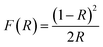Open Access Article
Open Access ArticleNovel hypophosphite hybrid perovskites of [CH3NH2NH2][Mn(H2POO)3] and [CH3NH2NH2][Mn(H2POO)2.83(HCOO)0.17] exhibiting antiferromagnetic order and red photoluminescence†
Mirosław Mączka *,
Anna Gągor
*,
Anna Gągor ,
Adam Pikul and
Dagmara Stefańska
,
Adam Pikul and
Dagmara Stefańska
Institute of Low Temperature and Structure Research, Polish Academy of Sciences, Box 1410, 50-950 Wrocław 2, Poland. E-mail: m.maczka@int.pan.wroc.pl
First published on 19th May 2020
Abstract
Hybrid perovskites based on hypophosphite ligands constitute an emerging family of compounds exhibiting unusual structures and offering a platform for construction of novel functional materials. We report the synthesis, crystal structure, and magnetic and optical properties of novel undoped and HCOO−-doped manganese hypophosphite frameworks templated by methylhydrazinium cations. The undoped compound crystallizes in a three-dimensional perovskite-like orthorhombic structure, space group Pnma, with ordered organic cations located in windows between the perovskite cages expanding along the a-direction. Both conventional anti-phase tilting, unconventional in-phase tilting and columnar shifts in the a-direction are present. Doping with HCOO− ions has a insignificant influence on the crystal structure but leads to a decrease of the unit cell volume. Magnetic studies indicate that these compounds order antiferromagnetically at TN = 6.5 K. Optical studies indicate that they exhibit red photoluminescence under 266 nm excitation with the activation energy for thermal quenching of 98 and 65 meV for the undoped and doped sample, respectively. For the undoped sample, the emission lifetime reaches 5.05 ms at 77 K but it decreases to 62.26 μs at 300 K. The low value of the activation energy and huge temperature dependence of photoluminescence intensity suggest a high potential of these hypophosphites for non-contact temperature sensing.
Introduction
Hybrid organic–inorganic compounds have been the subject of intense studies because their diverse structural and chemical variability offers unlimited opportunities for tuning their physical and chemical properties by chemical modification of the organic and/or inorganic part. One of the most important sub-groups of such hybrid compounds is halides, which have attracted a lot of attention in recent years due to their excellent optoelectronic properties.1–11 In particular, they are promising materials for applications in solar cells, which were shown to demonstrate power conversion efficiency of over 22%.1,6–9 They are also attractive materials exhibiting one-photon2–5,10 and multiphoton-excited upconversion photoluminescence.4,11 Another interesting class of compounds are molecular perovskites built of MIIO6 and MIIN6 octahedra (MII = Cd, Mg, Zn, Mn, Co, Fe, Cu, Ni) connected by short organic linkers such as formate (HCOO−), azide (N3−), cyanide (CN−), dicyanamide (N(CN)2−, dca−) and hypophosphite (H2PO2−), which possess large cavities occupied by organic cations.12–28 In this family of compounds, formates attracted a lot of attention due to their multiferroic properties14–16 while azides, dicyanamides and cyanides showed switchable dielectric behaviour, ferroelectric, luminescent, ferroelastic and non-linear optical properties.15–25 Dicyanamide-linked polymers were also shown to be promising barocaloric materials.26–28In contrast to widely studied formates, azides, dicyanamides and cyanides, family of hybrid perovskites based on hypophosphite ligand was discovered quite recently, in year 2017, and only six such compounds are known.29–32 The reported data show that this new emerging family of perovskites based on hypophosphite anion may show even more interesting physicochemical properties than formates, azides or dicyanamides. For instance, it was shown that whereas dimethylammonium (DMA+) cation is located in the center of the perovskite cage in multiferroic [DMA][Mn(HCOO)3], in the hypophosphite analogue this cation sits inside the windows between perovskite cages.30 This unusual behavior is related to different hydrogen bond network in formate and hypophosphite analogues, i.e., hydrogen bonds drive the structural distortion and off-centering in the hypophosphite.30 Furthermore, the known hypophosphites showed presence of unconventional tilts and columnar shifts.30,33 Thus, it has been argued that hypophosphites have higher than formates potential for symmetry breaking and this feature could lead to improper ferroelectricity or other functional properties.30 It is worth to add here that although magnetic data were reported only for two representatives, formamidinium (FA) and guanidinium (GUA) manganese hypophosphites, discovery of long-range magnetic order with a Néel temperature of 6.5 K for the latter compound29 proves that hypophosphite-based perovskites might also show interesting magnetic properties.
Since perovskite-like hypophosphites may exhibit various physicochemical properties, we have decided to search for novel perovskites constructed with H2POO− ligand. Herein, we show that a new hypophosphite can be synthesized using methylhydrazinium (MHy+) cations. We report studies of its structure as well as magnetic, optical and luminescent properties.
