Open Access Article
Open Access ArticleA novel CoFe2O4@Cr-MIL-101/Y zeolite ternary nanocomposite as a magnetically separable sonocatalyst for efficient sonodegradation of organic dye contaminants from water†
Meysam Sadeghi,
Saeed Farhadi * and
Abedin Zabardasti
* and
Abedin Zabardasti
Department of Chemistry, Lorestan University, Khorramabad, 68151-44316, Iran. E-mail: farhadi.s@lu.ac.ir; sfarhadi1348@yahoo.com; Fax: +98 6633120618; Tel: +98 6633120611
First published on 9th March 2020
Abstract
In this research, a novel magnetic sonocatalyst nanocomposite, CoFe2O4@Cr-MIL-101/Y zeolite, has been successfully fabricated employing a simple hydrothermal method. The as-prepared catalyst was thoroughly identified using Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), energy dispersive X-ray spectroscopy (EDS), EDS elemental dot-mapping, transmission electron microscopy (TEM), atomic force microscopy (AFM), vibrating sample magnetometer (VSM), and nitrogen Brunauer–Emmett–Teller (N2-BET) analyses. The procured CoFe2O4@Cr-MIL-101/Y nanocomposite was then assessed for the decomposition of three types of organic dyes namely methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) from water solution using ultrasound irradiation and subsequently monitored via UV-Vis absorption technique. The sonodecomposition reactions of organic dyes were accomplished in the presence of the H2O2 solution as a green oxidizing agent. Furthermore, the influence of various experimental independent factors such as irradiation time, process type, initial dye concentration, catalyst dosage, H2O2 concentration, scavenger type, and catalyst regeneration on the decomposition of MB, RhB and MO were surveyed. Additionally, a first order kinetic model was applied to investigate the sonodecomposition reactions of dye contaminants. The rate constant (k) and half-life (t1/2) data were gained as 0.0675 min−1 and 10.2666 min, respectively, for the decomposition of MB in the US/H2O2/CoFe2O4@Cr-MIL-101/Y system. Besides, evaluating the attained results, the distinctive performance of ˙OH as the radical scavenger originating from H2O2 throughout the sonodecomposition process is vividly approved.
1. Introduction
In recent years, the numbers of major health and environmental dilemmas have been growing from day to day due to the release of tremendous amounts of chemicals into the environmental zones like water, soil, air and so on.1 As industrial activities are spreading all over the world, it will be much harder to protect natural and environmental sources against drastic quantities of chemicals which plummet from these units. Also, it has been revealed that there are more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different kinds of dyes and pigments, approximately 700
000 different kinds of dyes and pigments, approximately 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of which are produced yearly.2 On the other hand, more than 200
000 tons of which are produced yearly.2 On the other hand, more than 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons (about 10–15%) of residual synthetic dyes are discarded from the textile mills dyeing processes and between 2 to 10% of them are directly evacuated into the sewage systems.3 The improper evacuation of organic dye sewages into water supplies and the environment without any remediation can cause serious and irreparable consequences for the human body and environmental species. The permanent exposure to such organic dye contaminants even at low concentrations has proven to be harmful owing to their bioaccumulation in living tissues through skin, eyes, inhalation and unclean drinking water.4 Therefore, these sorts of organic contaminants with carcinogenic and mutagenic effects referred to the existence of various aromatic compounds mostly naphthalene, benzidine within their structures, should be scrupulously disposed or removed from wastewater systems prior to their entrance into the environment.5–7 From the other point of view, there are diverse methodologies to eradicate contaminant dye molecules from aqueous solutions such as ultrasonic degradation,8 adsorption,9,10 photocatalytic treatment,11 biodegradation,12 electrochemical oxidation,13 etc. Among them, the sonodecomposition reaction has been exerted as a significant, effective and environmentally friendly process to eliminate dye contaminants through the formation of ˙OH (hydroxyl) and ˙H (proton) radicals and a series of other species like HOO˙ (perhydroxyl) radicals, O2, etc.14 Furthermore, the versatile sorts of catalysts, including CuFe2O4/MIL-101/Graphene,15 TiO2/montmorillonite,16 Yb, B and Ga dopted Er3+:Y3Al5O12/TiO2,17 CoFe2O4@ZnS,18 Au/NiGa2O4–Au–Bi2O3,19 CoFe2O4/CdS,20 InVO4/TiO2,21 CeO2/TiO2,22 MgO,23 WO3,24 AgBr,25 and some others, have been applied to remove dye contaminants through the sonodecomposition activities. In addition to this, metal–organic frameworks (MOFs) are introduced as one of the new kind of organic–inorganic materials.26 Among all types of MOFs, Cr-MIL-101 (with a band gap energy 2.06 eV)27 has attracted special attention because of its prominent chemical and physical features.28,29 Moreover, as it has been lucratively applied in different fields of research like gas sensing procedures, separation and catalysis,30–32 it counts as a promising candidate for the removal of organic dyes. It is also noticeable that the zeolites are known as a subgroup of hydrated crystalline aluminosilicate of alkali and alkaline-earth metals.33 Y zeolite displays the Faujasite (FAU) framework which has a 3D porous structure is known as one of the most accountable zeolites which has been employed for numerous research purposes so far.33 Y zeolite possesses distinctive features like high surface area, large pore volume, high hydrothermal stability and biocompatibility which is advantageous for its use in catalysis, development of adsorbents, etc.34–36 Elsewhere, in recent years, the inorganic spinel cobalt ferrite nanoparticles (CoFe2O4 NPs) with band gap energy 1.76 eV have grabbed much attention referred to their different properties such as coercivity, anisotropy, saturation, magnetization, etc.37,38 Thereupon, these salient and applicable features make magnetic CoFe2O4 NPs suitable for several fields for instance drug delivery,39 magnetic fluid hyperthermia,40 microwave devices,41 permanent magnets,42 catalysis,43 etc. The incorporation of various sorts of magnetic NPs over the surface of solid catalysts provides a high surface area to enhance the efficiency of the catalytic processes.44 In this scientific research, for the first time, CoFe2O4@Cr-MIL-101/Y zeolite as a novel magnetic catalyst nanocomposite was successfully fabricated using hydrothermal route and subsequently identified by several techniques including FTIR, XRD, FESEM, EDS, EDS dot-mapping, TEM, AFM, VSM, and BET analyses. The foregoing nanocomposite was then assessed for the sonodecomposition of the cationic and anionic organic dyes of methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) in water solution by employing H2O2 (hydrogen peroxide) as the hydroxyl radicals (˙OH) source. However, as far as we know, there are no scientific researches reporting the sonodecomposition reactions of organic dye contaminants using the CoFe2O4@Cr-MIL-101/Y zeolite catalyst nanocomposite in any previous study.
000 tons (about 10–15%) of residual synthetic dyes are discarded from the textile mills dyeing processes and between 2 to 10% of them are directly evacuated into the sewage systems.3 The improper evacuation of organic dye sewages into water supplies and the environment without any remediation can cause serious and irreparable consequences for the human body and environmental species. The permanent exposure to such organic dye contaminants even at low concentrations has proven to be harmful owing to their bioaccumulation in living tissues through skin, eyes, inhalation and unclean drinking water.4 Therefore, these sorts of organic contaminants with carcinogenic and mutagenic effects referred to the existence of various aromatic compounds mostly naphthalene, benzidine within their structures, should be scrupulously disposed or removed from wastewater systems prior to their entrance into the environment.5–7 From the other point of view, there are diverse methodologies to eradicate contaminant dye molecules from aqueous solutions such as ultrasonic degradation,8 adsorption,9,10 photocatalytic treatment,11 biodegradation,12 electrochemical oxidation,13 etc. Among them, the sonodecomposition reaction has been exerted as a significant, effective and environmentally friendly process to eliminate dye contaminants through the formation of ˙OH (hydroxyl) and ˙H (proton) radicals and a series of other species like HOO˙ (perhydroxyl) radicals, O2, etc.14 Furthermore, the versatile sorts of catalysts, including CuFe2O4/MIL-101/Graphene,15 TiO2/montmorillonite,16 Yb, B and Ga dopted Er3+:Y3Al5O12/TiO2,17 CoFe2O4@ZnS,18 Au/NiGa2O4–Au–Bi2O3,19 CoFe2O4/CdS,20 InVO4/TiO2,21 CeO2/TiO2,22 MgO,23 WO3,24 AgBr,25 and some others, have been applied to remove dye contaminants through the sonodecomposition activities. In addition to this, metal–organic frameworks (MOFs) are introduced as one of the new kind of organic–inorganic materials.26 Among all types of MOFs, Cr-MIL-101 (with a band gap energy 2.06 eV)27 has attracted special attention because of its prominent chemical and physical features.28,29 Moreover, as it has been lucratively applied in different fields of research like gas sensing procedures, separation and catalysis,30–32 it counts as a promising candidate for the removal of organic dyes. It is also noticeable that the zeolites are known as a subgroup of hydrated crystalline aluminosilicate of alkali and alkaline-earth metals.33 Y zeolite displays the Faujasite (FAU) framework which has a 3D porous structure is known as one of the most accountable zeolites which has been employed for numerous research purposes so far.33 Y zeolite possesses distinctive features like high surface area, large pore volume, high hydrothermal stability and biocompatibility which is advantageous for its use in catalysis, development of adsorbents, etc.34–36 Elsewhere, in recent years, the inorganic spinel cobalt ferrite nanoparticles (CoFe2O4 NPs) with band gap energy 1.76 eV have grabbed much attention referred to their different properties such as coercivity, anisotropy, saturation, magnetization, etc.37,38 Thereupon, these salient and applicable features make magnetic CoFe2O4 NPs suitable for several fields for instance drug delivery,39 magnetic fluid hyperthermia,40 microwave devices,41 permanent magnets,42 catalysis,43 etc. The incorporation of various sorts of magnetic NPs over the surface of solid catalysts provides a high surface area to enhance the efficiency of the catalytic processes.44 In this scientific research, for the first time, CoFe2O4@Cr-MIL-101/Y zeolite as a novel magnetic catalyst nanocomposite was successfully fabricated using hydrothermal route and subsequently identified by several techniques including FTIR, XRD, FESEM, EDS, EDS dot-mapping, TEM, AFM, VSM, and BET analyses. The foregoing nanocomposite was then assessed for the sonodecomposition of the cationic and anionic organic dyes of methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) in water solution by employing H2O2 (hydrogen peroxide) as the hydroxyl radicals (˙OH) source. However, as far as we know, there are no scientific researches reporting the sonodecomposition reactions of organic dye contaminants using the CoFe2O4@Cr-MIL-101/Y zeolite catalyst nanocomposite in any previous study.
2. Experimental
2.1. Materials
Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, 98%), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, 98%), terephthalic acid (H2BDC, 98%), chromium(III) nitrate nonahydrate (Cr(NO3)3·9H2O, 99%), aluminum hydroxide (Al(OH)3, 99%), sodium hydroxide (NaOH, 98%), hydrogen peroxide (H2O2, 30%), sodium silicate (Na2SiO3, 98%), ethanol (C2H5OH, 96%), dimethyl formamide (DMF, 99%), benzoquinone (BQ, 98%), isopropyl alcohol (IPA, 99%), disodium ethylenediaminetetraacetate (Na2-EDTA, 99%), methyl orange (MO, C14H14N3NaO3S, 99%), rhodamine B (RhB, C28H31ClN2O3, 99%), and methylene blue (MB, C16H18ClN3S, 99%) were all purchased from Alfa Aesar, Sigma-Aldrich and Merck companies.2.2. Instrumentation
The morphological, structural and magnetic properties of the as-prepared catalysts were evaluated using different analytical instruments. In this respect, the Fourier transform infrared (FTIR) was applied on a Schimadzu system spectrophotometer 8400S utilizing KBr disks in the wavelength between 400–4000 cm−1. The powder X-ray diffraction (XRD) patterns were surveyed by type XPERT Poro panalytical diffractometer along with Ni-filtered CuKα radiation at λ = 1.5406 Å (40 kV and 30 mA) and subsequently the related data were measured in the variation range of 2θ between 5° to 80° via a scanning speed of 2° min−1. Elsewhere, a field emission scanning electron microscopy along with energy-dispersive X-ray spectroscopy (FESEM-EDS) and X-ray dot-mapping (MIRA3 TESCAN) via an operating voltage of 15 kV, were exerted to investigate the shape and crystalline size of the understudy catalysts. Meantime, the atomic force microscopy (AFM, Noncontact mode, Ara-AFM, model Full plus) was operated and experiments were implemented at 25 ± 1 °C. The transmission electron microscopy (TEM) analysis was utilized to characterize the particles size and morphology of the as-prepared catalyst nanocomposite with type Philips CM120 electron microscope at an 80 kV voltage. For the investigation of the magnetic properties of as-synthesized catalysts, a vibrating sample magnetometer (VSM, Magnetic Daghigh Kavir, MDKB model) was applied. The nitrogen Brunauer–Emmett–Teller (N2-BET) with a PHSCHINA PHS1020 apparatus was used to study the specific surface area and pore size distributions of the catalysts at 77 K. Moreover, an ultrasonic device (Sonic 6MX; 100W output acoustic power and frequency of 37 kHz) was utilized to study all the sonodecomposition processes of organic dyes and the fabrication of the catalysts. A double-beam ultraviolet-visible (UV-Vis) spectrophotometer (Cary 100, Varian Company) was employed to evaluate the residual organic dyes concentrations from the supernatant aqueous solutions before and after the sonodecomposition reactions in the range wavelength between 200–800 nm.2.3. Synthesis of Y type zeolite
Typically, an adequate amount of NaOH (10 g) was poured into the deionized water (10 mL). Afterwards, the value of Al(OH)3 (9.75 g) was transferred into the NaOH solution and heated at temperature of 100 °C. Then, resultant suspension (10 g) was dissolved into the deionized water (61.2 mL) and subsequently NaOH (5.9 g) was added to this suspension until being dissolved to prepare the solution A. Meanwhile, Na2SiO3 (22 g) was gradually poured into the prepared solution containing NaOH (5.9 g) and deionized water (61.2 mL) until being dissolved to afford the solution B. The solution A was gently added to the solution B and acquired mixture was stirred for 40 min. The molar composition of gel was 4.3Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3
Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10SiO4
10SiO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180H2O. The mixture was placed into a Teflon-lined stainless steel autoclave and heated at 90 °C for 8 h. The product was filtered and washed by distilled water until the neutral pH was achieved. Consequently, the final product was dried at 100 °C.45
180H2O. The mixture was placed into a Teflon-lined stainless steel autoclave and heated at 90 °C for 8 h. The product was filtered and washed by distilled water until the neutral pH was achieved. Consequently, the final product was dried at 100 °C.45
2.4. Synthesis of Cr-MIL-101/Y zeolite
Firstly, as-prepared Y zeolite (0.2 g) was added into the deionized water (30 mL) and subsequently was dispersed under ultrasonic irradiation for 1 h. In the next step, the appropriate values of H2BDC (0.5 g) and Cr(NO3)3·9H2O (1.2 g) as the precursors were poured into the above specified solution and the solution was then rigorously agitated for 30 min at 25 ± 1 °C. Subsequently, the resultant suspension was transferred to a Teflon-lined stainless steel autoclave and heated at 200 °C overnight. The gained product was then left to be cooled down at 25 ± 1 °C. To achieve the extra purified sample, the resultant mixture was rinsed several times sequentially using the DMF and ethanol solvents to remove any possible impurities from the unreacted H2BDC on its surface. Finally, the obtained sample was dried at 150 °C for 12 h to achieve the Cr-MIL-101/Y zeolite composite.2.5. Synthesis of CoFe2O4@Cr-MIL-101/Y zeolite
A hydrothermal approach was employed to synthesize the magnetic CoFe2O4@Cr-MIL-101/Y zeolite catalyst nanocomposite as follows: the as-prepared Cr-MIL-101/Y composite (0.5 g) was appended into the deionized water (30 mL). Afterwards, the quantities of Fe(NO3)3·9H2O (1.32 g) and Co(NO3)2·6H2O (0.57 g) were added into the desired solution and subsequently stirred for 1 h at 25 ± 1 °C. Thereafter, as-prepared NaOH aqueous solution (1 mol L−1) was gradually added to the above cited suspension to achieve pH of 11 while continuously stirring for 1 h. Then, the immobilization reaction was ceased and the mixture was calcined at 180 °C for 12 h. Ultimately, the attained precipitate was magnetically separated and thoroughly rinsed using the deionized water and ethanol for multiple times and dried at 25 ± 1 °C.2.6. Sonodecomposition experiments
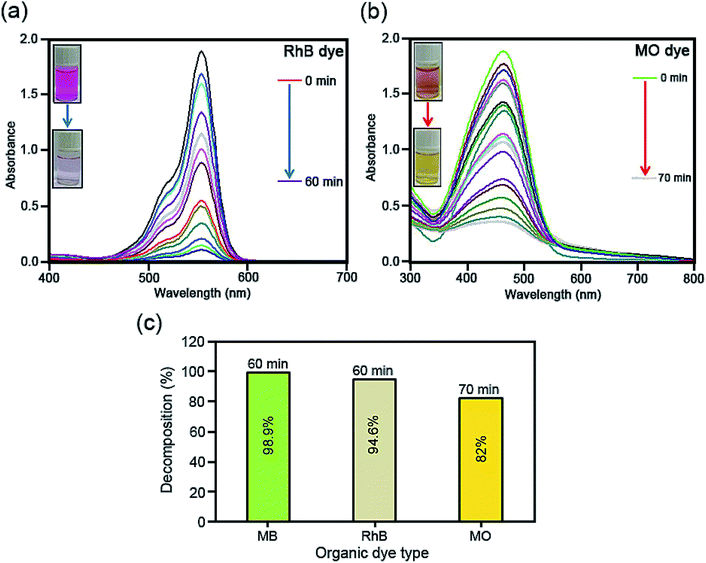
To scrutinize the sonocatalytic decomposition reactions of several organic cationic and anionic dyes including MB, RhB and MO over the desired catalysts from water solution, an ultrasonic device was applied. Typically, the amount of magnetic CoFe2O4@Cr-MIL-101/Y catalyst (0.5 g L−1) was transferred into the MB aqueous solution (25 mg L−1, 50 mL) and subsequently the resulting solution was shaken for 30 min in the darkness. This stirring step causes the formation an adsorption–desorption equilibrium among the catalyst surface and organic dye. In the next step, the reaction was accomplished using the H2O2 (40 mmol L−1, 2 mL). After the desired time intervals, the reaction process was halted; the quantity of the aqueous solution (2 mL) was taken out and then the CoFe2O4@Cr-MIL-101/Y nanocomposite was separated from the supernatant solution using an external magnetic field. At the end, the transparent supernatant solution of the organic dye was moved to the UV-Vis spectrophotometer instrument at λmax of 663 nm to evaluate the residual MB dye. In addition, the influence of several independent factors such as initial dye concentration (25, 35, 45 and 55 mg L−1), sonocatalyst kind (raw CoFe2O4, Y, Cr-MIL-101, Cr-MIL-101/Y and CoFe2O4@Cr-MIL-101/Y), sonocatalyst dosage (0.25, 0.5, 0.75 and 1 g L−1), initial H2O2 concentration (5, 10, 20, 30, 40 and 50 mmol L−1), and irradiation time (5, 10, 20, 30, 40, 50, and 60 min) over the sonodecomposition reactions of dyes were meticulously surveyed. Besides, to study the sonocatalytic performance of the CoFe2O4@Cr-MIL-101/Y nanocomposite to decompose and decontaminate other organic dyes namely RhB and MO from aqueous solution, the similar experimental conditions were employed and the acquired data were reported in the present research. The decomposition reaction efficiency% (DE%) was also investigated through the equation below (1):
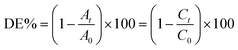 | (1) |
3. Result and discussion
3.1. FTIR analysis
The Fourier transform infrared spectroscopy (FTIR) spectra of the as-prepared catalysts namely raw Y zeolite (Fig. 1a), Cr-MIL-101/Y (Fig. 1b), CoFe2O4@Cr-MIL-101/Y (Fig. 1c), raw CoFe2O4 (Fig. 1d), and raw Cr-MIL-101 (Fig. 1e) have been exhibited in Fig. 1. As demonstrated in Fig. 1a–c, the bands located at 459 cm−1 and 578 cm−1 are ascribed to the internal TO4 (T = Al and/or Si elements) tetrahedral units and bending vibrations of the double six rings external linkage of Y zeolite, while the specified absorption bands at 719 cm−1 and 788 cm−1 can be assigned sequentially to the external linkage and vibrations O–T–O internal tetrahedral symmetrical stretching. Moreover, the observed bands near 1018 cm−1 and 1135 cm−1 are relevant to the O–Al–O and/or O–Si–O external connection and internal tetrahedral asymmetrical stretching vibrations of the Y zeolite. The bands at 1631 cm−1 and 3407 cm−1 can be attributed to the vibrations of hydroxyl groups and adsorbed H2O molecules bonds including O–H bonding and H–O–H bending sequentially in the structure of Y zeolite. Additionally, the bands at about 1512 cm−1 and 1398 cm−1 which have been indicated from Fig. 1b and c, can be pertained to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and carboxylate (O–C–O) bonds in H2BDC which referred to the successful incorporation of Cr-MIL-101 in the structure of CoFe2O4@Cr-MIL-101/Y. On the other hand, the absorption bands affiliated to the M–O–Si and/or M–O–Al (M = Co and/or Fe elements) from the incorporation of CoFe2O4 on the Cr-MIL-101/Y framework expected in the range of 400 to 1000 cm−1, were not illustrated because to the overlapping with the Y zeolite bands. The FTIR spectrum of the raw CoFe2O4 has been shown in Fig. 1d. The bands at about 3450 cm−1 and 1581 cm−1 are observed due to the adsorbed H2O molecules on the surface of CoFe2O4. The absorption bands between 400 cm−1 to 600 cm−1 were also recognized to the stretching vibrations of Co–O and Fe–O bonds. Also, Fig. 1e reveals FTIR spectrum of the raw Cr-MIL-101. The absorption bands in the range of 748 cm−1, 881 cm−1, 1016 cm−1, 1161 cm−1, and 1508 cm−1 are related to the C–H and C
C and carboxylate (O–C–O) bonds in H2BDC which referred to the successful incorporation of Cr-MIL-101 in the structure of CoFe2O4@Cr-MIL-101/Y. On the other hand, the absorption bands affiliated to the M–O–Si and/or M–O–Al (M = Co and/or Fe elements) from the incorporation of CoFe2O4 on the Cr-MIL-101/Y framework expected in the range of 400 to 1000 cm−1, were not illustrated because to the overlapping with the Y zeolite bands. The FTIR spectrum of the raw CoFe2O4 has been shown in Fig. 1d. The bands at about 3450 cm−1 and 1581 cm−1 are observed due to the adsorbed H2O molecules on the surface of CoFe2O4. The absorption bands between 400 cm−1 to 600 cm−1 were also recognized to the stretching vibrations of Co–O and Fe–O bonds. Also, Fig. 1e reveals FTIR spectrum of the raw Cr-MIL-101. The absorption bands in the range of 748 cm−1, 881 cm−1, 1016 cm−1, 1161 cm−1, and 1508 cm−1 are related to the C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively. The absorption bands around 1400 cm−1 to 1662 cm−1 are assigned sequentially to the asymmetric stretching vibrations and carboxylate (COO) symmetric stretching vibration from the H2BDC.
C bonds, respectively. The absorption bands around 1400 cm−1 to 1662 cm−1 are assigned sequentially to the asymmetric stretching vibrations and carboxylate (COO) symmetric stretching vibration from the H2BDC.
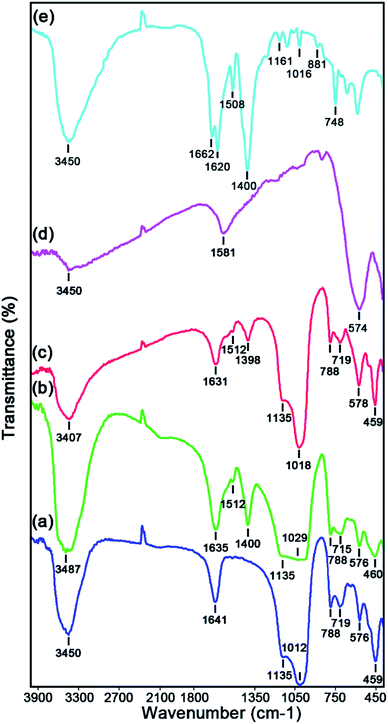 | ||
| Fig. 1 FTIR spectra of the as-synthesized (a) raw Y zeolite, (b) Cr-MIL-101/Y, (c) CoFe2O4@Cr-MIL-101/Y, (d) raw CoFe2O4, and (e) raw Cr-MIL-101. | ||
3.2. XRD analysis
Fig. 2 elucidates the X-ray diffraction (XRD) patterns of the raw Y zeolite (Fig. 2a), Cr-MIL-101/Y (Fig. 2b), CoFe2O4@Cr-MIL-101/Y (Fig. 2c), raw CoFe2O4 (Fig. 2d), and raw Cr-MIL-101 (Fig. 2e). As can be revealed from Fig. 2a–c, the several intense peaks belonged to the Y zeolite were determined at 2θ angles approximately 6.28°, 10.17°, 11.95°, 15.73°, 18.73°, 20.43°, 23.71°, 27.13°, and 32.54° sequentially recognized to the diffraction planes of (111), (220), (311), (331), (511), (440), (620), (533), and (733), which have been crystallized in the cubic structure with JCPDS card (41-0118). Besides, the main peaks of raw Cr-MIL-101 phase were observed at 2θ between 5° to 20° (Fig. 2d), which confirms the excellent crystalline framework of the fabricated sample. Also, by supporting of Cr-MIL-101 and CoFe2O4 subsequently over the Y zeolite surface to form the CoFe2O4@Cr-MIL-101/Y nanocomposite, no significant change was found in its crystalline structure. In this respect, the peaks attributed to the Cr-MIL-101 were demonstrated at scattering angles (2θ) approximately 8.62, 9.18°, 11.45° and 16.60°, respectively. Moreover, several new peaks affiliated to the presence of CoFe2O4 throughout the fabrication process of the nanocomposite were located at 2θ of 30.34°, 35.67°, 37.12°, 43.29°, 53.65°, 57.20°, 62.82°, 71.25°, 74.23° and 78.97° corresponded to the (220), (311), (222), (400), (422), (511), (440), (620), (533), and (444) (JCPDS card: 01-1121). The average crystalline size (dc) of the CoFe2O4 NPs over the Cr-MIL-101/Y was measured approximately 12 nm using the Debye–Scherrer eqn (2):
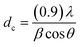 | (2) |
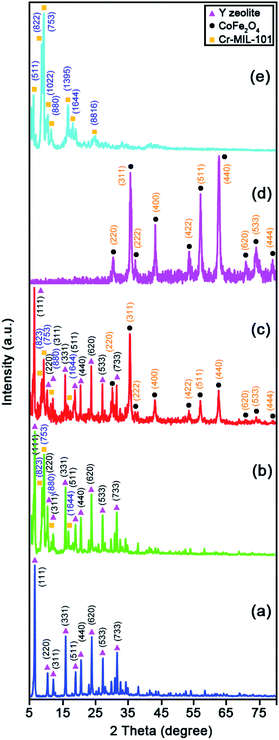 | ||
| Fig. 2 XRD patterns of the as-synthesized (a) raw Y zeolite, (b) Cr-MIL-101/Y, (c) CoFe2O4@Cr-MIL-101/Y, (d) raw CoFe2O4, and (e) raw Cr-MIL-101. | ||
3.3. FESEM analysis
To survey the structure, morphology and size of the as-prepared samples, a field emission scanning electron microscopy (FESEM) analysis was applied. The FESEM images of the raw Y zeolite (Fig. 3a and b), Cr-MIL-101/Y (Fig. 3c and d), CoFe2O4@Cr-MIL-101/Y (Fig. 3e and f), raw CoFe2O4 (Fig. 3g), and raw Cr-MIL-101 (Fig. 3h and i) with different resolutions were all respectively indicated in Fig. 3. The cubic crystals of the Y zeolite, the octahedral framework of the Cr-MIL-101 and also the quasi-spherical shape of the CoFe2O4 were displayed from the FESEM images. Based on the attained data, it can be inferred that by supporting the Cr-MIL-101 and CoFe2O4 subsequently over the surface of the Y zeolite, its morphology and framework remained intact. Moreover, the average particles size of the CoFe2O4 NPs as the guest molecules within the CoFe2O4@Cr-MIL-101/Y catalyst were found to be about 12 nm.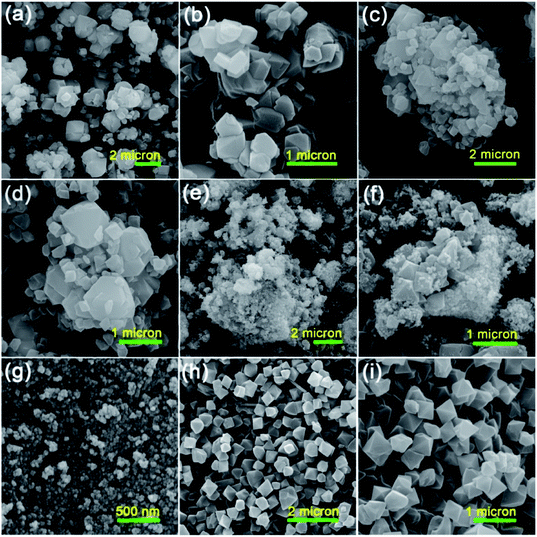 | ||
| Fig. 3 FESEM images of the as-synthesized (a and b) raw Y zeolite, (c and d) Cr-MIL-101/Y, (e and f) CoFe2O4@Cr-MIL-101/Y, (g) raw CoFe2O4, (h and i) raw Cr-MIL-101 with different resolutions. | ||
3.4. EDS and EDS elemental dot-mapping analyses
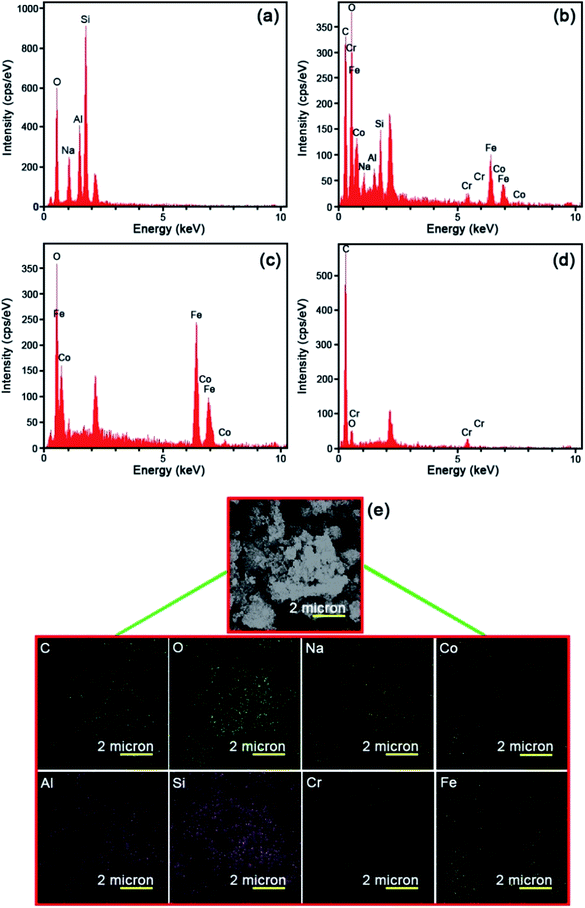
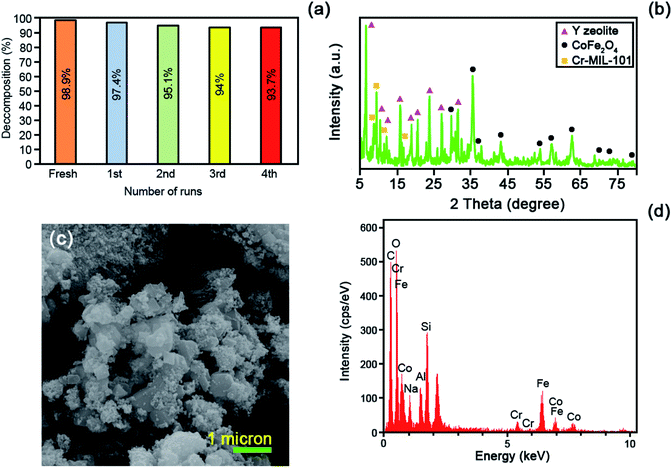
