Open Access Article
Open Access ArticleSynthesis of 2,5-diaryloxazoles through rhodium-catalyzed annulation of triazoles and aldehydes†
Jian Li *,
Shang-Rong Zhu,
Yue Xu,
Xue-Chen Lu,
Zheng-Bing Wang,
Li Liu and
De-feng Xu*
*,
Shang-Rong Zhu,
Yue Xu,
Xue-Chen Lu,
Zheng-Bing Wang,
Li Liu and
De-feng Xu*
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, School of Pharmaceutical Engineering & Life Sciences, Changzhou University, Changzhou, 213164, China. E-mail: lijianchem@cczu.edu.cn; markxu@cczu.edu.cn
First published on 30th June 2020
Abstract
An efficient synthesis of a variety of 2,5-diaryloxazole derivatives via a rhodium-catalyzed annulation of triazoles and aldehydes is achieved. Various oxazole derivatives could be obtained in good to excellent yields. A concise synthesis of antimycobaterial natural products balsoxin and texamine has been achieved using this method.
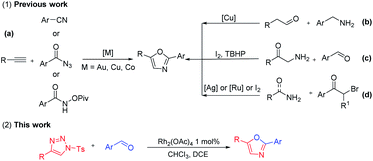
Oxazoles, an important class of heterocycles, are present in a wide range of natural products and biologically active molecules,1 as exemplified by salinazinone A,2 peptide alkaloid (−)-muscoride A,3 and antidiabetic agent AD-5061.4 In particular, 2,5-diaryl substituted oxazoles are found in pharmacologically active molecules such as antipancreatic cancer agent PC-046,5 antimycobaterial natural product texamine6 and balsoxin.7 Given this significant importance, several methodologies have been developed to access functionalized oxazole skeletons.8 Some groups reported a metal-catalyzed synthetic method for 2,5-diaryl substituted oxazoles through the reaction of alkynes and arylnitrile or aryl carbonyl azides or N-pivaloyloxyamides (Scheme 1(a)).9 In 2012, a copper-catalyzed oxidative dehydrogenative annulation method of alkynes and amines was developed by Jiao's group (Scheme 1(b)).10 Wang's group demonstrated a TBHP/I2-mediated tandem oxidative cyclization with 2-amino-1-arylethan-1-one and arylaldehyde to prepare 2,5-diaryl substituted oxazoles (Scheme 1(c)).11 In addition, α-bromoketones and arylamine or arlyamides derivatives were utilized by Moses,12 Zhang13 and Cho14 group for the synthesis of substituted oxazoles (Scheme 1(d)). Despite significant progress in this field, several limitation including low efficiency of activation, tedious side reactions and the lack of easy access to structurally diversity, have hampered their further application. Thus, the development of efficient and economy strategy for the synthesis of 2,5-diaryl substituted oxazoles has been both a challenge and a focus of synthetic chemistry. Herein, we report an approach that N-sulfonyl 1,2,3-triazoles react with aldehydes to give 2,5-diaryloxazoles via a cyclization.
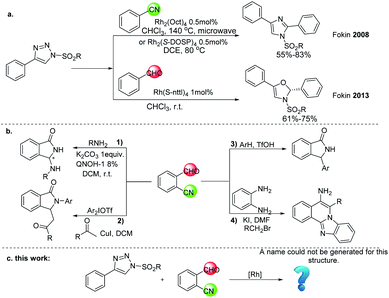
N-Sulfonyl-1,2,3-triazoles have recently emerged as structural motifs that are studied for synthesizing a variety of biologically active heterocycles,15 including pyrrole,16 imidazole,17 oxazoline,18 pyrroloindoline19 and others.20 In these transformations, Rh(II)-azavinylcarbene (Rh-AVC) was considered as the key intermediate, which derived from N-sulfonyl-1,2,3-triazoles under the treatment with rhodium(II) catalysts through denitrogenative reaction.21 Due to its dipolar nature, Rh-AVC could serve as an aza-[3C]-synthons in various [3 + 2] cycloaddition reactions. So far, a wide range of unsaturated chemical bonds, including aldehyde, nitrile, have been well explored in the [3 + 2] cycloadditions.22 2008, Fokin and co-workers exploited the reactivity of Rh-AVC to achieve imidazoles in good to excellent yields with N-sulfonyl 1,2,3-triazoles and nitriles.17 2013, they reported that Rh-AVC react with aldehydes to give 3-sulfonyl-4-oxazolines through an intramolecular cyclization (Scheme 2(a)).18 We were curious what would happen if o-cyanobenzaldehyde, containing aldehyde group and cyano group together, react with 1,2,3-triazoles? A variety of nitrogen-containing heterocycles,23 such as 3-amino-substituted isoindolinones (Scheme 2(b1)),23a 3-(2-oxopropyl)-2-arylisoindolinone (Scheme 2(b2)),23b 3-aryl isoindolinones (Scheme 2(b3))23c and benzo[4,5]imidazo[2,1-a]isoquinolines (Scheme 2(b4)),23d could be constructed with o-cyanobenzaldehyde. As a continuation of our research on cyano activation for the synthesis of nitrogen-containing heterocycles,24 we envisioned that the [3 + 2] cycloadditions between N-sulfonyl 1,2,3-triazoles and o-cyanobenzaldehyde would afford a novel approach to the synthesis of nitrogen-containing heterocyclic compounds (Scheme 2(c)).
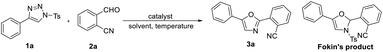
To test the hypothesis, we started the investigation by using 4-phenyl-1-tosyl-1H-1,2,3-triazole 1a and o-cyanobenzaldehyde 2a as model substrates in the presence of 1 mol% of Rh2(OAc)4 in CHCl3 at room temperature. Interestingly, we did not find the corresponding oxazoline product reported by Fokin's group. Instead, the oxazole 3a, which is the product of the sulfinic acid elimination was isolated in 14% yield after 12 hours, and the starting materials 2a was recovered in 68% yield. Due to the importance of oxazoles, we decided to optimize the reaction conditions with oxazoles as the target product. Increasing the temperature is beneficial to improve the yields of the products, the reactions afforded the desired product 3a in 24% yield at 80 °C and 79% yield at 120 °C (Table 1, entries 2, 3). From the experiment we could conclude that the product was easier to obtain at higher temperature, it seems that high temperatures help to the elimination of sulfinic acid. Other rhodium catalysts were tested, but no better results were obtained for this transformation (Table 1, entries 5–7). For Rh2(OAc)4 (1 mol%), the yields of the desired product 3a in various solvents were as follows: DCE (52%), toluene (11%) and CH3CN (7%), none or trace of product was found in other solvents (entries 8–14).
| Entry | Catalyst | Solvent | Temperature (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: 1a (0.6 mmol), 2a (0.3 mmol) in solvent (2.0 mL), 1 mol% of catalyst, 12 h.b Isolated yield. | |||||
| 1 | Rh2(OAc)4 | CHCl3 | rt | 12 | 14 |
| 2 | Rh2(OAc)4 | CHCl3 | 80 | 12 | 24 |
| 3 | Rh2(OAc)4 | CHCl3 | 120 | 12 | 79 |
| 4 | Rh2(OAc)4 | CHCl3 | 150 | 12 | 47 |
| 5 | Rh2(esp)2 | CHCl3 | 120 | 12 | 21 |
| 6 | Rh2(otc)4 | CHCl3 | 120 | 12 | 28 |
| 7 | Rh(Ph3P)3Cl | CHCl3 | 120 | 12 | — |
| 8 | Rh2(OAc)4 | DCE | 120 | 12 | 52 |
| 9 | Rh2(OAc)4 | Toluene | 120 | 12 | 11 |
| 10 | Rh2(OAc)4 | EtOH | 120 | 12 | — |
| 11 | Rh2(OAc)4 | CH3CN | 120 | 12 | 7 |
| 12 | Rh2(OAc)4 | DMF | 120 | 12 | — |
| 13 | Rh2(OAc)4 | THF | 120 | 12 | — |
| 14 | Rh2(OAc)4 | 1,4-Dioxane | 120 | 12 | Trace |
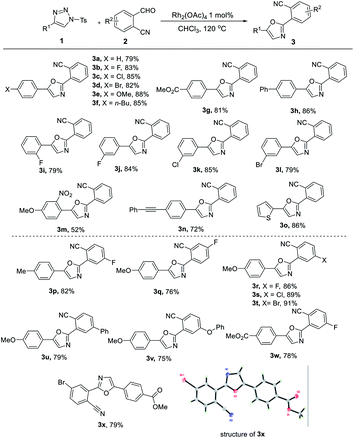
With an optimized set of reaction conditions in hand, we assessed the scope of these new reactions with various 4-phenyl-1-tosyl-1H-1,2,3-triazole 1 and o-cyanobenzaldehyde 2; the results are summarized in Table 2. As shown in Table 2, our cycloaddition reaction turned out to be widely applicable regardless of the electronic and steric properties of the aryl ring of the substrates 1. For 1,2,3-triazole substrates bearing 4-substituted 4-phenyl-1-tosyl-1H-1,2,3-triazoles (1a–1h; R1 = 4-XC6H4; X = F, Cl, Br, OMe, n-Bu, CO2Me, and Ph), rhodium-catalyzed reactions gave desired 3a–3h in 79–88% yields. The reaction was found to be marginally affected by the electronic nature of the substituents on the aryl ring as shown by the reactions of o-cyanobenzaldehyde 2a with triazoles bearing electron-donating substituents, which gave higher yields (3e and 3f). We tested the reaction with 2-substituted and 3-substituted 4-phenyl-1-tosyl-1H-1,2,3-triazoles, and the corresponding products 3i–3l were obtained in 79–85% yields. For disubstituted analogue 1m, the corresponding product 3m was produced in 52% yield. We also performed the reactions with reactants with 4-(phenylethynyl)-1-tosyl-1H-1,2,3-triazole 1n, which afforded alkynyl-containing compound 3n in 72% yield, this indicated that alkynyl group could be tolerated in the reaction conditions. The reaction was further compatible with heterocyclic substituted triazole (1o), yielding the desired thiophen product 3o in 86% yield.
We next assessed the scope of o-cyanobenzaldehyde 2. To our delight, with a series of substituents at different position of the o-cyanobenzaldehyde, including 4-F, 5-F, 5-Cl, 5-Br, 5-Ph and 5-OPh, the corresponding products 3p–3x were formed in 75–91% yields (Table 2). The molecular structure of compound 3x was confirmed by X-ray diffraction.
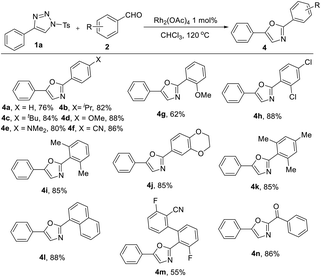
As cyano group was often employed as a ligand in transition metal-catalyzed reactions, we wondered whether the cyano group in o-cyanobenzaldehyde participated in the coordination with the rhodium metal in this reaction to promote the reaction. We chose unsubstituted benzaldehyde 2aa as a substrate to react with 4-phenyl-1-tosyl-1H-1,2,3-triazole 1a under the same conditions. It was surprising that the product 2,5-diphenyloxazole 4a was collected in 76% yield. To demonstrate the generality of the present rhodium-catalyzed reactions of benzaldehydes, a series of aromatic aldehydes were investigated under the optimized reaction conditions, the results are summarized in Table 3.
For the scope with respect to the aromatic aldehyde substrate, the corresponding 2,5-disubstituted oxazole products were obtained in good yields in all cases. In terms of electronic effect, both electron-rich substrates (2ab–2ad, 2ag, and 2ai–2ak) and electron-deficient substrate (2af) could be tolerated (yield from 62% to 88%, Table 3), including isopropyl group (4b), t-butyl group (4c), methoxy group (4d), methyl group (4i, 4k), and cyano group (3, 4f). Singly ortho-substitued (2ah, 2al) or doubly ortho-substitued (2ai, 2ak, 2am) aldehydes lead to the corresponding products smoothly. Complex substrate 3,3′-difluoro-2′-formyl-[1,1′-biphenyl]-2-carbonitrile 2am was prepared for this annulation, which proved to be applicable substrate and afforded compound 4m in 55% yield. The reaction was further compatible with 2-oxo-2-phenylacetaldehyde 2an, yielding the desired product 4n in 86%. Several cases of aliphatic aldehydes, such as butyraldehyde, isopropanal, phenylacetaldehyde were also investigated, but none of the corresponding products were found under the standard conditions.
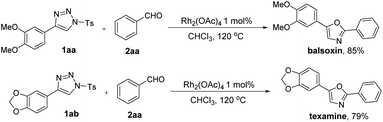
To demonstrate the synthetic utility of this protocol developed herein, we carried out the reaction with 4-(3,4-dimethoxyphenyl)-1-tosyl-1H-1,2,3-triazole 1aa and benzaldehyde 2aa in CHCl3 for the preparation of natural product balsoxin (Scheme 3). Gratifyingly, the desired product balsoxin could be obtained in 85% yield in one step. With the same strategy, natural product texamine, could be produced in 79% yield in 12 hours.
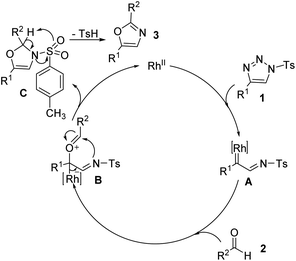
With literature precedents in hand, a reaction pathway is proposed in Scheme 4. 1,2,3-Triazole 1, upon reaction with Rh2(OAc)4, extrudes dinitrogen and forms Rh(II)-azavinyl carbene species A. Interaction of the carbonyl group with the carbene center leads to the formation of ylide B, which undergoes cyclization, leading to the formation of oxazoline C. Finally, removal of the p-toluenesulfonic acid followed afforded the desired oxazole 3.
In conclusion, a rhodium-catalyzed annulation of 1,2,3-triazoles and aldehydes has been developed. The developed methodology provides straightforward access to valuable 2,5-diaryl substituted oxazole derivatives. Applying this strategy, a concise synthesis of natural products balsoxin and texamine have been accomplished in good yields in one step. Further studies to explore the possibility for synthesis of various oxazoles are currently underway in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology (BM2012110), Advanced Catalysis and Green Manufacturing Collaborative Innovation Center in Changzhou University.Notes and references
- (a) P. Wipf, Chem. Rev., 1995, 95, 2115–2134 CrossRef CAS; (b) Oxazoles: Synthesis, Reactions and Spectroscopy, Part B, ed. D. C. Palmer, John Wiley & Sons, Hoboken, NJ, 2004 Search PubMed; (c) K. C. Nicolaou, J. Hao, M. V. Reddy, P. B. Rao, G. Rassias, S. A. Snyder, X. Huang, D. Y. K. Chen, W. E. Brenzovich, N. Giuseppone, P. Giannakakou and A. O'Brate, J. Am. Chem. Soc., 2004, 126, 12897 CrossRef CAS PubMed; (d) Z. Jin, Nat. Prod. Rep., 2011, 28, 1143 RSC; (e) J. Zhang and M. A. Ciufolini, Org. Lett., 2011, 13, 390 CrossRef CAS PubMed; (f) S. Imai, H. Kikui, K. Moriyama and H. Togo, Tetrahedron, 2015, 71, 5267 CrossRef CAS; (g) M. A. Chiacchio, G. Lanza, U. Chiacchio, S. V. Giofre, R. Romeo, D. Iannazzo and L. Legnani, Curr. Med. Chem., 2019, 41, 7337 Search PubMed.
- (a) M. C. Kim, J. H. Lee, B. Shin, L. Subedi, J. W. Cha, J.-S. Park, D.-C. Oh, S. Y. Kim and H. C. Kwon, Org. Lett., 2015, 17, 5024 CrossRef CAS PubMed; (b) P. Fu, S. La and J. B. MacMillan, J. Nat. Prod., 2016, 79, 455 CrossRef CAS PubMed.
- (a) A. Nagatsu, H. Kajitani and J. Sakakibara, Tetrahedron Lett., 1995, 36, 4097 CrossRef CAS; (b) A. Mattila, R. M. Andsten, M. Jumppanen, M. Assante, J. Jokela, M. Wahlsten, K. M. Mikula, C. Sigindere, D. H. Kwak, M. Gugger, H. Koskela, K. Sivonen, X. Y. Liu, J. Yli-Kauhaluoma, H. Iwai and D. P. Fewer, ACS Chem. Biol., 2019, 12, 2683 CrossRef PubMed.
- Y. Momose, T. Maekawa, T. Yamano, M. Kawada, H. Odaka, H. Ikeda and T. Sohda, J. Med. Chem., 2002, 45, 1518 CrossRef CAS PubMed.
- A. Y. Shaw, M. C. Henderson, G. Flynn, B. Samulitis, H. Han, S. P. Stratton, H.-H. S. Chow, L. H. Hurley and R. T. Dorr, J. Pharmacol. Exp. Ther., 2009, 331, 636 CrossRef CAS PubMed.
- X. A. Domínguez, G. D. L. Fuente, A. G. González, M. Reina and I. Timýan, Heterocycles, 1988, 27, 35 CrossRef.
- B. Burke, H. Parkins and A. M. Talbot, Heterocycles, 1979, 12, 349 CrossRef CAS.
- For reviews on the synthesis of oxazoles, see: (a) I. J. Turchi and M. J. S. Dewar, Chem. Rev., 1975, 75, 389 CrossRef CAS; (b) S. Bresciani and N. C. O. Tomkinson, Heterocycles, 2014, 89, 2479 CrossRef CAS; (c) A. Ibrar, I. Khan, N. Abbas, U. Farooq and A. Khan, RSC Adv., 2016, 6, 93016 RSCFor recently selected examples on the synthesis of oxazoles, see: (d) S. Samanta, R. R. Donthiri, M. Dinda and S. Adimurthy, RSC Adv., 2015, 5, 66718 RSC; (e) H. Peng, N. G. Akhmedov, Y.-F. Liang, N. Jiao and X. Shi, J. Am. Chem. Soc., 2015, 137, 8912 CrossRef CAS PubMed; (f) L. Chen, H. Li, P. Li and L. Wang, Org. Lett., 2016, 18, 3646 CrossRef CAS PubMed; (g) T. Soeta, A. Matsumoto, Y. Sakata and Y. Ukaji, J. Org. Chem., 2017, 82, 4930 CrossRef CAS PubMed; (h) X. Duan, K. Yang, J. Lu, X. Kong, N. Liu and J. Ma, Org. Lett., 2017, 19, 3370 CrossRef CAS PubMed; (i) R. J. Reddy, M. P. Ball-Jones and P. W. Davies, Angew. Chem., Int. Ed., 2017, 56, 13310 CrossRef CAS PubMed; (j) M. Mei, D. Anand and L. Zhou, Org. Lett., 2019, 21, 3548 CrossRef CAS PubMed.
- (a) W. He, C. Li and L. Zhang, J. Am. Chem. Soc., 2011, 133, 8482 CrossRef CAS PubMed; (b) . Cano, E. Álvarez, M. C. Nicasio and P. J. Pérez, J. Am. Chem. Soc., 2011, 133, 191 CrossRef PubMed; (c) E. Haldón, M. Besora, I. Cano, X. C. Cambeiro, M. A. Pericàs, F. Maseras, M. C. Nicasio and P. J. Pérez, Chem.–Eur. J., 2014, 20, 3463 CrossRef PubMed; (d) X. Yu, K. Chen, Q. Wang, W. Zhang and J. Zhu, Chem. Commun., 2018, 54, 1197 RSC.
- Z. Xu, C. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2012, 51, 11367 CrossRef CAS PubMed.
- C. Wan, L. Gao, Q. Wang, J. Zhang and Z. Wang, Org. Lett., 2010, 12, 3902 CrossRef CAS PubMed.
- D. J. Ritson, C. Spiteri and J. E. Moses, J. Org. Chem., 2011, 76, 3519 CrossRef CAS PubMed.
- W.-C. Gao, R.-L. Wang and C. Zhang, Org. Biomol. Chem., 2013, 11, 7123 RSC.
- T. Chatterjee, J. Y. Cho and E. J. Cho, J. Org. Chem., 2016, 81, 6995 CrossRef CAS PubMed.
- For leading reviews, see: (a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS; (b) F. Amblard, J. H. Cho and R. F. Schinazi, Chem. Rev., 2009, 109, 4207 CrossRef CAS PubMed; (c) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., 2012, 124, 886 (Angew. Chem., Int. Ed., 2012, 51, 862) CrossRef; (d) A. V. Gulevich and V. Gevorgyan, Angew. Chem., Int. Ed., 2013, 52, 1371 CrossRef CAS PubMed; (e) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed.
- (a) T. Miura, M. Yamauchi and M. Murakami, Chem. Commun., 2009, 45, 1470 RSC; (b) B. Chattopadhyay and V. Gevorgyan, Org. Lett., 2011, 13, 3746 CrossRef CAS PubMed; (c) Y. Shi and V. Gevorgyan, Org. Lett., 2013, 15, 5394 CrossRef CAS PubMed; (d) T. Miura, K. Hiraga, T. Biyajima, T. Nakamuro and M. Murakami, Org. Lett., 2013, 15, 3298 CrossRef CAS PubMed; (e) J. S. Alford, J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 11712 CrossRef CAS PubMed.
- T. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 14972 CrossRef CAS PubMed.
- M. Zibinsky and V. V. Fokin, Angew. Chem., 2013, 125, 1547 ( Angew. Chem., Int. Ed., 2013, 52, 1507) CrossRef.
- J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6802 CrossRef CAS PubMed.
- (a) T. Miura, T. Tanaka, K. Hiraga, S. G. Stewart and M. Murakami, J. Am. Chem. Soc., 2013, 135, 13652 CrossRef CAS PubMed; (b) S. Chuprakov, S. W. Kwok and V. V. Fokin, J. Am. Chem. Soc., 2013, 135, 4652 CrossRef CAS PubMed; (c) T. Miura, Y. Funakoshi and M. Murakami, J. Am. Chem. Soc., 2014, 136, 2272 CrossRef CAS PubMed.
- (a) H. M. L. Davies and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151 RSC; (b) P. Anbarasan, D. Yadagiri and S. Rajasekar, Synthesis, 2014, 46, 3004 CrossRef CAS; (c) Y. Jiang, R. Sun, X.-Y. Tang and M. Shi, Chem.–Eur. J., 2016, 22, 17910 CrossRef CAS PubMed.
- (a) E. E. Schultz and R. Sarpong, J. Am. Chem. Soc., 2013, 135, 4696 CrossRef CAS PubMed; (b) C. Kim, S. Park, D. Eom, B. Seo and P. H. Lee, Org. Lett., 2014, 16, 1900 CrossRef CAS PubMed; (c) R.-Q. Ran, J. He, S.-D. Xiu, K.-B. Wang and C.-Y. Li, Org. Lett., 2014, 16, 3704 CrossRef CAS PubMed.
- (a) A. Capobianco, A. D. Mola, V. Intintoli, A. Massa, V. Capaccio, L. Roiser, M. Waser and L. Palombi, RSC Adv., 2016, 6, 31861 RSC; (b) L. Liu, S.-H. Bai, Y. Li, X.-D. Ding, Q. Liu and J. Li, Adv. Synth. Catal., 2018, 360, 1617 CrossRef CAS; (c) J. Hu, H.-L. Qin, W. Xu, J. Li, F. Zhang and H. Zheng, Chem. Commun., 2014, 50, 15780 RSC; (d) A. L. Bagdasarian, H. H. Nguyen, T. A. Palazzo, J. C. Fettinger, M. J. Haddadin and M. J. Kurth, J. Org. Chem., 2016, 81, 3924 CrossRef CAS PubMed.
- (a) L. Liu, S.-H. Bai, Y. Li, L.-X. Wang, Y. Hu, H.-L. Sung and J. Li, J. Org. Chem., 2017, 82, 11084 CrossRef CAS PubMed; (b) L. Liu, J. Qiang, S.-H. Bai, H.-L. Sung, C.-B. Miao and J. Li, Adv. Synth. Catal., 2017, 359, 1283 CrossRef CAS; (c) J. Li, S.-H. Bai, Y. Li, Z.-B. Wang, X. Huo and L. Liu, J. Org. Chem., 2018, 83, 8780 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, additional spectroscopic data, and characterization of the new compounds. CCDC 1960749. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra03966g |
| This journal is © The Royal Society of Chemistry 2020 |