Open Access Article
Open Access ArticleDevelopment of a novel cellulose solvent based on pyrrolidinium hydroxide and reliable solubility analysis†
Elisabeth Rada Desideria Seiler ,
Yuko Takeoka
,
Yuko Takeoka ,
Masahiro Rikukawa
,
Masahiro Rikukawa and
Masahiro Yoshizawa-Fujita
and
Masahiro Yoshizawa-Fujita *
*
Department of Materials and Life Sciences, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554, Japan. E-mail: masahi-f@sophia.ac.jp
First published on 20th March 2020
Abstract
Cellulose processing remains a challenge as it is insoluble in water and common organic solvents. Ionic liquids (ILs) are organic salts with a melting point below 100 °C and are known for their excellent solvent properties. Unlike common organic solvents, which can form toxic or flammable vapours due to their high volatility, ILs can be considered as more environmentally friendly due to their negligible vapour pressure and flame retardant properties. We found that N-butyl-N-methylpyrrolidinium hydroxide enables rapid dissolution of up to 20 wt% Avicel® cellulose at 25 °C in aqueous solution (50 wt% water), making it the first pyrrolidinium-based salt capable of dissolving cellulose. Furthermore, solubility studies are currently carried out mainly with the naked eye, microscopy or spectroscopy. The former is a subjective method because it depends on the observer, and particles at the micro-level cannot be seen with the human eye. Microscopic and spectroscopic analyses are suitable for the verification of solubility; however, the acquisition costs of the instruments are high, and sample preparation is time-consuming. We propose that turbidity is a suitable measure for solubility, and investigated a simple and fast method to evaluate cellulose solubility in aqueous N-butyl-N-methylpyrrolidinium hydroxide by employing a turbidimeter which was compared with microscopy and ocular (eye) observation. In this study, we have not only found a promising new solvent for cellulose processing, but also offer a reliable solubility analysis.
1 Introduction
Utilization of renewable resources is an important step towards a more sustainable society. Cellulose is the most abundant and renewable biopolymer on earth with 1.5 × 1012 tons annually produced.1 It is mainly present in the primary cell wall of plants (biomass) and is broadly applicable due to biocompatibility and biodegradability. However, cellulose processing remains a challenge as it is insoluble in water and common organic solvents, due to strongly pronounced inter- and intramolecular hydrogen bonds. The potential of cellulose can only be fully exploited through simple and environmentally benign processing techniques. The first step is the introduction of a suitable solvent. Industrially, N-methylmorpholine N-oxide (NMMO) is widely used for cellulose processing.2 Up to 20 wt% cellulose can be dissolved in a solution with 10–18 wt% water.2 However, the temperature must be carefully controlled as NMMO exhibits thermal instability. It is also toxic and can react to form nitrogen oxides, which leads to air pollution. Thus, in moving towards a sustainable future, the discovery of improved solvents for biomass processing (here cellulose) is necessary.Ionic liquids (ILs) are organic salts with a melting point below 100 °C and are known for their excellent solvent properties.3 Swatloski et al. found that ILs enable the dissolution of cellulose.4 Compared to the organic solvents commonly used, they offer the advantage that they do not form toxic or highly flammable gases due to their extremely low vapour pressure. Previously reported ILs that can dissolve microcrystalline cellulose are based on imidazolium,4–9 ammonium,9–12 pyridinium,11 morpholium13 and phosphonium9,14 salts with a variety of ionic structures. It is proposed that ILs can break the inter- and intramolecular hydrogen bonds in cellulose by competing for hydrogen bonds.4 Especially, anions with high hydrogen bond accepting ability (basicity) can cleave the inter- and intramolecular hydrogen bonds of cellulose.15 The imidazolium salt with the highest ability to dissolve cellulose is 1-ethyl-3-methylimidazolium acetate which can dissolve up to 15 wt% Avicel® cellulose. However, it requires heating of 110 °C and such imidazolium-based salts cannot dissolve large amounts of cellulose in the presence of water.4,9 Since biomass typically contains water, any possibility to avoid a dehydration step would be advantageous. Thus, solvents with the ability to dissolve cellulose in the presence of water are desirable. Ammonium-12 and phosphonium-based14 ILs can dissolve cellulose in aqueous solution. Phosphonium-based ILs14 exhibit high cellulose solubility in aqueous solution; however, toxicity may cause environmental risks. Oulego et al. found that the toxicity of ILs is mainly controlled by the cationic structure.16 Phosphonium ILs are more toxic than ammonium and imidazolium ILs when testing with Vibrio fischeri and E. coli.16 Therefore, our goal was to find a new solvent which can dissolve high amounts of cellulose under mild conditions (ambient temperature) in aqueous solution with a less toxic cationic structure. Ammonium-based ILs can also dissolve high amounts of cellulose in aqueous solution.12 However, the cation stability under basic conditions can be problematic, especially for structures without ring closure. Tetraalkylammonium cations can undergo Hofmann-elimination when paired with a highly basic anion, e.g. hydroxide.17 Therefore, an IL with a cation structure which is stable under strongly basic conditions was sought. Sun et al. found that the pyrrolidinium cation, N,N-dimethylpyrrolidinium, is stable even paired with hydroxide anion and can be easily obtained from the halide form by anion exchange reaction.18 Taking advantage of the commercial availability of N-butyl-N-methylpyrrolidinium chloride, we synthesized N-butyl-N-methylpyrrolidinium hydroxide ([C4mpyr][OH]) and investigated the solubility of cellulose. The structure is shown in Fig. 1.
Solubility in general can be detected by microscope,13,19–23 UV-vis spectroscopy,2,24,25 and laser photometer.26,27 The corresponding designation of the substance as “soluble” or “insoluble” is made either by microscopic observation of particles20 or by employing turbidity analysis, measured by absorption of emitted light by a solution due to light scattering.2,26,27 Here, we note that in some solubility studies, although the phrase “optically/visually clear”5–8 is used for the dissolved state, the specific method of determination is not clearly described. There is ambiguity as to whether the determination is performed spectroscopically and visually with the eyes or a microscope. Considering specific phrases, one can only assume that this phrase refers to the UV spectrometer mentioned under “instruments” and thus refers to spectroscopic verification,6,7 or to the microscope mentioned in the ESI,†5 both without supporting documents (graphs, photographs). In some other solubility studies, the method of solubility determination is not mentioned.11,14 Therefore, it is unclear how solubility was detected and we assume that solubility was probably determined visually with the eyes by observation of turbidity. We propose that turbidity is a suitable measure for solubility; however, observation by eye is subjective. Microscopic and spectroscopic examinations are suitable for the determination of the dissolution state; however, they require high acquisition costs for high resolution and comparatively time-consuming sample preparation.
The aim of our study was to critically examine the methods of solubility analysis both microscope and ocular observation (i.e. by naked eye), and to compare them with a simple measuring method.
We employed a turbidity meter, also referred to as a turbidimeter or nephelometer, which is a handy, inexpensive tool that does not require sample preparation. In Fig. S1† a photograph of the turbidimeter and sample vial is shown (see ESI†). Similar to UV-vis spectroscopy, it measures the amount of light scattered by particles suspended in a liquid sample. We investigated a simple and rapid method to determine the solubility of cellulose using the turbidimeter. This method was applied to aqueous [C4mpyr][OH] and the solubility of cellulose was investigated by using different water contents at 25 °C. We have chosen room temperature (25 °C) to avoid energy costs by using a heat source, especially in view of future applications on a larger scale.
2 Materials and methods
2.1 Materials
Microcrystalline Avicel® PH-101 cellulose was purchased from Sigma-Aldrich and was dried at 80 °C under vacuum for 12 h before use. N-Butyl-N-methylpyrrolidinium chloride (>99%) was also obtained from Sigma-Aldrich. Silver oxide (99%) and activated charcoal powder from the Wako brand were used.2.2 Synthesis of [C4mpyr][OH] and sample preparation
Following the procedure of Sun et al., N-butyl-N-methylpyrrolidinium chloride (107 mmol, 1 eq.) and silver oxide (64 mmol, 0.6 eq.) were stirred in 200 mL deionized water for 6 h at room temperature (Sun et al., 2001).18 The obtained yellow liquid was purified with activated charcoal powder to remove the colour. After evaporation of water, a colourless viscous liquid was obtained. The water content was measured volumetrically by Karl-Fischer titration. Diluted [C4mpyr][OH] solutions were obtained by adding deionized water, and the resulting water content was also checked by Karl Fischer titration.2.3 Characterization of [C4mpyr][OH]
[C4mpyr][OH] was characterized by 1H NMR spectroscopy on a Bruker AVANCE III HD Nano Boy 400 MHz NMR spectrometer at room temperature. Mass spectra were measured on a Jeol JMS-SX 102A mass spectrometer. Water contents were determined in triplicates though Karl-Fischer titration using a Kyoto Electronics Manufacturing MKH-710M titrator, and the average was used. The titration performance was regularly checked with KEMAQUA Water Standard 10 containing 1% water to adjust the titration factor. Throughout the experiments, a Shimadzu AP 224X weighing scale (d = 0.1 mg) was used.2.4 pH measurement
The pH value of the diluted [C4mpyr][OH] solutions without cellulose were measured with the Horiba LAQUAtwin-pH-22B pH meter, after calibrating with pH 4 and pH 7 standard buffer solutions.2.5 Dissolution of microcrystalline cellulose
Dried microcrystalline cellulose (see above) was added stepwise into 2.0 g aqueous [C4mpyr][OH] solutions with water contents between 45–81 wt%. They were stirred at 25 °C in 9 mL glass vials on an EYELA RCH-1000 heat stirrer plate. After cellulose addition, the mixture was stirred at 300 rpm for 15 min and 2 h at 50 rpm at a constant temperature of 25 °C. The vials were left on the plate for 2 h to reduce air bubbles and detect solubility. The turbidity of each sample was checked with a turbidimeter (see below). The existence of cellulose crystals was checked under a microscope (see below). The absence of cellulose crystals was defined as a dissolved state. When the solution became, optically clear additional cellulose was added until crystals were detected under the microscope. The cellulose content at which the first crystals could be observed microscopically was defined as “insoluble”. The dissolution test was examined three to six times until the solubility limit was found. The maximum solubility values are expressed in weight percent (wt%), which is the mass of the maximum dissolved cellulose divided by the mass of the solvent.2.6 Turbidity measurement
Turbidity of the calibration standards diluted [C4mpyr][OH] solutions and cellulose-containing solutions were measured with a Thermo Scientific turbidimeter Eutech TN-100 (Fig. S1†). The turbidity was measured in Nephelometric Turbidity Units (NTU). The turbidimeter was calibrated and checked daily with Thermo Scientific TN100CALKIT turbidity standards, which contain styrene-divinylbenzene copolymer beads in water and refer to 800 NTU, 100 NTU, 20 NTU, and 0.02 NTU. Each vial was cleaned with a lint-free cloth. A thin film of silicone oil was applied and distributed evenly with a cloth to mask imperfections of the glass. For the measurement of the cellulose-containing solutions, the stirrer bar was carefully removed from the glass vial ground by attaching it to the top of the vial with a small magnet, so as not to alter the incidence of light. The turbidity was measured four times and the average was used.2.7 Microscopic observation
Diluted [C4mpyr][OH] solutions and cellulose-containing solutions were observed for the presence of impurities and cellulose crystals with an Olympus IX70 microscope by using an Olympus CPlanFL 10×/0.30 objective. Microscopic samples were prepared by applying a small drop of the analyte to a slide glass and cover glass previously cleaned with methanol. Photographs were taken with the Olympus SC35 type 12 microscope camera.3 Results and discussion
The pH of the aqueous [C4mpyr][OH] solutions between 45–81 wt% water was measured. It was found that the pH value was constantly above 14. Since a high basicity promotes the cleavage of hydrogen bonds in cellulose, it was suspected that the aqueous solution of [C4mpyr][OH] can dissolve cellulose and the solubility was investigated.Fig. 2 is a graphical representation of the maximum cellulose (Avicel® CH-101) solubility at various water contents at 25 °C. The mole ratio of water to IL can also be described as a hydration number in equivalents (eq.) and is the number of water molecules per ion pair. The “maximum solubility” describes the concentration when the solution is saturated. Complete dissolution was obtained because no crystals were visible under the microscope. When adding more cellulose, crystals were visible under the microscope. The data is included in Table S1† under the term “insolubility” and describes the presence of cellulose in the undissolved state (shown in the Fig. S2†). Strikingly, the dissolution of cellulose is highly dependent on the water content of the IL/water mixture. Between 45 wt% (7 eq.) water and 50 wt% (9 eq.) water, a sudden increase in cellulose solubility was observed. 20 wt% cellulose was dissolved in the mixture containing 50 wt% water, which was also the peak of the cellulose solubility. In total, cellulose exhibited some degree of solubility in solvent mixtures in the range of 50–74 wt% (9–26 eq.) water. Depending on the solution, the dissolution time was between 15 min and 2 h. Any further extension of stirring time had almost no impact on dissolution. The solutions with 45 wt% and 81 wt% water were stirred up to two weeks and were still insoluble. Below approximately 40 wt% water (6 eq.), the aqueous IL solution was highly viscous and solid-like; therefore, dissolution studies could not be performed below 45 wt% water.
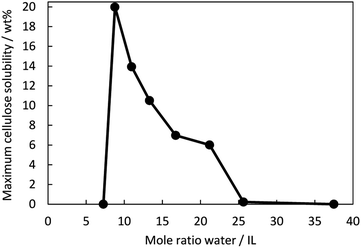 | ||
| Fig. 2 Maximum cellulose solubility in [C4mpyr][OH] at 25 °C as a function of the mole ratio of water to IL. | ||
It is known that the strength of the hydrogen bond interaction is directly related to the water content.28 Since the solubility of cellulose is attributed to the breaking of inter- and intramolecular hydrogen bonds by ILs,4 it can be assumed that there is a direct relationship between water content and solubility. One reason for the insolubility at 45 wt% water may be that the hydroxide anion attacks the pyrrolidinium cation, causing decomposition, as is the case with tetraalkylammonium cations.17 In that case, the number of intact pyrrolidinium and hydroxide ions that can hydrogen bond with cellulose would decrease, and hence the solubility decreases. The water content in aqueous ILs also corresponds to certain hydration states,29 which influences the strength of the interaction between water, ions, and cellulose and leads to different bulk properties (solubility behaviour of cellulose). Two hydration states can be roughly defined: bound water and free water.28 It is known that highly concentrated hydrated ILs exhibit no free water.30 When adding water, the ratio of free water to bound water increases, and the cellulose solubility decreases. The free water could lead to an interruption of ion–cellulose interaction.
When one [C4mpyr][OH] ion pair is surrounded by nine water molecules, corresponding to 50 wt% water, we can imagine that only strongly bound water exists, with no free water. It has been shown that in aqueous ILs, hydrated anions are strongly bound to a limited number of available water molecules, which increases the basicity of the oxygen atom of the water molecules, allowing it to react with other molecules.31 Therefore, the water molecules can not only stabilize the ionic structure but also act as a co-solvent to improve cellulose solubility.
When water content increases, the proportion of free water to bound water increases. For aqueous imidazolium ILs, it is reported that a disruption of the IL structure occurs because free water molecules compete with anions for contact with hydrogen atoms of the imidazolium cation.32 Another hypothesis is that free water molecules interact via hydrogen bonding with the anions,28 so the anion capacity to interact with cellulose is weakened, and the solubility decreases. It is noticeable that even the addition of two water molecules, comparing 50 (9 eq.) to 55% (11 eq.) water, leads to a reduction in cellulose solubility. These two additional water molecules per ion pair appear to interfere with the interaction between ions and cellulose and thus we speculate that these two molecules may correspond to free water. When the aqueous [C4mpyr][OH] reaches water contents of 74–81 wt% the ion pair is surrounded by 26–38 water molecules, which could form water clusters,33 shielding the ions from cellulose so that they can no longer interact with the hydroxyl groups of cellulose. Since cellulose is insoluble in water, higher water contents lead to the insolubility of cellulose. In further studies, we will investigate the interaction of IL, water and cellulose.
The turbidity, which was measured with a turbidimeter in Nephelometric Turbidity Units (NTU), of saturated cellulose solutions (maximum cellulose solubility) and when exceeding the solubility (insolubility), is given in Table S1.† In the literature, it is suggested that the turbidity remains reasonably constant in the soluble state and increases sharply when the solubility is exceeded.34 To assess the solubility of cellulose, turbidity measurements were complemented by microscopic observations to check the presence or absence of cellulose crystals (see below). The average turbidity of aqueous IL stock solutions without cellulose was 3 NTU, probably due to some small amounts of dust particles or invisible-to-the-eye scratches on the glass vial. It was recognized that the detected turbidity increased when adding cellulose. The increase of turbidity between saturated solution and insoluble solution is also mentioned in Table S1.† The smallest increase in turbidity was 17 NTU, and the average increase was 36 NTU ([C4mpyr][OH] with 50–74 wt% water). The turbidity more than doubled when the solubility was exceeded, which represent a sharp increase. The threshold between the dissolved and undissolved states was thus fixed at 33 NTU; above this limit, every solution was detected as undissolved by microscopic observation. As the particles in the sample increase, more light is scattered from the particles, and the intensity increases. It is noticeable that the two samples, 45 and 81 wt% water in which cellulose was completely insoluble, showed the highest increase in turbidity (205 NTU and 432 NTU). To investigate if the threshold of 33 NTU can be applied to other solvents for the dissolution of cellulose, further experiments will be conducted. Turbidity measurement with a turbidimeter is a simple and fast method that requires no additional sample preparation. To ensure sufficient signal intensity, sample sizes of 2–10 mL are suitable for this measurement. The accuracy can be increased by cleaning the outer wall of the glass vial and handling it carefully to avoid scratches.
Fig. 3 shows microscopic photographs of maximum dissolved cellulose and the first appearance of crystals (“undissolved cellulose”). In the ESI,† photographs for all tested solutions are provided (Fig. S2†). In the case of complete dissolution, no crystals were observed microscopically, and the solutions resembled the stock solutions of [C4mpyr][OH] without cellulose. When the solubility threshold was exceeded, cellulose crystals were observed. The microscopic analysis was used as a standard to judge between the “dissolved” and “not dissolved” states depending on the existence of cellulose crystals. Simultaneously with the turbidity measurements and microscopic analyses, we compared the data with the visual impression of turbidity by the naked eye. Fig. 4 compares the turbidity perceived by the eyes and the appearance of cellulose crystals under the microscope, measured in NTU using a turbidimeter. It was found that turbidity of up to 50 NTU is perceived as “dissolved” by the naked eye, even though crystals are visible under the microscope. This underlines the need for a further detection technique in solubility studies and a more accurate description of solubility analyses.
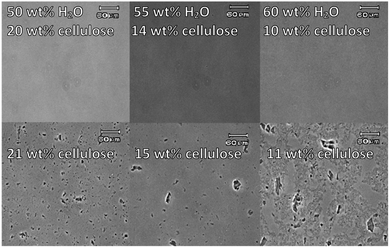 | ||
| Fig. 3 Microscopic photographs of dissolved (top) and undissolved cellulose (bottom) in [C4mpyr][OH] aqueous solution with different water concentrations. | ||
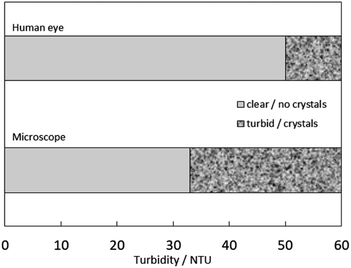 | ||
| Fig. 4 Comparison of two observation methods for estimating turbidity of aqueous [C4mpyr][OH] solutions, the human eye, and the microscope, which was measured with a turbidimeter. | ||
4 Conclusions
In past studies, no cellulose solubility was found in pyrrolidinium-based ILs (N-butyl-N-methylpyrrolidinium acetate) even when heated up to 110 °C.13 Thus, [C4mpyr][OH] is the first pyrrolidinium-based IL that can dissolve cellulose in aqueous solution. Up to 20 wt% cellulose could be dissolved in [C4mpyr][OH] with a water content of 50 wt% at 25 °C, which makes it a promising new solvent for the future cellulose processing. Abe et al. reported that aqueous tetrabutylphosphonium hydroxide solutions can also dissolve cellulose up to 20 wt% at room temperature.14 We assume that especially hydroxide anions promote cellulose solubility in aqueous media due to their high basicity. Furthermore, we have critically compared three methods for determining cellulose solubility. Microscopic observations require high acquisition costs but are nevertheless accurate to investigate solubility due to the visible particles. Turbidity is a suitable measure for solubility studies; however, the human eye is not a suitable instrument for determination. The eye cannot detect small amounts of undissolved particles at the micro-level and the determination of turbidity impression is subjective. We have utilized a simple, fast and inexpensive method to determine the solubility of cellulose using a turbidimeter, verified with a microscope as a standard. Below 26 NTU, complete dissolution of cellulose was observed. Above 33 NTU, cellulose was not completely dissolved and cellulose crystals were observed with the microscope. We conclude that turbidity measurement with a turbidimeter represents an alternative to the analytical techniques currently used and can provide initial indications of solubility. In order to increase the reproducibility for future solubility studies, we recommend specifying the method of determination.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a Sophia University Special Grant for Academic Research.References
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- Y. Kuo and J. Hong, Polym. Adv. Technol., 2005, 16, 425–428 CrossRef CAS.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- J. Vitz, T. Erdmenger, C. Haensch and U. S. Schubert, Green Chem., 2009, 11, 417–424 RSC.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44–46 RSC.
- Y. Fukaya, A. Sugimoto and H. Ohno, Biomacromolecules, 2006, 7, 3295–3297 CrossRef CAS PubMed.
- T. Erdmenger, C. Haensch, R. Hoogenboom and U. S. Schubert, Macromol. Biosci., 2007, 7, 440–445 CrossRef CAS PubMed.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Green Chem., 2008, 10, 696–705 RSC.
- W. Wei, F. Meng, Y. Cui, M. Jiang and Z. Zhou, Cellulose, 2017, 24, 49–59 CrossRef CAS.
- T. Heinze, K. Schwikal and S. Barthel, Macromol. Biosci., 2005, 5, 520–525 CrossRef CAS PubMed.
- K. Ohira, Y. Abe, M. Kawatsura, K. Suzuki and M. Mizuno, ChemSusChem, 2012, 8553, 388–391 CrossRef PubMed.
- B. Lu, A. Xu and J. Wang, Green Chem., 2014, 16, 1326–1335 RSC.
- M. Abe, Y. Fukaya and H. Ohno, Chem. Commun., 2012, 48, 1808–1810 RSC.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- P. Oulego, D. Blanco, D. Ramos, J. L. Vieca, M. Diaz and A. Hernandez Battez, J. Mol. Liq., 2018, 18, 1–35 Search PubMed.
- S. Chempath, J. M. Boncella, L. R. Pratt, N. Henson and B. S. Pivovar, J. Phys. Chem. C, 2010, 114, 11977–11983 CrossRef CAS.
- J. Sun, D. R. MacFarlane and M. Forsyth, J. Mater. Chem., 2001, 11, 2940–2942 RSC.
- J. Cai and L. Zhang, Macromol. Biosci., 2005, 5, 539–548 CrossRef CAS PubMed.
- M. Zavrel, D. Bross, M. Funke, J. Büchs and A. C. Spiess, Bioresour. Technol., 2009, 100, 2580–2587 CrossRef CAS PubMed.
- H. Zhang, J. Wu, J. Zhang and J. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS.
- D. Zhao, H. Li, J. Zhang, L. Fu, M. Liu, J. Fu and P. Ren, Carbohydr. Polym., 2012, 87, 1490–1494 CrossRef CAS.
- B. B. Y. Lau, T. Yeung, R. J. Patterson and L. Aldous, ACS Sustainable Chem. Eng., 2017, 5, 5320–5329 CrossRef CAS.
- N. H. C. S. Silva, A. Filipe, I. F. Almeida, P. C. Costa, C. Rosado, C. Pascoal, A. J. D. Silvestre and C. S. R. Freire, Carbohydr. Polym., 2014, 106, 264–269 CrossRef CAS PubMed.
- B. Li, S. Konecke, L. A. Wegiel, L. S. Taylor and K. J. Edgar, Carbohydr. Polym., 2013, 98, 1108–1116 CrossRef CAS PubMed.
- J. Zhou and L. Zhang, Polym. J., 2000, 32, 866–870 CrossRef CAS.
- M. Terbojevich, A. Cosani, G. Conio, A. Ciferri and E. Bianchi, Macromolecules, 1985, 18, 640–646 CrossRef CAS.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192–5200 RSC.
- H. Ohno, K. Fujita and Y. Kohno, Phys. Chem. Chem. Phys., 2015, 17, 14454–14460 RSC.
- K. Fujita and H. Ohno, Biopolymers, 2010, 93, 1093–1099 CrossRef CAS PubMed.
- R. Quinn, J. B. Appleby and G. P. Pez, J. Am. Chem. Soc., 1995, 117, 329–335 CrossRef CAS.
- E. P. Grishina, L. M. Ramenskaya, M. S. Gruzdev and O. V. Kraeva, J. Mol. Liq., 2013, 177, 267–272 CrossRef CAS.
- T. Mendez-Morales, J. Carrete, O. Cabeza, L. J. Gallego and L. M. Varela, J. Phys. Chem. B, 2011, 115, 6995–7008 CrossRef CAS PubMed.
- M. Mazza, D.-A. Catana, C. Vaca-Garcia and C. Cecutti, Cellulose, 2009, 16, 207–215 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and mass signals of [C4mpyr][OH]. Table of solubility data including turbidity, water and cellulose content. A photograph of the employed turbidity meter and microscopic photographs of dissolved and undissolved cellulose. See DOI: 10.1039/d0ra01486a |
| This journal is © The Royal Society of Chemistry 2020 |