Open Access Article
Open Access ArticleMicrowave roasting of blast furnace slag for carbon dioxide mineralization and energy analysis†
Zike Hana,
Jianqiu Gaoa,
Xizhi Yuana,
Yanjun Zhonga,
Xiaodong Mab,
Zhiyuan Chenc,
Dongmei Luoa and
Ye Wang *a
*a
aSchool of Chemical Engineering, Sichuan University, No. 24 South Section 1, Yihuan Road, Chengdu 610065, P. R. China. E-mail: wangye@scu.edu.cn
bSchool of Chemical Engineering, University of Queensland, Brisbane, Australia
cDepartment of Materials Science and Engineering, Delft University of Technology, The Netherlands
First published on 7th May 2020
Abstract
For both the waste treatment of large quantities of blast furnace (BF) slag and carbon dioxide (CO2) that are discharged in ironworks, mineral carbonation by BF slag was proposed in this decade. However, it has not been widely used due to its high energy consumption and low production efficiency. In this study, a microwave roasting method was employed to mineralize CO2 with BF slag, and the process parameters for the sulfation and energy consumption were investigated. A mixture of BF slag and recyclable ammonium sulfate [(NH4)2SO4] (mass ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was roasted in a microwave tube furnace, and then leached with distilled water at a solid
2) was roasted in a microwave tube furnace, and then leached with distilled water at a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (g mL−1). Under the optimized experiment conditions (T = 340 °C, holding time = 2 min), the best sulfation ratios of calcium (Ca), magnesium (Mg), aluminum (Al), and titanium (Ti) were 93.3%, 98.3%, 97.5%, and 80.4%, respectively. Compared with traditional roasting, the production efficiency of this process was more than 10 times higher, and the energy consumption for mineralizing 1 kg of CO2 could be reduced by 40.2% after simulation with Aspen Plus v8.8. Moreover, 236.1 kg of CO2 could be mineralized by one ton of BF slag, and a series of by-products with economic value could also be obtained. The proposed process offers an energy-efficient method with high productivity and good economy for industrial waste treatment and CO2 storage.
3 (g mL−1). Under the optimized experiment conditions (T = 340 °C, holding time = 2 min), the best sulfation ratios of calcium (Ca), magnesium (Mg), aluminum (Al), and titanium (Ti) were 93.3%, 98.3%, 97.5%, and 80.4%, respectively. Compared with traditional roasting, the production efficiency of this process was more than 10 times higher, and the energy consumption for mineralizing 1 kg of CO2 could be reduced by 40.2% after simulation with Aspen Plus v8.8. Moreover, 236.1 kg of CO2 could be mineralized by one ton of BF slag, and a series of by-products with economic value could also be obtained. The proposed process offers an energy-efficient method with high productivity and good economy for industrial waste treatment and CO2 storage.
1. Introduction
The reduction of carbon dioxide (CO2) emissions is a global problem related to the sustainable development of human society. The storage technology of CO2 can be divided into geologic storage, ocean storage, and mineralization.1 The mineralization technology has the advantages of a mild, safe reaction and stable products for the spontaneous reaction. For most minerals and some solid wastes (steel slag, coal slag, etc.), it can be given in a simplified form as:| Ca or Mg silicate (in minerals or solid waste) + CO2 → Ca or Mg carbonate + silica | (1) |
In 2018, global crude steel production was 1.808 billion tons, and 723 million tons of blast furnace (BF) slag were produced accordingly. Based on the mathematical model proposed by Ba-Shammakh,2 2.1 tons of CO2 per ton of crude steel will be produced; that is, 5.25 tons of CO2 will be generated for each ton of BF slag production. As the CO2 capture potential of BF slag can be 413 ± 13 kg CO2 per ton,3 mineral carbonation by BF slag was proposed to treat large quantities of steel industry waste: BF slag and CO2.4 Ca and Mg in BF slag were used to mineralize CO2 and a series of by-products with economic value can be obtained.5 The primary limiting factor of the mineralization process is the low productivity and economic efficiency.6
The extraction of Ca and Mg from BF slag is the key technology in the mineralization process. Both hydrometallurgical and pyrometallurgical methods have been reported with some extractants, such as acetic acid,7 ammonium acetate,8 hydrochloric acid,9 sulfuric acid,10,11 ammonium bisulfate,12–14 ammonium sulphate,15,16 and mono-ethanolamine.17 In the hydrometallurgical method, Eloneva et al.7 used acetic acid to leach Ca and Mg from BF slag. To mineralize 1 kg of CO2, 4.4 kg of BF slag, 3.6 L of acetic acid, and 3.5 kg of sodium hydroxide (NaOH) were consumed. However, the high power consumption for the regeneration of NaOH made the process unsuitable for CO2 storage. Chu et al.18 proposed a completely wet process, whereby sulfuric acid (H2SO4) and (NH4)2SO4 were mixed to generate ammonium bisulfate (NH4HSO4), and then BF slag was leached in NH4HSO4 solution at 80 °C in 20 min. The mass ratio of BF slag to NH4HSO4 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.25, and the leaching ratios of Ca, Mg, and Al reached 97.3%, 98.8%, and 96.4%, respectively. However, the corrosion of equipment and its unsuitability for Ti-bearing BF slag restrict the improvement of this method. In pyrometallurgical roasting, Hu et al.19 used (NH4)2SO4 to replace the high-cost NH4HSO4 as a recycling extractant, and the sulfation ratios of Ca, Mg, and Al were close to 100%. Compared with ordinary BF slag, the components of Ti-bearing BF slag are more difficult to be sulfated. Wang et al.20 roasted (NH4)2SO4 and Ti-bearing BF slag at 350 °C for 2 h and leached with dilute sulfuric acid. The sulfation ratios of Ca, Mg, Al, and Ti were 85%, 92.6%, 84.4%, and 87%, respectively. Whereas, the traditional roasting method needs to be heated for more than 2 h, which means a low production efficiency and high energy consumption.
3.25, and the leaching ratios of Ca, Mg, and Al reached 97.3%, 98.8%, and 96.4%, respectively. However, the corrosion of equipment and its unsuitability for Ti-bearing BF slag restrict the improvement of this method. In pyrometallurgical roasting, Hu et al.19 used (NH4)2SO4 to replace the high-cost NH4HSO4 as a recycling extractant, and the sulfation ratios of Ca, Mg, and Al were close to 100%. Compared with ordinary BF slag, the components of Ti-bearing BF slag are more difficult to be sulfated. Wang et al.20 roasted (NH4)2SO4 and Ti-bearing BF slag at 350 °C for 2 h and leached with dilute sulfuric acid. The sulfation ratios of Ca, Mg, Al, and Ti were 85%, 92.6%, 84.4%, and 87%, respectively. Whereas, the traditional roasting method needs to be heated for more than 2 h, which means a low production efficiency and high energy consumption.
To improve the production efficiency and reduce the energy consumption, microwave roasting technology was employed to mineralize CO2 with BF slag for two reasons: one, the Ti-bearing BF slag has a good microwave absorption performance in this process, and two, a larger microwave reactor has been developed that is more feasible for realizing industrialization. Moreover, with the existence of calcium titanate (CaTiO3), Ti-bearing BF slag is more difficult to sulfate than ordinary BF slag. In this study, microwave technology was used to extract valuable elements, such as Ca, Mg, Al, Fe, and Ti, from Ti-bearing BF slag with high efficiency. Ca and Mg can be used to mineralize CO2. Meanwhile, a series of by-products with economic value can also be obtained, such as titanium dioxide (TiO2) and ammonium alum (NH4Al(SO4)2·12H2O). The findings will be useful in reducing the energy consumption of CO2 mineralization and for enhancing the production efficiency of solid waste treatment, whereby the CO2 emission from steel and iron industry can be reduced. Moreover, this method is also expected to be applied to the CO2 mineral carbonation of other wastes.
2. Materials and methods
2.1 Materials
In this study, the BF slag was supplied by Panzhihua Iron and Steel Group Co., Ltd., including ordinary BF slag and water-quenched Ti-bearing BF slag. Moreover, the following chemicals were used in the experiment: analytically pure (NH4)2SO4 and H2SO4 (Chengdu Kelong Reagent Co., Ltd.) and nitrogen (N2, Chengdu Xuyuan Chemical Engineering Co., Ltd.) with a purity of >99.99%.In order to identify the phase analysis, X-ray diffraction (DX-1000, Dandong Oriental Circle Instrument Co., Ltd., China) was used, with a copper target (λ = 0.154056 nm) used as the target, test range of 2θ = 10–80°, tube voltage of 40 kV, and tube current of 40 mA. The elemental composition of BF slag was investigated by X-ray fluorescence spectrometry (XRF-1800, Shimadzu, Japan), with a Rh target used as the target.
In addition, the microstructure of the BF slag and leaching residue were observed by scanning electron microscopy (SEM, JSM-7500F, Japan Electronics Corporation, Japan) at an accelerating voltage of 10 kV. The relative elemental content of leaching residue was analyzed with combined energy-dispersive X-ray spectrometry (EDS, IS250, Oxford Instrument Company, UK). To measure the Ca, Mg, Al, and Ti concentrations in the leaching solution, inductively coupled plasma optical emission spectroscopy (ICP-OES, Spectro ARCOS ICP, Germany) was used.
To perform the energy analysis, Aspen Plus software v8.8 was applied to simulate the whole process. However, since the Aspen database lacks some thermodynamic data for some species, the missing thermodynamic data were retrieved from the HSC Chemistry 6.0 database. It is worthy to mention that the simulations of the CO2 capture and the mineralization process were based on our previous research.8,21–23
2.2 Experimental apparatus
Microwave roasting technology was employed in mineralizing CO2 with BF slag. The energy loss of the microwave in unit volume of material22 is:| P = 2πfε′ε′′E2 | (2) |
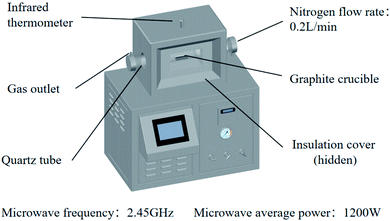
A microwave tube furnace (Fig. 1, HY-ZG, Hunan Huae Microwave Technology Co., Ltd.) was applied to proceed with the experimental procedure. A cylinder of pressurized N2 was attached to the quartz tube, and the injection rate of the inlet gas was measured by a rotameter (LZB-3, 60–600 mL min−1). The infrared thermometer, which was located on the top of the tube furnace, was used to measure the temperature, and the temperature interval set in this study was between 25–50 °C for reducing the error.26 In order to determine the range of temperature, the temperature dependence of the Gibbs free energy of the main reactions (R1–R5 from Table 1) during microwave roasting are shown in Fig. 2, with these thermodynamic data retrieved from the HSC Chemistry 6.0 database. The standard Gibbs free energy change of R1, R2, R3, R4, and R5 become negative from 200 °C, 100 °C, 200 °C, 200 °C, and 300 °C, respectively. Therefore, the experimental temperature range was set from 210 °C to 400 °C.
| No. | Reactions |
|---|---|
| R1 | CaMgSi2O6(s) + 2(NH4)2SO4(l) → CaSO4(s) + MgSO4(s) + 2SiO2(s) + 4NH3(g) + 2H2O(g) |
| R2 | CaAl2SiO6(s) + 4(NH4)2SO4(l) → CaSO4(s) + Al2(SO4)3(s) + SiO2(s) + 8NH3(g) + 4H2O(g) |
| R3 | Ca3Al2O6 + 6(NH4)2SO4(l) → 3CaSO4(s) + Al2(SO4)3(s) + 6H2O(g) + 12NH3(g) |
| R4 | CaTiO3(s) + 2(NH4)2SO4(l) → CaSO4(s) + TiOSO4(s) + 4NH3(g) + 2H2O(g) |
| R5 | (NH4)2SO4(l) → NH4HSO4(l) + NH3(g) |
| R6 | MO(s) + (NH4)2SO4(l) → MSO4(s) + H2O(g) + 2NH3(g) (M = Mg or Ca) |
| R7 | 2TiOSO4(s) → 2TiO2(s) + 2SO2(g) + O2(g) |
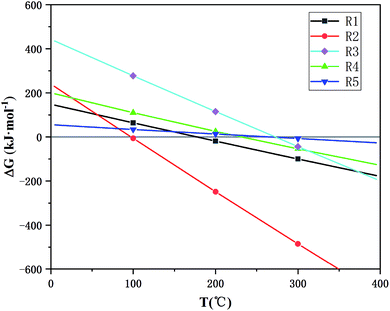 | ||
| Fig. 2 The temperature dependence of Gibbs free energy of the main reaction during microwave roasting. | ||
2.3 Experimental procedure
The experimental procedure in this study included two parts: microwave roasting and leaching. First, BF slag (100 mesh), ground by an agate mortar, was mixed uniformly with (NH4)2SO4 at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and then placed in a graphite crucible. The crucible was placed in a microwave tube furnace with a microwave frequency of 2.45 GHz, and average microwave power of 1200 W (at 340 °C). After several minutes of heating and a few minutes of heat preservation, the power supply was turned off and N2 was fed until the sample was cooled below 30 °C and then removed. The ammonia (NH3) generated during the reaction was absorbed by distilled water. The roasted product was leached with distilled water at a solid
2, and then placed in a graphite crucible. The crucible was placed in a microwave tube furnace with a microwave frequency of 2.45 GHz, and average microwave power of 1200 W (at 340 °C). After several minutes of heating and a few minutes of heat preservation, the power supply was turned off and N2 was fed until the sample was cooled below 30 °C and then removed. The ammonia (NH3) generated during the reaction was absorbed by distilled water. The roasted product was leached with distilled water at a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (g mL−1) for 1 h at 55 °C. The leached slurry was filtered and separated to obtain a leaching residue rich in calcium sulfate (CaSO4) and silica (SiO2) and a leaching solution rich in magnesium, aluminum, and titanium sulfates.
3 (g mL−1) for 1 h at 55 °C. The leached slurry was filtered and separated to obtain a leaching residue rich in calcium sulfate (CaSO4) and silica (SiO2) and a leaching solution rich in magnesium, aluminum, and titanium sulfates.
A direct leaching experiment was conducted to confirm the enhancement effect of the microwave roasting method. A mixture of BF slag and (NH4)2SO4 (mass ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was leached directly with 10% H2SO4 at a solid
2) was leached directly with 10% H2SO4 at a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (g mL−1) without microwave roasting, where the sulfation ratios of Mg, Al, and Ti were 49.6%, 25.2%, and 10.1%, respectively.
3 (g mL−1) without microwave roasting, where the sulfation ratios of Mg, Al, and Ti were 49.6%, 25.2%, and 10.1%, respectively.
According to R1–R4 in Table 1 and the compositions in Table 2, BF slag and (NH4)2SO4 have a theoretical mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.624. However, the actual mass ratio of BF slag to (NH4)2SO4 was 1
1.624. However, the actual mass ratio of BF slag to (NH4)2SO4 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, because an excess (NH4)2SO4 can not only promote the sulfation of BF slag, but also decompose into NH4HSO4 during microwave roasting process. With the help of NH4HSO4, the acidity of the solution can be increased, the consumption of sulfuric acid can be reduced, and the hydrolysis of Ti4+ can be inhibited during leaching. After microwave roasting and leaching, most of the Ca is transferred into the leaching residue in the form of CaSO4. Based on the composition of raw materials, the sulfation ratio of Ca is positively correlated with that of Mg, Al, and Ti. Therefore, only the sulfation ratios of Mg, Al, and Ti under different conditions were analyzed to determine the optimized microwave roasting conditions.
2, because an excess (NH4)2SO4 can not only promote the sulfation of BF slag, but also decompose into NH4HSO4 during microwave roasting process. With the help of NH4HSO4, the acidity of the solution can be increased, the consumption of sulfuric acid can be reduced, and the hydrolysis of Ti4+ can be inhibited during leaching. After microwave roasting and leaching, most of the Ca is transferred into the leaching residue in the form of CaSO4. Based on the composition of raw materials, the sulfation ratio of Ca is positively correlated with that of Mg, Al, and Ti. Therefore, only the sulfation ratios of Mg, Al, and Ti under different conditions were analyzed to determine the optimized microwave roasting conditions.
| Composition | O | Ca | Ti | Si | Al | Mg | Fe | Others |
|---|---|---|---|---|---|---|---|---|
| Ti-bearing BF slag | 42.81% | 18.24% | 11.88% | 11.3% | 5.63% | 4.07% | 2.08% | 3.99% |
| BF slag | 40.75% | 27.33% | 0.47% | 16.39% | 6.98% | 6.09% | 1.03% | 0.96% |
2.4 Calculation of the sulfation ratios
Since CaSO4 is slightly soluble in water, CaSO4 was also present in the leaching solution. The concentrations of Ca2+, Mg2+, Al3+, and Ti4+ in the leaching solution obtained under different roasting conditions were determined by ICP-OES. For determining the content of CaSO4 in the leaching residue, 0.500 g of leaching residue was dissolved in 250 mL distilled water at room temperature, and then the content of Ca in the solution was determined by ICP-OES.The sulfation ratios of Mg, Al, Ti (SMg, SAl, STi) were calculated using eqn (3):
| S1 = (c1 × V)/(m1 × w1) × 100% | (3) |
The sulfation ratio of Ca (SCa) was calculated using eqn (4):
| S2 = (c2 × V + m2 × w2)/(m1 × w3) × 100% | (4) |
3. Results and discussion
3.1 Physicochemical characterization of the BF slag
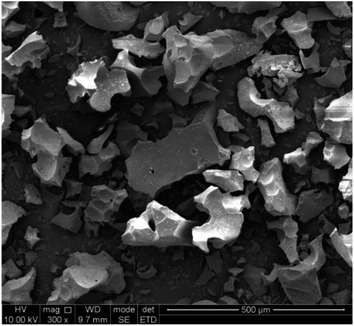
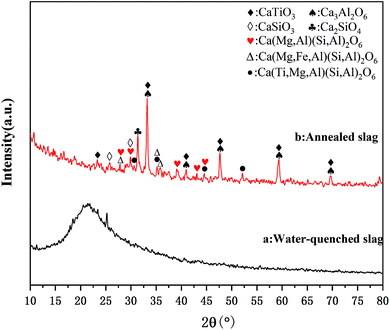
The chemical composition of the slag was analyzed by XRF, and the results are shown in Table 2. The results show that the content of Ti in the Ti-bearing BF slag was 11.88%, which has a high recycling value. A higher content of Ca and Mg is beneficial to the mineralization of CO2, whereby according to the content of Ca and Mg, it can be calculated that the CO2 capture potential of the Ti-bearing BF slag was 274.0 kg CO2 per ton (see ESI†). Furthermore, Al and Fe in the slag can be recovered in the subsequent process.The microstructure of BF slag observed by SEM is shown in Fig. 3. There is little difference in appearance between Ti-bearing BF slag and ordinary BF slag, which are irregular granular, where the particle size of treated Ti-bearing BF slag is less than 0.15 mm. According to the different cooling systems, BF slag can be divided into water-quenched slag and slow-cooling slag. Compared with slow-cooling slag, the particle size of water-quenched slag is much smaller and it is easy to crush. However, BF slag is quenched into a glassy, amorphous form. After nucleation at 780 °C for 1.5 h and crystallization at 850 °C for 2 h, XRD was performed and the analysis results are shown in Fig. 4, with the main constituents in the BF slag being perovskite (CaTiO3), calcium aluminate (Ca3Al2O6), diopside (Ca(Mg,Al)(Si,Al)2O6, Ca(Mg,Fe,Al)(Si,Al)2O6, Ca(Ti,Mg,Al)(Si,Al)2O6), and calcium silicate (CaSiO3).
3.2 Characterization of the leaching residue
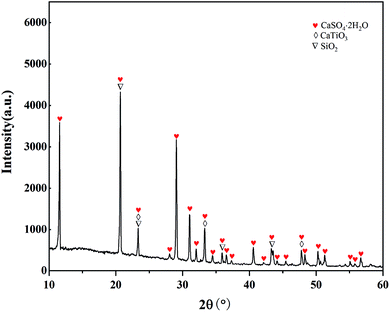
To understand the composition of the leaching residue and the reason for the low STi, the leaching residue was characterized by SEM. The morphology of the leaching residue is shown in Fig. 5(a). Dendritic and massive crystals and amorphous powders could be seen in the slag. Fig. 5(b) is a partial enlargement of Fig. 5(a). Combined with the EDS analysis results and XRD pattern in Fig. 6, it can be seen that the dendritic crystal in Fig. 5(b) is CaSO4, the bulk crystal is SiO2, a small amount of powder is CaTiO3. There was also a large number of amorphous powders, the main component of which is CaSO4, whereby most of the Ca in Ti-bearing BF slag is transferred into the leaching residue. A small amount of CaTiO3 also appeared in the leaching residue, which is one of the reasons for the low STi in this process. However, as the leaching residue may contain a small amount of amorphous titanium dioxide (produced by the decomposition of CaTiO3), the specific content of CaTiO3 was not obtained.3.3 Effect of the roasting temperature
In the study of the effect of the roasting temperature on the sulfation of the elements in BF slag, BF slag and (NH4)2SO4 were mixed uniformly at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The mixture was heated to the corresponding temperature in a microwave tube furnace, and kept there for 2 min (optimized holding time obtained in Section 3.4); then cooled to room temperature and leached with distilled water at a solid
2. The mixture was heated to the corresponding temperature in a microwave tube furnace, and kept there for 2 min (optimized holding time obtained in Section 3.4); then cooled to room temperature and leached with distilled water at a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
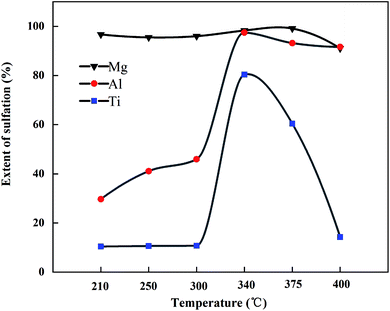
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (g mL−1) for 1 h at 55 °C. The sulfation ratios of Mg, Al, and Ti at different roasting temperatures are shown in Fig. 7. After microwave roasting, SMg was close to 100% at 210 °C, while Mg existed in the form of CaMgSi2O6 in the BF slag (as shown in Fig. 4), and the ΔG of R1 at 210 °C from Fig. 2 was consistent with the experimental results. In this process, STi was still 10.2% before 300 °C, which indicated that CaTiO3 did not react with (NH4)2SO4 under this condition. The XRD pattern of the roasted products at different temperatures is shown in Fig. 8 (there was no CO2 in the microwave process, so the carbonation reaction will be carried out in the mineralization process). From Fig. 8, when the temperature rose to 300 °C, the intermediate (NH4)3H(SO4)2 was formed, meanwhile, SAl and STi rose sharply under this condition. The sulfation ratio reached the maximum at 340 °C; at this point, the crystalline compositions of the product were NH4Fe(SO4)2, CaSO4, and (NH4)3H(SO4)2. Beyond 340 °C, STi decreased with the increase in temperature. When the temperature rose to 400 °C, STi was 14.3%, which was close to the STi before microwave roasting – this was attributed to thermal runaway.27
3 (g mL−1) for 1 h at 55 °C. The sulfation ratios of Mg, Al, and Ti at different roasting temperatures are shown in Fig. 7. After microwave roasting, SMg was close to 100% at 210 °C, while Mg existed in the form of CaMgSi2O6 in the BF slag (as shown in Fig. 4), and the ΔG of R1 at 210 °C from Fig. 2 was consistent with the experimental results. In this process, STi was still 10.2% before 300 °C, which indicated that CaTiO3 did not react with (NH4)2SO4 under this condition. The XRD pattern of the roasted products at different temperatures is shown in Fig. 8 (there was no CO2 in the microwave process, so the carbonation reaction will be carried out in the mineralization process). From Fig. 8, when the temperature rose to 300 °C, the intermediate (NH4)3H(SO4)2 was formed, meanwhile, SAl and STi rose sharply under this condition. The sulfation ratio reached the maximum at 340 °C; at this point, the crystalline compositions of the product were NH4Fe(SO4)2, CaSO4, and (NH4)3H(SO4)2. Beyond 340 °C, STi decreased with the increase in temperature. When the temperature rose to 400 °C, STi was 14.3%, which was close to the STi before microwave roasting – this was attributed to thermal runaway.27
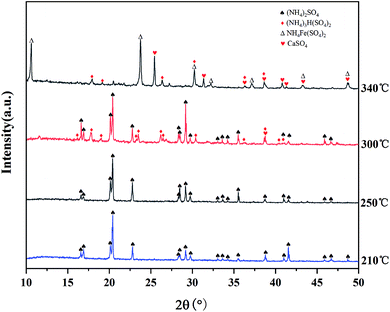 | ||
| Fig. 8 XRD patterns of the roasting products obtained at different roasting temperatures (holding time: 2 min). | ||
In this study, the dielectric constant and loss of main materials were measured by a network analyzer and probe method, as shown in Table 3, where it can also be seen that the dielectric constant and loss of roasted raw materials were similar to that of (NH4)2SO4, and the dielectric constant of Ti-bearing BF slag was about twice that of (NH4)2SO4. The dielectric constant and loss of CaTiO3 were much larger than that of other substances in the experiment. The P-value of CaTiO3 was much larger than other substances in the experiment so that it was easier to be heated. The electric and thermal fields produced by the microwave in the Ti-bearing BF slag were not uniform, because the Ti element in the Ti-bearing BF slag existed in the form of CaTiO3, whereby the different absorbing ability led to the nonuniform temperature distribution. The dielectric loss of CaTiO3 increased with the roasting temperature, resulting in a thermal runaway around CaTiO3, whereby TiOSO4 was decomposed into TiO2 (R7 in Table 1), which was difficult to react with dilute sulfuric acid, thus leading to the decrease in STi. The two Ti-related reactions in this process are shown in Table 1 as R4 and R6.
In the resistance furnace, the first step of (NH4)2SO4 decomposition to produce NH4HSO4 mainly occurs at >250 °C.28 In this work, the XRD peak of (NH4)3H(SO4)2 appeared at 300 °C, indicating that the (NH4)2SO4 began to decompose. The peak of (NH4)2SO4 disappeared and NH4Fe(SO4)2 began to appear when the temperature rose to 340 °C. Moreover, (NH4)2SO4 can react fully with minerals at this temperature, as seen from Fig. 7.
It should be noted that the sulfation ratio reached about 95% after 7 min of heating and 2 min of holding time using microwave roasting; whereas under the traditional roasting conditions, the sulfation ratio of ordinary BF slag in the first 10 min was lower,29 SMg and SAl were about 50% and about 5%, respectively. The conversion of Ti-bearing BF slag in the first 10 min was much lower.30 It was obvious that the reaction rate of microwave roasting was much faster than that of traditional roasting.
3.4 Effect of the holding time
In the study of the effect of different holding times on the sulfation of the elements in BF slag, BF slag and (NH4)2SO4 were mixed uniformly at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
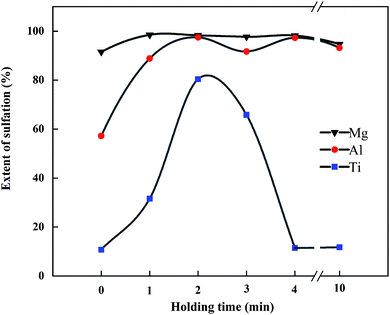
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The sulfation ratios of Mg, Al, and Ti at different holding times are shown in Fig. 9. Under this condition, SMg was close to 100%, and STi was about 10%. When the holding time was 1 min, SAl was over 80%, and SCa began to increase. When the holding time was 2 min, SMg, SAl, and STi all reached the maximum. SCa in the leaching residue was determined under this condition, and the sulfation ratios of Ca, Mg, Al, and Ti were 93.3%, 98.3%, 97.5%, and 80.4%, respectively.
