DOI:
10.1039/D0RA00390E
(Paper)
RSC Adv., 2020,
10, 6736-6742
Analysis of 17 elements in cow, goat, buffalo, yak, and camel milk by inductively coupled plasma mass spectrometry (ICP-MS)
Received
14th January 2020
, Accepted 6th February 2020
First published on 13th February 2020
Abstract
We analyzed the concentrations of 17 elements including arsenic (As), lead (Pb), cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), zinc (Zn), strontium (Sr), tin (Sn), aluminum (Al), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), sodium (Na), and selenium (Se) in cow, goat, buffalo, yak, and camel milk in China using inductively coupled plasma mass spectrometry. The concentrations of the elements varied and depended on the milk type. K, Ca, Na, and Mg were the most abundant elements. Fe and Zn concentrations ranged from 1 to 6 μg g−1, while Cu, Al, and Mn concentrations ranged from 0.1 to 1 μg g−1. Trace elements, especially toxic trace elements, were present at very low concentrations; however, Pb concentrations in cow milk reached the MRLs established by the Codex Alimentarius Commission. Data were analyzed by chemometrics to evaluate the correlations between elements in the milk samples. PCA and factor analysis highlighted the relationship between element distribution and milk type. The LDA model correctly identified most milk types. Element analysis combined with chemometrics can be used to distinguish milk types.
1. Introduction
Milk is a considerable source of nutrients, including protein, vitamins, and minerals. However, there are several safety risks associated with milk due to the presence of heavy metals, agricultural and veterinary drugs, and illegal additives.1 Milk is consumed worldwide, especially by infants and is economically important in many countries.2 Cow, goat, and sheep milk account for approximately 87% of the global milk production. However, milk from small dairy animals, such as buffalo, donkey, yak, horse and camel have important nutritional and economic value in specific areas.3 Medhammar et al. reported that milk from minor dairy species, such as goat, buffalo, camel and yak has high nutritional value. Therefore, minor dairy species milk is very popular in China.4
Elements are divided into major elements (concentrations > 100 μg g−1), minor elements (concentrations ranging between 0.1 μg g−1 and 100 μg g−1), and trace elements (concentrations < 0.1 μg g−1). Additionally, elements can be classified as minerals and toxic elements. Minerals are necessary in human growth and development. Mineral concentrations in milk are affected by nutrition, breed, animal species, stage of lactation, season, management, environmental conditions, locality, and health status of the udder, among others. Additionally, there are differences in major and minor elements between different milk types.5,6 The concentration of minerals in milk is an indicator of milk quality. Toxic elements such as lead, cadmium, chromium, and arsenic pose health risks.7,8 Toxic elements might originate from polluted water, metal ions, veterinary drug residues, residual detergents, and pesticides. Researchers have analyzed toxic element concentrations in milk and performed risk assessments.9–11
Prices are higher for minor dairy species milk than for cow milk. Higher-priced milk (e.g., yak and camel milk) is sometimes substituted with lower-priced milk (e.g., cow milk), which raises safety concerns among consumers due to possible cow milk allergies.3 Therefore, it is of utmost importance to identify milk from different animals. Multi-element analysis in milk can be an effective way to distinguish different milk types.
Different techniques have been used to determine elements in milk and milk-based products, such as flame and graphite furnace atomic absorption spectrometry (FAAS and GFAAS), atomic fluorescence spectrometry (AFS), inductively coupled plasma optical emission spectrometry (ICP-OES), and inductively coupled plasma mass spectrometry (ICP-MS). Tajkarimi et al. analyzed Pb in milk using GFAAS.11 Ca and Mg in dairy products have been determined by FAAS.12 The authors measured only a few elements, because multi-element analysis by FAAS and GFAAS requires multiple sample injections. Compared with other analytical methods, ICP-OES and ICP-MS are more suitable for multi-element analysis. Güler analyzed 24 minerals in goat milk and yoghurt using ICP-OES,13 and Benincasa et al. used ICP-MS to analyze 16 elements in cow and buffalo milk.14 Compared to ICP-MS, ICP-OES has a lower sensitivity; therefore, it is more difficult to analyze trace elements such as Cd and As by ICP-OES. ICP-MS has several advantages, such as fast scanning speed, short operation time, high sensitivity, low detection limit, and wide linear range. As a result, ICP-MS has been widely used for the analysis of trace elements in foods.
The objectives of this study were to (1) determine 17 elements, including As, Pb, Cd, Cr, Cu, Ni, Zn, Sr, Sn, Al, K, Ca, Mg, Fe, Mn, Na and Se in goat, cow, buffalo, yak, and camel milk by ICP-MS, and (2) compare the concentrations of the elements in the different milk types. Based on the elements and use of discriminant analysis, all milk types were characterized and classified.
2. Experimental
2.1 Samples
A total of 350 milk samples were collected from different provinces in China. There were 100 goat milk samples (50 from Shandong province and 50 from Shaanxi province), 100 cow milk samples (from Shandong province), 50 buffalo milk samples (from Guangxi province), 50 camel milk samples (from Xinjiang province), and 50 yak milk samples (from Sichuan province). The milk samples, which were untreated, were obtained from small farm cooperatives and large-scale farms. After stirring the milk in holding tanks, 100 mL of raw milk sample was removed from the upper third, 100 mL from the middle third, and 100 mL from the lower third. The collected milk (300 mL per holding tank) was stored in polyethylene bottles and kept at −20 °C for transportation and analysis.
2.2 Reagents and instrumentation
All solutions were prepared using ultrapure water (resistivity of 18 MΩ cm−1) obtained from a Milli-Q purification system (Millipore Corp., Bedford, MA, USA). Nitric acid (HNO3, trace metal grade, 67–70%, Thermo Fisher Scientific, USA) and hydrogen peroxide (H2O2; analytical reagent, 30%, Sinopharm Chemical Reagent, China) were used for sample digestion. Stock standard solutions of the elements (1000 mg L−1) were acquired from Inorganic Ventures (Lakewood, NJ, USA). All glass- and plastic-ware were decontaminated overnight with nitric acid (10%, v/v), rinsed with ultrapure water, and allowed to dry. All plastic and glass containers that came into contact with samples or standards were evaluated for contamination to avoid the release of metals. Certified reference materials (CRMs) consisting of milk powder (GBW10017) and wheat (GBW10011) were purchased from the Institute of Geophysical and Geochemical Exploration (China). A microwave digestion instrument (MARS5, CEM, USA) was used for the digestion of samples and CRMs. The simultaneous determination of elements was carried out by ICP-MS equipped with an autosampler (iCAP Q, Thermo, USA). Argon (purity of 99.999%) was used as an auxiliary gas and for plasma generation and nebulization.
2.3 Sample preparation and microwave digestion
Milk samples were defrosted overnight at 4 °C. Prior to digestion, the samples (2 g for milk samples and 0.3 g for powder CRMs) were treated overnight with 6 mL of concentrated HNO3 (65%) and 2 mL of concentrated H2O2 (30%) in tetrafluoroethylene containers. The process of microwave digestion is shown in Table 1. After cooling to room temperature, the samples were transferred into 25 mL polyethylene volumetric flasks and filled with ultrapure water to a final volume of 25 mL before ICP-MS. Blank samples and CRMs were prepared as described above.
Table 1 Microwave digestion process
| Stage |
Heating time (min) |
Target temperature (°C) |
Hold time (min) |
| 1 |
5 |
120 |
5 |
| 2 |
5 |
150 |
10 |
| 3 |
5 |
190 |
30 |
2.4 ICP-MS analysis and quality assurance
ICP-MS was used for the determination of Fe, Mn, Cu, Zn, K, Ca, Mg, Pb, Cd, Cr, As, Se, Sr, Al, Na, Ni, and Sn. The parameter conditions used in ICP-MS are summarized in Table 2. Blank samples were analyzed and subtracted from the sample measurements before the results were calculated. The limits of detection were calculated by measuring three times the standard deviation of the blank samples (Table 3). The limits of quantification were calculated based on the average sample volume and total volume analyzed. Each milk sample was measured three times, and the average value was used. CRMs (GBW10017 and GBW10011) were used for the assessment of both accuracy and precision in element analysis. The accuracy was calculated by comparing the results obtained for each element from CRMs analyzed to the certified value available from the manufacturer. It can be seen from the data presented in Table 3 that good agreement was achieved between the certified values and those determined by ICP-MS for the 17 elements reported. All the results obtained for the CRMs (GBW10011 and GBW10017) did not present a significant difference when compared with certified values (t test, 95% confidence level). The results were acceptable. The precision of the method was determined by analysis of the same CRM for three times. Relative standard deviation (RSD) values found to be within 0.7% to 12.5%.
Table 2 ICP-MS parameter conditions
| ICP-MS |
Parameter conditions |
| Radio frequency power (W) |
1550 |
| Cool gas flow (L min−1) |
14 |
| Auxiliary gas flow (L min−1) |
0.8 |
| Nebulizer gas flow (L min−1) |
1.08 |
| Peristaltic pump speed (rpm) |
40 |
| Sampling depth (mm) |
5 |
| Spray chamber temperature (°C) |
2.7 |
| Measurement mode |
Kinetic energy discrimination |
| Dwell time (ms) |
20 |
| Isotopes measured |
27Al, 75As, 44Ca, 111Cd, 52Cr, 65Cu, 57Fe, 39K, 24Mg, 55Mn, 23Na, 60Ni, 208Pb, 82Se, 118Sn, 88Sr, 66Zn |
Table 3 Validation parameters of the analytical method
| Element |
Calibration range (μg L−1) |
R2 |
LOD (mg kg−1) |
LOQ (mg kg−1) |
GBW10011 |
GBW10017 |
| Certified value |
Measured value |
Accuracy (%) |
Certified value |
Measured value |
Accuracy (%) |
| Al |
0.1–100 |
0.9999 |
0.033 |
0.101 |
104 ± 10 |
100 ± 6 |
96.2 |
10 |
10.3 ± 0.3 |
103.0 |
| As |
0.1–5 |
0.9999 |
0.0008 |
0.0024 |
0.031 ± 0.005 |
0.030 ± 0.003 |
96.8 |
0.031 ± 0.007 |
0.030 ± 0.004 |
96.8 |
| Ca |
1–1000 |
0.9995 |
0.89 |
2.67 |
340 ± 20 |
324 ± 12 |
95.3 |
9400 ± 300 |
9457 ± 91 |
100.6 |
| Cd |
0.1–2 |
0.9999 |
0.0001 |
0.0003 |
0.018 ± 0.004 |
0.018 ± 0.001 |
100.0 |
— |
— |
— |
| Cr |
0.1–50 |
0.9993 |
0.001 |
0.003 |
0.096 ± 0.014 |
0.101 ± 0.010 |
105.2 |
0.39 ± 0.04 |
0.41 ± 0.02 |
105.1 |
| Cu |
1–200 |
0.9996 |
0.012 |
0.036 |
2.7 ± 0.2 |
2.7 ± 0.1 |
100.0 |
0.51 ± 0.13 |
0.52 ± 0.3 |
102.0 |
| Fe |
1–500 |
0.9993 |
0.093 |
0.279 |
18.5 ± 3.1 |
17.8 ± 1.3 |
96.2 |
7.8 ± 1.3 |
7.3 ± 0.3 |
93.6 |
| K |
2–2000 |
0.9996 |
0.84 |
2.55 |
1400 ± 60 |
1420 ± 10 |
101.4 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 500 500 ± 500 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307 ± 289 307 ± 289 |
98.5 |
| Mg |
0.5–1000 |
0.9994 |
0.077 |
0.231 |
450 ± 70 |
442 ± 30 |
98.2 |
960 ± 70 |
924 ± 57 |
96.3 |
| Mn |
0.5–200 |
0.9994 |
0.009 |
0.027 |
5.4 ± 0.3 |
5.3 ± 0.2 |
98.1 |
0.51 ± 0.17 |
0.46 ± 0.06 |
90.2 |
| Na |
1–1000 |
0.9995 |
0.167 |
0.501 |
17 ± 5 |
16 ± 2 |
94.1 |
4700 ± 300 |
4528 ± 187 |
96.3 |
| Ni |
0.5–50 |
0.9997 |
0.008 |
0.024 |
0.06 ± 0.02 |
0.06 ± 0.005 |
100.0 |
0.18 |
0.17 ± 0.01 |
94.4 |
| Pb |
0.1–5 |
0.9999 |
0.003 |
0.009 |
0.065 ± 0.024 |
0.069 ± 0.013 |
106.2 |
0.07 ± 0.02 |
0.08 ± 0.01 |
114.3 |
| Se |
0.2–10 |
0.9991 |
0.008 |
0.024 |
0.053 ± 0.007 |
0.056 ± 0.004 |
105.7 |
0.11 ± 0.03 |
0.12 ± 0.01 |
109.1 |
| Sn |
0.5–50 |
0.9994 |
0.009 |
0.027 |
— |
— |
— |
— |
— |
— |
| Sr |
2–500 |
0.9998 |
0.021 |
0.063 |
2.5 ± 0.3 |
2.4 ± 0.2 |
96.0% |
5.3 ± 0.6 |
5.0 ± 0.3 |
94.3% |
| Zn |
5–1000 |
0.9992 |
0.072 |
0.216 |
11.6 ± 0.7 |
11.4 ± 0.4 |
98.3% |
34 ± 2 |
30 ± 3 |
88.2% |
2.5 Statistical analysis
Differences among samples were analyzed by one-way analysis of variance (ANOVA) and Duncan's multiple range test using the SPSS Statistics Software Version 23.0 (IBM, New York, USA). Statistical significance was set at p < 0.05. Factor and principal component analysis (PCA), correlation analysis, and linear discriminant analysis (LDA) were performed using SPSS 23.0.
3. Results and discussion
3.1 Differences in element concentrations of milk
The concentrations of the 17 elements in each milk type are shown in Table 4. There were differences (p < 0.05) in the concentrations of all elements with the exception of Mg (p = 0.115) and Na (p = 0.093).
Table 4 Element concentrations (μg g−1) in five milk types in Chinaa
| Element |
Goat milk |
Cow milk |
Buffalo milk |
Camel milk |
Yak milk |
| “*” concentration is expressed as ng g−1. Data are shown as mean ± standard deviation. Different superscripts indicate statistically significant differences among groups. |
| Al |
0.277 ± 0.155a |
0.493 ± 0.196d |
0.391 ± 0.176bc |
0.455 ± 0.287cd |
0.379 ± 0.181b |
| As* |
4.27 ± 3.93b |
4.61 ± 2.20b |
3.81 ± 2.26b |
8.06 ± 6.57c |
1.12 ± 0.57a |
| Ca |
520 ± 115a |
516 ± 78a |
750 ± 172b |
888 ± 358c |
803 ± 124b |
| Cd* |
0.425 ± 0.305a |
0.767 ± 0.558b |
0.676 ± 0.725b |
0.786 ± 1.191b |
0.254 ± 0.221a |
| Cr* |
11.7 ± 5.2c |
15.0 ± 9.2d |
7.94 ± 5.80b |
13.6 ± 5.3cd |
1.85 ± 0.63a |
| Cu |
0.208 ± 0.098b |
0.165 ± 0.058a |
0.209 ± 0.093b |
0.248 ± 0.055c |
0.522 ± 0.115d |
| Fe |
1.08 ± 0.38a |
1.45 ± 0.52b |
1.01 ± 0.24a |
1.29 ± 0.73b |
2.54 ± 0.64c |
| K |
1377 ± 325d |
1242 ± 279c |
698 ± 194a |
930 ± 99b |
1363 ± 200d |
| Mg |
92.5 ± 12.0 |
83.2 ± 10.3 |
58.5 ± 11.3 |
79.6 ± 33.2 |
96.7 ± 12.3 |
| Mn |
0.156 ± 0.031a |
0.187 ± 0.125a |
0.169 ± 0.049a |
0.188 ± 0.109a |
0.256 ± 0.061b |
| Na |
253 ± 55 |
292 ± 50 |
276 ± 66 |
428 ± 79 |
345 ± 59 |
| Ni* |
38.3 ± 26.3a |
81.9 ± 68.0b |
62.4 ± 47.4ab |
131 ± 148c |
66.8 ± 65.3b |
| Pb* |
7.97 ± 7.50a |
23.4 ± 13.8c |
17.3 ± 14.8b |
18.2 ± 7.1b |
4.31 ± 2.45a |
| Se* |
28.1 ± 10.4b |
37.2 ± 14.2c |
32.4 ± 11.0b |
29.4 ± 18.0b |
14.0 ± 5.3a |
| Sn* |
66.9 ± 28.3b |
97.6 ± 61.9c |
52.5 ± 16.7a |
50.6 ± 29.0a |
14.2 ± 30.5d |
| Sr |
1.66 ± 0.99c |
0.697 ± 0.157a |
0.695 ± 0.277a |
3.05 ± 0.85d |
1.14 ± 0.29b |
| Zn |
3.11 ± 0.81a |
4.36 ± 1.27b |
4.00 ± 0.84b |
5.81 ± 1.14d |
4.76 ± 1.49c |
3.1.1 Major elements. The major elements that were analyzed were K (698–1377 μg g−1), Ca (516–888 μg g−1), and Na (253–428 μg g; Table 4). K was the most predominant major element followed by Ca and Na. K regulates osmotic pressure and acid–base balance and participates in carbohydrate and protein metabolism. The highest K concentrations were identified in goat milk (1377 μg g−1), and significantly lower K concentrations were obtained in buffalo milk (698 μg g−1). Ca plays roles in the mineralization of bones and teeth and in several physiological and biochemical reactions in the human body. Milk is an important source of Ca. A study showed that women with low milk intakes during childhood and adolescence had low bone density during adulthood and high risk of fractures.15 The concentration of Ca was the highest in camel milk (888 μg g−1) followed by yak milk (803 μg g−1) and buffalo milk (750 μg g−1) In our study, the concentration of Ca in camel milk was lower than those reported by Haddadin et al. and Shamsia.5,16 Additionally, Ca concentration in goat and cow milk was 520 μg g−1 and 516 μg g−1, respectively, which was lower than that reported in previous studies (630 to 1970 μg g−1).17–20 Na is essential in muscle and nerve tissue. High Na levels were obtained in camel samples (428 μg g−1). Past studies have reported Na concentrations ranging from 235 to 815 μg g−1.17,20–22 In our study, Na concentrations were in agreement with those reported in the literature.
3.1.2 Minor elements. The minor elements that were analyzed were Mg (58.5–96.7 μg g−1), Zn (3.11–5.81 μg g−1), Fe (1.01–2.54 μg g−1), Sr (0.695–3.05 μg g−1), Al (0.277–0.493 μg g−1), Cu (0.165–0.522 μg g−1), and Mn (0.156–0.256 μg g; Table 4). The concentrations of Al, Mn, Mg and Zn were similar among the milk types. The Mg content of goat milk was lower in our study than in Güler's study (510 μg g−1). Güler reported that the high Mg content in goat milk may be due to sudden physiological changes in the animal body, mammary gland metabolism, diet, lactation stage, environmental temperature, and water intake.14 Sr was comparatively high in camel milk, while Fe and Cu were high in yak milk. In our study, Zn concentrations were similar to those previously reported in camel, cow, buffalo, and yak milk (1.24–6.2 μg g−1).22–25 Our values, however, were lower than the reported in goat milk (11.31 μg g−1) by Licata et al. The concentration of Al ranged from 0.277 to 0.397 μg g−1 in our study, while Al was not detected in Osorio's study. The concentrations of Cu, Mn, and Mg were in agreement with those reported in the literature.6,26,27 Sr concentrations were similar to those reported in milk from Cyprus.22
3.1.3 Trace elements. In order of decreasing concentrations, the seven trace elements analyzed were Ni > Sn > Se > Pb > Cr > As > Cd (Table 4). Ni ranged from 38.3 ng g−1 (goat milk) to 131 ng g−1 (camel milk), while Sn ranged from 14.2 ng g−1 (yak milk) to 97.6 ng g−1 (cow milk). There were similar Se concentrations among the milk types (14.2–37.2 ng g−1). Pb, Cr, As, and Cd are toxic; therefore, their concentrations in foods need to be carefully monitored. The concentrations (ng g−1) of these toxic trace elements were 7.97–23.4 ng g−1 Pb, 7.94–15.0 ng g−1 Cr, 3.81–8.06 ng g−1 As, and 0.425–0.786 ng g−1 Cd (Table 4). To guarantee the quality and safety of milk, the Codex Alimentarius Commission (CAC) has set the maximum residue level (MRL) of Pb in raw milk at 20 ng g−1 (Codex Stan 193-1995).28 The MRLs of Pb, Cr, and As are 50, 300, and 100 ng g−1, respectively, in China (GB2762-2017).29 The average concentration of Pb in cow milk was 23.4 ng g−1, which poses a human health risk according to CAC standards. The concentrations of the remaining trace elements were lower than the established MRLs. In our study, the concentrations of toxic trace elements were similar to those reported by Miedico et al. in goat milk.30 The contents of Ni, Pb, Cr, and Cd in goat milk were lower in our study than in Güler's study.14
3.2 Correlation analysis
Fig. 1 shows a correlation heatmap between elements. The correlations between the elements were verified, taking into consideration only those with significant coefficients (r > 0.6, p < 0.01). There were strong correlations between Mg and K (r = 0.755) and between Zn and Ca (r = 0.646). Fantuz reported moderate correlations between Mg and K in donkey milk.31 The secretion of Ca in milk is a very complex phenomenon with a wide variety of forms: casein-bound Ca, colloidal Ca phosphate, Ca citrate, and free ionized Ca. The majority of Ca (about 65%) is associated with casein micelles. Therefore, the number of casein phosphoserines in milk may determine Ca concentrations and possibly Zn concentrations, because the majority of Zn is also bound to casein micelles,32 which might explain the significant correlation between Zn and Ca. Cd was correlated with Pb (r = 0.723), Cr (r = 0.774), and As (r = 0.666), and Cr was correlated with Pb (r = 0.675) and As (r = 0.621). Milk may become contaminated with toxic elements as a result of environmental pollution, such as water, atmosphere, and soil, and from feed.10 Therefore, the correlations between trace elements are probably related to environmental factors.
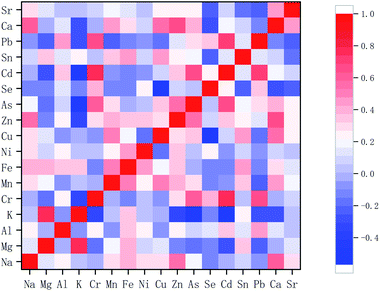 |
| | Fig. 1 Correlation heatmap of the elements. | |
3.3 Factor and principal component analysis
Statistical analysis of multi-element data principal component analysis (PCA) is the basic tool for data analysis. PCA is very important to gather an overview of data, especially in the preliminary steps of multivariate analysis. PCA constitutes a powerful visualization tool, provides a method of reducing the dimensionality of the data, and allows the elimination of unnecessary information. In our study, the first six factors explained 73% of the total variability (Table 5). Scatter plots for milk samples are shown in Fig. 2 using standardized scores of the first three factors. Based on the results, the milk samples could be divided into different clusters.
Table 5 Component matrix and cumulative contribution of variance of the first six factors
| Element |
Factors |
| 1 |
2 |
3 |
4 |
5 |
6 |
| Na |
0.152 |
0.037 |
−0.149 |
0.109 |
−0.14 |
0.251 |
| K |
0.028 |
−0.198 |
0.294 |
0.355 |
−0.007 |
0.234 |
| Ca |
0.194 |
0.036 |
−0.207 |
−0.095 |
−0.13 |
−0.026 |
| Mg |
0.151 |
−0.131 |
0.200 |
0.297 |
0.082 |
0.001 |
| Zn |
0.188 |
0.04 |
−0.087 |
0.101 |
0.012 |
−0.349 |
| Al |
0.03 |
0.17 |
0.256 |
−0.065 |
−0.341 |
0.28 |
| Cr |
0.01 |
0.148 |
0.135 |
0.281 |
0.165 |
0.108 |
| Mn |
0.162 |
0.015 |
0.136 |
−0.2 |
0.308 |
−0.046 |
| Fe |
0.178 |
−0.06 |
0.221 |
−0.2 |
−0.02 |
0.115 |
| Ni |
0.117 |
0.123 |
0.151 |
0.131 |
−0.147 |
−0.163 |
| Cu |
0.203 |
−0.069 |
−0.082 |
−0.155 |
−0.034 |
−0.167 |
| As |
0.037 |
0.175 |
−0.242 |
0.046 |
0.391 |
0.171 |
| Se |
−0.019 |
0.163 |
0.07 |
0.38 |
0.267 |
−0.395 |
| Cd |
0.014 |
0.273 |
0.06 |
−0.061 |
−0.2 |
0.043 |
| Sn |
0.031 |
0.046 |
0.125 |
−0.253 |
0.507 |
0.353 |
| Pb |
−0.026 |
0.239 |
0.215 |
−0.104 |
−0.058 |
−0.068 |
| Sr |
0.068 |
0.063 |
−0.29 |
0.34 |
−0.078 |
0.432 |
| Variance (%) |
24.361 |
17.845 |
9.930 |
7.447 |
7.031 |
6.507 |
| Cumulative variance (%) |
24.361 |
42.206 |
52.135 |
59.582 |
66.612 |
73.120 |
 |
| | Fig. 2 Scatter plots of different milk types for the regression factor score of the first three factors. | |
3.4 LDA
We randomly selected 20% of 350 milk samples for the validation set. Therefore, the validation set consisted of 70 samples: 20 goat milk samples, 20 cow milk samples, 10 yak milk samples, 10 buffalo milk samples, and 10 camel milk samples. The remaining 280 milk samples were used for the calibration set, which is used to establish the discriminant model, and the verification set is used for external verification. A stepwise LDA was used to identify the most useful variables and to remove non-essential information for the discrimination of milk types. LDA was carried out based on the 17 elements analyzed in the milk samples. A cross-validation procedure was used to evaluate this model. As a result, Na, Mg, K, Cr, Fe, Ni, Cu, Zn, As, Se, Pb, Ca, and Sr were selected to generate the discriminant model and classify milk types.
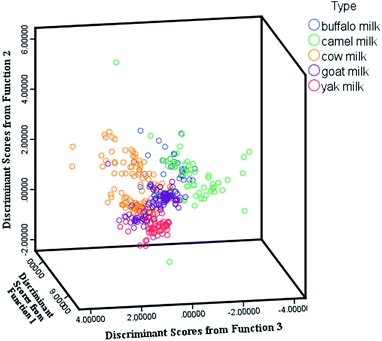
Four canonical discriminant functions explained 100% of the variance, and the first two functions explained 75.9% of the variance (function 1 explained 43.0% of the total variance, and function 2 explained 32.9% of the total variance). When examining LDA scatter plots in the space defined by the two functions, we noticed that there was a clear separation between milk samples (Fig. 3). It would appear that the element concentrations in cow milk and goat milk were similar. Buffalo milk samples were spread out and had more differences within the species. The milk samples were classified using this discriminant model, and the validity of the model was verified by the leave-one-out cross-validation method. Additionally, external verification was used to assess the validity of the model. The results showed that the overall discrimination of the milk samples was satisfactory. The overall accuracy of the back-substitution test and cross-validation of the single region was 98.6% and 96.4%, respectively. Overall, 95.7% of external-verification grouped cases were correctly classified (Table 6).
