Open Access Article
Open Access ArticleTransition metal- and catalyst-free one-pot green method for the synthesis of N-sulfonyl amidines via direct reaction of sulfonyl azides with amines†
Babak Kaboudin *a,
Saeed Torabia,
Foad Kazemi
*a,
Saeed Torabia,
Foad Kazemi a and
Hiroshi Aoyamab
a and
Hiroshi Aoyamab
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Gava Zang, Zanjan 45137-66731, Iran. E-mail: kaboudin@gmail.com; Tel: +98 24 3315 3220
bSchool of Pharmacy, Tokyo University of Pharmacy and Life Sciences, Horinouchi, Hachioji, Tokyo 192-0392, Japan
First published on 17th July 2020
Abstract
In this report, a green synthesis of N-sulfonyl amidines via the direct reaction of tertiary or secondary amines with sulfonyl azides is described. Transition metal- and catalyst-free conditions were used for the synthesis of biologically important N-sulfonyl amidines. Further studies showed that the reaction proceeded via in situ aerobic oxidation of amines under reflux conditions.
Introduction
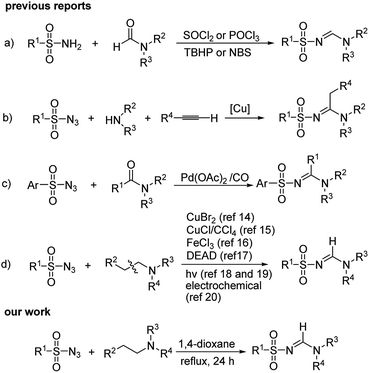
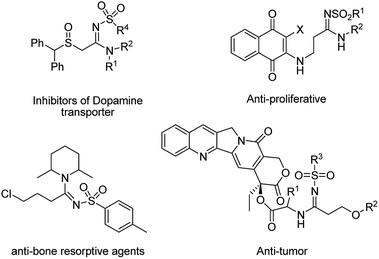
Amidines with N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds are known compounds in nature with an important role in pharmaceuticals and strong coordination affinity toward metal ions.1–4 Among the amidine compounds, N-sulfonyl amidines are of interest as they are effective in medicinal chemistry and synthesis of synthetic intermediates for drugs.5–12 A number of N-sulfonyl amidines with medicinal and biochemical applications are shown in Scheme 1. These compounds are bioactive pharmacophores with inhibitors of dopamine transporter,6 anti tumour,7 anti-bone resorptive agent8 and anti-proliferative9 properties (Fig. 1).
N bonds are known compounds in nature with an important role in pharmaceuticals and strong coordination affinity toward metal ions.1–4 Among the amidine compounds, N-sulfonyl amidines are of interest as they are effective in medicinal chemistry and synthesis of synthetic intermediates for drugs.5–12 A number of N-sulfonyl amidines with medicinal and biochemical applications are shown in Scheme 1. These compounds are bioactive pharmacophores with inhibitors of dopamine transporter,6 anti tumour,7 anti-bone resorptive agent8 and anti-proliferative9 properties (Fig. 1).
In the past years, various methods have been developed for the synthesis of N-sulfonyl amidines.13–21 These include: (a) condensation of amides with sulfonylamides,13,14 (b) Cu-catalyzed three-component coupling reactions of sulfonyl azides, alkynes and secondary amines,15 (c) Pd-catalyzed carbonylation/coupling of sulfonylazides and substituted amides,16 and (d) cross-coupling reaction of sulfonyl azides with tertiary amines17–21 (Scheme 1). Among them, cross-coupling of sulfonyl azides with tertiary amines has attracted much more attention. The key intermediate is enamine generated by oxidative dehydrogenation of amines in the presence of CuBr2,17 CuCl/CCl4,18 or FeCl3.19 On the other hand, Li and co-workers reported the cascade reaction of tertiary amines with sulfonyl azides in the presence of DEAD (diethyl azodicarboxylate) for the preparation of N-sulfonyl amidines.20
Recently, photocatalyzed cross-coupling of arylsulfonyl azides with tertiary amines in the presence of eosin Y and acridinium salts have been reported by Zeng et al.21 and Pan et al.22 respectively. An electrochemical synthesis of N-sulfonyl amidines from aliphatic amines and sulfonyl azides has been reported by Wang, Du and Zah.23 Syntheses of N-sulfonyl amidines based on the reaction between the sulfonyl azides and amines require harsh reaction conditions and stoichiometric or catalytic amounts of transition metal catalysts. Therefore, the development of an efficient, green, and novel method for the synthesis of N-sulfonyl amidines is thus highly desirable.
Recently we have reported catalyst-free synthesis of alkylaminophenols via Petasis-type reaction24 and decarboxylative P–C coupling reaction of amino acids.25 In continuation of our efforts in developing new green synthetic methods, herein we report an efficient, practical, and high yielding catalyst-free synthesis of N-sulfonylamidines via the direct reaction of tertiary or secondary amines with sulfonyl azides.
Results and discussion
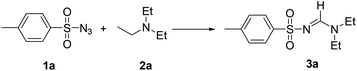
The cascade reaction of p-tosylazide (1a) with triethyl amine (2a) was chosen as the model reaction and screening results listed in Table 1. Initially, no reaction was observed when DMSO was used as a solvent at room temperature for 24 h (entry 1). Upon heating the reaction mixture, the compound 3a was obtained in 35% yield after 24 h at reflux condition (entry 2). The results for the reactions conducted in various solvents showed that using 1,4-dioxane as a solvent, the compound 3a was obtained in 66% isolated yield (entries 3–8). When the reaction was conducted with 2 eq. of the amine 2a, the compound 3a was obtained in 71% yield (entry 10). No reaction was observed when the reaction conducted under Ar or N2 atmosphere at reflux for 24 h (entry 11). With 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of azide to amine, the compound 3a was obtained in 25% yield (entry 12). The reaction afforded 3a in good yield at a reflux condition (entries 13–16). When the reaction was conducted in the presence of TEMPO (1 eq.), the compound 3a was obtained in 68% yield (entry 17). The reaction afforded 3a in 71% yield (same aerobic condition) in the presence of dioxygen at reflux condition (entry 18).
1 ratio of azide to amine, the compound 3a was obtained in 25% yield (entry 12). The reaction afforded 3a in good yield at a reflux condition (entries 13–16). When the reaction was conducted in the presence of TEMPO (1 eq.), the compound 3a was obtained in 68% yield (entry 17). The reaction afforded 3a in 71% yield (same aerobic condition) in the presence of dioxygen at reflux condition (entry 18).
| Entry | Solvent (2 mL) | T (°C) | Ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Yields refers to the isolated pure products after short column chromatography.b No reaction.c Reaction under Ar or N2.d Reaction in the presence of TEMPO (1 equiv.).e Reaction in the presence of dioxygen. | |||||
| 1 | DMSO | rt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | —b |
| 2 | DMSO | 110 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 35 |
| 3 | CHCl3 | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 55 |
| 4 | THF | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 51 |
| 5 | Toluene | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 62 |
| 6 | EtOH | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 40 |
| 7 | CH3CN | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 61 |
| 8 | 1,4-Dioxane | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 66 |
| 9 | 1,4-Dioxane | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
24 | 69 |
| 10 | 1,4-Dioxane | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
24 | 71 |
| 11 | 1,4-Dioxane | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
24 | —c |
| 12 | 1,4-Dioxane | Reflux | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 25 |
| 13 | 1,4-Dioxane | rt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
48 | —b |
| 14 | 1,4-Dioxane | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
48 | —b |
| 15 | 1,4-Dioxane | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
48 | 54 |
| 16 | 1,4-Dioxane | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
48 | 71 |
| 17 | 1,4-Dioxane | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
24 | 68d |
| 18 | 1,4-Dioxane | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
24 | 71e |
This result showed that the reaction mixture has no any radical intermediate under reaction condition. Therefore, further optimization on the amount of p-tosylazide (1a) and triethyl amine showed that treatment of azide 1a with 2 equiv. of triethyl amine for 24 h produced p-tosylamidine 3a in 71% yield (entries 9–18).
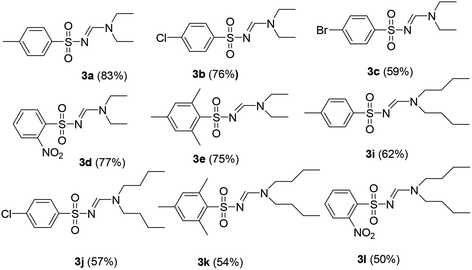
Under the optimized conditions, a wide range of sulfonyl azides were employed in the cascade reaction with tertiary amines for the synthesis of N-sulfonylamidines in good to modest yields as shown in Table 2. The arylsulfonyl azides bearing electron-donating and electron-withdrawing groups reacted with triethylamine to afford the corresponding N-sulfonylamidines in moderate to good yields (3a–3e). The steric hindrance did not apply considerable influence on the reaction. 2,4,6-Trimethyl-benzenesulfonyl azide reacted smoothly with triethyl amine 2a and the corresponding N-sulfonylamidines 3e was obtained in 70% yield.
| Entry | R1 | R2 | R3 | R4 | Product 3 | Yielda (%) |
|---|---|---|---|---|---|---|
| a Yields refers to the isolated pure products after short column chromatography.b Mixture of E and Z configurations.c Mixture of unknown compounds in the presence DABCO. | ||||||
| 1 | p-MeC6H4– | H | Et | Et | 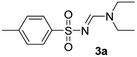 |
71 |
| 2 | p-ClC6H4– | H | Et | Et | 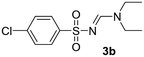 |
77 |
| 3 | p-BrC6H4– | H | Et | Et | 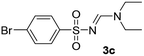 |
68 |
| 4 | o-NO2C6H4– | H | Et | Et | 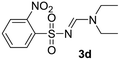 |
54 |
| 5 | 2,4,6-Me3C6H2– | H | Et | Et | 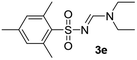 |
70 |
| 6 | Camphoryl | H | Et | Et | 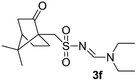 |
63 |
| 7 | Me– | H | Et | Et | 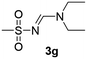 |
71 |
| 8 | p-MeC6H4– | Me | n-Pr | n-Pr | 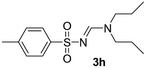 |
69 |
| 9 | p-MeC6H4– | Et | n-Bu | n-Bu | 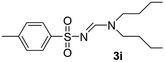 |
58 |
| 10 | p-ClC6H4– | Et | n-Bu | n-Bu | 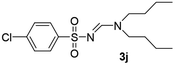 |
50 |
| 11 | 2,4,6-Me3C6H2– | Et | n-Bu | n-Bu | 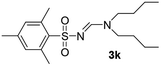 |
46 |
| 12 | o-NO2C6H4– | Et | n-Bu | n-Bu | 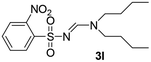 |
42 |
| 13 | p-MeC6H4– | H | i-Pr | i-Pr | 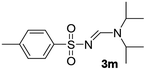 |
73 |
| 14 | p-BrC6H4– | H | i-Pr | i-Pr | 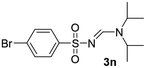 |
66 |
| 15 | 2,4,6-Me3C6H2– | H | i-Pr | i-Pr | 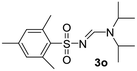 |
66 |
| 16 | p-MeC6H4– | H | Et | Ph | 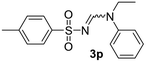 |
48b |
| 17 | p-MeC6H4– | — | — | — | — | —c |
The aliphatic sulfonyl azides, camphorsulfonyl azide and methylsulfonyl azide, afforded the corresponding N-sulfonylamidines 3f and 3g in 63% and 71% isolated yields respectively. Furthermore, the reaction of arylsulfonyl azides with tri-propyl and tributyl amines afforded corresponding N-sulfonylamidines (3h–3l) in moderate to good yield (entries 8–12). Tertiary amines with different alkyl groups, N,N-diisopropylethylamine and N,N-diethyl aniline, were also investigated and the corresponding N-sulfonylamidines were obtained for each tertiary amine in good yields (entries 13–16). The cascade reaction of DABCO as a cyclic tertiary amine with p-methylphenylsufonyl azide gave a mixture of unknown products (entry 17).
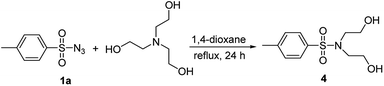
Interestingly, the corresponding sulfonamide 4 was obtained in 46% yield with using triethanol amine (Scheme 2). This experiment showed the product was obtained by the conversion of triethanol amine to its secondary amine followed by a nucleophilic substitution under optimized reaction condition.
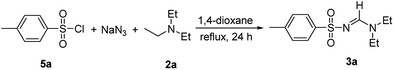
In other attempt, a one-pot three component reaction of sulfonyl chloride 5a with sodium azide (2 equiv.) and triethyl amine (2 equiv.) for the synthesis of N-sulfonylamidines was examined. The compound 3a was obtained in 78% yield in 1,4-dioxane at reflux for 24 h (Scheme 3).
In order to find the activity of secondary amines, diethylamine 6a was attempted to be employed in the reaction with sulfonyl azide 1a in dioxane at reflux for 24 h. Interestingly, the compound 3a was obtained in 83% yield as found for the triethylamine. A series of sulfonyl azides were used to react with secondary amines 6 to provide the same products as found for the tertiary amines (Table 3) with moderate to good yields.
| a Yields refers to the isolated pure products after short column chromatography. |
|---|
 |
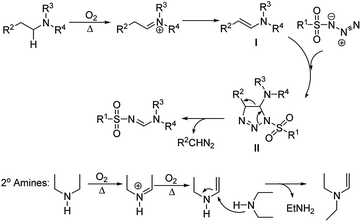
According to the literature reports and control experiments (Table 1, entries 10 and 16), a proposed mechanism was outlined in Scheme 4. Initially, the cascade reaction was started via aerobic oxidation–dehydration of C–H bond adjacent to nitrogen atom of tertiary amines. Then, the formed enamine I was reacted with sulfonyl azide 1 to yield the unstable cyclic compound II. Finally N-sulfonylamidine 3 was obtained by a retro cycloaddition of the compound II. With secondary amines, the reaction proceeded by the enamine I formation via a known conversion of diethylamine to enamine I (Scheme 4).14
Conclusion
In summary, we report a green synthesis of N-sulfonyl amidines via the direct reaction of tertiary or secondary amines with sulfonyl azides. Under transition metal- and catalyst-free condition, aromatic and aliphatic N-sulfonyl azides could be converted into N-sulfonyl amidines in modest to good yields. The reported method is easy to handle, readily available starting materials, air and moisture insensitive reagents, and a simple method compared with previously reported methods.Experimental section
General
NMR spectra were obtained on a 400 and 250 MHz NMR Spectrometer (1H NMR: 400 and 250 MHz and 13C NMR: 100 and 63 MHz). HRMS measurements were obtained on an ESI-TOF-Ms machine. Analytical TLC was carried out with plates pre-coated with silica gel 60 F254 (0.25 mm thick). Column chromatography was performed either with silica gel 60 (70–230 mesh) in common glass columns. All solvents were distillated before use.General procedure for the synthesis of N-sulfonyl amidines from sulfonyl azides
Sulfonyl azide (1 mmol) was added to a solution of amine (2 mmol) in 1,4-dioxane (2 mL). The mixture was stirred for 24 h at reflux (oil bath at 110 °C) without using any inert gas under air. Water (5 mL) was added to the reaction mixture and the mixture was washed with ethyl acetate (3 × 5 mL) and dried over sodium sulfate. The solvent was evaporated and the crude product was purified by a short column chromatography with n-hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to give compound 3 as the yellow viscous oil or white solid (the reaction was also examined with 5 mmol of sulfonyl azide of 1a with triethyl amine and a similar yield of 3a was obtained).
3) to give compound 3 as the yellow viscous oil or white solid (the reaction was also examined with 5 mmol of sulfonyl azide of 1a with triethyl amine and a similar yield of 3a was obtained).
(E)-N′-((4-Methylphenyl)sulfonyl)-N,N-diethylformimidamide (3a)17
Yellow oil (198 mg, 78% direct method from sulfonyl chloride); 1H NMR (400 MHz, CDCl3) δ: 8.17 (s, 1H), 7.79 (d, J = 8.3 Hz, 2H), 7.28 (d, J = 8.3 Hz, 2H), 3.50 (q, J = 7.2 Hz, 2H), 3.40 (q, J = 7.2 Hz, 2H), 2.43 (s, 3H), 1.28 (t, J = 7.2 Hz, 3H), 1.17 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 158.1, 142.3, 139.7, 129.3, 126.4, 47.1, 40.9, 21.5, 14.5, 12.1; HRMS (ESI): calcd for C12H19N2O2S [M + H]+: 255.1167, found: 255.1151.(E)-N′-((4-Chlorophenyl)sulfonyl)-N,N-diethylformimidamide (3b)17
Yellow oil (211 mg, 77%); 1H NMR (400 MHz, CDCl3) δ: 8.15 (s, 1H), 7.83 (d, J = 8.7 Hz, 2H), 7.44 (d, J = 8.7 Hz, 2H), 3.49 (q, J = 7.2 Hz, 2H), 3.41 (q, J = 7.2 Hz, 2H), 1.28 (t, J = 7.2 Hz, 3H), 1.15 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 158.1, 141.2, 138.0, 128.9, 127.9, 47.2, 41.1, 14.5, 12.1.(E)-N′-((4-Bromophenyl)sulfonyl)-N,N-diethylformimidamide (3c)22
Light yellow solid (216 mg, 68%); 1H NMR (400 MHz, CDCl3) δ: 8.14 (s, 1H), 7.76 (d, J = 8.6 Hz, 2H), 7.60 (d, J = 8.6 Hz, 2H), 3.48 (q, J = 7.2 Hz, 2H), 3.40 (q, J = 7.3 Hz, 2H), 1.27 (t, J = 7.2 Hz, 3H), 1.15 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 158.2, 141.7, 131.9, 128.0, 126.5, 47.2, 41.1, 14.5, 12.1.(E)-N′-((2-Nitrophenyl)sulfonyl)-N,N-diethylformimidamide (3d)17
Yellow oil (154 mg, 54%); 1H NMR (400 MHz, CDCl3) δ: 8.31 (d, J = 8.3 Hz, 1H), 8.13 (s, 1H), 7.75–7.64 (m, 3H), 3.45–3.55 (m, 4H), 1.36 (t, J = 7.2 Hz, 3H), 1.19 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 159.9, 147.7, 135.4, 132.7, 132.4, 130.7, 124.0, 47.4, 41.3, 14.4, 12.1.(E)-N′-((2,4,6-Trimethylphenyl)sulfonyl)-N,N-diethylformimidamide (3e)26
White solid (199 mg, 70%); 1H NMR (400 MHz, CDCl3) δ: 8.16 (s, 1H), 6.94 (s, 2H), 3.48 (q, J = 7.2 Hz, 2H), 3.38 (q, J = 7.3 Hz, 2H), 2.71 (s, 6H), 2.31 (s, 3H), 1.28 (t, J = 7.2 Hz, 3H), 1.17 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 157.4, 141.1, 138.4, 136.6, 131.4, 46.8, 40.8, 23.0, 20.9, 14.6, 12.1.(E)-N′-(((7,7-Dimethylbicyclo[2.2.1]hept-2-en-1-yl)methyl)sulfonyl)-N,N-diethylformimidamide (3f)22
Colorless viscous oil (192 mg, 61%); 1H NMR (400 MHz, CDCl3) δ: 8.00 (s, 1H), 3.51–3.31 (m, 4H), 3.02–2.88 (m, 1H), 2.59 (ddd, J = 14.8, 11.4, 4.0 Hz, 1H), 2.40–2.19 (m, 1H), 2.09–1.94 (m, 2H), 1.86 (d, J = 18.3 Hz, 1H), 1.69 (ddd, J = 13.9, 9.2, 4.5 Hz, 1H), 1.44–1.29 (m, 2H), 1.29–1.02 (m, 9H), 0.82 (d, J = 3.0 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 215.3, 158.7, 58.5, 50.6, 48.0, 46.9, 42.7, 42.6, 40.7, 27.0, 24.7, 19.9, 19.7, 14.5, 12.0.(E)-N′-(Methanesufonyl)-N,N-diethylformimidamide (3g)19
Colorless viscous oil (126 mg, 71%); 1H NMR (250 MHz, chloroform-d) δ: 8.05 (s, 1H), 3.47 (q, J = 7.2 Hz, 2H), 3.37 (q, J = 7.3 Hz, 2H), 2.94 (s, 3H), 1.29–1.22 (m, 3H), 1.22–1.14 (m, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 155.1, 44.0, 39.0, 37.8, 11.5, 9.0.(E)-N′-((4-Methylphenyl)sulfonyl)-N,N-dipropylformimidamide (3h)17
Yellow viscous oil (195 mg, 69%); 1H NMR (250 MHz, chloroform-d) δ 8.13 (s, 1H), 7.73 (d, J = 7.9 Hz, 2H), 7.23 (d, J = 8.1 Hz, 2H), 3.33 (t, J = 7.8 Hz, 2H), 3.24 (t, J = 7.3 Hz, 2H), 2.37 (s, 3H), 1.58 (dd, J = 14.6, 7.3 Hz, 4H), 0.86 (dt, J = 14.1, 7.4 Hz, 6H);13C{1H} NMR (63 MHz, CDCl3) δ: 155.9, 139.2, 136.8, 126.3, 123.3, 51.3, 44.8, 18.9, 18.5, 16.9, 8.2, 7.9.(E)-N′-((4-Methylphenyl)sulfonyl)-N,N-dibutylformimidamide (3i)17
Yellow oil (171 mg, 55%); 1H NMR (400 MHz, CDCl3) δ: 8.16 (s, 1H), 7.77 (d, J = 8.3 Hz, 2H), 7.28 (d, J = 8.0 Hz, 2H), 3.42 (t, J = 7.4, 2H), 3.31 (t, J = 7.4 Hz, 2H), 2.42 (s, 3H), 1.62–1.50 (m, 4H), 1.25–1.35 (m, 4H), 0.96 (t, J = 7.3 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 158.7, 142.2, 139.8, 129.2, 126.3, 52.3, 46.0, 30.7, 28.7, 21.5, 20.0, 19.7, 13.7, 13.6.(E)-N′-((4-Chlorophenyl)sulfonyl)-N,N-dibutylformimidamide (3j)17
Yellow oil (165 mg, 50%); 1H NMR (400 MHz, CDCl3); δ: 8.14 (s, 1H), 7.82 (d, J = 8.6 Hz, 2H), 7.44 (d, J = 8.6 Hz, 2H), 3.41 (t, J = 7.6 Hz, 2H), 3.32 (t, J = 7.4 Hz, 2H), 1.63–1.49 (m, 4H), 1.33–1.24 (m, 4H), 0.95 (t, J = 7.4 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 158.8, 141.3, 137.9, 128.9, 127.8, 52.4, 46.1, 30.7, 28.7, 19.9, 19.6, 13.7, 13.6.(E)-N′-((2,4,6-Trimethylphenyl)sulfonyl)-N,N-diethylformimidamide (3k)26
White solid (157 mg, 46%); 1H NMR (400 MHz, CDCl3) δ: 8.14 (s, 1H), 6.93 (s, 2H), 3.39 (t, J = 7.2 Hz, 2H), 3.29 (t, J = 7.4 Hz, 2H), 2.70 (s, 6H), 2.31 (s, 3H), 1.61–1.51 (m, 4H), 1.35–1.29 (m, 4H), 0.96 (t, J = 7.3 Hz, 3H), 0.91 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 157.9, 141.0, 138.3, 136.6, 131.4, 52.2, 46.1, 30.8, 28.7, 23.0, 20.9, 20.1, 19.6, 13.7, 13.6.(E)-N′-((2-Nitrophenyl)sulfonyl)-N,N-diethylformimidamide (3l)17
Yellow oil (143 mg, 42%); 1H NMR (400 MHz, CDCl3) δ: 8.29–8.26 (m, 1H), 8.11 (s, 1H), 7.73–7.64 (m, 3H), 3.40 (m, 4H), 1.70–1.62 (m, 2H), 1.58–1.51 (m, 2H), 1.40–1.27 (m, 4H), 0.98 (t, J = 7.3 Hz, 3H), 0.87 (t, J = 7.3 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 160.3, 147.7, 135.4, 132.7, 132.0, 130.7, 124.0, 52.7, 46.2, 30.6, 28.6, 19.8, 19.6, 13.6.(E)-N′-((4-Methylphenyl)sulfonyl)-N,N-diisopropylformimidamide (3m)17
Yellow oil (206 mg, 73%); 1H NMR (400 MHz, CDCl3) δ: 8.26 (s, 1H), 7.76 (d, J = 8.3 Hz, 2H), 7.26 (d, J = 8.0 Hz, 2H), 4.53 (p, J = 6.8 Hz, 1H), 3.70 (p, J = 6.8 Hz, 1H), 2.41 (s, 3H), 1.32 (d, J = 6.8 Hz, 6H), 1.22 (d, J = 6.8 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ: 156.4, 142.1, 139.9, 129.3, 126.2, 48.6, 47.9, 23.6, 21.5, 19.6.(E)-N′-((4-Bromophenyl)sulfonyl)-N,N-diisopropylformimidamide (3n)21
White solid (228 mg, 66%); 1H NMR (400 MHz, CDCl3) δ: 7.76 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 8.6 Hz, 2H), 4.54 (hept, J = 6.8 Hz, 1H), 3.73 (hept, J = 6.8 Hz, 1H), 1.35 (d, J = 6.8 Hz, 6H), 1.25 (d, J = 6.8 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ: 156.4, 141.9, 131.9, 127.9, 126.3, 48.8, 48.1, 23.6, 19.6.(E)-N′-((2,4,6-Trimethylphenyl)sulfonyl)-N,N-diisopropyl formimidamide (3o)21
White solid (205 mg, 66%); 1H NMR (400 MHz, CDCl3) δ: 8.29 (s, 1H), 6.93 (s, 2H), 4.53 (sep, J = 6.9 Hz, 1H), 3.69 (sep, J = 6.8 Hz, 1H), 2.69 (s, 6H), 2.30 (s, 3H), 1.33 (d, J = 6.8 Hz, 6H), 1.23 (d, J = 6.8 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ: 155.7, 141.0, 138.3, 136.6, 131.4, 48.2, 47.7, 23.7, 22.9, 20.9, 19.7.(E)-N′′-((p-Methylphenyl)sulfonyl)-N-ethyl-N′-phenylformimidamide (3p)26
White solid (139 mg, 46%); 1H NMR (400 MHz, CDCl3) δ: 8.48 (s, 1H), 7.85 (d, J = 8.3 Hz, 2H), 7.50–7.43 (m, 2H), 7.40–7.37 (m, 1H), 7.34–7.30 (m, 2H), 7.26–7.18 (m, 2H), 4.00 (q, J = 7.2 Hz, 2H), 2.45 (s, 3H), 1.21 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 158.1, 142.7, 141.9, 139.1, 129.9, 129.4, 127.7, 126.6, 123.6, 44.1, 21.5, 12.4.N,N-Bis(hydroxymethyl)-4methylbenzenesulfonamide (4)27
Colorless oil (143 mg, 46%); 1H NMR (400 MHz, CDCl3) δ: 7.72 (d, J = 8.1 Hz, 2H), 7.35 (d, J = 8.0 Hz, 2H), 4.12 (s, 2H), 3.88 (t, J = 4.9 Hz, 4H), 3.28 (t, J = 4.8 Hz, 4H), 2.45 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 143.8, 135.2, 129.9, 127.3, 62.3, 53.0, 21.5.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Institute for Advanced Studies in Basic Sciences is thanked for supporting this work. The authors also wish to thank Mr Haruhiko Fukaya, Tokyo University of Pharmacy and Life Sciences, for his help carrying out the HRMS measurements.References
- M. P. Coles, Application of neutral amidines and guanidines in coordination chemistry, Dalton Trans., 2006, 985–1001 RSC.
