Open Access Article
Open Access ArticleIn vitro studies of adhesion properties of six lactic acid bacteria isolated from the longevous population of China
Chengran Guana,
Xuan Chena,
Xinrong Jianga,
Ruifeng Zhaoa,
Yuan Yuana,
Dawei Chena,
ChenChen Zhanga,
Maolin Lua,
Zhen Lub and
Ruixia Gu *a
*a
aKey Lab of Dairy Biotechnology and Safety Control, College of Food Science and Engineering, Yangzhou University, Yangzhou, Jiangsu 225127, China. E-mail: 444306783@qq.com; Fax: +86-514-89788128; Tel: +86-514-89788128
bBloomage Biotechnology Corporation Limited, Ji'nan, Shandong 250000, China
First published on 25th June 2020
Abstract
Six lactic acid bacteria (LAB), isolated from the intestinal tract of the longevous population, were prominent for their strong bacteriostatic ability. In this study, the adhesion properties of the six strains were determined in vitro to explore their potential to be used as probiotics. The hydrophobicity and aggregation activity were firstly detected and were varied from 14.83% to 57.3% and 12.7% to 31%, respectively. Moreover, the adhesion activity to the intestinal crypt cells (IEC-6 cells) was proved to be varied from 5.4 to 21.7 bacteria numbers per cell. Furthermore, all the tested LAB samples could inhibit 3 Gram-positive and 3 Gram-negative indicator microorganisms to adhere to the IEC-6 cells. Meanwhile every sample was inclined to exclude rather than displace or compete to inhibit the indicator microorganisms to adhere to IEC-6 cells. Afterwards, the adhesion activities of the LAB were demonstrated to be highly affected by the surface proteins considering the treatments of heat, pepsin, trypsin and NaIO4. The surface proteins (8–14 kDa) of every sample were isolated and proved to be helpful to regain more than 30% of the adhesion activity for the corresponding samples. This study will be beneficial to examine the characteristics of these strains especially L. casei g9 when used as probiotics in dairy food products.
1. Introduction
Lactic acid bacteria (LAB) have long been of interest to the dairy and agriculture industries with the status of GRAS (generally regarded as safe), and some have been found as ubiquitous members of the intestinal epithelial mucosae of healthy subjects.1,2 Specific selected LAB strains have been increasingly introduced and characterized as probiotics for functional food products.3 The criteria suggested for the selection of probiotics are safety, tolerance to gastrointestinal conditions, ability to adhere to the gastrointestinal mucosa, and competitive exclusion of potential pathogens.4,5Adhesion to intestinal epithelial cells is considered to be the first step for the LAB strains performing beneficial effects on the health of the host, and high adhesive ability can promote the gut residence time of LAB strains, restrain potential pathogens and protect intestinal epithelial cells (IECs).6,7 Bacterial-mediated adhesion is initially based on non-specific physical interactions between two surfaces, which then enable specific interactions between adhesive components and complementary receptors.8 Hydrophobicity and aggregation activity play key roles in the first contact between the bacterial cell and the mucous or epithelial cells.9 Various surface determinants on the LAB strains are involved in the specific adhesion to the IECs. The balance of electrostatic, van der Waals interactions, the hydrophobic character of the cell surfaces and the aggregation ability have long been suggested to influence the adherence of lactobacilli to human epithelial cells.10,11
Specific adhesive properties of some LAB may inhibit the colonization of pathogenic bacteria resulting of antagonistic activity against adhesion of gastrointestinal pathogens.12 The term of “competitive exclusion” was firstly used to describe the scene that one species of bacteria was more vigorous than another species in competing for receptor sites in the intestinal tract. Exclusion refers that the probiotics inhibit pathogen adhesion with different mechanisms and properties, including the production of substances and the stimulation of IECs. Competitive exclusion means that the bacteria compete for available nutrients and mucosal adhesion sites mediated by the bacterium-to-bacterium interaction. Using human mucosal material, the effect of probiotic bacteria on the competitive exclusion of pathogens has been demonstrated.8,12 Based on the reported work, the potential adhesion activity should not be generalized because adhesion tend to be strain specific and the health benefit of adhesion attributed to one strain is not necessarily applicable to another strain even within one species.1
Previously, six LAB strains, isolated from the intestinal tract of longevous population of China, have been identified with some probiotic properties in our lab, such as tolerance to acid and bile and the antimicrobial activity against potential pathogenic bacteria. In this study, the adhesive characteristics of the six LAB were evaluated including the hydrophobicity, aggregation activity, adhesion activity and the related surface proteins of the cells. The aim of this study was to determine the capacity of the six LAB to colonize the gastrointestinal tract. This study could be helpful to apply the tested strains into dairy food products.
2. Materials and methods
2.1. Bacterial strains and culture conditions
The LAB strains of Lactobacillus rhamnosus grx19, L. acidophilus ss, L. plantarum s7, L. casei g9, Bifidobacterium animalis BLC and Streptococcus thermophiles 90–57 were isolated from the gut of subjects from Bama longevity, Guangxi province, China, in 2013. The strains were separately cultured in skim milk broth for 12 h at 37 °C. The bacteria and supernatant were separated by centrifugation. The harvested bacteria were suspended by saline to 1 × 108 CFU mL−1. The supernatant was filtered by 0.22 μm Millipore filtration for the assay.Escherichia coli CICC10899, Salmonella enterica WX29, Staphylococcus aureus CICC10201, Pseudomonas brenneri CICC10271, Clostridium difficile CICC22951, Bacillus subtilis CICC10012 were bought from China Center of Industrial Culture Collection (Beijing, China). All the strains were cultivated in Luria–Bertani broth at 37 °C except the Clostridium difficile CICC22951 cultivated in Reinforced Clostridium Medium broth. The bacteria were harvested after incubation for12 h and suspended to 1 × 107 CFU mL−1 by saline.
2.2. IEC-6 cell culture
IEC-6 cell was purchased from Maisha (Shanghai Biotech Co., Ltd., China). Cells were routinely cultured in the Dulbecco's modified Eagle's minimal essential medium (DMEM; Hyclone Laboratories, Inc., USA) supplement with 20% (v/v) inactivated fetal calf serum (Hyclone Laboratories, Inc., USA), 1% streptomycin (100 μg mL−1) and 1% penicillin (100 U mL−1) at 37 °C in an atmosphere of 5% CO2/95% air. And then the cells were digested by 0.25% trypsin for passage. The medium was replaced every 2 days, and all the adhesion assays were performed after passage for 5 times.2.3. Adhesion assays on IEC-6 cell
Bacterial adhesion tests on IEC-6 cells monolayer were carried out in 6-well (22 × 22 mm) tissue culture plates. The precultivated IEC-6 cells were seeded at a concentration of 2 × 104 cells per mL per well and cultivated in an atmosphere of 5% CO2/95% air at 37 °C until the formation of the dense cell layer. After washing the IEC-6 monolayer cells twice with phosphate buffer saline (PBS, pH 7.2), one milliliter bacterial suspension and 1 mL DMEM culture were added to each well and incubated for 60 min. Unattached cells were removed by PBS buffer for 5 times and then the attached cells fixed for 20 min in methanol then treated Gram staining. Adherent bacterial cells were then enumerated by microscopic examination. Twenty fields of microscope were randomly selected and the bacteria adhered to 100 cells were counted. The bacterial adherence value was defined as the number of the adhered bacteria per cell. Each assay was performed in triplicate.2.4. Surface hydrophobicity and aggregation activity of the LAB strains
The surface hydrophobicity and aggregation assay were performed according to the method described Xu with slight modification.13 Using PBS buffer as control, xylene (0.4 mL) mixed with bacteria suspension (4 mL) and then placed for 15 min obtaining the aqueous phase. The absorption value of the sample and the control were detected under 600 nm wavelength. Each assay was performed in triplicate with 10 parallel samples. The surface hydrophobicity was calculated by the equation.OD600 (control): the absorption value of the control, OD600 (test): the absorption value of the sample.
The bacteria suspension (0.1 mL) mixed with PBS buffer (2.9 mL) and then detected the absorption with the 600 nm wavelength. The aggregation activity was defined by the following equation.
2.5. Bacteria labeling and inhibition of indicator microorganisms
All the assays were determined in three independent experiments, and each assay was performed in quadruplicate.
2.6. Enzymatic and physico-chemical treatments of LAB strains
As to proteases treatment, the LAB bacteria suspension was separately treated by pepsin (400 U mL−1, dissolved in 0.05 M glycine-HCl with pH 2.2) and trypsin (400 U mL−1, dissolved in 0.2 M PBS buffer with pH 8.0) at 37 °C for 30 min.In the part of chemical treatment, the LAB bacteria suspension was treated by 5 M LiCl in ice bath for 30 min and NaIO4 (0.05 M, dissolved in the 0.1 M citric acid-phosphate buffer, pH 4.5) for 30 min at 37 °C, respectively. With regard to heat treatment, the LAB bacteria suspension was treated at 121 °C for 5 min.
The disposed strain was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 4 °C, 10 min), washed and resuspended in the PBS buffer. And then labeled by CFDA-SE, adhesion test to IEC-6 cells was performed by the usual procedures. The relative adhesion ratio was determined as the percentage of LAB strains bounded to the IEC-6 cells with and without the treatment.
000 × g, 4 °C, 10 min), washed and resuspended in the PBS buffer. And then labeled by CFDA-SE, adhesion test to IEC-6 cells was performed by the usual procedures. The relative adhesion ratio was determined as the percentage of LAB strains bounded to the IEC-6 cells with and without the treatment.
2.7. Surface protein extraction and cocultivation
The strain was cultivated as the usual procedure and then centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 4 °C, 15 min) and washed twice with PBS buffer. The bacteria were treated with 5 M LiCl for 30 min and then centrifuged (16
000 × g, 4 °C, 15 min) and washed twice with PBS buffer. The bacteria were treated with 5 M LiCl for 30 min and then centrifuged (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 4 °C, 10 min) to collect the supernatant to dialysis. The dialysate containing the surface protein was isolated in dialysis bag (molecular weight cut off 8000–14
000 × g, 4 °C, 10 min) to collect the supernatant to dialysis. The dialysate containing the surface protein was isolated in dialysis bag (molecular weight cut off 8000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) for 24 h. The IEC-6 cells were cultivated with the dialysate (37 °C, 2 h) and then washed by PBS buffer to construct the adhesion assays on IEC-6 cell as described above. The LAB strains treated with 5 M LiCl (30 min at 37 °C) to remove the surface protein were used as the control samples.
000 Da) for 24 h. The IEC-6 cells were cultivated with the dialysate (37 °C, 2 h) and then washed by PBS buffer to construct the adhesion assays on IEC-6 cell as described above. The LAB strains treated with 5 M LiCl (30 min at 37 °C) to remove the surface protein were used as the control samples.
2.8. Statistical analysis
Statistical analysis was done by SPSS 20.0 software (SPSS Inc., Chicago). Data were subjected to a one-way analysis of variance.3. Results and discussion
3.1. Hydrophobicity and aggregation activity of the LAB strains
Hydrophobicity and aggregation are important factors that greatly affect bacterial adhesion.8 It was reported that LAB strains possessing hydrophobic cell surface and aggregation capacity were more capable to adhere to the intestinal cells to perform beneficial effects.15,16 In this work, the hydrophobicity and aggregation activity of the six LAB strains L. rhamnosus grx19 (grx19), L. acidophilus ss (ss), L. plantarum s7 (s7), L. casei g9 (g9), B. animalis BLC (BLC), and S. thermophiles 90–57 (90–57) were separately determined by carbohydrate and spectrophotometer detection (Fig. 1).The cell surface hydrophobicity of the studied strains was significantly different with the hydrophobic spectrum ranging from 14.8% (90–57) to 57.3% (g9). Comparing with the other strains, strain g9 and s7 revealed higher surface hydrophobicity (Fig. 1). Though lots of researches have been done on the bacterial hydrophobicity, the hydrophobicity obtained in this work could not been strictly compared with the reported results owing to the respective methods.17 However, the results were in good accordance with some reported work that hydrophobicity was strain-specific.6,8 The different hydrophobicity exhibited among the tested strains might be mainly attributed to the structurally and chemically heterogeneous bacterial surface, such as the unique hydrophobic amino acids, polysaccharides and other constitutions on the cell surface. Moreover, the others conditions should also be considered in our future studies including environmental factors, cell growth phase and degree of pleomorphism.
The aggregation activity ranged from 12.7% (90–57) to 31% (g9). Likewise, strain g9 and s7 showed higher aggregation activity than the other strains, and strain 90–57 displayed the lowest activity. As aggregation activity of probiotic strains appeared to be necessary for the initial adhesion to intestinal mucosa, plenty of works were processed to gain information on the structural properties of the cell surface that were responsible for aggregation. For strain L. acidophilus M92, proteins were reasonably considered as mediators in the aggregation process because the aggregation activity was weakened by proteolytic treatment.8 The specific components responsible for the aggregation of the tested strains in this work would be further excavated.
3.2. Adhesion capacity of the LAB strains to the IEC-6 cells
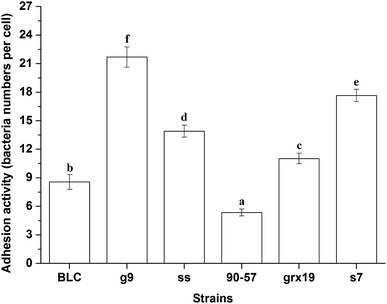
Adhesion to intestinal mucosa is regarded as a prerequisite for colonization and is important for the interaction between probiotic strains and the host to modulate the immune system and antagonize against indicator microorganisms.18,19 The specific LAB strain and IEC-6 cells were co-cultivated and the adhesion was microscopically examined (Fig. 2). All the tested samples were able to adhere to IEC-6 cells with different levels. The adhered number of strains varied from 5.4 (90–57) to 21.7 (g9) bacteria numbers per cell. In accordance with other previous studies, adhesion was clearly demonstrated to be strain-dependent property. Though the human intestinal epithelial cell lines seemed to better represent the in vivo situation, the IEC-6 cells were used as the in vitro models to test the adhesion properties because they grow in culture forming a homogeneous and polarized cell monolayer which was advanced in cellular interaction studies.20,21 The adhesion activity was different because of the specific applied models in vitro, nevertheless, the results were necessary to select the most promising strains prior to the definitive human clinical trials.22,23As the samples performed similar trend in hydrophobicity, aggregation activity and adhesion activity, the correlation analysis was adopted. It was noted that adhesion activity was positively correlated with the paralleled hydrophobicity (P < 0.05) and aggregation activity (P < 0.01). This result was in good accordance with the reported work that adhesion ability was correlated with hydrophobicity of lactobacilli surface as measured by microbial adhesion to hydrocarbons.13
3.3. Inhibition of the LAB strains to the indicator microorganisms to adhere to the IEC-6 cells
Pioneering studies have demonstrated that Lactobacillus strains owning adhesive properties could enable them to inhibit and/or prevent the colonization of epithelial cells by exogenous bacteria.7,18 Considering on these six strains showing adherent activity, 3 Gram-positive strains (S. aureus, B. subtilis, C. difficile) and 3 Gram-negative strains (E. coli, S. enterica WX29, P. brenneri) were used as indicator microorganisms to estimate the microbial inhibition activity of the LAB strains and the corresponding experiments were composed of exclusion, competition and displacement (Tables 1 and 2).| Exogenous bacteria | Inhibition assay | Adhesion inhibition (%) pathogens | |||||
|---|---|---|---|---|---|---|---|
| BLC | g9 | ss | 90–57 | grx19 | s7 | ||
| a Data are mean values ± SD (n = 3). (a–f) significant difference (P < 0.05) among all lactic acid bacteria strains within the same column. | |||||||
| S. aureus | Exclusion | 61.37 ± 0.35d | 67.2 ± 0.68c | 57.04 ± 0.38e | 44.04 ± 0.38f | 57.04 ± 0.38e | 69.04 ± 0.38b |
| Competition | 41.94 ± 0.41d | 64.27 ± 0.56a | 38.6 ± 0.17e | 34.6 ± 0.17f | 38.6 ± 0.17e | 51.6 ± 0.17b | |
| Displacement | 34.03 ± 0.17d | 47.03 ± 0.17a | 31.03 ± 0.17e | 21.03 ± 0.17fe | 20.03 ± 0.17f | 38.03 ± 0.17bc | |
| B. subtilis | Exclusion | 69.37 ± 0.35b | 66.37 ± 0.35c | 62.04 ± 0.38d | 34.03 ± 0.38f | 59.71 ± 0.27e | 72.37 ± 0.35a |
| Competition | 51.94 ± 0.41b | 52.7 ± 0.17b | 37.6 ± 0.17e | 24.6 ± 0.17f | 41.94 ± 0.41d | 55.6 ± 0.17a | |
| Displacement | 38.03 ± 0.17b | 39.36 ± 0.55a | 30.03 ± 0.25d | 19.36 ± 0.47f | 24.03 ± 0.17e | 31.03 ± 0.17d | |
| C. difficile | Exclusion | 67.04 ± 0.38a | 59.4 ± 0.35bc | 39.71 ± 0.27de | 24.37 ± 0.35g | 37.71 ± 0.27f | 45.06 ± 0.38e |
| Competition | 60.4 ± 0.17b | 43.6 ± 0.17a | 24.6 ± 0.17e | 22.6 ± 0.17f | 23.6 ± 0.17e | 31.27 ± 0.56d | |
| Displacement | 20.49 ± 0.41c | 34.36 ± 0.55a | 19.49 ± 0.41d | 15.49 ± 0.41f | 18.49 ± 0.41e | 20.69 ± 0.4c | |
| Exogenous bacteria | Inhibition assay | Adhesion inhibition (%) | |||||
|---|---|---|---|---|---|---|---|
| BLC | g9 | ss | 90–57 | grx19 | s7 | ||
| a Data are mean values ± SD (n = 3). (a–g) significant difference (P < 0.05) among all lactic acid bacteria strains within the same column. | |||||||
| E. coli | Exclusion | 64.63 ± 0.91d | 74.81 ± 0.57b | 59.97 ± 0.27e | 29.63 ± 0.61f | 66.97 ± 0.27c | 77.97 ± 0.27a |
| Competition | 49.0 ± 1.48e | 62.6 ± 0.17a | 41.34 ± 0.62f | 21.34 ± 0.62g | 52.7 ± 1.06cd | 59.27 ± 0.56b | |
| Displacement | 31.09 ± 0.86e | 44.36 ± 0.55a | 29.84 ± 0.53f | 13.64 ± 0.55g | 35.09 ± 0.83d | 42.69 ± 0.4b | |
| S.enterica WX29 | Exclusion | 71.55 ± 0.86a | 65.92 ± 0.61b | 60.38 ± 0.93e | 39.71 ± 0.84g | 57.38 ± 0.93f | 63.63 ± 0.38d |
| Competition | 51.27 ± 0.56a | 52.6 ± 0.17a | 37.1 ± 0.4e | 23.44 ± 0.8f | 40.1 ± 0.4cd | 42.6 ± 1.06c | |
| Displacement | 36.46 ± 1.04b | 42.79 ± 0.78a | 47.89 ± 0.48de | 10.79 ± 0.48g | 27.46 ± 1.04f | 34.46 ± 1.04c | |
| P. brenneri | Exclusion | 58.63 ± 0.61e | 68.7 ± 0.83 cd | 45.63 ± 0.38f | 44.63 ± 0.38g | 73.97 ± 0.27a | 71.3 ± 0.94ab |
| Exclusion | 36.12 ± 0.34e | 50.2 ± 0.74b | 31.79 ± 0.23f | 31.79 ± 0.23f | 55.7 ± 0.82a | 44.46 ± 0.54c | |
| Competition | 26.13 ± 0.83e | 37.46 ± 0.36c | 41.46 ± 0.59b | 10.79 ± 0.48f | 47.46 ± 0.36a | 30.79 ± 0.48d | |
Every LAB strain could significantly prevent the adhesion of the indicator microorganisms to the IEC-6 cells. All the studied strains were inclined to exclude rather than displace or compete to prevent the indicator microorganisms to adhere to the IEC-6 cells owing to the comprehensive evaluation of inhibition, replacement and competition. In exclusion assay, all the strains showed high inhibition activity ranged from 24.4% to 80%. Meanwhile, the strains (g9 and s7) with higher adhesion activity showed significantly higher inhibition activity, among which, strain g9 could inhibit more than 70% of S. aureus and E. coli. In competition assay, the indicator microorganisms inhibited to adhere to the IEC-6 cells were from 21.3% to 64.3%. Most of the inhibitive ratio of the competitive samples was over 50%, especially strain g9 to S. aureus and E. coli and strain BLC to C. difficile. In displacement assay, the adhesion of the indicator microorganisms was inhibited from 10.8% to 47.9%. More than 40% of S. aureus, E. coli and S. enterica WX29 could be inhibited by strain g9 to adhere to IEC-6 cells as well as strain s7 to E. coli and S. enterica WX29, strain ss to S. enterica WX29 and P. brenneri and the strain grx19 to P. brenneri.
Generally, combining the assay of exclusion, competition and displacement, the capacity of the studied LAB strains inhibiting the indicator microorganisms to adhere to the IEC-6 cells was in accordance with their corresponding adherent activity. The strain 90–57 with the lowest adherent activity showed the weakest inhibition activity to all the indicator microorganisms in each inhibitive assay. However, high adhesion ability was not always associated with high inhibition capacity against indicator microorganisms. Some commercial strains with low adhesive ability had better inhibition ability compared to strains with high adhesive activity.24–26 In addition, most of the LAB strains in this study showed weak inhibitive effect to Gram-positive bacterium C. difficile in the inhibition assay compared with the other two Gram-positive indicator bacteria. Therefore, specific components of the indicator microorganisms could exist to influence the inhibition process. A large number of researches have reported that the ability of inhibition varies with the Lactobacillus species and the potential pathogens, even the same Lactobacillus genus.27 In conclusion, the microbial inhibition process of LAB strains was complicated and various determinants were involved in preventing indicator microorganisms interacting with intestinal epithelial cells. The specific mechanism of the inhibition process would be studied in our followed work.
3.4. Tolerance ability of the LAB strains to pepsin, trypsin, LiCl, NaIO4 and heat
The microbial adhesion inhibition process of LAB strains was complicated and various surface determinants were involved in their interaction with intestinal epithelial cells, including passive forces, electrostatic interactions, hydrophobic, steric forces, lipoteichoic acids and specific structures.9,10 The cell surface components were tightly related to the adhesion activity of the LAB strains. To further understand the mechanism of the adhesion, the feasible elements were primarily explored by separately treating the LAB strains with protease, chemical and heat (Table 3).| Treatment | Relative adhesion ratio (%) | |||||
|---|---|---|---|---|---|---|
| BLC | g9 | ss | 90–57 | grx19 | s7 | |
| a Data are mean values ± SD (n = 3). (a–f) significant difference (P < 0.05) among all lactic acid bacteria strains within the same column. | ||||||
| Pepsin | 50.38 ± 0.99b | 50.02 ± 0.37b | 51.5 ± 1.38b | 54.87 ± 0.95c | 56.38 ± 0.79c | 51.43 ± 1.04b |
| Trypsin | 63.31 ± 0.4de | 68.15 ± 0.84f | 58.3 ± 0.27c | 54.8 ± 0.62b | 57.16 ± 1.12c | 61.41 ± 0.16d |
| LiCl | 33.24 ± 0.37b | 34.98 ± 0.84b | 36.89 ± 0.57c | 39.18 ± 0.94d | 39.62 ± 0.82d | 43.63 ± 0.83e |
| NaIO4 | 81.81 ± 1.27c | 83.72 ± 1.07d | 78.22 ± 1.03b | 80.8 ± 1.63bc | 73.79 ± 0.71a | 79.63 ± 0.81b |
| Heat | 17.83 ± 1.17ab | 18.93 ± 0.61b | 22.72 ± 0.47d | 18.02 ± 0.86b | 23.51 ± 0.54d | 21.11 ± 0.71c |
The adhesion activity of the LAB strains was decreased by 43.6–51.9%, 31.9–56.5%, 56.4–70.8%, 16–26.2% and 76.5–83.3% treated by pepsin, trypsin, LiCl, NaIO4 and heat, respectively. The adhesion activity reduced more than 50% with the treatment of LiCl or heat suggesting that the main elements mediating adhesion between bacteria and IEC-6 cells were proteinaceous.
The surface proteins (weighted from 8 to 14 kDa) of the six LAB were separately isolated and then co-cultivated with the corresponding LAB strains removing the surface proteins. All the tested strains regained more than 30% of the adhesion activity to the IEC-6 cells (Fig. 3). These results were in good accordance with the reported studies which indicated that the major components responsible for bacterial adhesion to the intestinal mucin types or epithelial cells were protein nature in the genera of Lactobacillus and Bifidobacterium.28,29
LiCl treatment is taken as the usual method to remove the surface proteins.6 However, the residual adhesion activity of the LAB treated by LiCl could still be as high as 43.6%. The results obtained from these treatments indicated that not only the surface proteins but also some other components on the cells were deemed to influence the adhesion process, such as some heat sensitive non-protein compound. It was reported that lipoteichoic acid and exopolysaccharides could also contributed to adhesion to the host epithelial cells and mucus as well as the extracellular appendages, such as flagella, fimbriae and pili.6,30,31 Hence, the specific components on the cell surface of the LAB strains relating to the adhesion activity would be identified in the followed work.
4. Conclusion
Adhesion of probiotics to the intestinal mucosa is also important for the interaction between probiotic strains and the hos as well as modulation of the immune system and antagonism against pathogens. Thus, adhesion has been one of the main selection criteria for new probiotic strains and has been related to certain beneficial effects of probiotics. In this work, the adhesion properties of the six strains were studied. The hydrophobicity, aggregation activity and adhesion capacity to IEC-6 cells of the six strains were prominent (especially L. casei g9) and performed strain specific. Moreover, all the LAB were inclined to exclude rather than displace or compete to inhibit the indicator microorganisms to adhere to IEC-6 cells. The surface proteins (8–14 kDa) played an important role in the adhesion activities of the LAB. These results suggested that the six strains could tightly adhere to the gastrointestinal tract and this study will be beneficial for these strains to be used as probiotics in dairy food products.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The investigation was supported by a project funded by the National Natural Science Foundation of China (31700079), the Natural Science Foundation of Jiangsu Province (BK20170496) and the fund of China Scholarship Council.References
- M. Bermudez-Brito, J. Plaza-Diaz, S. Munoz-Quezada, C. Gomez-Llorente and A. Gil, Probiotic mechanisms of action, Ann. Nutr. Metab., 2012, 61(2), 160–174 CrossRef CAS PubMed.
- F. Rodriguez-Gomez, V. Romero-Gil, F. N. Arroyo-Lopez, J. C. Roldan-Reyes, R. Torres-Gallardo, J. Bautista-Gallego, P. Garcia-Garcia and A. Garrido-Fernandez, Assessing the challenges in the application of potential probiotic lactic acid bacteria in the large-scale fermentation of spanish-style table olives, Front. Microbiol., 2017, 8, 915 CrossRef PubMed.
- C. G. Vinderola, P. Mocchiutti and J. A. Reinheimer, Interactions among lactic acid starter and probiotic bacteria used for fermented dairy products, J. Dairy Sci., 2002, 85(4), 721–729 CrossRef CAS.
- M. Gupta and B. K. Bajaj, Functional characterization of potential probiotic lactic acid bacteria isolated from kalarei and development of probiotic fermented oat flour, Probiotics Antimicrob., 2018, 10(4), 654–661 CrossRef CAS PubMed.
- M. P. Mokoena, T. Mutanda and A. O. Olaniran, Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages, Food Nutr. Res., 2016, 60, 29630 CrossRef PubMed.
- R. Wang, L. Jiang, M. Zhang, L. Zhao, Y. L. Hao, H. Y. Guo, Y. Sang, H. Zhang and F. Z. Ren, The adhesion of Lactobacillus salivarius REN to a human intestinal epithelial cell line requires s-layerproteins, Sci. Rep., 2017, 7, 44029 CrossRef PubMed.
- Z. L. Sun, L. H. Huang, J. Kong, S. M. Hu, X. W. Zhang and W. T. Kong, In vitro evaluation of Lactobacillus crispatus K313 and K243: high-adhesion activity and anti-inflammatory effect on Salmonella braenderup infected intestinal epithelial cell, Vet. Microbiol., 2012, 159(1–2), 212–220 CrossRef CAS PubMed.
- B. Kos, J. Suskovic, S. Vukovic, M. Simpraga, J. Frece and S. Matosic, Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92, J. Appl. Microbiol., 2003, 94(6), 981–987 CrossRef CAS PubMed.
- M. Polak-Berecka, A. Wasko, R. Paduch, T. Skrzypek and A. Sroka-Bartnicka, The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus, Anton Leeuw Int J G, 2014, 106(4), 751–762 CrossRef CAS PubMed.
- C. J. P. Boonaert and P. G. Rouxhet, Surface of lactic acid bacteria: relationships between chemical composition and physicochemical properties, Appl. Environ. Microbiol., 2000, 66(6), 2548–2554 CrossRef CAS PubMed.
- U. Schillinger, C. Guigas and W. H. Holzapfel, In vitro adherence and other properties of lactobacilli used in probiotic yoghurt-like products, Int. Dairy J., 2005, 15(12), 1289–1297 CrossRef CAS.
- A. L. Servin, Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens, FEMS Microbiol. Rev., 2004, 28(4), 405–440 CrossRef CAS PubMed.
- H. Xu, H. S. Jeong, H. Y. Lee and J. Ahn, Assessment of cell surface properties and adhesion potential of selected probiotic strains, Lett. Appl. Microbiol., 2009, 49(4), 434–442 CrossRef CAS PubMed.
- R. P. Logan, A. Robins, G. A. Turner, A. Cockayne, S. P. Borriello and C. J. Hawkey, A novel flow cytometric assay for quantitating adherence of Helicobacter pylori to gastric epithelial cells, J. Immunol. Methods, 1998, 213(1), 19–30 CrossRef CAS PubMed.
- M. A. Ehrmann, P. Kurzak, J. Bauer and R. F. Vogel, Characterization of lactobacilli towards their use as probiotic adjuncts in poultry, J. Appl. Microbiol., 2002, 92(5), 966–975 CrossRef CAS PubMed.
- A. L. Servin and M. H. Coconnier, Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens, Best Practice & Research Clinical Gastroenterology, 2003, 17(5), 741–754 Search PubMed.
- M. Nikolic, B. Jovcic, M. Kojic and L. Topisirovic, Surface properties of Lactobacillus and Leuconostoc isolates from homemade cheeses showing auto-aggregation ability, Eur. Food Res. Technol., 2010, 231(6), 925–931 CrossRef CAS.
- J. Hirano, T. Yoshida, T. Sugiyama, N. Koide, I. Mori and T. Yokochi, The effect of Lactobacillus rhamnosus on enterohemorrhagic Escherichia coli infection of human intestinal cells in vitro, Microbiol. Immunol., 2003, 47(6), 405–409 CrossRef CAS PubMed.
- G. Perdigon, C. M. Galdeano, J. C. Valdez and M. Medici, Interaction of lactic acid bacteria with the gut immune system, Eur. J. Clin. Nutr., 2002, 56, S21–S26 CrossRef CAS PubMed.
- G. L. Le Blay, I. Fliss and C. Lacroix, Comparative detection of bacterial adhesion to Caco-2 cells with ELISA, radioactivity and plate count methods, J. Microbiol. Methods, 2004, 59(2), 211–221 CrossRef CAS PubMed.
- E. Sanchez, I. Nadal, E. Donat, C. Ribes-Koninckx, M. Calabuig and Y. Sanz, Reduced diversity and increased virulence-gene carriage in intestinal enterobacteria of coeliac children, BMC Gastroenterol., 2008, 8, 50 CrossRef PubMed.
- V. Santarmaki, Y. Kourkoutas, G. Zoumpopoulou, E. Mavrogonatou, M. Kiourtzidis, N. Chorianopoulos, C. Tassou, E. Tsakalidou, C. Simopoulos and P. Ypsilantis, Survival, intestinal mucosa adhesion, and immunomodulatory potential of Lactobacillus plantarum strains, Curr. Microbiol., 2017, 74(9), 1061–1067 CrossRef CAS PubMed.
- M. Gagnon, A. Z. Berner, N. Chervet, C. Chassard and C. Lacroix, Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion, J. Microbiol. Methods, 2013, 94(3), 274–279 CrossRef CAS PubMed.
- K. Gao, L. Liu, X. X. Dou, C. Wang, J. X. Liu, W. M. Zhang and H. F. Wang, Doses Lactobacillus reuteri depend on adhesive ability to modulate the intestinal immune response and metabolism in mice challenged with lipopolysaccharide, Sci. Rep., 2016, 6, 28332 CrossRef CAS PubMed.
- H. Jensen, S. Roos, H. Jonsson, I. Rud, S. Grimmer, J. P. Van Pijkeren, R. A. Britton and L. Axelsson, Role of Lactobacillus reuteri cell and mucus-binding protein A (CmbA) in adhesion to intestinal epithelial cells and mucus in vitro, Microbiology, 2014, 160, 671–681 CrossRef CAS PubMed.
- S. Sandes, L. Alvim, B. Silva, L. Acurcio, C. Santos, M. Campos, C. Santos, J. Nicoli, E. Neumann and A. Nunes, Selection of new lactic acid bacteria strains bearing probiotic features from mucosal microbiota of healthy calves: Looking for immunobiotics through in vitro and in vivo alpproaches for immunoprophylaxis applications, Microbiology, 2017, 200, 1–13 CAS.
- W. Zhang, H. Wang, J. Liu, Y. Zhao, K. Gao and J. Zhang, Adhesive ability means inhibition activities for lactobacillus against pathogens and s-layer protein plays an important role in adhesion, Anaerobe, 2013, 22, 97–103 CrossRef CAS PubMed.
- E. Izquierdo, M. Medina, S. Ennahar, E. Marchioni and Y. Sanz, Resistance to simulated gastrointestinal conditions and adhesion to mucus as probiotic criteria for Bifidobacterium longum strains, Curr. Microbiol., 2008, 56(6), 613–618 CrossRef CAS PubMed.
- B. Sanchez, P. Bressollier and M. C. Urdaci, Exported proteins in probiotic bacteria: adhesion to intestinal surfaces, host immunomodulation and molecular cross-talking with the host, FEMS Immunol. Med. Microbiol., 2008, 54(1), 1–17 CrossRef CAS PubMed.
- S. Lebeer, J. Vanderleyden and S. C. J. De Keersmaecker, Genes and molecules of Lactobacilli supporting probiotic action, Microbiol. Mol. Biol. Rev., 2008, 72(4), 728–764 CrossRef CAS PubMed.
- M. P. Velez, S. C. J. De Keersmaecker and J. Vanderleyden, Adherence factors of Lactobacillus in the human gastrointestinal tract, FEMS Microbiol. Lett., 2007, 276(2), 140–148 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |