Open Access Article
Open Access ArticleRecent advancements in the production of rhamnolipid biosurfactants by Pseudomonas aeruginosa
Parisa
Eslami
a,
Hamidreza
Hajfarajollah
 *ab and
Shayesteh
Bazsefidpar
c
*ab and
Shayesteh
Bazsefidpar
c
aAmirkabir University of Technology, Chemical Engineering Department, Iran
bChemistry and Chemical Engineering Research Center of Iran, Chemical Engineering Department, Iran. E-mail: st_hajfarajollah@ccerci.ac.ir; Tel: +98 2122734406
cUniversity of Oviedo, Department of Physical and Analytical Chemistry, Spain
First published on 14th September 2020
Abstract
Rhamnolipid (RL) biosurfactant which is produced by Pseudomonas species is one of the most effective surface-active agents investigated in the literature. Over the years, many efforts have been made and an array of techniques has been developed for the isolation of RL produced strains as well as RL homolog characterization. Reports show that RL productivity by the best-known producer, Pseudomonas aeruginosa, is very diverse, from less than 1 gr/l to more than 200 g L−1. There are some major parameters that can affect RL productivity. These are culture conditions, medium composition, the mode of operation (batch, fed-batch and continuous), bioengineering/gene manipulation and finally extraction methods. The present paper seeks to provide a comprehensive overview on the production of rhamnolipid biosurfactant by different species of Pseudomonas bacteria. In addition, we have extensively reviewed their potential for possible future applications.
1. Background
Biosurfactants (BS) as one of the important bio-products have many applications in environmental, food, agriculture, petroleum, paper/pulp, cosmetics, and pharmaceutical industries. These natural compounds, which are produced by microbial cells,1 have several benefits compared to synthetic counterparts, including great biodegradability, low toxicity, better environmental compatibility, acceptable surface activity at extreme temperatures, pH and salinity, and the ability to be synthesized from renewable feedstocks.2,3 The main reason behind the current global interest in rhamnolipid production is due to their broad range of advantages as well as potential applications in various industries along with “eco-friendly” characteristics.Rhamnolipids (RLs), with the glycolipid-type structure, which are produced mainly by Pseudomonas aeruginosa, are the most intensively studied biosurfactants.4–6 This is because of their relatively high surface activities and high yields of production after relatively short incubation periods by a well-understood, easy to cultivate microorganism.
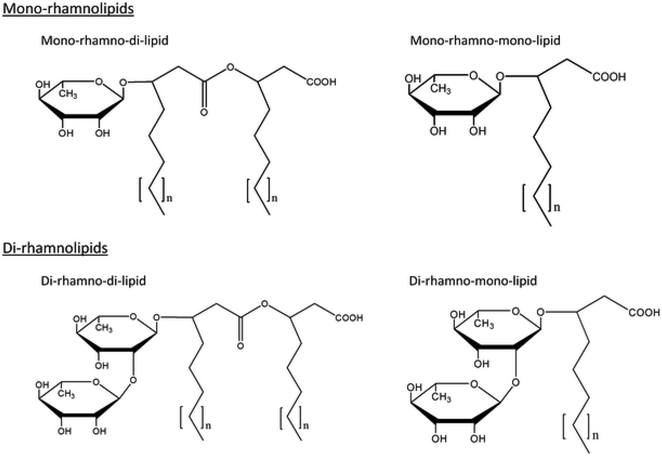
The discovery of RLs dates back to 1946 when an oily glycolipid, named pyolipic acid, was produced by Pseudomonas pyocyanea (P. aeruginosa) on glucose. L-Rhamnose and b-hydroxydecanoic acid were also reported as its structural units.7,8 The exact chemical nature of these biomolecules was unraveled by Jarvis et al.9 followed by Edwards et al.10 Since then, extensive investigations have been conducted covering various aspects of RL research. Fig. 1 shows various common structures of RLs.
Rhamnolipid structure varies due to the number of rhamnose moiety and the length and number of carbon chain. In view of the structures, RLs can be described as follows: they are glycosides composed of mainly two parts, first a rhamnose moiety (glycon part) and the second lipid moiety (aglycon part).11 The rhamnose moiety of RLs is composed of one or two rhamnose moieties linked to each other one which called mono-RLs and di-RLs, respectively. The lipid moiety, however, is composed of hydroxy fatty acid chains (saturated, mono-, or polyunsaturated and of chain length varying from C8 to C16) linked to each other. Abdel-Mawgoud et al.11 studied different structures of RLs and they concluded that variations in the chemical structures of RLs give rise to a large pool of RL homologues that approaches 60 structures. Their glycon and aglycon parts alterations cause homologues differences.
Although RLs have shown many advantages in their applications, there was no mass-production for them up to 2016 due to difficult process and low yield. However, the first company producing RL in large scale was Evonik Industry. They used recombinant Pseudomonas putida and butane to rhamnolipid production. Also, German biotech company Biotensidon GmbH is a leading and first company which develop cost-effective process to produce RL in industrial scale (annually 5000 tons). Other companies which are producing RLs in commercial plants are: TeeGene Biotech (UK), AGAE Technologies LLC (USA), Jeneil Biosurfactant (USA), Paradigm Biomedical Inc. (USA), and many Chinese companies such as Shaanxi Pioneer Biotech Company.
2. Detection and analysis
Detection and analysis of rhamnolipids is a base for better understanding of their different structures and functions. Several methods of analysis with variable precision and different purposes are generally employed which divided into qualitative and quantitative techniques.2.1. Qualitative methods
2.2. Quantitative methods
2.3. Chromatographic methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform). One of the plates put into a jar saturated with iodine vapors to detect lipids as yellow spots and another plate is sprayed evenly with the anthrone reagent and placed in an oven to detect the presence of rhamnose as blue-green spots.19
chloroform). One of the plates put into a jar saturated with iodine vapors to detect lipids as yellow spots and another plate is sprayed evenly with the anthrone reagent and placed in an oven to detect the presence of rhamnose as blue-green spots.19
2.4. Spectroscopic methods
All the mentioned methods can be used in different condition based on researchers' requires for detection and analysis as some of them are suitable for structural studies and not for quantification purposes. However, using both type of methods can be helpful to provide useful information for more accurate study.
3. Fermentation condition
In recent years, there are so many studies on the production of rhamnolipid biosurfactants, their characterizations, and evaluation of different aspects of these compounds. Due to a large number of researches performed in this field, different culture compounds and conditions are employed to produce rhamnolipid. Optimization plays an important role to improve rhamnolipid production. The solubility of a carbon source, the type of feeding, pH, temperature, aeration rate, dissolved oxygen, cell density and capability for removing the product in situ are the essential factors for setting up an efficient fermentation condition as these parameters can significantly impact on the rhamnolipid yield.27Researchers have always tried to improve rhamnolipid production by optimization routes. The parameters of fermentation such as the type of feeding of substrates, pH, temperature, aeration rate, dissolved oxygen, cell density and capability for removal the product in situ, are essential prerequisites for setting up an efficient fermentation condition as these parameters can significantly impact on the rhamnolipid yield.28,29
One of the important factors increasing the rhamnolipid yield is the solubility of carbon source in the culture media. For instance, palm oil and diesel, the insoluble carbon sources, generally produce more rhamnolipids in comparison with water-soluble carbon sources (e.g. glucose).30 Another important parameter is substrate feeding profile.29 To achieve the optimal microbial growth in lag and growth phase, the pH of the culture media should be adjusted around 7–7.5. In the stationery and death phase of fermentation, the slight acidity (pH 6–6.5) can effectively increase rhamnolipid production. The level of dissolved oxygen is also an effective parameter which leads to higher biosurfactant production.31
According to Table 1, medium and culture conditions are different for each strain. Table 2 also shows rhamnolipid production yield from different P. aeruginosa strains as well as surface tension and CMC values. Generally, 37 °C is the optimum temperature for P. aeruginosa growth; however, they are able to survive at the temperature between 4 °C and 42 °C. They can be stored at a temperature of 4 °C within a week. Moreover, some carbon sources along with nitrate as the terminal electron acceptor could be utilized as an anaerobic media for P. aeruginosa.32
| Strain | Source | Substrate and growth condition | Extraction procedure | Ref. |
|---|---|---|---|---|
| a NM: not mention. | ||||
| P. aeruginosa S6 | Oil-containing wastewater | - Nutrient medium | - Centrifugation and acidification | 98 |
| - 165 rpm | ||||
| - 30 °C | ||||
| - pH 7.5 | ||||
| - 48 h | ||||
| P. aeruginosa P20 | Institute of Medical Research (IMR), Malaysia | - Mineral slat medium | - Centrifugation and solvent extraction | 42 |
| - Carbon source: 1% (v/v) crude oil | ||||
| - 150 rpm | ||||
| - 40 °C | ||||
| - 7 d | ||||
| P. aeruginosa DR1 | Rice rhizosphere | - Mineral slat medium with different concentrations | - Centrifugation, acidification, and extraction with chloroform–methanol | 99 |
| - 30 °C | - Column chromatography for further purification | |||
| - Harvesting every 24 h till 120 h | ||||
| P. aeruginosa MA01 | Spoiled apples | - Culture medium (g L−1): NaNO3 3.0, KH2PO4 0.25, MgSO4·7H2O 0.25, yeast extract 1.0 and soybean oil 10 | - Acid precipitation and solvent extraction method | 24 |
| - 200 rpm | ||||
| - 30 °C | ||||
| - 7–10 d | ||||
| P. aeruginosa LBI | Petroleum-contaminated soil | - Mineral slat medium | - Centrifugation and absorption chromatographic column filled with a polystyrene resin | 100 |
| - 30 °C | ||||
| - 86 h | ||||
| P. aeruginosa M14808 | High magneto-gravitational environment | - Culture medium (g L−1): NaNO3 3.0, KH2PO4 2.0, K2HPO4 1.0, MgSO4·7H2O 0.50, KCl 0.1, CaCl2·2H2O 0.01, FeSO4·7H2O 0.01, yeast extract 0.01, vegetable oil 40, 0.05 mL trace element solution containing | - Centrifugation and solvent extraction using chloroform–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
101 |
| - 220 rpm | ||||
| - 30 °C | ||||
| - pH 7.0 ± 0.2 | ||||
| - 7 d | ||||
| P. aeruginosa ATCC 9027 | American Type Culture Collection | - PPGAS medium | - Centrifugation, acidification, and extraction with chloroform–ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) three times 1) three times |
102 |
| - 250 rpm | ||||
| - 37 °C | ||||
| - pH 7.2 | ||||
| P. aeruginosa #112 | Crude oil sample obtained from a Brazilian oil field | - Different culture media containing corn steep liquor (10%, v/v) and sugarcane molasses (10%, w/v), supplemented with olive mill wastewater at concentrations between 5% and 25% (v/v) | - Centrifugation and adsorption chromatography | 103 |
| - 180 rpm | ||||
| - 37 °C | ||||
| - pH 7.0 | ||||
| P. aeruginosa strain-PP2 | Soil contaminated with lube oil and distillery spent wash | - Crude whey | - Centrifugation | 104 |
| - 150 rpm | ||||
| - 30 °C | ||||
| - pH 7.0 | ||||
| - 96 h | ||||
| P. aeruginosa PA1 | NMa | - Culture medium (g L−1): NaNO3 1.0, KH2PO4 3.0, K2HPO4 7.0, MgSO4·7H2O 0.2, 0.5% yeast extract, peptone 0.5%, and 3% glycerol | - Centrifugation, using reverse osmosis process, and purification using purifying using a chloroform/methanol/culture medium mixture | 105 |
| - 170 rpm | ||||
| - 30 °C | ||||
| - 168 h | ||||
| P. aeruginosa BYK-2 KCTC 18012P | The southern sea of Korea | - Basal salts medium | - Centrifugation | 48 |
| - Fish oil and urea as the carbon source and nitrogen source | ||||
| - 180 rpm | ||||
| - 25 °C | ||||
| - 40 h | ||||
| P. aeruginosa HAK02 | Urban wastes of the Kahrizak site in the south of Tehran | - Culture medium (g L−1): sunflower oil (Nina, a local company) 20, NaNO3 (Merck, C 99.5%) 5, KH2PO4 (Merck, 99.5–100.5%) 0.2, and MgSO4·7H2O (Merck, C 98.0%) 0.2 | - Centrifugation, acidification, and extraction using ethyl acetate (99.5%) | 4 |
| - 180 rpm | ||||
| - 30 °C | ||||
| - 24 h | ||||
| P. aeruginosa SP4 | Petroleum-contaminated soil in Thailand | - Culture medium (g L−1): NaNO3 0.5, KH2PO4 0.5, K2HPO4 0.5, MgSO4·7H2O 0.5, KCl 0.1, and FeSO4·7H2O 0.01 | - Centrifugation, acidification, and extraction using a solvent (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3Cl–C2H5OH) 1 CH3Cl–C2H5OH) |
106 |
| P. aeruginosa SP4 | Petroleum-contaminated soil in Thailand | - Mineral medium + palm oil | NM | 107 |
| - 200 rpm | ||||
| - 37 °C | ||||
| - 22 h | ||||
| P. aeruginosa CPCL (GQ241355) | A petroleum contaminated site located in Chennai | - Mineral medium | - Acidification, extraction using CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH (2 CH3OH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and concentrating by a rotary evaporator 1), and concentrating by a rotary evaporator |
75 |
| - pH 7.0 ± 0.2 | ||||
| - 48 h | ||||
| P. fluorescens PMMD3 | The biofilm formed on metal coupons at Ennore port, Chennai (India) | - Minimal salt medium | - Acidification and extraction using equal volume of chloroform and ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture 1) mixture |
108 |
| - Paraffin as carbon source | ||||
| - 35 °C | ||||
| P. aeruginosa CPCL | A petroleum-contaminated soil, Chennai (India) | - 180 rpm | ||
| - 1 month under aerobic condition | ||||
| P. aeruginosa O-2-2 | The Ocean University of China | - Culture medium (g L−1): soybean oil 80, KH2PO4 4.0, K2HPO4 6.0, NaNO3 3.0, NaCl 1.1, KCl 1.1, MgSO4·7H2O 0.2, anhydrous CaCl2 0.2, anti-foam 1 mL L−1, trace elements solution 5 mL L−1 | - Centrifugation, acidification, and extraction using an equal volume of CHCl3/CH3OH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
29 |
| - 180, 350 and 500 rpm | - Concentrating by a rotary evaporator | |||
| - 30 °C | ||||
| - pH 7.0 | ||||
| - 96 h | ||||
| P. cepacia CCT669 | The culture collection of the André Tosello Research and technology Foundation in the city of Campinas | - Mineral medium | - Centrifugation, acidification | 109 |
| - 200 rpm | - Concentrating by a rotary evaporator | |||
| - 27 °C | ||||
| - pH 7 | ||||
| - 120 h | ||||
| P. aeruginosa ATCC 10145 | NM | - Liquid medium | - Centrifugation, acidification, and extraction using hexane | 110 |
| - Hexadecane (2% v/v) as the carbon source | ||||
| - 300 rpm (Shaker) | ||||
| - 600 rpm (fermenter) | ||||
| - 28 °C | ||||
| P. aeruginosa 57RP | Hydrocarbon contaminated soil | - Iron-limited mineral salts medium (MSM) supplemented with 2% (w/v) mannitol | - Centrifugation and filtration | 111 |
| - 200 rpm | - Adding an internal standard (hydroxyhexadecanoic acid) | |||
| - 30 °C | ||||
| P. aeruginosa ICP70 | Oily sludge | - Culture medium (per dm−3 of drinking water): glycerol, 30.5 cm3; MgSO4, 0.1 g; K2HPO4, 7 g; KH2PO4, 3 g; (NH4)2SO4 | - Thioglycolic acid method | 112 |
| - 140 rpm | ||||
| - 305 K | ||||
| - pH 6.5–7.0 | ||||
| P. aeruginosa ATCC 9027 | American Type Culture Collection | - Mineral salts medium | - Centrifugation, acidification, and extraction using 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of chloroform to methanol 1 ratio of chloroform to methanol |
113 |
| - 200 rpm | - Concentrating by rotoevaporation | |||
| - 37 °C | - Chromatography | |||
| - pH 7.0 | ||||
| - 96 h | ||||
| P. aeruginosa 6k11 | Soil contaminated with crude-oil (Talara, Peru) | - Mineral salt medium | - Centrifugation, acidification, and extraction using ethanol and chloroform | 34 |
| - 140 rpm | ||||
| - 37 °C | ||||
| - pH 6.8 | ||||
| - 250 hours | ||||
| P. aeruginosa PAO1 | NM | - BM2 minimal medium | - Solvent extraction | 36 |
| - 170 rpm | - Freezing and using subsequent phase separation | |||
| - 37 °C | ||||
| - 6 and 24 h | ||||
| P. aeruginosa PA1 | Brazilian petroleum exploring environment | - Culture medium (g L−1): NaNO3 1.0, KH2PO4 3.0, K2HPO4 0.7, MgSO4·7H2O 0.2, yeast extract 5.0, peptone 0.5, and glycerol 30 | - Centrifugation and acidification | 114 |
| - 170 rpm | ||||
| - 30 °C | ||||
| - pH 7.0 | ||||
| - 24 h | ||||
| P. SWP-4 | WCO-contaminated sludge samples | - Culture medium (g L−1): NH4NO3 2, NaCl 5, KH2PO4 1, K2HPO4 1, MgSO4·7H2O 0.3, FeSO4·7H2O 0.1, CaCl2 0.1, and WCO 20 | - Centrifugation, acidification, and extraction using ethyl acetate | 115 |
| - 150 rpm | ||||
| - 35 °C | ||||
| - 1 d | ||||
| P. aeruginosa PAO1 | M. Foglino, Marseille, France | - PPGAS medium | - Identifying and quantifying rhamnolipids using LC-MS | 35 |
| - 37 °C | ||||
| - pH 7.2 | ||||
| P. aeruginosa DN1 | Petroleum contaminated soil | - BPLM supplemented with palm oil as the carbon source and sodium nitrate as the nitrogen source | - Centrifugation, acidification, and extraction using ice-cold 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform and methanol ethyl acetate 1 chloroform and methanol ethyl acetate |
116 |
| - 7 d | ||||
| P. aeruginosa ATCC 9027 | The ATCC collection | - Mineral base | - Centrifugation and acidification | 117 |
| - Oleic acid as the carbon source | - Adsorption chromatography | |||
| - Sodium nitrate as the nitrogen sources | ||||
| - Phosphoric acid as the phosphorus sources | ||||
| - 150 rpm | ||||
| - 30 °C | ||||
| - 24 h | ||||
| P. aeruginosa NITT 6L | NM | - Culture medium (g L−1): glucose 40, sodium nitrate 3.5, magnesium sulphate 0.2, FeSO4 0.003, K2HPO4 5, NaCl 0.1. | - Solvent extraction using chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2 methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
118 |
| - 200 rpm | ||||
| - 37 °C | ||||
| - pH 7.0 | ||||
| - 144 h | ||||
| P. aeruginosa ATCC 9027 | American Type Culture Collection | - Culture medium (g L−1): MgSO4 0.2, NaCl 1, KCl 1, CaCl2 0.04, as well as corn oil 4.5% (v/v), H3PO4 (85%) (5 mL L−1), and 1 mL L−1 of trace element solution | - Centrifugation | 119 |
| - 260 rpm | ||||
| - pH 7.0 | ||||
| P. aeruginosa PAO1 | - 37 °C | |||
| - 6 d (batch) and 10 d (fed-batch) | ||||
| P. aeruginosa AB93066 | NM | - Culture medium (g L−1): COFCs 40, NaNO3 6, Na2HPO4·12H2O 1, KH2PO4 1, FeSO4·7H2O, 0.01 and MgSO4·7H2O 0.1 | - Centrifugation and acidification | 120 |
| - pH 7.0 | - Collecting crude RLs by vacuum evaporation | |||
| P. aeruginosa SG | NM | - Culture medium (g L−1): crude glycerol 60, KH2PO4 3.4, K2HPO4·3H2O 4.0, MgSO4·7H2O 0.8, NaNO3 3.5, KCl 0.5, CaCl2 0.05, NaCl 0.5 | - Centrifugation, acidification, and extraction using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform/methanol (v/v) 1 chloroform/methanol (v/v) |
33 |
| - 200 rpm | - Drying by vacuum rotary evaporation | |||
| - pH 6.8 | ||||
| - 37 °C | ||||
| P. aeruginosa E03-40 | Soil samples near a biodiesel plant | - Culture medium (g L−1): vegetable oil 100, NH4Cl 5.72, KH2PO4 6.0, NaCl 1.5, MgSO4·7H2O 0.9, FeSO4·7H2O 0.1, CaCl2·2H2O 0.03, MnCl2·4H2O 0.03, yeast extract 5.0, peptone 5.0, and 2 mL of a trace element solution | - Centrifugation and acidification | 43 |
| - 800 rpm | ||||
| - 32 °C | ||||
| - pH 7.0 | ||||
| P. aeruginosa MA01 | NM | - Culture medium (g L−1): sun flower oil 20, yeast extract 1.0, NaNO3 3.0, MgSO4·7H2O 0.25 and KH2PO4 0.25 | - Solvent extraction | 1 |
| - 200 rpm | - Column chromatography | |||
| - 30 °C | ||||
| - 5–6 d | ||||
| P. aeruginosa (ATCC 10145) | American Type Culture Collection | - Culture medium: 30 to 50% olive oil mill wastewater or whey | - Centrifugation, acidification, and extraction using ethyl acetate | 121 |
| - 100 or 200 rpm | ||||
| - 30 or 37 °C | ||||
| - PH 7.0 | ||||
| - 96 h | ||||
| P. aeruginosa ATCC 15692 | American Type Culture Collection | - Culture medium (g L−1): NaNO3 8.0, NaCl 1.0, KCl 1.0, MgSO4 0.25, CaCl2·2H2O 0.05, and H3PO4 (85%) 5 mL L−1, corn oil 7.5% (v/v) as well as 1 mL L−1 of a trace element solution | - Centrifugation | 44 |
| - 12% (v/v) of corn oil (batch culture) and 3% (v/v) of oil every 3 d after 5 d culture (fed-batch fermentation) | ||||
| - 240 rpm | ||||
| - 37 °C | ||||
| - pH 7.0 | ||||
| - 17–20 d | ||||
| P. aeruginosa USM AR2 | A local crude oil sample | - Culture medium: 0.6% (w/v) yeast extract, 0.05% (w/v) MgSO4·7H2O, 0.05% (v/v) Tween 80, and 30 mL diesel oil | - Centrifugation | 122 |
| - pH 5.0 | ||||
| - 27 °C | ||||
| P. sp. MIS38 | NM | - L broth: 1% Bacto tryptone, 0.5% yeast extract, 0.5% NaCl | - Centrifugation | 17 |
| - 27 °C | - Concentrating by ultra-filtration | |||
| - pH 7.2 | - Extraction using an equal volume of hexane | |||
| - 40 h | ||||
| P. aeruginosa 57RP | A hydrocarbon-contaminated soil | - Iron-limited mineral salts medium supplemented with 2% (w/v) mannitol | - Centrifugation and filtration | 21 |
| - 150 rpm | ||||
| - 30 °C | ||||
| - pH 6.7 | ||||
| - 359 h | ||||
| P. aeruginosa 47T2 NCIB 40044 | Oil contaminated soil sample | - Culture medium (g L−1): NaNO3 5, KH2PO4 2.0, K2HPO4 1.0, KCl 0.1, MgSO4·7H2O 0.5, CaCl2 0.01, FeSO4·7H2O 0.012, yeast extract 0.01 and 0.05 mL of a trace element solution | - Centrifugation | 73 |
| - 150 rpm | - Adsorption chromatography | |||
| - 30 °C | ||||
| - pH | ||||
| - 96 h | ||||
| P. aeruginosa strain ZJU211 (CCTCC M209237) | A heavily oil-contaminated soil | - Culture medium (g L−1): NaNO3 10.0, NaCl 1.0, KCl 1.0, CaCl2·2H2O 0.1, KH2PO4 6.5, Na2HPO4·12H2O 11.0, MgSO4 0.25, and 2 mL of a trace element as well as crude oil (from the Shengli oil field) 0.4% (w/v), | - Acidification, centrifugation, and extraction by chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2 methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
79 |
| - 300 rpm | ||||
| - 37 °C | ||||
| - 96 h | ||||
| P. aeruginosa | An oil-contaminated soil sample | - Medium with the following composition (g L−1): NaNO3 5, KH2PO4 2.0, K2HPO4 1.0, KCl 0.1, MgSO4·7H2O 0.5, CaCl2 0.01, FeSO4·7H2O 0.012, yeast extract 0.01, and 0.05 mL of a trace elements solution containing (g L−1): H3BO3 0.26, CuSO4·5H2O 0.5, MnSO4·H2O 0.5, MoNa2O4·2H2O 0.06, ZnSO4·7H2O 0.7 | - Centrifugation | 82 |
| 47T2 NCIB 40044 | - 150 rpm | - Adsorption chromatography | ||
| - 30 °C | ||||
| - pH 7.2 | ||||
| Microorganism | Fermentation mode | Min. STa (mN m−1) | CMC (mg L−1) | Max. yield (g L−1) | Ref. |
|---|---|---|---|---|---|
| a The second value is the surface tension of water in the defined condition of the experiments. | |||||
| P. aeruginosa S6 | Batch | 33.9 | 50 | 0.18 | 98 |
| P. aeruginosa (P20) | Batch | — | — | 7.5 | 42 |
| P. aeruginosa DR1 | Batch | 30 | 80 | 2.8 | 99 |
| P. aeruginosa MA01 | Batch | 32.5 | 10.1 | 12 | 24 |
| P. aeruginosa LBI | Batch | 24 | 120 | 15.8 | 100 |
| P. aeruginosa S2 | Fed-batch | 30 | — | 9.4 | 49 |
| P. aeruginosa ATCC 9027 | Batch | 30 | 40 | 0.3 | 123 |
| P. aeruginosa #112 | Batch | 30 | 13 | 5.1 | 103 |
| P. aeruginosa PA1 | Fed-batch | — | — | 16.9 | 124 |
| P. aeruginosa HR | Batch | 20 | 19 | 4.2 | 125 |
| P. aeruginosa PA1 | Batch | 27 | 25.7 | — | 105 |
| P. aeruginosa MR01 | Batch | 28 | — | 1.4 | 126 |
| P. aeruginosa SP4 | Batch | 28–30 | 150 | — | 106 |
| P. aeruginosa RS29 | Fed-batch | 26.3 | 90 | 0.80 | 127 |
| P. aeruginosa SP4 | Batch | 28–30 | 120 | 0.126 | 107 |
| P. aeruginosa KVD-HR42 | Batch | 30.14 | 100 | 5.09 ± 2.1 | 128 |
| P. aeruginosa | Batch | 19 | 25–30 | 16–17 | 108 |
| P. aeruginosa O-2-2 | Fed-batch | — | — | 70.56 | 29 |
| P. aeruginosa AT10 | Batch | — | — | 18.7 | 129 |
| P. cepacian CCT6659 | Batch | 27.57 | — | — | 109 |
| P. aeruginosa DSM2659 | Batch | 29 | — | 1.5 | 130 |
| P. aeruginosa HR | Batch | 20 | 19 | 4.2 | 131 |
| P. aeruginosa ATCC 9027 | Batch | — | — | 2.6 ± 0.26 | 113 |
| P. aeruginosa DS10-129 | Batch | 27.5 | 10 | — | 113 |
| P. aeruginosa 6K11 | Batch | — | — | 3.2904 | 34 |
| P. aeruginosa (P20) | Batch | — | — | 7.5 | 42 |
| P. aeruginosa DN1 | Batch | 25.88 | 50 | 25.9 | 116 |
| P. SWP-4 | Batch | 24.1 | 27 | 13.93 | 115 |
| P. aeruginosa ATCC 9027 | Batch | 29 | 30 mg L−1 | 0.9 | 132 |
| P. aeruginosa IFO 3924 | Batch | — | — | 32 | 133 |
| P. aeruginosa ATCC 9027 | Batch | — | — | 4.261 | 134 |
| P. aeruginosa NITT 6L | Batch | 27.5 | 11 | 7.65 | 118 |
| P. aeruginosa ATCC 9027 | Fed-batch | — | — | 43.3 | 119 |
| Batch | — | — | 61.2 | ||
| P. aeruginosa AB93066 | Batch | 25.3 | 45 | — | 120 |
| P. aeruginosa SG | Batch | 27.2 | 60 | 1.98 | 33 |
| P. aeruginosa PrhlAB | Batch | ≤ 30 | 80 | 2.87 | |
| P. stutzeri Rhl | Batch | ≤ 30 | 90 | 0.87 | |
| P. sp. SWP-4 | Batch | 22.7 | — | 6.87 | 86 |
| P. aeruginosa PA1 | Batch | 30 | 60 | 13.2 | 85 |
| P. aeruginosa PAO1 | Batch | 30 | — | — | 76 |
| P. aeruginosa HAK02 | Fed-batch | 30 | 500 | 240 | 4 |
| P. aeruginosa HAK02 | Batch | 30 | 500 | 22.5 | 4 |
| P. aeruginosa ATCC 15692TM | Fed-batch | 28 | 30 | 150 | 44 |
| P. aeruginosa YPJ-80 | Batch | — | — | 4.4 | 47 |
| P. aeruginosa MTCC 2297 | Batch | 24.02 | — | 1.975 ± 0.007 | 14 |
| P. aeruginosa 47T2 NCIB 400044 | Batch | 32.8 | 108 | 8.1 | 82 |
| P. aeruginosa FIN2 | Batch | 28.6 | 195 | — | 135 |
| P. aeruginosa EBN-8 | Batch | 28.5 | — | 8.5 | 56 |
Regarding culture media, LB broth also shows its ability in P. aeruginosa growth. A well-growing culture medium, which can be modified to study its impact on P. aeruginosa growth and virulence, includes crucial ingredients such as carbon and nitrogen sources.32
Zhao et al. have studied on three different rhamnolipid producers namely P. aeruginosa SG (the wild-type strain) and two recombinant strains, P. aeruginosa PrhlAB and P. stutzeri Rhl.33 The growth condition for all three strains was the same (37 °C, 200 rpm for 5 days) and crude glycerol, KH2PO4, K2HPO4·3H2O, MgSO4·7H2O, NaNO3, KCl, CaCl2 and NaCl were the main compounds in the medium. In another study, Hospinal et al. worked on P. aeruginosa 6k11 isolated from soil contaminated with crude-oil and inoculated in mineral salt medium (MSM).34 Furthermore, P. aeruginosa PAO1 (obtained from M. Foglino, Marseille, France) grown in PPGAS medium employed LC-MS to identify and quantify rhamnolipid from culture supernatants.35 Schmidberger et al. used BM2 minimal medium for P. aeruginosa PAO1.36 In this study, the effects of two changes on gene expression and rhamnolipid production were investigated: adding an extra amount of iron ions (Fe3+) as well as omitting them from BM2 medium and comparing with standard BM2.
Overly, the components of the substrates used for RL production can play an important role in increasing production yield. As carbon is the major component in deriving RLs, using low-cost waste containing sugar as the carbon source such as agricultural residues, whey products, etc. can be useful in reducing the cost of production. Also, it is noted that purification process is easier when using sugar (either commercial or waste containing sugar) as the carbon source. Proteins, amino acids, and lipids are also important in RL production which should be considered. Other factors such as type of strain, growth condition, feeding profile, pH, temperature, aeration rate, dissolve oxygen, and fermentation strategy should be optimized to explore the best procedure and suitable condition for industrial production.
4. Extraction and purification
Downstream processing plays an important role to determine the production cost of bioproducts, especially for those with high yields. It often allocates about 60–80% of the overall manufacturing costs. In addition, the difficulty of product extraction leads to the selection of the purification method which is mostly affected by the ionic charge and the metabolite types (intracellular or extracellular).28,37 Thus, the commercialization of the products necessarily needs the economic downstream producer.38 The compounds used in the fermentation broth namely salts, amino acids, proteins and etc. and their complexity are the main factors in the rhamnolipid purification (downstream process).28Table 3 shows extraction methods using in different research works for rhamnolipid production by P. aeruginosa.| Downstream process method | Biosurfactant feature for separation | Advantages | Ref. |
|---|---|---|---|
| Acid precipitation | Insolubility at low pH values | - Low cost | 38 and 42 |
| - Effective in the recovery of rhamnolipids | |||
| Centrifugation | Precipitating due to the centrifugal force | - Efficient in the recovery of crude rhamnolipids | 38 |
| - Reusable | |||
| Ammonium sulfate precipitation | Salting-out of the polymeric or protein rich biosurfactant | - Efficient in polymeric biosurfactants | 136 |
| Organic solvent extraction | Dissolving in organic solvents due to the hydrophobic ends | - Effective in the recovery of biosurfactants | 136 |
| - Reusable nature | |||
| Ion exchange chromatography | — | - High purity | 136 |
| - Reusability | |||
| - Fast recovery | |||
| Adsorption on wood active carbon | Absorption capability with organic solvents | - High pure biosurfactant | 136 |
| - Reusable | |||
| - Capability to recover from continues culture | |||
| Ultrafiltration | Forming aggregates above the CMC | - Inexpensive | 137 |
| - High purity of biosurfactant | |||
| Foam fractionation | Ability to form foam due to surface activity are able | - Continues recovery from fermentation | 138 |
| Adsorption chromatography | Adsorption capability of crude rhamnolipids on normal phase resin | - High quality purified rhamnolipid | 37 |
| - Economic method | |||
| - Low solvents for purification |
A few recovery methods for purification of rhamnolipids has been reported in recent years. Foam fraction, adsorption chromatography, ultrafiltration, and ion exchange chromatography are important strategies for rhamnolipid purification.38 Precipitation with acid or ammonium sulfate is the most common method for purification. This technique is commonly followed by centrifugation and solvent extraction.39 Anion exchange chromatography is another technique measuring the negative charge of the rhamnolipids at high pH. It is superior to acid precipitation method due to the lower losses.40 However, having the extracted mixture of rhamnolipid and some fatty acids is the main disadvantage of anion exchange chromatography.39 In addition, foam fraction is a type of downstream processing method for RLs purification41 used foam fraction in order to extract surfactin produced from fermentation broth. Shah et al. compared four different downstream recovery methods including acid precipitation, zinc sulfate precipitation, ammonium sulfate precipitation, and solvent extraction.42 Based on the results, the best method for rhamnolipid purification with the highest yield could be solvent extraction. Beuker et al. have studied the foam fraction method to purify the rhamnolipids driven from P. putida and the highly concentrated rhamnolipids were extracted from fermentation broth.39
Another downstream process method is adsorption chromatography developed for rhamnolipids purification and separation. In this method, the purification of rhamnolipid is carried out with normal phase resin and nonpolar solvents. It is claimed that the highest purification of rhamnolipids can be obtained being appropriate for food and medical applications. Moreover, purified rhamnolipids show noticeable antibacterial activity and can be applied in the formulations of cosmetics and skin care products.37 Invally et al. extracted rhamnolipid from fermentation broth using different unit operations.43 First, ethanol precipitation was used to remove biopolymers, followed by acid precipitation method. The separated rhamnolipids, then, were dissolved in neutral aqueous solution. Finally, calcium precipitation was used to enhance the purity of the product and remove residual impurities. It was shown that the percentage of purification reached around 87% by using this sequence. Moreover, this method has been reported as an eco-friendly technique.
5. Fermentation strategies
Batch, fed-batch and continuous modes are three main strategies for the fermentation process. In the batch cultivation process, all the nutrients required for growth of bacteria and production of desired metabolites are added to the culture medium before cultivation is started, and the product is only discharged from the fermenter at the end of the process. In the continuous microbial fermentation method, all the nutrients are continuously added to the fermenter and the components of the culture medium are removed from the fermenter at the same time in order to maintain a constant culture volume. Fed-batch is clearly similar to semi-batch. This technique is one of the most effective ways to enhance rhamnolipid productivity and yield by feeding more nutrients added to the fermenter during fermentation process.4,44,45 The fed-batch process is completely different. In this method, fresh culture medium and substrate are fed to the fermenter without removing the rhamnolipids produced in the fermenter. Therefore, efficient feeding strategy results in a significant increase in rhamnolipid production.28 Generally, there are two main types of feeding strategy including feedback control mechanism and without feedback control mechanism. A constant and increasing feeding rate are not involving feedback control. In the former, the rate of nutrient feed is constant during the fermentation, therefore, the specific growth rate drops with time by increasing nutrient consumption. In the latter, the specific growth rate is constant during the fermentation due to continuous feeding based on calculating the required feeding rate. In contrast, DO-stat and pH-stat cultivations are fed-batch processes with feedback control.46 A number of studies have been done to improve rhamnolipid production by different feeding strategies. However, there are a few works studied on the production of rhamnolipid biosurfactant in fed-batch mode. Table 4 shows fermentation modes as well as rhamnolipid production yield from different P. aeruginosa strains in different literatures. In one study, the yield of rhamnolipid derived from P. aeruginosa YPJ-80 by pH-stat fed-batch has reached reported 4.4 g L−1.47 Lee et al. reported the rhamnolipid concentration of 22.7 g L−1 by fed-batch cultivation of P. aeruginosa BYK-2 KCTC 18012P with feeding fish oil as a carbon source.48 In another study, a pH-stat feeding strategy was investigated and the maximum yield of rhamnolipid reached about 6 g L−1.49 Zhu et al. claimed yield of 70 g L−1 by pH-stat controlled fed-batch cultivation of P. aeruginosa O-2-2.29 Additionally, Invally et al. showed that P. aeruginosa E03-40 could produce 55.7 ± 2.6 g L−1 rhamnolipids in fed-batch cultivation by using vegetable oil as a carbon source.43 Bazsefidpar et al. reported the highest overall rhamnolipid production of 240 g L−1 with the productivity of 0.9 (g L−1 h−1) by feeding sunflower oil under tight DO control which showed 4.8-fold improvement compared to the batch cultivation.4 This is the highest rhamnolipid concentration has been ever reported from P. aeruginosa fermentation without genetic manipulation.| Strain | Maximum yield (g L−1) | Substrate | Feeding strategy | Downstream extraction producer | Ref. |
|---|---|---|---|---|---|
| P. aeruginosa YPJ-80 | 4.4 | Glucose | Fed-batch-pH-stat | — | 47 |
| P. aeruginosa BYK-2 KCTC 18012P | 22.7 | Fish oil | Fed-batch | Rosenberg method | 48 |
| P. aeruginosa S2 | 6.06 | Glucose | Fed-batch-pH-stat | Acid precipitation | 49 |
| Solvent extraction | |||||
| P. aeruginosa USM-AR2 | 2.61 | Diesel | Batch | Optimal density (OD540) | 30 |
| 18.9 | Diesel | Fed-batch-pulse-pause feeding of diesel | |||
| 23.6 | Diesel | Fed-batch-MSUR-based feeding of diesel | |||
| P. aeruginosa USM-AR2 | 2.35 | Diesel | Batch | Optimal density (OD421) | 139 |
| 3.13 | Diesel | Fed-batch-plus feeding of carbon source | |||
| P. aeruginosa (ATCC 53752) | 0.7 | Glycerol | Batch | Phenol-sulfuric acid method | 140 |
| 4.12 | Glycerol | Fed-batch- feeding of glycerol | |||
| P. aeruginosa | 55 | Soybean oil | Fed-batch- feeding of medium and substrate | Acid precipitation | 141 |
| Solvent extraction | |||||
| P. aeruginosa O-2-2 | 24.06 | Soybean oil | Batch | Acid precipitation | 29 |
| 70.56 | Soybean oil | Fed-batch-pH-stage-controlled | Solvent extraction | ||
| P. aeruginosa ATCC 15692 | 150 | Soybean oil | Sequential fed-batch | Acid precipitation | 44 |
| Solvent extraction | |||||
| P. aeruginosa E03-40 | 55.7 ± 2.6 | Vegetable oil | Fed-batch- feeding of vegetable oil | Ethanol precipitation | 43 |
| Acid precipitation | |||||
| Calcium precipitation | |||||
| P. aeruginosa HAK02 | 22.5 | Sunflower oil | Batch | Acid precipitation | 4 |
| 240 | Sunflower oil | Fed-batch cultivation under tight DO control | Solvent extraction |
In conclusion, an excellent fermentation strategy is critical factor to reduce the cost of production and increase productivity. The knowledge about metabolic pathway can help in selecting the best type of fermentation. In fed-batch fermentation method, the substrate inhibition is controlled, so developing fed-batch fermentation can effectively improve the RL yield as kinetic model for substrate utilization shows. In addition, the effect of nutrient concentration on yield and productivity in fed-batch cultivation is more than batch fermentation process. Type of feeding strategy also depends on the bacterial strains and desired metabolites.
6. Bioengineering
One of the efficient approaches for increasing biosurfactants production is bioengineering. In this context, two main strategies have been targeted to increase rhamnolipids production: (a) genetic engineering and (b) random mutagenesis. Genetic engineering has been widely used to generate a large number of modified strains, involving in gene expression. Random mutagenesis mostly is generated by UV-irradiation to randomly create fundamental changes in different Pseudomonas aeruginosa strains and do not focus on biosynthetic enzymes or genes. Table 5 shows the summary.| Microorganism | Host microorganism | Mutation method | Primary yield (g L−1) | Final yield (g L−1) | Ref. |
|---|---|---|---|---|---|
| P. aeruginosa PAO1 | E. coli | Genetic engineering | 0.227 | 0.121 | 52 |
| P. aeruginosa EMS1 | P. putida 1067 | Genetic engineering | 5 | 6.97 | 53 |
| P. aeruginosa SQ6 | P. stutzeri Rhl | Genetic engineering | 3.12 ± 0.11 | 4.37 ± 0.14 | 142 |
| P. aeruginosa 65E12 | E. coli | Genetic engineering | <0.1 | 0.85 | 143 |
| P. aeruginosa (ATCC 10145) | — | Genetic engineering | 9.6 | 13.3 | 121 |
| P. aeruginosa SG | P. aeruginosa PrhlAB | Genetic engineering | 1.98 | 2.87 | 25 |
| P. stutzeri Rhl | 0.87 | ||||
| P. aeruginosa EBN-8 | — | Random mutagenesis | — | 8.50 | 56 |
| P. aeruginosa | — | Random mutagenesis | — | 70–120 g L−1 | 58 |
| P. aeruginosa MM1011 | P. aeruginosa PTCC1637 | Random mutagenesis | 1.2 | 12.5 | 26 |
6.1. Genetic engineering for enhanced production of rhamnolipids
Genetic engineering or genetic modification is a manipulation of the selected organism's genome by employing biotechnological tools. In genetic engineering, alteration of genetic makeup is being performed through the transfer of genes across and within various species to develop improved or desired organism with a particular trait. The organism developed through genetic manipulation is considered as genetically modified and known as a genetically modified organism (GMO).In the last few years, many researchers worked on the metabolic engineering strategy to increase the production rate of rhamnolipids.50 On the other hand, understanding of the biosynthesis and genetic regulation systems of rhamnolipid production may help in the development of mutant strains with increased ability to produce rhamnolipids.28 The use of metabolic engineering tools may enable the development of bioprocesses that provide the necessary conditions for optimal synthesis of biosurfactants.51
3-(Hydroxyalkanoyloxy)alkanoic acid (HAA) and dTDP-l-rhamnose are two important precursors to rhamnolipid biosynthesize which the former is synthesized from β-hydroxydecanoyl-ACP. Due to the similarity of the biosynthesis pathway for both β-hydroxydecanoyl-ACP and dTDP-l-rhamnose in a large number of bacteria, the recombination of strains can be possible, and metabolic engineering can pave the way for constructing non-pathogenic recombinant strains by using different types of genes which are important in the biosynthesis of rhamnolipids. By introducing rhlA, rhlB, and rhlC into the target recombinant strains, both mono- and di-rhamnolipids can be produced.28
Cabrera-Valladares et al. worked on the production of the mono-rhamnolipids using biosynthesis methods.52 They used HAAs in a recombinant Escherichia coli strain and expressed it into P. aeruginosa rhlAB operon. This technique, accordingly, lead to a noticeable increase in rhamnolipid yield. In another study, Cha et al. have studied on the replacement of pathogen strains (P. aeruginosa) in a heterologous host (Pseudomonas putida) in order to produce a safe industrial strain.53 They used bioengineering techniques as well as the colonized rhlAB rhamnosyltransferase genes and the rhlRI quorum sensing system to produce mono-rhamnolipid in P. putida and the rhamnosyltransferase acted as a catalyzer. As the results showed, using this method lead to increase the rhamnolipid yield from 5.18 g L−1 (produced by P. aeruginosa EMS1) to 6.97 g L−1 (produced by P. putida 1067 (pNE2)). It should be noted that the rhamnolipid production from non-pathogen P. putida is ecologically more feasible than from pathogen P. aeruginosa and they are environmentally acceptable as they are not contaminated with toxins and pigments. The introduction of estA into P. aeruginosa PAO1 proposed by Wilhelm et al. cause an increase in rhamnolipid production.54
Similarly, the overexpression of rhlAB in B. kururiensis resulted in a noticeable increase in rhamnolipid production (0.78 to 5.76 g L−1). Likewise, by overexpressing rhlC in P. chlororaphis, it could produce both mono- and di-rhamnolipid while without using biosynthesis method, the strain was only able to produce mono-rhamnolipid.55
6.2. Improving rhamnolipid yield through random mutagenesis
The random mutagenesis technology which has been widely used to enhance microbial production is divided into chemical or radiation treatment. Through this technique, the rhamnolipid yield can be improved, however, it may lessen its productivity after some time.28 By subjecting the parent strain (P. aeruginosa S8) to the best gamma radiation dose, its mutant (P. aeruginosa EBN-8) was obtained which showed a better growth on oil refinery wastes and produced 8.5 g L−1 rhamnolipids.56 UV mutagenesis is another type of random mutagenesis technology being able to produce mutants and enhance rhamnolipid production. Husain et al. used this method to obtain P. fluorescens 29L which could produce mutants on pyrene and pyrene metabolism by P. fluorescens 29L is dependent on biosurfactants.57 In another study, mutagenesis of P. aeruginosa by using chemical mutagen could increase the rhamnolipid production from 70 to 120 g L−1.58 Dobler et al. used UV-radiation mutagenesis on a sample driven from soil. As a result, the generated mutant could produce rhamnolipid more than the parent strain.59As a conclusion, although both genetic engineering and random mutagenesis, have been improved the rhamnolipid production, generally there is not a significant change in production. These methods should be employed along with other feasible methods to enhance rhamnolipid productivity which represents a challenge and needs further understanding and exploring.
7. Applications
Low toxicity, biodegradability, pore-forming capacities, anti-adhesive, and anti-biofilm formation ability, anti-bacterial activity against a wide variety of bacteria, emulsification and de-emulsification activity are some RLs properties toward their great potential applications in many industries such as oil, cosmetics, special chemical foods, agriculture, medicine, etc. The potential applications of rhamnolipids in diverse industries have been presented in Table 6.| Application | Example | Ref. |
|---|---|---|
| Bioremediation | Desorption of contaminants from soil | 83 and 84 |
| Impacts on microbial adhesion/microbial mobility | 83 | |
| Bioremediation of petroleum | 71, 85, 144 and 145 | |
| Bioremediation of pesticides at agricultural fields | 83, 146 and 147 | |
| Remediation of oil-contaminated water | 148 | |
| Pest control | Enhancing the pesticide and agrochemical solubility | 83, 147 and 149 |
| Control plant diseases | 78–80 and 150 | |
| Oil recovery | Microbial enhanced oil recovery (MEOR) | 83, 86 and 151 |
| Increase amount of recoverable oil aided by rhamnolipid | 86, 87 and 149 | |
| Microbial de-emulsification of oil emulsions | 71 and 88 | |
| Oil-processing operations | 83 | |
| Medical use | Low toxicity, biocompatibility and digestibility | 71, 74 and 89 |
| Prevent biofilm formation | 76, 149 and 152 | |
| Anticancer agents | 149 and 152 | |
| Food processing | Improvement in the stability of dough, volume, texture and conservation | 72, 153 and 154 |
| As antimicrobial agent preventing food spoilage | 67–71 and 73 | |
| Mining processing | Enhanced metal extraction from the mining | 71 and 83 |
| Nanoparticles | Nanoparticle synthesis using microemulsion method | 91–96 and 155 |
| Drug delivery | 97 | |
| UF membranes cleaning | Great potential in industrial application as membrane cleaner | 153 |
| Microbial fuel cells | Promoting power density output of microbial fuel cells | 156 |
| Cosmetic and pharmacy | High emulsifying activity | 82 |
There are little publications strictly discussed on toxicity of biosurfactants. Biosurfactants are commonly considered as low- or non-toxic. Selected data on biosurfactants toxicitycab found on literature.60–62 Biosurfactants in comparison with synthetic surfactants pose haemolytic activity to human erythrocyte lower than cationic surfactants (CTAB, TTAB, BC) and anionic SDS. They do not pose detrimental effect to heart, lung, liver and kidney and interfere in blood coagulation in normal clotting time.60–62
The efficiency of surfactants and biosurfactant is expressed by some parameters mainly the critical micelle concentration (CMC) and emulsification index (E24). The CMC is the concentration limit of a biosurfactant after which the addition of more biosurfactant will not cause the surface tension to be further reduced. A biosurfactant with a low CMC is more efficient in lowering surface and interfacial tensions than a biosurfactant with a high CMC. Biosurfactant CMCs range from 1–200 mg L−1 (ref. 63) and are 10–40 fold less than that of synthetic surfactants.64 The CMC of surfactin has been reported to be as low as 21 mg L−1, while that of rhamnolipids has been reported to be around mg L−1.24 Also, Ferhat et al. showed a higher emulsification index than synthetic surfactants such as SDS, Tween 20, and Tween 80.65
Some of noticeable applications of RLs in the food industry are mostly due to their emulsification ability, antibacterial activity.66 Furthermore, they can act as preventive agents against contamination, food spoilage factors, and the transmission of diseases. The formation of biofilms is another concerning issue in the food industry. One effective solution is the pre-conditioning of surfaces, using biosurfactants, which can be helpful in avoiding adhesion.67,68 An investigation by ref. 69 showed the inhabitation ability of rhamnolipid produced by P. aeruginosa strain against both Gram-positive and Gram-negative bacterial strains, namely E. coli, B. subtilis, S. aureus, and S. epidermidis. In another study, the rhamnolipids produced by P. aeruginosa PAO1 could rapidly disrupt B. bronchiseptica biofilms on polystyrene.70 Furthermore, emulsions are an integral part of the food industry playing an important role in the quality of products such as mayonnaise, butter, cream, margarine, salad dressing, chocolates and hotdogs.71 On the other side, de-emulsification is breaking a stable emulsion and can be an effective process in the food industry especially when related to fat and oil products. RLs may stabilize (emulsify) or destabilize (de-emulsify) the emulsion. A recent invention by Van Haesendonck et al. have clearly investigated the effect of a sufficient amount of rhamnolipid on the stability of dough and texture of bakery products.72 In another study, Haba et al. P. aeruginosa 47T2 NCIB 40 could produce a rhamnolipid which showed acceptable results as an emulsifier.73 Finally, RLs are able to improve food quality by preserving them from contamination due to their antibacterial activities. Moreover, they could serve as a source of L-rhamnose having substantial potential in high-quality flavor compounds.
Biofilm is a complex community of microorganisms, and its formation is an important problem for many industries. It can be produced by microorganisms such as bacteria and fungi. Removing of biofilms are difficult since are resided within a polysaccharide and/or protein matrix. The resistance of biofilm to antimicrobial agents is becoming a global issue.74,75 Rhamnolipid as a natural surfactant regulated by quorum sensing has the inhibitory effect on biofilm formation.76 Davey et al. studied on the behavior of the purified rhamnolipid on cell-surface (on polyvinylchloride plastic) and cell–cell interaction (on pellicles of wild-type P. aeruginosa cells).77 As the results showed, biofilm formation on polyvinylchloride plastic significantly reduced by increasing in rhamnolipid concentration.
Moreover, adding rhamnolipid caused the disruption of cell-to-cell interactions. Wood et al. found that P. aeruginosa supernatants had a significant inhibitory effect on sulfate reducing bacteria (SRB) biofilm formation, which is the main reasons for metal corrosion in oil wells and drilling equipment.74
Another important application of rhamnolipid biosurfactants is in agriculture since they have shown their inhibitory effects against plant pathogens.
The first report on the rhamnolipid insecticidal activity was by Kim et al.78 They reported the ability of rhamnolipid produced by P. sp. EP-3 against Green Peach Aphid (Myzus persicae). In another study, the antifungal activity of rhamnolipids against seven plant pathogens has been surveyed. The results showed the high-level ability of rhamnolipid derived from Pseudomonas aeruginosa ZJU211 against two Oomycetes, three Ascomycota, and two Mucor spp. fungi.79 Similarly, rhamnolipid synthesized by P. aeruginosa DS9 were reported to show their antifungal ability against F. sacchari causing pokkah boeng disease.80
Since the chemical synthesis of surfactants reveals adverse effects on people's health, P. aeruginosa derived RLs play a noticeable role in cosmetic and pharmacy industries due to their emulsifying ability, solubilizing biodegradability, low toxicity, and detergency properties which can guarantee the cosmetics and drug delivery system safety. High emulsifying activity is the basis of the texture consistency of health care and cosmetic products such as antacids, acne pads, contact lens solutions, deodorants, and etc.81 Furthermore, the emulsifying ability of rhamnolipids synthesized by Pseudomonas aeruginosa 47T2 NCBIM 40044 was evaluated by Haba et al.82 Different types of oils were tested and it was found that only using linseed oil along with RL47T2 led to the formation of the strong and stable emulsion.82
Another application for RLs is their feasibility in bioremediation of heavy metals in soil or other media owing to their effect on the oil-water interface. It, consequently, enhances the degradation of such compounds in the environment. Because of rhamnolipid anionic nature, they widely use in removing heavy metals such as Ni and Cd from soils.83 Juwarkar et al. showed the ability of rhamnolipid biosurfactant by P. aeruginosa BS2 for bioremediation of multi-metal contaminated soil (Cr, Pb, Cd, Ni, and Cu).84 As a result of conducting column experiment, the feasibility of using rhamnolipid was proved although it was different for different metals. In another study, Santa et al. have concluded that rhamnolipid biosurfactant (extracted from P. aeruginosa PA1) had the ability to remove oil contamination from sandy soils.85
In recent years, many investigations have been carried out on the RL applications in oil recovery. RLs mostly have shown their feasibility in petroleum due to their high surface activity. They have been widely employed for heavy crude oil biodegradation and microbial enhanced oil recovery (MEOR). Lan et al. have indicated that the increase in rhamnolipid produced by P. sp. SWP-4 successfully could reduce the viscosity of crude oil and efficiently could enhance oil recovery.86 Li et al. showed the ability of rhamnolipid synthesized by P. aeruginosa (P-1) to increase the oil recovery by 11.2% and decreased crude oil viscosity by 38.5%.87 In another study, it was shown that rhamnolipid could recover over 98% of crude oil from the wastes using the demulsification process.88
Another potential application of rhamnolipids is devoted to biomedicine. Due to their antimicrobial activity against a wide range of bacterial, they are a safe alternative for the recovery of different illnesses. Thanomsub et al. evaluated the ability of rhamnolipid (derived from P. aeruginosa B189) isolated from milk factory waste in breast cancer therapy and insect cell line.89 Two rhamnolipids namely Rha–Rha–C10–C10 and Rha–Rha–C10–C12 produced by the mentioned strain showed inhibition activity in the spread of breast cancer at its minimum inhibitory concentration (MIC) (6.25 and 50 μg mL−1). However, the crude RL extract showed no antimicrobial activity. Also, successful treatment was reported by Piljac et al. for Decubitus Ulcer using di-rhamnolipid ointment.90 The wound was completely healed after 48 days while there was no evidence of improvement by the standard drugs.
In recent years, using rhamnolipids as an agent having the ability of the molecular self-assembly is a promising trend in nanotechnology. RLs could alter their self-assembled structures owing to their carboxylic acid on the headgroups. Consequently, they can be used in synthesizing nanoparticles and microemulsions,91 and they were named the natural green biosurfactants.92 Xie et al. investigated the effect of using rhamnolipid biosurfactants in silver nanoparticles stabilization in the liquid phase in the reverse micelles.92 The uniformity of obtained nanoparticles was analyzed and proved by the TEM and AFM. Moreover, in another study Xie et al. studied the difference between W/O and O/W microemulsion in a rhamnolipid/n-butanol/water/n-heptane system.93 In another research, microemulsion technique was employed to synthesize the nickel oxide nanoparticles by using, n-heptane, water, and rhamnolipids as biosurfactant. Besides, the effect of increasing pH on the size of nanoparticles was investigated. It was found that the nanoparticles were completely spherical in shape and in pH values of 11.6, 12.0, and 12.5, the size of nanoparticles was 86 ± 8 nm, 63 ± 6 nm and 47 ± 5 nm, respectively.94 Narayanan et al. used a novel method for synthesizing nanoparticles in aqueous condition by rhamnolipids.95 They have indicated the rhamnolipids ability as capping agents for capping ZnS nanoparticles and then they were evaluated by FT-IR, SAXS, HR-TEM to prove the formation of uniform nanoparticles. Furthermore, Farias et al. investigated the formation of silver nanoparticles using rhamnolipid produced by a strain of P. aeruginosa UCP0992 in a low-cost medium using microemulsion method.96 The size of the formed nanoparticle was about 1.13 nm and it could be stabilized for at least 3 months without adding passivator. Also, TEM was used to confirm the uniformity of particles.
Furthermore, improvement in making nanoparticles leads to the advancement in drug delivery. Recent researches have indicated that rhamnolipid nanoparticles have potential in imaging and nanomedicine. The only report on the application of rhamnolipid produced by P. aeruginosa in the intravenous injection of rhamnolipid nanoparticles for photodynamic therapy released by Yi et al.97 In this study, the rhamnolipid nanoparticles were prepared by pheophorbide having about 136.1 nm diameter and high water solubility. The results showed that after the injection of the loaded nanoparticle to SCC7 tumor-bearing mice model, tumor growth was prevented.
8. Conclusion
Generally, surfactants are surface active agents using to reduce surface tension between two surfaces in different industries. However, these surfactants are mostly allergic. They are not biodegradable and in some cases they can be toxic. All of these negative properties cause the researcher find a way to solve the problems and drive surfactants from various microorganisms named biosurfactant. Rhamnolipids and sophorolipid are two main group of biosurfactant. They not only can noticeably reduce the surface tension activity, but also they are biodegradable and environmentally friendly products. They also can decrease surface tension more than chemical surfactant at the same CMC.Introducing new products to the market is always a challenge. This is especially true for bio-materials such as rhamnolipids. Although every bio-products like biosurfactants exhibit many advantages over chemically synthesized counterparts, there are some bottlenecks for using them in commercial scale. They are not toxic in contact with human skin. However, it should be noted that they are toxic to microbial growth in certain concentrations. Scientists use this advantage of RLs for antimicrobial applications. Also, they are easily degraded in the environment by bacteria and other microscopic organisms; hence they are not considered a threat to the environment. In comparison with chemically synthesized surfactants, they show great surface activity great surface activity with very low CMC. In addition, waste and cheap raw materials (such as waste oil) which are available in large quantities can be used as the raw material to produce RLs. It can enhance economic efficiency in producing them. RLs can be efficiently used in bioremediation of contaminated soil, biodegradation, and detoxification of industrial effluents, preparation of industrial emulsions, and control of oil spills.
Despite the numerous advantages of RLs, there are still some challenges for their commercial production and application. The main issue is the high production cost. Many researchers tried to overcome this problem by optimization of culture condition and utilization of waste substrates. Due to the high cost of downstream processes (mainly RL purification), there is difficulty in obtaining pure substances. High purity compound is required and necessary in pharmaceutical, food and cosmetic applications. Another important factor for commercial production of RLs is high productivity strains of bacteria. As most of the bacteria used in the experiments display low productivity, they are not suitable for industrial purpose and economic production. In the other side, the mechanism of RL biosynthesis is not well understood. It seems that RL represents secondary metabolite regulation. So, more studies are needed to find the exact mechanism to design a liable and economic process for industrial purpose.
Conflicts of interest
There is no conflicts to declare.References
- H. Hajfarajollah, S. Mehvari, M. Habibian, B. Mokhtarani and K. A. Noghabi, RSC Adv., 2015, 5, 33089–33097 RSC.
- H. Hajfarajollah, P. Eslami, B. Mokhtarani and K. Akbari Noghabi, Biotechnol. Appl. Biochem., 2018, 65, 768–783 CrossRef CAS.
- S. Anvari, H. Hajfarajollah, B. Mokhtarani and K. A. Noghabi, RSC Adv., 2015, 5, 91836–91845 RSC.
- S. Bazsefidpar, B. Mokhtarani, R. Panahi and H. Hajfarajollah, Biodegradation, 2019, 1–11 Search PubMed.
- M. Partovi, T. B. Lotfabad, R. Roostaazad, M. Bahmaei and S. Tayyebi, World J. Microbiol. Biotechnol., 2013, 29, 1039–1047 CrossRef CAS.
- P. K. Rahman, G. Pasirayi, V. Auger and Z. Ali, Biotechnol. Appl. Biochem., 2010, 55, 45–52 CrossRef CAS PubMed.
- S. Bergstrom, H. Theorell and H. Davide, Arch. Biochem., 1946, 10, 165–166 CAS.
- S. Bergstrom, H. Theorell and H. Davide, Ark. Kemi, Mineral. Geol., 1947, 23, 1–12 Search PubMed.
- F. Jarvis and M. Johnson, J. Am. Chem. Soc., 1949, 71, 4124–4126 CrossRef CAS.
- J. R. Edwards and J. A. Hayashi, Arch. Biochem. Biophys., 1965, 111, 415–421 CrossRef CAS.
- A. M. Abdel-Mawgoud, F. Lépine and E. Déziel, Appl. Microbiol. Biotechnol., 2010, 86, 1323–1336 CrossRef CAS PubMed.
- H. Busscher, T. Neu and H. Van der Mei, Appl. Microbiol. Biotechnol., 1994, 41, 4–7 CrossRef CAS.
- K. Hayashi, Anal. Biochem., 1975, 67, 503–506 CrossRef CAS PubMed.
- S. George and K. Jayachandran, J. Appl. Microbiol., 2013, 114, 373–383 CrossRef CAS PubMed.
- T. Tugrul and E. Cansunar, World J. Microbiol. Biotechnol., 2005, 21, 851–853 CrossRef CAS.
- A. M. Elazzazy, T. Abdelmoneim and O. Almaghrabi, Saudi J. Biol. Sci., 2015, 22, 466–475 CrossRef CAS.
- M. Morikawa, Y. Hirata and T. Imanaka, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2000, 1488, 211–218 CrossRef CAS.
- M. G. Rikalovic, G. Gojgić Cvijović, M. M. Vrvic and I. Karadzic, J. Serb. Chem. Soc., 2012, 77, 27–42 CrossRef CAS.
- P. Das, S. Mukherjee and R. Sen, Bioresour. Technol., 2009, 100, 1015–1019 CrossRef CAS.
- N. M. Pinzon and L.-K. Ju, Biotechnol. Lett., 2009, 31, 1583–1588 CrossRef CAS.
- E. Déziel, F. Lépine, S. Milot and R. Villemur, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2000, 1485, 145–152 CrossRef PubMed.
- E. Déziel, F. Lépine, D. Dennie, D. Boismenu, O. A. Mamer and R. Villemur, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 1999, 1440, 244–252 CrossRef.
- T. B. Lotfabad, H. Abassi, R. Ahmadkhaniha, R. Roostaazad, F. Masoomi, H. S. Zahiri, G. Ahmadian, H. Vali and K. A. Noghabi, Colloids Surf., B, 2010, 81, 397–405 CrossRef CAS PubMed.
- H. Abbasi, M. M. Hamedi, T. B. Lotfabad, H. S. Zahiri, H. Sharafi, F. Masoomi, A. A. Moosavi-Movahedi, A. Ortiz, M. Amanlou and K. A. Noghabi, J. Biosci. Bioeng., 2012, 113, 211–219 CrossRef CAS PubMed.
- A. M. Abdel-Mawgoud, R. Hausmann, F. Lépine, M. M. Müller and E. Déziel, in Biosurfactants, Springer, 2011, pp. 13–55 Search PubMed.
- A. Tahzibi, F. Kamal and M. Mazaheri Assadi, Iran. Biomed. J., 2004, 8, 25–31 CAS.
- H. Zheng, S. Fan, W. Liu and M. Zhang, Chem. Eng. Process., 2020, 148, 107776 CrossRef CAS.
- H. Chong and Q. Li, Microb. Cell Fact., 2017, 16, 137 CrossRef.
- L. Zhu, X. Yang, C. Xue, Y. Chen, L. Qu and W. Lu, Bioresour. Technol., 2012, 117, 208–213 CrossRef CAS PubMed.
- N. Md Noh, S. Mohd Salleh and A. Yahya, Lett. Appl. Microbiol., 2014, 58, 617–623 CrossRef CAS.
- S. Joy, S. K. Khare and S. Sharma, Process Biochem., 2020, 90, 233–240 CrossRef CAS.
- A. E. LaBauve and M. J. Wargo, Curr. Protoc. Microbiol., 2012, 25, 6E Search PubMed.
- F. Zhao, H. Jiang, H. Sun, C. Liu, S. Han and Y. Zhang, RSC Adv., 2019, 9, 2885–2891 RSC.
- M. Hospinal, D. Martínez, K. Valladares, S. Gutierrez and F. Merino, World J. Microbiol. Biotechnol., 2015, 1–8 Search PubMed.
- A. Bazire, F. Diab, L. Taupin, S. Rodrigues, M. Jebbar and A. Dufour, Open Microbiol. J., 2009, 3, 128 CrossRef CAS PubMed.
- A. Schmidberger, M. Henkel, R. Hausmann and T. Schwartz, Appl. Microbiol. Biotechnol., 2014, 98, 6725–6737 CrossRef CAS.
- J. Jadhav, S. Dutta, S. Kale and A. Pratap, Prep. Biochem. Biotechnol., 2018, 48, 234–241 CrossRef CAS.
- S. Mukherjee, P. Das and R. Sen, Trends Biotechnol., 2006, 24, 509–515 CrossRef CAS.
- J. Beuker, A. Steier, A. Wittgens, F. Rosenau, M. Henkel and R. Hausmann, AMB Express, 2016, 6, 11 CrossRef PubMed.
- H. Reiling, U. Thanei-Wyss, L. Guerra-Santos, R. Hirt, O. Käppeli and A. Fiechter, Appl. Environ. Microbiol., 1986, 51, 985–989 CrossRef CAS.
- D. Davis, H. Lynch and J. Varley, Enzyme Microb. Technol., 2001, 28, 346–354 CrossRef CAS PubMed.
- M. U. H. Shah, M. Sivapragasam, M. Moniruzzaman and S. B. Yusup, Procedia Eng., 2016, 148, 494–500 CrossRef CAS.
- K. Invally, A. Sancheti and L.-K. Ju, Food Bioprod. Process., 2019, 114, 122–131 CrossRef CAS.
- N. He, T. Wu, J. Jiang, X. Long, B. Shao and Q. Meng, Colloids Surf., B, 2017, 157, 317–324 CrossRef CAS.
- T. Yamanè and S. Shimizu, in Bioprocess parameter control, Springer, 1984, pp. 147–194 Search PubMed.
- M. Salehmin, M. Annuar and Y. Chisti, Bioprocess Biosyst. Eng., 2013, 36, 1527–1543 CrossRef CAS.
- Y. Lee, S. Y. Lee and J.-W. Yang, Biosci., Biotechnol., Biochem., 1999, 63, 946–947 CrossRef CAS PubMed.
- K. M. Lee, S.-H. Hwang, S. D. Ha, J.-H. Jang, D.-J. Lim and J.-Y. Kong, Biotechnol. Bioprocess Eng., 2004, 9, 267–273 CrossRef CAS.
- S.-Y. Chen, Y.-H. Wei and J.-S. Chang, Appl. Microbiol. Biotechnol., 2007, 76, 67–74 CrossRef CAS.
- R. Kumar and A. J. Das, in Rhamnolipid Biosurfactant, Springer, 2018, pp. 43–50 Search PubMed.
- R. Lovaglio, V. Silva, H. Ferreira, R. Hausmann and J. Contiero, Biotechnol. Adv., 2015, 33, 1715–1726 CrossRef CAS.
- N. Cabrera-Valladares, A.-P. Richardson, C. Olvera, L. G. Treviño, E. Déziel, F. Lépine and G. Soberón-Chávez, Appl. Microbiol. Biotechnol., 2006, 73, 187–194 CrossRef CAS.
- M. Cha, N. Lee, M. Kim, M. Kim and S. Lee, Bioresour. Technol., 2008, 99, 2192–2199 CrossRef CAS PubMed.
- S. Wilhelm, A. Gdynia, P. Tielen, F. Rosenau and K.-E. Jaeger, J. Bacteriol., 2007, 189, 6695–6703 CrossRef CAS PubMed.
- L. F. Tavares, P. M. Silva, M. Junqueira, D. C. Mariano, F. C. Nogueira, G. B. Domont, D. M. Freire and B. C. Neves, Appl. Microbiol. Biotechnol., 2013, 97, 1909–1921 CrossRef CAS.
- Z. A. Raza, A. Rehman, M. S. Khan and Z. M. Khalid, Biodegradation, 2007, 18, 115–121 CrossRef CAS.
- S. Husain, World J. Microbiol. Biotechnol., 2008, 24, 2411 CrossRef CAS.
- C. Giani, D. Wullbrandt, R. Rothert and J. Meiwes, US Pat., US5501966A, 1997.
- L. Dobler, L. F. Vilela, R. V. Almeida and B. C. Neves, New Biotechnol., 2016, 33, 123–135 CrossRef CAS.
- I. Ivshina, M. Kuyukina, J. Philp and N. Christofi, World J. Microbiol. Biotechnol., 1998, 14, 711–717 CrossRef CAS.
- K. Das and A. K. Mukherjee, Appl. Microbiol. Biotechnol., 2005, 69, 192–199 CrossRef CAS.
- M. Kuyukina, I. Ivshina, S. Gein, T. Baeva and V. Chereshnev, Bull. Exp. Biol. Med., 2007, 144, 326–330 CrossRef CAS.
- J. D. Van Hamme, A. Singh and O. P. Ward, Biotechnol. Adv., 2006, 24, 604–620 CrossRef CAS PubMed.
- K. Clarke, F. Ballot and S. Reid, World J. Microbiol. Biotechnol., 2010, 26, 2179–2184 CrossRef CAS.
- S. Ferhat, S. Mnif, A. Badis, K. Eddouaouda, R. Alouaoui, A. Boucherit, N. Mhiri, N. Moulai-Mostefa and S. Sayadi, Int. Biodeterior. Biodegrad., 2011, 65, 1182–1188 CrossRef CAS.
- A. Flasz, C. Rocha, B. Mosquera and C. Sajo, Med. Sci. Res., 1998, 26, 181–185 CAS.
- P. Vatsa, L. Sanchez, C. Clement, F. Baillieul and S. Dorey, Int. J. Mol. Sci., 2010, 11, 5095–5108 CrossRef CAS.
- L. Magalhães and M. Nitschke, Food Control, 2013, 29, 138–142 CrossRef.
- D. H. Dusane, V. S. Pawar, Y. Nancharaiah, V. Venugopalan, A. R. Kumar and S. S. Zinjarde, Biofouling, 2011, 27, 645–654 CrossRef CAS PubMed.
- Y. Irie, G. A. O'toole and M. H. Yuk, FEMS Microbiol. Lett., 2005, 250, 237–243 CrossRef CAS PubMed.
- J. M. Campos, T. L. Montenegro Stamford, L. A. Sarubbo, J. M. de Luna, R. D. Rufino and I. M. Banat, Biotechnol. Prog., 2013, 29, 1097–1108 CrossRef CAS.
- I. Van Haesendonck and E. Vanzeveren, US Pat., US20060233935A1, 2006.
- E. Haba, S. Bouhdid, N. Torrego-Solana, A. Marqués, M. J. Espuny, M. J. García-Celma and A. Manresa, Int. J. Pharm., 2014, 476, 134–141 CrossRef CAS PubMed.
- T. L. Wood, T. Gong, L. Zhu, J. Miller, D. S. Miller, B. Yin and T. K. Wood, npj Biofilms Microbiomes, 2018, 4, 22 CrossRef.
- J. Arutchelvi, C. Joseph and M. Doble, Biochem. Eng. J., 2011, 56, 37–45 CrossRef CAS.
- S. Schooling, U. Charaf, D. Allison and P. Gilbert, Biofilms, 2004, 1, 91–99 CrossRef.
- M. E. Davey, N. C. Caiazza and G. A. O'Toole, J. Bacteriol., 2003, 185, 1027–1036 CrossRef CAS PubMed.
- S. K. Kim, Y. C. Kim, S. Lee, J. C. Kim, M. Y. Yun and I. S. Kim, J. Agric. Food Chem., 2010, 59, 934–938 CrossRef PubMed.
- R. Sha, L. Jiang, Q. Meng, G. Zhang and Z. Song, J. Basic Microbiol., 2012, 52, 458–466 CrossRef CAS.
- D. Goswami, P. J. Handique and S. Deka, J. Basic Microbiol., 2014, 54, 548–557 CrossRef CAS PubMed.
- N. Lourith and M. Kanlayavattanakul, Int. J. Cosmet. Sci., 2009, 31, 255–261 CrossRef CAS PubMed.
- E. Haba, A. Pinazo, O. Jauregui, M. Espuny, M. R. Infante and A. Manresa, Biotechnol. Bioeng., 2003, 81, 316–322 CrossRef CAS PubMed.
- A. Singh, J. D. Van Hamme and O. P. Ward, Biotechnol. Adv., 2007, 25, 99–121 CrossRef CAS.
- A. A. Juwarkar, K. V. Dubey, A. Nair and S. K. Singh, Indian J. Microbiol., 2008, 48, 142–146 CrossRef CAS.
- L. M. Santa Anna, A. U. Soriano, A. C. Gomes, E. P. Menezes, M. L. Gutarra, D. M. Freire and N. Pereira Jr, J. Chem. Technol. Biotechnol., 2007, 82, 687–691 CrossRef.
- G. Lan, Q. Fan, Y. Liu, Y. Liu, Y. Liu, X. Yin and M. Luo, Biochem. Eng. J., 2015, 103, 219–226 CrossRef CAS.
- Q. Li, C. Kang, H. Wang, C. Liu and C. Zhang, Biochem. Eng. J., 2002, 11, 197–199 CrossRef CAS.
- X. Long, G. Zhang, C. Shen, G. Sun, R. Wang, L. Yin and Q. Meng, Bioresour. Technol., 2013, 131, 1–5 CrossRef CAS PubMed.
- B. Thanomsub, W. Pumeechockchai, A. Limtrakul, P. Arunrattiyakorn, W. Petchleelaha, T. Nitoda and H. Kanzaki, Bioresour. Technol., 2006, 97, 2457–2461 CrossRef CAS.
- A. Piljac, T. Stipčević, J. Piljac-Žegarac and G. Piljac, J. Cutaneous Med. Surg., 2008, 12, 142–146 CrossRef CAS.
- T. T. Nguyen, A. Edelen, B. Neighbors and D. A. Sabatini, J. Colloid Interface Sci., 2010, 348, 498–504 CrossRef CAS PubMed.
- Y. Xie, R. Ye and H. Liu, Colloids Surf., A, 2006, 279, 175–178 CrossRef CAS.
- Y. Xie, R. Ye and H. Liu, Colloids Surf., A, 2007, 292, 189–195 CrossRef CAS.
- P. Palanisamy and A. M. Raichur, Mater. Sci. Eng., C, 2009, 29, 199–204 CrossRef CAS.
- J. Narayanan, R. Ramji, H. Sahu and P. Gautam, IET Nanobiotechnol., 2010, 4, 29–34 CrossRef CAS PubMed.
- C. B. Farias, A. Ferreira Silva, R. Diniz Rufino, J. Moura Luna, J. E. Gomes Souza and L. A. Sarubbo, Electron. J. Biotechnol., 2014, 17, 122–125 CrossRef CAS.
- G. Yi, J. Son, J. Yoo, C. Park and H. Koo, Nanomedicine, 2019, 19, 12–21 CrossRef CAS.
- H. Yin, J. Qiang, Y. Jia, J. Ye, H. Peng, H. Qin, N. Zhang and B. He, Process Biochem., 2009, 44, 302–308 CrossRef CAS.
- K. S. Reddy, M. Y. Khan, K. Archana, M. G. Reddy and B. Hameeda, Bioresour. Technol., 2016, 221, 291–299 CrossRef.
- M. Benincasa, A. Abalos, I. Oliveira and A. Manresa, Antonie van Leeuwenhoek, 2004, 85, 1–8 CrossRef CAS.
- J. Zhao, Y. Wu, A. T. Alfred, X. Xin and S. Yang, J. Chem. Pharm. Res., 2013, 5, 177–182 Search PubMed.
- J. S. Clifford, M. A. Ioannidis and R. L. Legge, J. Colloid Interface Sci., 2007, 305, 361–365 CrossRef CAS.
- E. J. Gudiña, A. I. Rodrigues, V. de Freitas, Z. Azevedo, J. A. Teixeira and L. R. Rodrigues, Bioresour. Technol., 2016, 212, 144–150 CrossRef.
- K. V. Dubey, P. N. Charde, S. U. Meshram, L. P. Shendre, V. S. Dubey and A. A. Juwarkar, Bioresour. Technol., 2012, 126, 368–374 CrossRef CAS.
- A. N. Mendes, L. A. Filgueiras, J. C. Pinto and M. Nele, J. Biomater. Nanobiotechnol., 2015, 6, 64 CrossRef.
- S. Pansiripat, O. Pornsunthorntawee, R. Rujiravanit, B. Kitiyanan, P. Somboonthanate and S. Chavadej, Biochem. Eng. J., 2010, 49, 185–191 CrossRef CAS.
- O. Pornsunthorntawee, S. Maksung, O. Huayyai, R. Rujiravanit and S. Chavadej, Bioresour. Technol., 2009, 100, 812–818 CrossRef CAS PubMed.
- N. Sakthipriya, M. Doble and J. S. Sangwai, J. Ind. Eng. Chem., 2015, 31, 100–111 CrossRef CAS.
- E. J. Silva, N. M. P. R. Silva, R. D. Rufino, J. M. Luna, R. O. Silva and L. A. Sarubbo, Colloids Surf., B, 2014, 117, 36–41 CrossRef CAS.
- C. Chayabutra, J. Wu and L. K. Ju, Biotechnol. Bioeng., 2001, 72, 25–33 CrossRef CAS.
- E. Deziel, F. Lepine, S. Milot and R. Villemur, Microbiology, 2003, 149, 2005–2013 CrossRef CAS PubMed.
- N.-G. Rosero, A.-L. Pimienta, F. Dugarte and F.-G. Carvajal, CT&F, Cienc., Tecnol. Futuro, 2003, 2, 35–42 Search PubMed.
- L. Zhang, T. A. Veres-Schalnat, A. Somogyi, J. E. Pemberton and R. M. Maier, Appl. Environ. Microbiol., 2012, 78, 8611–8622 CrossRef CAS.
- A. S. dos Santos, N. Pereira Jr and D. M. Freire, PeerJ, 2016, 4, e2078 CrossRef.
- G. Lan, Q. Fan, Y. Liu, C. Chen, G. Li, Y. Liu and X. Yin, Biochem. Eng. J., 2015, 101, 44–54 CrossRef CAS.
- K.-Y. Ma, M.-Y. Sun, W. Dong, C.-Q. He, F.-L. Chen and Y.-L. Ma, Biocatal. Agric. Biotechnol., 2016, 6, 144–151 CrossRef.
- S. Medina-Moreno, D. Jiménez-Islas, J. Gracida-Rodríguez, M. Gutiérrez-Rojas and I. Díaz-Ramírez, Int. J. Environ. Sci. Technol., 2011, 8, 471–482 CrossRef CAS.
- B. Vanavil, M. Perumalsamy and A. Seshagiri Rao, Chem. Biochem. Eng. Q., 2014, 28, 383–390 CrossRef CAS.
- T. Wu, J. Jiang, N. He, M. Jin, K. Ma and X. Long, J. Surfactants Deterg., 2019, 22, 395–402 CrossRef CAS.
- J. Wu, J. Zhang, H. Zhang, M. Gao, L. Liu and X. Zhan, Bioprocess Biosyst. Eng., 2019, 1–8 Search PubMed.
- A. K. Colak and H. Kahraman, Environ. Exp. Bot., 2013, 11, 125–130 Search PubMed.
- M. Salwa, M. N. Asshifa, A. Amirul and A. R. Yahya, Biotechnol. Bioprocess Eng., 2009, 14, 763–768 CrossRef CAS.
- Y. Zhang and R. M. Miller, Appl. Environ. Microbiol., 1992, 58, 3276–3282 CrossRef CAS PubMed.
- F. A. Kronemberger, C. P. Borges and D. M. Freire, Int. Rev. Chem. Eng., 2010, 2, 513–518 Search PubMed.
- H. Rashedi, M. Mazaheri Assadi, E. Jamshidi and B. Bonakdarpour, Iran. J. Chem. Chem. Eng., 2006, 25, 25–30 CAS.
- T. B. Lotfabad, M. Shourian, R. Roostaazad, A. R. Najafabadi, M. R. Adelzadeh and K. A. Noghabi, Colloids Surf., B, 2009, 69, 183–193 CrossRef CAS PubMed.
- R. R. Saikia, S. Deka, M. Deka and H. Sarma, J. Basic Microbiol., 2012, 52, 446–457 CrossRef CAS.
- K. Deepika, S. Kalam, P. R. Sridhar, A. R. Podile and P. Bramhachari, Biocatal. Agric. Biotechnol., 2016, 5, 38–47 CrossRef.
- A. Abalos, F. Maximo, M. A. Manresa and J. Bastida, J. Chem. Technol. Biotechnol., 2002, 77, 777–784 CrossRef CAS.
- L. Guerra-Santos, O. Käppeli and A. Fiechter, Appl. Environ. Microbiol., 1984, 48, 301–305 CrossRef CAS PubMed.
- H. Rashedi, E. Jamshidi, M. M. Assadi and B. Bonakdarpour, Iran. J. Chem. Chem. Eng., 2006, 25(1), 25–30 CAS.
- C. N. Mulligan, G. Mahmourides and B. F. Gibbs, J. Biotechnol., 1989, 12, 199–209 CrossRef CAS.
- K. Nakata, A. Yoshimoto and Y. Yamada, J. Ferment. Bioeng., 1998, 86, 608–610 CrossRef CAS.
- K. Clarke, F. Ballot and S. Reid, World J. Microbiol. Biotechnol., 2010, 26, 2179–2184 CrossRef CAS.
- J.-F. Liu, G. Wu, S.-Z. Yang and B.-Z. Mu, World J. Microbiol. Biotechnol., 2014, 30, 1473–1484 CrossRef CAS PubMed.
- S. K. Satpute, A. G. Banpurkar, P. K. Dhakephalkar, I. M. Banat and B. A. Chopade, Crit. Rev. Biotechnol., 2010, 30, 127–144 CrossRef CAS.
- A. Witek-Krowiak, J. Witek, A. Gruszczyńska, R. G. Szafran, T. Koźlecki and S. Modelski, World J. Microbiol. Biotechnol., 2011, 27, 1961–1964 CrossRef CAS.
- T. Sarachat, O. Pornsunthorntawee, S. Chavadej and R. Rujiravanit, Bioresour. Technol., 2010, 101, 324–330 CrossRef CAS PubMed.
- N. A. M. Noh, S. M. Salleh, A. A.-A. Abdullah and A. R. Mohd, J. Gen. Appl. Microbiol., 2012, 58(2), 153–161 CrossRef CAS.
- M. G. Avili, M. H. Fazaelipoor, S. A. Jafari and S. A. Ataei, Iran. J. Biotechnol., 2012, 10, 263–269 CAS.
- M. Sodagari and L. K. Ju, J. Surfactants Deterg., 2014, 17, 573–582 CrossRef CAS.
- F. Zhao, M. Mandlaa, J. Hao, X. Liang, R. Shi, S. Han and Y. Zhang, Lett. Appl. Microbiol., 2014, 59, 231–237 CrossRef CAS PubMed.
- U. A. Ochsner, A. K. Koch, A. Fiechter and J. Reiser, J. Bacteriol., 1994, 176, 2044–2054 CrossRef CAS.
- J. Bertrand, P. Bonin, M. Goutx, M. Gauthier and G. Mille, Res. Microbiol., 1994, 145, 53–55 CrossRef CAS PubMed.
- J. R. Bragg, R. C. Prince, E. J. Harner and R. M. Atlas, Nature, 1994, 368, 413 CrossRef CAS.
- Ł. Ławniczak, R. Marecik and Ł. Chrzanowski, Appl. Microbiol. Biotechnol., 2013, 97, 2327–2339 CrossRef.
- D. P. Sachdev and S. S. Cameotra, Appl. Microbiol. Biotechnol., 2013, 97, 1005–1016 CrossRef CAS PubMed.
- C. N. Mulligan, Environ. Pollut., 2005, 133, 183–198 CrossRef CAS PubMed.
- B. N. Paulino, M. G. Pessôa, M. C. R. Mano, G. Molina, I. A. Neri-Numa and G. M. Pastore, Appl. Microbiol. Biotechnol., 2016, 100, 10265–10293 CrossRef CAS PubMed.
- I. Mnif and D. Ghribi, J. Sci. Food Agric., 2016, 96, 4310–4320 CrossRef CAS PubMed.
- F. Zhao, J. Zhou, S. Han, F. Ma, Y. Zhang and J. Zhang, World J. Microbiol. Biotechnol., 2016, 32, 54 CrossRef.
- M. D. De Rienzo, P. Stevenson, R. Marchant and I. Banat, Appl. Microbiol. Biotechnol., 2016, 100, 5773–5779 CrossRef.
- X. Long, Q. Meng and G. Zhang, J. Membr. Sci., 2014, 457, 113–119 CrossRef CAS.
- M. Nitschke and S. Costa, Trends Food Sci. Technol., 2007, 18, 252–259 CrossRef CAS.
- G. S. Kiran, A. S. Ninawe, A. N. Lipton, V. Pandian and J. Selvin, Crit. Rev. Biotechnol., 2016, 36, 399–415 Search PubMed.
- T. Zheng, Y.-S. Xu, X.-Y. Yong, B. Li, D. Yin, Q.-W. Cheng, H.-R. Yuan and Y.-C. Yong, Bioresour. Technol., 2015, 197, 416–421 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |