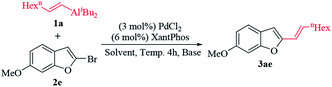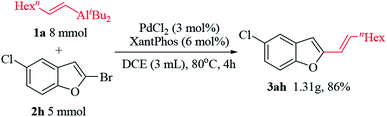Open Access Article
Open Access ArticlePalladium-catalyzed cross-coupling reaction of alkenyl aluminums with 2-bromobenzo[b]furans†
Chang Wen‡
,
Xin Jiang‡,
Kun Wu,
Ruiqiang Luo and
Qinghan Li *
*
College of Chemistry and Environmental Protection Engineering, Southwest University for Nationalities, Chengdu, 610041, China. E-mail: lqhchem@163.com; lqhchem@swun.cn; Fax: +86-28-8552-4382; Tel: +86-28-8592-8015
First published on 22nd May 2020
Abstract
Highly efficient and simple cross-coupling reactions of 2-bromobenzo[b]furans with alkenylaluminum reagents for the synthesis of 2-alkenylbenzo[b]furan derivatives using PdCl2 (3 mol%)/XantPhos (6 mol%) as catalyst are reported. Excellent yields (up to 97%) were obtained for a wide range of substrates at 80 °C for 4 h in DCE.
2-Substituted benzo[b]furans are important structural scaffolds found in many natural products and pharmaceutical products.1 Some of these compounds have been known to exhibit anti-inflammatory,2 antitumor,3 anticancer,4 and anti-fungal,5 antiplasmodial,6 antioxidant,7 anti-HIV,8 and estrogenic activities.9 In addition, they serve as building blocks for many organic transformations.10 Thus, their synthesis and applications have attracted considerable attention in the chemical and pharmaceutical industries over the past decades.11 Until now numerous effective synthetic methodologies of synthesis 2-substituted benzo[b]furans have been reported.12,13 Among these methods hitherto developed, the metal-catalyzed 2-halobenzo[b]furans coupling with organometallic nucleophiles is one of the most effective methods (Scheme 1).13 However, in most cases generally suffer from one or more drawbacks such as requirement co-catalyst like Cu salts, limited substrate scope, high catalyst loading, high temperature and poor chemoselectivity etc. Therefore, the development of more efficient and atom economical approaches for the preparation of 2-substituted benzo[b]furans remains as desirable work. Previous studies show that organoaluminum reagents are highly efficient nucleophiles for cross-coupling reactions with aromatic halides14 or benzylic halides,15 and the investigations have demonstrated that palladium is a good catalytic metal.16
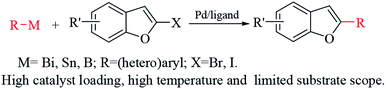 | ||
| Scheme 1 Palladium-catalyzed cross-coupling reactions of 2-halobenzo[b]furans derivatives with organometallic nucleophiles. | ||
At present, a variety of methods have been developed to prepare compounds containing olefin functional groups through hydrocarbon activation of olefins.17 To continue our effort to develop coupling reactions using reactive organoaluminum reagents,18 we herein report a palladium(II)-catalyzed, base free cross-coupling reactions of 2-bromo benzo[b]furans with alkenylaluminum reagents at 80 °C in short reaction time with good to excellent isolated yields for 2-alkenyl benzo[b]furans. The process was simple and easily performed, and it provides an efficient method for the synthesis of 2-alkenyl benzo[b]furans derivatives. Notably, in our procedure palladium is used as the single catalyst and base free.
Our initial studies used 2-bromo-6-methoxybenzo[b]furan (2e) with diethyl(oct-1-enyl)aluminum (1a) as model substrates. Treatment of compound 2e with the alkenylaluminum (1a) using PdCl2 (3 mol%)/XantPhos (6 mol%) as catalyst in toluene at 60 °C for 4 h, the coupled product 6-methoxy-2-(oct-1-enyl)benzo[b]furan (3ae) was obtained in 46% isolated yield (Table 1, entry 1). However, when using other palladium catalysts, such as Pd(OAc)2 and Pd(acac)2 the yield is lower than that of palladium dichloride(Table 1, entries 2 and 3). Some bases were investigated to further improve the yield of coupled products (3ae). When Et3N was used as a base, the reaction of compound 2e with alkenylaluminum (1a) produced the coupled product (3ae) with a 27% isolated yield only (Table 1, entry 4). While, the coupled product (3ae) could not obtain when K2CO3 and TMEDA were used as base (Table 1, entries 5 and 6). To further understand the nature of this catalysis, we tested the cross-coupling reaction of 1a with 2e under various solvents and the results revealed that DCE was the solvent of choice (Table 1, entry 9). In hexane or THF, the isolated yield of the coupled product (3ae) was low efficient (Table 1, entries 7 and 8). To our delighted, the isolated yield of the coupled product (3ae) increased from 74% to 85% when the reaction temperature was increased from 60 °C to 80 °C (Table 1, entries 9 and 10). Interestingly, the isolated yield of the coupled product (3ae) was almost unchanged when the alkenylaluminum loading was decreased from 1.0 mmol to 0.8 mmol (Table 1, entries 10 and 11). However, the isolated yield of the coupled product (3ae) is 53% only when the alkenylaluminum loading was decreased from 0.8 mmol to 0.6 mmol (Table 1, entries 11 and 12). Further studies indicated that the catalyst loading dramatically influenced the isolated yield of the coupled product (3ae). It was found that the most favorable catalyst loading is 3 mol% PdCl2/6 mol% XantPhos (Table 1, entry 11). Extensive screening showed that the optimized coupling conditions were 3 mol% PdCl2/6 mol% XantPhos, 0.8 mmol 1a, 0.5 mmol 2e in DCE at 80 °C for 4 h (Table 1, entry 11).
| Entry | Pd salt. | 1a (equiv.) | Base (x equiv.) | Solvent | 3aeb yield (%) |
|---|---|---|---|---|---|
| a 1a/2a/PdCl2/XantPhos = 1.0/0.5/0.03/0.06 mmol, 60 °C, 3 mL solvent, 4 h, Ar2.b Isolated yield of 3ae.c 80 °C.d 1a/2a/PdCl2/XantPhos = 0.8/0.5/0.02/0.04 mmol. | |||||
| 1 | PdCl2 | 1.0 | — | Toluene | 46 |
| 2 | Pd(OAc)2 | 1.0 | — | Toluene | 19 |
| 3 | Pd(acac)2 | 1.0 | — | Toluene | 10 |
| 4 | PdCl2 | 1.0 | Et3N (2.0) | Toluene | 27 |
| 5 | PdCl2 | 1.0 | K2CO3 (2.0) | Toluene | NR |
| 6 | PdCl2 | 1.0 | TMEDA (2.0) | Toluene | NR |
| 7 | PdCl2 | 1.0 | — | Hexane | 47 |
| 8 | PdCl2 | 1.0 | — | THF | 51 |
| 9 | PdCl2 | 1.0 | — | DCE | 74 |
| 10c | PdCl2 | 1.0 | — | DCE | 85 |
| 11c | PdCl2 | 0.8 | — | DCE | 84 |
| 12c | PdCl2 | 0.6 | — | DCE | 53 |
| 13c,d | PdCl2 | 0.8 | — | DCE | 49 |
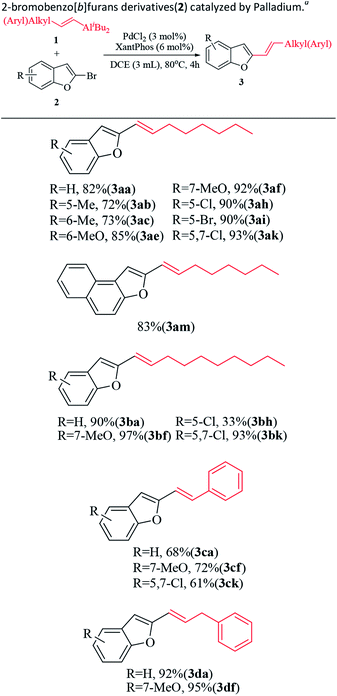