The energy dispersive X-ray (EDS) was operated to fully clarify the elemental composition of the as-fabricated catalysts. Fig. 4 represents the EDS analysis of the raw Y zeolite (Fig. 4a), CoFe2O4@Cr-MIL-101/Y (Fig. 4b), raw CoFe2O4 (Fig. 4c), and raw Cr-MIL-101 (Fig. 4d), respectively. The acquired data from the analysis elucidates the existence of the four main elements of Si, O, Al and Na in all of the samples including raw Y zeolite and CoFe2O4@Cr-MIL-101/Y. Also, Fig. 4b displays the presence of a series of other elements such as Co, Fe and O related to the CoFe2O4 NPs, and plus Cr, C and O corresponded to the Cr-MIL-101 within the framework of the CoFe2O4@Cr-MIL-101/Y ternary nanocomposite, which affirms the formation of the CoFe2O4 and Cr-MIL-101 over the Y zeolite (see Table 1). Also, as can be seen from Fig. 4b and d, composition analyses of the C and O elements clearly approved the presence of carboxylate within the Cr-MIL-101 frameworks. Further, the FESEM image affiliated to the EDS elemental dot-mappings of the CoFe2O4@Cr-MIL-101/Y were depicted in Fig. 4e. Evaluating the elemental mappings analysis represented in Fig. 4e, the uniform distribution of Si, O, Al, Na, Co, Fe, Cr and C elements on the nanocomposite is clearly verified.| Element type | Line | Weight% | Atomic% |
|---|---|---|---|
| C | Kα | 47.77 | 61.25 |
| O | Kα | 33.43 | 32.17 |
| Na | Kα | 1.51 | 1.01 |
| Al | Kα | 0.97 | 0.55 |
| Si | Kα | 2.03 | 1.11 |
| Cr | Kα | 0.88 | 0.26 |
| Fe | Kα | 8.81 | 2.43 |
| Co | Kα | 4.60 | 1.20 |
3.5. TEM analysis
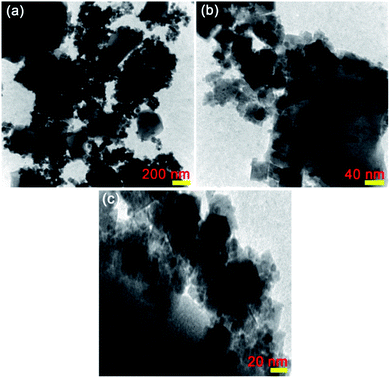
The TEM analysis was applied to further warrant the particle size and morphology of the as-prepared CoFe2O4@Cr-MIL-101/Y nanocomposite. The TEM images of CoFe2O4@Cr-MIL-101/Y with different resolutions are shown in Fig. 5a–c. Regarding the TEM images, it can be deduced that the both Cr-MIL-101 and CoFe2O4 NPs as the guest molecules were successfully incorporated over the Y zeolite structure. The mediocre particle diameter about 12 nm and plus the narrow particle size distribution between 8 to 16 nm were clearly identified using the TEM images. The abovementioned results were in good agreement with the mediocre particle diameter attained via the FESEM and XRD analyses.3.6. AFM analysis
The atomic force microscopy (AFM) is recognized as a suitable analytical method to study several important properties of the as-obtained catalysts such as surface roughness, size and topography. Fig. S1† represents AFM 2-dimensional (2D) and 3-dimensional (3D) images ascribed to the raw Y zeolite (Fig. S1a and b†), Cr-MIL-101/Y (Fig. S1c and d†) and CoFe2O4@Cr-MIL-101/Y (Fig. S1e and f†), respectively. By comparing the images, it is brightly indicated that the surface of the Y zeolite was mainly covered via the Cr-MIL-101 and CoFe2O4 NPs, which refers to the successful synthesis of the aforementioned guest molecules within the host framework. Besides, mean size of the composed CoFe2O4 NPs in the CoFe2O4@Cr-MIL-101/Y catalyst were approximately about 12.8 nm, which acquired data from this analysis are in good consistency with the FESEM, TEM and XRD.3.7. VSM analysis
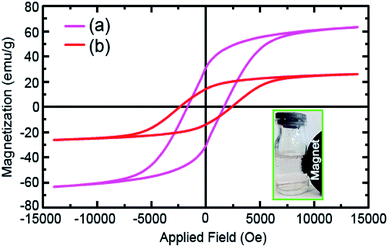
The vibrating sample magnetometer (VSM) analysis was utilized to thoroughly investigate the magnetic properties of the as-prepared samples at 25 ± 1 °C. Fig. 6 reveals the hysteresis loops of the raw CoFe2O4 NPs (Fig. 6a) and CoFe2O4@Cr-MIL-101/Y (Fig. 6b). Both raw CoFe2O4 NPs and CoFe2O4@Cr-MIL-101/Y illustrate the ferromagnetic features due to the existence of three parameters namely coercive force (Hc), remnant magnetization (Mr) and saturation magnetization (Ms). In this respect, the values of the Hc, Mr and Ms parameters of the raw CoFe2O4 NPs were determined approximately at about 1500 Oe, 33.94 emu g−1 and 63.46 emu g−1, whereas by supporting of the CoFe2O4 NPs on the surface of the Cr-MIL-101/Y, the abovementioned values decreased to 2500 Oe, 15.10 emu g−1 and 26.10 emu g−1, respectively. Consequently, it is deduced that the CoFe2O4@Cr-MIL-101/Y nanocomposite can be separated from the reaction solution in the shortest possible time using a simple external magnetic field.3.8. BET analysis
To meticulously unveil the structural outstanding of the as-prepared catalysts, BET analysis was utilized. Fig. 7 demonstrates the N2 adsorption–desorption isotherms and pore size distributions of raw Y zeolite (Fig. 7a) and CoFe2O4@Cr-MIL-101/Y (Fig. 7b), respectively. Pursuant to the acquired plot of isotherms of the understudy catalysts, it is obviously known that these plots can be assorted to type-IV isotherm with a H3 hysteresis loops (as per IUPAC recommendation), which affirmed the presence of mesoporous structure of the catalysts. The values of BET specific surface area for raw Y zeolite and CoFe2O4@Cr-MIL-101/Y were also measured about 494 and 222 m2 g−1 respectively and reflected in Table 2. As can be seen in Table 2, the acquired specific surface area, pore size distributions, Vme and Vmi of CoFe2O4@Cr-MIL-101/Y decreases in comparison with raw Y zeolite, which confirms the formation of the Cr-MIL-101 and CoFe2O4 on the Y zeolite framework.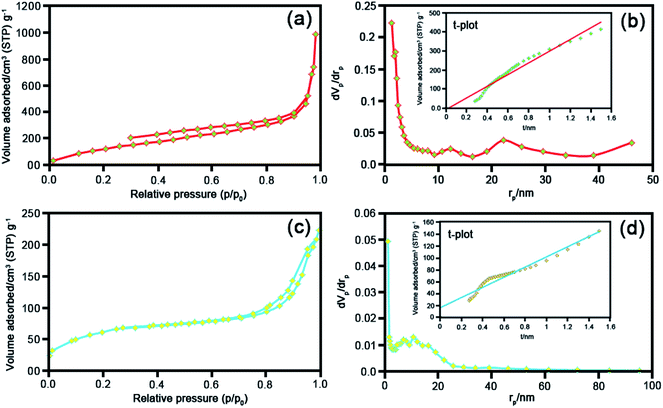 | ||
| Fig. 7 BET analyses, BJH and t plots of the as-synthesized (a) and (b) raw Y zeolite, (c) and (d) CoFe2O4@Cr-MIL-101/Y. | ||
| Sample | as, BET [m2 g−1] | Vme [cm3(STP) g−1] | Vmi [cm3(STP) g−1] | Total pore volume [cm3 g−1] | Average pore diameter [nm] |
|---|---|---|---|---|---|
| Y zeolite | 494 | 113 | 0.10 | 1.50 | 12.44 |
| CoFe2O4@Cr-MIL-101/Y | 222 | 51.06 | 0.02 | 0.34 | 6.11 |
3.9. Sonodecomposition processes investigation
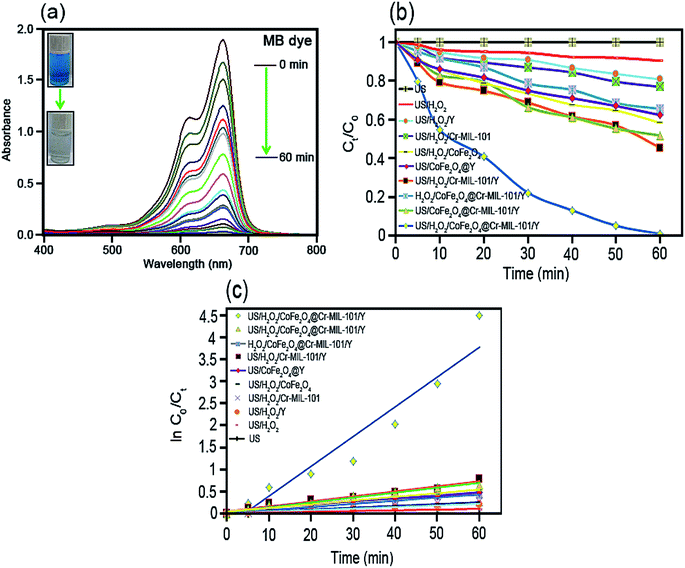
As it has been displayed in Fig. 8c, plotting ln initial dye concentration versus contact time (min) results in some curves which are of great significance for the investigation of the sonodecomposition reaction kinetics. Elsewhere, the decomposition rate constant symbolized as k (min−1), was calculated using the first order equation of
| ln(Co/Ct) = kt. | (3) |
| t1/2 = ln(2)/k. | (4) |
| Process type | Rate constant (k, min−1) | Half-life (t1/2, min) | Kinetic equation [ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co/Ct = at + b] Co/Ct = at + b] |
|---|---|---|---|
| US only | 0.0000 | 0.0000 | y = 0.0000 |
| US/H2O2 | 0.0016 | 433.1250 | y = 0.0016x + 0.0112 |
| US/H2O2/Y | 0.0034 | 203.8235 | y = 0.0034x + 0.0076 |
| US/H2O2/Cr-MIL-101 | 0.0042 | 165.0000 | y = 0.0042x + 0.0145 |
| US/H2O2/CoFe2O4 | 0.0071 | 97.6056 | y = 0.0071x + 0.0090 |
| US/CoFe2O4@Y | 0.0072 | 96.2500 | y = 0.0072x + 0.0486 |
| US/H2O2/Cr-MIL-101/Y | 0.0114 | 60.7894 | y = 0.0114x + 0.0525 |
| H2O2/CoFe2O4@Cr-MIL-101/Y | 0.0083 | 83.4939 | y = 0.0083x + 0.0451 |
| US/CoFe2O4@Cr-MIL-101/Y | 0.0109 | 63.5779 | y = 0.0109x + 0.0378 |
| US/H2O2/CoFe2O4@Cr-MIL-101/Y | 0.0675 | 10.2666 | y = 0.0675x − 0.2700 |
| No | Catalyst type | Irradiation type | Dye type | [H2O2]o (mmol L−1) | [Dye]o (mg L−1) | [Catalyst] (g L−1) | Time (min) | Eff.% | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cr-MIL-101@NiO/13X | Ultrasonic | MB | 40 | 25 | 0.5 | 80 | 97.2 | 26 |
| 2 | Cr-MIL-101@NiO/13X | Ultrasonic | RhB | 40 | 25 | 0.5 | 80 | 94.3 | 26 |
| 3 | TiO2@CNTs/AgNPs/C10 | Visible light | MB | 0.782 mol L−1 | 20 | 0.5 | 120 | 100 | 46 |
| 4 | Fe3O4 | Ultrasonic | RhB | 40 | 0.02 | 0.5 | 60 | 90 | 47 |
| 5 | Fe3O4/Al-B | Visible light | RhB | 50 | 40 | 0.5 | 300 | 95 | 48 |
| 6 | SCN/CoFe2O4 | Ultrasonic | MB | 4 vol% | 25 | 1 | 20 | 83 | 49 |
| 7 | SCN/CoFe2O4 | Ultrasonic | RhB | 4 vol% | 25 | 1 | 20 | 20 | 49 |
| 8 | CoFe2O4@Cr-MIL-101/Y | Ultrasonic | MB | 40 | 25 | 0.5 | 60 | 98.9 | This work |
| 9 | CoFe2O4@Cr-MIL-101/Y | Ultrasonic | RhB | 40 | 25 | 0.5 | 60 | 94.6 | This work |
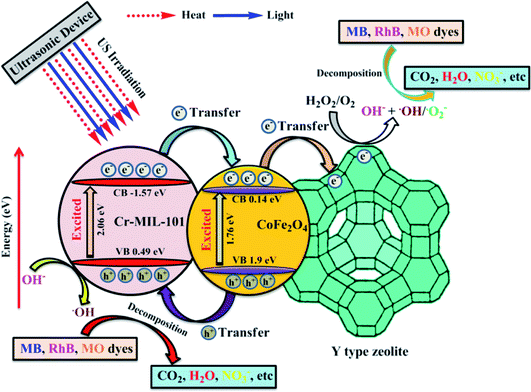
| (αhv)2 = A(hv − Eg) | (5) |
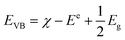 | (6) |
| ECB = EVB − Eg | (7) |
| US irradiation → heat–light (ref. 18) | (8) |
| CoFe2O4/Cr-MIL-101 + heat or light → (CoFe2O4/Cr-MIL-101)* (ref. 27) | (9) |
| (CoFe2O4)* → CoFe2O4 (hVB+) + CoFe2O4 (eCB−) (ref. 27) | (10) |
| (Cr-MIL-101)* → Cr-MIL-101 (hVB+) + Cr-MIL-101 (eCB−) (ref. 27) | (11) |
| Cr-MIL-101 (eCB−) + CoFe2O4 → Cr-MIL-101 + CoFe2O4 (eCB−) (ref. 27) | (12) |
| CoFe2O4 (eCB−) + Y → CoFe2O4 + Y (eCB−) (ref. 51) | (13) |
| Y (eCB−) + H2O2 and O2 → Y + OH− + ˙OH and ˙O2− (ref. 51) | (14) |
| Cr-MIL-101 (hVB+) + H2O/OH− → Cr-MIL-101 + ˙OH (ref. 27) | (15) |
| Dyes + ˙OH and ˙O2− → decomposition products | (16) |
4. Conclusion
For the very first time, we report a simple hydrothermal route to prepare the CoFe2O4@Cr-MIL-101/Y zeolite as a new magnetic catalyst nanocomposite. The morphological, structural and magnetic properties of the as-fabricated catalyst was characterized by several different techniques including FTIR, XRD, FESEM, EDS, EDS dot-mapping, TEM, AFM, VSM, and BET. The sonocatalytic decomposition experiments of cationic and anionic organic dyes contaminants from water solution over the CoFe2O4@Cr-MIL-101/Y were accomplished in the presence of H2O2 as the hydroxyl radial (˙OH) origin. The influence of various parameters like irradiation time, initial dye concentration, catalyst dosage, H2O2 concentration, scavenger type, process type, and catalyst regeneration over the decomposition of MB, RhB and MO were evaluated. The experiments data from UV-Vis analysis showed the maximum sonodecomposition efficiency of 98.9%, 94.6% and 82% for MB, RhB and MO in the presence of US/H2O2/CoFe2O4@Cr-MIL-101/Y system, respectively. Besides, the reaction kinetics study of MB was fulfilled based on the first order model. The values of rate constant (k) and half-life (t1/2) were determined as 0.0675 min−1 and 10.2666 min, respectively. In consequence, it is deduced that the CoFe2O4@Cr-MIL-101/Y exhibits particular and superior functionality to decompose the organic dyes compared to other catalysts such as Cr-MIL-101/Y, Y zeolite, Cr-MIL-101 and CoFe2O4.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors sincerely acknowledge all of the supports from Lorestan University, Khorramabad, Iran.References
- X. Liu, Y. Guan, Z. Ma and H. Zhou, Langmuir, 2004, 20, 10278–10282 CrossRef CAS PubMed.
- V. K. Gupta and Suhas, J. Environ. Manage., 2009, 90, 2313–2342 CrossRef CAS PubMed.
- H. S. Rai, M. S. Bhattacharyya, J. Singh, T. K. Bansal, P. Vats and U. C. Banerjee, Crit. Rev. Environ. Sci. Technol., 2005, 35, 219–238 CrossRef CAS.
- S. Sadettin and G. Dönmez, Process Biochem., 2006, 41, 836–841 CrossRef CAS.
- B. J. Brüschweiler and C. Merlot, Regul. Toxicol. Pharmacol., 2017, 88, 214–226 CrossRef PubMed.
- R. O. Alves de Lima, A. P. Bazoa, D. M. Fávero Salvadori, C. M. Rechb, D. de Palma Oliveira and G. de Aragão Umbuzeiro, Mutat. Res., 2007, 626, 53–60 CAS.
- D. P. Oliveira, P. A. Carneiro, M. K. Sakagami, M. V. B. Zanoni and G. A. Umbuzeiro, Mutat. Res., 2007, 626, 135–142 CAS.
- M. A. Behnajady, N. Modirshahla, S. Bavili Tabrizi and S. Molanee, J. Hazard. Mater., 2008, 152, 381–386 CrossRef CAS PubMed.
- Y. Zhang, L. Liang, Y. Chen, X. Chena and Y. Liu, Soft Matter, 2019, 15, 73–77 RSC.
- Y. Qin, M. Long, B. Tan and B. Zhou, Nano-Micro Lett., 2014, 6, 125–135 CrossRef CAS.
- M. Sboui, M. F. Nsib, A. Rayes, M. Swaminathan and A. Houas, J. Environ. Sci., 2017, 60, 3–13 CrossRef PubMed.
- H. Eslami, S. S. Khavidak, F. Salehi, R. Khosravi, R. A. Fallahzadeh, R. Peirovi and S. Sadeghi, J. Adv. Environ. Health Res., 2017, 5, 10–15 CAS.
- M. A. El Hajj Hassan and M. M. El Jamal, Port. Electrochim. Acta, 2012, 30, 351–359 CrossRef CAS.
- H. Ghodbane and O. Hamdaoui, Ultrason. Sonochem., 2009, 16, 593–598 CrossRef CAS PubMed.
- L. Nirumand, S. Farhadi, A. Zabardastia and A. Khataee, J. Taiwan Inst. Chem. Eng., 2018, 93, 674–685 CrossRef CAS.
- A. Khataee, M. Sheydaei, A. Hassani, M. Taseidifar and S. Karaca, Ultrason. Sonochem., 2015, 22, 404–411 CrossRef CAS PubMed.
- S. Li, C. Wei, J. Wang, L. Zhang, Y. Li, Y. Li and B. Wang, Mater. Sci. Semicond. Process., 2014, 26, 438–447 CrossRef CAS.
- S. Farhadi, F. Siadatnasab and A. Khataee, Ultrason. Sonochem., 2017, 37, 298–309 CrossRef CAS PubMed.
- G. Wang, Y. Huang, G. Li, H. Zhang, Y. Wang, B. Li, J. Wang and Y. Song, Ultrason. Sonochem., 2017, 38, 335–346 CrossRef CAS PubMed.
- S. Farhadi and F. Siadatnasab, Chin. J. Catal., 2016, 37, 1487–1495 CrossRef CAS.
- Y. Min, K. Zhang, Y. Chen and Y. Zhang, Ultrason. Sonochem., 2012, 19, 883–889 CrossRef CAS PubMed.
- J. Wang, Y. H. Lv, L. Q. Zhang, B. Liu, R. Z. Jiang, G. X. Han, R. Xu and X. D. Zhang, Ultrason. Sonochem., 2010, 17, 642–648 CrossRef CAS PubMed.
- R. Darvishi, C. Soltani, M. Safari and M. Mashayekhi, Ultrason. Sonochem., 2016, 30, 123–131 CrossRef PubMed.
- T. Li, L. Song and S. Zhang, Environ. Sci. Pollut. Res., 2018, 25, 7937–7945 CrossRef CAS PubMed.
- Y. Wu, L. Song, S. Zhang, X. Wu, S. Zhang, H. Tian and J. Ye, Catal. Commun., 2013, 37, 14–18 CrossRef CAS.
- M. Sadeghi, A. Zabardasti, S. Farhadi, S. Yekta and D. Mirzaei, J. Water Process Eng., 2019, 32, 100946 CrossRef.
- H. T. M. Thanh, N. T. T. Tu, N. P. Hung, T. N. Tuyen, T. X. Mau and D. Q. Khieu, J. Porous Mater., 2019, 26, 1699–1712 CrossRef CAS.
- M. L. Díaz-Ramírez, E. Sánchez-González, J. R. Álvarez, G. A. González-Martínez, S. Horike, K. Kadota, K. Sumida, E. González-Zamora, M. Springuel-Huet, A. Gutiérrez-Alejandre, V. Jancik, S. Furukawa, S. Kitagawa, I. A. Ibarra and E. Lima, J. Mater. Chem. A., 2019, 7, 15101–15112 RSC.
- X.-L. Liu, R. Wang, M.-Y. Zhang, Y.-P. Yuan and C. Xue, APL Mater., 2015, 3, 104403–104407 CrossRef.
- H. B. Tanh Jeazet, T. Koschine, C. Staudt, K. Raetzke and C. Janiak, Membranes, 2013, 3, 331–353 CrossRef PubMed.
- H. Belarbi, L. Boudjema, C. Shepherd, N. Ramsahye, G. Toquerd, J. Chang and P. Trens, Colloids Surf., A, 2017, 520, 46–52 CrossRef CAS.
- E. Niknam, F. Panahi, F. Daneshgar, F. Bahrami and A. Khalafi-Nezhad, ACS Omega, 2018, 3, 17135–17144 CrossRef CAS PubMed.
- M. Sadeghi, S. Yekta, H. Ghaedi and E. Babanezhad, Mater. Chem. Phys., 2017, 197, 113–122 CrossRef CAS.
- M. Dehghani, A. Tadjarodi and S. Chamani, ACS Omega, 2019, 4, 10640–10648 CrossRef CAS PubMed.
- M. Sadeghi, S. Yekta, D. Mirzaei, A. Zabardasti and S. Farhadi, J. Inclusion Phenom. Macrocyclic Chem., 2019, 93, 215–227 CrossRef CAS.
- H. Ramezani, S. N. Azizi and S. R. Hosseini, Sens. Actuators, B, 2017, 248, 571–579 CrossRef CAS.
- S. M. Moosavi, P. Molla-Abbasi and Z. Haji-Aghajani, J. Mater. Sci.: Mater. Electron., 2016, 5, 27–32 Search PubMed.
- P. Sonu, V. Dutta, S. Sharma, P. Raizada, A. Hosseini-Bandegharaei, V. K. Gupta and P. Singh, J. Saudi Chem. Soc., 2019, 23, 1119–1136 CrossRef.
- M. Amiri, A. Akbari, M. Ahmadi, A. Pardakhti and M. Salavati-Niasari, J. Mol. Liq., 2018, 249, 1151–1160 CrossRef CAS.
- A. Skumiel, J. Magn. Magn. Mater., 2006, 307, 85–90 CrossRef CAS.
- A. Hannour, D. Vincent, F. Kahlouche, A. Tchangoulian, S. Neveu and V. Dupuis, J. Magn. Magn. Mater., 2014, 353, 29–33 CrossRef CAS.
- B. Abraime, A. Mahmoud, F. Boschini, M. Ait Tamerd, A. Benyoussef, M. Hamedoun, Y. Xiao, A. El Kenz and O. Mounkachi, J. Magn. Magn. Mater., 2018, 467, 129–134 CrossRef CAS.
- F. Sadri, A. Ramazani, A. Massoudi, M. Khoobi, V. Azizkhani, R. Tarasi, L. Dolatyari and B.-K. Min, Bull. Korean Chem. Soc., 2014, 35, 2029–2032 CrossRef CAS.
- R. Tabit, O. Amadine, Y. Essamlali, K. Dânoun, A. Rhihila and M. Zahouily, RSC Adv., 2018, 8, 1351–1360 RSC.
- M. Rasouli, N. Yaghobi, S. Chitsazan and M. H. Sayyar, Chem. Eng. Res. Des., 2012, 90, 1407–1415 CrossRef CAS.
- E. M. S. Azzam, N. A. Fathy, S. M. El-Khouly and R. M. Sami, J. Water Process Eng., 2019, 28, 311–321 CrossRef.
- N. Wang, L. Zhu, M. Wang, D. Wang and H. Tang, Ultrason. Sonochem., 2010, 17, 78–83 CrossRef CAS PubMed.
- W. Li, D. Wan, G. Wang, L. Lu and X. Wei, Water Sci. Technol., 2016, 73, 2345–2352 CrossRef CAS PubMed.
- S. Kamal, G.-T. Pan, S. Chong and T. C.-K. Yang, Processes, 2020, 8, 104 CrossRef.
- S. Singh and N. Khare, RSC Adv., 2015, 5, 96562–96572 RSC.
- A. A. Hoseini, S. Farhadi, A. Zabardastiand and F. Siadatnasab, RSC Adv., 2019, 9, 24489–24504 RSC.
- M. Zendehdel, Z. Kalateh and Z. Mortezaii, J. Nov. Appl. Sci., 2014, 3, 135–141 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00877j |
| This journal is © The Royal Society of Chemistry 2020 |