Open Access Article
Open Access ArticlePalladium supported on triazolyl-functionalized hypercrosslinked polymers as a recyclable catalyst for Suzuki–Miyaura coupling reactions†
Cijie Liu‡
,
Lijuan Zheng‡,
Dexuan Xiang *,
Shasha Liu,
Wei Xu,
Qionglin Luo,
You Shu,
Yuejun Ouyang and
Hongwei Lin*
*,
Shasha Liu,
Wei Xu,
Qionglin Luo,
You Shu,
Yuejun Ouyang and
Hongwei Lin*
Hunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol (PVA) Fiber Material, Key Laboratory of Research and Utilization of Ethnomedicinal Plant Resources of Hunan Province, Huaihua University, Huaihua 418000, China. E-mail: dexuanxiang@126.com; linhongwei1968@163.com
First published on 1st May 2020
Abstract
A novel hypercrosslinked polymers-palladium (HCPs-Pd) catalyst was successfully prepared via the external cross-linking reactions of substituted 1,2,3-triazoles with benzene and formaldehyde dimethyl acetal. The preparation of HCPs-Pd has the advantages of low cost, mild conditions, simple procedure, easy separation and high yield. The catalyst structure and composition were characterized by N2 sorption, TGA, FT-IR, SEM, EDX, TEM, XPS and ICP-AES. The HCPs were found to possess high specific surface area, large micropore volume, chemical and thermal stability, low skeletal bone density and good dispersion for palladium chloride. The catalytic performance of HCPs-Pd was evaluated in Suzuki–Miyaura coupling reactions. The results show that HCPs-Pd is a highly active catalyst for the Suzuki–Miyaura coupling reaction in H2O/EtOH solvent with TON numbers up to 1.66 × 104. The yield of biaryls reached 99%. In this reaction, the catalyst was easily recovered and reused six times without a significant decrease in activity.
Heterogeneous catalysts are an attractive and versatile tool in industrial and academic research laboratories because of their unique properties,1 which include high reactivity, stability, easy separation, purification and good recyclability.2 Currently, heterogeneous catalysis represents an important field of research in green chemistry3 that receives frequent attention and witnessed constant development in recent years. However, heterogeneous catalysts normally have inferior catalytic efficiency compared to homogeneous systems because of their long diffusion pathways to catalytic sites and the difference in electron density on active sites.4 In addition, most recovered heterogeneous catalysts suffer from the sintering and leaching of metals, the loss of surface area and degradation after several reactions under typical reaction conditions.5 These drawbacks could be overcome by using appropriate catalyst supports that provide a large reaction surface with a proper porous structure.6
Hypercrosslinked polymers (HCPs), a type of microporous organic polymer (MOP), are good support materials for noble metals to form heterogeneous catalysts.6a,7 HCPs have a large number of permanent pores formed by extensive chemical crosslinking. Compared to other MOPs prepared by noble metal-based catalysts such as Pd, Pt and Ru, the preparation of HCPs is mainly based on acid-catalysed Friedel–Crafts alkylation reactions,8 which provide quick cross-linking to form strong linkages. These linkages lead to highly crosslinked networks with predominant porosity,9 resulting in extremely rigid networks that are difficult to collapse.10 Regarding the synthetic methodology, HCPs are mainly prepared by three methods.6a The first method involves post-crosslinked polymer precursors.11 The Davankov resins, which are the first examples of HCPs, were prepared by Davankov in the 1970s via post-crosslinking with external crosslinking agents.12 The second method is the one-step polycondensation of functional monomers.13 Recently, the design and synthesis of hypercrosslinked polystyrene and their applications have undergone rapid development. For example, Yang designed microporous polytriphenylamine networks via the oxidative polymerization of triphenylamine and DCX crosslinkers.14 The third method is external cross-linking reaction.15 Tan developed the knitting strategy using formaldehyde dimethyl acetal as an external crosslinker to combine with rigid aromatic building blocks through the Friedel–Crafts reaction.16 Recently, our group synthesized three HCPs based on pyridine-functionalized N-heterocyclic carbene via an external cross-linking reaction and applied them in Suzuki–Miyaura coupling reactions.17 We then prepared HCPs using the readily available raw material, phenanthroline, and used it to catalyse the Heck reaction with good effect.18 HCPs have been widely used in catalysis, gas storage and separation, and drug release because of the following merits:19 (i) large surface areas and pores; (ii) superior chemical and thermal stability; and (iii) low cost and simple and versatile synthetic approach.
1,2,3-Triazoles are one of the most important classes of N-heterocyclic compounds; they have been widely applied in pharmaceutical drugs,20 bioconjugation,21 catalysts,22 materials23 and synthetic organic chemistry.23 Due to the large dipole moment in the 1,2,3-triazole unit, many reports have found that 1,2,3-triazoles can coordinate with metals.24 This property gives 1,2,3-triazoles an important role in the field of catalysis, especially heterogeneous catalysis.25
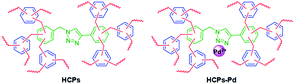
In this paper, we report the preparation of hypercrosslinked polymers-palladium (HCPs-Pd) catalysts based on substituted 1,2,3-triazoles via external cross-linking reaction (Fig. 1) along with the utilization of the HCPs-Pd in Suzuki–Miyaura coupling reactions as a recyclable catalyst with high TON number. HCPs-Pd is simply prepared with low cost, mild conditions and easy separation. The catalyst possesses recyclability, high activity and excellent yield, making it useful for Suzuki–Miyaura coupling reactions.
The surface area and pore structure of the HCPs and HCPs-Pd were investigated by nitrogen adsorption analyses at 77.3 K. The nitrogen adsorption and desorption isotherms of the HCPs exhibit type I adsorption–desorption isotherms (Fig. 2), similar to the isotherms of HCPs-Pd. The isotherms show steep nitrogen gas adsorption at low relative pressure (P/P0 < 0.05), which reflects abundant micropores in the polymers. Meanwhile, a slight hysteresis loop indicates the presence of some mesopores, and a sharp rise at high pressure (P/P0 = 0.9–1.0) implies a spot of macropores in these materials. The apparent Brunauer–Emmett–Teller surface area (SBET) of the HCPs (794 m2 g−1) is larger than that of HCPs-Pd (731 m2 g−1), which can be attributed to the partial filling of the pores in HCPs-Pd with metal (Table 1). These materials were further analyzed by pore-size distribution analysis (Fig. 2). The similar pore sizes of the HCPs and HCPs-Pd implies that the immobilization of palladium on the ESI† did not change the pore-size distribution. The presence of some mesopores and macropores in HCPs-Pd is also essential because they could enable the heterogeneous catalysts upon soaking in a certain solvent. As a result, the catalytically active sites would better contact the substrates. The content of Pd in HCPs-Pd was detected by ICP analysis (Table 1), indicating a Pd content of 1.58%.
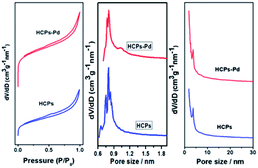 | ||
| Fig. 2 N2 adsorption–desorption isotherms and the corresponding pore size distributions of HCPs and HCPs-Pd. | ||
| Sample | SBETa [m2 g−1] | SMicrob [m2 g−1] | VMicroc [m3 g−1] | [Pd]d [wt%] |
|---|---|---|---|---|
| a Surface area calculated from the nitrogen adsorption isotherm using the BET method.b Micropore volume derived using a t-plot method based on the Halsey thickness equation.c Total pore volume at P/P0 = 0.99.d Data were obtained by ICP-AES. | ||||
| HCPs | 794 | 0 | 0.280 | — |
| HCPs-Pd | 731 | 547 | 0.292 | 1.58 |
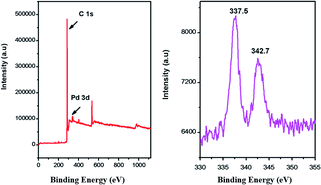
HCPs-Pd was then subjected to characterization by X-ray photoelectron spectroscopy (XPS) to investigate the coordination states of palladium species with the ligand triazoles. As shown in Fig. 3, the Pd 3d XPS spectrum of HCPs-Pd indicates that only one state of Pd (the +2 oxidation state rather than the metallic state) is present in the catalyst. This state corresponds to the binding energies of 337.5 and 342.7 eV, which are assigned to Pd2+ 3d5/2 and 3d3/2, respectively. Compared to the homogeneous counterpart PdCl2 (337.9 and 343.1 eV), the Pd2+ binding energy is negatively shifted by 0.4 eV for 3d5/2 and 3d3/2, which can be attributed to the coordination with triazoles immobilized on the hypercrosslinked polymers. The results show that Pd2+ was successfully immobilized successfully on the HCPs by coordination rather than by the physical adsorption of Pd2+ on the HCP surface.
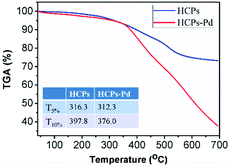
The thermal stability was assessed by TGA in the temperature range of 40–700 °C with a heating rate of 20 °C min−1 in nitrogen. The TGA traces of the HCPs and HCPs-Pd are shown in Fig. 4 along with the corresponding data analysis. Both materials exhibited good thermal stability up to at least 300 °C. The T5% and T10% values of HCPs-Pd were clearly lower than those of the HCPs.
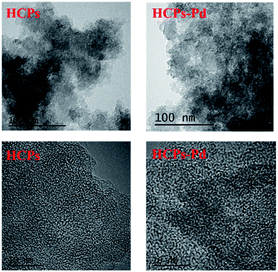
To investigate the surface morphologies of the HCPs and HCPs-Pd, these materials were subjected to scanning electron microscopy (SEM). As clearly shown in Fig. 5, numerous pores were distributed on the surfaces of the HCPs, and the surface morphology did not change remarkably after loading with palladium. The compositions of the HCPs and HCPs-Pd were then investigated by energy-dispersive X-ray spectroscopy (EDX; see ESI, Section III†). The results indicate that C, N, Pd and Cl were the major elements in HCPs-Pd. Meanwhile, Pd was distributed in the networks with a high degree of dispersion. Transmission electron microscopy (TEM) was also employed to study the hypercrosslinked polymers, and Pd nanoparticles were found to be uniformly distributed in HCPs-Pd with good dispersion (Fig. 6).
The catalytic activity of the HCPs-Pd catalyst was evaluated in the Suzuki–Miyaura coupling reaction with iodobenzene 1a and phenylboric acid 2a as a model substrate. Initially, we screened solvents including EtOH, DMSO, DMF, toluene, THF, CH3CN and H2O (Table 2, entries 1–7). When alcohol and DMF were used, the reaction proceeded with moderate yield, while using H2O as solvent only provided a yield of 18%. In our previous work, we found that the mixed solvent of water and ethanol is more favorable for this reaction; other studies have also indicated that the addition of some water to the solvent can speed up the conversion and enhance the yield.26 Next, a series of reactions with the feed volume ratio of EtOH to H2O (VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O) ranging from 4/1 to 1/4 was carried out (entries 8–11). The best yield was obtained at VEtOH
VH2O) ranging from 4/1 to 1/4 was carried out (entries 8–11). The best yield was obtained at VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 4/1. After screening the reaction solvent, a series of experiments was carried out to investigate the reaction conditions, including the base, reaction time, temperature, and the amount of catalyst; some of the results are summarized in Table 2, entries 12–17. Eventually, the optimal results were obtained when the reaction of 1a with 2a was carried out in the presence of NaOH with HCPs-Pd in EtOH/H2O (4/1, v/v) at 60 °C for 1.0 h to afford 3a in 99% yield (entry 8).
VH2O = 4/1. After screening the reaction solvent, a series of experiments was carried out to investigate the reaction conditions, including the base, reaction time, temperature, and the amount of catalyst; some of the results are summarized in Table 2, entries 12–17. Eventually, the optimal results were obtained when the reaction of 1a with 2a was carried out in the presence of NaOH with HCPs-Pd in EtOH/H2O (4/1, v/v) at 60 °C for 1.0 h to afford 3a in 99% yield (entry 8).