Under the optimized conditions, coupling reactions of aliphatic alkenylaluminum reagents, such as di-sec-butyl(oct-1-enyl)aluminum (1a) and di-sec-butyl(dec-1-enyl)aluminum (1b), proceed with electron-neutral, electron rich and electron-deficient 2-bromo benzo[b]furans derivatives affording the products in good yields (Table 2, 3(ae–ak), 3(ba–bk)). For example, 2-bromobenzo[b]furans containing methyl and methoxy affording the corresponding coupled products in 72–97% isolated yields (Table 2, 3(ab–af), 3bf). 2-Bromobenzo[b]furans containing chloro and bromo groups affording the corresponding coupled products in 33–95% isolated yields (Table 2, 3(ah–ak), 3(bh–bk)). Interestingly, 5,7-dichloro-2-bromobenzo[b]furan affording the corresponding coupled products (3ak) and (3bk) in 93% and 93% isolated yields, respectively (Table 2). Furthermore, the 2-bromonaphtho[2,3-b]furan was also produced the 2-(oct-1-enyl)naphtho[2,1-b]furan (3am) with isolated yield of 83% (Table 2). Besides aliphatic alkenylaluminums, aromatic alkenyl aluminums such as di-sec-butyl(styryl)aluminum (1c) and di-sec-butyl(3-phenylprop-1-enyl)aluminum (1d) also reacted smoothly to afford satisfactory isolated yields (61–95%) (Table 2, 3(ca–ck), 3(da,df)). Importantly, the coupling reactions with 2,5-dibromo benzo[b]furan, 2-bromo-5-chloro-benzo[b]furan and 2-bromo-5,7-dichlorobenzo[b]furan reacted regioselectivity at 2-position affording the corresponding 2-ynylbenzo[b]furans derivatives in 33–95% isolated yields (Table 2, 3(ah–ak), 3(bh,bk), 3ck). At the same time, the dehalogenation was not observed in the cross-coupling with 2-bromobenzo[b]furans derivatives containing halogen-substituents (Table 2, 3(ah–ak), 3(bh,bk), 3ck).
| a 1/2/PdCl2/XantPhos = 0.8/0.5/0.03/0.06 mmol, 80 °C, 4 h. Isolated yield of 3, two runs. |
|---|
 |
The reaction was also found to be effective in gram-scale synthesis, which indicated its potential for practical application (Scheme 2). 2-Substituted benzo[b]furans derivatives 3ah was synthesized in 1.31 gram using this methodology.
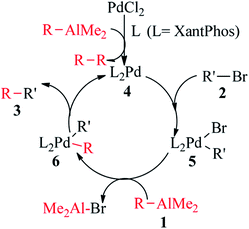
In order to further explore the reaction mechanism, control experiments were carried out (see the ESI†). We performed the reaction between 2-bromobenzo[b]furan (2a, 0.5 mmol) with di-sec-butyl(oct-1-enyl)aluminum (1a, 0.8 mmol) in the presence of PdCl2 (3 mol%)/XantPhos (6 mol%) in DCE at 80 °C for 4 h. The reaction mixture was analyzed by 31P NMR, it was found that the characteristic peak of 31P NMR appeared around at 22.98 ppm and 30.98 ppm. However, 31P NMR peak of pure XantPhos is −18.03 ppm. The results show that XantPhos work as a ligand of the palladium center. Thus, a proposed possible reaction mechanism for the cross-coupling reaction is shown in Scheme 3. The first step is the oxidative addition of 2-bromobenzo[b]furans (2) to Pd(0) phosphine complex (4) (which in turn from PdCl2 and RAlMe2 (1) reagents) to form the organopalladium(II) bromide intermediate (5). Transmetalation of RAlMe2 (1) with complex 5 gives R'PdR(II) intermediate (6) and Me2AlBr. Finally, complex 6 under goes reductive elimination to afford the desired coupling product of 2-alkenylbenzo[b]furans (3) and regenerate the active Pd(0) species for the next catalytic cycle.
Conclusions
A palladium-catalyzed the cross-coupling reactions of 2-bromobenzo[b]furans derivatives with alkenylaluminum reagents is reported. The cross-coupling reactions of aliphatic and aromatic alkenylaluminum reagents proceed with electron-neutral, electron rich and electron-deficient 2-bromobenzo[b]furans derivatives affording the coupled products 2-alkenyl benzo[b]furans in 33–97% isolated yields. More importantly, the reaction was found to be effective in gram-scale synthesis, and can be utilized as precursors for the synthesis of important bioactive compounds. The methodology provides useful procedure for the synthesis of 2-alkenylbenzo[b]furans derivatives. The coupling reactions with 2-bromo-5,7-dichlorobenzo[b]furan reacted regioselectivity at 2-position furnishing the corresponding 2-substituted benzo[b]furans derivatives in good yields. Further studies on the application of this catalytic system to synthesis of bioactive compounds are currently under way.Experimental
Melting points were determined with an XRC-1 micro melting point apparatus and uncorrected. 1H and 13C NMR spectra were recorded using a Varian 400 MHz spectrometer in CDCl3 with tetramethylsilane as internal standard. HRMS were recorded on a Bruker Micro TOF spectrometer equipped with an ESI ion source. Analytical thin-layer chromatography (TLC) was performed on silica 60F-254 plates. Flash column chromatography was carried out on silica gel (200–400 mesh). All reactions were carried out under an Argon gas atmosphere. The starting material 2-bromo benzo[b]furans was prepared according to literature.19 Alkenylaluminum reagents were prepared according to literature.15a Chemical reagents and solvents were purchased from Adamas-beta, Aldrich and XPKchem, and were used without further purification with the exception of these reagents: THF, hexane and toluene were distilled from sodium in mitrogen, and DCE was distilled from CaH2. Other reagents were commercially available and used as received.General producer for cross-coupling of 2-bromobenzo[b]furans with alkenylaluminum reagents
Under an atmosphere of Argon gas, PdCl2 (2.6 mg, 0.015 mmol), XantPhos (17.4 mg, 0.015 mmol), 2-bromobenzo[b]furans (98.0 mg, 0.5 mmol) and DCE (3 mL) were mixed in a Schlenk flask. Shortly afterwards, a solution of alkenylaluminums (0.8 mmol) was added with a syringe pump. At the end of the addition, the reaction mixture stirring was continued for 4 h at 80 °C. After completion the reaction, the mixture was diluted with 1 N aqueous HCl solution (10 mL) and extracted with EA (3 × 15 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and evaporated in vacuum. The residue was subjected to flash column chromatography on silica gel (hexane gradient) to afford the corresponding products.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors are grateful to the Fundamental Research Funds for the Central Universities, Southwest Minzu University (No. 2018NZD06) and the Sichuan Provincial Department of Science and Technology support program (No. 2015NZ0033) for financial support.Notes and references
- (a) M. C. Sill, A. K. Das, T. Itou, S. Karmakar, P. K. Mukherjee, H. Mizuguchi, Y. Kashiwada, H. Fukui and H. Nemoto, The isolation and synthesis of a novel benzofuran compound from Tephrosia purpurea, and the synthesis of several related derivatives, which suppress histamine H1 receptor gene expression, Bioorg. Med. Chem., 2015, 23, 6869–6874 CrossRef PubMed; (b) S. O. Simonetti, E. L. Larghi, A. B. J. Bracca and T. S. Kaufman, Angular tricyclic benzofurans and related natural products of fungal origin. Isolation, biological activity and synthesis, Nat. Prod. Rep., 2013, 30, 941–969 RSC.
- J. W. Hwang, D. H. Choi, J. H. Jeon, J. K. Kim and J. G. Jun, Facile preparation of 2-arylbenzo[b]furan molecules and their anti-inflammatory effects, Bull. Korean Chem. Soc., 2010, 31, 965–970 CrossRef CAS.
- S. Erber, R. Ringshandl and E. von Angerer, 2-Phenylbenzo[b] furans: relationship between structure, estrogen receptor affinity and cytostatic activity against mammary tumor cells, Anti-Cancer Drug Des., 1991, 6, 417–426 CAS.
- M. M. Ghorab, F. A. Ragab, H. I. Heiba and A. M. Soliman, Design and synthesis of some novel 4-chloro-N-(4-(1-(2-(2-cyanoacetyl)hydrazono)ethyl)phenyl) benzenesulfon amide derivatives as anticancer and radiosensitizing agents, Eur. J. Med. Chem., 2016, 117, 8–18 CrossRef CAS PubMed.
- S. N. Aslam, P. C. Stevenson, S. J. Phythian, N. C. Veitch and D. R. Hall, Synthesis of cicerfuran, an antifungal benzofuran, and some related analogues, Tetrahedron, 2006, 62, 4214–4226 CrossRef CAS.
- P. W. Bowyer, E. W. Tate, R. J. Leatherbarrow, A. A. Holder, D. F. Smith and K. A. Brown, N-Myristoyltransferase: a prospective drug target for protozoan parasites, ChemMedChem, 2008, 3, 402–408 CrossRef CAS PubMed.
- H. J. Tang, X. W. Zhang, L. Yang, W. Li, J. H. Li, J. X. Wang and J. Chen, Synthesis and evaluation of xanthine oxidase inhibitory and antioxidant activities of 2-arylbenzo [b]furan derivatives based on salvianolic acidc, Eur. J. Med. Chem., 2016, 124, 637–648 CrossRef CAS PubMed.
- J. Craigo, M. Callahan, R. C. C. Huang and A. L. DeLucia, Antiviral Res., 2000, 47, 19 CrossRef CAS PubMed.
- M. Halabalaki, X. Alexi, N. Aligiannis, M. N. Alexis and A. L. Skaltsounis, Ebenfurans IV-VIII from onobrychis ebenoides: evidence that C-prenylation is the key determinant of the cytotoxicity of 3-formyl-2-arylbenzofurans, J. Nat. Prod., 2008, 71, 1934–1938 CrossRef CAS PubMed.
- (a) X. C. Huang, F. Wang, Y. Liang and J. H. Li, Halopalladation/Decarboxylation/Carbon-carbon forming Domino process: synthesis of 5-halo-6-substituted benzo[b]naphtho[2,1-d] furans, Org. Lett., 2009, 11, 1139–1142 CrossRef CAS PubMed; (b) S. C. Yin, Q. Zhou, X. Y. Zhao and L. X. Shao, N-Heterocyclic carbene-palladium(II)-1-methylimidazole complex catalyzed direct C-H bond arylation of benzo[b] furans with aryl chlorides, J. Org. Chem., 2015, 80, 8916–8921 CrossRef CAS PubMed.
- (a) M. Somei and F. Yamada, Simple indole alkaloids and those with a nonrearranged monoterpenoid unit, Nat. Prod. Rep., 2004, 21, 278–311 RSC; (b) J. Zhang, L. Li, Y. Wang, W. Wang, J. Xue and Y. Li, A novel, facile approach to Frondosin B and 5-epi-Liphagal via a new [4+3]-cycloaddition, Org. Lett., 2012, 14, 4528–4530 CrossRef CAS PubMed.
- (a) H. M. Zhang, E. M. Ferreira and B. M. Stoltz, Direct oxidative Heck cyclizations: intramolecular Fujiwara-Moritani arylations for the synthesis of functionalized benzofurans and dihydrobenzofurans, Angew. Chem., Int. Ed., 2004, 43, 6144–6148 CrossRef CAS PubMed; (b) E. DiMauro and J. R. Vitullo, Microwave-assisted preparation of fused bicyclic heteroaryl boronates: application in one-Pot Suzuki couplings, J. Org. Chem., 2006, 71, 3959–3962 CrossRef CAS PubMed; (c) M. Nagamochi, Y. Q. Fang and M. Lautens, A general and practical method of alkynyl indole and benzofuran synthesis via tandem Cu- and Pd-catalyzed cross-couplings, Org. Lett., 2007, 7, 2955–2958 CrossRef PubMed; (d) J. Cao, Z. L. Chen, S. L. Li, G. F. Zhu, Y. Y. Yang, C. Wang, W. Z. Chen, J. T. Wang, J. Q. Zhang and L. Tang, Palladium-catalyzed regioselective C-2 arylation of benzofurans with N′-acyl arylhydrazines, Eur. J. Org. Chem., 2018, 22, 2774–2779 CrossRef; (e) L. M. Geary and P. G. Hultin, 2-Substituted benzo[b]furans from (E)-1,2-dichlorovinyl ethers and organoboron reagents: scope and mechanistic investigations into the one-pot Suzuki coupling/direct arylation, Eur. J. Org. Chem., 2010, 29, 5563–5573 CrossRef.
- (a) M. L. N. Rao, D. N. Jadhav and V. Venkatesh, Atom-efficient vinylic arylations with triarylbismuths as substoichiometric multicoupling reagents under palladium catalysis, Eur. J. Org. Chem., 2009, 25, 4300–4306 CrossRef; (b) N. T. Hung, M. Hussain, I. Malik, A. Villinger and P. Langer, Site-selective Suzuki cross-coupling reactions of 2,3-dibromo benzofuran, Tetrahedron Lett., 2010, 51, 2420–2422 CrossRef; (c) S. Y. Lin, C. L. Chen and Y. J. Lee, Total synthesis of ailanthoidol and precursor XH14 by Stille coupling, J. Org. Chem., 2003, 68, 2968–2971 CrossRef CAS PubMed; (d) S. Agasti, T. Pal, T. K. Achar, S. Maiti, D. Pal, S. Mandal, K. Daud, G. K. Lahiri and D. Maiti, Regioselective synthesis of fused furans by decarboxylative annulation of α,β-alkenyl carboxylic acid with cyclic ketone: synthesis of di-heteroaryl derivatives, Angew. Chem., Int. Ed., 2019, 58, 11039–11043 CrossRef CAS PubMed; (e) S. Agasti, S. Maity, K. J. Szabo and D. Maiti, Palladium-catalyzed synthesis of 2,3-disubstituted benzofurans: an approach towards the synthesis of deuterium labeled compounds, Adv. Synth. Catal., 2015, 357, 2331–2338 CrossRef CAS PubMed.
- (a) D. B. Biradar and H. M. Gau, Simple and efficient nickel-catalyzed cross-coupling reaction of alkynylalanes with benzylic and arylbromides, Chem. Commun., 2011, 47, 10467–10472 RSC; (b) K. Groll, T. D. Blümke, A. Unsinn, D. Haas and P. Knochel, Direct Pd-catalyzed cross-coupling of functionalized organo aluminum reagents, Angew. Chem., Int. Ed., 2012, 51, 11157–11161 CrossRef CAS PubMed; (c) Q. H. Li, X. B. Shao, Y. Ding, C. Wen and Z. G. Zhao, Research progress on cross-coupling reactions of alkenylaluminum with electrophiles reagents, Curr. Org. Chem., 2018, 22, 1523–1535 CrossRef CAS; (d) Q. H. Li, J. H. Wang, C. Wen, J. Xin, K. P. Cao, K. Wu and M. Liang, Research progress on cross-coupling reactions of alkynylaluminum with electrophiles reagents, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.02.028.