- J. Barker and M. Kilner, The coordination chemistry of the amidine ligand, Coord. Chem. Rev., 1994, 133, 219–300 CrossRef CAS.
- J. V. Greenhill and P. Lue, Amidines and guanidines in medicinal chemistry, Prog. Med. Chem., 1993, 30, 203–326 CrossRef CAS.
- P. Sienkiewich, K. Bielawski, A. Bielawska and J. Palka, Inhibition of collagen and DNA biosynthesis by a novel amidine analogue of chlorambucil is accompanied by deregulation of β1-integrin and IGF-I receptor signaling in MDA-MB 231 cells, Environ. Toxicol. Pharmacol., 2005, 20, 118–124 CrossRef PubMed.
- M. Wang, Y. Liu, L. Chang, C. Wang, Y. Zhao, X. Zhao, K. Qian, X. Nan, L. Yang, X. Yang, H. Hung, J. Yang, D. Kuo, M. Goto, S. Morris-Natschke, S. Pan, C. Teng, S. Kuo, T. Wu, Y. Wu and K. Lee, Design, Synthesis, Mechanisms of Action, and Toxicity of Novel 20(S)-Sulfonylamidine Derivatives of Camptothecin as Potent Antitumor Agents, J. Med. Chem., 2014, 57, 6008–6018 CrossRef CAS PubMed.
- T. Beryozkina, V. Bakulev, L. Dianova, V. Berseneva, P. Slepukhin, J. Leban, P. Kalaba, N. Y. Aher, M. Ilic, H. H. Sitte and G. Lubec, Design and Synthesis of N-Sulfonylamidines of Modafinic Acid, Synthesis, 2016, 48, 1046–1054 CrossRef CAS.
- Z. L. Song, H. L. Chen, Y. H. Wang, M. Goto, W. J. Gao, P. L. Cheng, S. L. Cheng Morris-Natschke, Y. Q. Liu, G. X. Zhu, M. J. Wang and K. H. Lee, Design and synthesis of novel PEG-conjugated 20(S)-camptothecin sulfonylamidine derivatives with potent in vitro antitumor activity via Cu-catalyzed three-component reaction, Bioorg. Med. Chem. Lett., 2015, 25, 2690–2693 CrossRef CAS PubMed.
- M. Y. Lee, M. H. Kim, J. Kim, S. H. Kim, B. T. Kim, I. H. Jeong, S. Chang, S. H. Kim and S.-Y. Chang, Synthesis and SAR of sulfonyl- and phosphoryl amidine compounds as anti-resorptive agents, Bioorg. Med. Chem. Lett., 2010, 20, 541–545 CrossRef CAS PubMed.
- T. D. Suja, K. V. L. Divya, L. V. Naik, A. R. Kumar and A. Kamal, Copper-catalyzed three-component synthesis of aminonaphthoquinone–sulfonylamidine conjugates and in vitro evaluation of their antiproliferative activity, Bioorg. Med. Chem. Lett., 2016, 26, 2072–2076 CrossRef CAS PubMed.
- L.-Y. Xie, T.-G. Fang, J.-X. Tan, B. Zhang, Z. Cao, L.-H. Yang and W.-M. He, Visible-light-induced deoxygenative C2-sulfonylation of quinoline N-oxides with sulfinic acids, Green Chem., 2019, 21, 3858–3863 RSC.
- S. Peng, Y.-X. Song, J.-Y. He, S.-S. Tang, J.-X. Tan, Z. Cao, Y.-W. Lin and W.-M. He, TsCl-promoted sulfonylation of quinoline N-oxides with sodium sulfinates in water, Chin. Chem. Lett., 2019, 30, 2287–2290 CrossRef CAS.
- L.-Y. Xie, S. Peng, F. Liu, G.-R. Chen, W. Xia, X. Yu, W.-F. Li, Z. Cao and W.-M. He, Metal-free deoxygenative sulfonylation of quinoline N-oxides with sodium sulfinates via a dual radical coupling process, Org. Chem. Front., 2018, 5, 2604–2609 RSC.
- S. Chen, Y. Xu and X. Wan, Direct Condensation of Sulfonamide and Formamide: NaI-Catalyzed Synthesis of N-Sulfonyl Formamidine Using TBHP as Oxidant, Org. Lett., 2011, 13, 6152–6155 CrossRef CAS PubMed.
- J. Chen, W. Long, S. Fang, Y. Yang and X. Wan, Interception of amide ylides with sulfonamides: synthesis of (E)-N-sulfonyl amidines catalyzed by Zn(OTf)2, Chem. Commun., 2017, 53, 13256–13259 RSC.
- I. Bae, H. Han and S. Chang, Highly Efficient One-Pot Synthesis of N-Sulfonylamidines by Cu-Catalyzed Three-Component Coupling of Sulfonyl Azide, Alkyne, and Amine, J. Am. Chem. Soc., 2005, 127, 2038–2039 CrossRef CAS PubMed.
- S. Chow and L. R. Odell, Synthesis of N-Sulfonyl Amidines and Acyl Sulfonyl Ureas from Sulfonyl Azides, Carbon Monoxide, and Amides, J. Org. Chem., 2017, 82, 2515–2522 CrossRef CAS PubMed.
- W.-Z. Bi, W.-J. Zhang, Z.-J. Li, X.-Y. Xia, X.-L. Chen, L.-B. Qu and Y.-F. Zhao, Air-induced one-pot synthesis of N-sulfonylformamidines from sulfonyl chlorides, NaN3 and tertiary/secondary amines, Eur. J. Org. Chem., 2019, 2019, 6071–6076 CrossRef CAS.
- X. Xu, Z. Ge, D. Cheng, C. Ma, L. Ma, C. Lu, Q. Zhang, N. Yao and X. Li, CuCl/CCl4-Promoted Convenient Synthesis of Sulfonyl Amidines from Tertiary Amines and Sulfonyl Azides, Org. Lett., 2010, 12, 897–899 CrossRef CAS PubMed.
- S. Wang, Z. Wang and X. Zheng, Facile synthesis of sulfonylamidines via carbon–nitrogen bond formation mediated by FeCl3, Chem. Commun., 2009, 7372–7374 RSC.
- X. Xu, X. Li, L. Ma, N. Ye and B. Weng, An Unexpected Diethyl Azodicarboxylate-Promoted Dehydrogenation of Tertiaryamine and Tandem Reaction with Sulfonyl Azide, J. Am. Chem. Soc., 2008, 130, 14048–14049 CrossRef CAS PubMed.
- J. Gui, H. Xie, H. Jian and W. Zeng, Visible-Light-Mediated Sulfonylimination of Tertiary Amines with Sulfonylazides Involving Csp3–Csp3 Bond Cleavage, Org. Lett., 2019, 21, 2804–2807 CrossRef CAS PubMed.
- R. Ding, H. Chen, Y.-L. Xu, H.-T. Tang, Y.-Y. Chen and Y.-M. Pan, Photoinduced Cascade Reaction of Tertiary Amines with Sulfonyl Azides: Synthesis of Amidine Derivatives, Adv. Synth. Catal., 2019, 361, 3656–3660 CrossRef.
- L. Zhang, J.-H. Su, S. Wang, C. Wan, Z. Zha, J. Du and Z. Wang, Direct electrochemical imidation of aliphatic amines via anodic oxidation, Chem. Commun., 2011, 47, 5488–5490 RSC.
- B. Kaboudin, A. Zangooei, F. Kazemi and T. Yokomatsu, Catalyst-free Petasis-type reaction: three-component decarboxylative coupling of boronic acids with proline and salicylaldehyde for the synthesis of alkylaminophenols, Tetrahedron Lett., 2018, 59, 1046–1049 CrossRef CAS.
- B. Kaboudin, L. Karami, J.-Y. Kato, H. Aoyama and T. Yokomatsu, A catalyst-free, three-component decarboxylative coupling of amino acids with aldehydes and H-dialkylphosphites for the synthesis of α-aminophosphonates, Tetrahedron Lett., 2013, 54, 4872–4875 CrossRef CAS.
- A. Rouzi, R. Hudabaierdi and A. Wusiman, Synthesis of N-sulfonylformamidines by tert-butyl hydroperoxide-promoted, metal-free, direct oxidative dehydrogenation of aliphatic amines, Tetrahedron, 2018, 74, 2475–2481 CrossRef CAS.
- M. Arisawa, C. Kato, H. Kaneko, A. Nishida and M. Nakagawa, Concise synthesis of azacycloundecenes using ring-closing metathesis (RCM), J. Chem. Soc., Perkin Trans. 1, 2000, 1873–1876 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra04545d |
| This journal is © The Royal Society of Chemistry 2020 |