Open Access Article
Open Access ArticleDevelopment and characterization of a babassu nut oil-based moisturizing cosmetic emulsion with a high sun protection factor
Michael Jackson Ferreira da Silvaa,
Alisson Mendes Rodrigues b,
Italo Rennan Sousa Vieira
b,
Italo Rennan Sousa Vieira c,
Gelmires de Araújo Nevesb,
Romualdo Rodrigues Menezes
c,
Gelmires de Araújo Nevesb,
Romualdo Rodrigues Menezes b,
Eloisa da Graça do Rosário Gonçalvesd and
Maria Célia Costa Pires
b,
Eloisa da Graça do Rosário Gonçalvesd and
Maria Célia Costa Pires *e
*e
aUniversidade Federal do Maranhão, Programa de Pós-Graduação em Saúde e Ambiente, Campus do Bacanga, 65085-580, São Luís, MA, Brazil
bUniversidade Federal de Campina Grande, Centro de Ciências e Tecnologia, Unidade Acadêmica de Engenharia de Materiais, 58109-970, Campina Grande, PB, Brazil
cUniversidade do Estado do Rio de Janeiro, Instituto de Química, Pavilhão Reitor Haroldo Lisboa da Cunha, Maracanã, 20550-900, Rio de Janeiro, RJ, Brazil
dUniversidade Federal do Maranhão, Departamento de Patologia, Centro de Ciências da Saúde, Campus do Bacanga, 65085-580, São Luís, MA, Brazil
eUniversidade Estadual do Maranhão, Departamento de Química, Campus Universitário Paulo VI, 65055-970, São Luís, MA, Brazil. E-mail: celiacosta@prof.elointernet.com.br
First published on 13th July 2020
Abstract
A stable moisturizing cosmetic emulsion was developed from babassu nut oil and high concentrations of sunscreens. Babassu nut oil was chosen because within the laboratory time-scale, this vegetable oil showed stable physicochemical properties (relative density, acidity index, and refracted index) and a good ratio between lauric and myristic fatty acids. The presence of these saturated fatty acids can confer specific activities to the cosmetic emulsion, such as antiviral, bactericidal, and anti-inflammatory activity. The prepared cosmetic emulsion, even after the centrifugation test (3000 rpm for 15 min), showed a creamy appearance with stable light-yellow coloration and the typical odor of babassu nut oil-based products. In the accelerated stability assays (pH, viscosity, and globule homogeneity), the cosmetic emulsion was kept at different temperatures (2.0 ± 0.2 °C (TG), 25 ± 2 °C (TA), and 40 ± 2 °C (TE)) and time durations (24 hours (t0), 7 days (t7), 15 days (t15), and 30 days (t30)). Finally, developed the cosmetic emulsion was investigated for occlusive properties and in vitro sun protection factor (SPF). Both were measured at room temperature and did not change significantly under the experimental conditions employed. The maximum experimental value measured in the in vitro occlusive test was equal to 34.2 ± 2.8, and the SPF was 39 ± 1.6 (t0) and 38 ± 2.9 (t30). In agreement with European and Brazilian legislations, the obtained babassu oil-based cosmetic emulsion is classified to have a high sun protection factor.
1 Introduction
Alterations in the components of the stratum corneum (SC), such as keratinocytes, fatty acids, lipid content, pH, and aquaporins, can lead to severe dehydration of the skin, giving it a rough and scaly appearance with a consequent loss of mechanical properties.1–3 Exogenous and endogenous factors are among the leading causes of these SC components alterations.4,5 Among the exogenous factors, climatic conditions (drought, cold, heat, sun exposure, etc.), environmental factors (central heating, air conditioning, etc.), and personal habits (excessive skin washing, cosmetics, etc.) are the most common. Hormonal imbalances such as menopause that causes changes in the lipids and glycosaminoglycans of skin, drugs such as retinoids, hypolipidemic agents, cimetidine, clofazimine, and diseases such as leprosy are the major endogenous factors.Therefore, the cosmetics industry seeks to develop emulsions with the potential to prevent and/or treat skin disorders.6,7 These cosmetic emulsions are topically administered and produced from emollient substances (substances capable of filling up the intercorneocytic slits, retaining water in this layer), humectants (which increase the water content in the epidermis), and occlusive substances (decreasing transepidermal water loss). Besides, these cosmetic emulsions allow the incorporation of other substances such as plant extracts and vitamins that provide photoprotective and antioxidative effects because they contain unsaturated fatty acids and lipids in their composition; vegetable oils are excellent emollients because, in addition to assisting in hydration, transepidermal reduction, and occlusive effect, they may also contain active substances responsible for the therapeutic effects. The main vegetable oils used as emollient substances in moisturizing emulsions are pequi fruit oil (Caryocar brasiliense),8 acai berry oil (Euterpe oleracea),9 babassu oil (Attalea speciosa),10 and sunflower oil (Helianthus annuus).11
Babassu oil is extracted from babassu nut and represents approximately 65% of the total almond mass. Babassu coconut is the generic name given to the fruit harvested from oil palm trees belonging to the Palmae family and members of the genera Orbignya and Attalea. This palm tree is found in Northeastern Brazil, Bolivia, and Suriname. Previous studies12–15 have shown that the babassu oil is composed of fatty acids such as lauric (44.0–46.0%), myristic (15.0–20.0%), oleic (12.0–18.0%), palmitic (6.0–9.0%), stearic (6.0%), caprylic (4.0–6.5%), capric (2.7–7.5%), caproic (0.2%), and arachidic (0.2–0.7%). The presence of saturated fatty acids in the composition of babassu oil, especially lauric and myristic, give this oil pharmacological (analgesic and anti-inflammatory), nutritive, hygienic, and cleansing (soap production) properties. The presence of unsaturated acids in the babassu coconut oil composition gives it a longer time of stability, thus favoring its long term storage.14,16
In this work, due to its compatibility with human skin, physicochemical properties, and the fatty acid composition, unfiltered babassu nut oil extracted by the cold pressing method has been used to develop a cosmetic emulsion. Unfiltered babassu nut oil was characterized in terms of acidity index, relative density, and refractive index. The fatty acid composition was determined by gas chromatography coupled to mass spectrometry (GC-MS). The development of babassu nut oil-based emulsion was carried out according to the emulsion inversion phase method.17,18 The cosmetic emulsion obtained was subjected to preliminary (centrifugation) and accelerated stability tests (pH, viscosity, and globule homogeneity), occlusion properties, and sun protection factor tests in vitro.
2 Materials and methods
2.1 Materials
Babassu nut oil was supplied by the Association of Babassu Coconut Breakers located in Penalva city, Maranhão state, Brazil. The cold-pressing method was used to extract the oil from babassu nuts. No filtration method was applied to the babassu oil used in this research. The other chemical reagents used for the preparation of the emulsion were cetostearyl alcohol ethoxylate, ammonium acryloyldimethyltaurate/VP copolymer, butylated hydroxytoluene (BHT), a blend of parabens, and phenoxyethanol (all purchased from Facial Pharmacy Compounding LTDA-ME). Also, the following sunscreens were used: benzophenone-3 (BZF-3), octyl methoxycinnamate (OMC), octocrylene (OCT), and diethylamino hydroxy benzoyl hexyl benzoate (DHHB) (all purchased from Facial Pharmacy Compounding LTDA-ME).2.2 Characterization of unfiltered babassu nut oil
The fatty acid profile was obtained using a Shimadzu GC gas chromatograph coupled to a Shimadzu mass spectrometer (model CGMS-QP2010) equipped with a ZBFFAP capillary column (30 m long × 0.25 mm i.d × 0.25 mm film thickness) polyethylene glycol (PEG) phase. The temperature was maintained at 120 °C for 2 min, heating ramp up to 180 °C (10 °C min−1), new heating ramp up to 230 °C (5 °C min−1), and maintained at the final temperature for 3 min more. The injector and detector temperatures were both 250 °C, the flow rate of helium (He) carrier gas was 1.60 mL min−1, and 50.0 splits.
2.3 Preparation of the cosmetic emulsion
The cosmetic emulsion was prepared by the phase inversion method17,18 with the components described in Table 1. For this, the aqueous phase and oil phase were heated separately at 75 °C. Then, the aqueous phase was dropped on the oil phase under constant agitation (1200 rpm) at 25 °C until complete homogenization. The sunscreens (BZF-3, OMC, OCT, and DHHB), which were called phase C, were added during the homogenization.| Reagents | Quantity (wt%) | Function |
|---|---|---|
| Aqueous phase A | ||
| Ammonium acryloyldimethyltaurate/VP copolymer | 0.6 | Stability and consistency |
| Distilled water | qsp(1). 100 | Vehicle |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Oil phase B | ||
| Cetostearyl alcohol ethoxylate | 6 | Emulsifier |
| Parabens and phenoxyethanol | 0.3 | Preservative |
| Babassu nut oil | 10 | Emollient active |
| BHT | 0.1 | Antioxidant |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Phase C | ||
| BZF-3 | 5 | UVA and UVB sunscreen |
| OMC | 10 | UVB sunscreen |
| OCT | 8 | UVA and UVB sunscreen |
| DHHB | 5 | UVA sunscreen |