| Entry | HCP-Pd | Solvent | Base | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (2.5 mmol), 2a (3.5 mmol), HCPs-Pd (1.0 mg, 1.5 × 10−4 mmol), 60 °C, 1.0 h.b Isolated yields.c No reaction. | ||||
| 1 | 1.0 | EtOH | NaOH | 84 |
| 2 | 1.0 | DMSO | NaOH | 55 |
| 3 | 1.0 | DMF | NaOH | 79 |
| 4 | 1.0 | Toluene | NaOH | 39 |
| 5 | 1.0 | THF | NaOH | NRc |
| 6 | 1.0 | CH3CN | NaOH | Trace |
| 7 | 1.0 | H2O | NaOH | 18 |
| 8 | 1.0 | EtOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NaOH | 99 |
| 9 | 1.0 | EtOH/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NaOH | 92 |
| 10 | 1.0 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NaOH | 89 |
| 11 | 1.0 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
NaOH | 51 |
| 12 | 1.0 | EtOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
K2CO3 | 96 |
| 13 | 1.0 | EtOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
K3PO4 | 98 |
| 14 | 1.0 | EtOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Cs2CO3 | 95 |
| 15 | 1.0 | EtOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Na2CO3 | 69 |
| 16 | 1.0 | EtOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Et3N | Trace |
| 17 | 0.5 | EtOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NaOH | 76 |
Two parallel reactions were conducted under the same conditions as in Table 2 (entry 8) to determine whether the real catalyst was the heterogeneous catalyst or the homogeneous Pd species dissolved in solvent. The two reactions were quenched after the reaction took place for 20 min, and the heterogeneous catalyst was separated from the reaction mixture via hot filtration. One of the reactions was separated by column chromatography, affording biphenyl in 75% yield, while the other reaction was allowed to react for another 40 min at the same temperature, affording biphenyl in 77% yield. The similar yields of biphenyl for the two reactions suggest that the real catalyst was the heterogeneous catalyst rather than the homogeneous Pd species leached from HCPs-Pd.
The results obtained in this work were compared with previous catalysts synthesized via external cross-linking reactions (Table 3). Various parameters such as base, solvent, temperature, yield and TON number were compared. As shown in Table 3, the yield of the product varied from 95% to 99%, indicating that microporous organic polymers synthesized by external cross-linking reactions are good platforms to support palladium species as catalysts for Suzuki–Miyaura reaction. This may be due to the large number of micropores in the microporous polymer, which can help improve the dispersion of palladium nanoparticles. However, the reported catalyst TON values range from 0.123 to 2.04 × 103 (Table 3, entries 1–6); the TON value in this work reached 16.6 × 103, indicating promising application potential.
| Entry | Catalyst | React condition | Yield | TONa (×103) |
|---|---|---|---|---|
| a TON number was calculated from the reported data. | ||||
| 1 | KAPs(Ph-PPh3)-Pd | K3PO4·3H2O, H2O/EtOH, 80 °C | 98% | 0.141 (ref. 7a) |
| 2 | Poly-NHC-2–Pd | K3PO4·3H2O, H2O/EtOH, 80 °C | 99% | 1.737 (ref. 8d) |
| 3 | Pd/SMP-PhPh3 | K3PO4·3H2O, H2O/EtOH, 80 °C | 99% | 2.04 (ref. 27) |
| 4 | HCP-Pd–I | K3PO4, H2O, 80 °C | 95% | 0.123 (ref. 17) |
| 5 | MOPs–Pd–I | K3PO4, H2O/EtOH, 80 °C | 97% | 0.206 (ref. 18) |
| 6 | HCPs-bipy-Pd–I | K2CO3, EtOH, 80 °C | 97% | 0.425 (ref. 28) |
| 7 | HCPs-Pd | NaOH, EtOH, 60 °C | 99% | 16.6 [this work] |
Having established the optimal conditions for the reaction, we investigated the reaction scope. Thus, a series of aryl boronic acids 2 were subjected to substrate 1; some of the results are summarized in Table 4. All the reactions proceeded smoothly to give the corresponding biphenyls 3b–l with excellent yields and TON values. Chlorobenzene was also applied in this reaction, but the result was not satisfactory. The TON values and isolated yields of the products demonstrate the practicality and efficiency of HCPs-Pd in the Suzuki–Miyaura reaction.
| Entry | R1 | X | R2 | 3 | Yieldb (%) | TON (×104) | |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (2.5 mmol), 2a (3.3 mmol), NaOH (3.3 mmol), HCPs-Pd (1.0 mg), EtOH/H2O (10 mL), 60 °C, 1.0 h.b Isolated yields.c The reaction time was 3.0 h. | |||||||
| 1 | H | I | H | 3a | 99 | 1.66 | |
| 2 | H | I | 2-Me | 3b | 96 | 1.61 | |
| 3 | H | I | 3-Me | 3c | 96 | 1.61 | |
| 4 | H | I | 4-Me | 3d | 99 | 1.66 | |
| 5 | H | I | 2-F | 3e | 94 | 1.58 | |
| 6 | H | I | 3-F | 3f | 95 | 1.60 | |
| 7 | H | I | 4-F | 3g | 92 | 1.55 | |
| 8 | H | I | 4-CN | 3h | 94 | 1.58 | |
| 9 | 4-Me | I | H | 3d | 99 | 1.66 | |
| 10 | 4-OMe | I | H | 3i | 97 | 1.63 | |
| 11c | 3,5-(Me)2 | I | H | 3j | 97 | 1.63 | |
| 12 | 4-CN | I | H | 3h | 93 | 1.56 | |
| 13 | H | Br | H | 3a | 93 | 1.56 | |
| 14 | H | Br | 4-Me | 3d | 94 | 1.60 | |
| 15 | H | Br | 4-F | 3g | 92 | 1.55 | |
| 16 | H | Br | 4-CN | 3h | 93 | 1.56 | |
| 17 | 4-Me | Br | H | 3d | 95 | 1.60 | |
| 18 | 2-Me | Br | H | 3b | 92 | 1.55 | |
| 19 | 3-Me | Br | H | 3c | 93 | 1.56 | |
| 20 | H | CH2Br | H | 3k | 91 | 1.53 | |
| 21 | H | I | 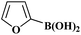 |
3l | 92 | 1.55 | |
Reusability test
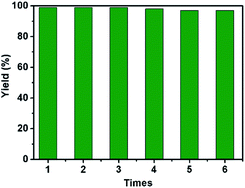
The reusability is a very important property of a supported catalyst.29 Thus, we investigated the recycling performance of HCPs-Pd in Suzuki–Miyaura reaction with 1a and 2a. First, the reaction was carried out in the presence of NaOH with HCPs-Pd as a catalyst under the optimized conditions. The catalyst was then recovered by filtering, washing with water and methanol, and drying in a vacuum oven for 3.0 h. The recovered HCPs-Pd was reused in the reaction six times, affording biphenyl in 99–95% yields with no remarkable decrease in yield (Fig. 7). The recovered catalyst was analyzed by SEM and EDX (see ESI†). The SEM images indicated that the recovered catalyst retained numerous pores on its surface. EDX revealed the Pd was still uniformly distributed on the HCPs without obvious agglomeration. In the last cycle, the palladium concentration in the filtrate was calculated by AAS to be 10 μg L−1. The very small amount of Pd in solution demonstrates that little metal leaching occurred in the reaction medium.Conclusion
In summary, we have prepared a novel heterogeneous catalyst supported on hypercrosslinked polymers. The catalyst was synthesized via the external cross-linking reaction of substituted 1,2,3-triazoles with benzene and formaldehyde dimethyl acetal. The structure and composition of the catalyst were characterized by FT-IR, N2 sorption, TGA, SEM, EDX, TEM, XPS and ICP-AES. The results show that HCPs-Pd exhibits high specific surface area, large microporous volume, chemical and thermal stability, and good dispersion of palladium chloride. The catalytic performance of HCPs-Pd was evaluated in Suzuki–Miyaura coupling reactions. The results show that HCPs-Pd is a highly active catalyst for the Suzuki–Miyaura coupling reaction, affording biaryl products with TON values reaching 1.66 × 104. In this reaction, the catalyst could be reused six times without any obvious loss of catalytic activity.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This research was financially supported by the Natural Science Foundation of Hunan Province (No. 2018JJ3409), Scientific Research Program of Huaihua University (No. HHUY2019-05), Scientific Research Fund of Hunan Provincial Education Department (17A166), the Foundation of Huaihua University Double First-rate Applied Characteristic Discipline Construction Projects of Materials Science and Engineering (No. 19CKA003), and the Foundation of Hunan Double First-rate Discipline Construction Projects (No. YYZW2019-02).Notes and references
- (a) Y. Kobayashi, Y. Tang, T. Kageyama, H. Yamashita, N. Masuda, S. Hosokawa and H. Kageyama, J. Am. Chem. Soc., 2017, 139, 18240–18246 CrossRef CAS PubMed; (b) L. C. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS PubMed; (c) C. Coperet, A. Comas-Vives, M. P. Conley, D. P. Estes, A. Fedorov, V. Mougel, H. Nagae, F. Nunez-Zarur and P. A. Zhizhko, Chem. Rev., 2016, 116, 323–421 CrossRef CAS PubMed; (d) J. L. Wang and Z. Y. Bai, Chem. Eng. J., 2017, 312, 79–98 CrossRef CAS; (e) H. Ishitani, K. Kanai, W. J. Yoo, T. Yoshida and S. Kobayashi, Angew. Chem., Int. Ed., 2019, 58, 13313–13317 CrossRef CAS PubMed; (f) H. Miyamura, F. Tobita, A. Suzuki and S. Kobayashi, Angew. Chem., Int. Ed., 2019, 58, 9220–9224 CrossRef CAS PubMed; (g) M. Tamura, N. Yuasa, J. Cao, Y. Nakagawa and K. Tomishige, Angew. Chem., Int. Ed., 2018, 57, 8058–8062 CrossRef CAS PubMed.
- (a) L. R. Chen, D. Leslie, M. G. Coleman and J. Mack, Chem. Sci., 2018, 9, 4650–4661 RSC; (b) T. Ichikawa, M. Mizuno, S. Ueda, N. Ohneda, H. Odajima, Y. Sawama, Y. Monguchi and H. Sajiki, Tetrahedron, 2018, 74, 1810–1816 CrossRef CAS; (c) Q. X. Liu, Z. L. Hu, S. C. Yu, Z. X. Zhao, D. C. Wei and H. L. Li, ACS Omega, 2018, 3, 4035–4047 CrossRef CAS PubMed; (d) A. Elhage, B. W. Wang, N. Marina, M. L. Marin, M. Cruz, A. E. Lanterna and J. C. Scaiano, Chem. Sci., 2018, 9, 6844–6852 RSC; (e) Z. Jia, K. Wang, B. Tan and Y. Gu, ACS Catal., 2017, 7, 3693–3702 CrossRef CAS.
- (a) E. Chiurchiu, S. Gabrielli, R. Ballini and A. Palmieri, Green Chem., 2017, 19, 4956–4960 RSC; (b) A. Kokel, C. Schafer and B. Torok, Green Chem., 2017, 19, 3729–3751 RSC; (c) S. Verma, R. B. N. Baig, M. N. Nadagouda, C. Len and R. S. Varma, Green Chem., 2017, 19, 164–168 RSC; (d) I. K. M. Yu, X. N. Xiong, D. C. W. Tsang, L. Wang, A. J. Hunt, H. Song, J. Shang, Y. S. Ok and C. S. Poon, Green Chem., 2019, 21, 1267–1281 RSC; (e) J. W. Zhong, Y. P. Xu and Z. M. Liu, Green Chem., 2018, 20, 2412–2427 RSC.
- (a) F. M. Wisser, P. Berruyer, L. Cardenas, Y. Mohr, E. A. Quadrelli, A. Lesage, D. Farrusseng and J. Canivet, ACS Catal., 2018, 8, 1653–1661 CrossRef CAS; (b) F. M. Wisser, Y. Mohr, E. A. Quadrelli, D. Farrusseng and J. Canivet, ChemCatChem, 2018, 10, 1778–1782 CrossRef CAS.
- (a) S. Kim, E. E. Kwon, Y. T. Kim, S. Jung, H. J. Kim, G. W. Huber and J. Lee, Green Chem., 2019, 21, 3715–3743 RSC; (b) M. D. Argyle and C. H. Bartholomew, Catalysts, 2015, 5, 145–269 CrossRef CAS; (c) P. Schwach, X. L. Pan and X. H. Bao, Chem. Rev., 2017, 117, 8497–8520 CrossRef CAS PubMed.
- (a) L. Tan and B. Tan, Chem. Soc. Rev., 2017, 46, 3322–3356 RSC; (b) K. Song, Z. Zou, D. Wang, B. Tan, J. Wang, J. Chen and T. Li, J. Phys. Chem. C, 2016, 120, 2187–2197 CrossRef CAS; (c) S. Farsadpour, L. T. Ghoochany, S. Shylesh, G. Dörr, A. Seifert, S. Ernst and W. R. Thiel, ChemCatChem, 2012, 4, 401–407 CrossRef CAS.
- (a) B. Li, Z. Guan, W. Wang, X. Yang, J. Hu, B. Tan and T. Li, Adv. Mater., 2012, 24, 3390–3395 CrossRef CAS PubMed; (b) J. Huang and S. R. Turner, Polym. Rev., 2018, 58, 1–41 CrossRef CAS; (c) Z. Jia, K. Wang, B. Tan and Y. Gu, Adv. Synth. Catal., 2017, 359, 78–88 CrossRef CAS; (d) J. J. Bi, Y. H. Dong, D. J. Zhu, W. Guo, D. Meng and T. Li, Appl. Surf. Sci., 2019, 495, 9 CrossRef.
- (a) Z. Jia, K. Wang, T. Li, B. Tan and Y. Gu, Catal. Sci. Technol., 2016, 6, 4345–4355 RSC; (b) C. Tang, Z. J. Zou, Y. F. Fu and K. P. Song, ChemistrySelect, 2018, 3, 5987–5992 CrossRef CAS; (c) S. J. Xu, Y. L. Luo and B. E. Tan, Macromol. Rapid Commun., 2013, 34, 471–484 CrossRef CAS PubMed; (d) S. Xu, K. Song, T. Li and B. Tan, J. Mater. Chem. A, 2015, 3, 1272–1278 RSC.
- (a) M. P. Tsyurupa and V. A. Davankov, React. Funct. Polym., 2006, 66, 768–779 CrossRef; (b) J. Germain, J. M. J. Frechet and F. Svec, J. Mater. Chem., 2007, 17, 4989–4997 RSC.
- R. Dawson, A. I. Cooper and D. J. Adams, Prog. Polym. Sci., 2012, 37, 530–563 CrossRef CAS.
- (a) V. A. Davankov, G. I. Timofeeva, M. M. Ilyin and M. P. Tsyurupa, J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 3847–3852 CrossRef CAS; (b) P. Veverka and K. Jeřábek, React. Funct. Polym., 1999, 41, 21–25 CrossRef CAS.
- V. A. Davankov, S. V. Rogoshin and M. P. Tsyurupa, J. Polym. Sci., Polym. Symp., 1974, 47, 95–101 CrossRef CAS.
- (a) C. D. Wood, B. Tan, A. Trewin, H. Niu, D. Bradshaw, M. J. Rosseinsky, Y. Z. Khimyak, N. L. Campbell, R. Kirk, E. Stöckel and A. I. Cooper, Chem. Mater., 2007, 19, 2034–2048 CrossRef CAS; (b) C. D. Wood, B. Tan, A. Trewin, F. Su, M. J. Rosseinsky, D. Bradshaw, Y. Sun, L. Zhou and A. I. Cooper, J. Adv. Mater., 2010, 20, 1916–1921 CrossRef.
- Y. Yang, Q. Zhang, S. Zhang and S. Li, Polymer, 2013, 54, 5698–5702 CrossRef CAS.
- (a) P. Cui, X.-F. Jing, Y. Yuan and G.-S. Zhu, Chin. Chem. Lett., 2016, 27, 1479–1484 CrossRef CAS; (b) M. Errahali, G. Gatti, L. Tei, G. Paul, G. A. Rolla, L. Canti, A. Fraccarollo, M. Cossi, A. Comotti and P. J. Sozzani, J. Phys. Chem. C, 2014, 118, 28699–28710 CrossRef CAS.
- B. Li, R. Gong, W. Wei, H. Xin, Z. Wang, H. Li, C. Hu and B. Tan, Macromolecules, 2011, 44, 2410–2414 CrossRef CAS.
- X. Liu, W. Xu, D. Xiang, Z. Zhang, D. Chen, Y. Hu, Y. Li, Y. Ouyang and H. Lin, New J. Chem., 2019, 43, 12206–12210 RSC.
- W. Xu, C. Liu, D. Xiang, Q. Luo, Y. Shu, H. Lin, Y. Hu, Z. Zhang and Y. Ouyang, RSC Adv., 2019, 9, 34595–34600 RSC.
- (a) W. Li, A. Zhang, H. Gao, M. Chen, A. Liu, H. Bai and L. Li, Chem. Commun., 2016, 52, 2780 RSC; (b) H. Gao, L. Ding, H. Bai, A. Liu, S. Li and L. Li, J. Mater. Chem. A, 2016, 4, 16490–16498 RSC; (c) Y. Xu, T. Q. Wang, Z. D. He, M. H. Zhou, W. Yu, B. Y. Shi and K. Huang, Appl. Catal., A, 2017, 541, 112–119 CrossRef CAS; (d) D. Z. Wang, X. M. Li, X. Q. Jin and Q. Jia, Sep. Purif. Technol., 2019, 216, 9–15 CrossRef CAS.
- I. Torres-Moya, B. Saikia, P. Prieto, J. R. Carrillo and J. W. Steed, CrystEngComm, 2019, 21, 2135–2143 RSC.
- C. J. Pickens, S. N. Johnson, M. M. Pressnall, M. A. Leon and C. J. Berkland, Bioconjugate Chem., 2018, 29, 686–701 CrossRef CAS PubMed.
- A. R. Hajipour and F. Mohammadsaleh, Catal. Lett., 2018, 148, 1035–1046 CrossRef CAS.
- S. Mukherjee, M. Das, A. Manna, R. Krishna and S. Das, J. Mater. Chem. A, 2019, 7, 1055–1068 RSC.
- (a) L. C. Moraes, G. P. de Souza, H. V. Fajardo, S. C. Luz, E. Alvarez, F. Lloret, R. M. Ribeiro-Viana, J. Rojo, H. O. Stumpf, R. C. Figueiredo and R. S. Correa, Inorg. Chim. Acta, 2019, 489, 93–99 CrossRef CAS; (b) L. Suntrup, M. Kleoff and B. Sarkar, Dalton Trans., 2018, 47, 7992–8002 RSC; (c) S. W. Zhang, W. Shi and P. Cheng, Coord. Chem. Rev., 2017, 352, 108–150 CrossRef CAS; (d) E. Kitteringham, Z. H. Zhou, B. Twamley and D. M. Griffith, Inorg. Chem., 2018, 57, 12282–12290 CrossRef CAS PubMed.
- (a) S. Sadjadi, M. M. Heravi, M. Malmir and F. Noritajer, Mater. Chem. Phys., 2019, 223, 380–390 CrossRef CAS; (b) P. Cruz, Y. Pérez and I. del Hierro, Eur. J. Inorg. Chem., 2018, 2018, 4206–4214 CrossRef CAS; (c) V. Sadhasivam, R. Balasaravanan and A. Siva, Appl. Organomet. Chem., 2019, 33, e4994 CrossRef.
- (a) Z. Guan, J. Hu, Y. Gu, H. Zhang, G. Li and T. Li, Green Chem., 2012, 14, 1964–1970 RSC; (b) Z. Guan, B. Li, G. Hai, X. Yang, T. Li and B. Tan, RSC Adv., 2014, 4, 36437–36443 RSC; (c) A. R. Hajipour and P. Abolfathi, Catal. Commun., 2018, 103, 92–95 CrossRef CAS.
- K. Song, P. Liu, J. Wang, B. Tan and T. Li, J. Porous Mater., 2016, 23, 725–731 CrossRef CAS.
- C. Liu, W. Xu, D. Xiang, Q. Luo, S. Zeng, L. Zheng, Y. Tan, Y. Ouyang and H. Lin, Catal. Lett., 2020 DOI:10.1007/s10562-020-03165-4.
- Á. Molnár and A. Papp, Catalyst recycling-A survey of recent progress and current status, Coord. Chem. Rev., 2017, 349, 1–65 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, spectral characterization data and supplementary figures. See DOI: 10.1039/d0ra01190h |
| ‡ Cijie Liu and Lijuan Zheng contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |