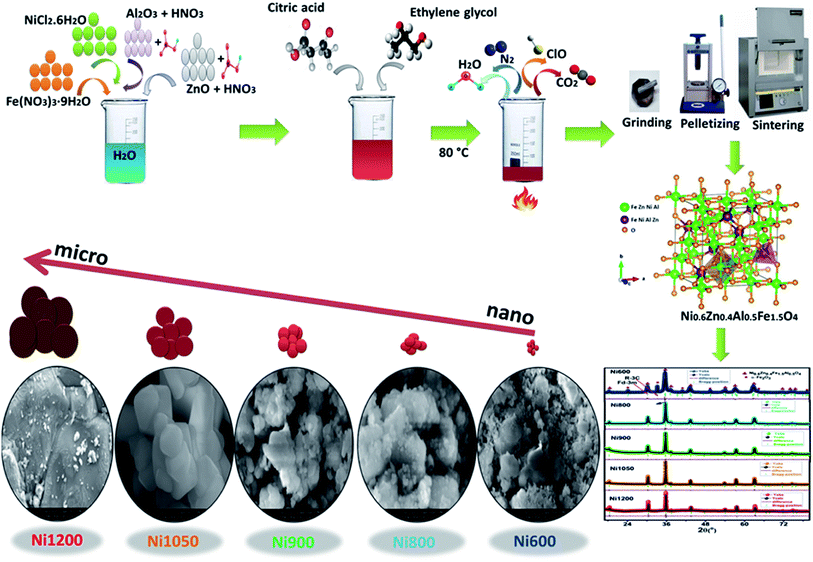
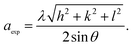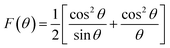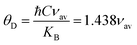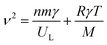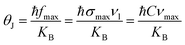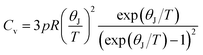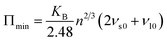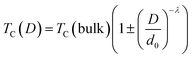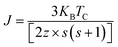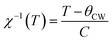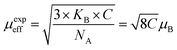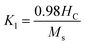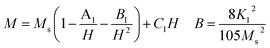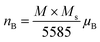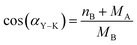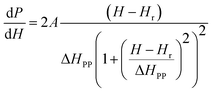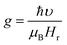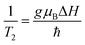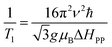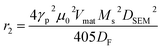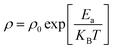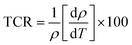Open Access Article
Open Access ArticleMagnetic and spectroscopic properties of Ni–Zn–Al ferrite spinel: from the nanoscale to microscale†
Jalel Massoudi *a,
Mourad Smari
*a,
Mourad Smari ab,
Kamel Nouric,
Essebti Dhahri
ab,
Kamel Nouric,
Essebti Dhahri a,
Kamel Khirounid,
Sylvain Bertainae,
Lotfi Bessaisc and
El Kebir Hlilf
a,
Kamel Khirounid,
Sylvain Bertainae,
Lotfi Bessaisc and
El Kebir Hlilf
aLaboratoire de Physique Appliquée, Faculté des Sciences, Université de Sfax, B. P. 1171, Sfax 3000, Tunisia. E-mail: jalel.massoudi@gmail.com
bDepartment of Materials and Ceramic Engineering, CICECO – Aveiro Institute of Materials, University of Aveiro, 3810-193 Aveiro, Portugal
cCMTR, ICMPE, UMR 7182 CNRS-UPEC, 2 Rue Henri Dunant, F-94320 Thiais, France
dLaboratoire de Physique des Matériaux et des Nanomatériaux Appliquée a` l'Environnement, Faculté des Sciences de Gabès Cité Erriadh, Université de Gabès, 6079 Gabès, Tunisia
eIM2NP, CNRS, Faculté des Sciences de Saint-Jérôme, Université d'Aix-Marseille, Case 142, 13397 Marseille, France
fInstitut Néel, CNRS, Université J. Fourier, B. P. 166, 38042 Grenoble, France
First published on 18th September 2020
Abstract
This article presents the annealing effect on the structural, elastic, thermodynamic, optical, magnetic, and electric properties of Ni0.6Zn0.4Fe1.5Al0.5O4 (NZFAO) nanoparticles (NPs). The samples were successfully synthesized by the sol–gel method followed by annealing of the as-synthesized at 600, 800, 900, 1050, and 1200 °C. This approach yielded the formation of a highly crystalline structure with crystallite size ranging from 17 nm to 40 nm. X-ray diffraction (XRD), scanning electron microscopy (SEM) techniques, as well as energy disperse spectroscopy (EDS), Fourier transform infrared (FTIR) and Raman spectroscopy, were used in order to determine the structural and morphological properties of the prepared samples. Rietveld XRD refinement reveals that Ni–Zn–Al ferrite nanoparticles crystallize in inverse cubic (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) spinel structure. Using FTIR spectra, the elastic and thermodynamic properties were estimated. It was observed that the particle size had a pronounced effect on elastic and thermodynamic properties. Magnetic measurements were performed up to 700 K. The prepared ferrite samples present the highest Curie temperature, which decreases with increasing particle size and which is consistent with finite-size scaling. The thickness of the surface shell of about 1 nm was estimated from size-dependent magnetization measurements using the core–shell model. Besides, spin resonance, magnetostriction, temperature coefficient of resistance (TCR), and electrical resistivity properties have been scientifically studied and appear to be different according to their size. The optical properties of synthesized NZFAO nanoparticles were investigated, and the differences caused by the particle sizes are discussed on the basis of the phonon confinement effect. This effect was also inspected by the Raman analysis. Tuning of the physical properties suggests that the Ni–Zn–Al ferrite samples may be promising for multifunctional diverse applications.
m) spinel structure. Using FTIR spectra, the elastic and thermodynamic properties were estimated. It was observed that the particle size had a pronounced effect on elastic and thermodynamic properties. Magnetic measurements were performed up to 700 K. The prepared ferrite samples present the highest Curie temperature, which decreases with increasing particle size and which is consistent with finite-size scaling. The thickness of the surface shell of about 1 nm was estimated from size-dependent magnetization measurements using the core–shell model. Besides, spin resonance, magnetostriction, temperature coefficient of resistance (TCR), and electrical resistivity properties have been scientifically studied and appear to be different according to their size. The optical properties of synthesized NZFAO nanoparticles were investigated, and the differences caused by the particle sizes are discussed on the basis of the phonon confinement effect. This effect was also inspected by the Raman analysis. Tuning of the physical properties suggests that the Ni–Zn–Al ferrite samples may be promising for multifunctional diverse applications.
Introduction
Over the past several years, ferrites spinels AFe2O4, where A is a divalent transition metal (Ni, Zn, Fe, Cr, Mn, Co, …) have attracted great interest due to their good biocompatibility, low toxicity and their magnetism.1,2 These materials are currently considered among the most successful magnetic nanoparticles (MNPs) for technological and medical applications e.g., magnetocaloric refrigerators, magnetic memory, solar water oxidation, electrochemical supercapacitor applications, biological applications, lithium-ion batteries, contrast enhancement in magnetic resonance imaging (MRI) and magnetic fluid hyperthermia.3–10 Therefore, an understanding of the structural and magnetic properties of spinels is of great importance from both a fundamental and an applied point of view. These properties can be controlled by synthesis methods, annealing temperature and/or by doping suitable elements into the A-site or B-site to change the structural parameters and/or the distribution cation.The ferrite spinel has a face-centered cubic (fcc) structure, which consists of a cubic close-packed oxygen lattice. The unit cell of a spinel ferrite consists of 16 trivalent iron ions, 8 divalent metal ions (M) and 32 oxygen ones. The spinel structure contains two interstitial sites, occupied by metal cations with octahedral (B) and tetrahedral (A) oxygen coordination. In general, the cationic distribution between the two sites is quantified by the inversion degree (γ), which is defined as the fraction of divalent ions in the octahedral sites. In the normal spinel configuration, the Fe3+ ions occupy the octahedral sites, while the tetrahedral sites contain the M2+, giving the empirical formula (M2+)A[Fe3+]BO4. On the other hand, in inverse spinels, half of the Fe3+ occupy the tetrahedral sites, while the octahedral sites contain the M2+ and the other half of Fe3+ ions, yielding the empirical formula (Fe3+)A[M2+Fe3+]BO4. The structure can also be mixed spinels. However, superexchange interactions between magnetic atoms located in the same kind of interstitial sites (JA–A and JB–B) lead to two ferromagnetically ordered sub-lattices. On the other hand, the dominant antiferromagnetic interactions between magnetic ions in the A and B sites (JA–B) induce a non-compensated antiferromagnetic order between the two sub-lattices inducing (ferrimagnetism). In the case of nanoparticles, the larger surface area creates surface relaxation, surface bond bending, surface effect and consequently spins canting which modifies the contribution of each interaction. The relation between structures and physical properties for nano ferrites requires a careful consideration. Therefore, various methods, including, forced hydrolysis in polyol, ball milling, coprecipitation, sol–gel auto-combustion and hydrothermal/solvothermal method etc.,3,11–14 have extensively been developed to synthesize nano-spinel ferrite with different sizes and shapes. Ball milling method is favorable to produce a highly crystalline nanostructure, but always leads to agglomeration in high reaction temperature and long time. By contrast, sol–gel method is preferred due to the better control over the shape and size for particle synthesis in low temperature and low time of process with remarkable purity and high amount. Also, this method offers an advantage due to its high quality production, its low cost.15
Ni–Zn ferrite is a member of the class of the spinel ferrites which is widely touted for its applications in magnetic, magnetooptical and magnetodielectric devices. Under normal conditions, spinel Ni–Zn ferrites is a typical ferrimagnetic material possessing mixed spinel structure (Fei3+M1−i2+)[Mi2+Fe2−i3+]O4,16,17 where i is the inversion degree, () and [] represent tetrahedral and octahedral positions respectively, which belongs to Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. The cation arrangement can vary between two extreme cases. One of them is the normal spinel (i = 0), where all the divalent elements occupy tetrahedral A sites and all the trivalent elements occupy octahedral B sites. The other one is the inverse spinel (i = 1), where all the divalent elements occupy octahedral B sites and all trivalent elements are equally distributed between tetrahedral A sites and octahedral B sites. Spinels with the cation distribution intermediate between normal and inverse (i.e., partially inverse spinels; 0 < i < 1) are also very frequent.18
m space group. The cation arrangement can vary between two extreme cases. One of them is the normal spinel (i = 0), where all the divalent elements occupy tetrahedral A sites and all the trivalent elements occupy octahedral B sites. The other one is the inverse spinel (i = 1), where all the divalent elements occupy octahedral B sites and all trivalent elements are equally distributed between tetrahedral A sites and octahedral B sites. Spinels with the cation distribution intermediate between normal and inverse (i.e., partially inverse spinels; 0 < i < 1) are also very frequent.18
Furthermore, Ni–Zn ferrites are characterized by high surface area, chemical stability, high saturation magnetization, high Curie temperature, low coercivity (soft magnetic), high permeability, high dielectric constant, low losses, semiconductor dielectric transition and they can find wide application in the high frequency region of electronics devices.19 On substitution of Zn2+ in Ni ferrite the Fe3+ ions migrate from (A) to [B] site and consequently the FeA–O–FeB interaction becomes feeble. Several studies have reported the dependence of magnetic properties of Ni–Zn ferrites on their microstructure, sintering temperature and preparation method, these properties can be changed and tuned by aluminum doping.20,21 The introduction of Al3+ ions leads to significant variations of cation distribution over the A and B sites depending on the method of synthesis and Al concentration, additional non-magnetic ions Al3+ on octahedral B site reduces saturation magnetization by increasing the resistivity.21
The electrical properties displayed macroscopically by the ferrite are a direct consequence of their cationic arrangement since both nickel and iron possess different electronic characters depending on the interstice they occupy inside the lattice. For nanometric Ni–Zn ferrites, it is possible to obtain good electric properties and high performance at relatively low sintering temperature. Recently, Li et al.22 examined the dielectric properties and direct-current resistivity of Al-substituted Ni–Zn–Co ferrite nanoparticles. Also, the magnetic properties of Ni–Zn ferrite nanocrystals depend on different sizes, shapes, and synthesis methods. Beji et al.11 prepared a series of polyol-made Ni–Zn ferrite nanoparticles with varying annealing temperatures to optimize the structural and magnetic properties and found that heating at different temperatures ranged from 400 to 800 °C provides changes of the magnetic properties of these particles. In our earlier work,23 we investigated the dielectric properties of Ni0.6Zn0.4Fe1.5Al0.5O4 annealed at 600, 900, and 1200 °C. It was found that the dielectric properties of the system improved on increasing the temperature. Moreover, K. Praveena et al.24 discussed the effect of annealing temperature in dynamic magnetic properties of Ni0.4Zn0.2Mn0.4Fe2O4 nanoparticles. However, their discussion was limited to the resonance field and g-value which are major imprints of the material characteristics, but other key spin resonance parameters that can provide an even more detailed understanding of the properties of materials required to enhance the performance of the device must be reported. To the best of our knowledge, there is no report performed to understand the effect of particle size on the relaxation mechanism, transverse relaxivity, magnetostrictive, thermodynamic, and elastic properties of Ni–Zn–Al ferrite in detail. A better understanding of these physical properties of Ni–Zn–Al ferrite is, therefore, highly beneficial to tune the properties for diverse technological applications.
Herein, the main aim of this framework is to synthesize and study nanoscale and bulk materials based on nickel–zinc ferrites. We are interested in determining the effects of particle size changes in the nano to micro-regime by annealing temperature at 600, 800, 900, 1050, and 1200 °C and characterized with different aspects such as structural, morphological, magnetic, elastic, thermodynamic, magnetostriction, optical and electric properties. A detailed investigation of the dynamic magnetic properties was carried out by an electron spin resonance (ESR) study and the spin resonance parameters were also estimated. Interestingly, the synthesized nanoparticles at low temperature possess excellent transverse relaxivity r2 and can be used as T2 contrast efficacy in imaging application. Hence, these synthesized materials will not only seem to be an ideal material for examining the size effect on the physical properties of nanomaterial but also have broad applications in the future.
Experimental section
Synthesis
All the chemical reactants in this work were purchased from Sigma-Aldrich and used without further purification. Ni0.6Zn0.4Fe1.5Al0.5O4 nanoparticles were successfully prepared through the sol–gel method. The process was described in detail elsewhere.23 In a typical preparation, analytical purity grade Fe(NO3)3·9H2O and NiCl2·6H2O were dissolved in distilled water. The oxides powders ZnO and Al2O3 of purity (99.99%) were dissolved in nitric acid. The obtained solution was added to the previous one. We mixed this solution with citric acid (C6H8O7) with molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 of metal cation (Ni + Fe + Zn + Al) to citric acid flowed by the addition of ethylene glycol (C2H6O2). The resulting solutions were heated to 80 °C with magnetic stirring to remove the excess water and to form a gel. To decompose the organic matter, the as prepared powder was annealed at 500 °C for 6 hours in an electrical muffle furnace and cooled slowly to room temperature. Finally, the obtained powder was then separated into five sets (Ni600), (Ni800), (Ni900), (Ni1050) and (Ni1200) were pelletized and annealed at 600, 800, 900, 1050 °C for 6 h and 1200 °C for 24 h, respectively. Chemical reaction of the prepared sample is shown in Fig. 1.
1.5 of metal cation (Ni + Fe + Zn + Al) to citric acid flowed by the addition of ethylene glycol (C2H6O2). The resulting solutions were heated to 80 °C with magnetic stirring to remove the excess water and to form a gel. To decompose the organic matter, the as prepared powder was annealed at 500 °C for 6 hours in an electrical muffle furnace and cooled slowly to room temperature. Finally, the obtained powder was then separated into five sets (Ni600), (Ni800), (Ni900), (Ni1050) and (Ni1200) were pelletized and annealed at 600, 800, 900, 1050 °C for 6 h and 1200 °C for 24 h, respectively. Chemical reaction of the prepared sample is shown in Fig. 1.
Characterizations and measurements
The structural characterization was carried out by X-ray diffraction at room temperature. The ferrite nanoparticles were characterized using a powder X-ray diffraction (XRD) with a Bruker D8 diffractometer with Cu Kα radiation (λ = 1.5406 Å) and a step size of 0.015° width from 15° to 80°. Morphological characterization was carried out using Merlin scanning electron microscopy (SEM) equipped with an energy-dispersive X-ray spectrometer (EDXS). The particle size was estimated by image processing of SEM's pictures using ImageJ software. Thermogravimetric analysis (TGA) and the differential thermal analysis (DTA) were carried out using the Setaram Instrument. The sample was heated up to 1000 °C with a heating rate 10 °C min−1 in argon atmosphere with nitrogen flow of 40 ml min−1. The Raman spectra were recorded using a SENTERRA spectrometer from Bruker Company with laser excitation of 532 nm at room temperature. Room temperature Fourier transform infrared (FTIR) spectra were obtained using Thermo Scientific Nicolet 6700 FTIR Spectrometer in the region 400–4000 cm−1 with diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) technique. Absorption spectra were registered on a UV-3600 Spectrophotometer (SHIMADZU, Japan) for the UV/visible/IR range. The magnetostriction measurements were made on the NZFAO pellets using the small angle rotation magnetization method. The magnetic isotherms M(H) were recorded at room temperature under a magnetic field up to 4 T using a SQUID magnetometer. Measurements of the intrinsic magnetic temperature (TC) were carried out using differential sample magnetometer MANICS in a field up to 1 kOe. Additionally, the electron spin resonance (ESR) measurements were carried out on a Bruker ESP-300E spectrometer operating in the X band 9.3 GHz and with a field modulation of 2 GHz. A thin silver film is deposited through a circular mask of 8 mm of diameter on both sides of the pellet by thermal evaporation. A plane capacitor configuration is obtained and permits the investigation of electrical properties of the synthesized material. Measurements are conducted with an Agilent 4294A analyzer under vacuum with signal amplitude of 20 mV.Result and discussion
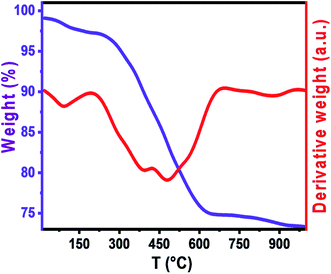
Thermal analysis
Thermal analysis is performed to understand the decomposition behavior of the Ni–Zn ferrite precursors and the formation of metal oxides, Fig. 2 shows the TGA and DTA curves of the as prepared sample which were performed up to temperature of 1000 °C at the heating rate 10 °C min−1 in argon gas to observe different changes in the form of endothermic and exothermic peaks which show the phase transition during the heat treatment. Moreover total weight loss is 24% which is the sum of two parts. The first broad weight loss of ∼2% in the temperature range below 200 °C is assigned to the dehydration of residual water. The second weight loss of ∼22%, between 200 °C and 630 °C on TGA curve, which may be due to the autocatalytic oxidation–reduction reaction between the nitrate and citrate acid. For temperature higher than 630 °C, the sample attains the stability in terms of weight and the weight loss at about this temperature possibly corresponds to the formation of metal oxide phase of ferrite Ni0.6Zn0.4Al0.5Fe1.5O4. We can conclude from TGA and DTA that the organic compounds were eliminated above 630 °C. Thus, the sample was further annealed at temperature from 600 °C.Structural analysis
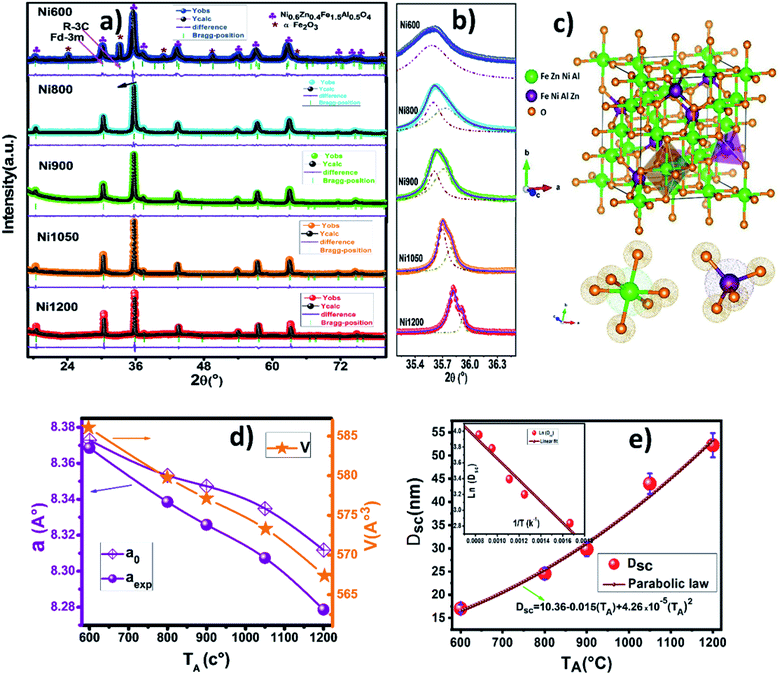
Fig. 3a depicts the Rietveld refined X-ray powder diffraction (XRD) patterns for Ni0.6Zn0.4Fe1.5Al0.5O4 samples annealed at 600, 800, 900, 1050 and 1200 °C using the FULLPROF program.25 The reflections from the atomic planes (111), (220), (311), (400), (422), (511), (440), (620), (533) and (622) are identified and confirm the formation of single phase cubic spinel structure with Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. For the sample annealed at 600 °C (Ni600), we notice the appearance of some peaks due to rhombohedral symmetry R
m space group. For the sample annealed at 600 °C (Ni600), we notice the appearance of some peaks due to rhombohedral symmetry R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c as a second phase (Fe2O3) designated by (*) which disappeared at higher annealing temperature. The hematite phase has been observed by several authors, up to the calcination temperature of 900 °C.26 No diffraction peaks of other structures were detected in the other samples, which indicated that high purity crystalline. The Rietveld refinement parameters are listed in Table S1 (ESI).† We have observed a low value of goodness of fit (χ2) which suggests that the refining of the samples is effective and that the obtained samples are of good quality. From Fig. 3b, it is observed that the width at half maximum of (311) peak decreases with increase in annealing temperature. Diffraction peaks become narrower and sharper with increasing temperature, thus demonstrating that the average size of the crystallites increases accordingly. The crystal structure of Ni0.6Zn0.4Fe1.5Al0.5O4 along with the different Wyckoff sites occupied by the Ni, Zn, Al, Fe and O atoms are shown in Fig. 3c.
c as a second phase (Fe2O3) designated by (*) which disappeared at higher annealing temperature. The hematite phase has been observed by several authors, up to the calcination temperature of 900 °C.26 No diffraction peaks of other structures were detected in the other samples, which indicated that high purity crystalline. The Rietveld refinement parameters are listed in Table S1 (ESI).† We have observed a low value of goodness of fit (χ2) which suggests that the refining of the samples is effective and that the obtained samples are of good quality. From Fig. 3b, it is observed that the width at half maximum of (311) peak decreases with increase in annealing temperature. Diffraction peaks become narrower and sharper with increasing temperature, thus demonstrating that the average size of the crystallites increases accordingly. The crystal structure of Ni0.6Zn0.4Fe1.5Al0.5O4 along with the different Wyckoff sites occupied by the Ni, Zn, Al, Fe and O atoms are shown in Fig. 3c.
The lattice constant (aexp) of Ni–Zn nanoparticles was determined from X-ray data analysis using the relation:12
The volume of the unit cell for a cubic system has been calculated from the following equation: V = aexp3. All the obtained lattice parameters and volume of the ferrite's compounds are plotted against annealing temperature in Fig. 3d. A decrease in the lattice parameter with increasing annealing temperature could be attributed to a decrease in particle surface stress and the higher crystal structure of larger particles. In addition, many metal oxides exhibit lattice parameter with a reduction in particle size which may be due to cation/anion vacancies, finite size effect, lattice stress etc. A similar variation of lattice parameter has been reported previously for Ni–Zn ferrite nanoparticles.11,21 The true values of the lattice parameter “a0” also are determined using the extrapolation function F(θ) by plotting the calculated “aexp” values of each diffraction peak versus the Nelson–Riley (N–R) function for each reflection of the studied Ni–Zn–Al ferrites:12
The values of the lattice constant “aexp” are depicted in the Fig. S1 (ESI)† as a function of F(θ) for all samples. As seen from the Fig. 3d, the true values of the lattice parameter are observed to be slightly higher than the experimental values. It is also evident that both “a0” and “aexp” and volume decreased as the size of particles increased. Lattice parameters (a0, aexp and aR measured from Rietveld refinement), density, porosity, crystallite size and surface area of all annealed samples are given in Table S1.† Density plays an important role in controlling the properties of polycrystalline ferrites. The X-ray density deduced from XRD data (ρX-ray) increases when increases annealing temperature rises, from 5.04 g cm−3 for Ni600 to 5.15 g cm−3 for Ni1200. Therefore, it has been noticed that the annealing temperature influences the densification process. In other words, during the sintering process, a force that is generated by the thermal energy drives the grains to grow over pores and as a result reduces the pores volume and their grain boundaries. The values of bulk density (ρth) also increase whereas the porosity (P) decreases with increasing the particles size, revealing that annealing temperature enhances the disorder of spinel ferrite system. The sol–gel process is known for synthesizing materials with high surface area (S). As it can be seen from Table S1,† the specific surface area varied in the range 69.8–22.2 (m2 g−1). Therefore, a high surface area of ferrite is needed for sensing and supercapacitor application.12
The crystallite size was calculated from the line broadening of the most intense (311) peak using the classical Scherer formula:12,21
where E is the activation energy, C is the specific reaction rate constant, T is the absolute temperature and R is the ideal gas constant. The crystallite growth activation energy of nickel ferrite synthesized by high-energy ball milling with Fe2O3 and NiO was found to be 64.4 kJ mol−1 as reported by Hajalilou et al.28 By comparison, this value is higher than the present value of 11.3 kJ mol−1. This means that the growth of Ni–Zn–Al ferrite crystallite prepared by the sol–gel method required less energy to overcome the kinetic barrier. Thus, it is one of the reasons for obtaining phase Ni–Zn–Al spinel ferrite at lower temperature.
The lattice strain (ε) was calculated from the Williamson–Hall method (figure not shown here). It is evident from Table S1† that strain is constantly decreasing with the increase in the crystallite size. This is evident because of the ratio of the number of atoms at the surface to the one of atoms inside the volume decreases when the crystallite size increases, reducing the number of broken bonds for surface atoms, hence reducing the strain.
The physical properties of ferrites are sensitive to the cation's nature, the valance state and their distribution on tetrahedral A and octahedral B sites of the spinel structure. Thus, understanding of the cation distribution is essential in understanding the intricate structural and physical property relationship. The distribution of cations in the various spinel ferrite systems has been estimated from Mossbauer, XPS, X-ray diffraction, and Raman measurements.29–31 Bestha et al.32 reported that the intensities of (220), (422), and (400) planes are sensitive to cations on tetrahedral (A-) and octahedral (B-) site. In our studies, the distribution of cations was estimated from XRD data by using the Bertaut method.32,33 From the best-matched intensity ratio, the cation distribution was estimated and the values are presented in Table S1.†
On the basis of estimated cation distribution, the value of ionic radius per molecule at the tetrahedral (rA) and octahedral (rB), theoretical lattice constant (ath), oxygen positional parameter (u), tetrahedral bond lengths (dAL), octahedral bond lengths (dBL), tetrahedral edge length (dAE), shared (dBE), unshared octahedral edge lengths (dBEU), jump length of the tetrahedral (LA) and octahedral (LB) for various samples has been determined using the relation (S5–S13) (ESI).† The values of all estimated structural parameters are shown in Table S1.† As shown in Table S1,† the variations of experimental (aexp) and theoretical lattice parameter (ath) with increasing annealing temperature are the same. Moreover, the theoretical lattice constant values are in close agreement with the experimentally derived values from XRD data. This result validates the estimated cation distribution from the XRD data. The oxygen positional parameter (u) is slightly greater than ideal value of 0.375. This has been attributed to the adjustment of the structure to accommodate differences in the relative effective radii of cations in the spinel structure.
LA and LB were observed to show a decreasing trend with an increase in the size of crystallites. The decreased behavior so observed can be correlated to the decrease in lattice parameter and to the decrease in distance between magnetic ions. It is also observed that LA > LB which indicates that the electron hopping between ions at A and B sites is less probable than that between B and B sites.
The strength of magnetic exchange interaction has direct dependence on bond angles and inverse dependence on interionic distances.34 Fig. S3 (ESI)† shows the interionic distances and bond angles between ions of spinel ferrites. The bond lengths and bond angles between the cations are calculated by the relations (S14–S27) (ESI) and are listed in Table S2.†
Morphological analysis
To obtain an accurate estimation of the microstructure and morphology of the nano-crystals, scanning electron microscopy has been performed. The SEM images of Ni0.6Zn0.4Fe1.5Al0.5O4 nanoparticles are shown in Fig. 4a–j, which demonstrates that the samples (Ni600, Ni800 and Ni900) are in nanoregime. At higher annealing temperature (>900 °C), a broad size of particle is observed for Ni1050 and Ni1200 samples. All samples showed a tendency towards agglomeration can be due to electrostatic effects as well as an artifact of the drying of aqueous suspensions and to magnetic interaction arising among of nanoparticles, revealing the formation of spherical and uniformly distributed nanoparticles. A similar behavior of nanoparticle agglomeration has been reported of Co3O4 nanoparticles.35 The observed asymmetric distribution can be described using a Lorentz distribution function as shown in Fig. 4k–o. Based on SEM images, the average grain size was found to be around 13, 20, 30, and 180 nm for Ni600, Ni800, Ni900 and Ni1050 samples respectively, and around 4 μm of the bulk sample (Ni1200). In fact, the annealing temperature increases the size of the grains. This increase is due to the coalescence of crystallites at increasing temperature. As shown, a clear difference between Ni600, Ni1050 and the bulk sample. The micrographs manifest grain boundaries and clear grains with non-uniform grain size distribution of Ni1200. The grains are almost homogeneously distributed throughout the sample surface. This observation is consistent with the results observed by XRD analysis, discussed earlier. Comparing the grain size obtained by SEM with crystallite size obtained from XRD analysis, it can be concluded that each grain of sample annealed at 600, 800 and 900 °C almost of single crystallite nature while the grains of sample annealed at 1050 and 1200 °C consist of agglomeration of many crystallites. The elemental analysis of the Fe600, Fe900 and Fe1200 samples, measured using EDX at room temperature, are shown in Fig. S3 (ESI).† The spectrum confirms the presence of all chemical elements Fe, Al, Zn, Ni and O in the synthesized Ni0.6Zn0.4Fe1.5Al0.5O4 samples, which confirms that there was no loss of any integrated elements and no contamination during the process of annealing. The result of EDX predicted the required proportion of the constituents as well as the chemical purity of the samples, denoting a homogeneous chemical composition.Raman analysis
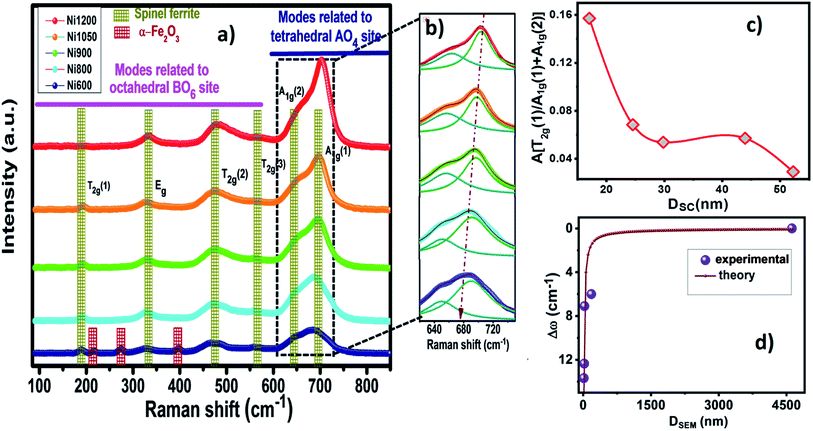
Further structural and complementary details can be obtained by the analysis of the Raman spectra. Confocal Raman spectroscopy has been widely used as an effective tool to investigate the cation disorder, the lattice dynamics and structure transition in ferrites due of their sensitivity and their high spatial resolution to explore atomic vibrations. In order to verify the chemical impurity and to confirm the formation of our simples, Raman spectra at room temperature in the region of 120 cm−1 to 850 cm−1 by using 540 nm laser excitation of Ni0.6Zn0.4Fe1.5Al0.5O4 nanoparticles and bulk synthesized by annealing at 600, 800, 900, 1050 and 1200 °C are presented in Fig. 5a. The factor group analysis predicts the following modes in spinel:36| ζ = A1g(R) + Eg(R) + T1g + 3T2g + 2A2u + 2Eu + 4T1u(IR) + 2T2u |
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group.14 The A1g Raman mode is associated to the symmetric stretching of the oxygen atom with respect to metal ion in tetrahedral sites. The Eg Raman mode corresponds to the symmetric bending of the oxygen atom with respect to the metal ion at the octahedral site and the T2g(3) and T2g(2) Raman modes are due to the symmetric and asymmetric bending of oxygen atom in M–O band at octahedral site. Finally, the T2g(1) phonon corresponds to the translation movement of the whole MO4 tetrahedral units.37 So accordingly, all the Raman active bands of NZFAO samples are assigned to the theoretical vibrational mode of Fd
m space group.14 The A1g Raman mode is associated to the symmetric stretching of the oxygen atom with respect to metal ion in tetrahedral sites. The Eg Raman mode corresponds to the symmetric bending of the oxygen atom with respect to the metal ion at the octahedral site and the T2g(3) and T2g(2) Raman modes are due to the symmetric and asymmetric bending of oxygen atom in M–O band at octahedral site. Finally, the T2g(1) phonon corresponds to the translation movement of the whole MO4 tetrahedral units.37 So accordingly, all the Raman active bands of NZFAO samples are assigned to the theoretical vibrational mode of Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group and illustrated in Table S3 (ESI).† The observed Raman modes are in good agreement with the literature reports.38 In the Ni600 nanoparticle sample, the peaks centered on 216, 274 and 397 cm−1 could be attributed to A1g, Eg(1) and Eg(2) vibrational modes of hematite α-Fe2O3,39 respectively, which is consistent with the results of the XRD. No additional peak corresponding to any other iron oxide phase is present for the other compounds, thus confirming the monophasic composition throughout. In order to determine lattice effect, the natural frequency, and the positions of the peak and its full width at half maximum (FWHM), Lorentzian line shape was used to fit the Raman spectra (Fig. S4, (ESI)†). In spinel ferrites, the modes above 600 cm−1 are belonged to the vibrations of the oxygen atoms in tetrahedral sites AO4 groups, whereas the modes below this frequency are related to the motion of the oxygen atoms in octahedral sublattice (O-modes).40 Additional shoulder mode are probably related to a breakdown of the momentum conservation rule since the particle diameter are significantly smaller than the wavelength of the exciting radiation.41 Focusing on the most intense A1g mode, a clear increase in intensity and blue shift along with the line broadening behavior can be seen with an increase of annealing temperature. The ratio of these signals is directly correlated to the degree of inversion of the spinel.42 As in spinel ferrite, the distribution of trivalent and divalent cations can change due to migration of metal ions from octahedral (B) to tetrahedral (A) sites and vice versa. The A1g splitting suggests the cation migration within both positions and the change in the area and intensity of the band related to the octahedral sites. In other words, the shift in the A1g modes due to a progressive change in inversion degree of the spinel lattice (see Fig. 5b).42 The obtained values of area ratio between the bands associated with the octahedral and tetrahedral sites are depicted in Fig. 5c. It shows that area ratio decreases with the increase in the size of crystallites indicating the decrease of the degree of inversion with increase crystallites size. These changes could be due to cation redistribution and a high degree of cation disorder induced by a crystallite size effect.43
m space group and illustrated in Table S3 (ESI).† The observed Raman modes are in good agreement with the literature reports.38 In the Ni600 nanoparticle sample, the peaks centered on 216, 274 and 397 cm−1 could be attributed to A1g, Eg(1) and Eg(2) vibrational modes of hematite α-Fe2O3,39 respectively, which is consistent with the results of the XRD. No additional peak corresponding to any other iron oxide phase is present for the other compounds, thus confirming the monophasic composition throughout. In order to determine lattice effect, the natural frequency, and the positions of the peak and its full width at half maximum (FWHM), Lorentzian line shape was used to fit the Raman spectra (Fig. S4, (ESI)†). In spinel ferrites, the modes above 600 cm−1 are belonged to the vibrations of the oxygen atoms in tetrahedral sites AO4 groups, whereas the modes below this frequency are related to the motion of the oxygen atoms in octahedral sublattice (O-modes).40 Additional shoulder mode are probably related to a breakdown of the momentum conservation rule since the particle diameter are significantly smaller than the wavelength of the exciting radiation.41 Focusing on the most intense A1g mode, a clear increase in intensity and blue shift along with the line broadening behavior can be seen with an increase of annealing temperature. The ratio of these signals is directly correlated to the degree of inversion of the spinel.42 As in spinel ferrite, the distribution of trivalent and divalent cations can change due to migration of metal ions from octahedral (B) to tetrahedral (A) sites and vice versa. The A1g splitting suggests the cation migration within both positions and the change in the area and intensity of the band related to the octahedral sites. In other words, the shift in the A1g modes due to a progressive change in inversion degree of the spinel lattice (see Fig. 5b).42 The obtained values of area ratio between the bands associated with the octahedral and tetrahedral sites are depicted in Fig. 5c. It shows that area ratio decreases with the increase in the size of crystallites indicating the decrease of the degree of inversion with increase crystallites size. These changes could be due to cation redistribution and a high degree of cation disorder induced by a crystallite size effect.43
Moreover, this blue shift can be understood quantitatively by estimating the effective force constant of the vibrating cation. Since the vibrational frequency of a species is proportional to its force constant (F), the following equations were used to calculate the force constants of the ions pertaining to tetrahedral (FT) and octahedral (FO) positions:32
| FT = 7.62 × MT × νT × 10−3 |
| FO = 10.62 × MO × νO × 10−3 |
The obtained FO values were found to rise and the FT values to decrease with an increase in crystallite size. The variation of FT and FO values with particle size can be understood as follows. In general, the force constant is inversely related to the bond length (viz., Fe–O, Ni–O, Zn–O and Al–O). Indeed, these bond lengths in ferrites will have an impact on the lattice parameter of the unit cell. Now, the lattice parameter was found to reduce with an increase crystallite size, and hence the Raman peak shifts towards the higher wavenumber side with increasing annealing temperature.
However, we can well understand the overall down shift of Raman bands in terms of size effect, because the size reduction leads to the increase of the micro-deformation and/or the dominance of phonon confinement.44 The small size of the crystallites hampers the propagation of phonons and thus induces a folding of the Brillouin zone making all Raman phonons active, leading to a broadening of the Raman spectrum band. The Raman shift and broadening of A1g peak can be explained by the bond-polarizability model described by Zi et al.45 Raman shift due to the confinement can be described by this model using the following relation:
| Δω = ω(D) − ω0 = A(a/D)δ |
Elastic and thermodynamic properties using FTIR analysis
The Fourier transform infrared spectroscopy spectrum was obtained in the wave number range of 400–4000 cm−1 at room temperature. The spectra of the investigated Ni–Zn–Al ferrite samples are shown in Fig. 6a. The peaks of metal–oxygen bonds at (557–581) cm−1 and (403–410) cm−1 correspond to characteristics of ferrites (see Fig. 6b). According to Waldron,47 these peaks are attributed to the stretching vibration of the cation–anion bond MA–O in tetrahedral sites (A) (νA) and the stretching vibration of the cation–anion bond MB–O in octahedral sites (B) (νB), respectively. Hence, the presence of these two peaks (νA) and (νB) confirms the formation of a spinel ferrite structure in the prepared nanoparticles. The positions of the vibrational bands of the Ni–Zn–Al ferrites are listed in Table S4 (ESI).† As can be seen in Fig. 6b, the peak (νA) slightly shifts towards a higher wavenumber with the increase in particle size, this shift is due to change in microstructure with increased annealing temperature, which may be the result of the transformation from the FeO6 octahedron to the FeO4 tetrahedron, and this transformation process can be accompanied by the enhanced covalency of Fe–O bonding. This confirms the results discussed in the Raman analysis.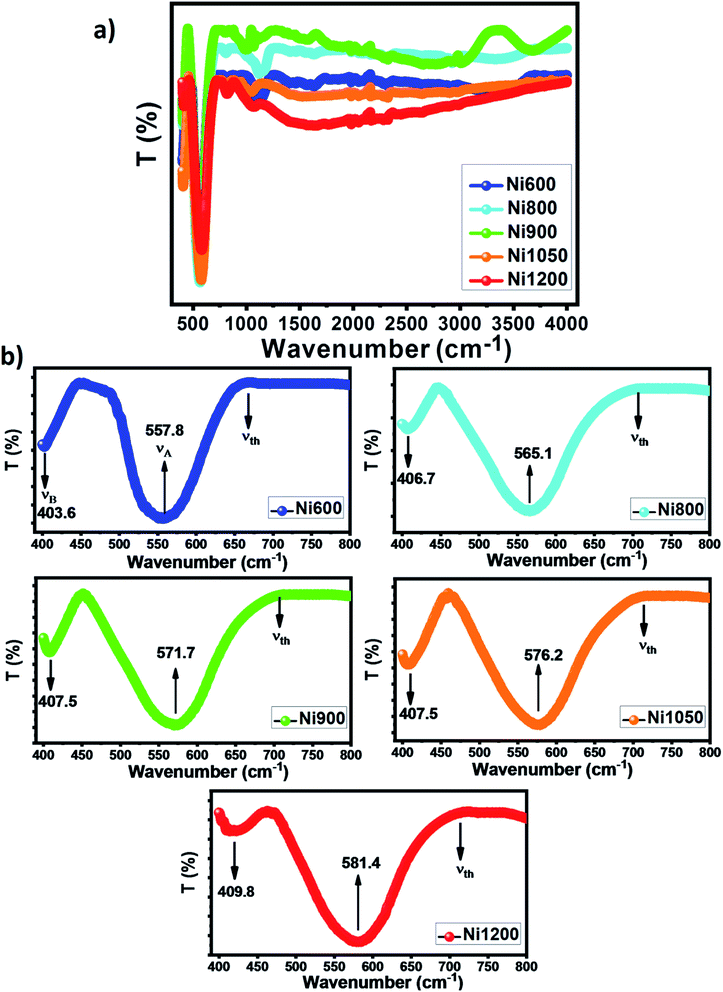 | ||
| Fig. 6 (a) FT-IR spectra of NZFAO samples. (b) Zoomed views in the wavenumber interval of 400–800 cm−1. | ||
The forces constants of the ions at the tetrahedral site (KT) and octahedral site (KO) are connected to the IR band frequencies (νA and νB) and to the molecular weight of cations on A and B sites which are expressed as:48
| KT = 7.62 × MT × νA × 10−3 |
| KO = 10.62 × MO × νB × 10−3 |
The force constant decreases for tetrahedral site while it increases for the octahedral site (Table S4†). The observed variation in the force constant values reflects changes in the Fe–O covalent with lattice contraction upon annealing temperature.48
The Debye temperature is an essential parameter to study lattice vibrations in solid-state physics, which is the temperature at which the maximum vibration of the network takes place and simplifies the integration of thermal capacity. The Debye temperature θD was determined using the following equation:12
Waldron have evidenced that the FTIR spectroscopy is a useful tool for calculating the threshold energy Eth associated with the electronic transition from the threshold frequency νth, which is determined from the maximum point of the absorption spectra, where it reaches a limiting value.47 The threshold energy can be calculated using the relation:
| Eth = ℏCνth |
The values of νth and Eth are shown in Table S4† and are comparable to those previously obtained in mechanically milled spinel ferrite by K. B. Modi et al.13 The Eth is found to decrease on increasing annealing temperature. This decrease in threshold energy may be due to the improvement of the electron hopping between the Fe2+ and Fe3+ ions in the octahedral coordination.
The elastic properties of materials are important for understanding the nature of binding forces in materials and can be used to measure the resistance of a crystal to an externally applied stress. The average force constant (Kav) is a product of lattice constant and stiffness constant (C11). The bulk modulus (B) of solids in terms of stiffness constants is defined as B = 1/3[C11 + 2C12]. According to Waldron,47 for the spinel ferrites, C11 = C12, hence B is simply given by C11. The value transverse wave velocity (νs), longitudinal wave velocity (νl), mean elastic wave velocity (νm), Young's modulus (E), rigidity modulus (G), Poisson's ratio (σ), and Debye temperature (θE) of the present system were calculated using the standard formulas available in the literature.12,13
The calculated values of elastic parameters for all the compositions (Table S4†) are in good agreement to those obtained from ultrasonic pulses transmission (UPT) technique.50 The values of elastic properties increase with the increase of annealing temperature, which indicates that the size of particles has large impact on elastic properties of spinel ferrite. However, the increase of Young's modulus E can be due to the rise of strength of inter-atomic bonding between the ions with an increase in annealing temperature.51 In addition, the Poisson's ratio σ (−1 < σ < 0.5) is generally used to quantify the stability of the crystal against shear. The higher the Poisson's ratio, the better the plasticity is. It can be noted that the value of the Poisson's ratio is constant for all the samples (σ = 0.35), which is in accordance with the theory of isotropic elasticity and implies a good elastic behavior. Furthermore, due to the strong effect of the porosity on the elastic properties, the values of elastic moduli have been also corrected to zero porosity using elastic theory and Ledbetter and Datta model52 and are listed in Table S5 (ESI).† From Table S5,† it can be observed that the Poisson's ratios increase with a raise of porosity and its existence can improve the plasticity. The elastic constants are similar to the obtained ones for Mn-doped Mg–Cu–Zn ferrites,51 LiZn2Fe3O8,12 and Ni0.5Zn0.5Fe2O4,13 which indicates that the elastic properties of our NZFAO samples have a high consistency. According to Pugh criteria,12 a useful value of bulk modulus to rigidity modulus (B0/G0) were used to distinguish if the materials under investigation are ductile or brittle in nature. It can be found that the value of B0/G0 is higher than 1.75, which means that the NZFAO compounds are of ductile nature. The high plasticity and ductility make our samples a potential candidate as materials in microelectro-mechanical systems (MEMS).
The lattice energy values (the bond strength values) for nanoparticles Ni–Zn–Al ferrite are estimated by Kadriavtsev's approach. Assuming the additive of internal energy, the velocity of sound wave (ν) in solid and liquid media is given by the standard thermodynamic equation:13,31,53
| UL = −3.108 × 10−3Mνm02 |
Table S6 (ESI)† lists the UL values for the different samples. In view of the Table S6,† our calculated values with this approach are close to those obtained by Catlow et al.54 from atomistic computer simulation.
Following Waldron's approach47 and according to Modi's work,13,31 the characteristic temperature of Debye θJ can be defined by:
where p is the number of atoms per chemical formula and R is the gas constant. The values of heat capacity are shown in Table S6.† In the case of spinel ferrite system of the type A2+B23+O4, according to Dulong and Petit law, the theoretically limiting value of heat capacity at constant volume and at T ≈ θ, Cv = 21, R = 174.5 J K−1 mol−1.13,31 The Cv values of our samples are in accord with this limiting value as obtained from Dulong and Petit law and to that obtained using Waldron's approach.47
The thermal conductivity of a material can be considered a fundamental property of the material. Using the Cahill's model,55 we have calculated the minimum lattice thermal conductivity at 300 K of annealed Ni–Zn–Al ferrite by the following relation:
Optical properties
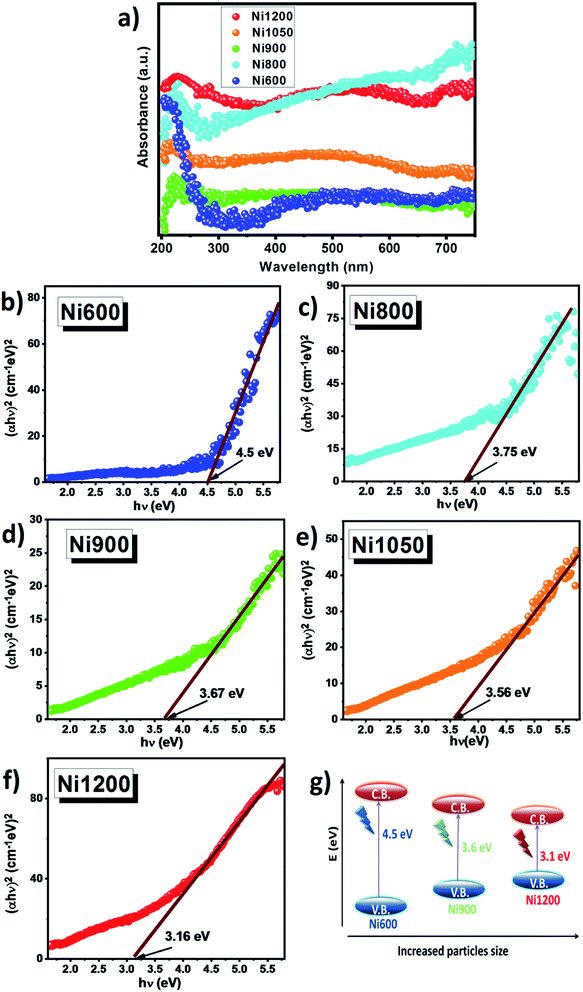
UV-visible absorption spectra were used to understand the optical properties of nickel–zinc ferrite samples. UV-visible absorbance spectra for all samples were recorded in the range 200–800 nm at room temperature and the absorbance spectra are shown in Fig. 7a. It is clear that Ni–Zn–Al ferrite shows high absorption intensity in the UV and visible light region. Absorbance spectra exhibit an absorption peak at around 300 nm. It is well known from past reports on ferrite spinel that, the absorption can be attributed to the photo-excitation of electrons from the valence band to the conduction band (ligand-to-metal (p–d) type charge transition O2p → Fe3d) or to the 3d5–3d44s1 transition in Fe3+ ions.12 Interestingly, a slightly redshift in the absorption edge was observed as the size of grain increases. This might be due to changes in their morphologies, particle size, carrier concentrations, surface microstructures, presence of very small amount of impurities, lattice strain and oxygen vacancy.57 Furthermore, the optical properties and band gaps of the samples sintered at 600, 800, 900, 1050 and 1200 °C are determined using absorption spectrum. The optical band gap (Eg) is the most important feature of materials that are good candidates for optoelectronic applications. The optical band gap (Eg) of the present Ni0.6Zn0.4Fe1.5Al0.5O4 nanoparticles can be calculated experimentally from the optical absorption edge using the Tauc's relation as follows:12,58| αhν = A[hν − Eg]n |
As ZnFe2O4 and NiFe2O4 (ref. 59 and 60) have direct gap, the synthesized compound has also a direct gap. So by plotting (αhν)2 vs. hν, the optical band gaps (Eg) of the samples were realized as the intercepts with the energy axis, as shown in Fig. 7b–f. The estimated band gap energies values were found to be 4.5, 3.75, 3.67, 3.56 and 3.16 eV for Ni600, Ni800, Ni900, Ni1050 and Ni1200, respectively, denoting redshift as a function of grain size. This might be due to sub-band gap energy level formation and the presence of defects in the nanoparticles as a result of annealing temperature. Similar effect has been observed for other ferrite compounds reported by Singh et al.60 (Eg = 4.28–3.80 eV for ZnFe2O4 nanoparticles) and Chand et al.59 (Eg = 3.54 eV for NiFe2O4 nanoparticles). For semiconductor nanoparticles, the quantum confinement effect is expected. In our case, it has been observed that the band gap energy decreases with an increase in the crystallite size. This decrease of band gap between the valence band and conduction band (red shift) with increase particle size can be attributed to weak quantum confinement effect.61 Additionally, the increase in annealing temperature decreased concentration of oxygen vacancy and may be a source of trapped exciton states that form a series of metastable energy levels within the energy gap, resulting in the red shift of the optical band gap and the absorption band moves to the upper wavelength region.62 These values of band gap are greater than the theoretical energy required for water splitting (λ > 1.23 eV) and this prominent red shift may be responsible for enhanced charge separation and increased photocatalytic activity under visible light (absorption capability in the visible range).12 Fig. 7g shows the change in band gap value and corresponding resultant schematic band structure. Furthermore, the reduction in the wide band gap with increasing particle size and transparency in the visible region makes these Ni–Zn ferrites samples a promising candidate for photodetectors and optoelectronic applications by harvesting more photons of light to excite the electrons from the valence band to the conduction band. In addition, to further investigate the pre-absorption edge, an Urbach tail analysis was also performed. The exponential absorption tails and Urbach energy is given in accordance with the following relation:63
α = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[hν/EU] exp[hν/EU] |
Urbach energy of samples is calculated by tacking the reciprocals of the slope of the liner portion in the lower photon energy of this curve by plotting the logarithm of the absorption coefficient, ln(α), against photon energy. The EU value of this exponential dependence on photon energy may arise due to the random fluctuations associated with the small structural disorder present within the system. It is clear from Table S7 (ESI)† that the band gap energy has a correlation with Urbach energy, and both red shifts with the increase annealing of the temperature. The observed red shift in EU arises because with increase of structural order variation with growth temperature, short range lattice disorder originates in the samples.63
The refractive index (n0) of semiconducting materials is very important in determining the optical and electric properties of the crystal. Knowledge of n is essential in the design of hetero-structure lasers in optoelectronic devices as well as in solar cell applications.64 The refractive index (n0) of ferrite nanostructures can be calculated from the value of Eg using Moss empirical relation:58,59
| Egn04 = 104 eV |
Furthermore, we have calculated the high frequency dielectric constant εα with a refractive index by using the following relation:58,59
| εα = n02 |
The values of the energy band gap, Urbach energy, refractive index, and high-frequency dielectric constant of Ni–Zn–Al ferrite samples are listed in Table S7.† Evidently, the values of the refractive index n0 and εα were increased with increasing the annealing temperature. The results can be discussed on the basis of the oxygen deficiency with enhanced annealing temperature.
Magnetostrictive properties
Fig. 8 shows the plots of longitudinal magnetostriction λ of Ni0.6Zn0.4Fe1.5Al0.5O4 samples sintered at 800, 900, 1050 and 1200 °C, measured at room temperature along the parallel directions to the applied magnetic field. It has been observed that annealing temperature in Ni–Zn–Al ferrite has a strong influence on the magnetostriction characteristics. The maximum value of magnetostriction increases with increasing grain size. Further, the highest magnitude of magnetostriction, λmax = −10 ppm, was achieved for sample annealed at 1200 °C. The magnetostrictive properties depends upon the processing parameters such as synthesis method, applied pressure while making pellets, sintering atmosphere, temperature and sintering time.65 However, the observed porous nature may be responsible for the decrease of magnetostrictive coefficient in nanoparticle samples. Also, another possibility is that the increase in the magnetostriction with sintering temperature can be due to the minor changes in the degree of inversion in the ferrite, which can be attributed to the increase super-exchange and the direction deflection of the magnetic domain would, therefore, result in a higher magnetostriction.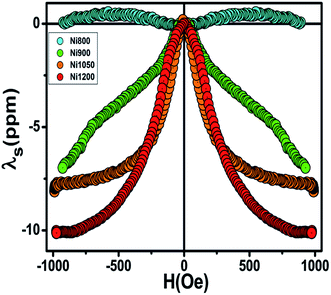 | ||
| Fig. 8 Magnetostriction of the annealed NZAFO samples at room temperature, as a function of magnetic field, measured along the parallel direction to the applied field. | ||
In the nickel ferrite, the unquenched orbital moment of Ni2+ leads to a large anisotropy and magnetostriction value (−26 ppm).66 This value of λmax for our samples are less than that reported for the pure bulk NiFe2O4, due to the addition of non-magnetic Zn and Al ions to nickel ferrite and to the difference in the microstructure associated with the different synthetic methods and processing conditions. The λmax obtained in the bulk sample Ni1200 is relatively larger than the reported values of magnesium and lithium spinel ferrite.67 Moreover, this value is similar to the ones of Ni0.5Zn0.5Fe2O4.68 Overall, the reasonable magnetostriction coefficient and the high Curie temperature in Ni1050 and Ni1200 samples makes this material attractive for the use in automotive stress-sensing sensors applications.
Magnetic properties
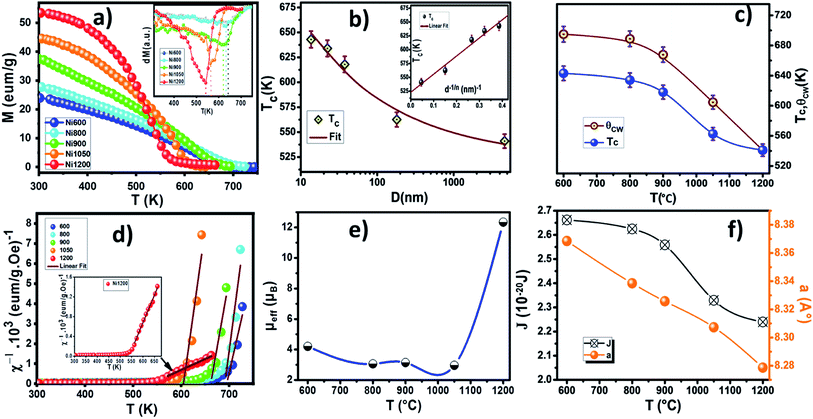
Curie temperature (TC) is known to be one of the main magnetic parameters of ferrite. So, to determine the Curie temperature of the spinel NZAFO nanoparticles, the temperature dependent magnetization was measured over a temperature range of 300–750 K under an external magnetic field of 1 kOe, as shown in Fig. 9a. All samples exhibit paramagnetic (PM) to ferrimagnetic (FiM) transition at Curie temperature TC. The TC was determined from The first point of inflection in the first derivative of the magnetization (inset of Fig. 9a) it appears that the FiM transition in the Ni600 and Ni800 nanoparticles samples are markedly sharper than the transition of the Ni900, Ni1050 and Ni1200 samples. Moreover, the maximum slope of the magnetization curves, i.e. the slope at TC, systematically decreases from 650 to 540 K with the grain size increases from 17 nm to 4 μm, indicating that a more direct relationship exists between the grain size and the broadening of the Curie transition of the NZFAO system.The obtained Curie temperature value, TC, of the samples are lower than that of polycrystalline NiFe2O4 (TC = 883 K)32 and NiFe2O4 nanoparticles (TC > 800 K)69 as well as Ni0.6Zn0.4Fe2O4 nanoparticles (TC = 663 K) reported by Gawas et al.70 The difference can be due to the replacement of magnetic Fe3+ ions by nonmagnetic Al3+ ions. The substitution of nonmagnetic ions reduces active magnetic linkages for magnetic ion per formula unit as a result Curie temperature decreases.
The decrease in Curie temperature can be explained usually on the basis of finite size effects, due to atomic defects at the surface, leading to disorder in nanocrystalline systems.71 To understand the strong size dependence of the Curie temperature, the finite-size-scaling theory was used to fit the behavior of TC as seen from Fig. 9b. According to this theory, the shift in the Curie temperature from that of the bulk is given by:72
Further, the values of exchange interaction J can be calculated using the formula:78
In general, a large magnetocaloric effect (MCE) is observed near the transition temperature and is closely related to the order of the corresponding magnetic phase transition. The field cooling (FC) curves show a reversible character of the magnetization around the Curie temperature. Furthermore, the small area of the hysteresis cycle indicates low energy loss during a magnetization–demagnetization process79 (see Fig. 10a). Therefore, the Ni–Zn–Al spinel ferrite can be considered as a candidate for magnetic refrigeration in a wide temperature range above room temperature and to transform industrial waste transformed into electrical energy using a thermomagnetic generator.80
The inverse magnetic susceptibilities are shown in Fig. 9d. The inverse susceptibility rise sharply when the magnetic state of the ferrite nanoparticles changes from ferrimagnetic to paramagnetic states. In the temperature range, well above TC, the susceptibility obeys the Curie–Weiss law:81
where NA is Avogadro number and KB is Boltzmann constant. Since the sign of θCW is positive (see Fig. 9c), then the ferrimagnetic interaction between spin in these compounds is highly expected. The obtained values of θCW are slightly higher than TC which refers basically to the presence of a magnetic inhomogeneity.82 Generally, the difference between TC and θCW is associated with the presence of short-range order slightly above TC (Fig. 9c). The increase in the experimental effective paramagnetic moment for the bulk sample can be explained by the existence of ferrimagnetic clusters within the paramagnetic phase (Fig. 9e).
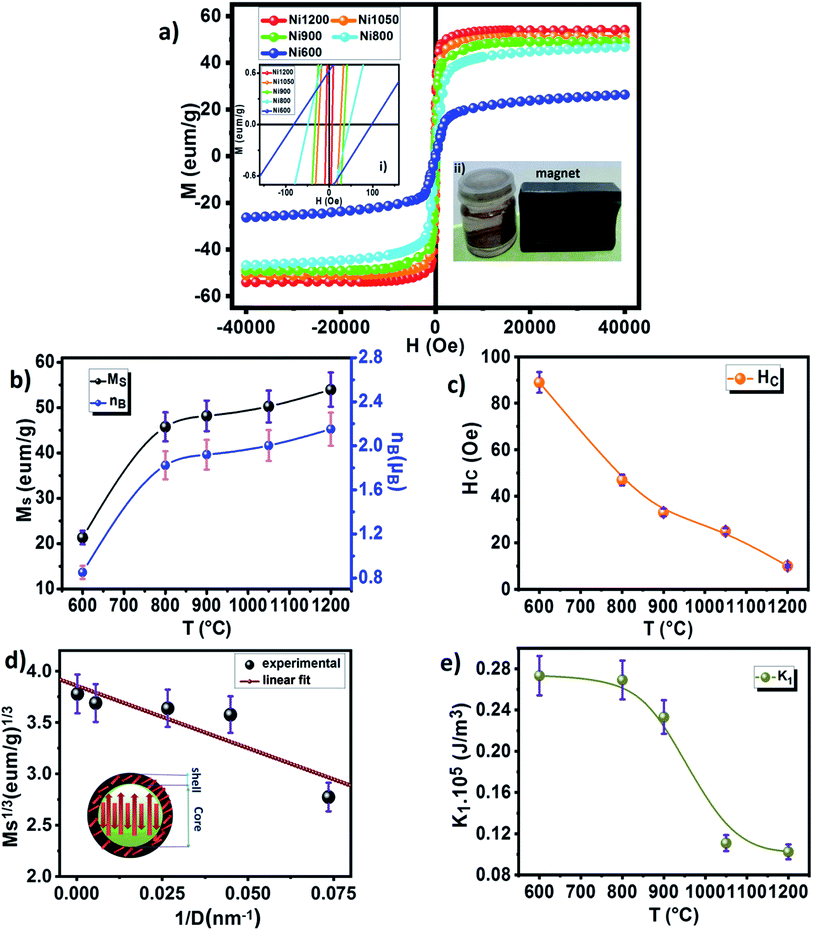
The room temperature magnetization M(H) of the nano-Ni0.6Zn0.4Fe1.5Al0.5O4 varying sizes is shown in Fig. 10a. The inset shows M versus H in a small field range. It could be observed from the hysteresis loop that all composition of nanoparticles shows negligible remanence and coercivity, which confirms the ferrimagnetism of the soft ferrite materials. This soft magnetic nature might be useful for designing multifunctional devices to switch magnetization with a small external magnetic field. The annealing temperature has an enormous impact on the magnetic performance parameters: as the annealing temperature increases (i.e., increasing grain size) the saturated magnetization increases, while the coercivity decreases. The variation of coercivity (Hc) as a function of annealing temperature is summarized in Fig. 10c. The observed decrease in coercivity in the samples has been due to multi-domain nature of particles.83 Recently, Das et al.84 reported the variation of coercivity (Hc) with grain size. They found that it increases initially up to 40 nm and then decreases for CoFe2O4 nanoparticles. The decreasing trend of Hc with grain size beyond 40 nm gives a hint of transforming single domain into a multidomain particle. In our studied samples, the continuous decrease of coercivity with the increase in grain size indicates that the NZFAO particles do not undergo from single magnetic domain to multi-domain in this range of particle size. Similar variation of Hc with increasing particle size from 10 to 200 nm in NiFe2O4 is also reported by Yadav et al.85
According to Stoner–Wohlfarth theory, the anisotropy constant value is related to the coercivity by the following relation:86
The increasing of coercivity observed in the Ni600 and Ni800 samples can be related to frustrated magnetic structure developing in the surface layer of nanoparticles.29 Further, the non-zero Hc suggests presence of some blocked particles in the nanoparticles samples and that the samples had both ferrimagnetic and superparamagnetic components together. In addition, the Ni600 sample is magnetically well ordered at 300 K and it displays a substantial amount of coercive field (Hc = 90 Oe) and this has been noticed only in very few nanometric ferrites. The hight coercivity (280 Oe) at room temperature is also reported by S. Dey et al.87 in Ni0.5Zn0.5Fe2O4 nanoparticle with 8–20 nm particle size.
Therefore, further investigation of the observed behavior of Hc is desired. The high saturated magnetization (Ms) values were determined by fitting the high field magnetization data using the saturation approach law given by:88
From Table S2,† the cation distribution results clearly indicate that Zn2+ has strong preference for the tetrahedral sites, whereas Ni2+ ions mainly occupy the octahedral sites and Al3+ and Fe3+ ions have a preference for both the available A and B sites but the Al3+ have strong preference for the octahedral site. As the sintering temperature increases, most of the Fe3+ ions occupy the octahedral site and the majority of the Al3+ ions occupy the tetrahedral site which increases the net magnetic moment and hence the increase of the saturation magnetization values.
The magnetic moments calculated per the formula unit are chows in Fig. 10b.32
The magnetic moment of nanoparticles at room temperature increases remarkably with increasing particle size from nB = 0.85 μB (Ni600) to nB = 2.15 μB for Ni1200 sample. This enhancement is attributed to increase in exchange interactions between A and B sites, which resultantly increases net magnetization as well as magnetic moment per formula unit.
The net magnetization of a unit cell in spinel ferrites is considered to be proportional to the difference between magnetic moment of the A and B sites. According to Neel's collinear model, the magnetic moment per formula unit for such a system can be given as:92
| nth = MB − MA |
The value of αY–K angle is in the range 53–65.8°, which confirms the existence of a triangular (canted) spin arrangement in all samples. The estimated values of canting angle are close to the value 53.64° for Ni0.6Zn0.4Fe2O4.93 It is clear that the non-zero αY–K angle suggest that the magnetization behavior for all samples cannot be explained on the basis of Neel two-sublattice model due to non-collinearity of spins on B site. Therefore, we may conclude that our NZFAO samples show Y–K magnetic ordering. Furthermore, the results indicated cation migration as the annealing temperature or the grain size increased. The migration of cation causes strains and it may break the surface exchange bonds to yield a canted spin structure. Thus, the canted spin structure weakens the A–B exchange interactions to reduce the saturation magnetization as the particle size decreases.
For the nanosized materials, the decrease in the saturation magnetization is due to the increasing surface area to volume ratio. It is assumed that a magnetically dead layer is formed at the surface of the particles due to the uncompensated exchange interactions in a surface layer of thickness t which is composed by canted spins that do not contribute to the net magnetic moment of the particle. We can then propose a model of the particles in which the outer part contains defects in the crystallographic structure and oxygen defects which will lead to a magnetically disordered state but the internal part will have the same properties as the bulk compound. Assuming a spherical shape of NZFAO nanoparticles, we will estimate the thickness of the shell (t) using the experimentally determined saturation magnetization (Ms) and particle size (D) values of the samples annealed at various temperatures. The variation of the saturation magnetization versus the diameter of nanoparticles has been fitted according to a core–shell model and shown in Fig. 10d. In this core–shell model, the magnetization of the particles can be expressed as:94
| Ms1/3 = M01/3[1 − 2t/D] |
Using the saturation approach law, we have determined the anisotropy constant K1 for different sizes of Ni–Zn–Al ferrite. K1 values are plotted as a function of the annealing temperature in the Fig. 10e. It is clear that the anisotropy constant decreases with the increase in particle size. This may be due to the finite size effects and the collective fraction of broken lattice symmetry accompanying atoms residing on the surface. These values are slightly greater as compared to the other magnetic nanoparticles, e.g., Fe2O3 (ref. 96) and CoFe2O4.65
In order to check if our material can be useful for high-frequency microwave applications, the microwave frequency (ωm), of the present NZFAO NPs has been calculated by the following equation:97
| ωm = γ18π2Ms |
Electron spin resonance analysis
Electron spin resonance (ESR) spectra (first derivative of the power absorption curve) were recorded to further investigate the magnetic features and spin related phenomena of the all annealed samples. The X-band ESR spectra of the Ni–Zn–Al ferrite nanoparticles with different crystallite sizes at room temperature are shown in Fig. 11. All main ferrite spectra are well described by a single Lorentz line function in its field-derivative form:where A is its amplitude factor, Hr is the resonance magnetic field and ΔHPP is the half width at half maximum of the absorbance line. As can be easily seen from Fig. 11, the ESR spectra are characterized by a broad, single and slightly asymmetric resonance line, which becomes narrower as the annealing temperature increases from 600 to 1200 °C. The broad curves indicate that the existence of dipolar–dipolar interactions among particles and randomly oriented magnetic anisotropy axes, especially for the Ni600, Ni800 and Ni900 nanoparticles samples, which is the characteristic property of superparamagnetic materials. In accordance with this observation, K. Praveena et al.24 have reported a similar behavior in Ni0.4Zn0.2Mn0.4Fe2O4 nano powders annealed at different temperatures. In the case of Ni1050 and Ni1200, the reduced value of ΔHPP can be due to the strong super exchange interaction between magnetic ions through oxygen anions. According to resonance condition of the ESR spectra, we estimated the effective factor g value from ESR spectra by using the formula:
where, υ is the microwave frequency, μB is the Bohr magneton, Hr is the resonance field and ℏ is the Planck constant. The resonance line width (ΔHPP), the resonance filed (Hr), and the effective g-factor are listed in Table S8 (ESI).†
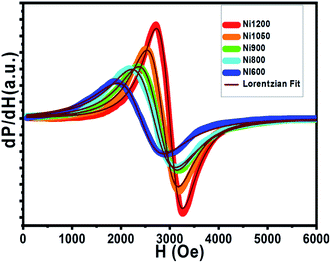 | ||
| Fig. 11 First derivative (dP/dH vs. H) of resonance signals of different annealing temperatures NZFAO nanoferrites. | ||
The g factors for the low temperature annealed samples Ni600, Ni800 and Ni900 are very close to that for polycrystalline Ni–Zn ferrite.99 The high value of g and the largest ΔHPP, indicate the strong interaction between small NZAFO NPs. Increasing the annealing temperature to 1200 °C, the g value decreased to 2.64 accompanied by the smallest value of ΔHPP of 405 Oe, reflecting the presence of the strong exchange interaction in bulk sample, indicating exchange interaction between Fe3+ and Ni2+ ions. This decrease can be due to the isotropic alignment of magnetic moments with the increase in particle size. ESR signals show that when the size of particles increases, the resonance field increases, but the peak to peak line width decreases. This means that the net magnetization of the samples increases with the increase of annealing temperature. Therefore, with the increase of grain size, migrate of Ni2+ and Fe3+ ions from the surface/interface site to bulk one. The octahedral and tetrahedral sites should also alter the interaction of magnetic ions. Moreover, the shell is visualized to be the zone in which created vacancies and the super exchange interaction has been destroyed. The shell has a smaller magnetization compared to the core and the magnetization of this zone decreases with increasing size of grain. Therefore, the decrease in resonance field was observed.
ESR spectroscopy was used to study the relaxation mechanism. The spin relaxation process is a function of static magnetic field and depends on the rate at which microwave energy can be absorbed and dissipated via relaxation processes either by spin–lattice (T1) or spin–spin relaxation (T2) process. The spin–spin relaxation process is the energy difference (ΔE) transferred to neighboring electrons and the relaxation time (T2) can be determined from the ΔHPP line width according to following equation:99
where, the spin–lattice relaxation involves the thermal equilibrium of the spin system with the lattice or environment of the spin. Hence, the super-paramagnetic behavior of the samples can be explained on the basis of spin–lattice interaction. Neel superparamagnetic is due to thermally induced fluctuations of the direction of magnetization in single domain particles below a certain critical size. However, the completion of g and ΔHPP parameters leads to an increase of the relaxation time (T2) from 2.9 × 10−11 s for Ni600 to 6.4 × 10−11 s for Ni1200 (Table S8†). It is well know that the specific loss power (SLP) value for hyperthermia treatment depends inversely to the relaxation time; thus, the observed minimum value of T2 for Ni600, Ni800 and Ni900 makes them a good candidate for use in hyperthermia treatment.101 For these samples, T1 has a lower magnitude than T2 showing the dominance of spin–spin interaction. Super-exchange magnetic interaction is dominated in Ni1200 sample where the relaxation rate ratio R2/R1 is 1.7 × 10−2 and dipolar magnetic interactions is dominated in Ni600 sample with highest the relaxation rate ratio R2/R1 = 8.6 × 10−2 (Table S8†).
For magnetic resonance imaging (MRI) T2-contrast agent, the transverse relaxivity r2 can be estimated at the magnetic field assuming the magnetic average regime (MAR) to interpret the interaction of nanoparticles with the protons using the following equation:78
Electric properties
Let us now turn to the effect of annealing temperature on the electrical properties. Fig. 12a shows the temperature dependence of direct-current resistivity regime (ρdc) in the range 220–600 K for the NZFAO nanoparticles (Ni600, Ni900 and Ni1200), the resistivity decreases continuously with increasing temperature for all samples, which reflects a semiconductor behavior. In general, this could be ascribed to the increase in the drift mobility of electric charge carriers which are thermally activated with increasing temperature.103 The electrical resistivity of the Ni–Zn nano ferrite decreases from 7 × 106 to 4.7 × 103 Ω cm at 360 K with the increase in crystallite particles size from 17 to 40 nm. This can be due to the increase of compactness of the specimens assured by increasing the annealing temperature. The observed variations in resistivity with composition can be explained by Verwey's hopping mechanism.104 The electronic conduction in ferrites is principally due to hopping of electrons between the ions of the same element present in more than one valence state (Fe2+ and Fe3+ ions), distributed randomly over crystallographically equivalent lattice sites.105 For the Ni600 and Ni900 nanoparticles with such high resistivity that led to very low eddy current losses are desirable in transformers, electronic inductors, electromagnets and at high frequency application,106 while the Ni1200 sample having with higher conductivity may be desirable in the use in solid oxide fuel cells.103 The relation between resistivity and temperature describes the well-known Arrhenius equation and may be expressed as:where ρ is the dc electrical resistivity at temperature T, KB is Boltzmann constant, Ea is activation energy and ρ0 is the pre-exponential constant. Typical plots of (log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρdc) versus 1000/T for the samples sintered at 600, 900 and 1200 °C are shown in Fig. S5 (ESI).† For the Ni600 and Ni900 samples, a change in slope was observed in the resistivity curve that divides the curve into two regions or one break, whereas in the Ni1200 sample, which the studied temperature range was extended to a few degrees upper the Curie temperature, the plot shows three zones, i.e. two breaks. The second break occurs in the vicinity of the Curie temperature (TC = 540 K) and this has been attributed to the influence of magnetic order on the conduction mechanism.107 The Curie temperature obtained from the resistivity is in agreement with the TC obtained by the magnetic measurements. The calculated activation energies (E1 and E2) for the three compositions corresponding to the two regions (Tr1 and Tr2) have been estimated and are shown in inset of Fig. S5.† The activations energies in Tr2 region (E2) were higher than those in Tr1 region (E1). Increasing activation energy with increasing temperature can be ascribed to the presence of different carriers' traps. For the low temperature process, the dominant conduction mechanism would be an exchange of electrons between nearest neighbor Fe2+ ↔ Fe3+ pairs, with associated activation energy of the order of 0.1 eV.108 While for the high-temperature process (Tr2 region), the conduction mechanism is expected via Ni2+ or Fe3+ neighbors, i.e. Ni2+ + Fe3+ ↔ Fe2+ + Ni3+. Similar results to the present study were obtained in the case of a number of mixed ferrites such as Ni–Zn,107 and Mn–Ni–Zn.109
ρdc) versus 1000/T for the samples sintered at 600, 900 and 1200 °C are shown in Fig. S5 (ESI).† For the Ni600 and Ni900 samples, a change in slope was observed in the resistivity curve that divides the curve into two regions or one break, whereas in the Ni1200 sample, which the studied temperature range was extended to a few degrees upper the Curie temperature, the plot shows three zones, i.e. two breaks. The second break occurs in the vicinity of the Curie temperature (TC = 540 K) and this has been attributed to the influence of magnetic order on the conduction mechanism.107 The Curie temperature obtained from the resistivity is in agreement with the TC obtained by the magnetic measurements. The calculated activation energies (E1 and E2) for the three compositions corresponding to the two regions (Tr1 and Tr2) have been estimated and are shown in inset of Fig. S5.† The activations energies in Tr2 region (E2) were higher than those in Tr1 region (E1). Increasing activation energy with increasing temperature can be ascribed to the presence of different carriers' traps. For the low temperature process, the dominant conduction mechanism would be an exchange of electrons between nearest neighbor Fe2+ ↔ Fe3+ pairs, with associated activation energy of the order of 0.1 eV.108 While for the high-temperature process (Tr2 region), the conduction mechanism is expected via Ni2+ or Fe3+ neighbors, i.e. Ni2+ + Fe3+ ↔ Fe2+ + Ni3+. Similar results to the present study were obtained in the case of a number of mixed ferrites such as Ni–Zn,107 and Mn–Ni–Zn.109
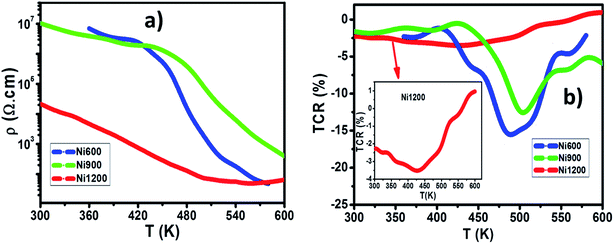 | ||
| Fig. 12 (a) The temperature dependence of direct-current resistivity for Ni600, Ni900 and Ni1200 samples. (b) The TCR as a function of temperature for Ni600, Ni900, and Ni1200. | ||
The value of the temperature coefficient of resistance TCR is very useful for determining the sensitivity of the infrared detectors (IR) for night vision bolometer technologies.110
Conclusion
In this study, we described an investigation on the influence of the particle size in the structural, optical, elastic, thermodynamic, magnetic and electrical properties of Ni0.6Zn0.4Fe1.5Al0.5O4 nanoparticles. The sol–gel technique was successfully used to obtain nano and micro-powders of Ni0.6Zn0.4Fe1.5Al0.5O4. Heat-treatment in the temperature ranges of 600–1200 °C yields to an average grain size ranging from 17 nm to 4 μm. We arrived at the following conclusion:(1) Rietveld refinement of the XRD patterns confirmed the high crystalline quality of the particles obtained particles. The grain size and strain were observed to increase with increasing annealing temperature. FTIR and Raman spectroscopy analyses confirmed the presence of tetrahedral and octahedral sites in the prepared samples. The nanometric and micrometric nature of Ni–Zn–Al ferrite was confirmed by SEM microscopy.
(2) The compositional dependence of elastic constants can be explained on the basis of inter-atomic bonding between the ions. The Debye temperature and molar heat capacity values determined from FTIR spectral analysis are in agreement with the reported ones in literature. The increase of size results in an increase of both Debye temperature and thermal conductivity but it decreases of the molar heat capacity. The Ni–Zn–Al ferrite samples can be used for switch-mode power supply.
(3) The optical properties of the synthesized Ni0.6Zn0.4Fe1.5Al0.5O4 samples were also investigated. The decrease of size results in a decrease of the band gap energy and in an increase of refractive index n0. Hence, the annealed Ni–Zn–Al ferrite samples display enhanced optical absorption capability in the visible range.
(4) The high temperature magnetic measurements and hysteresis loops at RT indicate that the increase in annealing temperature leads to significant shift in Curie temperature consistent with finite size scaling formula and to an increase of the fraction of the superparamagnetic phase at the expense of the ferrimagnetic one. Ms consistently increases with the increasing of the annealing temperature and this can be explained by an interface/surface region of the nano-spinel ferrite. High values of microwave frequency are calculated (from C to X band) which indicates that it is a promising infrared detector (IR) for night vision bolometer material. Ni–Zn–Al ferrites may have potential in industrial and technological applications e.g. magnetic refrigeration, biomedical applications and microwave frequency (C and X) band applications.
(5) The room temperature ESR spectra reveal that peak width ΔHPP was reduced and the spin–spin relaxation T2 increases as the annealing temperature increases due to the increase in the dipole–dipole interaction. Moreover, the high values of the transverse relaxivity r2 of Ni600, Ni800, and Ni900 make them a potential candidate for use in hyperthermia treatment. In contrast, the reasonable magnetostriction coefficient (λmax = −10 ppm), which was achieved for bulk sample (Ni1200) makes this material attractive for the use in automotive stress-sensing sensors applications.
(6) The electrical resistivity of the Ni–Zn nanoferrites decrease with the increase in particle size. The temperature coefficient of resistance TCR of three typical samples was calculated. Ni600 (with the highest electrical resistivity) had a highest temperature coefficient of resistance than Ni900 and Ni1200 samples and could reach −14%/K at 550 K, which indicates that it is a promising infrared detector (IR) for night vision bolometer material.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the Tunisian Ministere de l'Enseignement Superieur et de la Recherche Scientifique, the PHCMaghreb 15 MAG07 program.References
- Y. Zhang, Z. Yang, D. Yin, Y. Liu, C. Fei, R. Xiong, J. Shi and G. Yan, Composition and magnetic properties of cobalt ferrite nano-particles prepared by the co-precipitation method, J. Magn. Magn. Mater., 2010, 322, 3470–3475 CrossRef CAS.
- M. Gharibshahian, O. Mirzaee and M. S. Nourbakhsh, Evaluation of superparamagnetic and biocompatible properties of mesoporous silica coated cobalt ferrite nanoparticles synthesized via microwave modified Pechini method, J. Magn. Magn. Mater., 2017, 425, 48–56 CrossRef CAS.
- B. Rabi, A. Essoumhi, M. Sajieddine, J. M. Greneche, E. K. Hlil, A. Razouk and M. A. Valente, Structural, magnetic and magnetocaloric study of Ni0.5Zn0.5Fe2O4 spinel, Appl. Phys. A, 2020, 126, 174 CrossRef CAS.
- A. C. Gandhi, P. M. Reddy, T.-S. Chan, Y.-P. Ho and S. Y. Wu, Memory effect in weakly-interacting Fe3O4 nanoparticles, RSC Adv., 2015, 5, 84782–84789 RSC.
- N. Guijarro, P. Bornoz, M. Prévot, X. Yu, X. Zhu, M. Johnson, X. Jeanbourquin, F. Le Formal and K. Sivula, Evaluating spinel ferrites MFe2O4 (M = Cu, Mg, Zn) as photoanodes for solar water oxidation: prospects and limitations, Sustainable Energy Fuels, 2018, 2, 103–117 RSC.
- A. M. Popov, T. A. Labutin, A. E. Goldt, O. V. Usovich, S. E. Bozhenko and N. B. Zorov, Determination of lithium in lithium-ionic conductors by laser-enhanced ionization spectrometry with laser ablation, J. Anal. At. Spectrom., 2014, 29, 176–184 RSC.
- M. Colombo, S. Carregal-Romero, M. F. Casula, L. Gutiérrez, M. P. Morales, I. B. Böhm, J. T. Heverhagen, D. Prosperi and W. J. Parak, Biological applications of magnetic nanoparticles, Chem. Soc. Rev., 2012, 41, 4306 RSC.
- A. E. Reddy, T. Anitha, C. V. V. Muralee Gopi, S. S. Rao, B. Naresh and H.-J. Kim, Construction of novel nanocomposite ZnO@CoFe2O4 microspheres grown on nickel foam for high performance electrochemical supercapacitors, Anal. Methods, 2018, 10, 223–229 RSC.
- J. Xie, K. Chen, H.-Y. Lee, C. Xu, A. R. Hsu, S. Peng, X. Chen and S. Sun, Ultrasmall c(RGDyK)-Coated Fe3O4 Nanoparticles and Their Specific Targeting to Integrin αvβ3-Rich Tumor Cells, J. Am. Chem. Soc., 2008, 130, 7542–7543 CrossRef CAS.
- E. Céspedes, J. M. Byrne, N. Farrow, S. Moise, V. S. Coker, M. Bencsik, J. R. Lloyd and N. D. Telling, Bacterially synthesized ferrite nanoparticles for magnetic hyperthermia applications, Nanoscale, 2014, 6, 12958–12970 RSC.
- Z. Beji, L. S. Smiri, N. Yaacoub, J.-M. Grenèche, N. Menguy, S. Ammar and F. Fiévet, Annealing Effect on the Magnetic Properties of Polyol-made Ni–Zn Ferrite Nanoparticles, Chem. Mater., 2010, 22, 1350–1366 CrossRef CAS.
- D. Bouokkeze, J. Massoudi, W. Hzez, M. Smari, A. Bougoffa, K. Khirouni, E. Dhahri and L. Bessais, Investigation of the structural, optical, elastic and electrical properties of spinel LiZn2Fe3O8 nanoparticles annealed at two distinct temperatures, RSC Adv., 2019, 9, 40940–40955 RSC.
- K. B. Modi, S. J. Shah, N. B. Pujara, T. K. Pathak, N. H. Vasoya and I. G. Jhala, Infrared spectral evolution, elastic, optical and thermodynamic properties study on mechanically milled Ni0.5Zn0.5Fe2O4 spinel ferrite, J. Mol. Struct., 2013, 1049, 250–262 CrossRef CAS.
- G. Datt, M. Sen Bishwas, M. Manivel Raja and A. C. Abhyankar, Observation of magnetic anomalies in one-step solvothermally synthesized nickel–cobalt ferrite nanoparticles, Nanoscale, 2016, 8, 5200–5213 RSC.
- C. J. Brinker and G. W. Scherer, Sol–Gel Science, Academic Press, New York, 1990 Search PubMed.
- M. Tan, Y. Köseoğlu, F. Alan and E. Şentürk, Overlapping large polaron tunneling conductivity and giant dielectric constant in Ni0.5Zn0.5Fe1.5Cr0.5O4 nanoparticles (NPs), J. Alloys Compd., 2011, 509, 9399–9405 CrossRef CAS.
- S. K. Pradhan, S. Bid, M. Gateshki and V. Petkov, Microstructure characterization and cation distribution of nanocrystalline magnesium ferrite prepared by ball milling, Mater. Chem. Phys., 2005, 93, 224–230 CrossRef CAS.
- P. Dolcet, K. Kirchberg, A. Antonello, C. Suchomski, R. Marschall, S. Diodati, R. Muñoz-Espí, K. Landfester and S. Gross, Exploring wet chemistry approaches to ZnFe2O4 spinel ferrite nanoparticles with different inversion degrees: a comparative study, Inorg. Chem. Front., 2019, 6, 1527–1534 RSC.
- S. Atiq, M. Majeed, A. Ahmad, S. K. Abbas, M. Saleem, S. Riaz and S. Naseem, Synthesis and investigation of structural, morphological, magnetic, dielectric and impedance spectroscopic characteristics of Ni-Zn ferrite nanoparticles, Ceram. Int., 2017, 43, 2486–2494 CrossRef CAS.
- B. R. Babu, K. V. Ramesh, M. S. R. Prasad and Y. Purushotham, Structural, Magnetic, and Dielectric Properties of Ni0.5Zn0.5AlxFe2−xO4 Nanoferrites, J. Supercond. Novel Magn., 2016, 29, 939–950 CrossRef CAS.
- M. Hashim, S. K. Alimuddin, S. Ali, B. H. Koo, H. Chung and R. Kumar, Structural, magnetic and electrical properties of Al3+ substituted Ni–Zn ferrite nanoparticles, J. Alloys Compd., 2012, 511, 107–114 CrossRef CAS.
- L.-Z. Li, X.-X. Zhong, R. Wang, X.-Q. Tu, L. He, R.-D. Guo and Z.-Y. Xu, Structural, magnetic and electrical properties in Al-substituted NiZnCo ferrite prepared via the sol–gel auto-combustion method for LTCC technology, RSC Adv., 2017, 7, 39198–39203 RSC.
- R. Lahouli, J. Massoudi, M. Smari, H. Rahmouni, K. Khirouni, E. Dhahri and L. Bessais, Investigation of annealing effects on the physical properties of Ni0.6Zn0.4Fe1.5Al0.5O4 ferrite, RSC Adv., 2019, 9, 19949–19964 RSC.
- K. Praveena, K. Sadhana, S. Matteppanavar and H.-L. Liu, Effect of sintering temperature on the structural, dielectric and magnetic properties of Ni0.4Zn0.2Mn0.4Fe2O4 potential for radar absorbing, J. Magn. Magn. Mater., 2017, 423, 343–352 CrossRef CAS.
- J. Rodríguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction, Phys. B, 1993, 192, 55–69 CrossRef.
- J. Azadmanjiri, S. A. Seyyed Ebrahimi and H. K. Salehani, Magnetic properties of nanosize NiFe2O4 particles synthesized by sol–gel auto combustion method, Ceram. Int., 2007, 33, 1623–1625 CrossRef CAS.
- R. L. Coble, Sintering Crystalline Solids. I. Intermediate and Final State Diffusion Models, J. Appl. Phys., 1961, 32, 787–792 CrossRef CAS.
- A. Hajalilou, M. Hashim, R. Ebrahimi-Kahrizsangi and H. Mohamed Kamari, Influence of evolving microstructure on electrical and magnetic characteristics in mechanically synthesized polycrystalline Ni-ferrite nanoparticles, J. Alloys Compd., 2015, 633, 306–316 CrossRef CAS.
- I. S. Lyubutin, S. S. Starchikov, A. O. Baskakov, N. E. Gervits, C.-R. Lin, Y.-T. Tseng, W.-J. Lee and K.-Y. Shih, Exchange-coupling of hard and soft magnetic sublattices and magnetic anomalies in mixed spinel NiFe0.75Cr1.25O4 nanoparticles, J. Magn. Magn. Mater., 2018, 451, 336–343 CrossRef CAS.
- V. K. Mittal, P. Chandramohan, S. Bera, M. P. Srinivasan, S. Velmurugan and S. V. Narasimhan, Cation distribution in NixMg1−xFe2O4 studied by XPS and Mössbauer spectroscopy, Solid State Commun., 2006, 137, 6–10 CrossRef CAS.
- K. B. Modi, P. Y. Raval, S. J. Shah, C. R. Kathad, S. V. Dulera, M. V. Popat, K. B. Zankat, K. G. Saija, T. K. Pathak, N. H. Vasoya, V. K. Lakhani, U. Chandra and P. K. Jha, Raman and Mossbauer Spectroscopy and X-ray Diffractometry Studies on Quenched Copper–Ferri–Aluminates, Inorg. Chem., 2015, 54, 1543–1555 CrossRef CAS.
- K. K. Bestha, J. J. Abraham, J. A. Chelvane and V. Gorige, Influence of cation distribution on magnetic response of polycrystalline Co1−xNixFe2O4 (0 ≤ x ≤ 1) ferrites, Phys. Scr., 2020, 95, 085802 CrossRef.
- E. F. Bertaut, Structures des boroferrites, Acta Crystallogr., 1950, 3, 473–474 CrossRef.
- A. Hajalilou, H. M. Kamari and K. Shameli, Dielectric and electrical characteristics of mechanically synthesized Ni-Zn ferrite nanoparticles, J. Alloys Compd., 2017, 708, 813–826 CrossRef CAS.
- S. R. Gawali, A. C. Gandhi, S. S. Gaikwad, J. Pant, T.-S. Chan, C.-L. Cheng, Y.-R. Ma and S. Y. Wu, Role of cobalt cations in short range antiferromagnetic Co3O4 nanoparticles: a thermal treatment approach to affecting phonon and magnetic properties, Sci. Rep., 2018, 8, 249 CrossRef.
- J. P. Singh, R. C. Srivastava, H. M. Agrawal and R. Kumar, Micro-Raman investigation of nanosized zinc ferrite: effect of crystallite size and fluence of irradiation, J. Raman Spectrosc., 2011, 42, 1510–1517 CrossRef CAS.
- P. Chirawatkul, S. Khoonsap, S. Phumying, C. Kaewhan, S. Pinitsoontorn and S. Maensiri, Cation distribution and magnetic properties of CoxMg1−xFe2O4 nanoparticles, J. Alloys Compd., 2017, 697, 249–256 CrossRef CAS.
- S. Aliyeva, S. Babayev and T. Mehdiyev, Raman spectra of Ni1-XZnXFe2O4 nanopowders, J. Raman Spectrosc., 2018, 49, 271–278 CrossRef CAS.
- M. I. Dar and S. A. Shivashankar, Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity, RSC Adv., 2014, 4, 4105–4113 RSC.
- D. K. Pradhan, S. Kumari, V. S. Puli, P. T. Das, D. K. Pradhan, A. Kumar, J. F. Scott and R. S. Katiyar, Correlation of dielectric, electrical and magnetic properties near the magnetic phase transition temperature of cobalt zinc ferrite, Phys. Chem. Chem. Phys., 2017, 19, 210–218 RSC.
- B. K. Chatterjee, K. Bhattacharjee, A. Dey, C. K. Ghosh and K. K. Chattopadhyay, Influence of spherical assembly of copper ferrite nanoparticles on magnetic properties: orientation of magnetic easy axis, Dalton Trans., 2014, 43, 7930–7944 RSC.
- J. R. Scheffe, M. D. Allendorf, E. N. Coker, B. W. Jacobs, A. H. McDaniel and A. W. Weimer, Hydrogen Production via Chemical Looping Redox Cycles Using Atomic Layer Deposition-Synthesized Iron Oxide and Cobalt Ferrites, Chem. Mater., 2011, 23, 2030–2038 CrossRef CAS.
- P. Yaseneva, M. Bowker and G. Hutchings, Structural and magnetic properties of Zn-substituted cobalt ferrites prepared by co-precipitation method, Phys. Chem. Chem. Phys., 2011, 13, 18609–18614 RSC.
- D. Zakutna, I. Matulkova, E. Kentzinger, R. Medlin, Y. Su, K. Nemkovski, S. Disch, J. Vejpravova and D. Niznansky, Dispersible cobalt chromite nanoparticles: facile synthesis and size driven collapse of magnetism, RSC Adv., 2016, 6, 107659–107668 RSC.
- J. Zi, H. Büscher, C. Falter, W. Ludwig, K. Zhang and X. Xie, Raman shifts in Si nanocrystals, Appl. Phys. Lett., 1996, 69, 200–202 CrossRef CAS.
- P. Chandramohan, M. P. Srinivasan, S. Velmurugan and S. V. Narasimhan, Cation distribution and particle size effect on Raman spectrum of CoFe2O4, J. Solid State Chem., 2011, 184, 89–96 CrossRef CAS.
- R. D. Waldron, Infrared Spectra of Ferrites, Phys. Rev., 1955, 99, 1727–1735 CrossRef CAS.
- L. Wang, B. K. Rai and S. R. Mishra, Structural and magnetic study of Al3+ doped Ni0.75Zn0.25Fe2−xAlxO4 nanoferrites, Mater. Res. Bull., 2015, 65, 183–194 CrossRef CAS.
- S. A. Mazen, S. F. Mansour, E. Dhahri, H. M. Zaki and T. A. Elmosalami, The infrared absorption and dielectric properties of Li–Ga ferrite, J. Alloys Compd., 2009, 470, 294–300 CrossRef CAS.
- R. A. Pawar, S. S. Desai, S. M. Patange, S. S. Jadhav and K. M. Jadhav, Inter-atomic bonding and dielectric polarization in Gd3+ incorporated Co-Zn ferrite nanoparticles, Phys. B, 2017, 510, 74–79 CrossRef CAS.
- A. Bhaskar and S. R. Murthy, Effect of sintering temperatures on the elastic properties of Mn (1%) added MgCuZn ferrites, J. Magn. Magn. Mater., 2014, 355, 100–103 CrossRef CAS.
- P. U. Sharma and K. B. Modi, Effect of Fe 3+ substitution on elastic properties of yttrium iron garnet, Phys. Scr., 2010, 81, 015601 CrossRef.
- K. B. Modi, M. C. Chhantbar and H. H. Joshi, Study of elastic behaviour of magnesium ferri aluminates, Ceram. Int., 2006, 32, 111–114 CrossRef CAS.
- H. Donnerberg and C. R. A. Catlow, Atomistic computer simulations of yttrium iron garnet (YIG) as an approach to materials defect chemistry. I. Intrinsic defects, J. Phys.: Condens. Matter, 1993, 5, 2947–2960 CrossRef CAS.
- D. G. Cahill, S. K. Watson and R. O. Pohl, Lower limit to the thermal conductivity of disordered crystals, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6131–6140 CrossRef CAS.
- Q. Tariq, A. S. Saleemi, M. Saeed and M. Anis-ur-Rehman, Effect of Sintering on Co-precipitated Nanoparticles of Mn Ferrite, J. Supercond. Novel Magn., 2015, 28, 971–975 CrossRef CAS.
- T. M. Hammad, J. K. Salem, A. A. Amsha and N. K. Hejazy, Optical and magnetic characterizations of zinc substituted copper ferrite synthesized by a co-precipitation chemical method, J. Alloys Compd., 2018, 741, 123–130 CrossRef CAS.
- A. O. Turky, M. Mohamed Rashad, A. E.-H. Taha Kandil and M. Bechelany, Tuning the optical, electrical and magnetic properties of Ba0.5Sr0.5TixM1−xO3 (BST) nanopowders, Phys. Chem. Chem. Phys., 2015, 17, 12553–12560 RSC.
- P. Chand, S. Vaish and P. Kumar, Structural, optical and dielectric properties of transition metal (MFe2O4; M = Co, Ni and Zn) nanoferrites, Phys. B, 2017, 524, 53–63 CrossRef CAS.
- J. P. Singh, R. C. Srivastava, H. M. Agrawal, P. K. Giri, D. K. Goswami, A. Perumal and A. Chattopadhyay, Optical Behaviour of Zinc Ferrite Nanoparticles, AIP Conf. Proc., 2010, 1276, 137 CrossRef.
- A. Amiri Zarandi, A. A. Sabbagh Alvani, R. Salimi, H. Sameie, S. Moosakhani, D. Poelman and F. Rosei, Self-organization of an optomagnetic CoFe2O4–ZnS nanocomposite: preparation and characterization, J. Mater. Chem. C, 2015, 3, 3935–3945 RSC.
- M. Chakraborty, R. Thangavel, A. Biswas and G. Udayabhanu, Facile synthesis, and the optical and electrical properties of nanocrystalline ZnFe2O4 thin films, CrystEngComm, 2016, 18, 3095–3103 RSC.
- J. Melsheimer and D. Ziegler, Band gap energy and Urbach tail studies of amorphous, partially crystalline and polycrystalline tin dioxide, Thin Solid Films, 1985, 129, 35–47 CrossRef CAS.
- L. Hannachi and N. Bouarissa, Band parameters for cadmium and zinc chalcogenide compounds, Phys. B, 2009, 404, 3650–3654 CrossRef CAS.
- P. N. Anantharamaiah and P. A. Joy, Enhancing the strain sensitivity of CoFe2O4 at low magnetic fields without affecting the magnetostriction coefficient by substitution of small amounts of Mg for Fe, Phys. Chem. Chem. Phys., 2016, 18, 10516–10527 RSC.
- K. Kamala Bharathi, K. Balamurugan, P. N. Santhosh, M. Pattabiraman and G. Markandeyulu, Magnetocapacitance in Dy-doped Ni ferrite, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 172401 CrossRef.
- S. Chikazumi and C. D. Graham, Physics of Ferromagnetism, Oxford University Press, Oxford, New York, 2nd edn, 1997 Search PubMed.
- S. R. Murthy and T. S. Rao, Magnetostriction of Ni-Zn and Co-Zn ferrites, Phys. Status Solidi A, 1985, 90, 631–635 CrossRef CAS.
- S. M. Patange, S. E. Shirsath, G. S. Jangam, K. S. Lohar, S. S. Jadhav and K. M. Jadhav, Rietveld structure refinement, cation distribution and magnetic properties of Al3+ substituted NiFe2O4 nanoparticles, J. Appl. Phys., 2011, 109, 053909 CrossRef.
- U. B. Gawas, V. M. S. Verenkar and S. C. Mojumdar, Synthesis and characterization of Ni0.6Zn0.4Fe2O4 nano-particles obtained by auto catalytic thermal decomposition of carboxylato-hydrazinate complex, J. Therm. Anal. Calorim., 2011, 104, 879–883 CrossRef CAS.
- V. M. Andrade, R. J. C. Vivas, S. S. Pedro, J. C. G. Tedesco, A. L. Rossi, A. A. Coelho, D. L. Rocco and M. S. Reis, Magnetic and magnetocaloric properties of La0.6Ca0.4MnO3 tunable by particle size and dimensionality, Acta Mater., 2016, 102, 49–55 CrossRef CAS.
- C.-b. Rong, D. Li, V. Nandwana, N. Poudyal, Y. Ding, Z. L. Wang, H. Zeng and J. P. Liu, Size-Dependent Chemical and Magnetic Ordering in L10-FePt Nanoparticles, Adv. Mater., 2006, 18, 2984–2988 CrossRef CAS.
- M. Farle, K. Baberschke, U. Stetter, A. Aspelmeier and F. Gerhardter, Thickness-dependent Curie temperature of Gd(0001)/W(110) and its dependence on the growth conditions, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 11571–11574 CrossRef CAS.
- J. P. Chen, C. M. Sorensen, K. J. Klabunde, G. C. Hadjipanayis, E. Devlin and A. Kostikas, Size-dependent magnetic properties of MnFe2O4 fine particles synthesized by coprecipitation, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 9288–9296 CrossRef CAS.
- B. Barbara, Louis Néel: His multifaceted seminal work in magnetism, C. R. Phys., 2019, 20, 631–649 CrossRef CAS.
- R. Sharma, P. Thakur, M. Kumar, N. Thakur, N. S. Negi, P. Sharma and V. Sharma, Improvement in magnetic behaviour of cobalt doped magnesium zinc nano-ferrites via co-precipitation route, J. Alloys Compd., 2016, 684, 569–581 CrossRef CAS.
- M. Mozaffari, J. Amighian and E. Darsheshdar, Magnetic and structural studies of nickel-substituted cobalt ferrite nanoparticles, synthesized by the sol–gel method, J. Magn. Magn. Mater., 2014, 350, 19–22 CrossRef CAS.
- K. V. Chandekar and K. M. Kant, Relaxation phenomenon and relaxivity of cetrimonium bromide (CTAB) coated CoFe2O4 nanoplatelets, Phys. B, 2018, 545, 536–548 CrossRef CAS.
- M. Wali, R. Skini, M. Khlifi, E. Dhahri and E. K. Hlil, A giant magnetocaloric effect with a tunable temperature transition close to room temperature in Na-deficient La0.8Na0.2−x□xMnO3 manganites, Dalton Trans., 2015, 44, 12796–12803 RSC.
- S. Dash, A. V. Lukoyanov, Y. V. Knyazev, Y. I. Kuz'min, E. D. Baglasov, B. Weise, P. Kumar, M. Vasundhara and A. K. Patra, Impression of magnetic clusters, critical behavior and magnetocaloric effect in Fe3Al alloys, Phys. Chem. Chem. Phys., 2019, 21, 10823–10833 RSC.
- M. Jeddi, H. Gharsallah, M. Bejar, M. Bekri, E. Dhahri and E. K. Hlil, Magnetocaloric study, critical behavior and spontaneous magnetization estimation in La0.6Ca0.3Sr0.1MnO3 perovskite, RSC Adv., 2018, 8, 9430–9439 RSC.
- M. Nasri, M. Triki, E. Dhahri, M. Hussein, P. Lachkar and E. K. Hlil, Investigation of structural, magnetocaloric and electrical properties of La0.6Ca0.4−xSrxMnO3 compounds, Phys. B, 2013, 408, 104–109 CrossRef CAS.
- K. Srinivasa Rao, S. V. Ranga Nayakulu, M. Chaitanya Varma, G. S. V. R. K. Choudary and K. H. Rao, Controlled phase evolution and the occurrence of single domain CoFe2O4 nanoparticles synthesized by PVA assisted sol-gel method, J. Magn. Magn. Mater., 2018, 451, 602–608 CrossRef CAS.
- A. Das, K. Kumar Bestha, P. Bongurala and V. Gorige, Correlation between size, shape and magnetic anisotropy of CoFe2O4 ferrite nanoparticles, Nanotechnology, 2020, 31, 335716 CrossRef CAS.
- R. S. Yadav, I. Kuřitka, J. Vilcakova, J. Havlica, J. Masilko, L. Kalina, J. Tkacz, V. Enev and M. Hajdúchová, Structural, magnetic, dielectric, and electrical properties of NiFe2O4 spinel ferrite nanoparticles prepared by honey-mediated sol-gel combustion, J. Phys. Chem. Solids, 2017, 107, 150–161 CrossRef CAS.
- S. E. Shirsath, S. S. Jadhav, B. G. Toksha, S. M. Patange and K. M. Jadhav, Influence of Ce4+ ions on the structural and magnetic properties of NiFe2O4, J. Appl. Phys., 2011, 110, 013914 CrossRef.
- S. Dey, R. Gomes, R. Mondal, S. K. Dey, P. Dasgupta, A. Poddar, V. R. Reddy, A. Bhaumik and S. Kumar, Stable room temperature magnetic ordering and excellent catalytic activity of mechanically activated high surface area nanosized Ni0.45Zn0.55Fe2O4, RSC Adv., 2015, 5, 78508–78518 RSC.
- V. Blanco-Gutiérrez, E. Climent-Pascual, R. Sáez-Puche and M. J. Torralvo-Fernández, Temperature dependence of superparamagnetism in CoFe2O4 nanoparticles and CoFe2O4/SiO2 nanocomposites, Phys. Chem. Chem. Phys., 2016, 18, 9186–9193 RSC.
- T. Sarkar, A. K. Raychaudhuri, A. K. Bera and S. M. Yusuf, Effect of size reduction on the ferromagnetism of the manganite La1−xCaxMnO3 (x =0.33), New J. Phys., 2010, 12, 123026 CrossRef.
- V. Šepelák, M. Myndyk, R. Witte, J. Röder, D. Menzel, R. H. Schuster, H. Hahn, P. Heitjans and K.-D. Becker, The mechanically induced structural disorder in barium hexaferrite, BaFe12O19, and its impact on magnetism, Faraday Discuss., 2014, 170, 121–135 RSC.
- L. Hao, Y. Zhao, Q. Jiao and P. Chen, Synthesis of zinc–nickel ferrite nanorods and their magnetic properties, RSC Adv., 2014, 4, 15650–15654 RSC.
- A. B. Nawale, N. S. Kanhe, S. A. Raut, S. V. Bhoraskar, A. K. Das and V. L. Mathe, Investigation of structural, optical and magnetic properties of thermal plasma synthesized Ni-Co spinel ferrite nanoparticles, Ceram. Int., 2017, 43, 6637–6647 CrossRef CAS.
- T. Slatineanu, A. R. Iordan, M. N. Palamaru, O. F. Caltun, V. Gafton and L. Leontie, Synthesis and characterization of nanocrystalline Zn ferrites substituted with Ni, Mater. Res. Bull., 2011, 46, 1455–1460 CrossRef CAS.
- S. Daengsakul, C. Thomas, C. Mongkolkachit and S. Maensiri, Effects of crystallite size on magnetic properties of thermal-hydro decomposition prepared La1−xSrxMnO3 nanocrystalline powders, Solid State Sci., 2012, 14, 1306–1314 CrossRef CAS.
- P. Heitjans, M. Masoud, A. Feldhoff and M. Wilkening, NMR and impedance studies of nanocrystalline and amorphous ion conductors: lithium niobate as a model system, Faraday Discuss., 2007, 134, 67–82 RSC.
- F. Bødker and S. Mørup, Size dependence of the properties of hematite nanoparticles, Europhys. Lett., 2000, 52, 217–223 CrossRef.
- M. N. Akhtar, M. Saleem and M. A. Khan, Al doped spinel and garnet nanostructured ferrites for microwave frequency C and X-band applications, J. Phys. Chem. Solids, 2018, 123, 260–265 CrossRef CAS.
- M. N. Akhtar, M. Babar, S. Qamar, Z. ur Rehman and M. A. Khan, Structural Rietveld refinement and magnetic features of prosademium (Pr) doped Cu nanocrystalline spinel ferrites, Ceram. Int., 2019, 45, 10187–10195 CrossRef CAS.
- K. H. Wu, Y. C. Chang, H. B. Chen, C. C. Yang and D. N. Horng, Variable temperature electron paramagnetic resonance studies of the NiZn ferrite/SiO2 nanocomposite, J. Magn. Magn. Mater., 2004, 278, 156–163 CrossRef CAS.
- B. Vigneashwari, S. Dash, A. K. Tyagi and S. A. Suthanthiraraj, Synthesis, Characterization, And Assembly Of CdSe Quantum Dot Array, Int. J. Nanosci., 2008, 7, 9–19 CrossRef CAS.
- J.-H. Lee, J. Jang, J. Choi, S. H. Moon, S. Noh, J. Kim, J.-G. Kim, I.-S. Kim, K. I. Park and J. Cheon, Exchange-coupled magnetic nanoparticles for efficient heat induction, Nat. Nanotechnol., 2011, 6, 418–422 CrossRef CAS.
- H. Y. Carr and E. M. Purcell, Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments, Phys. Rev., 1954, 94, 630–638 CrossRef CAS.
- E. Stefan, P. A. Connor, A. K. Azad and J. T. S. Irvine, Structure and properties of MgMxCr2−xO4 (M = Li, Mg, Ti, Fe, Cu, Ga) spinels for electrode supports in solid oxide fuel cells, J. Mater. Chem. A, 2014, 2, 18106–18114 RSC.
- E. J. W. Verwey and J. H. de Boer, Cation arrangement in a few oxides with crystal structures of the spinel type, Recl. Trav. Chim. Pays-Bas, 2010, 55, 531–540 CrossRef.
- A. Lakshman, P. S. V. S. Rao, B. P. Rao and K. H. Rao, Electrical properties of In3+ and Cr3+ substituted magnesium–manganese ferrites, J. Phys. D: Appl. Phys., 2005, 38, 673–678 CrossRef CAS.
- M. Raghasudha, D. Ravinder, P. Veerasomaiah, K. M. Jadhav, M. Hashim, P. Bhatt and S. S. Meena, Electrical resistivity and Mössbauer studies of Cr substituted Co nano ferrites, J. Alloys Compd., 2017, 694, 366–374 CrossRef CAS.
- B. P. Rao and K. H. Rao, J. Mater. Sci., 1997, 32, 6049–6054 CrossRef CAS.
- E. J. W. Verwey and P. W. Haayman, Electronic conductivity and transition point of magnetite (“Fe3O4”), Physica, 1941, 8, 979–987 CrossRef CAS.
- A. A. Sattar, H. M. El-Sayed and M. M. El-Tabey, The effect of Al-substitution on structure and electrical properties of Mn-Ni-Zn ferrites, J. Mater. Sci., 2005, 40, 4873–4879 CrossRef CAS.
- H. Chouaya, M. Smari, I. Walha, E. Dhahri, M. P. F. Graça and M. A. Valente, The effect of bismuth on the structure, magnetic and electric properties of Co2MnO4 spinel multiferroic, J. Magn. Magn. Mater., 2018, 451, 344–350 CrossRef CAS.
- R. Hamdi, A. Tozri, M. Smari, E. Dhahri and L. Bessais, Structural, magnetic, magnetocaloric and electrical studies of Dy0.5(Sr1−xCax)0.5MnO3 manganites, J. Magn. Magn. Mater., 2017, 444, 270–279 CrossRef CAS.
- A. J. Miller, A. Rotaru, D. C. Arnold and F. D. Morrison, Effect of local A-site strain on dipole stability in A6GaNb9O30 (A = Ba, Sr, Ca) tetragonal tungsten bronze relaxor dielectrics, Dalton Trans., 2015, 44, 10738–10745 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Relations and formulae used to calculate various structural parameters, deconvoluted Raman spectra at 300 K of NZFAO samples annealed at different temperature fitted with the Lorentzian function, representative fitted high field magnetization of annealed Ni–Zn–Al ferrite at room temperature to the saturation approach law, representative fitted resistivity curve of Ni600, Ni900, and Ni1200 samples to Arrhenius equation. See DOI: 10.1039/d0ra05522k |
| This journal is © The Royal Society of Chemistry 2020 |