2.4 Stability assays
100 mg of the cosmetic emulsion was allocated to poly(ethylene terephthalate) jars and kept at different temperatures (room temperature, 2 °C (freezer), and estufa 40 °C) for different time durations (24 hours-t0, 7 days-t7, 15 days-t15, and 30 days-t30). After that, the following parameters were evaluated, namely, phase separation after centrifugation, pH, viscosity, and globule homogeneity. All the measurements were performed in triplicate.2.5 Occlusion factor
30 mL of distilled water was added to a glass cup (40 mL volume and diameter of 4.6 cm) and covered with a qualitative cellulose filter paper (Whatman, number 6, 90 mm diameter). The cosmetic emulsion samples were spread on the cellulose filter paper surface (13.3 mg cm−2) and stored in an oven at 40 °C for different time durations (6, 24, and 48 hours). A glass cup covered with a cellulose filter paper without the samples was used as the blank. The occlusion factor (F) was calculated with the aid of eqn (1):21,22
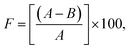 | (1) |
2.6 In vitro sun protection factor (SPF) assessment
In vitro SPF measurements were accomplished using a UV transmittance analyzer (Labsphere® UV-2000 S) and quartz plates with an area of 25 cm2 covered by a Transpore™ tape. A certain amount (1.2 mg cm−2) of the cosmetic emulsion was deposited on the support and manually spread to obtain a uniform film layer. Vaseline was spread in the support as the reference for 100% of transmission. SPF, UVA/UVB ratio, and critical wavelength (λc) results were determined in triplicate.233 Results and discussion
3.1 Physicochemical parameters
For industrial purposes, it is essential that the physicochemical properties of unfiltered babassu nut oil, such as the acidity index, relative density, and refractive index, do not change significantly after extraction and in the subsequent storage. A marked change in the physicochemical properties can be a consequence of the decrease in the amount of saturated fatty acids present in the oil; therefore, a reduction in the occlusive and emollient potential of the cosmetic emulsion can occur. Table 2 compares the acidity index, relative density, and refractive index measured from the unfiltered babassu nut oil with standard values founded on Codex Alimentarius.24| Parameters | UBO (this work) | Codex Alimentarius24 |
|---|---|---|
| Acidity index (mg KOH per g oil) | 3.582 ± 0.084 | Up to 4.0 |
| Relative density (g mL−1) at 25 °C ref. water 25 °C | 0.923 ± 0.001 | 0.914–0.917 |
| Refracted index (nD40) | 1.447 ± 0.001 | 1.448–1.451 |
The acidity index measured from the UBO sample was 3.582 ± 0.084 mg KOH per g oil, which is in agreement with Codex Alimentarius since the value maximum allowed is up to 4.0 mg per gram of vegetable oil. It is known that this parameter reflects the amount of free fatty acids present in the UBO samples. A high acidity value suggests the presence of hydrolysis and oxidation reactions at a level that is high enough to initiate the decomposition of the fatty acids.25 In vegetable oils, hydrolysis and oxidation reactions are catalyzed by lipase enzymes, which are synthesized on exposure to light and heat. Free fatty acids are the products of these reactions and can modify the acidity index and some organoleptic properties, i.e., taste and odor.26,27 During the extraction and storage, no changes were observed in the color, taste, and smell of unfiltered babassu nut oil.
The refractive index measured for the UBO (1.447 ± 0.000 nD40) is within the standards set by the Codex Alimentarius (1.448–1.451 nD40). The refractive index varies with the fatty acid chain length, molecular weight, and saturation level, and similar to the relative density, it is a physical–chemical parameter that determines the oil identity and can serve as a quality control. The relative density measured for UBO was 0.923 ± 0.001 g mL−1, which is slightly above the standard value (0.914–0.917 g mL−1).24 Oliveira et al.13 studied the oil extracted from Orbignya spp. and also reported a high relative density value compared to CODEX. This behavior is probably related to the characteristic of the species itself, as it has a high index of saturated compounds (see Table 3).
| Fatty acids | Codex Alimentarius (2015) | |
|---|---|---|
| Fatty acids | % (Fatty acid) | |
| UBO | ||
| C 10:0 (capric) | 7.29 | 1.2–7.6 |
| C 12:0 (lauric) | 26.31 | 40.0–55.0 |
| C 14:0 (myristic) | 19.93 | 11.0–27.0 |
| C 16:0 (palmitic) | 12.79 | 5.2–11.0 |
| C 18:0 (stearic) | 5.60 | 1.8–7.4 |
| C 18:1 (oleic) | 18.83 | 9.0–20.0 |
| C 18:2 (linoleic) | 2.38 | 1.4–6.6 |
| Other | — | — |
| Σ saturated | 71.92 | 61.90–115.4 |
| Σ monounsaturated | 18.83 | 9.0–20.0 |
| Σ polyunsaturated | 2.38 | 1.4–6.6 |
3.2 Fatty acid composition
The percentage of fatty acids in unfiltered babassu nut oil was determined from the transesterification reactions and analysed by the chromatographic elution profile obtained by GC-MS. Table 3 summarizes the main fatty acids (capric, lauric, myristic, palmitic, stearic, oleic, and linoleic) identified in unfiltered babassu nut oil. The lauric and myristic fatty acids presented the most significant percentages, viz., 26.31% and 19.93%, respectively. The presence of lauric acid gives antiviral, bactericidal, and anti-inflammatory properties to unfiltered babassu nut oil,28–30 and consequently, to the cosmetic emulsion. On the other hand, myristic acid is used as a cleansing, surfactant, and opacifying agent in cosmetics and personal care products.12,13 These fatty acids can also be found in other vegetable sources such as coconut oil, linseed oil, grape seed oil, and palm oil.24,31 However, their concentration in unfiltered babassu nut oil is more balanced (see Fig. 1). Besides the above-mentioned fatty acids, it also was possible to obtain the concentration of the monounsaturated (18.83%) and polyunsaturated fatty acids (2.38%) (see Table 3).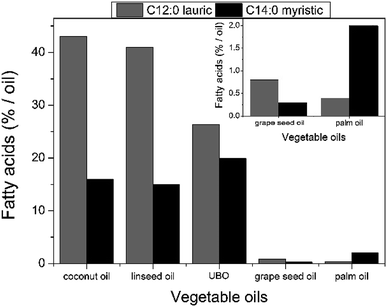 | ||
| Fig. 1 Comparison of the lauric and myristic fatty acids percentages measured in unfiltered babassu nut oil with those measured in other vegetable oils.33 | ||
3.3 Characterization of the unfiltered babassu nut oil-based emulsion
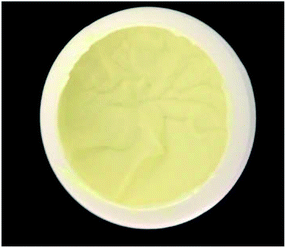
As mentioned in Section 2, the cosmetic emulsion was prepared with the aid of the phase-inversion method.17,18 After 24 hours of preparation, the cosmetic emulsion was subjected to a centrifugation test (3500 rpm for 15 min (ref. 32)). The purpose of this test is to monitor the physical instabilities, such as creaming and phase separation. The centrifugation test was carried out in triplicate and no evidence of the physical instabilities was observed. It is important to note that in the babassu nut oil-cosmetic emulsion, the separation of the phases is one of the most important phenomena to be monitored since it can affect important properties such as pH, viscosity, and occlusion.33 Finally, it is essential to highlight that the creamy appearance, light-yellow coloration, and the typical odor of babassu nut oil products (Fig. 2) observed in the developed cosmetic emulsion in this work were stable throughout the analysis period (90 days).3.3.1.1 pH test. Marked changes in the pH of the stratum corneum can damage the skin defense mechanism against infection, thus influencing the cutaneous bacterial flora.34,35 Therefore, if the cosmetic emulsion topically administered will significantly change the pH of the skin, it also will affect its homeostasis. Table 4 shows the pH values measured from the UBO samples kept at different temperatures (2.0 ± 0.2 °C (TG), 25 ± 2 °C (TA), and 40 ± 2 °C (TE)) and durations (24 hours, 7 days, 15 days, and 30 days (t0, t7, t15, and t30, respectively)). For all the temperatures and times investigated, the pH values measured were stable, indicating that hydrolysis and oxidation reactions did not occur at the laboratory time-scale employed. The minimum and maximum emulsion pH values (4.686 ± 0.02 and 5.136 ± 0.06, respectively) were observed at room temperature for 30 days (TA experimental condition). These pH values are in agreement with those found for human skin, which can vary between 4.6 to 5.8, depending on the body region studied.34
| Days | TA | TG | TE |
|---|---|---|---|
| T0 | 4.686 ± 0.02 | — | — |
| T7 | 4.835 ± 0.03 | 4.724 ± 0.01 | 4.798 ± 0.02 |
| T15 | 4.922 ± 0.02 | 4.722 ± 0.01 | 4.771 ± 0.00 |
| t30 | 5.136 ± 0.06 | 4.701 ± 0.02 | 4.770 ± 0.02 |
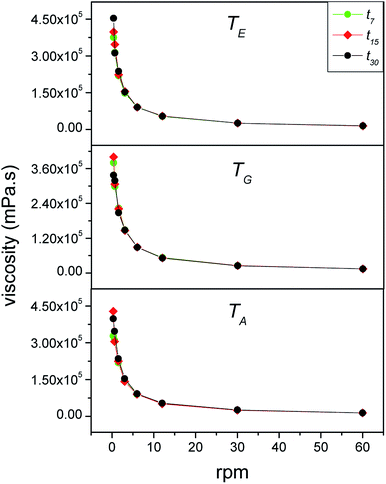
3.3.1.2 Viscosity measurements. Fig. 3 shows the rotation speed-dependence of the viscosity of the babassu nut oil-based cosmetic emulsion at different temperatures (TG, TA, and TE) and time durations (t0, t7, t15, and t30). For all the time durations investigated, the viscosity decreases with the rotation speed. This behavior is characteristic of non-Newtonian fluids, more precisely, the thixotropic type. For cosmetic emulsions, thixotropic behavior is required because the products become more fluid when subjected to external pressure, spreading easily and recovering the initial viscosity after application, which prevents the product from dripping.
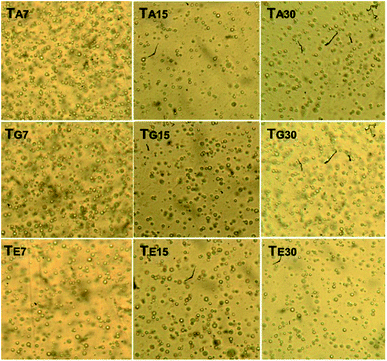
3.3.1.3 Globule homogeneity. Fig. 4 shows the optical micrographs (40×) captured from the babassu nut oil-based cosmetic emulsion at different experimental conditions. The oil globules observed were small and homogeneously dispersed on the water phase. This is a positive behavior; according to Vieira et al.,10 the smaller and more homogeneous the globules, the higher the tendency to form a stable emulsion. Emulsions with a large number of small globules are highly efficient as pharmaceuticals and/or cosmetics.10,36
By qualitatively analyzing Fig. 4, it is possible to see that the size of the globules immersed in the aqueous phase does not vary significantly over time or temperature. Regarding the number of globules immersed in the aqueous phase, the TA7 and TG7 samples showed a higher number than the TE7 sample, indicating that higher temperatures negatively influence the density of the globules. However, all the samples showed similar numbers of globules for the time duration of 30 days. It is important to note that this variation in the number of globules did not affect the creamy appearance, permanent light-yellow coloration, the typical odor of babassu nut oil products, pH, and viscosity. Therefore, in this case, the variation in the number of oil globules immersed in the aqueous phase was not enough to affect the stability of the developed cosmetic emulsion.
3.4. Occlusive properties
The in vitro occlusive test was accomplished to verify the skin hydration potential of the babassu nut oil-based cosmetic emulsion. Fig. 5 shows the occlusive test results measured for the cosmetic emulsion samples kept at room temperature for different time durations (6, 24, and 48 hours). The results show that there was no significant difference in the occlusive properties under the investigated experimental conditions. The occlusive potential of a cosmetic emulsion is based on the formation of a film after application on skin and strongly depends on the concentration of fatty acids, size of the globules, volume of the applied sample, and type of colloidal system.21,33 The cosmetic emulsion developed in this work has a potential emollient characteristic since the active ingredient (babassu nut oil) is rich in fatty acids (see Table 3). As described by Costa et al.,37 emollients are rich in substances capable of “filling the cracks” and are interorneocytic, i.e., they retain water in the layer.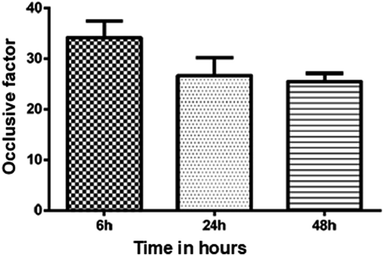 | ||
| Fig. 5 The in vitro occlusive results measured for the cosmetic emulsions kept at room temperature for 6, 24, and 48 hours; (p = 0.1249), ANOVA (p < 0.05). | ||
3.5 SPF assessment
As described before, sunscreens (BZF-3, OMC, OCT, and DHHB) were added to the babassu nut oil-based cosmetic emulsion during the homogenization step. In vitro SPF experiments were performed with the samples kept at room temperature for 24 hours and for other different time durations (see Table 5). As already seen for the accelerated stability assays and occlusive factor, the in vitro SPF values also did not change under the investigated experimental conditions, and the values were 39 ± 1.6 and 38 ± 2.9 at t0 and t30, respectively. In agreement with the SPF categories claimed in Europe and Brazilian legislation, the babassu nut oil-based cosmetic emulsion developed in this work is classified to have a high protection SPF.23,38,39 It is important to note that the SPF measured is mainly the result of sunscreens added to the cosmetic emulsion (phase C, see Table 1). A cosmetic emulsion with a high SPF value is recommended for skin disease treatments (with damaged stratum corneum) since it protects against UVA and UVB rays, thus avoiding harmful effects such as skin irritation, burns, and cancer.As shown in Table 5, the UVA/UVB ratio was 0.521 and 0.561 for the t0 and t30 samples, respectively. The UVA/UVB ratio is an indication of the UVA protection (anti-UVA).40 Therefore, in addition to UVB protection, the formulation showed satisfactory UVA protection values, which were classified as commercially acceptable since they are within the range (0.4 < UVA/UVB < 0.6).38,40 UVB rays are partially absorbed by the ozone layer and have a mean wavelength (λ = 290–320 nm).
3.6 Protection mechanism of the skin via babassu nut oil-based cosmetic emulsion
As babassu nut oil has stable physico-chemical properties during the extraction and storage processes, the cosmetic emulsion developed in this work has the potential to be used in topical treatments for human skins that have compromised their ability to protect and hydrate. Fig. 6 shows the cross-sectional view of human skin (stratum corneum, epidermis, and dermis) with and without the application of the cosmetic emulsion based on babassu nut oil. In the region where the cosmetic emulsion was topically applied (stratum corneum), it is possible to identify greater hydration, while in the region without the cosmetic emulsion, there was a severe loss of water. The greater hydration observed in the region treated with the cosmetic emulsion is the result of a sum of factors, such as the occlusive potential, moisturization, and protection against UVA and UVB rays. Another positive characteristic that unfiltered babassu nut oil gives to the cosmetic emulsion is the emollient capacity.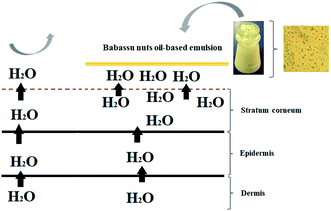 | ||
| Fig. 6 Mechanism of action of the cosmetic emulsion based on babassu coconut oil applied on human skin. | ||
4 Conclusions
A stable cosmetic emulsion with a high sun protection factor was developed from unfiltered babassu nut oil. The physicochemical properties (acidity index, relative density, and refractive indexes) of unfiltered babassu oil did not change during the laboratory time-scale; therefore, it was considered acceptable to develop a commercially competitive cosmetic emulsion. Also, the balanced amounts of lauric and myristic fatty acids identified in the oil sample give it potential antiviral, bactericidal, and anti-inflammatory activities. The cosmetic emulsion developed has a creamy appearance with a light yellow color and a typical odor of the active base (babassu nut oil). The accelerated stability tests showed that the developed cosmetic emulsion has a pH range of 4.686 ± 0.02 and 5.136 ± 0.06, thixotropic viscosity, and small oil globules homogeneously dispersed in the aqueous phase. The occlusive factor of the cosmetic emulsion was measured at different temperatures and time durations, and the maximum value obtained was approximately 34.2 ± 2.8. The in vitro SPF values were 39 ± 1.6 and 38 ± 2.9, which confer competitive photoprotective factor to the cosmetic emulsion developed.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by financial support in the purchase of equipment from the Foundation for Research and Scientific and Technological Development of Maranhão (FAPEMA No. 40/2014 – UNIVERSAL/2015). From the Macromolecules and Natural Products laboratories of the State University of Maranhão to the Biofuels, Catalysis and Environmental Center of the Federal University of Maranhão and the Galenic Development Laboratory of the Federal University of Rio de Janeiro.References
- T. M. Weber, M. Kausch, F. Rippke, A. M. Schoelermann and A. W. Filbry, Treatment of xerosis with a topical formulation containing glyceryl glucoside, natural moisturizing factors, and ceramide, Journal of Clinical and Aesthetic Dermatology, 2012, 5, 29–39 Search PubMed.
- J. Parker, R. Scharfbillig and S. Jones, Moisturisers for the treatment of foot xerosis: a systematic review, Journal of Foot and Ankle Research, 2017, 10, 9, DOI:10.1186/s13047-017-0190-9.
- F. Boralevi, A.-P. Meledie N'Djong, P. Yao Yoboue, O. Faye, M. T. Dieng, S. Coniquet, F. Atadokpede, P. A. Niamba, A. Delarue and C. Cazeau, Regression of cutaneous xerosis with emollient treatment in sub-Saharan African patients, International Journal of Dermatology, 2017, 56, 467–473, DOI:10.1111/ijd.13454.
- A. Pons-Guiraud, Dry skin in dermatology: a complex physiopathology, Journal of the European Academy of Dermatology and Venereology, 2007, 21, 1–4, DOI:10.1111/j.1468-3083.2007.02379.x.
- C. Paul, S. Maumus-Robert, J. Mazereeuw-Hautier, C. N. Guyen, X. Saudez and A. M. Schmitt, Prevalence and risk factors for xerosis in the elderly: A cross-sectional epidemiological study in primary care, Dermatology, 2011, 223, 260–265, DOI:10.1159/000334631.
- W. Harding and S. Rawlings, Dry skin, moisturization and corneodesmolysis, International Journal of Cosmetic Science, 2000, 22, 21–52, DOI:10.1046/j.1467-2494.2000.00001.x.
- H. Kim, J. T. Kim, S. Barua, S.-Y. Yoo, S.-C. Hong, K. Bin Lee and J. Lee, Seeking better topical delivery technologies of moisturizing agents for enhanced skin moisturization, Expert Opinion on Drug Delivery, 2018, 15, 17–31, DOI:10.1080/17425247.2017.1306054.
- A. R. Pianovski, C. G. Lima, V. Franco, M. Carvalho, C. R. De Musis, S. Regina and P. Machado, Use of pequi oil (Caryocar brasiliense) in cosmetics emulsions: development and evaluate of physical stability, Brazilian Journal of Pharmaceutical Sciences, 2008, 44, 249–259 CAS.
- M. Ferrari and P. A. da Rocha-Filho, Multiple emulsions containing amazon oil: açaí oil (Euterpe oleracea), Revista Brasileira de Farmacognosia, 2011, 21, 737–743, DOI:10.1590/S0102-695X2011005000093.
- I. R. Sousa Vieira, J. Silva Sales, C. dos Santos Cerqueira-Coutinho, T. Hellmann, B. F. Silva de Sousa, T. L. Joaquim, A. L. Camara, M. C. Pires Costa and E. P. dos S. Eduardo Ricci-Júnior, Development and in vivo evaluation of the moisturising potential of cosmetic formulations containing Babassu (Orbignya phalerata Martius) oily extract, Journal Biomedical and Biopharmaceutical Research, 2017, 14, 204–219, DOI:10.19277/bbr.14.2.163.
- K. Banerjee, N. Thiagarajan and P. Thiagarajan, Formulation and characterization of a Helianthus annuus - alkyl polyglucoside emulsion cream for topical applications, Journal of Cosmetic Dermatology, 2019, 18, 628–637, DOI:10.1111/jocd.12756.
- D. Urioste, M. B. A Castro, F. C. Biaggio Heizir and F. de Castro, Synthesis of chromatographic standards and establishment of a method for the quantification of the fatty ester composition of biodiesel from babassu oil, Quim. Nova., 2008, 31, 407–412 CrossRef CAS.
- L. R. De Oliveira, J. A. Neves and M. De Jesus Marques Da Silva, Evaluation of physico-chemical quality crude oil almond babassu (Orbigny spp), Comunicata Scientiae, 2013, 4, 161–167 Search PubMed.
- D. S. Santos, I. G. da Silva, B. Q. Araújo, C. A. Lopes Júnior, N. B. N. Monção, A. M. das, G. L. Citó, M. H. S. L. de Souza, M. do D. S. B. Nascimento and M. C. P. Costa, Extraction and Evaluation of Fatty Acid Compositon of Orbignya phalerata Martius Oils (Arecaceae) from Maranhão State, Brazil, Journal of the Brazilian Chemical Society, 2013, 24, 355–362, DOI:10.5935/0103-5053.20130045.
- F. A. F. da Ponte, J. S. Rodrigues, J. Q. Malveira, J. A. S. Ramos Filho and M. C. G. Albuquerque, Physical-chemical evaluation of babassu oil (Orbinya speciosa) and coconut oil (Cocos nucife) with high acidity and fatty acids (C6 to C16), Scientia Plena, 2017, 13, 1–8, DOI:10.14808/sci.plena.2017.085301.
- C. L. Costa, E. T. R. França, D. S. Santos, M. C. P. Costa, M. C. L. Barbosa and M. D. S. B. Nascimento, Caracterização físico-química de óleos fixos artesanais do coco babaçu (Orbibnya phalerata) de regiões ecológicas do estado do Maranhão, Brasil, Pesquisa Em Foco, 2015, vol. 1, pp. 27–38, http://ppg.revistas.uema.br/index.php/PESQUISA_EM_FOCO/article/view/711 Search PubMed.
- O. D. H. dos Santos, J. V. Miotto, J. M. de Morais, P. A. da Rocha-Filho and W. P. de Oliveira, Attainment of emulsions with liquid crystal from marigold oil using the required HLB method, Journal of Dispersion Science and Technology, 2005, 26, 243–249, DOI:10.1081/DIS-200045610.
- K. P. Boock, J. M. de Morais, D. S. Masson, O. D. H. dos Santos and P. Rocha Filho, Development of O/W emulsion with cupuaçu butter containing liquid crystal by the HLB system, Internacional of Congress of Pharmaceutical Science (CIFARP., 2005, 41, 206 Search PubMed.
- S. S. Arya, S. Ramanujam and P. K. Vijayaraghavan, Refractive index as an objective method for evaluation of rancidity in edible oils and fats, Journal of the American Oil Chemists Society, 1969, 46, 28–30, DOI:10.1007/BF02632705.
- K. R. M. Moura, K. S. R. Brandão, S. D. F. Freire, A. P. Maciel, A. G. Souza and F. C. Silva, Otimização Do Processo De Produção De Biodiesel Metílico Do Sebo Bovino Aplicando Delineamento Composto Central, Revista Científica - Cadernos de Pesquisa, 2009, 438, 31–36 Search PubMed.
- S. A. Wissing and R. H. Müller, The influence of the crystallinity of lipid nanoparticles on their occlusive properties, International Journal of Pharmaceutics, 2002, 242, 377–379, DOI:10.1016/S0378-5173(02)00220-X.
- E. B. Souto, S. A. Wissing, C. M. Barbosa and R. H. Müller, Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery, International Journal of Pharmaceutics, 2004, 278, 71–77, DOI:10.1016/j.ijpharm.2004.02.032.
- S. Miksa, D. Lutz and C. Guy, New approach for a reliable in vitro sun protection factor method Part I: Principle and mathematical aspects, International Journal of Cosmetic Science, 2015, 37, 555–566, DOI:10.1111/ics.12226.
- Codex Alimentarius Commission, Codex Standards for Named Vegetable Oils, CODEX- STAN 210-1999, Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO), Rome, Italy, revised in 2019 Search PubMed.
- E. A. Canesin, C. C. de Oliveira, M. Matsushita, L. Felicidade Dias, M. Reghiany Pedrão and N. E. de Souza, Characterization of residual oils for biodiesel production, Electronic Journal of Biotechnology, 2014, 17, 39–45, DOI:10.1016/j.ejbt.2013.12.007.
- L. N. de Lima, M. W. N. Carvalho and J. C. O. Santos, Thermal and oxidative characterization of biodiesel derived from cotton oil, Química Do Brasil, 2008, 2, 91–96 CAS.
- R. Stachiw, S. B. Ribeiro, M. A. G. Jardim, D. Possimoser, W. D. C. Alves and W. C. S. Cavalheiro, Potencial de produção de biodiesel com espécies oleaginosas nativas de Rondônia, Brasil, Acta Amazonica, 2016, 46, 81–90, DOI:10.1590/1809-4392201501151.
- N. L. P. Martins, O. Malafaia, J. M. Ribas-Filho, M. Heibel, R. N. Baldez, P. R. L. De Vasconcelos, H. Moreira, M. Mazza, P. A. N. Nassif and T. Z. Wallbach, Análise comparativa da cicatrização da pele com o uso intraperitoneal de extrato aquoso de Orbignya phalerata (babaçu). Estudo controlado em ratos, Acta Cirurgica Brasileira, 2006, 21, 66–75, DOI:10.1590/S0102-86502006000900010.
- X.-R. Yang, H.-Y. Zheng, Y.-Y. Dong and J.-B. Wang, Assessment of the skin irritation hazard of 5 cosmetics using the shell-less hen's egg test chorioallantoic membrane assay, Journal of Clinical Rehabilitative Tissue Engineering Research, 2009, 13, 5673–5676, DOI:10.3969/j.issn.1673-8225.2009.29.016.
- D. S. Santos, I. G. Da Silva, L. B. M. Do Carmo, S. B. N. M. Do Desterro and M. C. P. Costa, Parâmetros de qualidade físico-química de óleos e análise morfométrica de frutos e sementes da espécie Orbignya phalerata Martius por regiaõ ecológica, Ecletica Quimica, 2016, 41, 74–84, DOI:10.26850/1678-4618eqj.v41.1.2016.p74-84.
- F. Shahidi, Bailey's Industrial Oil and Fat Products, 6th edn, 2005, vol. 1–6, pp. 303–332. DOI:10.1002/047167849X.
- Agência Nacional de Vigilância Sanitária, Guia de Estabilidade de Produtos Cosméticos, Brasília, 2004. http://www.anvisa.gov.br/divulga/public/series/cosmeticos.pdf Search PubMed.
- V. C. Casteli, C. C. Mendonça, I. C. L. da Silva, K. A. Rodrigues, M. A. L. de Campos and S. R. P. Machado, Development and Preliminary Stability Evaluations of O/W emulsion containing Ketoconazole 2.0%, Acta Scientiarum. Health Science, 2008, 30, 121–128, DOI:10.4025/actascihealthsci.v30i2.812.
- M. H. Schmid-Wendtner and H. C. Korting, The pH of the skin surface and its impact on the barrier function, Skin Pharmacology and Physiology, 2006, 19, 296–302, DOI:10.1159/000094670.
- T. Oranges, V. Dini and M. Romanelli, Skin Physiology of the Neonate and Infant: Clinical Implications, Advances in Wound Care, 2015, 4, 587–595, DOI:10.1089/wound.2015.0642.
- D. Vasiljevic, G. Vuleta and M. Primorac, The characterization of the semi-solid W/O/W emulsions with low concentrations of the primary polymeric emulsifier, International Journal of Cosmetic Science, 2005, 27, 81–87, DOI:10.1111/j.1467-2494.2004.00247.x.
- A. Costa, M. C. Pires, L. H. Z. Fabrício, L. B. de O. Torloni, S. Langen and E. B. Botero, Multicenter clinical study to evaluate safety and clinical efficacy of a body moisturizer based on ceramides, omegas, glycerin, imperata cylindrica, erythritol, and homarine, Surgical and Cosmetic Dermatology, 2014, 6, 32–38 Search PubMed.
- S. Schalka, D. Steiner, F. N. Ravelli, T. Steiner, A. C. Terena, C. R. Marçon, E. L. Ayres, F. A. S. Addor, H. A. Miot, H. Ponzio, I. Duarte, J. Neffá, J. A. J. da Cunha, J. C. Boza, L. D. P. Samorano, M. D. P. Corrêa, M. Maia, N. Nasser, O. M. R. Ribeiro Leite, O. S. Lopes, P. D. Oliveira, R. L. B. Meyer, T. Cestari, V. M. S. dos Reis and V. R. P. de Almeida Rego, Brazilian consensus on photoprotection, Anais Brasileiros de Dermatologia, 2014, 89, 1–74, DOI:10.1590/abd1806-4841.20143971.
- S. Miksa, D. Lutz, C. Guy and E. Delamour, Sunscreen sun protection factor claim based on in vivo interlaboratory variability, International Journal of Cosmetic Science, 2016, 38, 541–549, DOI:10.1111/ics.12333.
- A. Springsteen, R. Yurek, M. Frazier and K. F. Carr, In vitro measurement of sun protection factor of sunscreens by diffuse transmittance, Analytica Chimica Acta, 1999, 380, 155–164, DOI:10.1016/S0003-2670(98)00577-7.
| This journal is © The Royal Society of Chemistry 2020 |