Open Access Article
Open Access ArticleUltrasound-assisted coagulation for Microcystis aeruginosa removal using Fe3O4-loaded carbon nanotubes†
Xiaoge Wu ab,
Guofeng Xua and
Juanjuan Wang*a
ab,
Guofeng Xua and
Juanjuan Wang*a
aEnvironment Science and Engineering College, Yangzhou University, Yangzhou, Jiangsu 225009, China. E-mail: wangjuanjuan@yzu.edu.cn; Fax: +86 514 87978626; Tel: +86 514 89791109
bJiangsu Provincial Laboratory of Water Environmental Protection Engineering, School of Environmental Science and Engineering, Yangzhou University, Yangzhou 225127, Jiangsu, China
First published on 2nd April 2020
Abstract
Harmful cyanobacterial blooms are increasing environmental issues and require novel removal technology since the required doses of algaecides may cause further environmental pollution or treatment facility damage. Herein, we firstly introduce the combination of ultrasound and Fe3O4/CNTs as an alternative strategy to enhance coagulation for the removal of Microcystis aeruginosa cells in water. It remarkably enhanced cyanobacterial cell removal and microcystins control, compared with sonication alone (40 kHz ultrasonic bath, 4.2 mJ mL−1). 94.4% cyanobacterial cells were removed using 20 second sonication with 20 mg L−1 Fe3O4/CNTs, Al2(SO4)3 coagulation (20 μM). Both sonication time and catalyst dose significantly influenced the cyanobacterial removal. Ultrasound with Fe3O4/CNTs only induced a slight increase of cell permeability, which may contribute to the effective control of DOC and microcystins' release in water. The enhanced settlement of the cyanobacterial cells may result from the moderate oxidation on the cell surface. This study suggested a novel ultrasound-Fe3O4/CNT process to promote cyanobacteria removal with efficient DOC and microcystin release control, which is a green and safe technology for drinking water treatment.
Introduction
Harmful cyanobacterial cells have been considered as a threat to drinking water safety. In particular, lysis of cyanobacterial cells may occur during cyanobacterial cell removal, leading to the leakage of odor, cyanobacterial toxins and other organic metabolites.1,2 An ideal treatment should be effective at removing the intact cells without cell lysis.3,4 Traditional coagulation and sedimentation employs coagulants to remove intact cyanobacteria cells as well as the intracellular toxins within cells.5 But the coagulation process could be interfered with by algal organic matter.6 In fact, it is still challenging to achieve high removal efficiency of cyanobacterial cells without significant cell lysis, and the overdosed coagulant may cause secondary pollution for the subsequent mainstream processes.7–9 Therefore, developing novel technologies to reduce cyanobacterial cells' threat to drinking water quality is important and necessary.Pre-oxidation has been widely considered as a pre-treatment process to enhance the removal cyanobacterial cells.10 Free radicals generated from advanced oxidation process can rapidly react with organic compounds. In this regard, pre-oxidation is capable of reducing the concentration of negative charge organic matters, leading to a higher efficiency of coagulation on cyanobacterial cells.7 Preoxidation processes using chlorine,8,11 ozone,11,12 Fenton agents7 and Fenton-like agents,13,14 have been test whether they could increase the coagulation efficiency for the removal of cyanobacterial cells. Pre-chlorination was found to increase the Microcystis aeruginosa cells in water after coagulation–filtration, while pre-oxidation using ozone enhanced the cyanobacterial removal.11 Recently, physical UV irradiation is considered as a part of the preoxidation. For example, UV/chlorine could change the surface conditions of cyanobacterial cells and promote the coagulation efficiency.8 UV/H2O2 was applied to enhance Fe(II) coagulation, and the total microcystins could be reduced with the decreasing of cyanobacterial cells and AOM.7 In order to improve cyanobacterial removal efficiency and control the release of microcystins, the dosage of oxidants, pre-oxidation time and settled period should be carefully optimized.
Compared with UV irradiation, ultrasound is an alternative technology less affected by turbidity of water and it has been investigated for cyanobacteria cells removal via coagulation.15 Currently, iron based sonication is an advanced oxidation process, which is widely investigated in water treatment.16 The combination with Fe-based catalyst and ultrasound forms ultrasonic Fenton-like reactions, which could improve the removal efficiency of organic pollutants and reduce the energy cost. Furthermore, with the inhibition of Fe3O4 particle aggregation, the Fe3O4/MWCNTs hybrid catalysts would be a promising preoxidation strategy.17 In this work, we synthesized Fe3O4/CNTs by ultrasound and proved that it has an effective catalytic effect on the removal of Microcystis aeruginosa via coagulation. This study discussed the removal effect of Fe3O4/CNTs on cyanobacteria cells, and the specific objectives are: (1) to evaluate the removal effect of Fe3O4/CNTs on cyanobacteria by coagulation–sedimentation method; (2) to analyze the permeability of cyanobacteria cells treated with Fe3O4/CNTs; (3) to evaluate the water quality after Fe3O4/CNTs and Fe3O4 treatment, including concentration of algal toxin and dissolved organic carbon. (4) To explore the removal mechanism using ultrasound and Fe3O4/CNT. This study demonstrated that ultrasound with Fe3O4/CNTs is effective on the cyanobacterial removal as well as microcystins release control, which may provide a green and promising strategy without negative effects on drinking water quality.
Materials and methods
Material and reagents
Microcystis aeruginosa cells was purchased from Culture Collection of Algae and Protozoa. It was cultured using BG11 medium. Algal suspensions were cultured at 25 °C under 12 hour light illumination every day. For catalyst fabrication, all the reagents were purchased from Yangtai Company (Yangzhou, China), and they were at analytical reagent grade without further purification. For the experiments, the aqueous solutions were prepared using deionized water.Ultrasonic fabrication and characterization of Fe3O4/CNTs
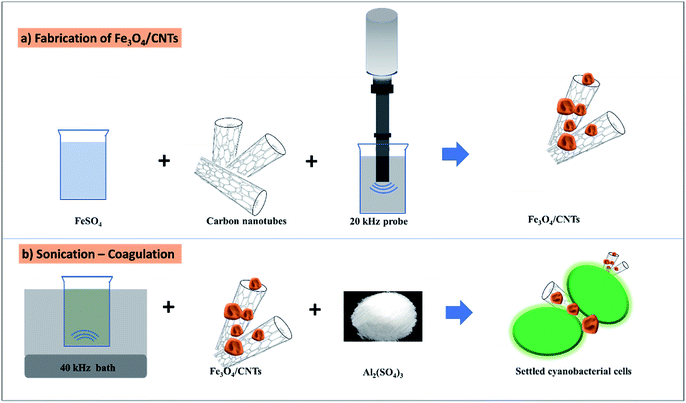
Fe3O4/CNTs were synthesized by ultrasonic oxidation and modified precipitation method (Fig. 1a).18 Following the reported method, 20 mg CNTs were sonicated in 40 mL water/ethylene glycol (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 2 minutes. 40 mg FeCl3·6H2O and 1.52 g sodium acetate were then added into the CNTs solution. The whole reaction system was sonicated for 1 hour using 20 kHz probe (VCX130, Sonics Vibra cell™, Stanford, USA). The temperature during sonication was controlled to be 40–45 °C using a cooling system. The final products were separated and washed by distilled water and ethanol. The obtained Fe3O4/CNTs were dried at 60 °C for 24 hours. The morphology of Fe3O4/CNTs were detected using a transmission electron microscopy (TEM, JEM-200CX), which is equipped with a FEI Tecnai G2 F30 S-TWIN electron microscope. X-ray photoelectron spectroscopy (XPS) data were collected using an ESCALAB 250Xi X-ray photoelectron spectrometer (Thermo Fisher).
1) for 2 minutes. 40 mg FeCl3·6H2O and 1.52 g sodium acetate were then added into the CNTs solution. The whole reaction system was sonicated for 1 hour using 20 kHz probe (VCX130, Sonics Vibra cell™, Stanford, USA). The temperature during sonication was controlled to be 40–45 °C using a cooling system. The final products were separated and washed by distilled water and ethanol. The obtained Fe3O4/CNTs were dried at 60 °C for 24 hours. The morphology of Fe3O4/CNTs were detected using a transmission electron microscopy (TEM, JEM-200CX), which is equipped with a FEI Tecnai G2 F30 S-TWIN electron microscope. X-ray photoelectron spectroscopy (XPS) data were collected using an ESCALAB 250Xi X-ray photoelectron spectrometer (Thermo Fisher).
Pre-oxidation experiments
As shown in Fig. 1b, the experimental tests on the preoxidation of Microcystis aeruginosa cells from water were performed in 200 mL glass beakers. For sonication–coagulation treatment, cyanobacterial cells were sonicated in the glass beaker using a 40 kHz ultrasonic bath (3L, Ultrasonic Cleaner SB-200DTD, Yimaneili Company, Nanjing, China). The ultrasonic intensity of the 40 kHz ultrasonic system was 0.78 W mL−1, which was determined by calorimetry.The sonication time was set as 0, 5, 10, 20, 30, seconds (corresponding to 0 mJ mL−1, 1.1 mJ mL−1, 2.1 mJ mL−1, 4.2 mJ mL−1, 6.3 mJ mL−1) to evaluate the effects of ultrasound doses on cyanobacterial removal. The dosage of Fe3O4/CNTs was 20 mg L−1 with the different sonication time during the treatment. To investigate the effects of different Fe3O4/CNTs concentrations on cyanobacterial removal, the 200 mL cyanobacterial cells without the addition of Fe3O4/CNTs were set as control. 0, 10 mg L−1, 20 mg L−1, 30 mg L−1 and 50 mg L−1 Fe3O4/CNTs were added in the deoxygenated cyanobacterial suspension, respectively. The treated cyanobacterial suspensions were cultured in the incubator without stirring.
A coagulation process was performed on the obtained cyanobacterial suspension using a programmable jar tester with 20 μM Al2(SO4)3 solution, which was followed from Qi's work.19 Water samples were collected at 2 cm below the water surface. The residual Microcystis aeruginosa samples were divided into two subgroups, the first group was assessed by cell density and cell integrity. The second group was immediately filtered for dissolved organic carbon (DOC) and microcystins analysis.
Analysis methods
For each sample, (i) the density of cyanobacterial cells was counted using a haemocytometer. (ii) The membrane integrity of the cyanobacterial cells remained in water suspension were analyzed by the flow cytometry (Becton Dickinson, USA). A LIVE/DEAD Baclight bacterial viability kit (L10316, Invitrogen, USA) was used to measure the percentage of intact/viable (Syto-9 stained) to damage/nonviable (propidium iodide stained) cells in a sample.20 (iii) K+ leaking from cyanobacterial were measured following the reported methods of Gu et al., using an inductively coupled plasma optical emission spectrometer (ICP-OES, OPTIMA 8000, PerkinElmer, USA).21 (iv) The microcystins concentration from water samples was measured using the microcystins ELISA Kit (Beacon Analytical Systems Inc. USA).22 The detection limit of the ELISA kit was is 0.1 μg L−1. (v) The DOC content was analyzed using a TOC-VCPN analyzer (Shimadzu, Japan).23Statistical analysis
Each treatment was conducted with three replications. Statistical analyses were performed using Microsoft Excel (Microsoft, USA) and the results were presented as mean ± SD (standard deviation).Results and discussion
Effect of 40 kHz sonication with Fe3O4/CNTs on enhanced coagulation for cyanobacterial cells removal
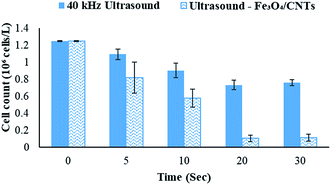
Fe3O4/CNTs were ultrasonically fabricated following our pervious method (data shown in ESI, Fig. S1†).18 Fig. 2 demonstrates the effects of ultrasonic time from 0 to 30 seconds to M. aeruginosa removal after coagulation–sedimentation process. The Fe3O4/CNTs dosage was 20 mg L−1, which was added into the cyanobacterial suspension at the beginning of sonication. The cyanobacterial cell number decreased from 1.25 × 106 cells per mL to 0.11 × 106 cells per mL with the increasing of sonication time from 0 to 20 seconds. After 30 second sonication, the cyanobacterial cells number was slightly higher than that of 20 second sonication. In contrast, only a small amount of M. aeruginosa cells were settled using sonication alone. The results suggested that the Fe3O4/CNTs catalyzed ultrasonic effects on the removal of Microcystis aeruginosa cells. For the ultrasound – Fe3O4/CNTs preoxidation process, the sonication time play vital roles for cyanobacterial removal.Similar results were reported that different ultrasonic time has different impact on cyanobacterial cells. Li et al. suggested that in a short duration of ultrasonic irradiation, the increasing of ultrasonic power input may result in a small amount of algal organic matter (AOM) release, promoting coagulation (eqn (1) and (2)).15 The formation of free radical was measured using potassium iodide dosimetry (Fig. S3†).20 But in this work, the effect of ultrasound was further catalyzed by iron(II, III) oxide (Fe3O4) (eqn (3) and (4)),16 which could produce more free radicals, to react with extracellular organic matter (EOM) on the cell wall (eqn (5) and (6)).24 In fact, the EOM play critical roles on coagulation since it could control the charge of cyanobacterial cells, which is an essential factor for the electrostatic attraction between cyanobacterial cells and coagulants.25,26 Furthermore, Li et al. suggested sonication within 5 minutes could release EOM to enhance the coagulation via adhesion bridging.15 Although the mechanism is unclear yet, our work also confirmed that using moderate peroxidation, cells could be well settled down after the coagulation process.
| H2O → H+ + HO˙ | (1) |
| HO˙ + HO˙ → H2O2 | (2) |
| Fe2+ + H2O2 → Fe3+ + OH˙ + OH− | (3) |
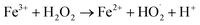 | (4) |
| OH˙ + cell → cell** + AOM | (5) |
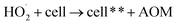 | (6) |
The preoxidation has been applied for cyanobacterial removal, and the reaction time varies between a several minutes and tens of minutes.5–9 Recently, a study reported a photocatalytic pretreatment by 0.05 g L−1 Zn-doped Fe3O4 particles with 360 minutes visible-light irradiation, followed by 20 μM Al2(SO4)3 for coagulation and sedimentation.19 The enhanced coagulation efficiency was 96%, suggesting that a moderate oxidation of cyanobacterial cells may provide great potential for algae removal via coagulation. Jia et al. reported that 94.7% algae cells were removed using 5 min UV/H2O2 pre-treatment, followed with 125 μmol L−1 FeSO4 for coagulation and sedimentation, and the 94.7% cyanobacterial cells were removed.7 In this work, 94.4% cyanobacterial cells were removed via preoxidation (20 second sonication and 20 mg L−1 Fe3O4/CNTs), followed by coagulation (20 μM Al2(SO4)3). It is suggested that the combination of 40 kHz sonication and 20 mg L−1 Fe3O4/CNTs on cyanobacterial removal is comparable to these reported preoxidation–coagulation treatment.
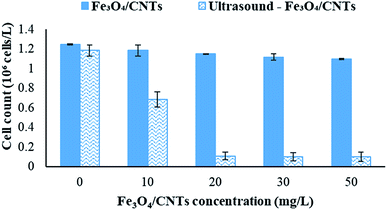
To investigate the effects of sonocatalysis by Fe3O4/CNTs on the removal of M. aeruginosa in water via sedimentation, the catalysis dosage was controlled from 0 to 50 mg L−1. As shown in Fig. 3, the addition of Fe3O4/CNTs without sonication barely decreased the cyanobacterial cells number. The cyanobacterial cell number decreased slightly from 1.25 × 106 cells per mL to 1.10 × 106 cells per mL with increasing Fe3O4/CNTs doses to 50 mg L−1. In the present of 20 second sonication, the cyanobacterial cell number decreased slightly from 1.18 × 106 cells per mL to 0.11 × 106 cells per mL with increasing Fe3O4/CNTs doses to 20 mg L−1. But the cells number of M. aeruginosa did not further reduced when the Fe3O4/CNTs dosage increased to 30 mg L−1. It is observed that the optimum Fe3O4/CNTs dose was 20 mg L−1 in this work.
Nano iron particles has received increasing considerations for cyanobacterial removal since cells attached to magnetic nanoparticles can be easily removed by followed sedimentation.25,27–29 Generally, magnetic nanoparticles remove algae cells by electrostatic adsorption.27 However, the microalgae flocs formed by adding Fe3O4 alone need high concentration of coagulant, which may due to the agglomeration of magnetic particles.17 Thus, to increase the effective utilization of nano Fe3O4 particles, carbon porous materials are usually used to support the nanoparticles loading, which could avoid the agglomeration of magnetic nanoparticles and improve the dispersion of materials in water. In addition, carbon nanotubes (CNTs) have effective absorption ability due their morphology and surface chemistry.30–32 CNTs have been used for the removal of contaminants in water, and they were found to have a strong ability in the adsorption of MCs.33 However, no reported work has involved in the application of cyanobacterial cells removal using CNTs. In this work, the combination of Fe3O4 and CNTs has been considered as a useful and effective strategy to overcome the agglomeration of Fe3O4 particles. Therefore, it is worth to develop a strategy using Fe3O4/CNTs for Microcystis aeruginosa cells removal from water.
Cell permeability of M. aeruginosa cells after sonication with Fe3O4/CNTs
The cell permeability of M. aeruginosa cells remained in water suspension was test after sonication with Fe3O4/CNTs followed with 3 hour cultivation. For ultrasonic treatment, the population of the cell intact cells was around 90.33% after 20 second sonication (data shown in ESI†). The results are in agreements of previous studies that 40 kHz ultrasound may increase the algal cell permeability within 5 minute treatment. But our work suggested that the cells could be recovered after 3 hours. For the addition of Fe3O4/CNTs, most cells showed intact cell integrity, indicating that the nano-material offers high biocompatibility. For the combination of ultrasound and Fe3O4/CNTs, no data were obtained for the percentage of intact cells since there were no enough cells in water suspension to be counted using flow cytometry. The superior removal efficiency suggested that there seems low risk of intracellular toxin releasing from cyanobacterial cells remained in water after sonication–coagulation treatment (Fig. 4).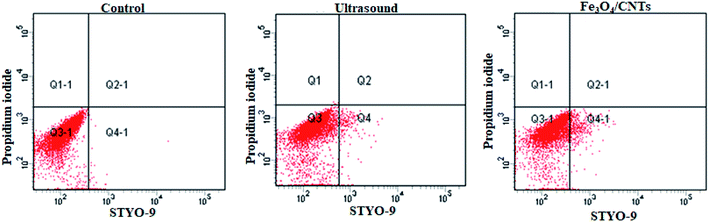 | ||
| Fig. 4 The change of the cell permeability of Microcystis aeruginosa cells in cyanobacterial suspension treated with 20 second sonication and 20 mg L−1 Fe3O4/CNTs, after coagulation–sedimentation. | ||
The intact cell population of M. aeruginosa cells in the sediment was also analyzed using microscopy. Neither sonication nor Fe3O4/CNTs addition enhanced the population of the damaged cells. The pre-treatment of 20 second sonication and 20 mg L−1 Fe3O4/CNTs did not enhance the cyanobacterial cells' permeability. These results indicated that the sonocatalytic effect of Fe3O4/CNTs did not damage the cyanobacterial cells. To remove cyanobacterial cells in water, sonication has been applied in many reported works.34,35 But the removal mechanism usually involved the destruction of algal cells, which may lead to the release of microcystins as well as other algal organic matter.15,36 It has been acknowledged that maintaining the cellular integrity is important for the control of the toxin releasing from harmful cyanobacterial cells. An ideal treatment should be effective at removing the intact cells without cell lysis.4 Compared with the previous ultrasonic studies, the cyanobacterial cells remained intact in water and in sediment, suggesting this novel preoxidation strategy is safe for cyanobacterial cells removal.
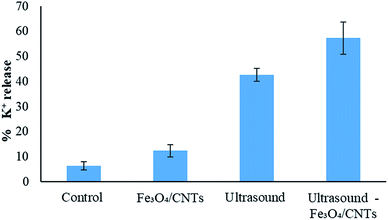
K+ is a key indicator for the cell membrane integrity of M. aeruginosa, since K+ could be released from cells if the cell permeability is enhanced.7 In Fig. 5, the damage of cell membrane by ultrasound, Fe3O4/CNTs and the combination of ultrasound with Fe3O4/CNTs was indicated using K+ release. Compared with the control sample, all the three methods increased the K+ content in cyanobacterial suspension. The highest damage to the cyanobacterial cells was caused by sonication with Fe3O4/CNTs. It is reported that 62.0% K+ released using UV/H2O2 pre-treatment,7 which is in agreement with this work (57.3% K+ released in water).
Effect of sonication with Fe3O4/CNTs on AOM release
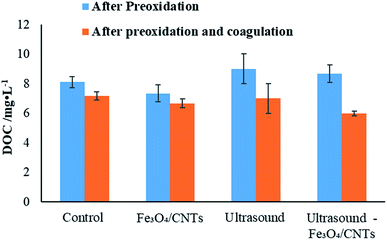
DOC valve is an important parameter for water quality. Fig. 6 shows the DOC values in the water samples before and after coagulation. After pre-treatment, the lowest DOC value was observed with Fe3O4/CNTs alone, suggested that the iron nanoparticles did not release AOM. While ultrasound-Fe3O4/CNTs increased the DOC valve slightly (8.7 mg L−1), showing the AOM release is limited after the ultrasonic Fenton-like treatment. The results indicated this pre-oxidation could produce limited damage on the surface of cyanobacterial cells. After preoxidation and coagulation, ultrasound alone could not effectively control the AOM level in water, meanwhile the DOC values were 6.7 and 6.0 mg L−1 using Fe3O4/CNTs and ultrasound-Fe3O4/CNTs treatment, respectively.Pre-oxidation has been reported to increase the DOC concentration during cyanobacterial removal processes.10,23 It is suggested that moderate pre-oxidation could oxidize and remove the organic compounds attached on the surface of Microcystis aeruginosa cells, leading to a reasonable enhancement of DOC concentration in water.7 While stronger oxidation could break up cyanobacterial cells, which could cause the releasing of intracellular organic matters into the water and lead to a concern on water quality.3 In this work, we did not observe an obvious declining trend of DOC, which may due to a relatively shorter sonication time (20 seconds). Considering the Chinese standard level for DOC in drinking water 1.5 mg L−1 (GB5749-2006),37 further processes such as enhanced coagulation, air floating and filtrating are required.
The concentration of microcystins in water before and after coagulation are shown in Fig. 7. Compared with the results from preoxidation, it could be observed that the coagulation did not promote the reduction of microcystins level in water. After coagulation, the level of microcystins in cyanobacterial suspension increased using sonication, while the addition of Fe3O4/CNTs slightly reduced the MCs level in water. Furthermore, after the co-treatment, microcystins were degraded or settled down after coagulation. Our results suggested that the potential risks from microcystins in water could be prevented using peroxidation–coagulation.
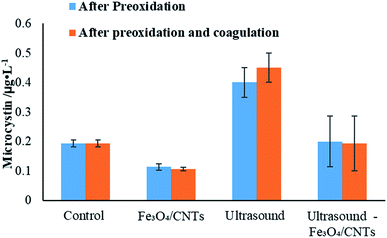 | ||
| Fig. 7 The change of microcystins concentration using different pre-treatments before and after coagulation. | ||
Microcystins are produced by M. aeruginosa cells, and they could be released into water when the cyanobacterial cells were damaged or dead.38 According to flow cytometry results, a few cells were damaged in water suspension after sonication, which may lead to a releasing of microcystins (data shown in ESI†). It was observed that the microcystins concentration with Fe3O4/CNTs and sonication-Fe3O4/CNTs were lower than that of sonication. It could be explained as that the system of ultrasound and Fe3O4/CNTs generated free radicals, which could reduce the concentration of cyanobacterial toxins in water via oxidation. The hypothesis is supported by pervious reports that pre-oxidation could degrade microcystins.7
During the sonication with Fe3O4/CNTs, ultrasound accelerates the Fenton reaction by accelerating the H2O2 production. The Fenton reaction may change the extracellular conditions of cyanobacterial cells,22 leading to an enhanced the coagulation efficiency. Meanwhile, the iron ions could be released from Fe3O4 particles, leading to adsorption of iron ions onto the surface of cyanobacterial cells, which also could promote the removal efficiency via coagulation and sedimentation.29 The sonication with Fe3O4/CNTs also removed the MCs and AOM via degradation during Fenton reaction and/or adsorption using Fe3O4 particles and CNTs due to their porosity structure.16 Our work suggested that moderate preoxidation using sonication with Fe3O4/CNTs could efficiently remove cyanobacterial cells showing a small amount of damaged cells, and the microcystins valve fit to the standard of drinking water without secondary pollution.
Conclusion
The 40 kHz sonication with Fe3O4/CNTs is demonstrated to be an effective strategy for the preoxidation on Microcystis aeruginosa cells, and the cyanobacterial cells were removed via followed coagulation without secondary pollution. The novel moderate preoxidation process showed that a reduced sonication time combined with a relatively dosage of iron achieved to 90% removal rate of M. aeruginosa cells. At the same time, without harsh oxidation, the cyanobacterial cells were not destroyed seriously, which prevented the release of algal organic matter including microcystins. The enhanced settlement of the cyanobacterial cells may due to the moderate oxidation on cell surface. The work demonstrated that the combination of 40 kHz sonication and Fe3O4/CNTs is a promising strategy for drinking water treatment. Further investigation is needed to assess the potential for scale-up application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Provincial Laboratory for Water Environmental Protection Engineering (Grant No. W180815). Natural Science Foundation of Jiangsu Province (BK20180938). Natural Science Foundation of Jiangsu Province (BK20160468), Natural Science Foundation of China (41701093). Jiangsu Modern Agricultural Industry Technology System Project (JATS[2018]313).References
- D. M. Anderson, Ocean Coast. Manage., 2009, 52, 342 CrossRef PubMed.
- I. Chorus, M. Cavalieri, J. Bartram and G. Rees, Geneva Cyanobacteria and Algae, Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management, World Health Organization (WHO), 2000 Search PubMed.
- J. J. Fan, L. Ho, P. Hobson and J. Brookes, Water Res., 2013, 47, 5153 CrossRef CAS PubMed.
- M. Mucci, N. P. Noyma, L. D. Magallháes, M. Miranda, F. V. Oosterhout, I. A. Guedes, V. L. M. Huszar, M. M. Marinho and M. Lrling, Water Res., 2017, 118, 121 CrossRef CAS PubMed.
- J. L. Lin, L. C. Hua, Y. T. Wu and C. Huang, Water Res., 2016, 89, 261 CrossRef CAS PubMed.
- J. H. Zhou, Z. W. Zhao, J. Liu, W. Peng, Y. T. Han and P. Xiao, Korean J. Chem. Eng., 2019, 36, 1587 CrossRef CAS.
- P. L. Jia, Y. P. Zhou, X. F. Zhang, Y. zhang and R. H. Dai, Water Res., 2018, 131, 122 CrossRef CAS PubMed.
- J. L. Sun, L. J. Bu, L. Deng, Z. Shi and S. Q. Zhou, Chem. Eng. J., 2018, 349, 408 CrossRef CAS.
- J. An, N. Li, S. Wang, C. Liao, L. Zhou, T. Li, X. Wang and Y. Feng, J. Hazard. Mater., 2018, 365, 650 CrossRef PubMed.
- P. C. Xie, Y. Q. Chen, J. Ma, X. Zhang, J. Zou and Z. P. Wang, Chemosphere, 2016, 155, 550 CrossRef CAS PubMed.
- M. Ma, M. Wang, X. Cao, Y. Li and J. Gu, J. Hazard. Mater., 2019, 364, 762 CrossRef CAS PubMed.
- J. D. Plummer and J. K. Edzwald, J. Water Supply: Res. Technol.--AQUA, 2002, 51, 307 CrossRef CAS.
- B. Liu, F. S. Qu, W. Chen, H. Liang, T. Y. Wang, X. X. Cheng, H. R. Yu, G. B. Li and B. V. D. Bruggen, Water Res., 2017, 125, 72 CrossRef CAS PubMed.
- J. Qi, H. C. Lan, S. Y. Miao, Q. Xu, R. P. Liu, H. J. Liu and J. H. Qu, Water Res., 2016, 88, 127 CrossRef CAS PubMed.
- Y. T. Li, X. D. Shi, Z. Zhang and Y. Z. Peng, Ultrason. Sonochem., 2019, 55, 232 CrossRef CAS PubMed.
- N. Zhang, G. Xian, X. M. Li, P. Y. Zhang, G. M. Zhang and J. Zhu, Front. Chem., 2018, 6, 12 CrossRef PubMed.
- T. Aya, M. G. Alalm and E. Mohamed, Process Saf. Environ. Prot., 2019, 128, 273 CrossRef.
- X. G. Wu, G. F. Xu and J. J. Zhu, Ultrason. Sonochem., 2019, 58, 104634 CrossRef CAS PubMed.
- J. Qi, H. C. Lan, R. P. Liu, H. J. Liu and J. H. Qu, Water Res., 2019, 171, 115448 CrossRef PubMed.
- X. G. Wu, A. G. Babu, B.-L. Kim, J.-O. Kim, J. H. Shin and D.-P. Kim, Algal Res., 2016, 15, 210 CrossRef.
- N. Gu, Y. X. Wu, J. L. Gao, X. Y. Meng, P. Zhao, H. H. Qin and K. T. Wang, Ecol. Eng., 2017, 99, 290 CrossRef.
- X. G. Wu, J. L. Liu and J. J. Zhu, Ultrason. Sonochem., 2019, 53, 68 CrossRef CAS PubMed.
- F. Sun, H. B. Cong, X. Y. Jiang, W. J. Chen, S. T. Xu and Y. J. Liu, J. Environ. Eng., 2019, 145, 04019085 CrossRef.
- Y. Jin, H. Z. Xu, C. X. Ma, J. M. Sun, H. M. Li, S. S. Zhang and H. Y. Pei, Water Res., 2018, 143, 550 CrossRef CAS PubMed.
- L. Xu, C. Guo, F. Wang, S. Zheng and C. Z. Liu, Bioresour. Technol., 2011, 102, 10047 CrossRef CAS PubMed.
- Z. Yu, H. Y. Pei, Q. J. Hou, C. L. Nie, L. J. Zhang, Z. G. Yang and X. D. Wang, Bioresour. Technol., 2018, 267, 192 CrossRef CAS PubMed.
- C. Jiang, R. Wang and W. Ma, Colloids Surf., A, 2010, 369, 260 CrossRef CAS.
- S. K. Wang, A. R. Stiles, C. Guo and C. Z. Liu, Algal Res., 2015, 9, 178 CrossRef.
- J. J. Fan, Y. B. Hu and X. Y. Li, ACS Sustainable Chem. Eng., 2018, 6, 15135 CrossRef CAS.
- W. Jiang, F. Xiao, D. S. Wang, Z. C. Wang and Y. H. Cai, RSC Adv., 2015, 5, 35461 RSC.
- L. G. Delogu, G. Vidili, E. Venturelli, C. Ménard-Moyon, M. A. Zoroddu, G. Pilo, P. Nicolussi, C. Ligios, D. Bedognetti, F. Sgarrella, R. Manetti and A. Bianco, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 16612 CrossRef CAS PubMed.
- X. L. Yang, C. J. Li, J. F. Huang, Y. Y. Liu, W. Chen, J. H. Shen, Y. H. Zhu and C. Z. Li, RSC Adv., 2017, 7, 15168 RSC.
- H. Yan, A. J. Gong, H. S. He, J. Zhou, Y. X. Wei and L. Lv, Chemosphere, 2006, 62, 142 CrossRef CAS PubMed.
- X. Tan, X. Q. Shu, J. J. Guo, K. Parajuli, X. D. Zhang and Z. P. Duan, Bull. Environ. Contam. Toxicol., 2018, 101, 117 CrossRef CAS PubMed.
- X. G. Wu, E. M. Joyce and T. J. Mason, Water Res., 2012, 46, 2851 CrossRef CAS PubMed.
- H. Liang, J. Nan, W. J. He and G. Li, Desalination, 2009, 239, 191 CrossRef.
- The Ministry of Health of the P. R. China and Standardization Administration of the P. R. China, GB5749-2006. Standards for drinking water quality, the national standard of the P. R. China, China Standard Press, Beijing, 2007, p. 2 Search PubMed.
- X. C. Huo, D. W. Chang, J. H. Tseng, M. D. Burch and T. F. Lin, Environ. Sci. Technol., 2015, 49, 5502 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01530j |
| This journal is © The Royal Society of Chemistry 2020 |