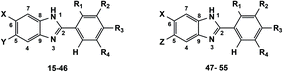Open Access Article
Open Access ArticleSynthesis and insight into the structure–activity relationships of 2-phenylbenzimidazoles as prospective anticancer agents†
Thi-Kim-Chi Huynhab,
Thi-Hong-An Nguyena,
Thi-Cam-Thu Nguyenac and
Thi-Kim-Dung Hoang *ab
*ab
aInstitute of Chemical Technology – VAST, 01 Mac Dinh Chi Str., Dist. 1, Ho Chi Minh City, Vietnam. E-mail: hoangthikimdung@gmail.com
bGraduate University of Science and Technology – VAST, 18 Hoang Quoc Viet Str., Cau Giay Dist., Hanoi, Vietnam
cTon Duc Thang University, 19 Nguyen Huu Tho Str., Dist. 7, Ho Chi Minh City, Vietnam
First published on 1st June 2020
Abstract
In order to explore and develop new anticancer agents, three series of 2-phenylbenzimidazoles, 15–46, were condensed under simple and mild conditions using sodium metabisulfite as an oxidation agent and another series, 47–55, were obtained via a reduction reaction using sodium borohydride. All the compounds synthesized were evaluated for their in vitro anticancer activities against three human cancer cell lines. The novel compound 38 was found to be the most potent multi cancer inhibitor against A549, MDA-MB-231, and PC3 cell lines (IC50 values 4.47, 4.68 and 5.50 μg mL−1, respectively). In addition, compound 40 exhibited the best IC50 value of 3.55 μg mL−1 against the MDA-MB-231 cell line. The results demonstrated that introducing a new substituent to compounds 37–55 could improve their antiproliferative activities.
1. Introduction
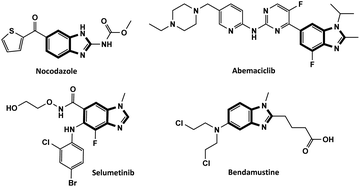
Cancer is a phenomenon that involves abnormal cell growth invading or spreading to other parts of the organism. There are more than 100 types of cancers which affect humans and that has led to the discovery of many options for cancer treatment such as chemotherapy, surgery, radiation therapy, targeted therapy, immunotherapy, and so on. In particular, chemotherapy is required in the treatment, either alone or in combination with the other therapies depending on type of cancer, its location, and its grade. For example, chemotherapy could reduce the size of an inoperable tumor, which could then be operated on in the future. However, the increase in multidrug resistance (MDR) to chemotherapy is a contributing factor that influences the treatment of cancer1 and side effects can also limit the effectiveness of anticancer drugs such as nausea, arthralgia, fatigue, osteoporosis, and so on.2 Therefore, the exploration and development of new anticancer drugs that have better efficacy and fewer side effects are required.Benzimidazole structures are well-known heterocyclic compounds that possess a wide range of antiviral, anti-histaminic, antifungal, antiallergic, anticoagulant, antihypertensive, and antiparasitic properties.3–12 In particular, some of the commercial anticancer drugs contain the benzimidazole scaffold (Fig. 1), so benzimidazole is one of the most promising structures used against several human cancer cell lines. Many studies have reported that benzimidazole derivatives that contain functional groups at the 1, 2, 5 and/or 6-positions on their skeleton show the most efficient anticancer activity.13–17 However, previous studies18–21 that evaluated the antiproliferative activity of 2-phenylbenzimidazoles had a small number of desired products which led to a lack of conclusion on the structure–activity relationship (SAR), the effect of the presence of substituents into both the C-5/6 and C-2 positions on benzimidazole structure and also the bioactivity. In addition, there are two general pathways to synthesize benzimidazole derivatives. The first method involves the condensation of o-phenylenediamines and carboxylic acids (or their derivatives such as nitriles, chlorides and orthoesters) in the presence of acid as we have previously reported.22 The second one is conducted by using o-phenylenediamines in combination with aldehydes instead of the carboxylic acids and oxidation agents that should have been required for this process.23–30 According to our latest publication, a large number of benzimidazoles were synthesized from o-phenylenediamines and aldehydes under simple and mild conditions using sodium metabisulfite (Na2S2O5) as the oxidative reagent and a solvent which was a mixture of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (9
water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) in high yields.30 Thus, it was recognized that this pathway is convenient to synthesize benzimidazoles that contain a variety of substituents on the C-2, C-5 and C-6 positions of the benzimidazole scaffold without undesired products. Continuing with our plans to develop new anti-tumor agents,22,31,32 in this research, we planned to synthesize a large number of new 2-phenylbenzimidazole derivatives with a diverse range of substituents on the C-2, C-5 and C-6 positions via condensation of o-phenylenediamines and various aldehydes under the conditions mentioned previously and then to elucidate their SAR against three cancer cell lines: A549, MDA-MB-231, and PC3. The aim was that the presence of substituents such as carbonyl and hydroxyl groups in the C-5 position together with substituents at the C-2 position would affect the bioactivity of targeted products and their presence could significantly enhance the anticancer activity of the novel compounds.
1 v/v) in high yields.30 Thus, it was recognized that this pathway is convenient to synthesize benzimidazoles that contain a variety of substituents on the C-2, C-5 and C-6 positions of the benzimidazole scaffold without undesired products. Continuing with our plans to develop new anti-tumor agents,22,31,32 in this research, we planned to synthesize a large number of new 2-phenylbenzimidazole derivatives with a diverse range of substituents on the C-2, C-5 and C-6 positions via condensation of o-phenylenediamines and various aldehydes under the conditions mentioned previously and then to elucidate their SAR against three cancer cell lines: A549, MDA-MB-231, and PC3. The aim was that the presence of substituents such as carbonyl and hydroxyl groups in the C-5 position together with substituents at the C-2 position would affect the bioactivity of targeted products and their presence could significantly enhance the anticancer activity of the novel compounds.
2. Results and discussion
2.1. Chemistry
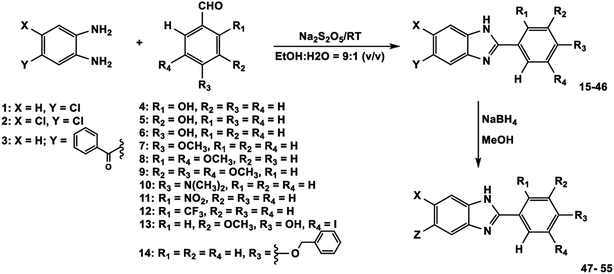
The preparation of the target 2-phenylbenzimidazoles 15–55 is shown in Scheme 1. Firstly, the ortho-phenylenediamine derivatives were sequentially condensed with various benzaldehydes that contain different substituents (–OH, –OCH3, –NO2, –N(CH3)2, –CF3, –I, –O-CH2-Ph). This reaction was carried out under mild conditions using a mixture of solvents: ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (ratio of 9
water (ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and Na2S2O5 as an oxidation agent to produce a series of benzimidazoles, 15–46, that have substituent groups such as hydro, chloro, phenyl methanonyl at the 5, 6 positions, with yields ranging from 32% to 98%. Next, the carbonyl group in compounds 37–46 was reduced to a corresponding hydroxyl group in the presence of NaBH4 in methanol to give the desired benzimidazole derivatives 47–55 with yields of 45% to 98%. The structures of the synthesized compounds are shown in the Table 1. All the synthesized compounds 15–55 were carefully characterized using FTIR, 1H-NMR, 13C-NMR spectroscopic and ESI-HRMS spectrometric methods. The FTIR bands at 3256, 3025, 1613 cm−1 confirmed the presence of –NH, C–H and C
1 v/v) and Na2S2O5 as an oxidation agent to produce a series of benzimidazoles, 15–46, that have substituent groups such as hydro, chloro, phenyl methanonyl at the 5, 6 positions, with yields ranging from 32% to 98%. Next, the carbonyl group in compounds 37–46 was reduced to a corresponding hydroxyl group in the presence of NaBH4 in methanol to give the desired benzimidazole derivatives 47–55 with yields of 45% to 98%. The structures of the synthesized compounds are shown in the Table 1. All the synthesized compounds 15–55 were carefully characterized using FTIR, 1H-NMR, 13C-NMR spectroscopic and ESI-HRMS spectrometric methods. The FTIR bands at 3256, 3025, 1613 cm−1 confirmed the presence of –NH, C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functionalities, respectively, of the most potent compound 38. The 1H-NMR spectrum showed a multiplet at δ 6.95–7.94 ppm confirming the presence of aromatic protons and benzimidazole protons. Similarly, in the 13C-NMR spectrum of compound 38, the carbonyl carbon was at δ 195.58 ppm and the remaining carbons all belonged to aromatic carbons which appeared in the range of δ 113.53–157.79 ppm. The fashion in the FTIR, 1H-NMR, and 13C-NMR spectra similar patterns were observed for the other compounds 37–46. Moreover, the appearance of carbon which was eliminated from a carbonyl group (compounds 37–46) to form a hydroxyl group (compounds 47–55) was recognized by 13C-NRM high field signals at δ 73.77–74.58 ppm. The HRMS (ESI) spectra of all the compounds showed the pseudo-molecular ion [M + H]+ or [M − H]− peaks corresponding to their respective molecular weights. The spectroscopic data (FTIR, 1H-NMR, 13C-NMR and ESI-HRMS) of all the newly synthesized benzimidazoles 24, 35, 39, 41, 43–45 and 47–55 were in agreement with the corresponding structures illustrated in the Experimental section.
N functionalities, respectively, of the most potent compound 38. The 1H-NMR spectrum showed a multiplet at δ 6.95–7.94 ppm confirming the presence of aromatic protons and benzimidazole protons. Similarly, in the 13C-NMR spectrum of compound 38, the carbonyl carbon was at δ 195.58 ppm and the remaining carbons all belonged to aromatic carbons which appeared in the range of δ 113.53–157.79 ppm. The fashion in the FTIR, 1H-NMR, and 13C-NMR spectra similar patterns were observed for the other compounds 37–46. Moreover, the appearance of carbon which was eliminated from a carbonyl group (compounds 37–46) to form a hydroxyl group (compounds 47–55) was recognized by 13C-NRM high field signals at δ 73.77–74.58 ppm. The HRMS (ESI) spectra of all the compounds showed the pseudo-molecular ion [M + H]+ or [M − H]− peaks corresponding to their respective molecular weights. The spectroscopic data (FTIR, 1H-NMR, 13C-NMR and ESI-HRMS) of all the newly synthesized benzimidazoles 24, 35, 39, 41, 43–45 and 47–55 were in agreement with the corresponding structures illustrated in the Experimental section.
| Cpd no. | X | Y | Z | R1 | R2 | R3 | R4 | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 15 | H | Cl | — | OH | H | H | H | 68 |
| 16 | H | Cl | — | H | OH | H | H | 54 |
| 17 | H | Cl | — | H | H | OH | H | 41 |
| 18 | H | Cl | — | H | H | OCH3 | H | 63 |
| 19 | H | Cl | — | OCH3 | H | H | OCH3 | 84 |
| 20 | H | Cl | — | H | OCH3 | OCH3 | OCH3 | 81 |
| 21 | H | Cl | — | H | H | N(CH3)2 | H | 81 |
| 22 | H | Cl | — | NO2 | H | H | H | 67 |
| 23 | H | Cl | — | CF3 | H | H | H | 83 |
| 24 | H | Cl | — | H | OCH3 | OH | I | 98 |
| 25 | H | Cl | — | H | H | –O-CH2-Ph | H | 48 |
| 26 | Cl | Cl | — | OH | H | H | H | 85 |
| 27 | Cl | Cl | — | H | OH | H | H | 78 |
| 28 | Cl | Cl | — | H | H | OH | H | 78 |
| 29 | Cl | Cl | — | H | H | OCH3 | H | 87 |
| 30 | Cl | Cl | — | OCH3 | H | H | OCH3 | 82 |
| 31 | Cl | Cl | — | H | OCH3 | OCH3 | OCH3 | 90 |
| 32 | Cl | Cl | — | H | H | N(CH3)2 | H | 73 |
| 33 | Cl | Cl | — | NO2 | H | H | H | 32 |
| 34 | Cl | Cl | — | CF3 | H | H | H | 52 |
| 35 | Cl | Cl | — | H | OCH3 | OH | I | 85 |
| 36 | Cl | Cl | — | H | H | –O-CH2-Ph | H | 68 |
| 37 | H | Ph-CO– | — | OH | H | H | H | 75 |
| 38 | H | Ph-CO– | — | H | OH | H | H | 78 |
| 39 | H | Ph-CO– | — | H | H | OH | H | 84 |
| 40 | H | Ph-CO– | — | H | H | OCH3 | H | 84 |
| 41 | H | Ph-CO– | — | H | OCH3 | OCH3 | OCH3 | 83 |
| 42 | H | Ph-CO– | — | H | H | N(CH3)2 | H | 98 |
| 43 | H | Ph-CO– | — | NO2 | H | H | H | 54 |
| 44 | H | Ph-CO– | — | CF3 | H | H | H | 53 |
| 45 | H | Ph-CO– | — | H | OCH3 | OH | I | 98 |
| 46 | H | Ph-CO– | — | H | H | –O-CH2-Ph | H | 76 |
| 47 | H | — | Ph-CH(OH)– | OH | H | H | H | 56 |
| 48 | H | — | Ph-CH(OH)– | H | OH | H | H | 72 |
| 49 | H | — | Ph-CH(OH)– | H | H | OH | H | 45 |
| 50 | H | — | Ph-CH(OH)– | H | H | OCH3 | H | 84 |
| 51 | H | — | Ph-CH(OH)– | H | OCH3 | OCH3 | OCH3 | 92 |
| 52 | H | — | Ph-CH(OH)– | H | H | N(CH3)2 | H | 64 |
| 53 | H | — | Ph-CH(OH)– | CF3 | H | H | H | 53 |
| 54 | H | — | Ph-CH(OH)– | H | OCH3 | OH | I | 98 |
| 55 | H | — | Ph-CH(OH)– | H | H | –O-CH2-Ph | H | 57 |
2.2. In vitro anticancer activities and structure–activity relationships (SAR)
All the synthesized benzimidazole derivatives 15–55 were tested for their cytotoxicity in vitro against human cancer cell lines: A549 (human lung adenocarcinoma epithelial cell line), MDA-MB-231 (human breast cancer cell line) and PC3 (human prostate cancer cell line), using camptothecin as a standard drug and the IC50 values (μg mL−1) are detailed in Table 2. Notably, the tested compounds showed a moderate to good antiproliferative activity on selected cancer cell lines. From the overall results, there were 11 compounds such as 16, 19, 21, 22, 23, 24, 28, 38, 45, 47, and 49 which displayed IC50 values less than 100 μg mL−1 on the three cancer cell lines. There were five compounds (23, 25, 28, 38, 39), two compounds (23, 38) and one compound (38) that displayed IC50 values of less than 8 μg mL−1 on the MDA-MB-231, PC3, and A549 cell lines, respectively. From a scientific point of view, the MDA-MB-231 human breast cancer cell line was found to be more sensitive towards the synthesized 2-phenylbenzimidazoles than the PC3 and A549 cell lines.| Cpd no. | IC50 ± SD (μg mL−1) | ||
|---|---|---|---|
| A549a | MDA-MB-231b | PC3c | |
| a Human lung adenocarcinoma epithelial cell line.b Human breast cancer cell line.c Human prostate cancer cell line.d Positive contrast drug. All the values are showed as mean ± SD in which each treatment was calculated from at least three independent experiments. ND = not determined. | |||
| 15 | >100 | 43.65 ± 1.58 | >100 |
| 16 | 12.02 ± 0.56 | 22.39 ± 1.05 | 18.20 ± 1.85 |
| 17 | 31.62 ± 1.46 | >100 | 36.56 ± 1.67 |
| 18 | >100 | >100 | >100 |
| 19 | 44.67 ± 1.69 | 12.02 ± 0.75 | 85.11 ± 2.67 |
| 20 | 11.48 ± 0.74 | >100 | 21.88 ± 1.59 |
| 21 | 9.12 ± 0.55 | 10.72 ± 1.11 | 18.62 ± 0.46 |
| 22 | 38.02 ± 1.89 | 30.2 ± 1.53 | 33.11 ± 1.43 |
| 23 | 45.71 ± 1.75 | 7.08 ± 0.86 | 6.92 ± 0.62 |
| 24 | 39.81 ± 1.03 | 37.15 ± 1.73 | 39.26 ± 1.59 |
| 25 | >100 | 6.61 ± 0.83 | 19.95 ± 1.08 |
| 26 | >100 | >100 | >100 |
| 27 | >100 | >100 | >100 |
| 28 | 64.57 ± 2.68 | 6.92 ± 0.98 | 60.26 ± 2.12 |
| 29 | >100 | >100 | >100 |
| 30 | 85.11 ± 2.12 | >100 | 69.18 ± 2.09 |
| 31 | >100 | 83.18 ± 2.05 | >100 |
| 32 | 52.48 ± 2.34 | >100 | 60.26 ± 2.42 |
| 33 | >100 | >100 | >100 |
| 34 | >100 | 45.71 ± 1.56 | >100 |
| 35 | ND | ND | ND |
| 36 | 60.26 ± 1.25 | >100 | >100 |
| 37 | >100 | 44.67 ± 1.69 | >100 |
| 38 | 4.47 ± 0.33 | 4.68 ± 0.42 | 5.50 ± 0.28 |
| 39 | >100 | >100 | >100 |
| 40 | >100 | 3.55 ± 0.35 | >100 |
| 41 | >100 | 66.07 ± 2.12 | >100 |
| 42 | >100 | >100 | >100 |
| 43 | >100 | >100 | >100 |
| 44 | >100 | >100 | >100 |
| 45 | 42.66 ± 1.62 | 46.77 ± 1.50 | 54.95 ± 1.96 |
| 46 | >100 | >100 | >100 |
| 47 | 14.13 ± 0.57 | 14.79 ± 1.08 | 17.78 ± 1.45 |
| 48 | 12.88 ± 0.46 | >100 | 17.38 ± 0.78 |
| 49 | 38.90 ± 2.17 | 30.2 ± 2.04 | 33.88 ± 1.98 |
| 50 | 53.70 ± 1.68 | >100 | >100 |
| 51 | 22.39 ± 1.07 | >100 | 44.67 ± 1.77 |
| 52 | >100 | >100 | >100 |
| 53 | 47.86 ± 1.73 | 54.95 ± 1.84 | >100 |
| 54 | >100 | 61.66 ± 1.83 | 79.43 ± 1.62 |
| 55 | >100 | 15.85 ± 0.56 | 22.91 ± 0.24 |
| Camptothecind | 0.2 ± 0.06 | 0.47 ± 0.04 | 0.87 ± 0.11 |
The difference in the electron donating and electron withdrawing groups in the 2-phenyl ring affected the activity of the synthesized compounds and this effect was found to be related to the substituents in the C-5 position. To clarify this point, the activities of compounds containing the hydroxyl group (electron donating group) were compared to those of compounds containing the trifluoromethyl group (electron withdrawing group) at the R1-position such as 15 vs. 23 (containing one chloro atom at the C-5 position), 26 vs. 34 (containing two chloro atoms at the C-5 and C-6 positions), 37 vs. 44 (containing a carbonyl group at the C-5 position) and 47 vs. 53 (containing a hydroxyl group at the C-5 position) on the MDA-MB-231 cell line and it was realized that the activity of compounds 15/26 and 23/34 also showed the same trend. The appearance of a hydroxyl group as an electron donating group at the R1-position decreased the activity of compound 15/26 in comparison to that of 23/34 containing trifluoromethyl as a withdrawing electron group (IC50 values of compounds 15, 26, 23 and 34 were 43.65 μg mL−1, >100, 7.08 and 45.71 μg mL−1, respectively). In contrast to this, introducing a hydroxyl group into the R1-position boosted the anticancer effect of 37/47 more than that of 44/53 which consisted of a trifluoromethyl group (IC50 values of compounds 37, 47, 44 and 53 were 44.67, 14.79, >100 and 54.95 μg mL−1, respectively).
To demonstrate that the chloro atoms on the benzimidazole skeleton make a significant contribution to the anticancer activity, compounds 15–25 and 26–36 were synthesized that contain one and two chloro atoms in the 5- and 6-positions, respectively. The compounds containing one chloro atom at the 5-position such as 21, 23 and 25 reached IC50 values of 10.7, 7.08 and 6.61 μg mL−1 with the MDA-MB-231 cell line, respectively. Unfortunately, for all three cancer cell lines of compounds, the anti-tumor activity of compounds that have two chloro atoms in the 5-, and 6-positions was considerably decreased and was less than that of compounds with one chloro atom in the 5-position, for example, 21 vs. 32, 23 vs. 34 and 25 vs. 36, but excluding compound 28, which had an IC50 value of 6.92 μg mL−1 for the MDA-MB-231 cell line. In the further studies, other halogen atoms (such as fluoro or bromo atoms) will be investigated at the 5-, and 6-positions in the benzimidazole structure so that those products may improve the antiproliferative activity, for example, against A549 cell lines, as reported previously in the literature.21
To find prospective anticancer agents, the phenyl methanonyl group was introduced into the benzene ring at the 5-position and compounds 37–46 were obtained. Nevertheless, this replacement lead to a decrease in the activity, compounds 39 and 42–46, when compared to other compounds that had one chloro atom in the 5-position (compounds 17 and 21–25). Surprisingly, there was a remarkable increase in the antiproliferative activity of benzimidazoles 38 and 40. In detail, the IC50 value of compound 40 with the MDA-MB-231 cell line reached 3.55 μg mL−1 which had decreased dramatically from more than 100 μg mL−1 – the IC50 value of compound 18 (without a phenyl methanonyl group but with a chloro atom at 5-position). Specifically, compound 38 acted as a potential multi cancer inhibitor against A549, MDA-MB-231 and PC3 cell lines with IC50 of 4.47 μg mL−1, 4.68 μg mL−1 and 5.50 μg mL−1, respectively.
To explore the effect of the presence of a carbonyl group in compounds 37–42 and 44–46 on their bioactivity, the carbonyl group was converted to a hydroxyl group by a reduction reaction and produced the novel compounds 47–55. Basically, the bioactivity on A549 and PC3 cell lines of compounds 47–55 was better than that of compounds 37–46. This was shown by the fact that there were six compounds (47–51 and 53) which displayed IC50 values less than 100 μg mL−1 on the A549 cell line instead of only two compounds (38 and 45). Conversely, that modification gave no considerable change of the activity of compounds 50–54, but compound 55 had an IC50 of 15.85 μg mL−1 with the MDA-MB-231 cell line.
In detail, the IC50 values of compound 48 on the three cancer cell lines were slightly increased, in contrast to that of compound 38 which did not have carbonyl group reduced to a hydroxyl group. Considering the SARs of compound 47, 37, 26 and 15, the presence of substituent containing hydroxyl group at the R1-position greatly increased the antiproliferative effect of compound 47, in comparison to those of the remained compounds (IC50 values ranged from 43.65 μg mL−1 to more than 100 μg mL−1 on the three cancer cell lines). This was shown by the fact that the IC50 values of compound 47 dropped to 14.13, 14.79 μg mL−1 and 17.78 μg mL−1 with the A549, MDA-MB-231 and PC3 cell lines, respectively. After the analysis of the SARs, it was hypothesized that it was necessary to introduce the carbonyl group into the benzimidazole structure at C-5 position and this adjustment markedly improved the anticancer activity of compounds 38 and 40.
3. Conclusion
In summary, 41 2-phenylbenzimidazoles with various substituents in the 5-, 6-positions and a 2-phenyl ring, including 17 novel derivatives, were synthesized under mild conditions using a mixture of solvents: ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (9
water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to increase the solubility of reactants (step 1) and NaBH4 in methanol (step 2) and the products were obtained in high yields. All of synthesized compounds were evaluated the antiproliferative activity against A549, MDA-MB-231 and PC3 human cancer cell lines. Compound 40 expressed a high selectivity towards the breast cancer cell line MDA-MB-231 (IC50 = 3.55 μg mL−1). Whereas compound 38 with the most potent anticancer activity showed IC50 values of 4.47, 4.68 and 5.50 μg mL−1 for the A549, MDA-MB-231, and PC3 cell lines, respectively, and shows promise as a new multi-cancer inhibitor. Benzimidazoles and the 2-phenylbenzimidazole derivatives are also topoisomerase I inhibitors.18,33–35 The studies to clarify the modes of action of the synthesized 2-phenylbenzimidazoles binding on the topo I and DNA complex are currently in progress.
1 v/v) to increase the solubility of reactants (step 1) and NaBH4 in methanol (step 2) and the products were obtained in high yields. All of synthesized compounds were evaluated the antiproliferative activity against A549, MDA-MB-231 and PC3 human cancer cell lines. Compound 40 expressed a high selectivity towards the breast cancer cell line MDA-MB-231 (IC50 = 3.55 μg mL−1). Whereas compound 38 with the most potent anticancer activity showed IC50 values of 4.47, 4.68 and 5.50 μg mL−1 for the A549, MDA-MB-231, and PC3 cell lines, respectively, and shows promise as a new multi-cancer inhibitor. Benzimidazoles and the 2-phenylbenzimidazole derivatives are also topoisomerase I inhibitors.18,33–35 The studies to clarify the modes of action of the synthesized 2-phenylbenzimidazoles binding on the topo I and DNA complex are currently in progress.
4. Experimental section
4.1. Materials
All reagents and solvents were purchased from Acros Organics (Belgium), Sigma-Aldrich (USA) and Xilong (China) and used without further purification.4.2. Characterization of synthesized benzimidazoles
Reactions were monitored by thin-layer chromatography (TLC) which was performed on silica gel 60 F254 plates (Merck). The synthesized compounds were visualized by UV light (254 nm). All of the melting points (MPs) were determined on an IA 9000 series digital melting point apparatus (Electrothermal) and were uncorrected. The FTIR spectra were recorded using KBr pellets with an Equinox 55 IR spectrometer (Bruker, Germany) and the absorption bands were expressed in wavenumbers (cm−1). The 1H-NMR and 13C-NMR spectra were recorded at 500 MHz (1H-NMR) and 125 MHz (13C-NMR) on an AM0 FT-NMR spectrometer (Bruker) in (CD3)2SO. Tetramethylsilane (TMS) was used as an internal standard and the chemical shifts were expressed in δ (ppm) and the coupling constants (J) in hertz (Hz). The signal multiplicities were expressed by standard abbreviations as follows: s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublet, m = multiplet. High-resolution mass spectra were recorded using electrospray ionization on a X500R QTOF quadrupole time-of-flight (QTOF) system (Sciex).4.3. General procedure for the synthesis of compounds 15–46
A mixture of o-phenylenediamines 1–3 (2 mmol) and benzaldehyde derivatives 4–14 (2 mmol) were stirred in 10–20 mL of ethanol and water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) (until both dissolved) and then Na2S2O5 (2 mmol) was added. The suspension was stirred constantly at room temperature for 2 h and the reaction was monitored by TLC. After completion of the reaction, the precipitate was removed by filtering and the filtrate was evaporated under reduced pressure to obtain the crude product. The crude product was washed three times with water and n-hexane, and then dried in a vacuum at 80 °C to achieve the final product. The purification was performed on a column chromatography system or by crystallization from solvents with the desired method as listed in the information for each product.
1 v/v) (until both dissolved) and then Na2S2O5 (2 mmol) was added. The suspension was stirred constantly at room temperature for 2 h and the reaction was monitored by TLC. After completion of the reaction, the precipitate was removed by filtering and the filtrate was evaporated under reduced pressure to obtain the crude product. The crude product was washed three times with water and n-hexane, and then dried in a vacuum at 80 °C to achieve the final product. The purification was performed on a column chromatography system or by crystallization from solvents with the desired method as listed in the information for each product.
2-(5-Chloro-1H-benzoimidazol-2-yl)-phenol (15): isolated by silica gel column chromatography with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (98
methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) to obtain a light yellow powder; yield: 68%; mp (°C): 240–241; FTIR (KBr, ν (cm−1)): 3330 (N–H), 3057 (C–H), 1633 (C
2, v/v) to obtain a light yellow powder; yield: 68%; mp (°C): 240–241; FTIR (KBr, ν (cm−1)): 3330 (N–H), 3057 (C–H), 1633 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1489 (C
N), 1489 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1257 (C–N), 738 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.02–8.06 (m, 7H, CHAr), 12.74 (s, 1H, OH), 13.23 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 112.51–157.79 (CHAr); ESI-HRMS (DMSO): m/z = 245.0463 [M + H]+.
C), 1257 (C–N), 738 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.02–8.06 (m, 7H, CHAr), 12.74 (s, 1H, OH), 13.23 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 112.51–157.79 (CHAr); ESI-HRMS (DMSO): m/z = 245.0463 [M + H]+.
3-(5-Chloro-1H-benzoimidazol-2-yl)-phenol (16): light yellow powder; yield: 54%; mp (°C): 236–237; FTIR (KBr, ν (cm−1)): 3250 (N–H), 3060 (C–H), 1591 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1466 (C
N), 1466 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1231 (C–N), 784 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.93–7.22 (m, 7H, CHAr), 9.75 (s, 1H, OH), 12.99 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 113.42–157.77 (CHAr); ESI-HRMS (DMSO): m/z = 245.0479 [M + H]+.
C), 1231 (C–N), 784 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.93–7.22 (m, 7H, CHAr), 9.75 (s, 1H, OH), 12.99 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 113.42–157.77 (CHAr); ESI-HRMS (DMSO): m/z = 245.0479 [M + H]+.
4-(5-Chloro-1H-benzoimidazol-2-yl)-phenol (17): slightly brown powder; yield: 41%; mp (°C): 259–260; FTIR (KBr, ν (cm−1)): 3227 (N–H), 3070 (C–H), 1609 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1461 (C
N), 1461 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1251 (C–N), 729 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.93–8.00 (m, 7H, CHAr), 10.04 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 114.55–159.53 (CHAr); ESI-HRMS (DMSO): m/z = 245.0482 [M + H]+.
C), 1251 (C–N), 729 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.93–8.00 (m, 7H, CHAr), 10.04 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 114.55–159.53 (CHAr); ESI-HRMS (DMSO): m/z = 245.0482 [M + H]+.
5-Chloro-2-(4-methoxy-phenyl)-1H-benzoimidazole (18): slightly yellow powder; yield: 63%; mp (°C): 174–175; FTIR (KBr, ν (cm−1)): 3007 (C–H), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1492 (C
N), 1492 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1258 (C–N), 1180 (O–CH3), 738 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.38 (s, 3H, O–CH3), 6.67–7.67 (m, 7H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.30 (O–CH3), 114.38–160.88 (CHAr); ESI-HRMS (DMSO): m/z = 259.0633 [M + H]+.
C), 1258 (C–N), 1180 (O–CH3), 738 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.38 (s, 3H, O–CH3), 6.67–7.67 (m, 7H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.30 (O–CH3), 114.38–160.88 (CHAr); ESI-HRMS (DMSO): m/z = 259.0633 [M + H]+.
5-Chloro-2-(2,5-methoxy-phenyl)-1H-benzoimidazole (19): slightly yellow powder; yield: 84%; mp (°C): 139–140; FTIR (KBr, ν (cm−1)): 3218 (N–H), 3008 (C–H), 1614 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1490 (C
N), 1490 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1223 (C–N), 1176 (O–CH3), 740 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.80 (s, 3H, O–CH3), 3.98 (s, 3H, O–CH3), 7.22–7.86 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.50, 56.17 (O–CH3), 113.50–153.18 (CHAr); ESI-HRMS (DMSO): m/z = 289.0734 [M + H]+.
C), 1223 (C–N), 1176 (O–CH3), 740 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.80 (s, 3H, O–CH3), 3.98 (s, 3H, O–CH3), 7.22–7.86 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.50, 56.17 (O–CH3), 113.50–153.18 (CHAr); ESI-HRMS (DMSO): m/z = 289.0734 [M + H]+.
5-Chloro-2-(3,4,5-trimethoxy-phenyl)-1H-benzoimidazole (20): slightly gray powder; yield: 81%; mp (°C): 233–234; FTIR (KBr, ν (cm−1)): 3063 (C–H), 1588 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1464 (C
N), 1464 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1240 (C–N), 1127 (O–CH3), 731 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.74 (s, 3H, O–CH3), 3.90 (s, 6H, 2 × O–CH3), 7.23–7.64 (m, 5H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.04, 60.11 (O–CH3), 104.00–153.21 (CHAr); ESI-HRMS (DMSO): m/z = 319.08466 [M + H]+.
C), 1240 (C–N), 1127 (O–CH3), 731 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.74 (s, 3H, O–CH3), 3.90 (s, 6H, 2 × O–CH3), 7.23–7.64 (m, 5H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.04, 60.11 (O–CH3), 104.00–153.21 (CHAr); ESI-HRMS (DMSO): m/z = 319.08466 [M + H]+.
4-(5-Chloro-1H-benzoimidazol-2-yl)-N,N-dimethylaniline (21): light yellow powder; yield: 81%; mp (°C): 233–234; FTIR (KBr, ν (cm−1)): 3074 (C–H), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1501 (C
N), 1501 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1367 (C–N), 1127 (N–(CH3)2), 740 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.00 (s, 6H, 2 × CH3), 6.84–7.99 (m, 7H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 79.11 (CH3), 111.74–153.55 (CHAr); ESI-HRMS (DMSO): m/z = 272.0949 [M + H]+.
C), 1367 (C–N), 1127 (N–(CH3)2), 740 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.00 (s, 6H, 2 × CH3), 6.84–7.99 (m, 7H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 79.11 (CH3), 111.74–153.55 (CHAr); ESI-HRMS (DMSO): m/z = 272.0949 [M + H]+.
5-Chloro-2-(2-nitro-phenyl)-1H-benzoimidazole (22): slightly yellow powder; yield: 67%; mp (°C): 103–104; FTIR (KBr, ν (cm−1)): 3197 (N–H), 3078 (C–H), 1613 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1529 (C
N), 1529 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1348 (C–N), 753 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.28–8.04 (m, 7H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 122.78–148.83 (CHAr); ESI-HRMS (DMSO): m/z = 274.0387 [M + H]+.
C), 1348 (C–N), 753 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.28–8.04 (m, 7H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 122.78–148.83 (CHAr); ESI-HRMS (DMSO): m/z = 274.0387 [M + H]+.
5-Chloro-2-(2-trifluoromethyl-phenyl)-1H-benzoimidazole (23): slightly gray powder; yield: 83%; mp (°C): 173–174; FTIR (KBr, ν (cm−1)): 3053 (C–H), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1552 (C
N), 1552 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1433 (C–N), 1129 (C–F), 769 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.26–7.95 (m, 7H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 120.41–150.8 (CF3, CHAr); ESI-HRMS (DMSO): m/z = 297.04084 [M + H]+.
C), 1433 (C–N), 1129 (C–F), 769 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.26–7.95 (m, 7H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 120.41–150.8 (CF3, CHAr); ESI-HRMS (DMSO): m/z = 297.04084 [M + H]+.
4-(5-Chloro-1H-benzoimidazole-2-yl)-2-iodo-6-methoxy-phenol (24): slightly gray powder; yield: 98%; mp (°C): 204–205; FTIR (KBr, ν (cm−1)): 3453 (OH), 3098 (C–H), 1624 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1539 (C
N), 1539 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1464 (C–N), 1275 (O–CH3), 724 (C–Cl), 593 (C–I); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.93 (s, 3H, O–CH3), 7.21–8.1 (m, 5H, CHAr), 10.14 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.20 (O–CH3), 84.59 (CAr–I), 110.03–151.57 (CHAr); ESI-HRMS (DMSO): m/z = 400.9527 [M + H]+.
C), 1464 (C–N), 1275 (O–CH3), 724 (C–Cl), 593 (C–I); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.93 (s, 3H, O–CH3), 7.21–8.1 (m, 5H, CHAr), 10.14 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.20 (O–CH3), 84.59 (CAr–I), 110.03–151.57 (CHAr); ESI-HRMS (DMSO): m/z = 400.9527 [M + H]+.
2-(4-(Benzyloxy)phenyl)-5-chloro-1H-benzoimidazole (25): brown powder; yield: 48%, mp (°C): 170–171; FTIR (KBr, ν (cm−1)): 3030 (C–H), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1552 (C
N), 1552 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1314 (C–N), 1129 (C–O–C ether), 769 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.2 (s, 2H, O–CH2), 7.19–7.60 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 69.37 (O–CH2), 115.22–159.93 (CHAr); ESI-HRMS (DMSO): m/z = 335.09497 [M + H]+.
C), 1314 (C–N), 1129 (C–O–C ether), 769 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.2 (s, 2H, O–CH2), 7.19–7.60 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 69.37 (O–CH2), 115.22–159.93 (CHAr); ESI-HRMS (DMSO): m/z = 335.09497 [M + H]+.
5,6-Dichloro-2-(4-trimethoxy-phenyl)-1H-benzoimidazole (26): brown powder; yield: 85%; mp (°C): 228–229; FTIR (KBr, ν (cm−1)): 3331 (OH), 3098 (C–H), 1636 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1488 (C
N), 1488 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1369 (C–N), 752 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.02–8.07 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 112.48–157.64 (CHAr); ESI-HRMS (DMSO): m/z = 279.0069 [M + H]+.
C), 1369 (C–N), 752 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.02–8.07 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 112.48–157.64 (CHAr); ESI-HRMS (DMSO): m/z = 279.0069 [M + H]+.
3-(5,6-Dichloro-1H-benzoimidazol-2-yl)phenol (27): white powder; yield: 78%; mp (°C): 260–261, FTIR (KBr, ν (cm−1)): 3228 (OH), 3032 (C–H), 1650 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1469 (C
N), 1469 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1232 (C–N), 7.18 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.94–7.82 (m, 6H, CHAr), 9.76 (s, 1H, OH), 13.13 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 113.53–157.77 (CHAr); ESI-HRMS (DMSO): m/z = 279.0088 [M + H]+.
C), 1232 (C–N), 7.18 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.94–7.82 (m, 6H, CHAr), 9.76 (s, 1H, OH), 13.13 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 113.53–157.77 (CHAr); ESI-HRMS (DMSO): m/z = 279.0088 [M + H]+.
4-(5,6-Dichloro-1H-benzoimidazol-2-yl)phenol (28): yellow powder; yield: 78%; mp (°C): 209–210; FTIR (KBr, ν (cm−1)): 3200 (OH), 3032 (C–H), 1609 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1454 (C
N), 1454 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1250 (C–N), 743 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.93–8.00 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 115.76–159.69 (CHAr); ESI-HRMS (DMSO): m/z = 279.0088 [M + H]+.
C), 1250 (C–N), 743 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.93–8.00 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 115.76–159.69 (CHAr); ESI-HRMS (DMSO): m/z = 279.0088 [M + H]+.
5,6-Dichloro-2-(4-methoxy-phenyl)-1H-benzoimidazole (29): slightly yellow powder; yield: 87%; mp (°C): 200–201; FTIR (KBr, ν (cm−1)): 3019 (C–H), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1493 (C
N), 1493 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1261 (C–N), 1181 (O–CH3), 734 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.85 (s, 3H, O–CH3), 7.13–8.11 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.39 (O–CH3), 114.49–161.14 (CHAr); ESI-HRMS (DMSO): m/z = 293.02468 [M + H]+.
C), 1261 (C–N), 1181 (O–CH3), 734 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.85 (s, 3H, O–CH3), 7.13–8.11 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.39 (O–CH3), 114.49–161.14 (CHAr); ESI-HRMS (DMSO): m/z = 293.02468 [M + H]+.
5,6-Dichloro-2-(2,5-dimethoxyphenyl)-1H-benzo[d]imidazole (30): yellow powder; yield: 82%; mp (°C): 173–174; FTIR (KBr, ν (cm−1)): 3086 (C–H), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1483 (C
N), 1483 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1307 (C–N), 1169 (O–CH3), 731 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.80 (s, 3H, O–CH3), 3.98 (s, 3H, O–CH3), 7.10–7.86 (m, 5H, CHAr), 12.29 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.52, 56.25 (O–CH3), 104.18–153.79 (CHAr); ESI-HRMS (DMSO): m/z = 323.0368 [M + H]+.
C), 1307 (C–N), 1169 (O–CH3), 731 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.80 (s, 3H, O–CH3), 3.98 (s, 3H, O–CH3), 7.10–7.86 (m, 5H, CHAr), 12.29 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.52, 56.25 (O–CH3), 104.18–153.79 (CHAr); ESI-HRMS (DMSO): m/z = 323.0368 [M + H]+.
5,6-Dichloro-2-(3,4,5-trimethoxy-phenyl)-1H-benzoimidazole (31): yellow powder; yield: 90%; mp (°C): 252–253; FTIR (KBr, ν (cm−1)): 3290 (N–H), 2990 (C–H), 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1412 (C
N), 1412 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1375 (C–N), 1127 (O–CH3), 763 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.75 (s, 3H, O–CH3), 3.90 (s, 6H, 2 × O–CH3), 7.51–7.92 (m, 4H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.07, 60.13 (O–CH3), 104.18–153.79 (CHAr); ESI-HRMS (DMSO): m/z = 353.04543 [M + H]+.
C), 1375 (C–N), 1127 (O–CH3), 763 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.75 (s, 3H, O–CH3), 3.90 (s, 6H, 2 × O–CH3), 7.51–7.92 (m, 4H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.07, 60.13 (O–CH3), 104.18–153.79 (CHAr); ESI-HRMS (DMSO): m/z = 353.04543 [M + H]+.
4-(5,6-Dichloro-1H-benzo[d]imidazol-2-yl)-N,N-dimethylaniline (32): yellow powder; yield: 73%; mp (°C): 265–266; FTIR (KBr, ν (cm−1)): 3422 (NH), 3033 (C–H), 1612 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1503 (C
N), 1503 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1372 (C–N), 1208 (N–(CH3)2), 735 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.01 (s, 6H, 2 × CH3), 6.84–7.96 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 39.33 (CH3), 111.72–154.68 (CHAr); ESI-HRMS (DMSO): m/z = 306.0568 [M + H]+.
C), 1372 (C–N), 1208 (N–(CH3)2), 735 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.01 (s, 6H, 2 × CH3), 6.84–7.96 (m, 6H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 39.33 (CH3), 111.72–154.68 (CHAr); ESI-HRMS (DMSO): m/z = 306.0568 [M + H]+.
5,6-Dichloro-2-(2-nitrophenyl)-1H-benzoimidazole (33): orange powder; yield: 32%; mp (°C): 218–219; FTIR (KBr, ν (cm−1)): 2924 (C–H), 1612 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1526 (C
N), 1526 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1349 (C–N), 784 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.80–8.07 (m, 6H, CHAr), 13.38 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 123.62–150.09 (CHAr); ESI-HRMS (DMSO): m/z = 307.9926 [M + H]+.
C), 1349 (C–N), 784 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.80–8.07 (m, 6H, CHAr), 13.38 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 123.62–150.09 (CHAr); ESI-HRMS (DMSO): m/z = 307.9926 [M + H]+.
5,6-Dichloro-2-(2-trifluoromethyl-phenyl)-1H-benzoimidazole (34): slightly yellow powder; yield: 52%; mp (°C): 266–267; FTIR (KBr, ν (cm−1)): 3007 (C–H), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1582 (C
N), 1582 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1314 (C–N), 1134 (C–F), 772 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.84–7.98 (m, 6H, CHAr), 13.14 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 115.13–150.68 (CF3, CHAr); ESI-HRMS (DMSO): m/z = 331.0035 [M + H]+.
C), 1314 (C–N), 1134 (C–F), 772 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.84–7.98 (m, 6H, CHAr), 13.14 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 115.13–150.68 (CF3, CHAr); ESI-HRMS (DMSO): m/z = 331.0035 [M + H]+.
4-(5,6-Dichloro-1H-benzoimidazol-2-yl)-2-iodo-6-methoxy-phenol (35): isolated by silica gel column chromatography with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (98
methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as a slightly yellow powder; yield: 85%; mp (°C): 226–227; FTIR (KBr, ν (cm−1)): 3454 (OH), 3224 (NH), 2999 (C–H), 1699 (C
2) as a slightly yellow powder; yield: 85%; mp (°C): 226–227; FTIR (KBr, ν (cm−1)): 3454 (OH), 3224 (NH), 2999 (C–H), 1699 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1481 (C
N), 1481 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1279 (C–N), 1100 (O–CH3), 731 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.93 (s, 3H, O–CH3), 7.76–8.11 (m, 4H, CHAr), 10.17 (s, 1H, OH), 13.08 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.20 (O–CH3), 84.60 (CAr–I), 110.09–152.86 (CHAr); ESI-HRMS (DMSO): m/z = 434.91745 [M + H]+.
C), 1279 (C–N), 1100 (O–CH3), 731 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.93 (s, 3H, O–CH3), 7.76–8.11 (m, 4H, CHAr), 10.17 (s, 1H, OH), 13.08 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.20 (O–CH3), 84.60 (CAr–I), 110.09–152.86 (CHAr); ESI-HRMS (DMSO): m/z = 434.91745 [M + H]+.
2-(4-Benzyloxy-phenyl)-5,6-dichloro-1H-benzoimidazole (36): isolated by silica gel column chromatography with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (98
methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as a slightly yellow powder; yield: 68%; mp (°C): 262–263; FTIR (KBr, ν (cm−1)): 3291 (N–H), 3031 (C–H), 1608 (C
2) as a slightly yellow powder; yield: 68%; mp (°C): 262–263; FTIR (KBr, ν (cm−1)): 3291 (N–H), 3031 (C–H), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1494 (C
N), 1494 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1252 (C–N), 1174 (C–O–C ether), 743 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.20 (s, 2H, O–CH2), 7.22–8.11 (m, 11H, CHAr), 13.06 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 69.39 (O–CH2), 112.34–160.18 (CHAr); ESI-HRMS (DMSO): m/z = 369.05330 [M + H]+.
C), 1252 (C–N), 1174 (C–O–C ether), 743 (C–Cl); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.20 (s, 2H, O–CH2), 7.22–8.11 (m, 11H, CHAr), 13.06 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 69.39 (O–CH2), 112.34–160.18 (CHAr); ESI-HRMS (DMSO): m/z = 369.05330 [M + H]+.
[2-(2-Hydroxy-phenyl)-1H-benzoimidazol-5-yl]-phenyl-methanone (37): slightly yellow powder; yield: 75%; mp (°C): 262–263; FTIR (KBr, ν (cm−1)): 3296 (N–H), 3025 (C–H), 1613 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1446 (C
N), 1446 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1294 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.06–8.08 (m, 12H, CHAr), 12.68 (s, 1H, OH), 13.38 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 112.51–195.45 (CHAr); ESI-HRMS (DMSO): m/z = 315.11349 [M + H]+.
C), 1294 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.06–8.08 (m, 12H, CHAr), 12.68 (s, 1H, OH), 13.38 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 112.51–195.45 (CHAr); ESI-HRMS (DMSO): m/z = 315.11349 [M + H]+.
[2-(3-Hydroxy-phenyl)-1H-benzoimidazol-5-yl]-phenyl-methanone (38): yellow powder; yield: 78%; mp (°C): 254–255; FTIR (KBr, ν (cm−1)): 3445 (OH), 3256 (NH), 3025 (C–H), 1613 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1461 (C
N), 1461 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1276 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.95–7.94 (m, 12H, CHAr), 9.79 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 113.53–195.58 (CHAr); ESI-HRMS (DMSO): m/z = 315.11315 [M + H]+.
C), 1276 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.95–7.94 (m, 12H, CHAr), 9.79 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 113.53–195.58 (CHAr); ESI-HRMS (DMSO): m/z = 315.11315 [M + H]+.
[2-(4-Hydroxy-phenyl)-1H-benzoimidazol-5-yl]-phenyl-methanone (39): slightly yellow powder; yield: 84%; mp (°C): 295–296; FTIR (KBr, ν (cm−1)): 3421 (OH), 3250 (NH), 3063 (C–H), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1454 (C
N), 1454 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1282 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.96–8.02 (m, 12H, CHAr), 12.77 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 115.44–195.20 (CHAr); ESI-HRMS (DMSO): m/z = 315.11300 [M + H]+.
C), 1282 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 6.96–8.02 (m, 12H, CHAr), 12.77 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 115.44–195.20 (CHAr); ESI-HRMS (DMSO): m/z = 315.11300 [M + H]+.
[2-(4-Methoxy-phenyl)-1H-benzoimidazol-5-yl]-phenyl-methanone (40): slightly yellow powder; yield: 84%; mp (°C): 295–296; FTIR (KBr, ν (cm−1)): 3273 (NH), 3061 (C–H), 1640 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1493 (C
N), 1493 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1318 (C–N), 1177 (O–CH3); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.85 (s, 3H, O–CH3), 7.16–8.16 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.39 (O–CH3), 114.53–195.53 (CHAr); ESI-HRMS (DMSO): m/z = 329.12917 [M + H]+.
C), 1318 (C–N), 1177 (O–CH3); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.85 (s, 3H, O–CH3), 7.16–8.16 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.39 (O–CH3), 114.53–195.53 (CHAr); ESI-HRMS (DMSO): m/z = 329.12917 [M + H]+.
Phenyl-[2-(3,4,5-trimethoxy-phenyl)-1H-benzoimidazol-5-yl]-methanone (41): slightly brown powder; yield: 83%; mp (°C): 220–221; FTIR (KBr, ν (cm−1)): 3094 (C–H), 1639 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1585 (C
N), 1585 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1293 (C–N), 1177 (O–CH3); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.74 (s, 3H, OCH3), 3.90 (s, 6H, OCH3), 7.53–7.95 (m, 10H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.09–60.18 (O–CH3), 104.22–195.54 (CHAr); ESI-HRMS (DMSO): m/z = 389.15027 [M + H]+.
C), 1293 (C–N), 1177 (O–CH3); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.74 (s, 3H, OCH3), 3.90 (s, 6H, OCH3), 7.53–7.95 (m, 10H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.09–60.18 (O–CH3), 104.22–195.54 (CHAr); ESI-HRMS (DMSO): m/z = 389.15027 [M + H]+.
[2-(4-Dimethylamino-phenyl)-1H-benzoimidazol-5-yl](phenyl)methanone (42): yellow powder; yield: 98%; mp (°C): 216–217; FTIR (KBr, ν (cm−1)): 3302 (NH), 3062 (C–H), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1502 (C
N), 1502 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1287 (C–N), 1198 (N–(CH3)2); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.01 (s, 6H, CH3), 6.86–8.03 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 39.33 (CH3), 111.79–195.54 (CHAr); ESI-HRMS (DMSO): m/z = 342.16067 [M + H]+.
C), 1287 (C–N), 1198 (N–(CH3)2); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.01 (s, 6H, CH3), 6.86–8.03 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 39.33 (CH3), 111.79–195.54 (CHAr); ESI-HRMS (DMSO): m/z = 342.16067 [M + H]+.
[2-(2-Nitrophenyl)-1H-benzoimidazol-5-yl](phenyl)methanone (43): isolated by silica gel column chromatography with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (98
methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as a slightly yellow powder; yield: 54%; mp (°C): 194–195; FTIR (KBr, ν (cm−1)): 3216 (NH), 3062 (C–H), 1600 (C
2) as a slightly yellow powder; yield: 54%; mp (°C): 194–195; FTIR (KBr, ν (cm−1)): 3216 (NH), 3062 (C–H), 1600 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1537 (C
N), 1537 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1286 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.57–8.09 (m, 12H, CHAr), 13.44 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 123.92–195.66 (CHAr); ESI-HRMS (DMSO): m/z = 344.1011 [M + H]+.
C), 1286 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.57–8.09 (m, 12H, CHAr), 13.44 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 123.92–195.66 (CHAr); ESI-HRMS (DMSO): m/z = 344.1011 [M + H]+.
Phenyl(2-(2-(trifluoromethyl)phenyl)-1H-benzo[d]imidazol-5-yl)methanone (44): recrystallized from ethyl acetate to obtain a brown solid; yield: 53%; mp (°C): 114–115; FTIR (KBr, ν (cm−1)): 3279 (NH), 3076 (C–H), 1650 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1554 (C
N), 1554 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1311 (C–N), 1128 (C–F); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.58–7.99 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 122.55–195.65 (CF3, CHAr); ESI-HRMS (DMSO): m/z = 367.1052 [M + H]+.
C), 1311 (C–N), 1128 (C–F); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 7.58–7.99 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 122.55–195.65 (CF3, CHAr); ESI-HRMS (DMSO): m/z = 367.1052 [M + H]+.
(2-(4-Hydroxy-3-iodo-5-methoxyphenyl)-1H-benzoimidazol-5-yl)(phenyl)methanone (45): yellow powder; yield: 98%; mp (°C): 164–165; FTIR (KBr, ν (cm−1)): 3491 (OH), 3256 (NH), 3060 (C–H), 1616 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1544 (C
N), 1544 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1281 (C–N), 1116 (O–CH3), 582 (C–I); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.95 (s, 3H, O–CH3), 7.57–8.15 (m, 10H, CHAr), 10.17 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.21 (O–CH3), 84.65 (C–I), 110.12–195.46 (CHAr); ESI-HRMS (DMSO): m/z = 471.02093 [M + H]+.
C), 1281 (C–N), 1116 (O–CH3), 582 (C–I); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.95 (s, 3H, O–CH3), 7.57–8.15 (m, 10H, CHAr), 10.17 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.21 (O–CH3), 84.65 (C–I), 110.12–195.46 (CHAr); ESI-HRMS (DMSO): m/z = 471.02093 [M + H]+.
(2-(4-(Benzyloxy)phenyl)-1H-benzoimidazol-5-yl)(phenyl)methanone (46): slightly yellow powder; yield: 76%; mp (°C): 198–199; FTIR (KBr, ν (cm−1)): 3246 (NH), 3060 (C–H), 1612 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1495 (C
N), 1495 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1248 (C–N), 1179 (C–O–C ether); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.23 (s, 2H, O–CH2), 7.24–8.17 (m, 2H, CHAr, 17H), 13.11 (s, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 69.41 (O–CH2), 115.31–195.53 (CHAr); HRMS (m/z): 405.16112 [M + H]+.
C), 1248 (C–N), 1179 (C–O–C ether); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.23 (s, 2H, O–CH2), 7.24–8.17 (m, 2H, CHAr, 17H), 13.11 (s, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 69.41 (O–CH2), 115.31–195.53 (CHAr); HRMS (m/z): 405.16112 [M + H]+.
4.4. General procedure for the synthesis of the final compounds 47–55
The benzimidazoles 37–46 (1 mmol) in 5 mL of methanol were stirred in a 2-neck round-bottom flask until they were completely dissolved, then 2 mmol of NaBH4 was added. The reaction mixture was stirred at room temperature for 1 hand monitored by TLC using a mixture of ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (95
hexane (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or 9
5 or 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as eluting solvent. Then, the solvent was evaporated under reduced pressure and the residue was washed with water to obtain the product. The purification was performed on a column chromatography system or by crystallization from solvents using the desired method, as listed in the information for each product.
1 v/v) as eluting solvent. Then, the solvent was evaporated under reduced pressure and the residue was washed with water to obtain the product. The purification was performed on a column chromatography system or by crystallization from solvents using the desired method, as listed in the information for each product.
2-(5-(Hydroxy(phenyl)methyl)-1H-benzo[d]imidazol-2-yl)phenol (47): isolated by silica gel column chromatography with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (98
methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as a white powder; yield: 56%; mp (°C): 161–162; FTIR (KBr, ν (cm−1)): 3361 (NH), 3056 (C–H), 1593 (C
2) as a white powder; yield: 56%; mp (°C): 161–162; FTIR (KBr, ν (cm−1)): 3361 (NH), 3056 (C–H), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1486 (C
N), 1486 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1255 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.86 (s, 1H, C–H), 5.94 (s, 1H, OH), 7.03–8.03 (m, 12H, CHAr), 13.12 (s, 2H, NH and OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 74.41 (C–H), 112.61–157.92 (CHAr); ESI-HRMS (DMSO): m/z = 315.1128 [M − H]−.
C), 1255 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.86 (s, 1H, C–H), 5.94 (s, 1H, OH), 7.03–8.03 (m, 12H, CHAr), 13.12 (s, 2H, NH and OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 74.41 (C–H), 112.61–157.92 (CHAr); ESI-HRMS (DMSO): m/z = 315.1128 [M − H]−.
3-(5-(Hydroxy(phenyl)methyl)-1H-benzo[d]imidazol-2-yl)phenol (48): isolated by silica gel column chromatography with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (98
methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as a white powder; yield: 72%; mp (°C): 146–147; FTIR (KBr, ν (cm−1)): 3240 (NH), 3061 (C–H), 1600 (C
2) as a white powder; yield: 72%; mp (°C): 146–147; FTIR (KBr, ν (cm−1)): 3240 (NH), 3061 (C–H), 1600 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1472 (C
N), 1472 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1232 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.81 (s, 1H, C–H), 5.85 (s, 1H, OH), 6.88–7.55 (m, 12H, CHAr), 12.65 (s, 1H, NH), 9.66 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 74.46 (C–H), 108.85–157.67 (CHAr); ESI-HRMS (DMSO): m/z = 315.1136 [M − H]−.
C), 1232 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.81 (s, 1H, C–H), 5.85 (s, 1H, OH), 6.88–7.55 (m, 12H, CHAr), 12.65 (s, 1H, NH), 9.66 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 74.46 (C–H), 108.85–157.67 (CHAr); ESI-HRMS (DMSO): m/z = 315.1136 [M − H]−.
4-(5-(Hydroxy(phenyl)methyl)-1H-benzo[d]imidazol-2-yl)phenol (49): isolated by silica gel column chromatography with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (98
methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as a soft white powder; yield: 45%; mp (°C): 147–148; FTIR (KBr, ν (cm−1)):3212 (NH), 3031 (C–H), 1612 (C
2) as a soft white powder; yield: 45%; mp (°C): 147–148; FTIR (KBr, ν (cm−1)):3212 (NH), 3031 (C–H), 1612 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1466 (C
N), 1466 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1278 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.81 (s, 1H, CH), 5.84 (s, 1H, OH), 6.91–7.97 (m, 12H, CHAr), 12.53 (s, 1H, NH), 9.91 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 74.58 (C–H), 115.60–158.99 (CHAr); ESI-HRMS (DMSO): m/z = 315.1134 [M − H]−.
C), 1278 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.81 (s, 1H, CH), 5.84 (s, 1H, OH), 6.91–7.97 (m, 12H, CHAr), 12.53 (s, 1H, NH), 9.91 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 74.58 (C–H), 115.60–158.99 (CHAr); ESI-HRMS (DMSO): m/z = 315.1134 [M − H]−.
[2-(4-Methoxy-phenyl)-1H-benzoimidazol-5-yl]-phenyl-methanol (50): recrystallized from methanol to obtain a slightly yellow solid; yield: 84%; mp (°C):157–158; FTIR (KBr, ν (cm−1)): 3410 (NH), 3082 (C–H), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1485 (C
N), 1485 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1252 (C–N), 1177 (O–CH3); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.81 (m, 1H, C–H), 5.83 (m, 1H, OH), 3.83 (s, 3H, O–CH3), 7.10–8.08 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.29 (O–CH3), 74.56 (C–H), 114.31–160.51(CHAr); ESI-HRMS (DMSO): m/z = 329.1291 [M − H]−.
C), 1252 (C–N), 1177 (O–CH3); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.81 (m, 1H, C–H), 5.83 (m, 1H, OH), 3.83 (s, 3H, O–CH3), 7.10–8.08 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 55.29 (O–CH3), 74.56 (C–H), 114.31–160.51(CHAr); ESI-HRMS (DMSO): m/z = 329.1291 [M − H]−.
Phenyl-[2-(3,4,5-trimethoxy-phenyl)-1H-benzoimidazol-5-yl]-methanol (51): recrystallized from methanol to obtain a white solid; mp (°C): 231–232; yield: 92%, FTIR (KBr, ν (cm−1)): 3359 (NH), 3094 (C–H), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1593 (C
N), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1263 (C–N), 1131 (O–CH3); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.79–3.94 (s, 9H, O–CH3), 5.93 (s, 1H, C–H), 7.22–7.88 (m, 10H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.62–60.28 (O–CH3), 73.77 (C–H), 106.00–153.40 (CHAr); ESI-HRMS (DMSO): m/z = 391.1660 [M + H]+.
C), 1263 (C–N), 1131 (O–CH3); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.79–3.94 (s, 9H, O–CH3), 5.93 (s, 1H, C–H), 7.22–7.88 (m, 10H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.62–60.28 (O–CH3), 73.77 (C–H), 106.00–153.40 (CHAr); ESI-HRMS (DMSO): m/z = 391.1660 [M + H]+.
[2-(4-Dimethylamino-phenyl)-1H-benzoimidazol-5-yl]-phenyl-methanol (52): recrystallized from methanol to obtain a slightly yellow solid; yield: 64%; mp (°C): 269–270; FTIR (KBr, ν (cm−1)): 3312 (NH), 3010 (C–H), 1604 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1514 (C
N), 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1220 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.06 (s, 6H, CH3), 5.89 (s, 1H, C–H), 6.93–8.15 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 73.83 (CH3), 108.39–153.11 (CHAr); ESI-HRMS (DMSO): m/z = 342.1743 [M − H]−.
C), 1220 (C–N); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.06 (s, 6H, CH3), 5.89 (s, 1H, C–H), 6.93–8.15 (m, 12H, CHAr); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 73.83 (CH3), 108.39–153.11 (CHAr); ESI-HRMS (DMSO): m/z = 342.1743 [M − H]−.
Phenyl(2-(2-(trifluoromethyl)phenyl)-1H-benzo[d]imidazol-5-yl)methanol (53): recrystallized from ethyl acetate to obtain a soft white powder; yield: 53%; mp (°C): 154–155; FTIR (KBr, ν (cm−1)): 3357 (NH), 3057 (C–H), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1486 (C
N), 1486 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1255 (C–N), 1018 (C–F); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.84 (s, 1H, C–H), 5.87 (s, 1H, OH), 7.21–7.93 (m, 12H, CHAr), 12.65 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 74.54 (C–H), (CF3, CHAr); ESI-HRMS (DMSO): m/z = 369.1207 [M + H]+.
C), 1255 (C–N), 1018 (C–F); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.84 (s, 1H, C–H), 5.87 (s, 1H, OH), 7.21–7.93 (m, 12H, CHAr), 12.65 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 74.54 (C–H), (CF3, CHAr); ESI-HRMS (DMSO): m/z = 369.1207 [M + H]+.
4-[5-(Hydroxy-phenyl-methyl)-1H-benzoimidazol-2-yl]-2-iodo-6-methoxy-phenol (54): recrystallized from methanol to obtain a soft white solid; yield: 98%; mp (°C): 228–229; FTIR (KBr), ν/cm−1: 3389 (NH), 3032 (C–H), 1629 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1485 (C
N), 1485 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1294 (C–N), 511 (C–I); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.98 (s, 3H, O–CH3), 5.92 (s, 1H, OH), 7.22–8.33 (m, 10H), 10.81 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.85 (O–CH3), 73.78 (C–H), 84.97 (C–I), 110.54–150.86 (CHAr); ESI-HRMS (DMSO): m/z = 471.0206 [M − H]−.
C), 1294 (C–N), 511 (C–I); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 3.98 (s, 3H, O–CH3), 5.92 (s, 1H, OH), 7.22–8.33 (m, 10H), 10.81 (s, 1H, OH); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 56.85 (O–CH3), 73.78 (C–H), 84.97 (C–I), 110.54–150.86 (CHAr); ESI-HRMS (DMSO): m/z = 471.0206 [M − H]−.
[2-(4-Benzyloxy-phenyl)-1H-benzoimidazol-5-yl]-phenyl-methanol (55): recrystallized from methanol to obtain a slightly brown solid; yield: 57%; mp (°C): 126–127; FTIR (KBr, ν (cm−1)): 3353 (NH), 3030 (C–H), 1610 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1492 (C
N), 1492 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1249 (C–N), 1130 (C–O–C ether); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.19 (s, 2H, O–CH2), 5.82 (s, 1H, OH), 7.20–8.1 (m, 17H); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 69.35 (O–CH2), 74.58 (C–H), 115.13–159.55 (CHAr); ESI-HRMS (DMSO): m/z = 405.1595 [M − H]−.
C), 1249 (C–N), 1130 (C–O–C ether); 1H-NMR (500 MHz, DMSO-d6, δ ppm): 5.19 (s, 2H, O–CH2), 5.82 (s, 1H, OH), 7.20–8.1 (m, 17H); 13C-NMR (125 MHz, DMSO-d6, δ ppm): 69.35 (O–CH2), 74.58 (C–H), 115.13–159.55 (CHAr); ESI-HRMS (DMSO): m/z = 405.1595 [M − H]−.
4.5. Cytotoxicity assay
All of the target compounds 15–55 were tested against A549 (human lung adenocarcinoma epithelial cell line), MDA-MB-231 (human breast cancer cell line) and PC3 (human prostate cancer cell line). Dr Jeong-Hyung Lee (College of Natural Sciences, Kangwon National University) provided the A549, MDA-MB-231 and PC3 cell lines to the Vietnam Academy of Sciences and Technology (VAST). The benzimidazole derivatives 15–55 were dissolved in DMSO to obtain various concentrations. Camptothecin and DMSO were used as the reference compound and blank controls, respectively. All the cancer cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U mL−1 of penicillin and 100 μg mL−1 of streptomycin in a 5% CO2 atmosphere for 48 h. Next, the cells were suspended in the culture medium added to well plates at a concentration of 104 cells/well. After 24 h of incubation, the cells were treated with various concentrations of the test compounds for a period of 72 h. Then, 0.5 mg mL−1 of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added and incubated at 37 °C, in 5% CO2 for 4 h followed by the procedure described by Mosmann.36 The formazan crystals were dissolved by adding acidified isopropanol and mixed thoroughly to produce the purple solution. This solution was spectrophotometrically measured using a Multiskan™ microplate reader (ThermoFisher Scientific) at 570 nm. Each concentration of tested benzimidazoles was examined in triplicate. The percentage of cell survival (CS) was calculated using the formula: CS (%) = 100 × (ODsample − ODday 0)/(ODDMSO − ODday 0). The IC50 values (μg mL−1) were calculated for tested compounds, which had CS values < 50% in the initial screening, using the non-linear regression analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.01-2017.335.References
- J.-P. Gillet and M. M. Gottesman, in Multi-drug resistance in cancer, Springer, 2010, pp. 47–76 Search PubMed.
- D. Basile, V. G. Pelizzari, M. G. Vitale and F. Puglisi, VI European conference on Cured and Chronic Cancer Patients, Italy, 2016 Search PubMed.
- A. Spasov, I. Yozhitsa, L. Bugaeva and V. Anisimova, Pharm. Chem. J., 1999, 33, 232–243 CrossRef CAS.
- C. Kavitha, K. M. Hosamani and H. Seetharamareddy, Eur. J. Med. Chem., 2010, 45, 2048–2054 CrossRef PubMed.
- J. r. Bauer, S. Kinast, A. Burger-Kentischer, D. Finkelmeier, G. Kleymann, W. A. Rayyan, K. Schröppel, A. Singh, G. n. Jung and K.-H. Wiesmüller, J. Med. Chem., 2011, 54, 6993–6997 CrossRef CAS PubMed.
- H. B. El-Nassan, Eur. J. Med. Chem., 2012, 53, 22–27 CrossRef CAS PubMed.
- B. Garudachari, M. Satyanarayana, B. Thippeswamy, C. Shivakumar, K. Shivananda, G. Hegde and A. M. Isloor, Eur. J. Med. Chem., 2012, 54, 900–906 CrossRef CAS PubMed.
- Y.-F. Li, G.-F. Wang, P.-L. He, W.-G. Huang, F.-H. Zhu, H.-Y. Gao, W. Tang, Y. Luo, C.-L. Feng and L.-P. Shi, J. Med. Chem., 2006, 49, 4790–4794 CrossRef CAS PubMed.
- A. T. Mavrova, D. Vuchev, K. Anichina and N. Vassilev, Eur. J. Med. Chem., 2010, 45, 5856–5861 CrossRef CAS PubMed.
- C. S. Mizuno, A. G. Chittiboyina, F. H. Shah, A. Patny, T. W. Kurtz, H. A. Pershadsingh, R. C. Speth, V. T. Karamyan, P. B. Carvalho and M. A. Avery, J. Med. Chem., 2010, 53, 1076–1085 CrossRef CAS PubMed.
- C. G. Neochoritis, T. Zarganes-Tzitzikas, C. A. Tsoleridis, J. Stephanidou-Stephanatou, C. A. Kontogiorgis, D. J. Hadjipavlou-Litina and T. Choli-Papadopoulou, Eur. J. Med. Chem., 2011, 46, 297–306 CrossRef CAS PubMed.
- R. V. Patel, P. K. Patel, P. Kumari, D. P. Rajani and K. H. Chikhalia, Eur. J. Med. Chem., 2012, 53, 41–51 CrossRef CAS PubMed.
- Y. Bansal and O. Silakari, Bioorg. Med. Chem., 2012, 20, 6208–6236 CrossRef CAS PubMed.
- C. Karthikeyan, V. R. Solomon, H. Lee and P. Trivedi, Arabian J. Chem., 2017, 10, S1788–S1794 CrossRef CAS.
- H. S. Elzahabi, Eur. J. Med. Chem., 2011, 46, 4025–4034 CrossRef CAS PubMed.
- K. Starčević, M. Kralj, K. Ester, I. Sabol, M. Grce, K. Pavelić and G. Karminski-Zamola, Bioorg. Med. Chem., 2007, 15, 4419–4426 CrossRef PubMed.
- M. Rangarajan, J. S. Kim, S.-P. Sim, A. Liu, L. F. Liu and E. J. LaVoie, Bioorg. Med. Chem., 2000, 8, 2591–2600 CrossRef CAS PubMed.
- J. S. Kim, Q. Sun, B. Gatto, C. Yu, A. Liu, L. F. Liu and E. J. LaVoie, Bioorg. Med. Chem., 1996, 4, 621–630 CrossRef CAS PubMed.
- A. W. White, N. J. Curtin, B. W. Eastman, B. T. Golding, Z. Hostomsky, S. Kyle, J. Li, K. A. Maegley, D. J. Skalitzky and S. E. Webber, Bioorg. Med. Chem. Lett., 2004, 14, 2433–2437 CrossRef CAS PubMed.
- J.-F. Liu, Y.-L. Huang, W.-H. Yang, C.-S. Chang and C.-H. Tang, Int. J. Mol. Sci., 2012, 13, 16472–16488 CrossRef CAS PubMed.
- A. Kamal, V. Srinivasulu, M. Sathish, Y. Tangella, V. L. Nayak, M. N. Rao, N. Shankaraiah and N. Nagesh, Asian J. Org. Chem., 2014, 3, 68–76 CrossRef CAS.
- T. K. C. Huynh, T. C. T. Ly, A. T. Le, N. D. Ngo, T. H. T. Do and T. K. D. Hoang, Vietnam J. Chem., 2018, 56, 336–341 Search PubMed.
- C. Rathod, R. Rajurkar and S. Thonte, Indo Am. J. Pharm. Res., 2013, 2323–2329 Search PubMed.
- S. Özbey, F. B. Kaynak, C. Kuš and H. Göker, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2002, 58, o1062–o1064 CrossRef.
- H. T. B. Bui, Q. T. K. Ha, W. K. Oh, D. D. Vo, Y. N. T. Chau, C. T. K. Tu, E. C. Pham, P. T. Tran, L. T. Tran and H. Van Mai, Tetrahedron Lett., 2016, 57, 887–891 CrossRef CAS.
- Y. Venkateswarlu, S. R. Kumar and P. Leelavathi, Bioorg. Med. Chem. Lett., 2013, 3, 7 CrossRef PubMed.
- R. Srinivasulu, K. R. Kumar and P. V. V. Satyanarayana, Green Sustainable Chem., 2014, 4, 33 CrossRef CAS.
- P. Aniket, D. Shantanu, O. Anagha and P. Ajinkya, Int. J. ChemTech Res., 2015, 8, 496–500 Search PubMed.
- V. Patil and K. Patil, Int. J. ChemTech Res., 2015, 8, 457–465 CAS.
- T. K. C. Huynh, N. H. S. Tran and T. K. D. Hoang, Vietnam J. Chem., 2019, 57, 350–354 Search PubMed.
- T. K.-D. Hoang, T. K.-C. Huynh and T.-D. Nguyen, Bioorg. Chem., 2015, 63, 45–52 CrossRef CAS PubMed.
- T. K.-D. Hoang, T. K.-C. Huynh, T. H.-T. Do and T.-D. Nguyen, Chem. Pap., 2018, 72, 1399–1406 CrossRef CAS.
- J. S. Kim, B. Gatto, C. Yu, A. Liu, L. F. Liu and E. J. LaVoie, J. Med. Chem., 1996, 39, 992–998 CrossRef CAS PubMed.
- S. Bansal, S. Sur and V. Tandon, Biochemistry, 2018, 58, 809–817 CrossRef PubMed.
- U. Issar, R. Arora, T. Kumari and R. Kakkar, Struct. Chem., 2019, 30, 1185–1201 CrossRef CAS.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02282a |
| This journal is © The Royal Society of Chemistry 2020 |