Open Access Article
Open Access ArticleHydrogen peroxide-mediated synthesis of 2,4-substituted quinazolines via one-pot three-component reactions under metal-free conditions†
Khang H. Trinh‡
 ab,
Khang X. Nguyen‡
ab,
Khang X. Nguyen‡ ab,
Phuc H. Pham
ab,
Phuc H. Pham ab,
Tung T. Nguyen
ab,
Tung T. Nguyen ab,
Anh N. Q. Phan*ab and
Nam T. S. Phan
ab,
Anh N. Q. Phan*ab and
Nam T. S. Phan *ab
*ab
aFaculty of Chemical Engineering, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam. E-mail: pnqanh@hcmut.edu.vn; ptsnam@hcmut.edu.vn; Fax: +84 8 38637504; Tel: +84 8 38647256, ext. 5681
bVietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc District, Ho Chi Minh City, Vietnam
First published on 13th August 2020
Abstract
An efficient metal-free synthesis of 2,4-substituted quinazolines via a hydrogen peroxide-mediated one-pot three-component reaction of 2-aminoaryl ketones, aldehydes, and ammonium acetate has been developed. The transformation proceeded readily under mild conditions in the presence of commercially available hydrogen peroxide. The significant advantages of this approach are (1) the readily available atom-efficient and green hydrogen peroxide as oxidant; (2) no transition metal catalyst is required; (3) mild reaction conditions; and (4) wide substrate scope. To the best of our knowledge, utilizing hydrogen peroxide as an atom-efficient and green oxidant for the synthesis of 2,4-substituted quinazolines has not previously been reported in the literature. This method is complementary to previous protocols for the synthesis of 2,4-substituted quinazolines.
1. Introduction
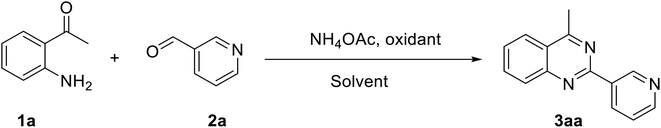
Quinazolines and their derivatives are a privileged class of nitrogen-containing heterocyclic compounds, normally occurring in a variety of natural products and synthetic chemicals with a wide spectrum of biological and medicinal activities.1–4 Due to their pharmaceutical applications and significant biological value, a variety of methodologies have been explored for the synthesis of these structures. Typical approaches started from ortho-functionalized anilines and derivatives, such as 2-carbonyl anilines,5 2-aminobenzylamines,6 and 2-aminobenzonitriles.7 Recently, several new strategies from diversified starting materials using transition metal catalysts have been developed for the construction of substituted quinazolines.8–11 Additionally, metal-free synthetic pathways to these heterocyclic compounds have attracted significant interest. Zhang et al. previously achieved quinazolines via a one-pot three-component reaction of 2-aminoaryl ketones, aldehyde, and ammonium acetate in mixtures of maltose-dimethylurea (DMU)–NH4Cl.12 Zhao et al. demonstrated a KI-catalyzed three-component synthesis of quinazolines utilizing 2-aminophenyl ketones, methylarenes, and ammonium acetate.13 Saikia et al. achieved quinazolines by a trimethylsilyltrifluoromethane sulfonate-catalyzed activation of aliphatic and aromatic nitriles.14 Chen et al. prepared quinazolines from DMSO-mediated transformation of anilines, aromatic aldehydes, and ammonium iodide under oxygen atmosphere.15Hydrogen peroxide has been considered as an ideal oxidant for liquid-phase green chemistry as it is non-toxic, environmentally benign, and atom efficient, producing only water as theoretical by-product.16–18 Additionally, utilizing hydrogen peroxide as oxidant normally does not require temperatures over 100 °C for oxidation transformations.17 In organic synthesis, low efficiency of hydrogen peroxide has limited its applications, and organic peroxides are preferred in many cases. There has been growing interest in employing hydrogen peroxide as green oxidant in many synthetic strategies. Doherty et al. successfully replaced strong oxidants by hydrogen peroxide for sulfoxidation reactions.19 Devi et al. previously employed hydrogen peroxide instead of organic peroxides for the synthesis of N-(pyridin-2-yl)benzamides.20 Zhou et al. described hydrogen peroxide-mediated transformations of enamides to generate substituted α-acyloxyketones.21 Lindhorst et al. conducted oxidations of aromatic hydrocarbons using hydrogen peroxide as green oxidant.22 Yaremenko et al. converted 1,5-diketones to ozonides in the presence of hydrogen peroxide.23 Qiu et al. demonstrated an efficient oxidation of methylene C–H in oxindoles or 2,3-dihydroquinolin-4-ones by utilizing hydrogen peroxide.24 In this work, we would like to report a metal-free synthesis of 2,4-substituted quinazolines via hydrogen peroxide-mediated one-pot three-component reaction of 2-aminoaryl ketones, aldehydes, and ammonium acetate (Scheme 1). High yields were achieved under mild reaction conditions.
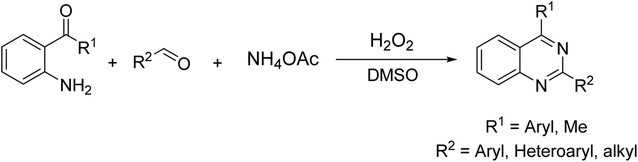 | ||
| Scheme 1 Synthesis of 2,4-substituted quinazolines via hydrogen peroxide-mediated one-pot three-component reactions. | ||
2. Experimental
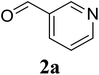
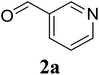
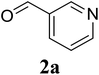
In a representative experiment, a mixture of 2′-aminoacetophenone (13.6 mg, 0.1 mmol), 3-pyridinecarboxaldehyde (21.5 mg, 0.2 mmol), NH4OAc (23.2 mg, 0.3 mmol), and diphenyl ether (17.1 mg, 0.1 mmol) as an internal standard in dimethylsulfoxide (DMSO; 1 mL, 14 mmol) was added into a 8 mL screw-cap vial. The reaction mixture was magnetically stirred for 2 min to disperse entirely reactants in the liquid phase, followed by the addition of hydrogen peroxide (H2O2; 30 wt% in H2O; 0.4 mmol, 41.2 μL). The resulting mixture was subsequently stirred at 60 °C for 6 h. The reaction yield monitored by withdrawing aliquots from the reaction mixture, and quenched with brine. The organic components were consequently extracted into ethyl acetate (2 mL), dried over anhydrous Na2SO4, and analyzed by GC with reference to diphenyl ether. To isolate the desired product, after disappearance of the reactant (monitored by TLC), the mixture was slowly cooled to room temperature and diluted with ethyl acetate (20 mL), washed with saturated NaHCO3 solution (3 × 5 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The crude product was purified by column chromatography on silica gel with hexane/ethyl acetate solvent system to give pure product. The product structure was confirmed by 1H NMR, and 13C NMR.3. Results and discussion
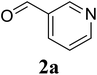
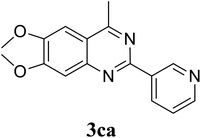
Initially, the three-component reaction between 2′-aminoacetophenone (1a), 3-pyridinecarboxaldehyde (2a), and ammonium acetate to produce 4-methyl-2-(pyridin-3-yl)quinazoline (3aa) was investigated (Table 1). Preliminary studies indicated that an oxidant was required for this transformation. Reaction conditions were screened to maximize the yield of 3aa, concerning temperature, reactant molar ratio, solvent, ammonium acetate amount, oxidant, and oxidant amount. The reaction was conducted in DMSO for 24 h, using 2 equivalents of 2a, 3 equivalents of ammonium acetate, and 4 equivalents of hydrogen peroxide, at temperature ranging from ambient to 120 °C (entries 1–6). Best result was obtained for the reaction conducted at 60 °C with 84% yield of 3aa being detected (entry 3). Increasing the temperature resulted in lower yields, with only 44% yield being recorded for the reaction performed at 120 °C. It should be noted that decreasing the reaction time from 24 h to 6 h led to 90% yield of 3aa (entry 12). Indeed, experimental results indicated that pyridine-3-carboxylic acid was produced as by-product at high temperature or after long reaction time. Additionally, the reactant molar ratio exhibited a noticeable influence on the formation of 3aa (entries 7–17). Utilizing excess amounts of 2a favored the transformation, while significantly lower yields were detected in the presence of excess amounts of 1a.| Entry | Temperature (°C) | 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a (mol 2a (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mol) |
Solvent | NH4OAc amount (equiv.) | Oxidant | Oxidant amount (equiv.) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 2′-aminoacetophenone (0.1 mmol); solvent (1 mL); under air; 6 h. DMSO: dimethyl sulfoxide; DMF: N,N-dimethylformamide; DMAc: N,N-dimethylacetamide; DCB: 1,2-dichlorobenzene; NMP: N-methyl-2-pyrrolidone; DTBP: di-tert-butylperoxide; aqTBHP: tert-butyl hydroperoxide in water; dcTBHP: tert-butyl hydroperoxide in decane.b GC yield.c The reaction was carried out in 24 h. | |||||||
| 1 | RTc | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 4 | 42 |
| 2 | 40c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 4 | 45 |
| 3 | 60c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 4 | 84 |
| 4 | 80c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 4 | 83 |
| 5 | 100c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 4 | 62 |
| 6 | 120c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 4 | 44 |
| 7 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DMSO | 3 | H2O2 | 4 | 51 |
| 8 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DMSO | 3 | H2O2 | 4 | 58 |
| 9 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
DMSO | 3 | H2O2 | 4 | 71 |
| 10 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
DMSO | 3 | H2O2 | 4 | 78 |
| 11 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8 |
DMSO | 3 | H2O2 | 4 | 81 |
| 12 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 4 | 90 |
| 13 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
DMSO | 3 | H2O2 | 4 | 59 |
| 14 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
DMSO | 3 | H2O2 | 4 | 31 |
| 15 | 60 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DMSO | 3 | H2O2 | 4 | 54 |
| 16 | 60 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DMSO | 3 | H2O2 | 4 | 59 |
| 17 | 60 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DMSO | 3 | H2O2 | 4 | 60 |
| 18 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DCB | 3 | H2O2 | 4 | 17 |
| 19 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
PhCl | 3 | H2O2 | 4 | 28 |
| 20 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Dioxane | 3 | H2O2 | 4 | 42 |
| 21 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMF | 3 | H2O2 | 4 | 62 |
| 22 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMAc | 3 | H2O2 | 4 | 71 |
| 23 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
NMP | 3 | H2O2 | 4 | 72 |
| 24 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 1 | H2O2 | 4 | 44 |
| 25 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 2 | H2O2 | 4 | 73 |
| 26 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 4 | H2O2 | 4 | 84 |
| 27 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 5 | H2O2 | 4 | 80 |
| 28 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | TEMPO | 4 | 22 |
| 29 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | Air | — | 55 |
| 30 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | O2 | — | 69 |
| 31 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | DTBP | 4 | 61 |
| 32 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | K2S2O8 | 4 | 67 |
| 33 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | aqTBHP | 4 | 83 |
| 34 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 0 | 55 |
| 35 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 1 | 72 |
| 36 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 2 | 75 |
| 37 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 3 | 85 |
| 38 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMSO | 3 | H2O2 | 5 | 80 |
With these results in hand, we subsequently investigated the impact of solvent on the formation of 3aa. The reaction was carried out at 60 °C for 6 h, using 2 equivalents of 2a, 3 equivalents of ammonium acetate, and 4 equivalents of hydrogen peroxide, in different solvents including chlorobenzene, 1,2-dichlorobenzene, dioxane, DMF, DMSO, NMP, and DMAc, respectively (entries 18–23). It was noted that the solvent exhibited a significant impact on the reaction, and DMSO emerged as the best option (entry 12). The transformation was also controlled by the amount of the nitrogen source (entries 24–27). Using 1 equivalent of ammonium acetate afforded 44% yield (entry 24), while 90% yield was obtained in the presence of 2 equivalents of ammonium acetate (entry 12). Nevertheless, extending the amount of ammonium acetate resulted in lower yields. Next, a series of oxidants were tested for the reaction, including both organic and inorganic oxidants (entries 28–33). The reaction conducted under air without any additional oxidant afforded 55% yield (entry 29), and this value was improved to 69% for the reaction carried out under molecular oxygen (entry 30). Interestingly, utilizing the readily available hydrogen peroxide afforded 90% yield of 3aa (entry 12). Organic peroxides such as di-tert-butylperoxide or tert-butyl hydroperoxide in water exhibited lower efficiency than hydrogen peroxide. Indeed, besides air and molecular oxygen, hydrogen peroxide should be utilized as the most atom-efficient and green oxidant. Furthermore, the transformation was controlled by the amounts of hydrogen peroxide (entries 34–38), and utilizing 4 equivalents offered best yield of 3aa (entry 12).
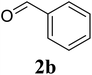
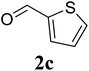
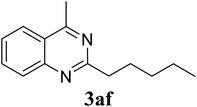
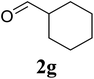
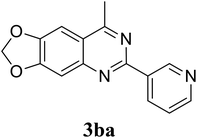
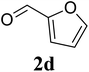
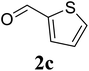
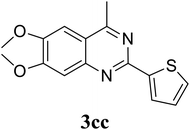
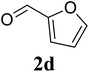
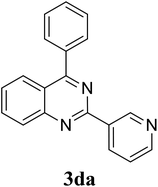
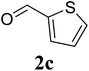
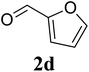
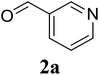
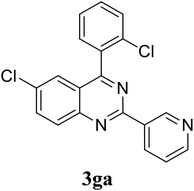
Based on these results, the scope was then extended to the synthesis of many substituted quinazolines via the hydrogen peroxide-mediated three-component reaction of 2-aminoaryl ketones, aldehydes, and ammonium acetate (Table 2). The reaction was carried out in DMSO at 60 °C for 6 h, with 2 equivalents of the aldehyde, using 3 equivalents of ammonium acetate, and 4 equivalents of hydrogen peroxide. Products were consequently purified by column chromatography. In the first series, several aromatic aldehydes were utilized for the reaction with 2′-aminoacetophenones and ammonium acetate. Under these conditions, 3aa, 3ab, 3ac, and 3ad were achieved in 88%, 89%, 75%, and 74% yields, respectively (entries 1–4). Coupling with aliphatic aldehydes was also feasible, yielding 2,4-dialkylsubstituted quinazolines 3ae, 3ag, 3ah in good yields (entries 5–7). Next, 1-(6-aminobenzo[d][1,3]dioxol-5-yl)ethan-1-one was employed for the reaction with different aldehydes, producing 3ba, 3bb, 3bc, and 3bd in 74%, 78%, 75%, and 72% yields, respectively (entries 8–11). Moving to the transformation of 1-(2-amino-4,5-dimethoxyphenyl)ethan-1-one with several aldehydes, 3ca, 3cb, 3cc, and 3cd were generated in 72%, 70%, 75%, and 69% yields, respectively (entries 12–15). In the second series, many 2-aminobenzophenones were used for the three-component reaction with various aldehydes. It was found that 2-aminobenzophenones were also reactive towards the transformation. Under standard conditions, 3db, 3dc, and 3dd were obtained in 85%, 78%, and 68% yields, respectively (entries 16–18). Similarly, 2-aminobenzophenone derivatives containing different substituents were employed for the reaction, producing corresponding products in reasonable yields (3ea, 3fa, 3ga, and 3ha) (entries 19–23).
| Entry | Reactant 1 | Reactant 2 | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: reactant 1 (0.1 mmol); reactant 2 (0.2 mmol); NH4OAc (0.3 mmol); H2O2 (0.4 mmol); DMSO (1 mL); 60 °C; under air; 6 h.b Isolated yields. | ||||
| 1 | 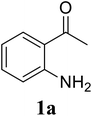 |
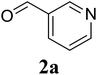 |
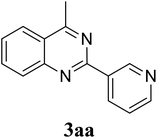 |
88 |
| 2 | 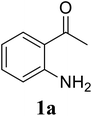 |
 |
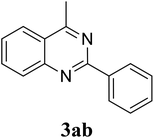 |
89 |
| 3 | 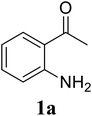 |
 |
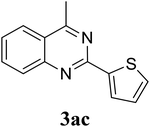 |
75 |
| 4 | 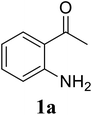 |
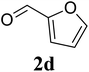 |
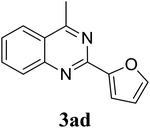 |
74 |
| 5 | 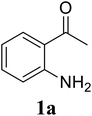 |
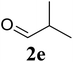 |
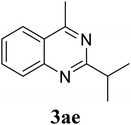 |
70 |
| 6 | 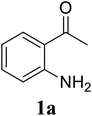 |
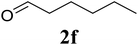 |
 |
75 |
| 7 | 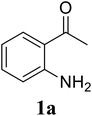 |
 |
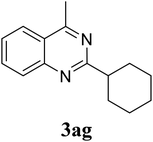 |
85 |
| 8 | 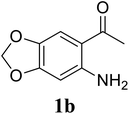 |
 |
 |
74 |
| 9 | 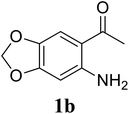 |
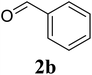 |
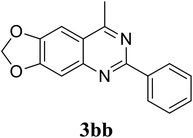 |
78 |
| 10 | 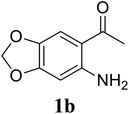 |
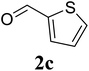 |
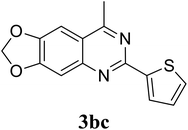 |
75 |
| 11 | 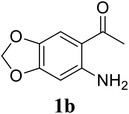 |
 |
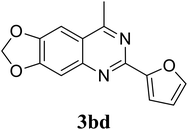 |
72 |
| 12 | 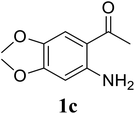 |
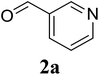 |
 |
72 |
| 13 | 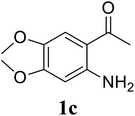 |
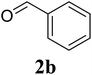 |
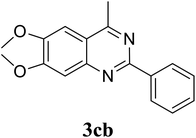 |
75 |
| 14 | 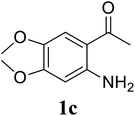 |
 |
 |
70 |
| 15 |  |
 |
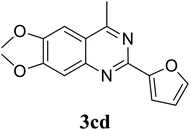 |
69 |
| 16 | 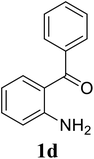 |
 |
 |
84 |
| 17 | 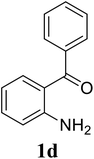 |
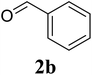 |
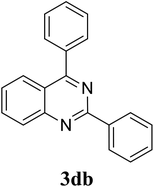 |
85 |
| 18 | 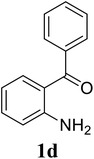 |
 |
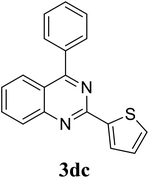 |
78 |
| 19 | 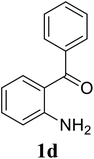 |
 |
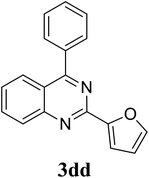 |
68 |
| 20 | 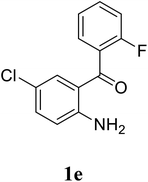 |
 |
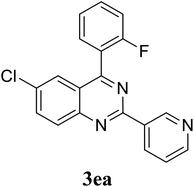 |
69 |
| 21 | 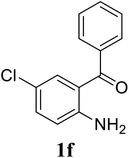 |
 |
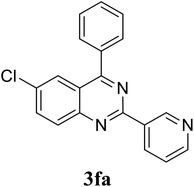 |
70 |
| 22 | 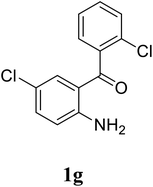 |
 |
 |
68 |
| 23 |  |
 |
 |
61 |
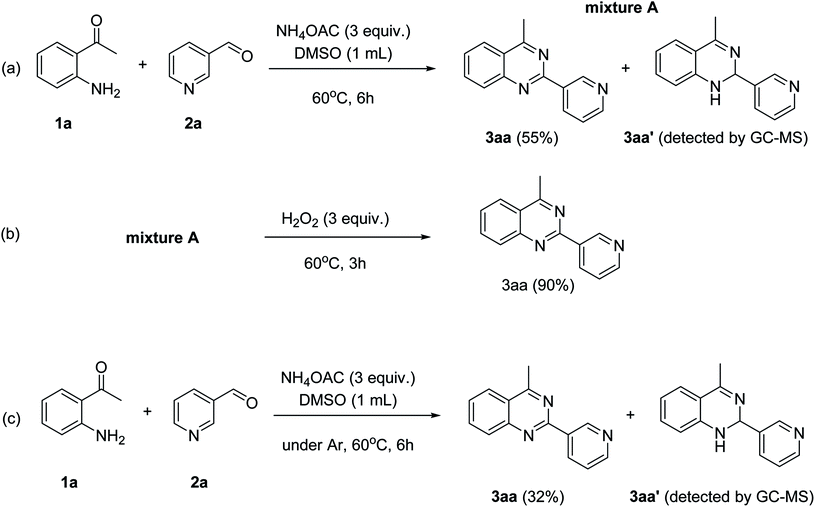
To gain insight into the possible mechanism on the formation of the quinazoline products, several control experiments were conducted. The reaction between 1a and 2a was performed in the absence of hydrogen peroxide. Except for the generation of desired product 3aa in 55% yield, the dihydroquinazoline intermediate 3aa′ was observed by GC-MS analysis in trace amount (Scheme 2a). In another control experiment, hydrogen peroxide was subsequently added into the reaction mixture depicted in Scheme 2a and the resulting solution was stirred for a further 3 h (Scheme 2b). As expected, 90% yield of target product 3aa was obtained in the presence of 3 equivalents of hydrogen peroxide. It should be noted that 64% and 78% yields of 3aa was detected when 1 and 2 equivalents of hydrogen peroxide were utilized, respectively. These observations suggested that compound 3aa′ was the reaction intermediate. The similar result was achieved when the reaction between 1a and 2a was conducted under argon atmosphere (Scheme 2c). These observations confirmed the essential role of active oxygen species for the transformation.
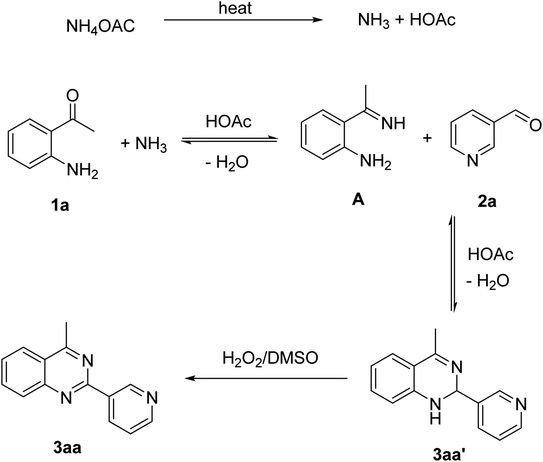
Based on experimental observations and previous reports,6,12,15 the proposed pathway for the formation of quinazoline derivatives was shown in Scheme 3. Firstly, ammonium acetate was decomposed to NH3 and AcOH by heating. Condensation between 2′-aminoacetophenone 1a with NH3 occurred to give imine A. Subsequent condensation between the amino moiety of imine A with aldehyde 2a, followed by nucleophilic cyclization, generated the dihydroquinazoline intermediate 3aa′. The key intermediate 3aa′ can be rapidly oxidized to the final product 3aa in the presence of hydrogen peroxide and DMSO.
4. Conclusions
In summary, we have developed an efficient metal-free synthesis of 2,4-substituted quinazolines via hydrogen peroxide-mediated one-pot three-component reaction of 2-aminoaryl ketones, aldehydes, and ammonium acetate. The transformation proceeded readily under mild conditions in the presence of commercially available hydrogen peroxide. The nature of solvent was critical for the one-pot three-component reaction, and DMSO emerged as the best option for the formation of 2,4-substituted quinazolines. Reaction conditions were compatible with numerous functionalities. Aromatic, heteroaromatic and aliphatic aldehydes were reactive towards this transformation, affording corresponding 2,4-substituted quinazolines in good yields. The significant advantages of this approach are the (1) easily available atom-efficient and green hydrogen peroxide as oxidant; (2) no transition metal catalyst is required; (3) mild reaction conditions; and (4) wide substrate scope. To the best of our knowledge, utilizing hydrogen peroxide as atom-efficient and green oxidant for the synthesis of 2,4-substituted quinazolines was not previously reported in the literature. This strategy provides an efficient and environmentally benign route for the synthesis of 2,4-substituted quinazolines, and would be interested to the chemistry community.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the Viet Nam National Foundation for Science and Technology Development (NAFOSTED) for financial support under Project code 104.05-2018.330 (PI: Anh N. Q. Phan). Ms Thu T. H. Nguyen is acknowledged for her help in taking samples.References
- J. Zheng, S. Jeon, W. Jiang, L. F. Burbulla, D. Ysselstein, K. Oevel, D. Krainc and R. B. Silverman, J. Med. Chem., 2019, 62, 1218–1230 CrossRef CAS PubMed.
- R. Ilmi, E. Tseriotou, P. Stylianou, Y. A. Christou, I. Ttofi, N. Dietis, C. Pitris, A. D. Odysseos and S. N. Georgiades, Mol. Pharm., 2019, 16, 4260–4273 CrossRef CAS PubMed.
- K. Zhang, F. Lai, S. Lin, M. Ji, J. Zhang, Y. Zhang, J. Jin, R. Fu, D. Wu, H. Tian, N. Xue, L. Sheng, X. Zou, Y. Li, X. Chen and H. Xu, J. Med. Chem., 2019, 62, 6992–7014 CrossRef CAS PubMed.
- P. Sang, Y. Xie, J. Zou and Y. Zhang, Org. Lett., 2012, 14, 3894–3897 CrossRef CAS PubMed.
- Z.-H. Zhang, X.-N. Zhang, L.-P. Mo, Y.-X. Li and F.-P. Ma, Green Chem., 2012, 14, 1502–1506 RSC.
- T. Chatterjee, D. I. Kim and E. J. Cho, J. Org. Chem., 2018, 83, 7423–7430 CrossRef PubMed.
- K. Hu, Q. Zhen, J. Gong, T. Cheng, L. Qi, Y. Shao and J. Chen, Org. Lett., 2018, 20, 3083–3087 CrossRef PubMed.
- P. T. Kirinde Arachchige and C. S. Yi, Org. Lett., 2019, 21, 3337–3341 CrossRef PubMed.
- K. Das, A. Mondal, D. Pal and D. Srimani, Org. Lett., 2019, 21, 3223–3227 CrossRef PubMed.
- G. Satish, A. Polu, L. Kota and A. Ilangovan, Org. Biomol. Chem., 2019, 17, 4774–4782 RSC.
- R. Lai, X. Wu, S. Lv, C. Zhang, M. He, Y. Chen, Q. Wang, L. Hai and Y. Wu, Chem. Commun., 2019, 55, 4039–4042 RSC.
- Z.-H. Zhang, X.-N. Zhang, L.-P. Mo, Y.-X. Li and F.-P. Ma, Green Chem., 2012, 14, 1502–1506 RSC.
- D. Zhao, Q. Shen and J.-X. Li, Adv. Synth. Catal., 2015, 357, 339–344 CrossRef CAS.
- U. P. Saikia, G. Borah and P. Pahari, Eur. J. Org. Chem., 2018, 2018, 1211–1217 CrossRef CAS.
- J. Chen, D. Chang, F. Xiao and G.-J. Deng, Green Chem., 2018, 20, 5459–5463 RSC.
- R. Ciriminna, L. Albanese, F. Meneguzzo and M. Pagliaro, ChemSusChem, 2016, 9, 3374–3381 CrossRef CAS.
- T. Cousin, G. Chatel, N. Kardos, B. Andrioletti and M. Draye, Catal. Sci. Technol., 2019, 9, 5256–5278 RSC.
- J. Přech, R. E. Morris and J. Čejka, Catal. Sci. Technol., 2016, 6, 2775–2786 RSC.
- S. Doherty, J. G. Knight, M. A. Carroll, J. R. Ellison, S. J. Hobson, S. Stevens, C. Hardacre and P. Goodrich, Green Chem., 2015, 17, 1559–1571 RSC.
- E. Sankari Devi, A. Alanthadka, A. Tamilselvi, S. Nagarajan, V. Sridharan and C. U. Maheswari, Org. Biomol. Chem., 2016, 14, 8228–8231 RSC.
- X. Zhou, H. Ma, J. Cao, X. Liu and G. Huang, Org. Biomol. Chem., 2016, 14, 10070–10073 RSC.
- A. C. Lindhorst, J. Schütz, T. Netscher, W. Bonrath and F. E. Kühn, Catal. Sci. Technol., 2017, 7, 1902–1911 RSC.
- I. A. Yaremenko, G. d. P. Gomes, P. S. Radulov, Y. Y. Belyakova, A. E. Vilikotskiy, V. A. Vil', A. A. Korlyukov, G. I. Nikishin, I. V. Alabugin and A. O. Terent'ev, J. Org. Chem., 2018, 83, 4402–4426 CrossRef CAS.
- B. Qiu, D. Xu, Q. Sun, J. Lin and W. Sun, Org. Lett., 2019, 21, 618–622 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05040g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |