Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Role of alkan-1-ol solvents in the synthesis of yellow luminescent carbon quantum dots (CQDs): van der Waals force-caused aggregation and agglomeration†
Kenta Hagiwaraa,
Hiroshi Uchida a,
Yumiko Suzuki
a,
Yumiko Suzuki a,
Takashi Hayashita
a,
Takashi Hayashita a,
Kanjiro Torigoe
a,
Kanjiro Torigoe b,
Tetsuya Kida
b,
Tetsuya Kida c and
Satoshi Horikoshi
c and
Satoshi Horikoshi *a
*a
aDepartment of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioicho, Chiyodaku, Tokyo 102-8554, Japan. E-mail: horikosi@sophia.ac.jp
bDepartment of Pure and Applied Chemistry, Faculty of Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan
cDivision of Materials Science, Faculty of Advanced Science and Technology, Kumamoto University, Kumamoto 860-8555, Japan
First published on 7th April 2020
Abstract
Carbon quantum dots (CQDs; luminescent carbon nanoparticles, size < 10 nm) have attracted much attention with respect to their eco-friendliness and multi-functionality. The solvent-dependent photoluminescence of CQDs has been well investigated to optimize the synthesis process and homogeneous dispersion. Although some alkan-1-ol solvents, such as ethanol, have been well utilized empirically as good solvents when synthesizing highly photoluminescent CQDs, the role of alkan-1-ol solvents, particularly long-chain alkan-1-ols (e.g., 1-nonanol, 1-decanol), has not yet been clarified. Herein, we demonstrate a method for the synthesis of strongly yellow emitting CQDs using solvothermal treatment and elucidate the role of alkan-1-ol solvents in the photoluminescence of CQDs. These CQDs have been characterized using theoretical calculations, ex situ morphological observations using transmission electron microscopy (TEM) and dynamic light scattering (DLS), and 500 MHz 1H nuclear magnetic resonance (NMR) and 13C NMR spectroscopy. A comparative study of alkan-1-ol solvents suggests a mechanism for the agglomeration and aggregation of carbon precursors, intermediates, and CQDs, which is expected to lead to further synthesis studies on highly luminescent CQDs.
Introduction
Carbon quantum dots (CQDs), which are photoluminescent carbon nanoparticles with sizes below 10 nm have raised much expectation for industrial applications such as bio-imaging,1,2 heavy-metal ion detection,2–4 and carbon Q-LED lighting sources5–10 due to advantageous features such as their eco-friendliness and multi-functionality.11 Since the discovery of CQDs in 2004,12 various bottom-up synthetic protocols have been reported (e.g., carbonization,13,14 ultrasonication,15–17 solvothermal treatment,3,5–7,9,10,18,19 and microwave chemistry8,20,21) from various carbon precursors (e.g., juice,9,22 fruit peel,23 vegetables,9,24 paper,20 organic acids and bases,6–8,15,18,20,25–28 and, not least, aromatic compounds5,10,19,28,29). Technological improvements in the synthesis of multi-color emitting CQDs have been widely reported with respect to the solvent,18,19,29 eluent,28 surfactant,27 dispersing agent,10 and doping,30 and these reports have sustained the recent progress in the synthesis of CQDs. In the solvothermal method, the selection of solvent plays a significant role in the quantum yields (QYs) and the emission color of CQDs due to the reactivity of the solvent with CQDs and the fragility of the solvent under high-temperature/pressure conditions. Zhan et al. revealed the importance of the solvent on the emission color and size control of CQDs produced from a 1,3,6-trinitropyrene carbon precursor.29 Along similar lines, Ding and coworkers reported solvent-dependent multi-color CQDs using a solvothermal treatment.18 The selection of an appropriate solvent in the synthesis of well-functionalized multi-color emitting CQDs is therefore important.Comparing previous reports, alcohol solvents (e.g., ethanol) have been empirically well utilized as a good solvent and surface-functionalizer to produce CQDs with high QYs. Along this line, Yuan et al. reported the importance of ethanol in the synthesis of highly photoluminescent CQDs that exhibit high QYs (66% (blue), 72% (green), 62% (yellow), and 54% (red)).5 Kuwahara and his research group similarly discovered a QY enhancement by esterification with benzyl alcohol (more than 100-fold photoluminescent than non-functionalized CQDs). Therefore, alcohol groups can be one of the key factors to synthesize CQDs with the high QY.31
In contrast, long-chain alcohols (e.g., 1-nonanol and 1-decanol) are not often used in the synthesis of CQDs for unknown reasons. Long-chain solvents (or surfactants such as 1-octadecene and 1-oleic acid) are typically utilized in the synthesis of semiconductor quantum dots (QDs) to prevent nanoparticle agglomeration;32–35 therefore, such long-chain alcohols could play an important role in the synthesis of CQDs. To clarify the role of the alcohol solvent and/or surfactant in the synthesis of CQDs, well-utilized alcoholic solvents (e.g., ethanol and 1-propanol) and long-chain alkan-1-ols (e.g., 1-nonanol and 1-decanol) should be precisely compared for the optimization of CQD syntheses. In this report, alkan-1-ol solvents (CnH2n+1OH: n = 1–10) were selected as model solvents to reveal their effect on CQD synthesis with consideration of the different characteristics of each alkan-1-ol e.g. viscosity, aggregation of CQDs, pKa (reducing force), boiling point (inner pressure) and comparison of the morphological and chemical difference of the synthesized carbon products.
Experimental
Materials
Anthracene, aqueous nitric acid solution (HNO3), sodium hydroxide (NaOH), methanol, ethanol, 1-propanol, 1-butanol, 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, 1-nonanol, and 1-decanol reagents were used as received from Fujifilm-Wako Pure Chem. Ind. Ltd. Dimethyl sulfoxide (DMSO-d6) and D2O solvents for 1H NMR and 13CNMR analyses were purchased from Kanto Chemical Co., Inc. and Fujifilm-Wako Pure Chem. Ind. Ltd., respectively.Synthesis
Results and discussion
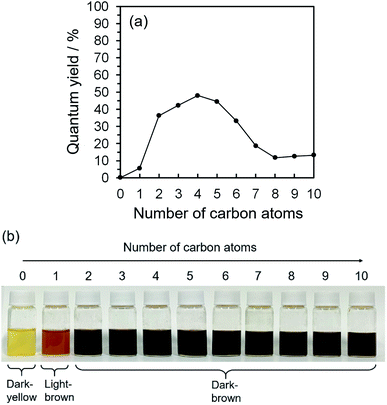
2D excitation–emission spectra, PL spectra, and ultraviolet-visible light (UV-Vis) spectroscopic analysis were all taken into account for the characterization of the PL ability of the CQDs. UV-Vis spectroscopy measurements showed the main absorbance peaks at 251.0, 275.1, 321.5, 327.9, 332.3, 336.4, 367.7, 374.6, and 385.9 nm (Fig. S.2(a-1)–(a-10)†); in accordance with 2D excitation–emission spectra, the strong excitation peak areas were from 250–300 and 360–500 nm (Fig. S.2(b-1)–(b-10)†). What is more, UV-Vis and 2D excitation–emission spectroscopy measurements specified the best excitation wavelength as 275 nm, which brought the maximum PL and QYs of CQDs synthesized by alkan-1-ol solvents (CnH2n+1OH: n = 1–10). 2D excitation–emission spectra also highlight that CQDs synthesized with and dispersed in 1-butanol (n = 4) showed the strongest PL intensity, and the maximum PL peak was at 580 nm in all the alkan-1-ol solvents, which corresponds to the PL spectra (Fig. S.2(c-1)–(c-10)†). Automatically calculated QYs of CQDs showed an interesting correlation between the number of carbon atoms (CnH2n+1OH: n = 1–10) of the alkan-1-ol solvents and the QYs of the CQDs (Fig. 1(a) and Table S.1†). The QYs of CQDs showed a significant increase from methanol (n = 1, QY: 5.6%) to ethanol (n = 2, QY: 36.3%), a stabilization at the maximum of the QYs (1-propanol (n = 3), QY: 44.2%; 1-butanol (n = 4), QY: 48.0%; 1-butanol (n = 4), QY: 44.5%), and an extreme decrease from 1-hexanol (n = 6, QY: 33.15%) to 1-octanol (n = 8, QY: 11.7). It should be noted that water (n = 0) was also utilized as a non-alcoholic solvent, and CQDs in water only showed a QY of 0.16%. It should also be noted that only a slight difference of the QYs (11.7–13.2%) was observed from 1-octanol (n = 8) to 1-decanol (n = 10).Why is this photoluminescent difference apparent? There may be significant and decisive factor(s) that cause this conspicuous disharmony of the QYs. We surmised that this obvious difference could be explained by four main reasons, thermodynamic influence (e.g., supercritical fluidized solvent and liquid–gas equilibrium of solvent), morphology (shape and/or size distribution), physical and/or electrochemical forces (e.g., van der Waals force, steric hindrance, zeta potential), and chemical structural difference (on the surface and/or in the core of CQDs).
To investigate the thermodynamic influences, the reaction temperature of 210 °C was compared with the critical temperatures of each solvent. Consequently, no supercritical fluid was produced with even the lowest boiling point solvent, methanol (n = 1) (Table 1). Next, our focus relied on the liquid–gas equilibrium of each alkan-1-ol solvent. It was surmised that an excessive tendency to vaporization of a solvent would adversely affect the quantitative relation and reactions (e.g., reduction reaction, dehydration condensation) between carbon precursor and each solvent in the liquid phase, which might directly relate with the QYs of the CQDs. The Antoine equation was used for the theoretical calculation of the liquid–gas equilibriums; however, no decisive difference between alkan-1-ol solvents was determined (Table 1). Therefore, the apparent photoluminescent difference is not due to thermodynamic influences.
| Number of carbon atoms (n) | Critical temperaturea/K | A (const.)a | B (const.)a | C (const.)a | Vapor pressureb/atm | Volume of vaporc/mL |
|---|---|---|---|---|---|---|
a Information obtained from the National Institutes of Standards and Technology (NIST) website (NIST Chemistry Webbook): https://webbook.nist.gov/chemistry/.b Vapor pressure calculated with the Antoine equation: 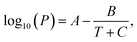 where A, B, and C are solvent-dependent constants. T and P represent the temperature and inner pressure at a specified temperature, respectively.c The volume of vaporized solvent was determined using the ideal gas law. where A, B, and C are solvent-dependent constants. T and P represent the temperature and inner pressure at a specified temperature, respectively.c The volume of vaporized solvent was determined using the ideal gas law. |
||||||
| 0 | 647.0 | 3.55959 | 643.748 | −198.043 | 19.78 | 0.27 |
| 1 | 513.0 | 5.15853 | 1569.613 | −34.846 | 44.84 | 1.37 |
| 2 | 514.0 | 4.92531 | 1432.526 | −61.819 | 33.08 | 1.46 |
| 3 | 536.9 | 4.59871 | 1300.491 | −86.364 | 20.67 | 1.17 |
| 4 | 562.0 | 4.42921 | 1305.001 | −94.676 | 11.59 | 0.80 |
| 5 | 580.0 | 3.97383 | 1106.11 | −134.578 | 6.24 | 0.51 |
| 6 | 610.5 | 4.41271 | 1422.031 | −107.706 | 4.16 | 0.39 |
| 7 | 633.0 | 3.97940 | 1256.783 | −133.487 | 2.40 | 0.26 |
| 8 | 655.0 | 3.96451 | 1350.263 | −129.565 | 1.38 | 0.16 |
| 9 | 672.0 | 3.96157 | 1373.417 | −139.182 | 0.92 | 0.12 |
| 10 | 690.0 | 3.85752 | 1373.019 | −147.727 | 0.57 | 0.08 |
In contrast, ex situ TEM observation revealed an apparent morphological difference of the carbon products. In particular, methanol (n = 1) as a solvent provided large line-shaped (350–400 nm, >10 μm) and sphere-shaped (500–1000 nm) carbon particles (Fig. 2(a)). Note that relatively small sphere particles (CQDs; ca. 10 nm) were also observed when methanol was used as a solvent (Fig. S.3†). However, with the alkan-1-ol (CnH2n+1OH: n = 2–5) solvents, CQDs (spherical, <10 nm) were mainly observed, although agglomerated particles that possibly do not show high emission in the yellow wavelength region (565–590 nm) were also obtained (Fig. 2(b), (c), S.4 and S.5†).
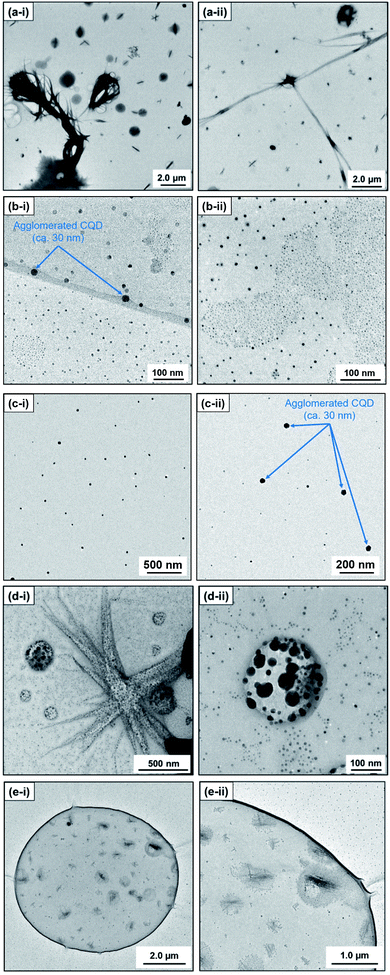 | ||
| Fig. 2 TEM images of carbon products synthesized with alkan-1-ols; (a) methanol (n = 1), (b) 1-propanol (n = 3), (c) 1-butanol (n = 4), (d) 1-octanol (n = 8), and (e) 1-nonanol (n = 9). | ||
Consequently, agglomerated CQDs with a scale of 20–50 nm were mainly observed in the use of 1-hexanol (n = 6, Fig. S.6†) and 1-heptanol (n = 7, Fig. S.7†). A long-chain solvent (e.g., 1-octanol and 1-nonanol) resulted in carbon products with different structures than those obtained in short-chain solvents (CnH2n+1OH: n = 1–7); inclusion complexes (sizes: 200–6000 nm) surrounded by small spherical particles (CQDs; ca. 10 nm) were mainly observed (Fig. 2(d) and (e)). The inclusion complexes indicated that phase separation between water (byproduct) and the long-chain solvent, which produced a water-in-oil (W/O) emulsion-like structure throughout the solvothermal treatment.
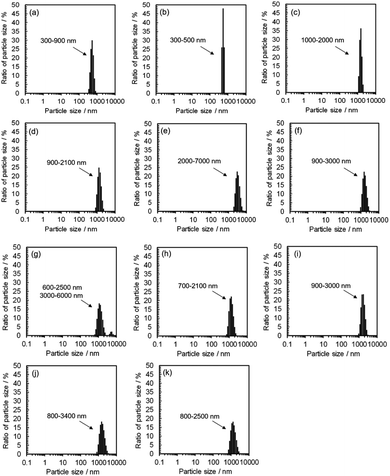
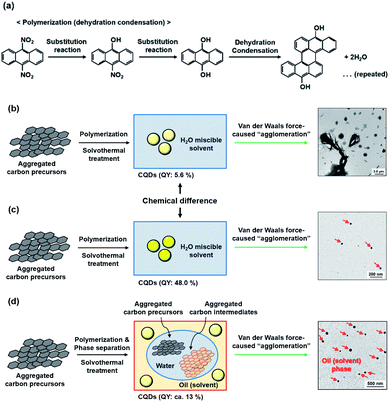
This morphological difference may originate from intermolecular physical forces such as van der Waals force. As the number of carbon atoms in the alkan-1-ol solvents increases, intermolecular forces between alkan-1-ol molecules increase, which causes aggregation of the carbon precursors and/or intermediates (e.g., 1-octanol (n = 8), 1-nonanol (n = 9), and 1-decanol (n = 10)). On the other hand, self π–π stacking between CQDs becomes more robust, which causes agglomeration of CQDs when the number of carbon atoms in the alkan-1-ol solvents is smaller (e.g., methanol (n = 1)). Therefore, van Therefore, van der Waals forces of solvents possibly impede the mono-dispersion of CQDs, and leads to the aggregation and agglomeration of carbon precursors, intermediates, and/or CQDs. (Note: in this report, the word, aggregation, was used to express an assembly of carbon precursors and intermediates, and the word, agglomeration, was used to express a mass of carbon quantum dots, in accordance with the previous report.38) Along this line, Yuan and co-workers recently reported that particle sizes of highly bright CQDs are within 4.0 nm with a narrow size distribution.5 Likewise, Yeh et al. highlighted the existence of a quantum effect in yellow luminescent 5.4 nm CQDs. Therefore, the selection of an appropriate solvent is important to produce a mono-dispersion of CQDs (size: <10 nm) that contributes to a high QY in the yellow emitting region.39 A second possibility is the difference in the reaction scheme wherein carbon products including CQDs are produced. For a step-by-step analysis of the difference in the reaction scheme, we first analysed the aggregation of carbon precursors prior to synthesis by DLS measurements. The DLS measurement indicates that carbon precursors are aggregated in the solvent due to robust π–π stacking between molecules (Fig. S.8†). However, from the perspective of size distribution, the carbon precursors were sized in the ranges of 250–400 nm in water (relatively small aggregation), 300–500 nm in methanol (n = 1) (relatively small aggregation), and 500–2600 nm in alkan-1-ol solvents (CnH2n+1OH: n = 2–10) (relatively large aggregation). This result is consistent with visual observations; undissolved carbon precursors were visually observed in water (Fig. 3(a)). Therefore, strong π–π stacking between carbon precursors may be stabilized in relatively long-chain solvents. A base was also added into the solution of aggregated carbon precursor prior to the synthesis of the CQDs. Throughout the visual observation, an obvious colour difference was observed; the base-added carbon precursor mixed solution turned dark-yellow in water (all the carbon precursor was also dissolved in water), light-brown in methanol (n = 1), and dark-brown in alkane-1-ol solvents (CnH2n+1OH: n = 2–10) (Fig. 1(b)).
 | ||
| Fig. 3 Photographs of mixtures of 9,10-dinitroanthracene and each solvent (water or alkan-1-ol solvents (CnH2n+1OH: n = 1–10)). | ||
This colour difference suggests a chemical difference in the base-added carbon precursors, which varied their absorption peaks as a result. According to the UV-Vis spectra (Fig. S.9†), a specific broad peak at 300–360 nm was obtained only in the methanol (n = 1)-dispersed mixed solution, which indicates an apparent chemical difference of NaOH-added carbon precursors in methanol (n = 1). The chemical difference of the carbon precursors prior to synthesis adversely affected the QYs of the CQDs synthesized with methanol. In this regard, 1H and 13C NMR analyses clarified that CQDs synthesized with methanol have weak sp2 carbon bonds in the core and weak C–O bonds on the surface of the CQDs, compared to that obtained with 1-butanol (Fig. S.10–S.13†), which indicates low PL of the CQDs.31
DLS observation showed that the size range of carbon precursors was changed from 250–400 nm to 300–900 nm in water, stabilized in 300–500 nm in methanol (n = 1) (relatively small aggregation), and also stabilized in 600–3000 nm in alkan-1-ol solvents (CnH2n+1OH: n = 2–10) (relatively large aggregation) (Fig. 4). This apparent difference in size distribution was due to both the chemical difference and degree of dispersion of the carbon precursors in each solvent. This result also possibly indicates the formation of a W/O (water: byproduct, oil: e.g., 1-octanol and 1-nonanol) emulsion during the synthesis; the size of carbon precursors (200–700 nm) in water measured by DLS corresponded to that determined by TEM observation of the carbon products synthesized with 1-nonanol (n = 9; 300–900 nm) (Fig. 2(e)), while the size was inconsistent with 1-octanol (n = 8; 20–50 nm) (Fig. 2(d)), which suggests the presence of aggregated carbon intermediates/agglomerated CQDs in water (byproduct).
The different perspective of physical forces, the probability density functions, and highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) band gaps of solvents could be the third possibility for the obvious photoluminescent difference, and these were also theoretically simulated with a semi-empirical molecular orbital method using Winmostar™,40 which indicated no evident difference in the solvents (Fig. S.14–S.16†). This indicates that a solvent-driven force to a reaction, such as the reduction force and steric hindrance, may not be the main influences on the chemical reaction schemes wherein CQDs or other carbon products are produced.
Consequently, the apparent photoluminescent difference was considered to be caused by the differences in either the chemical structure (methanol, n = 1) or van der Waals force-induced agglomeration of the CQDs in alkan-1-ol solvents (CnH2n+1OH; n = 1–10), or aggregations of the carbon precursors/intermediates (n = 8–10). Furthermore, phase separation, which may result in a W/O emulsion, was clearly observed with the use of such long-chain solvents as 1-octanol and 1-nonanol. A plausible reaction model is inferred herein from comparisons made in this study. In the first instance, the aggregated carbon precursors (9,10-dinitroanthracene) were denitrated via a substitution reaction in the alkaline media (OH−) to yield 9,10-dihydroxyanthracene (Fig. 5(a)). Subsequent polymerization throughout the repeated dehydration and condensation under the solvothermal treatment5 led to the formation of CQDs in water-miscible alkan-1-ol solvents such as methanol and 1-butanol, which cause an agglomeration of the CQDs by the van der Waals forces of the solvents (Fig. 5(b) and (c)), whereas the formation of a W/O emulsion-like phase separation between carbon precursors or intermediates in water (byproduct) and CQDs in oil (solvent) would prevent the formation of highly photoluminescent CQDs (Fig. 5(d)). It is therefore important to select an appropriate solvent for optimization of the QY of photoluminescent CQDs.
Conclusions
Throughout the solvothermal syntheses of CQDs using alkan-1-ol (CnH2n+1OH: n = 1–10) solvents, it was revealed that the van der Waals forces of solvents has an adverse effect on the mono-dispersity of the CQDs due to aggregation (between carbon precursors and/or carbon intermediates) and agglomeration (CQDs). In a model CQD synthesis demonstrated herein, 1-butanol solvent (n = 4) yielded an optimum QY for the resultant CQDs, out of all the alkan-1-ol solvents examined. Precise particle size control of the CQDs plays an important role in the production of highly luminescent CQDs; therefore, the role of the alkan-1-ol solvents examined herein is important for the optimization of CQD synthesis. Solvent-dependent morphological influences on the synthesis of CQDs revealed here can be expected to undergo further investigation concerning solvent effects such as to the size control and the luminescence of CQDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dr Satoshi Horikoshi is grateful to the Japan Society for the Promotion of Science (JSPS) for financial support through a Grant-in-aid for Scientific Research (No. 19K22316).Notes and references
- Y. F. Wu, H. C. Wu, C. H. Kuan, C. J. Lin, L. W. Wang, C. W. Chang and T. W. Wang, Sci. Rep., 2016, 6, 21170 CrossRef CAS PubMed.
- M. J. Molaei, RSC Adv., 2019, 9, 6460–6481 RSC.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- M. Li, T. Chen, J. J. Gooding and J. Liu, ACS Sens., 2019, 4, 1732–1748 CrossRef CAS PubMed.
- F. Yuan, T. Yuan, L. Sui, Z. Wang, Z. Xi, Y. Li, X. Li, L. Fan, Z. Tan, A. Chen, M. Jin and S. Yang, Nat. Commun., 2018, 9, 2249 CrossRef PubMed.
- C. Sun, Y. Zhang, K. Sun, C. Reckmeier, T. Zhang, X. Y. Zhang, J. Zhao, C. Wu, W. W. Yu and A. L. Rogach, Nanoscale, 2015, 7, 12045–12050 RSC.
- X. Miao, D. Qu, D. Yang, B. Nie, Y. Zhao, H. Fan and Z. Sun, Adv. Mater., 2018, 30, 1704740 CrossRef PubMed.
- B. Chen and J. Feng, J. Phys. Chem. C, 2015, 119, 7865–7872 CrossRef CAS.
- P. K. Sarswat and M. L. Free, Phys. Chem. Chem. Phys., 2015, 17, 27642–27652 RSC.
- M. Wu, J. Zhan, B. Geng, P. He, K. Wu, L. Wang, G. Xu, Z. Li, L. Yin and D. Pan, Nanoscale, 2017, 9, 13195–13202 RSC.
- P. Zuo, X. Lu, Z. Sun, Y. Guo and H. He, Microchim. Acta, 2016, 183, 519–542 CrossRef CAS.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- A. Chae, Y. Choi, S. Jo, Nur'aeni, P. Paoprasert, S. Y. Park and I. In, RSC Adv., 2017, 7, 12663–12669 RSC.
- S. Li, S. Zhou, Y. Li, X. Li, J. Zhu, L. Fan and S. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 22332–22341 CrossRef CAS PubMed.
- Y. Zhang, M. Park, H. Y. Kim, B. Ding and S. J. Park, Sci. Rep., 2017, 7, 45086 CrossRef CAS PubMed.
- S. Zhuo, M. Shao and S. T. Lee, ACS Nano, 2012, 6, 1059–1064 CrossRef CAS PubMed.
- S. Y. Park, H. U. Lee, E. S. Park, S. C. Lee, J. W. Lee, S. W. Jeong, C. H. Kim, Y. C. Lee, Y. S. Huh and J. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 3365–3370 CrossRef CAS PubMed.
- H. Ding, J. S. Wei, P. Zhang, Z. Y. Zhou, Q. Y. Gao and H. M. Xiong, Small, 2018, 14, 1800612 CrossRef PubMed.
- D. Zhao, X. Liu, Z. Zhang, R. Zhang, L. Liao, X. Xiao and H. Cheng, Nanomaterials, 2019, 9, 1556 CrossRef CAS PubMed.
- D. Rodríguez-Padrón, M. Algarra, L. A. C. Tarelho, J. Frade, A. Franco, G. De Miguel, J. Jiménez, E. Rodríguez-Castellón and R. Luque, ACS Sustainable Chem. Eng., 2018, 6, 7200–7205 CrossRef.
- F. Arcudi, L. Dordevic and M. Prato, Angew. Chem., Int. Ed., 2016, 55, 2107–2112 CrossRef CAS PubMed.
- E. M. Schneider, A. Bärtsch, W. J. Stark and R. N. Grass, J. Chem. Educ., 2019, 96, 540–545 CrossRef CAS.
- J. Zhou, Z. Sheng, H. Han, M. Zou and C. Li, Mater. Lett., 2012, 66, 222–224 CrossRef CAS.
- B. Yin, J. Deng, X. Peng, Q. Long, J. Zhao, Q. Lu, Q. Chen, H. Li, H. Tang, Y. Zhang and S. Yao, Analyst, 2013, 138, 6551–6557 RSC.
- Y. Choi, S. Jo, A. Chae, Y. K. Kim, J. E. Park, D. Lim, S. Y. Park and I. In, ACS Appl. Mater. Interfaces, 2017, 9, 27883–27893 CrossRef CAS PubMed.
- W. Kasprzyk, T. Świergosz, S. Bednarz, K. Walas, N. V. Bashmakova and D. Bogdał, Nanoscale, 2018, 10, 13889–13894 RSC.
- W. Kwon, S. Do, J. H. Kim, M. S. Jeong and S. W. Rhee, Sci. Rep., 2015, 5, 12604 CrossRef CAS PubMed.
- K. Holá, M. Sudolská, S. Kalytchuk, D. Nachtigallová, A. L. Rogach, M. Otyepka and R. Zbořil, ACS Nano, 2017, 11, 12402–12410 CrossRef PubMed.
- J. Zhan, B. Geng, K. Wu, G. Xu, L. Wang, R. Guo, B. Lei, F. Zheng, D. Pan and M. Wu, Carbon, 2018, 130, 153–163 CrossRef CAS.
- S. Yang, J. Sun, X. Li, W. Zhou, Z. Wang, P. He, G. Ding, X. Xie, Z. Kang and M. Jiang, J. Mater. Chem. A, 2014, 2, 8660–8667 RSC.
- S. Tachi, H. Morita, M. Takahashi, Y. Okabayashi, T. Hosokai, T. Sugai and S. Kuwahara, Sci. Rep., 2019, 9, 14115 CrossRef PubMed.
- M. A. Hines and G. D. Scholes, Adv. Mater., 2003, 15, 1844–1849 CrossRef CAS.
- I. Moreels, K. Lambert, D. Smeets, D. De Muynck, T. Nollet, J. C. Martins, F. Vanhaecke, A. Vantomme, C. Delerue, G. Allan and Z. Hens, ACS Nano, 2009, 3, 3023–3030 CrossRef CAS PubMed.
- D. E. Nam, W. S. Song and H. Yang, J. Colloid Interface Sci., 2011, 361, 491–496 CrossRef CAS PubMed.
- S. B. Brichkin, Colloid J., 2015, 77, 393–403 CrossRef CAS.
- M. Fu, F. Ehrat, Y. Wang, K. Z. Milowska, C. Reckmeier, A. L. Rogach, J. K. Stolarczyk, A. S. Urban and J. Feldmann, Nano Lett., 2015, 15, 6030–6035 CrossRef CAS PubMed.
- W. H. Wright, Chem. Rev., 1967, 67, 581–597 CrossRef CAS PubMed.
- G. Nichols, S. Byard, M. J. Bloxham, J. Botterill, N. J. Dawson, A. Dennis, V. Diart, N. C. North and J. D. Sherwood, J. Pharm. Sci., 2002, 91, 2103–2109 CrossRef CAS PubMed.
- T. F. Yeh, W. L. Huang, C. J. Chung, I. T. Chiang, L. C. Chen, H. Y. Chang, W. C. Su, C. Cheng, S. J. Chen and H. Teng, J. Phys. Chem. Lett., 2016, 7, 2087–2092 CrossRef CAS PubMed.
- Winmostar V9, X-Ability Co. Ltd., Tokyo, Japan, 2019 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01349h |
| This journal is © The Royal Society of Chemistry 2020 |