2. The sulfation ratios of Mg, Al, and Ti at different holding times are shown in Fig. 9. Under this condition, SMg was close to 100%, and STi was about 10%. When the holding time was 1 min, SAl was over 80%, and SCa began to increase. When the holding time was 2 min, SMg, SAl, and STi all reached the maximum. SCa in the leaching residue was determined under this condition, and the sulfation ratios of Ca, Mg, Al, and Ti were 93.3%, 98.3%, 97.5%, and 80.4%, respectively.
After 2 min of heat preservation, SMg and SAl tended to be stable with the increase in time, with figures close to 100%. Affected by the phenomenon of “thermal runaway”, STi decreased sharply with the increase in holding time, and the color of the product transited from black to white; whereby when the holding time exceeded 4 min, the color of the product completely turned white. The decomposition product TiO2 does not react with dilute sulfuric acid, thus resulting in a decrease in STi.
As can be seen from Fig. 10, the only crystalline substance in the raw material was (NH4)2SO4. There were no new diffraction peaks during heating from room temperature to 340 °C. The diffraction peaks of (NH4)2SO4 disappeared after 2 min of heat preservation at 340 °C, and the main crystalline components of the product were (NH4)3H(SO4)2 and (NH4)3Fe(SO4)3. After 2 min of heat preservation, the diffraction peaks of (NH4)3Fe(SO4)3 disappeared and the peaks of NH4Fe(SO4)2 and CaSO4 appeared. It could be inferred that the following reactions took place:
| 2(NH4)2SO4(l) → (NH4)3H(SO4)2(l) + NH3(g) | (5) |
| 3(NH4)3H(SO4)2(l) + Fe2O3(s) → 2(NH4)3Fe(SO4)3(s) + 3H2O(g) + 3NH3(g) | (6) |
| 2(NH4)3Fe(SO4)3(s) → 2NH4Fe(SO4)2(s) + (NH4)3H(SO4)2(l) + NH3(g) | (7) |
| (NH4)3H(SO4)2(l) + 2CaO(s) → 2CaSO4(s) + 2H2O(g) + 3NH3(g) | (8) |
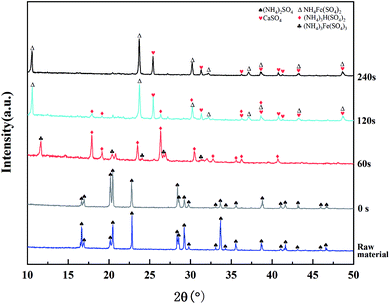 | ||
| Fig. 10 XRD patterns of the roasting products obtained at different holding times (roasting temperature: 340 °C). | ||
When the holding time was extended to 4 min, the peak of (NH4)3H(SO4)2 disappeared, indicating that it had been completely decomposed, and the STi clearly decreased. The decomposition reaction of TiOSO4 and (NH4)3H(SO4)2 was unfavorable for the process, because it not only reduced the yield of Ti, but also increased the consumption of acid in the leaching process. Therefore, the optimized holding time was 2 min.
4. Energy analysis
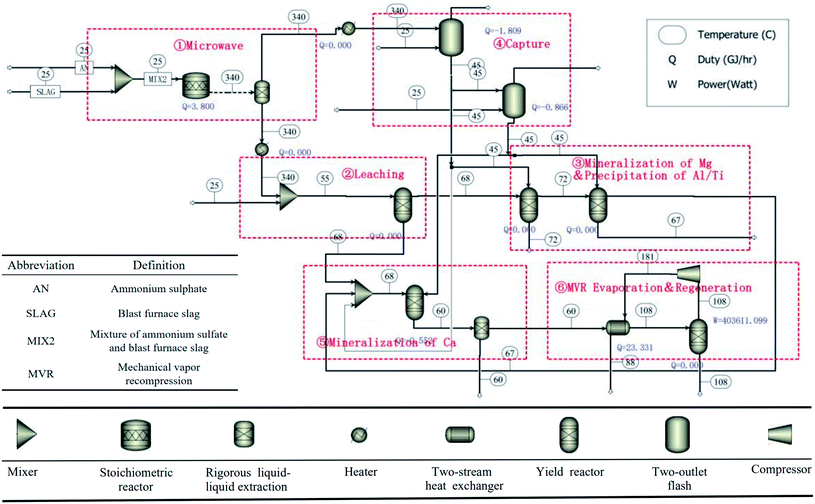
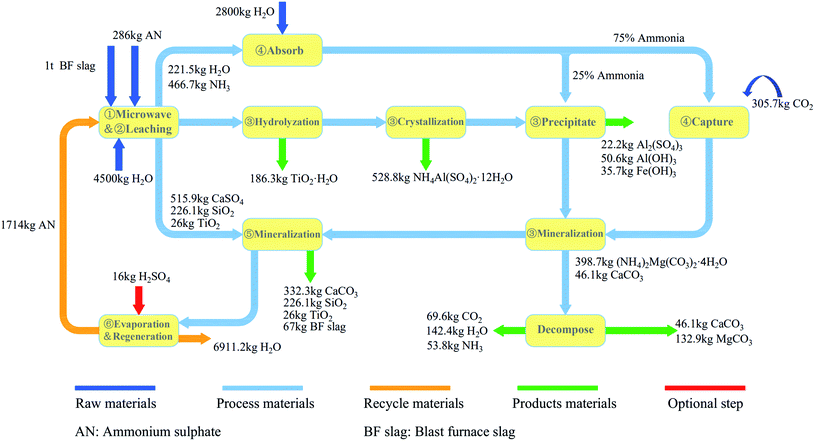
Based on the above analysis, it can be concluded that the microwave roasting process has a shorter roasting time and higher sulfation ratio than the traditional roasting process. Therefore, the treatment efficiency of BF slag can be significantly improved. In order to further illustrate the advantages of the process in terms of the energy consumption, based on the experimental results and previous experimental data, the processes of microwave roasting and traditional roasting were simulated by Aspen Plus v8.8 software, respectively, and the energy consumption of the microwave roasting process was analyzed. The whole process could be divided into six steps as shown in Fig. 11. The main chemical reaction equations involved in the process are shown in Table 1. The specific process material relations are listed in Fig. 12.Combined with Fig. 11 and 12, the whole process is as follows:
Step 1: A mixture of BF slag and (NH4)2SO4 was roasted in the microwave tube furnace at 340 °C for 2 min.
Step 2: The roasted material was leached at a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (g ml−1), and the leaching process was carried out in a water bath at 55 °C and the leaching time was 1 h. The leaching solution was acidic due to the NH4HSO4 decomposed by excessive (NH4)2SO4, so Ti4+ did not hydrolyze. It is worth mentioning that the roasting products in this process were leached with water instead of dilute sulfuric acid, compared with previous studies,20 so about 57 kg sulfuric acid per ton of BF slag could be saved.
3 (g ml−1), and the leaching process was carried out in a water bath at 55 °C and the leaching time was 1 h. The leaching solution was acidic due to the NH4HSO4 decomposed by excessive (NH4)2SO4, so Ti4+ did not hydrolyze. It is worth mentioning that the roasting products in this process were leached with water instead of dilute sulfuric acid, compared with previous studies,20 so about 57 kg sulfuric acid per ton of BF slag could be saved.
Step 3: The leaching solution was hydrolyzed at 102 °C for 4 h, and Ti4+ was precipitated in the form of TiO2·H2O. 186.3 kg of TiO2·H2O could be obtained with 1 ton of BF slag, and after calcination, 98 wt% of TiO2 could be obtained from the precipitation. After filtration, the leaching solution was directly put into a 10 °C water bath for 12 h, and 62% of Al was precipitated in the form of NH4Al(SO4)2·12H2O (purity of 99%),31 and 528.8 kg of NH4Al(SO4)2·12H2O could be obtained with 1 ton of BF slag. Then the pH value was adjusted by ammonia produced in the first step, and the remaining Al3+ and Fe3+ were precipitated.
Step 4: CO2 was captured by ammonia gas from the roasting process to produce ammonium carbonate.
Step 5: Ammonium carbonate was added to the solution to react with Ca2+, Mg2+, and CaSO4 in leaching residue to produce CaCO3 and MgCO3.
Step 6: (NH4)2SO4 was recovered from the solution by evaporation.
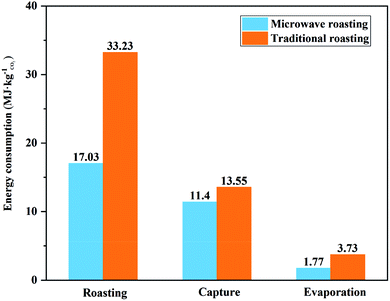
The simulation calculation of the process showed that 236.1 kg of CO2 could be mineralized by one ton of BF slag, which was 86% of the CO2 capture potential. The main components of the solid products from the leaching residue mineralization were CaCO3 and SiO2, which could be used as raw materials for cement. The final solid products from the leaching solution mineralization were CaCO3 and MgCO3, which could replace natural dolomite mining. Through the simulation of the process flow, it could be concluded that the energy consumption of the process was mainly distributed in three processes: roasting, the recovery of (NH4)2SO4, and the capture of CO2. Here, the energy consumption of these three processes was transformed into the energy consumption for mineralizing 1 kg of CO2, to analyze whether microwave roasting could allow saving process energy. The energy consumption of mineralizing 1 kg of CO2 in microwave roasting was compared with that in traditional roasting, as shown in Fig. 13, where it can be seen that microwave roasting could accelerate the roasting reaction rate and increase the sulfation ratios of BF slags. Moreover, it could significantly reduce the energy consumption of treating 1 kg of CO2 in each unit of the whole process, and the energy consumption for mineralizing 1 kg of CO2 could decrease by 20.3 MJ, which represented a 40.2% reduction compared with traditional roasting.
5. Conclusion
This study was focused on microwave roasting and the leaching of BF slag. Under the optimized experimental conditions (T = 340 °C, holding time = 2 min), the best sulfation ratios of Ca, Mg, Al, and Ti were 93.3%, 98.3%, 97.5%, and 80.4%, respectively. The main advantage of employing microwave technology was due to the significant reduction in the roasting time and energy saving. The valuable elements in the BF slag were leached out with distilled water, whereby about 57 kg sulfuric acid per ton of BF slag could be saved, and by-products with economic value, such as TiO2 (purity of 98%) and NH4Al(SO4)2·12H2O (purity of 99%), could be obtained. Furthermore, 236.1 kg of CO2 could be mineralized by one ton of BF slag. Compared with traditional roasting, the production efficiency of microwave roasting was increased by more than 10 times. Moreover, after simulation with Aspen Plus v8.8 software, the energy consumption for mineralizing 1 kg of CO2 could be reduced by 40.2% compared with traditional roasting.This process could reduce CO2 emissions from iron and steel plants, whereby even though all BF slags are used for mineralizing CO2, only a small proportion of CO2 emissions from iron and steel industry can be offset. It is difficult for the iron and steel industry to meet the requirements of negative emission in the short term, but the CO2 emissions could be further reduced by carbon sequestration in other alkaline wastes (basic oxygen slag, coal ash, etc.). The microwave roasting of BF slag for CO2 mineralization was proved to be an energy-efficient method with high productivity and economy, which has broad application prospects in industrial waste treatment and CO2 storage.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Science and Technology Cooperation Program of Sichuan University and Panzhihua, China [No. 2018CDPZH-24]; the Post doctoral interdisciplinary innovation initiation fund, China [No. 0030704153019]; and the Key Research and Development Program of Sichuan, China [No. 18ZDYF]; the Science and Technology Benefiting Project of Chengdu, China [No. 2016-HM01-00399-SF].References
- M. H. Ibrahim, M. H. El-Naas, A. Benamor, S. S. Al-Sobhi and Z. Zhang, Processes, 2019, 7, 1–21 Search PubMed.
- M. S. Ba-Shammakh, Energy Fuels, 2019, 33, 11439–11445 CrossRef.
- P. Renforth, Nat. Commun., 2019, 10, 1401 CrossRef PubMed.
- S. Lee, J. W. Kim, S. Chae, J. H. Bang and S. W. Lee, J. CO2 Util., 2016, 16, 336–345 CrossRef.
- Q. Liu, W. Liu, J. Hu, L. Wang, J. Gao, B. Liang, H. Yue, G. Zhang, D. Luo and C. Li, J. Cleaner Prod., 2018, 197, 242–252 CrossRef.
- H. J. Ho, A. Iizuka and E. Shibata, Ind. Eng. Chem. Res., 2019, 58, 8941–8954 CrossRef.
- S. Eloneva, S. Teir, J. Salminen, C. J. Fogelholm and R. Zevenhoven, Energy, 2008, 33, 1461–1467 CrossRef.
- S. Eloneva, A. Said, C. J. Fogelholm and R. Zevenhoven, Appl. Energy, 2012, 90, 329–334 CrossRef.
- S. Teir, S. Eloneva, C. J. Fogelholm and R. Zevenhoven, Appl. Energy, 2009, 86, 214–218 CrossRef.
- Q. Zhao, C. J. Liu, M. F. Jiang, H. Saxén and R. Zevenhoven, Miner. Eng., 2015, 79, 116–124 CrossRef.
- M. M. Maroto-Valer, D. J. Fauth, M. E. Kuchta, Y. Zhang and J. M. Andrésen, Fuel Process. Technol., 2005, 86, 1627–1645 CrossRef.
- X. Wang and M. M. Maroto-Valer, Fuel, 2011, 90, 1229–1237 CrossRef.
- A. Sanna, A. Lacinska, M. Styles and M. M. Maroto-Valer, Fuel Process. Technol., 2014, 120, 128–135 CrossRef.
- A. Sanna, X. Wang, A. Lacinska, M. Styles, T. Paulson and M. M. Maroto-Valer, Miner. Eng., 2013, 49, 135–144 CrossRef.
- J. Fagerlund, E. Nduagu, I. Romaoãc and R. Zevenhoven, Proc. 23rd Int. Conf. Effic. Cost, Optim. Simulation, Environ. Impact Energy Syst. ECOS 2010, 2010, vol. 4, pp. 67–75 Search PubMed.
- J. Fagerlund, E. Nduagu, I. Romão and R. Zevenhoven, Front. Chem. Eng. China, 2010, 4, 133–141 CrossRef.
- O. Rahmani, J. CO2 Util., 2020, 35, 265–271 CrossRef.
- G. Chu, C. Li, W. Liu, G. Zhang, H. Yue, B. Liang, Y. Wang and D. Luo, Energy, 2019, 166, 1314–1322 CrossRef.
- J. Hu, W. Liu, L. Wang, Q. Liu, F. Chen, H. Yue, B. Liang, L. Lü, Y. Wang, G. Zhang and C. Li, J. Energy Chem., 2017, 26, 927–935 CrossRef.
- L. Wang, W. Liu, J. Hu, Q. Liu, H. Yue, B. Liang, G. Zhang, D. Luo, H. Xie and C. Li, Chin. J. Chem. Eng., 2018, 26, 583–592 CrossRef.
- J. Gao, C. Li, W. Liu, J. Hu, L. Wang, Q. Liu, B. Liang, H. Yue, G. Zhang, D. Luo and S. Tang, Chin. J. Chem. Eng., 2019, 27, 157–167 CrossRef.
- M. Al-Harahsheh and S. W. Kingman, Hydrometallurgy, 2004, 73, 189–203 CrossRef.
- H. Li, Z. Zhao, C. Xiouras, G. D. Stefanidis, X. Li and X. Gao, Renewable Sustainable Energy Rev., 2019, 114, 109316 CrossRef.
- C. Lucas-Torres, A. Lorente, B. Cabañas and A. Moreno, J. Cleaner Prod., 2016, 138, 59–69 CrossRef.
- Y. Yuan, Y. Zhang, T. Liu, P. Hu and Q. Zheng, J. Cleaner Prod., 2019, 234, 494–502 CrossRef.
- S. Waheed Ul Hasan and F. N. Ani, Ind. Eng. Chem. Res., 2014, 53, 12185–12191 CrossRef CAS.
- C. Yan, N. Yoshikawa and S. Taniguchi, ISIJ Int., 2005, 45, 1232–1237 CrossRef CAS.
- J. Highfield, H. Lim, J. Fagerlund and R. Zevenhoven, RSC Adv., 2012, 2, 6535–6541 RSC.
- J. Hu, Research of the process of CO2 mineral carbonation with Al2O3 by-product using blast furnace slag and kinetics, Sichuan University, 2018 Search PubMed.
- L. Wang, A study on the indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of high value-added products of titanium and aluminum, Sichuan University, 2018 Search PubMed.
- C. Wang, Lab. Sci., 2013, 16, 4–6 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02846k |
| This journal is © The Royal Society of Chemistry 2020 |