Experimental details
Materials and instrumentation
Manganese (II) carbonate (99.9%, Sigma-Aldrich), hypophosphorous acid (50% w/w aqueous solution, Supelco), methylhydrazine (98%, Sigma-Aldrich) and formic acid (98%, Sigma-Aldrich) were commercially available and used without further purification. Elemental analysis (C, H, N) was performed on a Elementar CHN/O FlashSmart analyzer. Powder X-ray diffraction was collected on X'Pert PRO powder diffractometer operating with Cu Kα radiation. Magnetization of a large number of freely oriented single crystals of [MHy][Mn(H2POO)3] (about 120 mg in total) and [MHy][Mn(H2POO)2.83(HCOO)0.17] (90 mg) was measured using a commercial SQUID magnetometer in the temperature range 1.7–300 K and in external magnetic fields up to 50 kOe. The background coming from a weakly diamagnetic sample holder was found to be negligible in comparison to the total signal, hence its subtraction was omitted. For measurements of the absorption spectra, the Varian Cary 5E UV-Vis-NIR spectrophotometer was used. Temperature-dependent emission spectra under 266 nm excitation from a diode laser (10 mW power) were measured with the Hamamatsu photonic multichannel analyzer PMA-12 equipped with a BT-CCD linear image sensor. The temperature was controlled applying Linkam THMS 600 Heating/Freezing Stage. Decay profiles were recorded with a Lecroy digital oscilloscope and the Nd:YAG laser as an excitation source.Synthesis
In order to grow single crystals of [MHy][Mn(H2POO)3], manganese carbonate (0.575 g, 5 mmol) was dissolved in hypophosphorous acid (6.46 mL, 60 mmol). The solution was heated to 50 °C and stirred. Then 10 mmol of methylhydrazine (0.5 mL) was added and the mixture was left at 50 °C in air. Faint pink crystals that grew overnight were separated from the mother liquid, washed with methanol and dried at room temperature. Anal. Calcd for [MHy][Mn(H2POO)3] (%): C, 4.03; H, 4.42; N, 9.29; found (%): C, 4.04; H, 4.38; N, 9.43. Comparison of the powder XRD pattern with the calculated one based on the single-crystal data attests phase purity of powdered sample (Fig. S1 in the ESI†).We also tried to obtain mixed formate–hypophosphite crystals using the same procedure and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of hypophosphorous and formic acid. Unfortunately, the obtained crystals contained small amount of HCOO−. Chemical composition found for [MHy][Mn(H2POO)3−x (HCOO)x] (%): C, 4.77; H, 4.43; N, 9.51. From C/N ratio, x = 0.17.
1 molar ratio of hypophosphorous and formic acid. Unfortunately, the obtained crystals contained small amount of HCOO−. Chemical composition found for [MHy][Mn(H2POO)3−x (HCOO)x] (%): C, 4.77; H, 4.43; N, 9.51. From C/N ratio, x = 0.17.
Single-crystal X-ray diffraction
The single-crystal X-ray diffraction was performed for pure [MHy][Mn(H2POO)3] at room temperature (RT) and 100 K, and for formate doped crystals of [MHy][Mn(H2POO)2.83(HCOO)0.17] formula at RT. The four-circle Xcalibur diffractometer operating with Mo Kα radiation source and CCD Atlas detector was used for data collection. Absorption was corrected by multi-scan methods in CrysAlis PRO 1.171.38.43 (Rigaku Oxford Diffraction, 2015). The details of the crystals, data collections and refinements are given in Table S1.† The low-temperature refinement confirmed the stability of the orthorhombic Pnma symmetry down to 100 K. Table S2† shows selected geometrical parameters. For all structures, H-atoms were introduced at calculated positions and refined with constrained parameters. In the formate doped crystals the dopant amount was constrained to the value obtained from the elemental analysis. However, free refinement gave very similar results. Formate groups were localized only in one of two symmetry allowed sites, at the position of hypophosphite ligand linking two Mn2+ centers in the evident anti–anti bonding.Results and discussion
Single-crystal X-ray diffraction
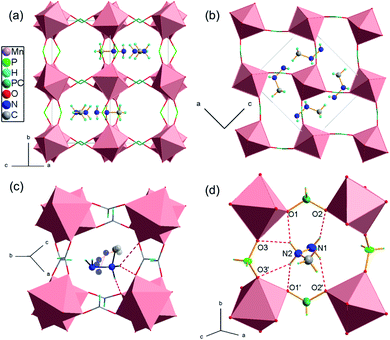
[MHy][Mn(H2POO)3] crystallizes in the orthorhombic system, in the Pnma space group. The inorganic part of the structure comprises one symmetry independent metal center and two distinct hypophosphite ligands, one H2POO− unit of C1 symmetry that connects neighbouring Mn2+ ions in (101) planes through anti–anti bonding, with two P–O–Mn angles of 127° and 130°, and one-half of another hypophosphite group of Cs symmetry that acts as a linker along [010] direction with P–O–Mn angles around 157°. This flexible binding capability in conjunction with the H-bonding-driven off-centering of cations leads to a considerably different structure compared to the formate analogue. In [MHy][Mn(HCOO)3] the planar anti–anti connection modes in combination with the narrow range of binding angles, which is characteristic to formates, prevent large framework distortions and cations displacements from the centers of perovskite cages, where they perform thermally induced rotations.14 The adjustable hypophosphite framework in [MHy][Mn(H2POO)3] accommodates notable off-center shifts of MHy+ (Fig. 1a and b). The high obtuse P–O–Mn angle opens the window between the perovskite cages and allows for location of MHy+ inside these windows expanding along the a-direction. The off-center placement of amines is driven by the N–H⋯OH-bonding and results in the lower symmetry of hypophosphite framework (Pnma) compared to the formate (R3c at RT). Similar, strongly off-centered distribution of protonated amines and lowering of symmetry was encountered in [DMA][Mn(H2POO)3] and [GUA][Mn(H2POO)3].29,30 Opposite to formate analogue, MHy+ cations are ordered at RT and interact with hypophosphite oxygen atoms via hydrogen bond interactions of medium strength (Fig. 1c and d). It is worth noting that both amine groups are involved in H-bonds, opposite to the formate analogue, where only the middle NH2 groups may interact with the framework at RT. As a result, the H-bonds in the hypophosphite are strong enough to overcome thermally induced rotational motions of MHy+ and these cations are ordered. The hydrogen bonds parameters are given in Table 1. Fig. S2† shows the placement of MHy+ cations in the structure in the space feeling model.| D–H⋯A | D–H (Å) | H⋯A (Å) | D⋯A (Å) | D–H⋯A (°) |
|---|---|---|---|---|
| a Symmetry code(s): (i) x, −y + 1/2, z; (ii) −x + 1/2, y − 1/2, z + 1/2; (iii) −x + 1/2, −y + 1, z + 1/2. | ||||
| I | ||||
| N2–HB⋯O1i | 0.89 | 2.08 | 2.875 (2) | 148.4 |
| N2–HB⋯O3ii | 0.89 | 2.53 | 3.176 (5) | 130.5 |
| N2–HC⋯O1 | 0.89 | 2.08 | 2.875 (2) | 148.4 |
| N2–HC⋯O3iii | 0.89 | 2.53 | 3.176 (5) | 130.5 |
| N1–HA⋯O2i | 0.87 | 2.24 | 3.001 (3) | 146.8 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| II | ||||
| N2–HB⋯O1i | 0.89 | 2.05 | 2.8461 (18) | 147.7 |
| N2–HB⋯O3ii | 0.89 | 2.48 | 3.122 (3) | 129.6 |
| N2–HC⋯O1 | 0.89 | 2.05 | 2.8461 (18) | 147.7 |
| N2–HC⋯O3iii | 0.89 | 2.48 | 3.122 (3) | 129.6 |
| N1–HA⋯O2i | 0.86 | 2.20 | 2.977 (2) | 150.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| III | ||||
| N2–HB⋯O1i | 0.89 | 2.08 | 2.871 (2) | 148.0 |
| N2–HB⋯O3ii | 0.89 | 2.51 | 3.164 (4) | 130.6 |
| N2–HC⋯O1 | 0.89 | 2.08 | 2.871 (2) | 148.0 |
| N2–HC⋯O3iii | 0.89 | 2.51 | 3.164 (4) | 130.6 |
| N1–HA⋯O2i | 0.87 | 2.21 | 2.994 (2) | 150.1 |
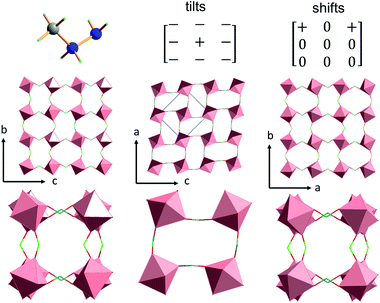
The inorganic substructure yields R5− distortion mode and propagation vector k = [½, ½, ½]. Fig. 2 shows tilt and shift matrixes together with the structure viewed along the normal to the three pseudo-perovskite cubic axes. The conventional anti-phase tilting is present down the cubic a- and b-direction and in all (100), (010), (001) planes. Toward the b-direction, the structure accommodates unconventional in-phase tilting. The shift matrix is similar to that found in [GUA][Mn(H2POO)3]. There are active shifts in the a-direction with in-phase correlation in the c-direction.
In the formate doped [MHy][Mn(H2POO)3], the formate groups seem to locate exclusively in the position of C1 symmetry that links two Mn2+ centres via pronounced anti–anti coordination mode with two P–O–Mn angles of 128° and 130°. Such a preference is in line with the fact that formates strongly prefer anti–anti bonding in the perovskite topology.33 The relatively low concentration of HCOO− groups has insignificant influence on the crystal structure; however, it slightly disturbs the crystal volume that decreases from 1078 Å3 in pure hypophosphite to 1071 Å3 in doped one.
Magnetic studies
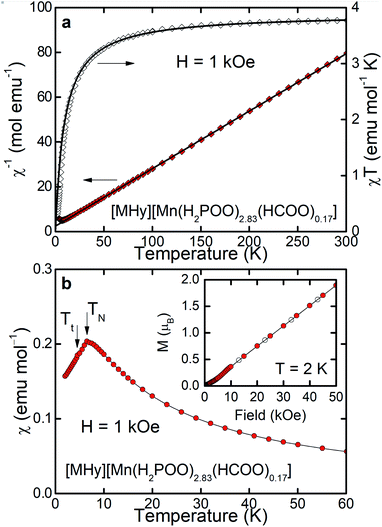
As can be inferred from Fig. 3a, [MHy][Mn(H2POO)2.83(HCOO)0.17] exhibits at elevated temperatures paramagnetic behavior with linear T-dependence of χ−1 above about 10 K. The experimental curve can be described in this temperature range by the conventional Curie–Weiss law χ(T) = C(T − θp), where C is the Curie constant and θp is the paramagnetic Curie–Weiss temperature. Least-squares fitting procedure yielded the values C = 3.89(1) emu mol−1 K and θp = −9.4(1) K (see the thick solid line in Fig. 3a, left axis).The effective magnetic moment μeff derived from the Curie constant as 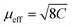 is equal to 5.58(1) μB. This value is lower than (but still close to) the theoretical value calculated for Mn2+ ions with the electron configuration 3d5: the effective magnetic moment calculated for such a configuration within the Russel–Saunders coupling scheme (S = 5/2, L = 0, J = 5/2 and gJ = 2) is equal to
is equal to 5.58(1) μB. This value is lower than (but still close to) the theoretical value calculated for Mn2+ ions with the electron configuration 3d5: the effective magnetic moment calculated for such a configuration within the Russel–Saunders coupling scheme (S = 5/2, L = 0, J = 5/2 and gJ = 2) is equal to 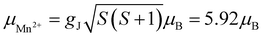 (for details see e.g. ref. 34).
(for details see e.g. ref. 34).
The negative value of θp indicates presence of predominant antiferromagnetic coupling between the magnetic moments of Mn2+, which can be inferred also from the overall behavior of the product χT (Fig. 3a, right axis). In particular, at RT χT achieves a value of about 3.8 emu mol−1 K, which is close to the experimental value of the Curie constant (i.e. 3.89 emu mol−1 K) and noticeably lower that the theoretical value of the Curie constant expected for non-interacting Mn2+ ions, i.e. 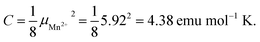 As a consequence of the postulated antiferromagnetic coupling, the χT(T) curve bends towards lower values upon decreasing temperature.
As a consequence of the postulated antiferromagnetic coupling, the χT(T) curve bends towards lower values upon decreasing temperature.
As can be inferred from Fig. 3b, the compound orders antiferromagnetically at TN = 6.5 K, absolute value of which is of the same order of magnitude as θp derived from the Curie–Weiss fit. Linear field dependence of the magnetization M(H) of [MHy][Mn(H2POO)2.83(HCOO)0.17] (see the inset to Fig. 3b) and lack of any hysteresis in M(H) confirm the latter hypothesis. At the highest field applied M achieves a value of 1.89 μB, which is far from the saturated magnetic moment expected for Mn2+, i.e. μsat = gJ = 5 μB, corroborating once more the antiferromagnetic character of the ordering.
It must be noted, however, that in the magnetically ordered range another anomaly in χ(T) is hardly visible at Tt = 4.5 K, which can be associated with a tiny deviation of M(H) from linearity at about 5–10 kOe. We suppose that this anomaly manifests presumably some small rearrangement of antiferromagnetically ordered moment of Mn2+ ions, but making final conclusion is of course difficult without more sophisticated experiments.
Results of magnetization measurements carried out for [MHy][Mn(H2POO)3] are gathered in Fig. 4. As can be seen, its magnetic properties are very similar to those observed for the HCOO−-doped compound. In particular, the Curie–Weiss fit performed for [MHy][Mn(H2POO)3] (see the solid lines in Fig. 4a) yielded the parameters: C = 4.08(1) emu mol−1 K and θp = −9.3(1) K (see the thick solid line in Fig. 4a, left axis) – very close to those obtained for [MHy][Mn(H2POO)2.83(HCOO)0.17]. The effective magnetic moment derived from C is of about 5.71 μB, which is even closer to the theoretical one (5.92 μB). The RT value of χT (i.e. 3.95 emu mol−1 K) is close to the experimental value of the Curie constant (4.08 emu mol−1 K) and the χT(T) curve bends towards lower values upon cooling down the sample. The long-range antiferromagnetic order occurs in the [MHy][Mn(H2POO)3] compound at the Néel temperature of 6.5 K and is followed by a tiny anomaly at Tt = 4.5 K (see the inset to Fig. 4b), being exactly the same as in [MHy][Mn(H2POO)2.83(HCOO)0.17]. The antiferromagnetic order of [MHy][Mn(H2POO)3] is confirmed by linear variation of M(H) (see the inset to Fig. 4b) with hardly visible deviation at about 5–10 kOe, presumably associated with the spin-rearrangement detected at Tt. It is worth adding that the same Néel temperature and very similar Weiss constant of −9.3(1) K were previously reported also [GUA][Mn(H2POO)3] whereas [FA][Mn(H2POO)3] do not order magnetically down to 2 K.29 It should also be noticed that the formate analogue, [MHy][Mn(HCOO)3], also exhibits antiferromagnetic order but with slightly larger TN = 9.0 K.14 This behavior is consistent with stronger superexchange interactions in the formate compared to hypophosphite due to shorter Mn⋯Mn distances in [MHy][Mn(HCOO)3] (6.0297–6.1187 Å at 100 K)14 compared to [MHy][Mn(H2POO)3] (6.277 and 6.6302 Å at 100 K).
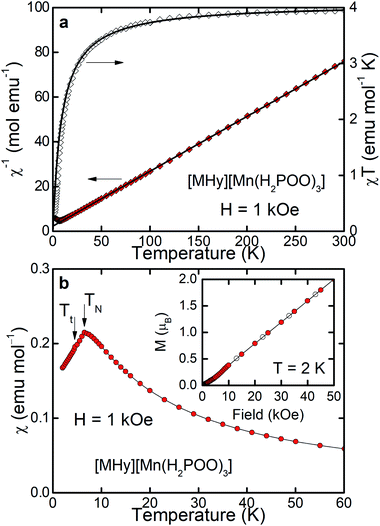 | ||
| Fig. 4 Magnetic properties of [MHy][Mn(H2POO)3] measured and displayed in the same way as properties of [MHy][Mn(H2POO)2.83(HCOO)0.17] shown in Fig. 3., i.e., panel (a) shows inverse magnetic susceptibility χ−1 and product χT whereas panel (b) illustrates temperature dependence of χ. | ||
The observed similarity of the magnetic properties of the systems studied is not a big surprise. Although the crystal structures of the parent systems [MHy][Mn(H2POO)3] and [MHy][Mn(HCOO)3] are considerably different, the concentration of the HCOO groups in [MHy][Mn(H2POO)2.83(HCOO)0.17] is very small, leading to very small modification of the crystal structure. Therefore, one can expect that the leading factors governing the magnetic ground state of [MHy][Mn(H2POO)2.83(HCOO)0.17], i.e. the oxidation state (and hence the electron configuration) of the manganese ions, their environment (local symmetry and ligands), and the exchange interactions between Mn2+ (depending on i.a. Mn–Mn distances and P–O–Mn angles), remain nearly the same as in [MHy][Mn(H2POO)3].
Optical studies
Diffuse reflectance spectra of the studied samples are shown in Fig. S3 and S4.† The spectra consist of an intense band centered at 220 nm (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455 cm−1) associated with matrix absorption. Above 250 nm the broad tail ascribed to overlapped O2−–Mn2+ charge transfer (CT) band with the host absorption band was detected. Moreover, a few peaks with smaller intensity are observed in the 300–550 nm range that are characteristic for Mn2+ ions in octahedral coordination (Fig. S3 and S4†). These bands are more clearly observed for [MHy][Mn(H2POO)2.83(HCOO)0.17] and they can be attributed to the electron transitions from 6A1g(S) ground level to 4T1g(P) (312 nm), 4E(D) (339 nm), 4T2g(D) (357 nm), 4A1g (401 nm), 4Eg(G) (404 nm), 4T2g(G) (440 nm) and 4T1g(G) (527 nm) excited states.35–37
455 cm−1) associated with matrix absorption. Above 250 nm the broad tail ascribed to overlapped O2−–Mn2+ charge transfer (CT) band with the host absorption band was detected. Moreover, a few peaks with smaller intensity are observed in the 300–550 nm range that are characteristic for Mn2+ ions in octahedral coordination (Fig. S3 and S4†). These bands are more clearly observed for [MHy][Mn(H2POO)2.83(HCOO)0.17] and they can be attributed to the electron transitions from 6A1g(S) ground level to 4T1g(P) (312 nm), 4E(D) (339 nm), 4T2g(D) (357 nm), 4A1g (401 nm), 4Eg(G) (404 nm), 4T2g(G) (440 nm) and 4T1g(G) (527 nm) excited states.35–37
Using the RT diffuse reflectance spectra, we determined the energy bandgap (Eg) of investigated hypophosphites with the Kubelka–Munk relation:38
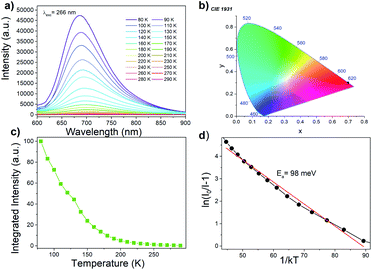
[MHy][Mn(H2POO)3] exhibits red emission at 80 K with maximum at 686 nm (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 577 cm−1, CIE chromacity (0.7, 0.3)) (Fig. 5a and b) that can be attributed to the 4T1g(G) → 6A1g(S) transition of Mn2+ ions. This emission is red-shifted compared to Mn-based dicyanamides (maximum near 630 nm) and perovskite-type pyrrolidinium manganese halides with octahedral coordination of Mn2+ ions (maximum near 615–645 nm).28,37,39 Since the energy of the 4T1g(G) → 6A1g(S) transition decreases with increasing crystal field strength,40 the observed red shift of the emission points to higher crystal field strength in the studied hypophosphites.
577 cm−1, CIE chromacity (0.7, 0.3)) (Fig. 5a and b) that can be attributed to the 4T1g(G) → 6A1g(S) transition of Mn2+ ions. This emission is red-shifted compared to Mn-based dicyanamides (maximum near 630 nm) and perovskite-type pyrrolidinium manganese halides with octahedral coordination of Mn2+ ions (maximum near 615–645 nm).28,37,39 Since the energy of the 4T1g(G) → 6A1g(S) transition decreases with increasing crystal field strength,40 the observed red shift of the emission points to higher crystal field strength in the studied hypophosphites.
The Mn2+ emission quenches very quickly (Fig. 5c), much faster than emission of tetrapropylammonium manganese and tributylammonium manganese discyanamides.28,37 Such pronounced temperature dependence of emission intensity suggests that [MHy][Mn(H2POO)3] has high potential for noncontact temperature sensing. The energy activation for thermal quenching, Ea, was calculated using Arrhenius formula:41
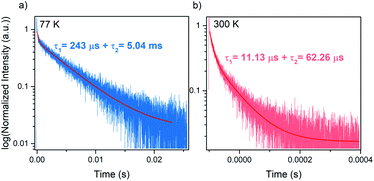
Luminescence decay can be can be best fitted by bi-exponential decay function with τ1 = 243 μs and τ2 = 5.05 ms at 77 K (Fig. 6a). The shorter life time can be most likely attributed to nonradiative processes. The emission lifetime strongly decreases with increasing temperature and at 300 K equals to τ1 = 11.13 μs and τ2 = 62.26 μs (Fig. 6b). The significant shortening of the decay time with temperature indicates that nonradiative processes play an important role in the investigated hypophosphite.42 It is worth adding that significantly shorter lifetime was reported for tetrapropylammonium manganese dicyanamide (5.88 μs at 300 K), for which the luminescence decay was monoexponential.37
Very similar emission is also observed for [MHy][Mn(H2POO)2.83(HCOO)0.17] (Fig. S7†). However, doping with HCOO− ions leads to decrease of the activation energy for thermal quenching to 65 meV (Fig. S7d†). Furthermore, the lifetime of the emission measured at 77 K is approximately two times shorter compared to the undoped sample (Fig. S8†).
A possible mechanism of Mn2+ luminescence in the investigated samples is presented in Fig. S9.† Excitation at UV region (266 nm) leads to transfer of the electron to the O2−–Mn2+ CT band. Subsequently, nonradiative depopulation of the CT band leads to the population of the 4T1(G) excited state of Mn2+. Similar excitation path was recently reported by Lin et. al.43 With the increasing temperature, the 4T1(G) excited state of Mn2+ is depopulated due to the intercrossing of its parabola with the parabola of 6A1g(S) ground state. This process is thermally dependent with the activation energy of 98 and 65 meV for [MHy][Mn(H2POO)3] and [MHy][Mn(H2POO)2.83(HCOO)0.17], respectively.
Conclusions
We report that MHy+ cations can be used for construction of a new manganese hypophosphite perovskite-like framework. [MHy][Mn(H2POO)3] crystallizes in the orthorhombic system, in the Pnma space group. This is the highest symmetry reported up to now for any hypophosphite framework templated by monovalent cations. The characteristic feature of the crystal structure is strongly off-centered distribution of MHy+ cations. Studies of magnetic properties reveal that this compound orders antiferromagnetically at 6.5 K. Thus, [MHy][Mn(H2POO)3] is the second perovskite-type hypophosphite exhibiting magnetic order.We also report studies of optical properties of [MHy][Mn(H2POO)3] and [MHy][Mn(H2POO)2.83(HCOO)0.17] samples. To the best of our knowledge, this is the first report on such studies for of any hypophosphite. These studies show that both samples exhibit red emission at 77 K with the energy activation for thermal quenching of 98 and 65 meV for undoped and HCOO−-doped sample, respectively. The emission decay time at 77 K is of ms order and it decreases for the doped sample, indicating enhancement of nonradiative processes on HCOO− doping. Interestingly, in contrast to manganese dicyanamide frameworks, exhibiting relatively weak temperature dependence of the emission intensity, the Mn2+ emission of [MHy][Mn(H2POO)3] and [MHy][Mn(H2POO)2.83(HCOO)0.17] quenches very quickly suggesting a high potential of these hypophosphites for non-contact temperature sensing.
Conflicts of interest
There are no conflicts of interest to declareAcknowledgements
This research was supported by the National Science Center (Narodowe Centrum Nauki) in Poland under project no. 2018/31/B/ST5/00455.References
- W. Li, Z. Wang, F. Deschler, S. Gao, R. H. Friend and A. K. Cheetham, Nature Rev. Mater., 2017, 2, 16099 CrossRef.
- B. Saparov and D. B. Mitzi, Chem. Rev., 2016, 116, 4558–4596 CrossRef CAS PubMed.
- M. Mączka, M. Ptak, A. Gągor, D. Stefańska and A. Sieradzki, Chem. Mater., 2019, 31, 8563–8575 CrossRef.
- M. Mączka, M. Ptak, A. Gągor, D. Stefańska, J. K. Zaręba and A. Sieradzki, Chem. Mater., 2020, 32, 1667–1673 CrossRef.
- M. Mączka, A. Gagor, J. K. Zaręba, D. Stefanska, M. Drozd, S. Balciunas, M. Šimėnas, J. Banys and A. Sieradzki, Chem. Mater., 2020, 32, 4072–4082 CrossRef.
- X. Zeng, T. Zhou, C. Leng, Z. Zang, M. Wang, W. Hu, X. Tang, S. Lu, L. Fang and M. Zhou, J. Mater. Chem. A, 2017, 5, 17499–17505 RSC.
- M. Wang, Z. Zang, B. Yang, X. Hu, K. Sun and L. Sun, Sol. Energy Mater. Sol. Cells, 2018, 185, 117–123 CrossRef CAS.
- M. Wang, H. Wang, W. Li, X. Hu, K. Sun and Z. Zang, J. Mater. Chem. A, 2019, 7, 26421–26428 RSC.
- T. Zhou, M. Wang, Z. Zang and L. Fang, Adv. Energy Mater., 2019, 1900664 CrossRef.
- X. Zhao, J. D. A. Ng, R. H. Friend and Z.-K. Tan, ACS Photonics, 2018, 5, 3866–3875 CrossRef CAS.
- W. Chen, S. Bhaumik, S. A. Veldhuis, G. Xing, Q. Xu, M. Grätzel, S. Mahaisalkar, N. Mathews and T. C. Sum, Nature Commun, 2017, 8, 15198 CrossRef CAS PubMed.
- R. Shang, S. Chen, S. M. Wang and S. Gao, in Metal-Organic Framework Materials, ed. R. L. MacGillivray and C. M. Lukehart, John Wiley & Sons Ltd, 2014, pp. 221–238 Search PubMed.
- W.-J. Xu, Z.-Y. Du, W.-X. Zhang and X.-M. Chen, CrystEngComm, 2016, 18, 7915–7928 RSC.
- M. Mączka, A. Gągor, M. Ptak, W. Paraguassu, T. A. da Silva, A. Sieradzki and A. Pikul, Chem. Mater., 2017, 29, 2264–2275 CrossRef.
- Y. Tian, A. Stroppa, Y. Chai, L. Yan, S. Wang, P. Barone, S. Picozzi and Y. Sun, Sci. Rep., 2014, 4, 6062 CrossRef CAS PubMed.
- L. C. Gómez-Aguirre, B. Pato-Doldán, J. Mira, S. Castro-García, M. A. Señarís-Rodríguez, M. Sánchez-Andújar, J. Singleton and V. S. Zapf, J. Am. Chem. Soc., 2016, 138, 1122–1125 CrossRef PubMed.
- W.-J. Xu, P.-F. Li, Y.-Y. Tang, W.-X. Zhang, R.-G. Xiong and X.-M. Chen, J. Am. Chem. Soc., 2017, 139, 6369–6375 CrossRef CAS PubMed.
- M. Trzebiatowska, A. Gagor, L. Macalik, P. Peksa and A. Sieradzki, Dalton Trans., 2019, 48, 15830–15840 RSC.
- Z.-Y. Du, T.-T. Xu, B. Huang, Y.-J. Su, W. Xue, C.-T. He, W.-X. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2015, 54, 914–918 CrossRef CAS PubMed.
- M. Trzebiatowska, M. Mączka, M. Ptak, L. Giriunas, S. Balciunas, M. Simenas, D. Klose and J. Banys, J. Phys. Chem. C, 2019, 123, 11840–11849 CrossRef CAS.
- F.-J. Geng, L. Zhou, P.-P. Shi, X.-L. Wang, X. Zheng, Y. Zhang, D.-W. Fu and Q. Ye, J. Mater. Chem. C, 2017, 5, 1529–1536 RSC.
- M. Mączka, M. Ptak, A. Gągor, A. Sieradzki, P. Peksa, G. Usevicius, M. Simenas, F. Furtado Leite and W. Paraguassu, J. Mater. Chem. C, 2019, 7, 2408–2420 RSC.
- M. M. Zhao, L. Zhou, P.-P. Shi, X. Zheng, X.-G. Chen, J.-X. Gao, L. He, Q. Ye, C.-M. Liu and D.-W. Fu, Chem.–Eur. J., 2019, 25, 6447–6454 CrossRef CAS PubMed.
- M. Mączka, A. Gągor, M. Ptak, D. Stefańska and A. Sieradzki, Phys. Chem. Chem. Phys., 2018, 20, 29951–29958 RSC.
- M. Mączka, I. E. Collings, F. Furtado Leite and W. Paraguassu, Dalton Trans., 2019, 48, 9072–9078 RSC.
- (a) J. M. Bermúdez-Garcia, M. Sánchez-Andújar, S. Castro-García, J. López-Beceiro, R. Artiaga and M. A. Señarís-Rodríguez, Nature Comm, 2017, 8, 15715 CrossRef PubMed; (b) J. M. Bermúdez-Garcia, M. Sánchez-Andújar and M. A. Señarís-Rodríguez, J. Phys. Chem. Lett., 2017, 8, 4419–4423 CrossRef PubMed.
- J. M. Bermúdez-Garcia, S. Yáñez-Vilar, A. Garcia-Fernández, M. Sánchez-Andújar, S. Castro-García, J. López-Beceiro, R. Artiaga, M. Dilshad, X. Moya and M. A. Señarís-Rodríguez, J. Mater. Chem. C, 2018, 6, 9867–9874 RSC.
- M. Mączka, A. Gągor, M. Ptak, D. Stefańska, L. Macalik, A. Pikul and A. Sieradzki, Dalton Trans., 2019, 48, 13006–13016 RSC.
- Y. Wu, S. M. Shaker, F. Brivio, R. Murugavel, P. D. Bristowe and A. K. Cheetham, J. Am. Chem. Soc., 2017, 139, 16999–17002 CrossRef CAS PubMed.
- Y. Wu, T. Binford, J. A. Hill, S. Shaker, J. Wang and A. K. Cheetham, Chem. Commun., 2018, 54, 3751–3754 RSC.
- Y. Wu, D. M. Halat, F. Wei, T. Binford, I. D. Seymour, M. W. Gaultois, S. Shaker, J. Wang, C. P. Grey and A. K. Cheetham, Chem. European J., 2018, 44, 11309–11313 CrossRef PubMed.
- M. Ptak and M. Mączka, Spectrochim. Acta A, 2020, 230, 118010 CrossRef CAS PubMed.
- H. L. B. Boström, CrysEngComm, 2020, 22, 961–968 RSC.
- S. Blundell, Magnetism in Condensed Matter (Oxford Master Series in Condensed Matter Physics), Oxford University Press, New York, 2001 Search PubMed.
- P. G. Manning, Can. Mineral., 1968, 9, 348–357 CAS.
- M. Li, V. Smetana, M. Wilk-Kozubek, Y. Mudryk, T. Alammar, V. K. Pecharsky and A.-V. Mudring, Inorg. Chem., 2017, 56, 11104–11112 CrossRef CAS PubMed.
- M. Mączka, D. Stefańska, J. K. Zaręba, M. Nyk and A. Sieradzki, J. Alloys Compds., 2020, 821, 153464 CrossRef.
- P. Kubelka and F. Munk, Z. Tech. Phys., 1931, 12, 593–601 Search PubMed.
- C. Jiang, H. Fu, Y. Han, D. Li, H. Lin, B. Li, X. Meng, H. Peng and J. Chu, Cryst. Res. Technol., 2019, 54, 1800236 CrossRef.
- S. Sugano, Y. Tanabe and Y. H. Kamimura, Multiplates of Transition-Metal Ions in Crystals, Academic, New York, 1970 Search PubMed.
- A. Watras, A. Matraszek, P. Godlewska, I. Szczygieł, J. Wojtkiewicz, B. Brzostowski, G. Banach, J. Hanuza and P. J. Dereń, Phys. Chem. Chem. Phys., 2014, 16, 5581–5588 RSC.
- M. Ptak, A. Gągor, A. Sieradzki, B. Bondzior, P. Dereń, A. Ciupa, M. Trzebiatowska and M. Mączka, Phys. Chem. Chem. Phys., 2017, 19, 12156–12166 RSC.
- S. Lin, H. Lin, C. Ma, Y. Cheng, S. Ye, F. Lin, R. Li, J. Xu and Y. Wang, Light Sci. Appl., 2020, 9, 22 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1 and S2: crystallographic data. Fig. S1–S9: XRD patterns, the position of organic cations in the structure, absorption spectra, temperature-dependent photoluminescence, colour coordinate and emission decay curves for HCOO−-doped compound, schematic illustration of luminescence mechanism. CCDC 1989064–1989066. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra03397a |
| This journal is © The Royal Society of Chemistry 2020 |