 Open Access Article
Open Access ArticleIonic liquids as potentially new antifungal agents against Alternaria species†
Maja Karaman‡
 *a,
Milan Vraneš‡
*a,
Milan Vraneš‡ a,
Aleksandar Tot
a,
Aleksandar Tot a,
Snežana Papovića,
Dragana Miljaković
a,
Snežana Papovića,
Dragana Miljaković b,
Slobodan Gadžurić
b,
Slobodan Gadžurić a and
Maja Ignjatovb
a and
Maja Ignjatovb
aFaculty of Science, University of Novi Sad, Trg Dositeja Obradovića 3, 21000 Novi Sad, Serbia. E-mail: maja.karaman@dbe.uns.ac.rs; Fax: +381 21 450 620; Tel: +381 21 485 2682
bInstitute of Field and Vegetable Crops, MaksimaGorkog 30, 21000 Novi Sad, Serbia
First published on 12th June 2020
Abstract
The fungal genus Alternaria Nees 1816 includes the most prevalent pathogenic species that can cause crop diseases such as blight, black spot, and dark leaf spot. In accordance with the aim of developing modern sustainable approaches in agriculture for the replacement of synthetic and toxic substances with environmentally friendly alternatives, the objective of this study was to examine the in vitro antifungal activities of 18 newly synthesized ionic liquids (ILs) against three Alternaria strains: A. padwickii, A. dauci and A. linicola. The antifungal activities of the ILs were estimated via a microdilution method to establish minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) values. The results confirmed that 17 of the 18 ILs showed strain specificity, including good antifungal activity toward Alternaria strains, with MIC and MFC values in the range of 0.04 to 0.43 mol dm−3. The strongest antifungal effects toward all analyzed Alternaria strains were displayed by the compounds with long alkyl chains: [omim][Cl] (MIC/MFC: 0.042 mol dm−3), [dmim][Cl] (MIC/MFC: 0.043 mol dm−3), [ddmim][Cl] (MIC/MFC: 0.053 mol dm−3), [ddTSC][Br] (MIC/MFC: 0.053 mol dm−3), and [Allyl-mim][Cl] (MIC/MFC: 0.054 mol dm−3). The introduction of oxygen as a hydroxyl group resulted in less-pronounced toxicity towards Alternaria compared to the introduction of an ether group, while the contribution of the hydroxyl group was shown to be a more determining factor than the prolongation of the side-chain, resulting in overall fungicidal activity decrease. Our results indicate the possibility that the most effective ILs ([Allyl-mim][Cl], [omim][Cl], [dmim][Cl], [ddmim][Cl], [bTSC][Br], [hTSC][Br], [oTSC][Br], [dTSC][Br], and [ddTSC][Br]) could be applied to the control of plant diseases caused by Alternaria species, based on their potential as an environmentally friendly crop protection approach. Since salts based on TSC cations are significantly cheaper to synthesize, stable under mild conditions, and environmentally friendly after degradation, thiosemicarbazidium-based ILs can be a suitable replacement for commercially available imidazolium ILs.
Introduction
Seed-borne pathogens have a significant influence on the plant production and food industries because they can reduce seed germination and cause contamination with mycotoxins that have a harmful impact and cause health risks to humans and animals.1 Among them, members of the genus Alternaria Nees 1816, which are significant producers of toxins like alternariol and tenuazonic acids, are the most common pathogens affecting the seeds or seedlings of various plant species, including vegetables, spice plants, cereals, fruits and soybeans.2–5 Among the many existing Alternaria species, the species A. padwickii (Ganguly) M. B. Ellis 1971, A. dauci (J. G. Kühn) J. W. Groves & Skolko 1944, and A. linicola J. W. Groves & Skolko 1944 are well known as seed-borne pathogens of rice, carrots and linseed, with a great impact that decreases germination and seed emergence.6 Alternaria dauci causes a significant decrease in seed germination and a reduction in the number of emerged seedlings.7 Alternaria linicola is a commonly occurring pathogen of linseed (Linum usitatissimum L.) that causes the death of seedlings pre- and post-emergence, and decreases seed yields by up to 35% with effects on oil quantity and quality.8 Alternaria padwickii is widespread in several rice-growing countries. It is considered as one of the primary fungi responsible for the kernel discoloration of rice seeds.9Protection against seed-borne pathogens, like Alternaria fungi, is gained through the application of different chemicals or biocides to the seeds, which can prevent or reduce harmful damage. These treatments include fungicides (e.g., metalaxyl, captan, thiram, carboxin, fludioxonil, tebuconazole, boscalid, pyraclostrobin, and trifloxystrobin), bioproducts, antagonistic organisms and, occasionally, antibiotics.2 However, common pathogenic fungal strains often express resistance towards fungicides.10 Hence many novel chemicals have been developed recently, including flutianil, pyriofenone, fenpyrazamine, tolprocarb, tebufloquin, and picarbutrazox, to reduce the presence and impact of seed-borne pathogens.11 Considering this, ionic liquids (ILs) and salts with melting points below 100 °C, which are well known to manifest antimicrobial activity, can also be used in the development of new sources of antimicrobial agents, such as antiseptics, biocides and antifungal agents12–15 with a focus on sustainable environmental strategies. ILs have already been reported as alternative, “green”, solvents for a wide range of reactions and technological processes.16 Considering the biodegradability and environmental persistence of ILs17 and the requirements for low toxicity and environmentally safe chemicals, within studies of any novel ILs, efforts are made to reduce the toxicity of ILs via fine-tuning the essential properties of the IL molecular structures.18
Aspects such as the biodegradability and (eco)toxicity of ILs must be considered before designating a specific IL as a green solvent. The unclear hazard status of ILs and uncertainty surrounding many aspects of related green assessments result in the need to apply dedicated tools to their full characterisation; therefore, investigations of their toxicity towards various species are necessary.19
Considering the importance of research in the discovery of new environmentally friendly disease control agents, the objective of this study was to examine the in vitro antifungal activities of novel ILs against three Alternaria species: A. padwickii, A. dauci and A. linicola, which were isolated from rice, carrot and linseed seeds, respectively.
Materials and methods
Preparation of fungal strains
The analyzed fungi were obtained from carrot AD-1 (A. dauci), linseed AL-12 (A. linicola) and rice AP-3 (A. padwickii), and were kept in the Culture Collection of the Laboratory for Seed Testing at the Institute of Field and Vegetable Crops, Novi Sad. Cultures were grown on PDA (potato dextrose agar, Sigma-Aldrich), which was proved to promote fast growth and sporulation, within 7 days at 27 °C. After incubation under sterile conditions, a sample of the cultivated fungi was taken and suspended in sterile distilled water. Suspensions of the spores of AD-1, AL-12, and AP-3 were adjusted to 1.5 × 106 cells per ml using a Bürker-Türk chamber (hemocytometer) and a microscope (Olympus, BX51, Japan) to obtain adequate inoculum turbidity and the desired density. The number of spores in a chamber is used to calculate the concentration or spore density of a suspension, using the formula:| Mixture cell concentration = [(no. of counted cells)/(no. of chambers) × (chamber volume)] × 1000. |
Preparation of IL solutions
A summary of the provenance and purity of the ILs is given in Table S1 in the ESI.† ILs based on the imidazolium (IM) cation with different alkyl chain lengths and chloride anion were purchased from IoLiTec. Before use, ILs were gently heated under vacuum (p = 10 Pa) for 24 h at a temperature T = 373.15 K. The ILs functionalized with oxygen, as well as with imidazolium chloride, were synthesized according to detailed procedures from the work of Tot et al.20Synthesis of thiosemicarbazidium (TSC) salts with bromide as the anion was performed via one-step reactions in ethanol. After the completion of the reaction, the pale white solid compound was purified via solid–liquid extraction using ethyl acetate. Afterwards, the remaining ethyl acetate was removed using a vacuum pump. The final, powdery product was gently heated under vacuum (p = 10 Pa) until a constant mass was achieved, and it was stored with P2O5 under vacuum for the next 72 h. Synthesis reactions like this are suitable due to the simple purification procedure, high conversion rate (98%), and low reaction temperature. Also, the reaction solvent, ethanol, could be regenerated afterwards.
For the newly synthesized salts, nuclear magnetic resonance (NMR) data were recorded in D2O at 298.15 K using a Bruker 300 DRX spectrometer (Coventry, UK), and the solvent peak was used as a reference. Infrared spectra were recorded in the form of neat samples from 4000 to 650 cm−1 using a Thermo-Nicolet Nexus 670 spectrometer fitted with a Universal ATR Sampling Accessory. The obtained NMR and IR spectra with adequate assignments are given in Fig. S1–S12, ESI.† The ILs and salts were stored in a dry box under a nitrogen atmosphere. The structures of the tested ILs and salts are presented in Table S1 of the ESI.†
Antifungal activities of the ILs via micro-dilution assays
The antifungal activities of the selected ILs were determined with sterile polypropylene 96-well microtiter plates (Spektar, Čačak, Serbia) using in vitro microdilution assays for the determination of the minimal inhibitory and minimal fungicide concentration (MIC and MFC) values. 50 μl of MB (malt broth, Torlak, Serbia) was added into each well along with 1 μl of fungal spore suspension at 4 different solution concentrations (100%, 50%, 25%, and 12.50%). The microtiter plates were incubated for 72 h at 27 °C, and the results were read and interpreted visually. The lowest concentration of the tested IL without visible growth was taken as the minimal inhibitory concentration (MIC). The minimal fungicide concentration (MFC) was determined after reading the MIC value, transferring the total volume of suspension onto a malt agar plate (Torlak, Serbia) and incubating for 72 h at 27 °C.Statistical analysis
Data were subjected to analysis of variance (ANOVA) using STATISTICA 12.6 software (Statsoft, Tulsa, Oklahoma, USA). Means were separated using Tukey's HSD (honest significant difference) test at the p < 0.05 level. Using the same software, nonparametric principal component analysis (PCA) was applied to the IL antifungal activity results based on the obtained MIC and MFC values towards the tested Alternaria strains.Results and discussion
The results obtained are presented in Table 1, showing that 17 out of the 18 tested ILs and salts have good antifungal activity, in the range from 0.041 mol dm−3 to 0.434 mol dm−3. Only in the case of [emim][Cl] were no antifungal effects observed on all tested fungi, and for [OHC2OC2mIm][Cl], an antifungal effect on A. dauci was not observed. Among the tested ILs, the strongest antifungal effects on all analyzed Alternaria strains were displayed when using the compounds with long alkyl chains: [omim][Cl] (MIC/MFC: 0.042 mol dm−3), [dmim][Cl] (MIC/MFC: 0.043 mol dm−3), [ddmim][Cl] (MIC/MFC: 0.053 mol dm−3), [ddTSC][Br] (MIC/MFC: 0.053 mol dm−3), and [Allyl-mim][Cl] (MIC/MFC: 0.054 mol dm−3). Compared with A. padwickii and A. dauci, A. linicola showed higher sensitivity to the ILs, which was also proved by statistical analysis (Table 1 and Fig. 1). All tested ILs, except [emim][Cl], showed strain-specific and good antifungal activities toward A. linicola, with MIC and MFC values detected as being in the range from 0.041 mol dm−3 to 0.168 mol dm−3.| Ionic liquids | Fungi | |||||
|---|---|---|---|---|---|---|
| A. padwickii | A. dauci | A. linicola | ||||
| MIC (mol dm−3) | MFC (mol dm−3) | MIC (mol dm−3) | MFC (mol dm−3) | MIC (mol dm−3) | MFC (mol dm3) | |
| a nd: not detected (in the range of tested concentrations: 0.04 to 0.43 mol dm−3); IL (chemical name): [emim][Cl] – 1-ethyl-3-methylimidazolium chloride, [bmim][Cl] – 1-butyl-3-methylimidazolium chloride, [hmim][Cl] – 1-hexyl-3-methylimidazolium chloride, [omim][Cl] – 1-octyl-3-methylimidazolium chloride, [dmim][Cl] – 1-decyl-3-methylimidazolium chloride, [ddmim][Cl] – 1-dodecyl-3-methylimidazolium chloride, [im][Cl] – imidazolium chloride, [Allyl-im][Cl] – 1-allyl-3-methylimidazolium chloride, [OHC3mim][Cl] – 1-(3-hydroxypropyl)-3-methylimidazolium chloride, [OHC2mim][Cl] – 1-(2-hydroxyethyl)-3-methylimidazolium chloride, [C2OC2mim][Cl] – N-ethyl-(2-etoxy)-3-methylimidazolium chloride, [OHC2OC2mim][Cl] – 1-(4-hydroxy-2-oxy)butyl-3-methylimidazolium chloride, [eTSC][Br] – S-ethylthiosemicarbazidium bromide, [bTSC][Br] – S-butylthiosemicarbazidium bromide, [hTSC][Br] – S,S-hexylthiosemicarbazidium bromide, [oTSC][Br] – S-octylthiosemicarbazidium bromide, [dTSC][Br] – S-decylthiosemicarbazidium bromide, [ddTSC][Br] – S-dodecylthiosemicarbazidium bromide. | ||||||
| [emim][Cl] | nd | nd | nd | nd | nd | nd |
| [bmim][Cl] | 0.106 | 0.106 | 0.106 | 0.106 | 0.053 | 0.053 |
| [hmim][Cl] | 0.084 | 0.084 | 0.042 | 0.167 | 0.042 | 0.042 |
| [omim][Cl] | 0.042 | 0.042 | 0.042 | 0.042 | 0.042 | 0.042 |
| [dmim][Cl] | 0.043 | 0.043 | 0.043 | 0.043 | 0.043 | 0.043 |
| [ddmim][Cl] | 0.053 | 0.053 | 0.053 | 0.053 | 0.053 | 0.053 |
| [im][Cl] | 0.217 | 0.434 | 0.217 | 0.434 | 0.054 | 0.109 |
| [Allyl-mim][Cl] | 0.054 | 0.054 | 0.054 | 0.054 | 0.054 | 0.054 |
| [OHC3mim][Cl] | 0.434 | 0.434 | 0.434 | 0.434 | 0.054 | 0.054 |
| [OHC2mim][Cl] | 0.434 | 0.434 | 0.434 | 0.434 | 0.054 | 0.054 |
| [C2OC2mim][Cl] | 0.054 | 0.109 | 0.054 | 0.109 | 0.054 | 0.054 |
| [OHC2OC2mim][Cl] | 0.425 | nd | nd | nd | 0.053 | 0.053 |
| [eTSC][Br] | 0.213 | 0.425 | 0.213 | 0.213 | 0.053 | 0.053 |
| [bTSC][Br] | 0.042 | 0.084 | 0.042 | 0.084 | 0.042 | 0.084 |
| [hTSC][Br] | 0.041 | 0.041 | 0.041 | 0.082 | 0.041 | 0.041 |
| [oTSC][Br] | 0.084 | 0.084 | 0.042 | 0.042 | 0.042 | 0.042 |
| [dTSC][Br] | 0.084 | 0.084 | 0.084 | 0.084 | 0.042 | 0.042 |
| [ddTSC][Br] | 0.053 | 0.053 | 0.053 | 0.053 | 0.053 | 0.053 |
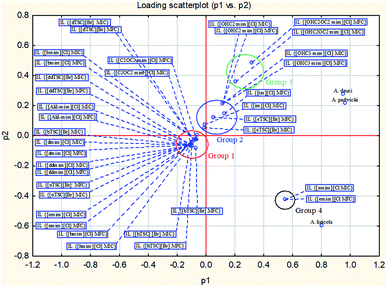 | ||
| Fig. 1 Positions of clusters after PCA analysis of the examined ILs based on the obtained MIC and MFC values against the tested Alternaria strains. | ||
Based on the positions of the clusters obtained after statistical analysis, the classification of the examined ILs into four specific groups was proposed (Fig. 1). Generally, A. padwickii and A. dauci are separated from A. linicola, probably due to it having the highest susceptibility towards all the ILs. Furthermore, the ILs were grouped according to the MIC and MFC values as follows. Group 1 predominately consists of imidazolium (IM) salts with chloride as the anion and TSC salts with bromide as the anion, and it is concentrated at the center of the X and Y axes ([im][Cl]; [eTSC][Br]; [bmim][Cl]; [hmim][Cl]; [omim][Cl]; [dmim][Cl]; [ddmim][Cl]; [Allyl-mim][Cl]; [C2OC2mim][Cl]; [bTSC][Br]; [hTSC][Br]; [oTSC][Br]; [dTSC][Br]; and [ddTSC][Br]). Group 2 predominately consists of salts with added OH groups, and it is concentrated in the positive zone of the X and Y axes ([OHC3mim][Cl]; [OHC2mim][Cl]; [im][Cl]; and [eTSC][Br]). Group 3 is concentrated in the positive zone of the X axis with only one IL ([OHC2OC2mim][Cl]) and group 4, with only one IL, is located in the negative zone of the X-axis ([emim][Cl]).
According to current knowledge, it appears that the toxicity of an IL is much more affected by the structure of the cation than the anion,21 whereas the alkyl chain length, which determines the IL hydrophobicity, seems to be the main factor influencing its toxicity.22–29 So far, the observed effects of different anionic moieties on toxicity are negligible. Hence, only the toxicity effects of cations on Alternaria strains are discussed in this work. IL toxicity mechanisms are not yet fully understood, but it has been proposed that the mode of toxic action occurs through membrane disruption due to the structural similarities of ILs with detergents, pesticides and antibiotics.30–32 Specifically, long alkyl chains in the cation increase the lipophilic nature of these compounds, thus increasing the possibility that they will interact with cell membrane phospholipid bilayers and the hydrophobic domains of membrane proteins, leading to the disruption of membrane physiological functions and, consequently, cell death.33,34 Based on these observations, the influences of different cations, the length of the alkyl side chain, and the functionalization of the side-chain with polar groups on the growth of Alternaria strains were studied.
It can be noted from the obtained results (Table 1) that a longer alkyl side chain, both on imidazolium (IM) and thiosemicarbazidium (TSC) cations, increases toxicity towards A. padwickii and A. dauci strains. In the case of A. linicola, all investigated ILs and salts showed similar fungicidal and inhibitory effects. This observation showed that A. linicola had a different response to the presence of ILs and salts compared to the other two fungal strains, pointing out the importance of species specificity. Furthermore, all presented discussion concerns the A. padwickii and A. dauci strains.
Analyzing results for IM ILs without oxygen in the side chain, it can be noted that toxicity towards the investigated moulds was not detected in the case of [emim][Cl]. The IL without a side chain, [im][Cl], expresses lower toxicity towards the Alternaria strains than IM ILs with longer alkyl chains. As can be seen from Table 1, a longer alkyl side chain significantly decreases the MIC and MFC values, up to a hexyl side chain. It is interesting to point out that further elongation of the side chain did not affect the MIC and MFC values as expected. Comparing the obtained results from IM ILs with thiosemicarbazidium, it can be noted that the MIC and MFC values for TSC-based salts were similar. Almost constant MIC and MFC values for TSC salts were obtained for alkyl chains that were longer than butyl. This leads to the conclusion that the additional increase of the lipophilic domain of the cation does not influence the toxicity towards Alternaria strains, showing that an increase of the alkyl side chain to decyl or even dodecyl is not necessary to obtain optimal fungicidal performance. Furthermore, when comparing [dTSC][Br] and ILs with shorter alkyl chains, it can be seen that there is a change in the toxicity tendency, since elongation of the alkyl chain leads to a decrease in the IL toxicity (Table 1). It has also been described in the literature that the increasing relationship between the alkyl chain length and toxicity no longer applies for very long alkyl chains. At a certain chain length, the toxicity cannot be increased any further because of the “cut-off” effect.35 Explanations for this phenomenon are proposed based on either insufficient solubility or kinetics aspects (the uptake is slowed because of steric effects from compounds with a large molecular size).
The similarity of the obtained inhibitory and fungicidal values for ILs with different aromatic and aliphatic groups implies that thiosemicarbazidium-based ILs can be a suitable replacement for commercially available IM ILs. Namely, salts based on the TSC cation are more easily synthesized, the starting compound is significantly cheaper, and the entire process of synthesis can be conducted under mild conditions. Further degradation of IM ILs can produce more toxic compounds, which can contaminate the environment. On the other hand, the degradation of the thiosemicarbazidium group produces more benign and environmentally friendly compounds.36
The introduction of oxygen into the side chain of IM ILs suggests that the nature of the functional group is responsible for lowering the toxicity. Namely, with the introduction of oxygen as a hydroxyl group, toxicity towards Alternaria is less pronounced compared to ether group addition. The obtained similar values for [OHC3mim][Cl], [OHC2mim][Cl], and [OHC2OC2mim][Cl] indicate that the contribution of the hydroxyl group is a more determining factor than the elongation of the side-chain, resulting in an overall decrease in the fungicidal activity, which is in accordance with previously detected antimicrobial mechanisms related to different naturally originating compounds.37 On the other hand, after the introduction of oxygen in the form of an ether group, the MIC and MFC values were similar or higher than the non-functionalized ILs. Specifically, the less pronounced lipophilic nature of the compounds decreases the possibility that they will interact with cell membrane phospholipid bilayers and the hydrophobic domains of membrane proteins, leading to negligible disruption of membrane physiological functions and, consequently, to decreased toxicity. In our case, the introduction of the oxygen into the alkyl side chain increases the polarity of the cation, while the lipophilicity concurrently decreases. This weakens the interactions between the lipid components of the cell membrane and the IL, significantly reducing the toxicity. The IL that shows high antifungal activity is 1-allyl-3-methylimidazolium chloride (Table 1). These results suggest that the existence of a double bond, due to the higher electron density around it, increases toxicity towards fungal cells. Fujisawa and associates38 proved, using NMR and differential scanning calorimetry, that the allyl group forms hydrophobic interactions with dipalmitoylphosphatidylcholine, which represents the main component of the cell membrane. In that manner, molecules with allyl groups strongly interact with the lipid bilayer and have a negative effect on the cell membrane permeability and physiology of the cell.38
1-Butyl-3-methylimidazolium chloride ([bmim][Cl]) is already known for its many good qualities and usability but also, unfortunately, for its toxicity.39 According to Desai et al.,40 who tested newly synthesized compounds for antifungal activity against Aspergillus niger and A. clavatus at 4 different concentrations (1000, 500, 200 and 100 μg ml−1), compounds 3b (3-Cl–C6H4), 3d (4-Cl–C6H4), 3h (4-NO2–C6H4) and 3j (3-OH–C6H4) possess good activity against the fungal strain A. niger, while compounds 3c (3-Cl–C6H4), 3f (4-F–C6H4), 3g (3-NO2–C6H4), and 3l (–C5H4N) possess good activity against A. clavatus.
Conclusions
The obtained results confirmed that 17 out of the 18 tested ILs were found positive for antifungal activity via in vitro testing against the fungal strains A. padwickii, A. dauci, and A. linicola. Furthermore, the anion influence on IL toxicity is subordinate to the cation effect. The antifungal activity results from imidazolium ILs and thiosemicarbazidium salts indicate that the cation, the length of the alkyl side chain, and the polar group side-chain functionality all influence the effects on Alternaria strains. Additionally increasing the lipophilic domain of the cation does not influence toxicity towards Alternaria strains, pointing out that an increase in the alkyl side chain length to decyl or even dodecyl is not necessary to obtain optimal fungicidal performance. The contribution of a hydroxyl group is a more determining factor than the elongation of the side-chain for affecting the overall fungicidal activity and decreasing the toxicity. The oxygenation of the alkyl side chain primarily with an –OH group can be used for fine-tuning and controlling the IL toxicity, thus leading to fungicidal effects. Due to their similar antifungal effects, salts based on the TSC cation can be suggested as a replacement for commercially available IM ILs, especially due to the fact that they are more environmentally friendly, are significantly cheaper, are easier to synthesise, and can be kept under mild conditions. These results might lead to a dramatic future reduction in the application of fungicides, and they also could provide a good starting point for research into IL usage in crop protection.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All authors are thankful for the technical assistance with the experimental procedures from PhD students Nemanja Spremo MSc and Nataša Bešir MSc. This research work was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. III43002 and No. 451-03-68/2020-14/200032) and by the Provincial Secretariat for Higher Education and Scientific Research of APV under project contract 142-451-2766/2018-01/02.References
- M. U. Ukwuru, C. G. Ohaegbu and A. Muritala, J. Biochem. Microb. Toxicol., 2017, 1, 101 Search PubMed.
- H. B. Lee, A. Patriarca and N. Magan, Microbiology, 2015, 43, 93 Search PubMed.
- T. T. M. S. Rodrigues, M. L. Berbee, E. G. Simmons, C. R. Cardoso, A. Reis, L. A. Maffia and E. S. G. Mizubuti, New Dis. Rep., 2010, 22, 28 CrossRef.
- D. Petrović, M. Ignjatov, M. Vujaković, Z. Nikolić, K. Taški-Ajduković and D. Jovičić, Ratarstvo i povrtarstvo, 2011, 48, 173 CrossRef.
- J. Lević, S. Stanković, V. Krnjaja, A. Bočarov-Stančić and D. Ivanović, Pestic. Phytomed., 2012, 27, 33 CrossRef.
- A. Mamgain, R. Roychowdhury and J. Tah, Res. J. Biol., 2013, 1, 1 Search PubMed.
- A. Bulajić, I. Djekić, N. Lakić and B. Krstić, Arch. Biol. Sci., 2009, 61, 871 CrossRef.
- E. Gruzdevienė, A. Mankevičienė, A. Lugauskas and J. Repečkienė, Ekologija, 2006, 3, 64 Search PubMed.
- L. E. Jayachandran, R. Kumari and P. S. Rao, J. Stored Prod. Res., 2019, 83, 14 CrossRef.
- B. J. Goates, Plant Dis., 2012, 96, 361 CrossRef PubMed.
- A. Leadbeater, Plant Prot. Sci., 2015, 51, 163 CrossRef CAS.
- A. Busetti, D. E. Crawford, M. J. Earle, M. Gilea, B. F. Gilmore, S. P. Gorman, G. Laverty, A. F. Lowry, M. McLaughlin and K. R. Seddon, Green Chem., 2010, 12, 420 RSC.
- F. Walkiewicz, K. Materna, A. Kropacz, A. Michalczyk, R. Gwiazdowski, T. Praczyk and J. Pernak, New J. Chem., 2010, 34, 2281 RSC.
- V. Z. Bergamo, R. K. Donato, D. F. Dalla Lana, K. J. Donato, G. G. Ortega, H. S. Schrekker and A. M. Fuentefria, Lett. Appl. Microbiol., 2015, 60, 66 CrossRef CAS PubMed.
- A. Koziróg, A. Wysocka-Robak and K. Przybysz, Fibres Text. East. Eur., 2015, 23, 134 Search PubMed.
- G. Tao, L. He, W. Lui, L. Xu, W. Xiong, T. Wang and Y. Kou, Green Chem., 2006, 8, 639 RSC.
- E. Liwarska-Bizukojc, C. Maton and C. V. Stevens, Biodegradation, 2015, 26, 453 CrossRef CAS PubMed.
- M. Vraneš, A. Tot, S. Jovanović-Šanta, M. Karaman, S. Dožić, K. Tešanović, V. Kojić and S. Gadžurić, RSC Adv., 2016, 6, 96289 RSC.
- M. Bystrzanowska, F. Pena-Pereira, Ł. Marcinkowskiand and M. Tobiszewski, Ecotoxicol. Environ. Saf., 2019, 174, 455–458 CrossRef CAS PubMed.
- A. Tot, Č. Podlipnik, M. Bešter-Rogač, S. Gadžurić and M. Vraneš, Arabian J. Chem., 2020, 13(1), 1598–1611 CrossRef CAS.
- C. Bubalo, M. Hanousek, K. Radošević, K. Gaurina, V. Srček, T. Jakovljević and R. I. Redovniković, Ecotoxicol. Environ. Saf., 2014, 101, 116 CrossRef PubMed.
- P. Liu, Y. Ding, H. Liu, L. Sun, X. Li and J. Wang, J. Environ. Sci., 2010, 22, 1974 CrossRef CAS.
- T. P. Pham, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352 CrossRef CAS PubMed.
- M. H. Fatemiand and P. Izadiyan, Chemosphere, 2011, 84, 553 CrossRef PubMed.
- B. Peric, E. Martí, J. Sierra, R. Cruañas, M. Iglesias and M. A. Garau, Environ. Toxicol. Chem., 2011, 30, 2802 CrossRef CAS PubMed.
- M. Petkovic, K. R. Seddon, L. P. Rebelo and C. S. Pereira, Chem. Soc. Rev., 2011, 40, 1383 RSC.
- B. Zhang, X. Li, D. Chen and J. Wang, Protoplasma, 2012, 250, 103 CrossRef PubMed.
- S. P. M. Ventura, A. M. M. Gonçalves, T. Sintra, J. L. Pereira, F. Gonçalves and J. A. P. Coutinho, Ecotoxicology, 2013, 22, 1 CrossRef CAS PubMed.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132 CrossRef CAS PubMed.
- C. Pretti, C. Chiappe, I. Baldetti, S. Brunini, G. Monni and L. Intorre, Ecotoxicol. Environ. Saf., 2009, 72, 1170 CrossRef CAS PubMed.
- H. Li, C. Yu, R. Chen, J. Li and J. Li, Colloids Surf., A, 2012, 395, 116 CrossRef CAS.
- D. Wang, H. J. Galla and P. Drücker, Biophys. Rev., 2018, 10, 735 CrossRef CAS PubMed.
- A. Romero, A. Santos, J. Tojo and A. Rodrıguez, J. Hazard. Mater., 2008, 151, 268 CrossRef CAS PubMed.
- M. Petkovic, D. O. Hartmann, G. Adamová, K. R. Seddon, L. p. N. Rebelo and C. S. Pereira, New J. Chem., 2012, 36, 56 RSC.
- M. Matzke, J. Arning, J. Ranke, B. Jastorff and S. Stolte, Green Chem., 2010, 6, 235 CAS.
- S. P. M. Ventura, C. S. Marques, A. A. Rosatella, C. A. M. Afonso, F. Gonçalves and J. A. P. Coutinho, Ecotoxicol. Environ. Saf., 2012, 76, 162 CrossRef CAS PubMed.
- M. M. Cowan, Clin. Microbiol. Rev., 1999, 12, 564 CrossRef CAS PubMed.
- S. Fujisawa, Y. Kadoma and Y. Komoda, J. Dent. Res., 1988, 67, 1438 CrossRef CAS PubMed.
- C. Zhang, Y. Shao, L. Zhu, J. Wang, J. Wang and Y. Guo, Environ. Toxicol. Pharmacol., 2017, 51, 131–137 CrossRef CAS PubMed.
- N. C. Desai, A. S. Maheta, K. M. Rajpara, V. V. Joshi, H. V. Vaghani and H. M. Satodiya, J. Saudi Chem. Soc., 2014, 18, 963 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Table S1 showing the provenance, purity and structures of the tested ILs; Fig. S1–S6 showing 1H and 13C NMR spectra of the ILs; and Fig. S7–S12 showing IR spectra of the ILs. See DOI: 10.1039/d0ra02475a |
| ‡ Both first and second author contributed equally to this research and manuscript writing. |
| This journal is © The Royal Society of Chemistry 2020 |