- (a) D. B. Biradar and H. M. Gau, An efficient nickel-catalyzed alkenylation of functionalized benzylic halides with alkenyl aluminum reagents, Org. Biomol. Chem., 2012, 10, 4243–4248 RSC; (b) X. Chen, L. M. Zhou, Y. M. Li, T. Xie and S. L. Zhou, Synthesis of heteroaryl compounds through cross-coupling reaction of aryl bromides or benzyl halides with thienyl and pyridyl aluminum reagents, J. Org. Chem., 2014, 79, 230–235 CrossRef CAS PubMed.
- (a) J. Blum, D. Gelman, W. Baidossi, E. Shakh, A. Rosenfeld, Z. Aizenshtat, B. C. Wassermann, M. Frick, B. Heymer, S. Schutte, S. Wernik and H. Schumann, Palladium-catalyzed methylation of aryl and vinyl halides by stabilized methylaluminum and methylgallium complexes, J. Org. Chem., 1997, 62, 8681–8686 CrossRef CAS; (b) S. Baba and E. Negishi, A novel stereospecific alkenyl-alkenyl cross-coupling by a palladium- or nickel-catalyzed reaction of alkenylalanes with alkenyl halides, J. Am. Chem. Soc., 1976, 98, 6729–6731 CrossRef CAS.
- (a) T. K. Achar, X. L. Zhang, R. Mondal, M. S. Shanavas, S. Maiti, S. Maity, N. Pal, R. S. Paton and D. Maiti, Palladium-catalyzed directed meta-selective C−H allylation of arenes: unactivated internal olefins as allyl surrogates, Angew. Chem., Int. Ed., 2019, 58, 10353–10360 CrossRef CAS PubMed; (b) M. Bera, A. Maji, S. K. Sahoo and D. Maiti, Palladium(II)-catalyzed meta-C-H olefination: constructing multisubstituted arenes through homo-diolefination and sequential hetero-diolefination, Angew. Chem., Int. Ed., 2015, 54, 8515–8519 CrossRef CAS PubMed; (c) T. Patra, S. Bag, R. Kancherla, A. Mondal, A. Dey, S. Pimparkar, S. Agasti, A. Modak and D. Maiti, Palladium-catalyzed directed para C−H functionalization of phenols, Angew. Chem., Int. Ed., 2016, 56, 7751–7755 CrossRef PubMed; (d) M. Brochetta, T. Borsari, S. Bag, S. Jana, S. Maiti, A. Porta, D. B. Werz, G. Zanoni and D. Maiti, Direct meta-C−H perfluoroalkenylation of arenes enabled by a cleavable pyrimidine-based template, Chem.–Eur. J., 2019, 25, 10323–10327 CrossRef CAS PubMed; (e) T. K. Achar, K. Ramakrishna, T. Pal, S. Porey, P. Dolui, J. P. Biswas and D. Maiti, Regiocontrolled remote C−H olefination of small heterocycles, Chem.–Eur. J., 2018, 24, 17906–17910 CrossRef CAS PubMed; (f) A. Deb and D. Maiti, Emergence of unactivated olefins for the synthesis of olefinated arenes, Eur. J. Org. Chem., 2017, 1239–1252 CrossRef CAS; (g) N. Thrimurtulu, A. Dey, A. Singh, K. Pal, D. Maiti and C. M. R. Volla, Palladium catalyzed regioselective C4-arylation and olefination of indoles and azaindoles, Adv. Synth. Catal., 2019, 361, 1441–1446 CrossRef CAS; (h) A. Deb, A. Hazra, Q. Peng, R. S. Paton and D. Maiti, Detailed mechanistic studies on palladium-catalyzed selective C–H olefination with aliphatic alkenes: a significant influence of proton shuttling, J. Am. Chem. Soc., 2017, 39, 763–775 CrossRef PubMed; (i) S. Agasti, B. Mondal, T. K. Achar, S. K. Sinha, A. S. Suseelan, K. J. Szabo, F. Schoenebeck and D. Maiti, Orthogonal selectivity in C–H olefination: synthesis of branched vinylarene with unactivated aliphatic substitution, ACS Catal., 2019, 9, 9606–9613 CrossRef CAS; (j) K. Seth, M. Bera, M. Brochetta, S. Agasti, A. Das, A. Gandini, A. Porta, G. Zanoni and D. Maiti, Incorporating unbiased, unactivated aliphatic alkenes in Pd(II)-catalyzed olefination of benzyl phosphonamide, ACS Catal., 2017, 7, 7732–7736 CrossRef CAS; (k) M. Bera, A. Modak, T. Patra, A. Maji and D. Maiti, Meta-selective arene C–H bond olefination of arylacetic acid using a nitrile-based directing group, Org. Lett., 2014, 16, 5760–5763 CrossRef CAS PubMed; (l) T. K. Achar, J. P. Biswas, S. Porey, T. Pal, K. Ramakrishna, S. Maiti and D. Maiti, Palladium-catalyzed template directed C-5 selective olefination of thiazoles, J. Org. Chem., 2019, 84, 8315–8321 CrossRef CAS PubMed; (m) U. Dutta, S. Maiti, S. Pimparkar, S. Maiti, L. R. Gahan, E. H. Krenske, D. W. Lupton and D. Maiti, Rhodium catalyzed template-assisted distal para-C–H olefination, Chem. Sci., 2019, 10, 7426–7432 RSC; (n) S. Maity, P. Dolui, R. Kancherla and D. Maiti, Introducing unactivated acyclic internal aliphatic olefins into a cobalt catalyzed allylic selective dehydrogenative Heck reaction, Chem. Sci., 2017, 8, 5181–5185 RSC; (o) A. Modak, A. Mondal, R. Watile, S. Mukherjee and D. Maiti, Remote meta C–H bond functionalization of 2-phenethylsulphonic acid and 3-phenylpropanoic acid derivatives, Chem. Commun., 2016, 52, 13916–13919 RSC; (p) T. Patra, R. Watile, S. Agasti, T. Naveen and D. Maiti, Sequential meta-C–H olefination of synthetically versatile benzyl silanes: effective synthesis of meta-olefinated toluene, benzaldehyde and benzyl alcohols, Chem. Commun., 2016, 52, 2027–2030 RSC; (q) S. Agasti, U. Sharma, T. Naveen and D. Maiti, Orthogonal selectivity with cinnamic acids in 3-substituted benzofuran synthesis through C–H olefination of phenols, Chem. Commun., 2015, 51, 5375–5378 RSC; (r) S. Maity, E. Hoque, U. Dhawa and D. Maiti, Palladium catalyzed selective distal C–H olefination of biaryl systems, Chem. Commun., 2016, 52, 14003–14006 RSC; (s) S. Bag and D. Maiti, Palladium-Catalyzed Olefination of Aryl C–H Bonds by Using Directing Scaffolds, Synthesis, 2016, 48, 804–815 CrossRef CAS.
- (a) S. Mo, X. B. Shao, G. Zhang and Q. H. Li, Highly efficient synthesis of unsymmetrical 1,3-diynes from organoalanes reagents and alkynyl bromides mediated by nickel catalyst, RSC Adv., 2017, 7, 27243–27247 RSC; (b) X. B. Shao, X. Jiang, Q. H. Li and Z. G. Zhao, Highly efficient synthesis of 1,2-disubstituted acetylenes derivatives from the cross-coupling reactions of 1-bromoalkynes with organoalane reagents, Tetrahedron, 2018, 74, 6063–6070 CrossRef CAS; (c) G. Zhang, K. Wu, C. Wen and Q. H. Li, Nickel-catalyzed cross-coupling of organoaluminum reagents with alkynylhalides for the synthesis of symmetrical and unsymmetrical conjugated 1,3-diynes derivatives, J. Organomet. Chem., 2020, 906, 121040 CrossRef CAS.
- S. G. Newman, V. Aureggi, C. S. Bryan and M. Lautens, Intramolecular cross-coupling of gem-dibromoolefins: a mild approach to 2-bromo benzofused heterocycles, Chem. Commun., 2009, 35, 5236–5238 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02984j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |