Open Access Article
Open Access ArticleRetracted Article: Structural characterization and anticancer potency of centipede oligopeptides in human chondrosarcoma cancer: inducing apoptosis
Yuebing Ren,
Haibo Song,
Yuanpeng Wu,
Xiaochun Ma,
Xuezhong Yu,
Jia Liu,
Jianhui Sun and
Zhicheng Zhang *
*
Department of Cardiology, Dongying People's Hospital, Dongying, Shandong 257091, China. E-mail: zczhang_2019@163.com; Fax: +86-546-83311902; Tel: +86-546-83311902
First published on 13th August 2020
Abstract
Anticancer oligopeptides are rarely studied because they are often present in very low concentrations in a complex matrix. In the current study, twelve oligopeptides were isolated and the amino acid sequence identified from the centipede. MTT results indicated that Trp–Gly–His–Glu (CO-10) showed excellent anti-proliferative potency against chondrosarcoma cells in vitro. Further study showed that CO-10 induced SW1353 cells apoptosis and blocked cell cycle in the G0/G1 phase. Further, results demonstrate that the apoptotic and cytotoxic effects of CO-10 are mediated by the intrinsic mitochondria-mediated apoptotic pathway, which in turn causes the release of cytochrome c and the activation of caspases. This study will be important for the development of pharmaceutical anticancer peptides from natural products as anticancer agents against chondrosarcoma.
1 Introduction
Chondrosarcoma is one of the most common malignancies and is the second most common primary malignancy of bone after osteosarcoma. It's usually due to overproduction of chondrocytes and cartilage matrix by a heterogeneous group of neoplasms. The majority of the chondrosarcoma is mild and slow growing, however it still constitutes a significant morbidity risk.1,2 The most effective treatment for the chondrosarcoma is surgical excision, traditional chemotherapy and radiotherapy. However, the properties of the extra-cellular matrix, preferential location deeper in the pelvis, proximal femur, proximal humerus, distal femur or the ribs, low percentage of dividing cells and poor vascularity make it difficult to cure.3,4 Therefore, novel methods and agents for chondrosarcoma treatment are significantly required.Pharmaceutical peptides have been recognized for decades with their special biological properties of inhibiting tumor angiogenesis, tumor growth and metastasis, and anti-tumor peptides become a hot spot in cancer treatment.5 Researchers have screened dozens of anti-tumor peptides and proved that they can inhibit growth or occurrence of the tumors significantly.5 For example, tyroserleutide can increase the concentration of Ca2+ in human hepatoma BEL-7402 cells, induce calcium overload and reduce the mitochondrial transmembrane potential, but has no effect on normal liver cells.6 Tyroservaltide can induce necrosis and apoptosis of cancer cells by altering the ultrastructure, down-regulating the expression of proliferating cell nuclear antigen (PCNA).7 Thymopentin can promote the reconstruction of immune function, inhibit the proliferation, spread and metastasis of tumor, reduce the side effects and improve the quality of life.8 Further, both the herb and medicinal animals provide a significant resource, which can be exploited for potential anticancer agents.
The centipede is widely used in traditional Chinese and Korean medicine against sorts of diseases, including cancer,9 stroke-induced hemiplegia,10 apoplexy, and epilepsy.11 Studies reported that water-soluble centipede extract can significantly induce apoptosis and improve the immune activity of tumor-bearing mice.12 However, to date, no work focused on anti-tumor capability of centipede oligopeptides, and the underlie mechanism is seldom explored. To illustrate the anticancer potency of the centipede oligopeptides and expand their clinical application, in this study, a series of centipede oligopeptides were isolated and amino acid sequence identified. Further, the anti-proliferative activity and the potential anticancer mechanism was also explored.
2 Materials and methods
2.1 Materials and cell culture
The centipede (Scolopendra subspinipes mutilans L. Koch) was obtained from Ertiantang Pharmacy (Guangzhou, China) and identified by Professor Wang (Jinan University, Guangzhou, China). The reagents PI and JC-1 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). PierceTM BCA Protein Assay Kit was obtained from Thermo Fisher Scientific (Rockford, IL, USA). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT), TUNEL apoptosis detection kit, dithiothreitol (DTT), nuclear and cytoplasmic extraction kit, RIPA buffer and RNase were purchased from Beyotime (Shanghai, China). All the antibodies were purchased from cell signaling technology (CST, Beverly, MA, USA). All other chemicals were obtained from Sigma-Aldrich (Sigma-Aldrich, USA) without any further purification.All the cell lines (A549, HepG2, MCF-7, SW1353, Caco-2) were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). All the cancer cell lines were grown in specific media supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (Invitrogen, Carlsbad, CA, USA). The cells were grown in a 5% CO2 humidified atmosphere in incubators maintained at 37 °C.
2.2 Isolation and identification of the oligopeptides
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (w/v) and stirred for 4 h. The iso-propanol was replaced every 0.5 h. The supernatant was removed, and the sediment was freeze-dried and stored at −20 °C.13
8 (w/v) and stirred for 4 h. The iso-propanol was replaced every 0.5 h. The supernatant was removed, and the sediment was freeze-dried and stored at −20 °C.13The defatted precipitate (100 g) was dissolved (10%, w/v) in 0.5 M phosphate buffer solution (PBS, pH 7.3) then a KQ-250B ultrasonic cleaner (Shanghai, China) was used to ultrasound for 1 h. After centrifugation (9000 × g, 20 min), the supernatant was collected as total protein and was fractionated by salting-out with increasing concentrations of (NH4)2SO4, and the resulting supernatant was freeze-dried and stored at −20 °C.
Hydrophobic chromatography. The CO-α was dissolved in 1.4 M (NH4)2SO4 prepared with 30 mM phosphate buffer (pH 7.3) and loaded onto a Phenyl Sepharose CL-4B hydrophobic chromatography column (2.2 cm × 100 cm) which had previously been equilibrated with the above buffer. A stepwise elution was carried out with decreasing concentrations of (NH4)2SO4 (1.4, 0.7 and 0 M) dissolved in 30 mM phosphate buffer (pH 7.3) at a flow rate of 2.0 mL min−1. Each fraction was collected at a volume of 75 mL and was monitored at 300 nm. Fractions were then lyophilized and anticancer activity was evaluated. The fraction performed the strongest anticancer activity was collected and prepared for anion-exchange chromatography.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v) and acetonitrile–trifluoroacetic acid (solvent B; 100
0.1, v/v) and acetonitrile–trifluoroacetic acid (solvent B; 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v). The peptide was separated using a gradient elution from 30% to 70% of solvent B for 60 min at a flow rate of 1.0 mL min−1. Detection wavelength was set at 300 nm.
0.1, v/v). The peptide was separated using a gradient elution from 30% to 70% of solvent B for 60 min at a flow rate of 1.0 mL min−1. Detection wavelength was set at 300 nm.HPLC-ESI-MS was evaluated on a SCIEX X500R Q-TOF mass spectrometer (Framingham, U.S.A.). And the MS conditions were as follows: ESI-MS analysis was performed using a SCIEX X500R Q-TOF mass spectrometer equipped with an ESI source. The mass range was set at m/z 100–1000. The Q-TOF MS data were acquired in positive mode and conditions of MS analysis were as follows: CAD gas flow-rate, 7 L min−1; drying gas temperature, 550 °C; ion spray voltage, 5500 V; Declustering potential, 80 V. Software generated data file: SCIEX OS 1.0.
2.3 MTT and cell apoptosis assays
The MTT assay is used to assess cell viability.13 After the CO-10 treatments for 48 h, cells were washed with PBS. MTT (0.5 mg mL−1) was added to each well and the mixture was incubated at 37 °C for half an hour. The absorbance of each well was determined at 550 nm with a microplate reader (Bio-Tek, USA).The cell apoptosis was evaluated by Annexin V and PI staining according to the manufacturer's protocol. Briefly, 1 mL of 2 × 105 cells per mL was incubated with CO-10 at 0, 12.4, 24.7 and 49.4 μM for 48 h. The cells were collected and suspended in 100 μL Annexin V binding buffer and 5 μL each of Annexin V and PI were added, then, incubated for 30 min at room temperature and detected by flow cytometry (BD FACS Calibur, Franklin Lakes, CA, USA). All the tests were repeated at least 3 times.
2.4 Cell cycle analysis
The cell cycle distribution was detected by PI (propidium iodide) staining through flow cytometry. Briefly, the SW1353 cells were exposed to CO-10 at 0, 12.4, 24.7 and 49.4 μM for 48 h, respectively. After that, cells were harvested and fixed in 70% ethanol and stored at −20 °C overnight. The cells were washed with PBS and stained with PI and analyzed by flow cytometry (BD FACS Calibur, Franklin Lakes, CA, USA). All the tests were repeated at least 3 times.2.5 Determinations of ROS and mitochondrial transmembrane potential (MTP)
Dichlorodihydrofluorescein diacetate (DCFH-DA) could pass through the cell membrane to produce DCFH by hydrolysis of the intracellular esterase. The ROS can oxidize DCFH to produce fluorescent DCF. So, the DCF fluorescence detection could tell the ROS level.13 The levels of intracellular ROS were explored by the fluorogenic probe, dichlorodihydrofluorescein (CM-H2DCFDA). Cells were plated at a density of 2 × 105 cells per mL and were exposed to CO-10 at 0, 12.4, 24.7 and 49.4 μM for 48 h, or 24.7 μM for 2, 4, 8, 12, 24 and 48 h, respectively. Afterwards, the cells were stained with CM-H2DCFDA for 20 min at 37 °C, and the fluorescence intensity was determined by flow cytometry and fluorescence microscopy.The MTP collapse induced by CO-10 was detected by JC-1 staining according to manufacturer's protocol (Beyotime, Suzhou). Briefly, 2 mL of 1 × 106 mL−1 cells were treated with CO-10 at 0, 12.4, 24.7 and 49.4 μM for 48 h, or 24.7 μM for 2, 4, 8, 12, 24 and 48 h, respectively. Then, cells were collected, washed triple with cold PBS and incubated with 1 μg mL−1 of JC-1 at 37 °C for 20 min without light. Remove the supernatant and wash the cells with cold PBS before the flow cytometry analysis (BD FACS Calibur, Franklin Lakes, CA, USA). All the tests were repeated at least 3 times.
2.6 Western blot analysis
Cells were plated in 6-well culture dishes, grown to confluence, and treated with CO-10 for 48 h as experimental design. After incubation, cells were washed with ice-cold PBS, scraped, pelleted and lysed in a radioimmunoprecipitation assay (RIPA) buffer (protease inhibitor cocktail and phosphatase inhibitor). After incubation for 1 h on ice, cell lysates were centrifuged at 3000 g for half an hour at 4 °C. Lysate protein concentrations were determined by a BCA protein assay kit (Thermo Scientific, USA) and the lysates were adjusted with a lysis buffer. Proteins were resolved on a 10–15% SDS-PAGE and transferred to immobilon polyvinyldifluoride (PVDF) membranes. The blots were blocked with blocking buffer for 10 min at r.t. and probed with the antibodies for 1 h at room temperature. Then, the blots were incubated with a peroxidase conjugated secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 dilution) for 1 h at room temperature.14 Protein bands were visualized using enhanced chemiluminescence detection reagents (Bio-Rad, USA).
3000 dilution) for 1 h at room temperature.14 Protein bands were visualized using enhanced chemiluminescence detection reagents (Bio-Rad, USA).
2.7 Statistical analysis
Data analysis was performed with Graphpad Prism 5 (San Diego, USA). Statistical comparison between control and treatments were performed by the Student's t-test. All the experiments were performed at least three times. Data are presented as mean ± SD. p < 0.05 is considered statistically significant.3 Results and discussion
3.1 Isolation and identification of centipede oligopeptides
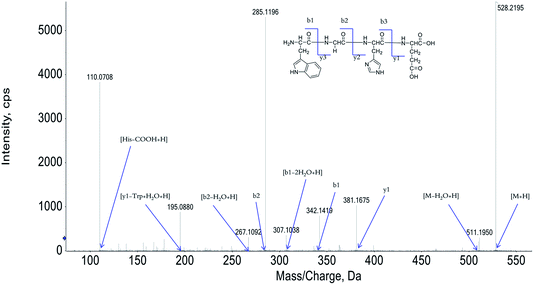
The peptide is usually protonated under ESI-MS/MS conditions, and fragmentations mostly occur at the amide bonds because it is difficult to break the chemical bonds of the side chains at such low energy. Therefore, the b and y ions are the main fragment ions when the collision energy is <200 eV.13 We have successfully isolated twelve centipede oligopeptides. Their structures were identified by HPLC-ESI-MS analysis. The centipede oligopeptide-10 (CO-10) was analyzed by HPLC-ESI-MS for molecular mass determination and peptide characterization (Fig. 1). The molecular mass of the peptide was determined to be 528.2195 Da. The ion fragment m/z 511.1950 was regarded as the [M–H2O + H]+ ion, while m/z 381.1675 was regarded as the y1 ion, m/z 342.1419 was regarded as the b1 ion, and m/z 307.1038 was regarded as the [b1–2H2O + H]+ ion. The ion (m/z 285.1186) was the b2 ion, m/z 267.1092 was regarded as the [b2–H2O + H]+ ion, and m/z 195.0880 was regarded as the [y1–Trp + H2O + H]+ ion. m/z 110.0708 was the typical fragment [His–COOH + H]+. On the basis of this, we concluded that the sequence of the peptide was Trp–Gly–His–Glu. The rest of the COs were identified, and the amino acid sequences are list in Table 1.| COs | Amino acid sequences | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| A549 | HepG2 | MCF-7 | SW1353 | Caco-2 | ||
| a IC50 values are shown as mean ± standard error of the mean (SD), from at least three independent experiments. | ||||||
| CO-1 | GEYHSHE | 165 ± 16.6 | 131 ± 10.2 | >200 | 82.1 ± 9.23 | 117 ± 12.5 |
| CO-2 | RHSHSWD | 142 ± 13.4 | 96.7 ± 7.82 | 122 ± 11.4 | 92.5 ± 9.09 | 84.6 ± 9.07 |
| CO-3 | EAHGHSF | >200 | 70.2 ± 7.34 | 86.6 ± 9.23 | >200 | >200 |
| CO-4 | KYAVHS | 123 ± 11.7 | 98.2 ± 8.53 | >200 | 82.3 ± 8.16 | 91.7 ± 10.5 |
| CO-5 | DSHTS | >200 | 105 ± 10.1 | >200 | >200 | >200 |
| CO-6 | SHAYSH | 171 ± 17.7 | 115 ± 8.16 | 81.1 ± 8.18 | 70.8 ± 7.27 | 86.5 ± 8.23 |
| CO-7 | GEFTRS | 71.3 ± 6.02 | 136 ± 9.21 | 132 ± 12.9 | 76.3 ± 8.12 | 93.8 ± 9.02 |
| CO-8 | FWHG | >200 | 112 ± 11.1 | 136 ± 13.5 | 101 ± 10.7 | > 200 |
| CO-9 | YEHSHG | >200 | 177 ± 18.8 | 125 ± 13.4 | >200 | >200 |
| CO-10 | WGHE | 56.3 ± 4.37 | 65.2 ± 6.32 | 51.7 ± 5.65 | 24.7 ± 2.20 | 56.3 ± 5.69 |
| CO-11 | DRHSFL | 74.3 ± 7.58 | 99.1 ± 7.50 | >200 | 74.3 ± 7.04 | 82.6 ± 9.10 |
| CO-12 | TYKRHS | 91.4 ± 6.21 | 93.7 ± 8.72 | 103 ± 11.2 | 104 ± 10.5 | >200 |
3.2 COs inhibit the growth of cancer cells
It's the common sense that lung cancer, liver cancer, breast cancer, chondrosarcoma and colorectal adenocarcinoma are very popular cancer types, this is the reason why human lung cancer A549, human liver HepG2, human breast MCF-7, chondrosarcoma cell SW1353 and human colorectal adenocarcinoma Caco-2 cancer cell lines were chosen as materials in the current study. After the treatments of COs, the MTT assay was used to detect the anti-proliferative activity of COs against the experimental cancer cells and the results were list in Table 1.Of interest, most of the COs performed anti-proliferative potency in the experimental cancer cells. However, more excitingly, the CO-10 exhibited significant inhibitory activity against the tested cancer cell lines, and showed the best anti-proliferative effect in the SW1353 cell, with an IC50 of 24.7 ± 2.20 μM. These results indicate that the COs show a broad range of growth inhibition effects in the tested cancer cell lines and the CO-10 exhibited significant anti-proliferative activity in SW1353 cells. So, further study of mechanism was performed on CO-10 in SW1353 cells.
The current results support that the CO-10 is probably a promising anti chondrosarcoma therapy. Therefore, the cytotoxicity in normal cells from healthy tissues must be evaluated. The testing method is same as that applied to cancer cell lines. It was found that the cytotoxicity of CO-10 in normal cells is far less than that in cancer cell lines. The IC50 value is 3.04 ± 0.25 mM (for 48 h) against human liver cell line HL-7702; while the IC50 values are 3.29 ± 0.36 mM (for 48 h), 4.01 ± 0.43 mM (for 48 h) against mouse embryo fibroblast cell line NIH/3T3 and primary human articular chondrocytes. Compared the IC50 value in a same incubation time (48 h), the cytotoxicity in primary human articular chondrocytes cells is much less than that in SW1353 cells. Definitely, CO-10 significantly showed attenuated cytotoxicity in normal cells. Hence, we speculated that CO-10 deserves a potentiality for a further research.
3.3 CO-10 induces apoptosis in human chondrosarcoma cells
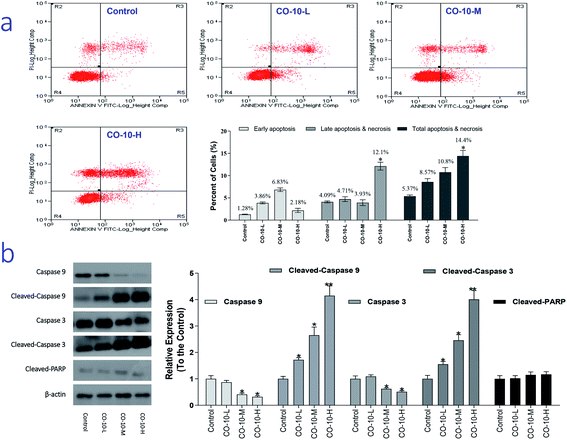
It is recognized that chondrosarcoma is one of the most common cancers in clinical. Severe adverse effects are commonly observed in chondrosarcoma patients.15 So, development of novel improved chemotherapy is an urgent work. As we know, necrosis is a progress of traumatic cell death that results from acute injury; while apoptosis, the process of programmed cell death, is an important therapy target and different from the normal cells with certain distinct morphological features, such as cytoplasmic shrinkage, membrane blebbing and chromatin condensation.16 Interestingly, CO-10 is active against SW1353 cell lines. Therefore, we chose SW1353 cells to explore the mechanism involved in CO-10 induce apoptosis. In order to investigate how the CO-10 induce the SW1353 cells apoptosis, flow cytometry with propidium iodide (PI) and Annexin-V in SW1353 cells was performed. After incubated with the CO-10 for 48 h, the cells were labeled with the two dyes and the resulting mixture was monitored.As shown in Fig. 2a, at the dose of 12.4 μM, CO-10 induced 8.57% total apoptosis/necrosis and 3.86% early apoptosis, where 2.89% of cell death caused by necrosis and 4.71% by late apoptosis; At the dose of 24.7 μM, CO-10 caused 10.8% total apoptosis/necrosis and 6.83% early apoptosis, where 2.13% by necrosis and 3.93% by late apoptosis. Interestingly, at 49.4 μM, the CO-10 had induced a higher total apoptosis/necrosis rate (14.4%), particularly, the late apoptosis (12.1%) also had a big contribution to cell death.
Caspases are a family of cysteine protease enzymes that play an essential role in programmed cell death. The proteolytic cascade of caspases mediates cell apoptosis.17 We therefore examined the involvement of caspases in CO-10-induced apoptosis. In cells treated with CO-10, the levels of cleaved-PARP, cleaved-caspase-3 and cleaved-caspase-9 significantly increased (Fig. 2b). Meanwhile, caspase-3 and caspase-9 activities declined significantly, as shown that treatment with CO-10 regulated caspase-3 and caspase-9 activity in chondrosarcoma cells. These results demonstrate that CO-10 induces apoptosis in chondrosarcoma cells may via caspase dependent pathways.
3.4 CO-10 induces cell cycle arresting
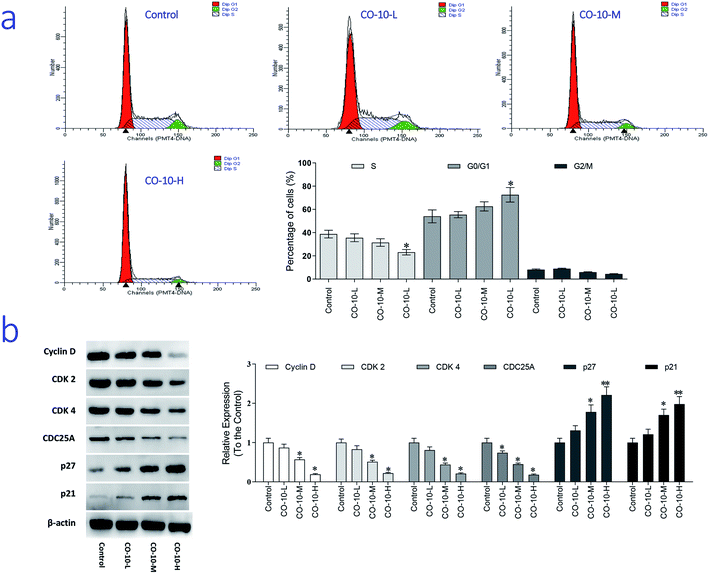
The main property of tumor cells is uncontrollable and the cell cycle arresting becomes a pivotal therapy target in remedies. To establish whether CO-10 inhibited cell growth by blocking the cell cycle, cellular DNA was analyzed and the profiles were list in Fig. 3a.Compared to the control group, CO-10 clearly blocked the SW1353 cells in G0/G1 phase, treatment with CO-10 at 12.4, 24.7 and 49.4 μM induced the accumulations of 55.4%, 62.5% and 72.5% at G0/G1 phase, respectively. Usually, G0/G1 is the prophase of DNA synthesis. If DNA can't be replicated, cell cycle will be stopped and cells can't transform to S phase and M phase, slow growth and proliferation activity decline would happen.18 Based on the results, it was clearly that CO-10 inhibited the DNA replication and blocked the cell cycle in G0/G1 phase. This fact suggests that the cell cycle arresting is also the primary mechanism responsible for the anticancer activities of CO-10.
To further investigate the molecular basis by which CO-10 inhibited the G0/G1 transition in tumor cells, we treated cells with CO-10 and then analyzed the expression of proteins involved in cell cycle regulation. We found that CO-10 treatment inhibited cyclin D expression and reduced the expression of CDK2 and CDK4; in contrast, p21 and p27 were increased in HepG2 cells (Fig. 3b). The expression level of cell division cycle 25A (CDC25A), which acts as an upstream regulator of the CDK/cyclin complex, was significantly inhibited by CO-10.
3.5 CO-10 increased the ROS generation and MTP dysfunction in human chondrosarcoma cells
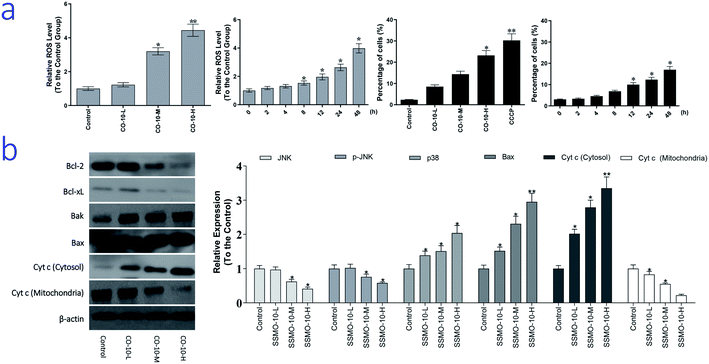
As we know, ROS is generated as a by-product of normal mitochondrial activity. If out of controlled, severe damages to cellular macromolecules, especially the DNA, and oxidative stress would be caused by the ROS. While, oxidative stress refers to elevated intracellular ROS level that cause damages to DNA, and subsequently induce apoptosis.19 To examine the intracellular ROS level, we checked it by DCFH-DA method.The normal group was regarded as the control group and the relative expression of ROS was regarded as 1, and the results were list in Fig. 4a. Compared with the control group, CO-10 induced the generation of ROS in a dose dependent manner. After the 12.4, 24.7 and 49.4 μM CO-10 treatments for 48 h, the ROS levels were up-regulated by 22.4%, 220% and 344%, respectively. Further, CO-10 also up-regulated the ROS level in a time dependent manner.
As reported previously, oxidative stress is considered to be an important pathogenic mechanism. Oxidative stress is an imbalance between free radical generation and antioxidant defenses. Oxidative stresses refer to elevate the levels of intracellular ROS that cause damages to lipids, proteins and DNA and other macromolecules that can regulate initiation of apoptotic signaling.19 ROS level in chondrosarcoma cells after CO-10 treatment was evaluated. The results showed that treatment of chondrosarcoma cells with CO-10 induced the accumulation of ROS clearly and also demonstrated that ROS is a crucial factor in the induction of apoptosis and act upstream signaling molecules to initiate cell apoptosis.
The apoptosis contains two major apoptotic pathways, the intrinsic pathway (mitochondrial-mediated) and the extrinsic pathway (death receptor-mediated).20 The extrinsic or death receptor pathway activates the Fas ligand (FasL) receptor and recruits Fas-Associated protein with Death Domain (FADD) and caspase-8.21 The intrinsic mitochondrial pathway may be activated by multiple stimuli that converge at the mitochondrion and induce mitochondrial dysfunction and subsequently induce the release of pro-apoptotic mitochondrial proteins into cytosol. The pro-apoptotic proteins include proteins that can activate the caspases dependent pathway. Release of cytochrome c (cyt c) activates Apaf-1, which subsequently activates a downstream caspase program. Bcl-2 prevents the release of cyt c into the cytoplasm and thus blocks cyt c from promoting Apaf-1 mediated caspase-9 activation, leading some to hypothesize that Bcl-2 and its homologues function to keep mitochondrial membranes intact.22
Here, we hypothesized that mitochondrial initiated ROS activation mediates CO-10 inducing cell death. Disruption of MTP results in release of cyt c from mitochondria into the cytoplasm. In our experiment, the loss of MTP and increased cyt c release (Fig. 4b) confirmed our hypothesis.
ROS induce cell apoptosis by regulating the proapoptotic Bcl-2 family proteins, such as Bcl-2 associated X protein (Bax) and Bcl-2-antagonistic/killer (Bak), resulting in increased mitochondrial membrane permeabilization and cyt c released into the cytosol.23 We observed a decrease in expression of Bcl-2 and Bcl-xL, as well as an increase in expression of Bax and Bak, in chondrosarcoma cells after treatment CO-10 (Fig. 4b). MTP was detected with the mitochondria sensitive fluorescent dye, JC-1, by flow cytometry. CO-10 treatments marked changes in MTP. Furthermore, CO-10 could induce depolarization of the inner mitochondrial membrane in a dose and time-dependent manner (Fig. 4a). Therefore, we inferred that the mitochondrial dysfunction is also involved in CO-10-mediated cell apoptosis.
The mitochondrial pathway of apoptosis involves signaling by mitochondrial related apoptotic proteins, including B-cell lymphoma 2 (Bcl-2), Bcl-x L, Bak, Bax and the mitochondrial release of cyt c into the cytosol.23 CO-10 treatments increased Bax and Bak expressions and reduced Bcl-xL and Bcl-2 expressions (Fig. 4b), which increased the ratio of pro-apoptotic/anti-apoptotic Bcl-2.
Further, CO-10 enhanced cytosolic cyt c expression and reduced mitochondrial cyt c expression (Fig. 4b). These data suggest that CO-10 induced cell apoptosis may through the mitochondrial dysfunction in human chondrosarcoma cells.
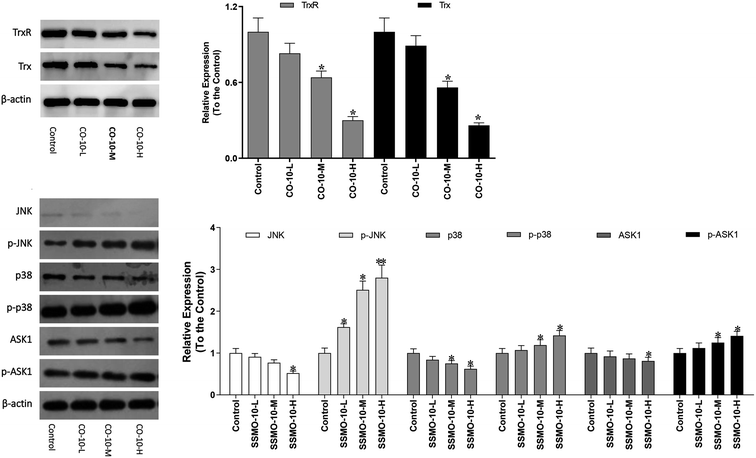
3.6 CO-10 activates ASK1 and MAPK pathways
The Trx system is an important antioxidant system of keeping the intracellular redox state balance. Studied reported that Trx/TrxR is often over-expressed in tumor cells.24 Hence, the effect of CO-10 in Trx and TrxR expressions were detected in SW1353 cells.As shown in Fig. 5, the both the Trx and TrxR expressions were down-regulated. The ASK1/MAPK is involved in many cellular and immune responses, such as cell cycle regulation and apoptosis. ASK1 is activated by various types of stress, including the accumulation of ROS. The reduced type of Trx is a significant inhibitor of ASK1. When Trx is oxidized, it dissociates from ASK1, which is then activated by the auto-phosphorylation of the kinase domain, leading to the downstream activation of the JNK and p38 MAPK pathways.25,26 In order to detect whether the CO-10 increase the cellular ROS levels by inhibiting the Trx system and activating ASK1 and MAPKs, we detect their expressions.
As shown in Fig. 5, CO-10 activated the endogenous ASK1 in a dose-dependent manner in SW1353 cells. Next, we investigated how CO-10 affected the phosphorylation statuses of the two MAPKs (p38, JNK). As shown in Fig. 5b, p38 and JNK were dose-dependently activated after the CO-10 treatments, which indicated that CO-10 may induce the Δψm collapse by activating ASK1 and MAPK pathways.
4 Conclusion
In this study, we showed that CO-10 effectively inhibited the growth of human chondrosarcoma SW1353 cells. Further, CO-10 induced cell apoptosis with mitochondria dysfunction and caspases, ASK1-MAPKs pathway activation.Conflicts of interest
The authors declare that they have no conflicts of interest concerning.Acknowledgements
We thank Professor Chen (Jinan University, Guangzhou, China) for technical assistance as well as critical editing of the manuscript.References
- C. Y. Lin, S. W. Wang and Y. L. Chen, Brain-derived neurotrophic factor promotes VEGF-C-dependent lymphangiogenesis by suppressing miR-624-3p in human chondrosarcoma cells, Cell Death Dis., 2017, 8(8), e2964 CrossRef CAS PubMed.
- F. Y. Lee, et al., Chondrosarcoma of Bone: An Assessment of Outcome, J. Bone Jt. Surg., Am. Vol., 1999, 81(3), 326–338 CrossRef CAS PubMed.
- H. Gelderblom, et al., The Clinical Approach Towards Chondrosarcoma, Oncologist, 2008, 13(3), 320–329 CrossRef PubMed.
- C. Lin, et al., Hypoxia induces HIF-1Œ± and VEGF expression in chondrosarcoma cells and chondrocytes, J. Orthop. Res., 2004, 22(6), 1175–1181 CrossRef CAS PubMed.
- V. P. Torchilin, Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery, Pept. Sci., 2010, 90(5), 604–610 CrossRef PubMed.
- R. Lu, et al., Experimental study of the inhibition of human hepatocarcinoma Bel7402 cells by the tripeptide tyroserleutide (YSL), Cancer Chemother. Pharmacol., 2006, 57(2), 248–256 CrossRef CAS PubMed.
- J. Jia, et al., Preliminary investigation of the inhibitory effects of the tyroservaltide (YSV) tripeptide on human hepatocarcinoma BEL-7402, Cancer Biol. Ther., 2005, 4(9), 993–997 CrossRef CAS PubMed.
- Z. Q. Wang, et al., Effect of synthetic thymopentin on immune function in chick, Chin. Vet. Sci., 2009, 39(6), 555–560 Search PubMed.
- W. Ma, et al., Potential roles of Centipede Scolopendra extracts as a strategy against EGFR-dependent cancers, Am. J. Transl. Res., 2015, 7(1), 39–52 CAS.
- C. E. Levy, et al., Functional MRI Evidence of Cortical Reorganization in Upper-Limb Stroke Hemiplegia Treated with Constraint-Induced Movement Therapy, J. Phys. Med. Rehabil., 2001, 80(1), 4–12 CrossRef CAS PubMed.
- L. Chen, et al., Influences of “Spasmolytic Powder” on Pgp Expression of CoriariaLactone-Kindling Drug-Resistant Epileptic Rat Model, J. Mol. Neurosci., 2013, 51(1), 1–8 CrossRef CAS PubMed.
- X. L. Xu, et al., Effects of Centipede Extracts on Normal Mouse and S180, H22 Bearing Mouse, Zhongyaocai, 2010, 33(4), 499–503 Search PubMed.
- H. Cao, et al., Structural characterization of peptides from Locusta migratoria manilensis (Meyen, 1835) and anti-aging effect in Caenorhabditis elegans, RSC Adv., 2019, 9(16), 9289–9300 RSC.
- J. Liu, et al., Synthesis of xanthone derivatives and studies on the inhibition against cancer cells growth and synergistic combinations of them, Eur. J. Med. Chem., 2017, 133, 50–61 CrossRef CAS PubMed.
- X. Lai and S. Chen, Identification of novel biomarker candidates for immunohistochemical diagnosis to distinguish low-grade chondrosarcoma from enchondroma, Proteomics, 2015, 15(13), 2358–2368 CrossRef CAS PubMed.
- M. Ankarcrona, Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function, Neuron, 1995, 15(4), 961–973 CrossRef CAS PubMed.
- Y. Ibuki and R. Goto, Role of Redox Controls of Caspase Activities in Regulation of Cell Death, Curr. Enzyme Inhib., 2005, 1(3), 281–285 CrossRef.
- K. Sankaranarayanan, et al., Ionizing radiation and genetic risks. XVII. Formation mechanisms underlying naturally occurring DNA deletions in the human genome and their potential relevance for bridging the gap between induced DNA double-strand breaks and deletions in irradiated germ c, Mutat. Res., Rev. Mutat. Res., 2013, 753(2), 114–130 CrossRef CAS PubMed.
- M. Schieber and N. S. Chandel, ROS Function in Redox Signaling and Oxidative Stress, Curr. Biol., 2014, 24(10), R453–R462 CrossRef CAS PubMed.
- M. Mittelbronn, S. Fulda and D. Kogel, Therapeutic Exploitation of Apoptosis and Autophagy for Glioblastoma, Anti-Cancer Agents Med. Chem., 2010, 10(6), 438–449 CrossRef PubMed.
- H.-Y. Ryu and, Environmental Chemical-Induced Bone Marrow B Cell Apoptosis: Death Receptor-Independent Activation of a Caspase-3 to Caspase-8 Pathway, Mol. Pharmacol., 2005, 68(4), 1087–1096 CrossRef PubMed.
- Z. Hua, et al., An APAF-1 Cytochrome c Multimeric Complex is a Functional Apoptosome That Activates Procaspase-9, J. Biol. Chem., 1999, 274(17), 11549–11556 CrossRef PubMed.
- Y. A. Mekori, et al., Human Mast Cell Apoptosis Is Regulated Through Bcl-2 and Bcl-XL, J. Clin. Immunol., 2001, 21(3), 171–174 CrossRef CAS PubMed.
- J. Liu, et al., Inhibition of thioredoxin reductase by auranofin induces apoptosis in adriamycin-resistant human K562 chronic myeloid leukemia cells, Pharmazie, 2011, 66(6), 440–444 CAS.
- A. Matsuzawa and H. Ichijo, Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling, Biochim. Biophys. Acta, Gen. Subj., 2008, 1780(11), 1325–1336 CrossRef CAS PubMed.
- R. Jin, et al., Trx1/TrxR1 system regulates post-selected DP thymocytes survival by modulating ASK1-JNK/p38 MAPK activities, Immunol. Cell Biol., 2015, 93(8), 744–752 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |