactants to allow sufficient time for the reaction while maintaining the temperature at 50 °C. Then, the solution was removed from the water bath and was allowed to gradually cool to room temperature while stirring. The resulting Au/TiO2 nanocomposite was centrifuged at 7000 rpm for 10 minutes to remove the excess, unreacted reagents and was re-suspended in ultrapure water to ensure the original TiO2 concentration (i.e. 250 ppm). The as-purified Au/TiO2 nanocomposite was stored at 4 °C and was used as needed for characterization and analysis.Microscopic characterization
A drop of the Au/TiO2 nanocomposite dispersion was casted on a holey carbon copper grid and was allowed to dry at room temperature. Electron microscopic images were obtained using a high-resolution transmission electron microscope (HRTEM) (Jeol JEM 2100) operated at accelerating voltage of 200 kV. The elemental distribution on the sample area was analyzed using an energy dispersive X-ray (EDX) spectroscopy using a detector (EDAX-Ametek) attached to the HRTEM and the elemental composition was analyzed with elemental mapping in scanning transmission electron microscopic (STEM) mode at an accelerating voltage of 200 kV. Furthermore, the electron energy loss spectroscopic (EELS) image of gold nanoparticles was acquired with an EELS spectrometer (GATAN 963 EELS spectrometer) attached to the HRTEM with an energy resolution of 0.25 eV per channel in STEM spectral imaging mode.Dye adsorption on Au/TiO2
MB solutions of different known concentrations between 2–100 ppm were prepared. Each MB solution was mixed with a portion of the Au/TiO2 nanocomposite suspension (250 ppm) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio, followed by incubation in darkness for 1 hour under continuous stirring. The resulting solutions were centrifuged to precipitate all solid material, along with any MB adsorbed on Au/TiO2 nanocomposites. Then, the supernatant was analyzed using UV-visible spectroscopy to quantify the unadsorbed MB remaining in solution.
1 volume ratio, followed by incubation in darkness for 1 hour under continuous stirring. The resulting solutions were centrifuged to precipitate all solid material, along with any MB adsorbed on Au/TiO2 nanocomposites. Then, the supernatant was analyzed using UV-visible spectroscopy to quantify the unadsorbed MB remaining in solution.
Photocatalytic dye degradation on Au/TiO2
Equal volumes of Au/TiO2 nanocomposite suspension (250 ppm) and a standard MB solution were mixed and incubated in darkness for ∼1 hour under continuous stirring. Then, identical volumes of the MB–nanocomposite mixture were transferred into Petri dishes and the samples were irradiated with UV-C light for a known duration. The resulting solutions were then centrifuged at a high centrifugal force to precipitate all solid material, along with any adsorbed MB, and the supernatant was analyzed using UV-visible spectroscopy to quantify the unadsorbed and unphotoreacted MB remaining in solution. The experiment was repeated for different durations of UV irradiation and for bare TiO2.Results and discussion
Here, a novel method was developed for the facile modification catalytic titania with AuNPs via an entirely green synthetic approach mediated by green tea. The green tea extract contained a mixture of polyphenols such as catechin, epicatechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, epigallocatechin methylgallate, gallocatechin, and gallocatechin gallate, as well as caffeine, gallic acid, L-theanine, theobromine, and theophylline, similar to previous reports on green tea.19,20 The conditions used for the reduction of Au3+ into the AuNPs were previously optimized in a separate synthetic approach that was conducted in the absence of any TiO2 in the synthesis medium such as to produce significantly stable AuNPs of uniform size and morphology.Notably, doping of the titania surface with gold nanoparticles has been extensively studied. According to Shibata et al. scanning transmission electron microscopy investigations along with density functional theory calculations have indicated that AuNPs preferentially attach to specific sites on the TiO2 surface forming an epitaxial and coherent heterointerface.21 Additionally, Matthey et al. have theoretically shown strong adhesion of gold clusters on TiO2.22 Hence, it was hypothesized that if the synthesis of AuNPs was conducted in the presence of TiO2 particles, the nucleation of AuNPs could occur on the TiO2 surface. Hence, eventually, a procedure was conducted (vide supra) using the optimized synthetic conditions to repeat the synthesis of AuNPs in the presence of TiO2 particles suspended in the synthetic medium, where Au3+ is reduced to form AuNPs that systematically deposit decorating the TiO2 surface.
The facile green synthetic approach adopted for Au/TiO2 nanocomposite here involved only the Au precursor and the green tea extract being concurrently added to a titania suspension, where the oxidation-prone polyphenols present in the extract are likely responsible for the reducing action.16,23,24 Typically, synthesis of AuNPs requires the presence of stabilizing agents to allow the produced nanoparticles to be stably dispersed in solution. Additionally, many synthetic approaches for AuNPs, including the well-known Turkevich method utilize harsh reaction conditions such as boiling temperatures in order for the chemical reactivity to prevail.25 In contrast, green tea here serves dual roles of a reducing and a stabilizing agent, hence precluding the need for any auxiliary chemical species, while the synthesis efficiently took place at 50 °C; a relatively mild temperature compared to the typical boiling conditions employed during the synthesis of AuNPs.
However, regardless of the absence of any strong reducing and/or stabilizing agent and at the mild temperature used, it was observed that the Au3+ reduced forming a pertinent nanoparticle decoration on the titania surface as evidenced by the clear reddish hue. This observation was akin to that expected in a chemical-mediated synthetic method, indicating the notable atom economy of the synthetic approach. Additionally, this color was stable during purification via centrifugation and under storage for prolonged durations, indicating the remarkable stability of the nanoparticles. Further, the Au/TiO2 nanocomposite could be efficiently recovered from the synthetic medium via centrifugation, leaving only a negligible trace of AuNPs in the supernatant, indicating the significant efficacy and atom economy in decorating the titania surface with AuNPs. The reddish milky suspension of particles obtained post-purification was stored until further use. Gravitational settling was visible in the sample with time and the suspension was homogenized prior to being used in the analysis as described below.
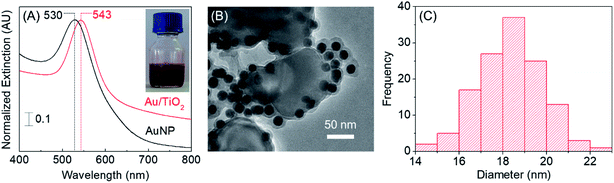
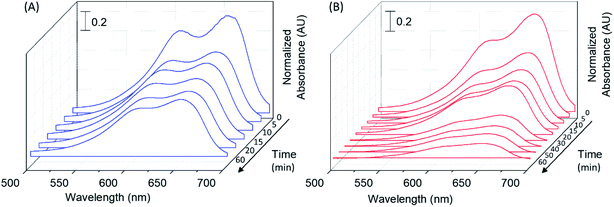
The Localized Surface Plasmon Resonance (LSPR) spectra of as-synthesized Au/TiO2 nanocomposite and bare AuNPs prepared in an identical procedure, but in the absence of TiO2, are shown in Fig. 1(A), while the inset shows a photograph of the clear red colored Au/TiO2 nanocomposite. As can be seen, the wavelength of maximum extinction for the Au/TiO2 nanocomposite is ∼13 nm red shifted compared to bare AuNPs, being in good agreement with previous reports.26 The wavelength of maximum extinction for AuNPs have been previously reported to be dependent upon the size of the nanoparticles.27 Hence, it could be hypothesized that both the variation in the particle size of AuNPs as well as a change in the local refractive index of the AuNPs that takes place in the presence of TiO2, in combination are responsible for this shift in extinction maximum wavelength.
Further, the shift in the LSPR spectrum of Au/TiO2, specifically at the higher wavelengths can be attributable to the photoinduced charge separation at the plasmonic AuNP–TiO2 interface. Similar behavior has been previously reported for composite materials of Au and TiO2.28,29 Of note, such charge separation plays a prominent role in enhancing the quantum photocatalytic efficiency of a material.30 Hence, the LSPR spectrum of Au/TiO2 provided the initial evidence in support of the hypothesis on which the novel composite catalyst was developed herein.
Electron micrographs of the Au/TiO2 nanocomposite are shown in Fig. 1(B) and S1 included in the ESI.† These indicate the spherical and homogeneous AuNPs formed in the presence of TiO2, creating a pertinent decoration of AuNPs on the titania surface. Additionally, a coating likely resulting from the phytochemicals present in the green tea extract is visible around the AuNPs. The remarkable stability of the Au/TiO2 nanocomposite produced herein could be attributed to the presence of the above coating on the AuNPs, which is likely to reduce the propensity for any interaction driven particle coalescence. The average diameter of AuNPs was determined to be 18 ± 2 nm based on the measurements taken on randomly selected AuNPs (n > 100) present on TiO2 as shown in Fig. 1(C).
Electron micrograph of a single AuNP is indicated in Fig. 2(A), with the fast Fourier Transform (FFT) pattern of the same is indicated as the inset. This indicates the near-perfect spherical geometry and the poly-crystallinity of the green-synthesized AuNPs on the titania surface. Additionally, Fig. 2(B) indicates the intensity profile of the area indicated in blue on the nanoparticle in the high-resolution transmission electron microscopic (HRTEM) image. Accordingly, the interatomic layer distance of gold in the green-synthesized AuNPs is found to be 2.4 Å. This indicates good agreement with the value previously reported for gold,31 thus offering credit to the sustainable approach developed herein.
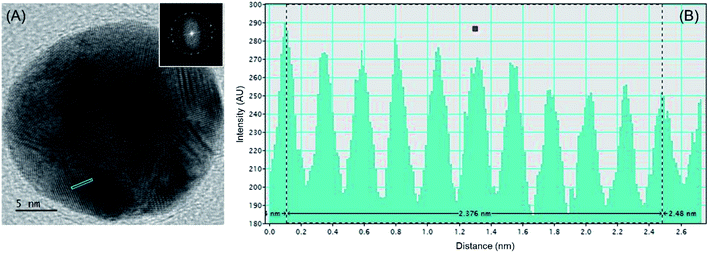 | ||
| Fig. 2 (A) HRTEM image of a single AuNP with the fast Fourier Transform (FFT) pattern indicated as the inset and (B) the intensity profile of the area indicated in blue on the nanoparticle. | ||
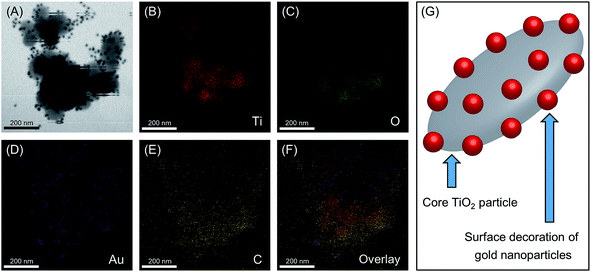
The HRTEM images were analyzed further to confirm the presence and to investigate the distribution of AuNPs on the TiO2 surface. Fig. 3(A) shows the scanning transmission electron microscopic (STEM) image used for the energy dispersive X-ray spectroscopic (EDX) analysis of the Au/TiO2 nanoparticles, and panels (B), (C), (D), and (E) indicates the elemental distribution maps for Ti (red), O (green), Au (purple), and C (yellow) within the area indicated on the STEM image, while panel (F) indicates the overlay of the above elemental distribution maps. The EDX spectrum obtained for the above analysis is included in the ESI (Fig. S2†). The presence of a decoration of AuNP on TiO2 structures can be confirmed by the overlap of the elemental maps indicated in Fig. 3, where the results clearly show the localized presence of AuNPs on the TiO2 surface Hence, it is visible that the AuNPs are embedded on the TiO2 surface mimicking a plum-pudding or decorated composite architecture, as illustrated in Fig. 3(G) and as initially hypothesized here. Although comparatively smaller in number, the EDAX image also shows the presence of free AuNPs, other than those bound on TiO2. However, given that such free AuNPs observed during centrifugation was negligible (vide supra), the above observation is likely a consequence of sample preparation for HRTEM. Additionally, the presence of Au was confirmed with the EELS spectrum observed with Au M edge at 2206 eV, which is shown in Fig. S3 in ESI.†
Overall, the synthesis of these composite nanostructures could be conducted without the involvement of any chemical species other than the metal precursor and green tea, while maintaining mild temperatures throughout the procedure. Additionally, the process indicated notable atom economy, akin to that observed in a typical chemical mediated synthetic procedure. The resulting AuNPs indicate spherical and monodisperse nature while the Au/TiO2 nanocomposites indicate appreciable stability during processing as well as prolonged storage. From the detailed characterization conducted, it could be inferred that this green method results in a pertinent coating of spherical and homogeneous AuNPs on the titania surface via a facile and entirely sustainable approach. Collectively, therefore, the above factors offer notable credibility to the sustainability of the green synthetic approach presented herein.
With the strong electromagnetic coupling likely to occur at the interface between titania and surface-bound AuNPs, the developed Au/TiO2 nanocomposite can be used in wide variety of photo-mediated applications such as photocatalysis. Notably, the role of a photocatalyst is to enhance the rate of chemical reactivity under photo irradiation to facilitate any chemical degradation, resulting in improved photoremediation. However, as previously shown by Rodriguez et al. via synchrotron-based high-resolution photoemission and first-principles density-functional slab calculations, the deposition of gold nanoparticles on TiO2 produces a system with an extraordinary ability to adsorb and dissociate SO2, making Au/TiO2 much more chemically active than metallic gold or stoichiometric titania.32 Interactions between AuNPs and titania are likely to electronically perturb gold, making it more chemically active, while the same may facilitate the migration of O vacancies from the bulk to the surface of the oxide, enhancing the reactivity of titania.32
Therefore, the Au/TiO2 nanocomposite developed here would indicate two distinct and independent pathways for the efficient removal of contaminants from aqueous environments; namely (1) surface adsorption and (2) photocatalysis. Further, the proximity of the contaminants to the substrate, resulting via the adsorption of contaminants on the AuNP decorated titania, would facilitate efficient electron transfer thereby enhancing the photocatalytic activity. Hence, it was hypothesized that surface adsorption and photocatalysis in combination would produce a synergetic effect in the efficient removal of hazardous chemicals from contaminated waters. Accordingly, the ability of Au/TiO2 nanocomposite to be used in environmental remediation via enhanced surface adsorptions and ensuing photocatalysis was investigated using methylene blue (MB) as a model dye.
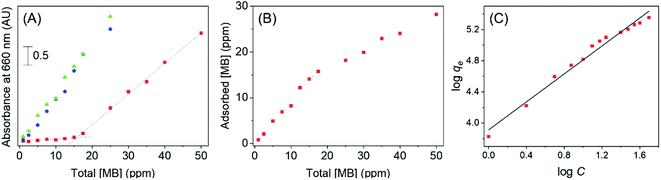
First, a dye adsorption study was conducted to quantify the extent of MB that can be effectively adsorbed onto the Au/TiO2 nanocomposite. A sample of bare TiO2 was analyzed in a similar procedure to determine any significant variations in adsorption capacity resulting from the AuNP decoration. Here, the UV-visible absorbance of free MB in solution was measured after complete dye adsorption onto the catalyst surface for both Au/TiO2 and bare TiO2. As seen from Fig. 4(A), the absorbance of free MB increased linearly in the presence of bare TiO2 with increasing initial MB concentration. This indicates that MB is only sparingly adsorbed onto the bare TiO2 surface. In fact, these values of absorbance at each MB concentration was very close to that of MB in the absence of any adsorbent, thus implying the significantly low or even negligible adsorption capacity of bare TiO2.
In contrast, for Au/TiO2, there was no significant absorbance in the supernatant until the MB concentration reached ∼17 ppm. This result suggest that MB will be completely adsorbed onto the Au/TiO2 catalytic surface at concentrations of MB below 17 ppm; a result attributable to the increased interactions arising due to the AuNPs on the surface of titania. Hence, the Au/TiO2 nanocomposite clearly indicates a significantly higher affinity towards MB compared to bare TiO2. Beyond the threshold concentration, the free MB concentration indicates a linear progression as a function of concentration, contrary to the surface saturation that one may expect to observe at high MB concentrations. This result was further analyzed by determining the concentration of adsorbed MB as indicated in Fig. 4(B). Accordingly, the adsorption onto Au/TiO2 shows a significant linear increase with the MB concentration up to ∼17 ppm, beyond which the adsorption continues to occur, but at a slower rate of increase.
Although it is typical for a sorbent surface to saturate upon the formation of a monolayer, the continual increase in adsorption suggests that the gold decoration on TiO2 promotes the formation of multi layers of MB on the sorbent surface. It is hypothesized that the initial rapid increase in MB adsorption on Au/TiO2 as seen in Fig. 4(B) is due to the monolayer formation, during which the dye molecules have the ability to adsorb onto the Au surface through the N or S atoms present in methylene blue. However, π–π stacking of MB molecules may allow further dye molecules to adsorb onto the catalyst surface, subsequent to monolayer formation, as have been previously observed with MB.33 Clearly, this behavior is attributable to notable enhancement in the overall adsorption capacity for MB on the Au/TiO2 nanocomposite developed here.
To better understand the adsorption process, the results indicated in Fig. 4(B) were fit to the Freundlich isotherm model which has the form;
The possibility of photosensitization of titania via gold by injection of electrons into the conduction band of the latter has been reported in previous literature. Tsukamoto et al. have confirmed the efficient transfer of electrons from photoactivated Au particles to titania by electron spin resonance (ESR) analysis of catalysts under visible-light irradiation.37 According to Su et al. the high photocatalytic activity could be attributed to the (1) plasmonic effect of gold nanoparticles, which enhances the visible light absorption, (2) increased surface area, (3) efficient charge separation, and (4) high carrier mobility of the titania.38 According to diffuse reflectance spectroscopic measurements reported by Bian et al., a significant proportion of electrons are injected from the AuNPs to the titania through the surface plasmon excitation.7 Further, the close resemblance of the incident photon conversion efficiency spectrum to the plasmonic features of the Au/TiO2 system have suggested that the observed photocurrents originate from the plasmonic excitation of the AuNPs, indicating the prominent role of the plasmonic excitations of the AuNPs towards enhanced photocatalysis.7
Thus, the photocatalytic activity of fabricated nanocomposite was studied by measuring the rate of MB degradation under UV irradiation as indicated in Fig. 5. Here, Au/TiO2 nanocomposite and bare TiO2 samples, containing known concentrations of MB were irradiated with UV radiation for known durations. As can be seen on Fig. 5(A) and (B), the MB oxidizes via photoreactivity on both bare TiO2 as well as the Au/TiO2 nanocomposite, as evidenced by the decrease in absorption due to MB observed as a function of irradiation time. However, in the presence of Au/TiO2 nanocomposite, the MB solution turned clear within ∼30 min of UV irradiation, whereas, the blue color in the presence of bare TiO2 remained even after one hour of photo irradiation.
The results were further analyzed to obtain the time-dependent decrease in MB absorption and hence the rate constants for both bare TiO2 and the Au/TiO2 nanocomposite as shown in Fig. 6(A) and (B) respectively. Notably, it is observed that the photocatalytic rate constants of TiO2 and Au/TiO2 for the degradation of MB were 1.8 × 10−2 and 7.5 × 10−2 min−1, respectively, which indicates that the photocatalytic activity is enhanced by ∼4.2 times when TiO2 was decorated with AuNPs compared to bare TiO2. Similar enhancements in terms of MB degradation rate constants have been previously reported for Au and TiO2 composites.39
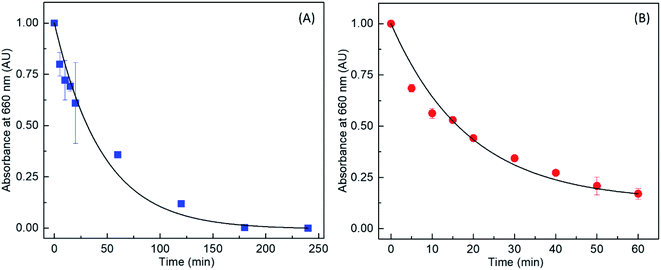 | ||
| Fig. 6 Variation in absorbance at 660 nm (due to free MB in solution) as a function of UV irradiation time, in the presence of (A) TiO2 and (B) Au/TiO2. | ||
According to Quinones et al. the half-life of MB degradation over Au/Pd-modified TiO2 was 2.8 times smaller than pure TiO2 pure.39 Khalil et al. have reported the pseudo-first order reaction rate of MB over Au–TiO2 heterostructures to be 0.1570 min−1.40 Sangpour et al. have reported a degradation rate of 11.4 × 10−3 min−1 over a titania film containing AuNPs prepared by radio frequency reactive magnetron cosputtering.41 In their recent work, Yulizar et al. have reported that the photocatalytic activity of Au/TiO2 nanocomposite prepared via a plant extract mediated synthesis was 2.17 times higher than titania.42 However, the increase in reaction rate observed here via the surface decoration with AuNPs is significantly higher compared to those previously reported. Hence, this green synthesized Au/TiO2 nanocomposite shows promising potential for environmental remediation via efficient photocatalysis.
Hence, in summary, the Au/TiO2 nanocomposite developed here via the facile and sustainable green route indicates (1) prominent atom economy in preparation, (2) remarkable stability during preparation, processing, as well as prolonged storage, (3) superior adsorption capacity of 8185 mg g−1 for MB, demonstrating significant potential for efficient contaminant capture, and (4) significantly enhanced photocatalytic degradation rate of MB compared to bare TiO2. Additionally, given the widespread use of TiO2 based materials in environmental applications, and the facetious gravitational settling of this nanocomposite, efficient post-treatment removal of the material is possible from environmental or perhaps even other systems. Hence, collectively the improved capacity to capture as well as degrade MB in solution indicates that the nanocomposite has outstanding potential to serve in pronounced and sustainable environmental remediation.
Conclusion
Here, the development of a novel, facile, and green chemical synthetic approach to prepare Au/TiO2 nanocomposites for sustainable environmental remediation is reported. The synthesis involved only the metal precursor and green tea, obviating the need for any solvents and/or harsh chemical reducing or stabilizing agents, and was conducted at mild conditions, allowing notable sustainability to prevail in the preparation of the novel catalyst. Experimental evidence indicated that the Au/TiO2 nanocomposite show a superior adsorption capacity of 8185 mg g−1 towards MB in solution, while the photocatalytic rate constants for the degradation of MB on the substrate indicated 4.2-fold improvement compared to bare TiO2. Adsorption capacity as well as the increase in reaction rate observed here were significantly higher compared to those previously reported, while there being no previous accounts of akin sustainable synthetic procedures for the preparation of composite nanomaterials of this nature. Hence, this novel green synthesized Au/TiO2 nanocomposite shows promising potential for environmental remediation via efficient contaminant capture, and subsequent photocatalysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors would like to express gratitude towards the Sri Lanka Institute of Nanotechnology (SLINTEC) and Professor Gehan Amaratunga for the assistance provided for this research work.References
- Y. Zhao, C. Li, X. Liu, F. Gu, H. Jiang, W. Shao, L. Zhang and Y. He, Synthesis and optical properties of TiO2 nanoparticles, Mater. Lett., 2007, 61, 79–83 CrossRef CAS.
- C. Xu, R. Killmeyer, M. L. Gray and S. U. M. Khan, Photocatalytic effect of carbon-modified n-TiO2 nanoparticles under visible light illumination, Appl. Catal., B, 2006, 64, 312–317 CrossRef CAS.
- X. Li, D. Wang, G. Cheng, Q. Luo, J. An and Y. Wang, Preparation of polyaniline-modified TiO2 nanoparticles and their photocatalytic activity under visible light illumination, Appl. Catal., B, 2008, 81, 267–273 CrossRef CAS.
- M. Ni, M. K. H. Leung, D. Y. C. Leung and K. Sumathy, A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production, Renewable Sustainable Energy Rev., 2007, 11, 401–425 CrossRef CAS.
- C. Gomes Silva, R. Juárez, T. Marino, R. Molinari and H. García, Influence of Excitation Wavelength (UV or Visible Light) on the Photocatalytic Activity of Titania Containing Gold Nanoparticles for the Generation of Hydrogen or Oxygen from Water, J. Am. Chem. Soc., 2011, 133, 595–602 CrossRef CAS PubMed.
- A. Tanaka, S. Sakaguchi, K. Hashimoto and H. Kominami, Preparation of Au/TiO2 exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation from organic and inorganic compounds under irradiation of visible light, Catal. Sci. Technol., 2012, 2, 907–909 RSC.
- Z. Bian, T. Tachikawa, P. Zhang, M. Fujitsuka and T. Majima, Au/TiO2 Superstructure-Based Plasmonic Photocatalysts Exhibiting Efficient Charge Separation and Unprecedented Activity, J. Am. Chem. Soc., 2014, 136, 458–465 CrossRef CAS.
- A. Primo, A. Corma and H. García, Titania supported gold nanoparticles as photocatalyst, Phys. Chem. Chem. Phys., 2011, 13, 886–910 RSC.
- Y. Ide, M. Matsuoka and M. Ogawa, Efficient Visible-Light-Induced Photocatalytic Activity on Gold-Nanoparticle-Supported Layered Titanate, J. Am. Chem. Soc., 2010, 132, 16762–16764 CrossRef CAS PubMed.
- L. Guo, K. Liang, K. Marcus, Z. Li, L. Zhou, P. D. Mani, H. Chen, C. Shen, Y. Dong, L. Zhai, K. R. Coffey, N. Orlovskaya, Y.-H. Sohn and Y. Yang, Enhanced Photoelectrocatalytic Reduction of Oxygen Using Au@TiO2 Plasmonic Film, ACS Appl. Mater. Interfaces, 2016, 8, 34970–34977 CrossRef CAS PubMed.
- H. Li, Z. Bian, J. Zhu, Y. Huo, H. Li and Y. Lu, Mesoporous Au/TiO2 Nanocomposites with Enhanced Photocatalytic Activity, J. Am. Chem. Soc., 2007, 129, 4538–4539 CrossRef CAS PubMed.
- K. Hashimoto, H. Irie and A. Fujishima, TiO2 Photocatalysis: A Historical Overview and Future Prospects, Jpn. J. Appl. Phys., 2005, 44, 8269–8285 CrossRef CAS.
- S. L. Smitha, D. Philip and K. G. Gopchandran, Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth, Spectrochim. Acta, Part A, 2009, 74, 735–739 CrossRef CAS PubMed.
- S. Aswathy Aromal and D. Philip, Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity, Spectrochim. Acta, Part A, 2012, 97, 1–5 CrossRef CAS PubMed.
- V. G. Kumar, S. D. Gokavarapu, A. Rajeswari, T. S. Dhas, V. Karthick, Z. Kapadia, T. Shrestha, I. A. Barathy, A. Roy and S. Sinha, Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata, Colloids Surf., B, 2011, 87, 159–163 CrossRef CAS PubMed.
- H. Wang, H. Gao, M. Chen, X. Xu, X. Wang, C. Pan and J. Gao, Microwave-assisted synthesis of reduced graphene oxide/titania nanocomposites as an adsorbent for methylene blue adsorption, Appl. Surf. Sci., 2016, 360, 840–848 CrossRef CAS.
- J. Ma, F. Yu, L. Zhou, L. Jin, M. Yang, J. Luan, Y. Tang, H. Fan, Z. Yuan and J. Chen, Enhanced Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution by Alkali-Activated Multiwalled Carbon Nanotubes, ACS Appl. Mater. Interfaces, 2012, 4, 5749–5760 CrossRef CAS PubMed.
- T. S. Natarajan, H. C. Bajaj and R. J. Tayade, Preferential adsorption behavior of methylene blue dye onto surface hydroxyl group enriched TiO2 nanotube and its photocatalytic regeneration, J. Colloid Interface Sci., 2014, 433, 104–114 CrossRef CAS PubMed.
- S. P. J. Namal Senanayake, Green tea extract: chemistry, antioxidant properties and food applications – a review, J. Funct. Foods, 2013, 5, 1529–1541 CrossRef CAS.
- M. Farahat, F. Abdallah, T. Abdel-hamid and A. Hernandez-Santana, Effect of supplementing broiler chicken diets with green tea extract on the growth performance, lipid profile, antioxidant status and immune response, Br. Poult. Sci., 2016, 57, 714–722 CAS.
- N. Shibata, A. Goto, K. Matsunaga, T. Mizoguchi, S. D. Findlay, T. Yamamoto and Y. Ikuhara, Interface Structures of Gold Nanoparticles on TiO2(110), Phys. Rev. Lett., 2009, 102, 136105 CrossRef CAS PubMed.
- D. Matthey, J. Wang, S. Wendt, J. Matthiesen, R. Schaub, E. Lægsgaard, B. Hammer and F. Besenbacher, Enhanced Bonding of Gold Nanoparticles on Oxidized TiO2(110), Science, 2007, 315, 1692–1696 CrossRef CAS PubMed.
- S. K. Nune, N. Chanda, R. Shukla, K. Katti, R. R. Kulkarni, S. Thilakavathi, S. Mekapothula, R. Kannan and K. V Katti, Green Nanotechnology from Tea: Phytochemicals in Tea as Building Blocks for Production of Biocompatible Gold Nanoparticles, J. Mater. Chem., 2009, 19, 2912–2920 RSC.
- S. Boruah, P. Boruah, P. Sarma, C. Medhi and O. Kumar, Green synthesis of gold nanoparticles using Camellia sinensis and kinetics of the reaction, Adv. Mater. Lett., 2012, 3(6), 481–486 CrossRef.
- J. Kimling, M. Maier, B. Okenve, V. Kotaidis, H. Ballot and A. Plech, Turkevich Method for Gold Nanoparticle Synthesis Revisited, J. Phys. Chem. B, 2006, 110, 15700–15707 CrossRef CAS PubMed.
- Y. Chen, J. Bian, L. Qi, E. Liu and J. Fan, Efficient Degradation of Methylene Blue over Two-Dimensional Au/TiO2 Nanosheet Films with Overlapped Light Harvesting Nanostructures, J. Nanomater., 2015, 2015, 1–10 CAS.
- W. Haiss, N. T. K. Thanh, J. Aveyard and D. G. Fernig, Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra, Anal. Chem., 2007, 79, 4215–4221 CrossRef CAS PubMed.
- A. Kathiravan, P. S. Kumar, R. Renganathan and S. Anandan, Photoinduced electron transfer reactions between meso-tetrakis(4-sulfonatophenyl)porphyrin and colloidal metal-semiconductor nanoparticles, Colloids Surf., A, 2009, 333, 175–181 CrossRef CAS.
- E. Kazuma and T. Tatsuma, In Situ Nanoimaging of Photoinduced Charge Separation at the Plasmonic Au Nanoparticle-TiO2 Interface, Adv. Mater. Interfaces, 2014, 1, 1400066 CrossRef.
- J. Chen, F. Qiu, W. Xu, S. Cao and H. Zhu, Recent Progress in Enhancing Photocatalytic Efficiency of TiO2-based Materials, Appl. Catal., A, 2015, 495, 131–140 CrossRef CAS.
- C. Untiedt, A. I. Yanson, R. Grande, G. Rubio-Bollinger, N. Agraït, S. Vieira and J. M. van Ruitenbeek, Calibration of the length of a chain of single gold atoms, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 85418 CrossRef.
- J. A. Rodriguez, G. Liu, T. Jirsak, J. Hrbek, Z. Chang, J. Dvorak and A. Maiti, Activation of Gold on Titania: Adsorption and Reaction of SO2 on Au/TiO2(110), J. Am. Chem. Soc., 2002, 124, 5242–5250 CrossRef CAS PubMed.
- C.-H. Weng and Y.-F. Pan, Adsorption characteristics of methylene blue from aqueous solution by sludge ash, Colloids Surf., A, 2006, 274, 154–162 CrossRef CAS.
- J. Li, J. Feng and W. Yan, Excellent adsorption and desorption characteristics of polypyrrole/TiO2 composite for Methylene Blue, Appl. Surf. Sci., 2013, 279, 400–408 CrossRef CAS.
- L. Xiong, Y. Yang, J. Mai, W. Sun, C. Zhang, D. Wei, Q. Chen and J. Ni, Adsorption behavior of methylene blue onto titanate nanotubes, Chem. Eng. J., 2010, 156, 313–320 CrossRef CAS.
- Y. Bulut and H. Aydın, A kinetics and thermodynamics study of methylene blue adsorption on wheat shells, Desalination, 2006, 194, 259–267 CrossRef CAS.
- D. Tsukamoto, Y. Shiraishi, Y. Sugano, S. Ichikawa, S. Tanaka and T. Hirai, Gold Nanoparticles Located at the Interface of Anatase/Rutile TiO2 Particles as Active Plasmonic Photocatalysts for Aerobic Oxidation, J. Am. Chem. Soc., 2012, 134, 6309–6315 CrossRef CAS PubMed.
- F. Su, T. Wang, R. Lv, J. Zhang, P. Zhang, J. Lu and J. Gong, Dendritic Au/TiO2 nanorod arrays for visible-light driven photoelectrochemical water splitting, Nanoscale, 2013, 5, 9001–9009 RSC.
- C. Quiñones, J. Ayala and W. Vallejo, Methylene blue photoelectrodegradation under UV irradiation on Au/Pd-modified TiO2 films, Appl. Surf. Sci., 2010, 257, 367–371 CrossRef.
- M. Khalil, E. S. Anggraeni, T. A. Ivandini and E. Budianto, Exposing TiO2 (001) crystal facet in nano Au-TiO2 heterostructures for enhanced photodegradation of methylene blue, Appl. Surf. Sci., 2019, 487, 1376–1384 CrossRef CAS.
- P. Sangpour, F. Hashemi and A. Z. Moshfegh, Photoenhanced Degradation of Methylene Blue on Cosputtered M:TiO2 (M = Au, Ag, Cu) Nanocomposite Systems: A Comparative Study, J. Phys. Chem. C, 2010, 114, 13955–13961 CrossRef CAS.
- Y. Yulizar, Sudirman, D. O. B. Apriandanu and A. P. Wibowo, Plant extract mediated synthesis of Au/TiO2 nanocomposite and its photocatalytic activity under sodium light irradiation, Composites Communications, 2019, 16, 50–56 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05607c |
| ‡ Current address: National Institute of Fundamental Studies, Hantana Road, Kandy, Sri Lanka. |
| This journal is © The Royal Society of Chemistry 2020 |