 |
| | Fig. 3 Scatter plot of discriminant functions 1 and 2 of milk. | |
Table 6 LDA of milk samples
| |
Types |
Predicted group membership |
| Goat milk |
Cow milk |
Buffalo milk |
Camel milk |
Yak milk |
Total |
| Cross validation was performed only for those cases in the analysis. In cross validation, each case was classified by the functions derived from all cases other than that case. 98.6% of original grouped cases were correctly classified. 96.4% of cross-vilification grouped cases were correctly classified. 95.7% of external-verification grouped cases were correctly classified. |
| Originalb |
Goat milk |
77 |
3 |
0 |
0 |
0 |
80 |
| Cow milk |
1 |
79 |
0 |
0 |
0 |
80 |
| Buffalo milk |
0 |
0 |
40 |
0 |
0 |
40 |
| Camel milk |
0 |
0 |
0 |
40 |
0 |
40 |
| Yak milk |
0 |
0 |
0 |
0 |
40 |
40 |
| Correct (%) |
96.3 |
98.8 |
100 |
100 |
100 |
|
| Cross-verificationa,c |
Goat milk |
76 |
3 |
0 |
1 |
0 |
80 |
| Cow milk |
4 |
74 |
2 |
0 |
0 |
80 |
| Buffalo milk |
0 |
0 |
40 |
0 |
0 |
40 |
| Camel milk |
0 |
0 |
0 |
40 |
0 |
40 |
| Yak milk |
0 |
0 |
0 |
0 |
40 |
40 |
| Correct (%) |
95 |
92.5 |
100 |
100 |
100 |
|
| External-verificationd |
Goat milk |
19 |
1 |
0 |
0 |
0 |
20 |
| Cow milk |
2 |
18 |
0 |
0 |
0 |
20 |
| Buffalo milk |
0 |
0 |
10 |
0 |
0 |
10 |
| Camel milk |
0 |
0 |
0 |
10 |
0 |
10 |
| Yak milk |
0 |
0 |
0 |
0 |
10 |
10 |
| Correct (%) |
95 |
90 |
100 |
100 |
100 |
|
4. Conclusions
We analyzed 17 elements in goat, cow, camel, buffalo, and yak milk using ICP-MS. The concentrations of the elements varied and depended on the milk type. K, Ca, Na, and Mg were the most abundant elements with concentrations > 10 μg g−1. The average concentrations of toxic trace elements such as Cd, As, and Cr were very low. However, Pb may pose health risks in consumers based on CAC standards. Correlation analysis showed that there was a strong correlation between individual elements in milk samples. PCA and factor analysis highlighted the relationship between element distribution and milk type. The LDA model correctly identified milk type in most cases. Element analysis combined with chemometrics may be used to identify/distinguish milk types.
Conflicts of interest
There are no conflicts to declare.
References
- S. K. Kailasa and H. Wu, J. Ind. Eng. Chem., 2015, 21, 138–144 CrossRef CAS.
- D. Bakircioglu, N. Topraksever, S. Yurtsever, M. Kizildere and Y. B. Kurtulus, Microchem. J., 2018, 136, 133–138 CrossRef CAS.
- Y. Yang, D. Bu, X. Zhao, P. Sun, J. Wang and L. Zhou, J. Proteome Res., 2013, 12, 1660–1667 CrossRef CAS PubMed.
- E. Medhammar, R. Wijesinha-Bettoni, B. Stadlmayr, E. Nilsson, U. R. Charrondiere and B. Burlingame, J. Sci. Food Agric., 2012, 92, 445–474 CrossRef CAS PubMed.
- M. S. Haddadin, S. I. Gammoh and R. K. Robinson, J. Dairy Res., 2008, 75, 8–12 CrossRef CAS PubMed.
- O. S. F. Khalil, Journal of Food and Dairy Sciences, 2018, 9, 289–296 CrossRef.
- M. Navarro-Alarcon, C. Cabrera-Vique, M. D. Ruiz-Lopez, M. Olalla, R. Artacho, R. Gimenez, V. Quintana and T. Bergillos, Food Chem., 2011, 129, 1126–1131 CrossRef CAS PubMed.
- F. Qin and W. Chen, Bull. Environ. Contam. Toxicol., 2007, 79, 247–250 CrossRef CAS PubMed.
- R. G. l. O. Simsek, O. Öksüz and S. Kurultay, Nahrung, 2000, 44, 360–363 CrossRef PubMed.
- X. Y. Qu, N. Zheng, X. W. Zhou, S. L. Li, J. Q. Wang and W. J. Zhang, Biol. Trace Elem. Res., 2018, 183, 92–101 CrossRef CAS PubMed.
- M. Tajkarimi, M. Ahmadi Faghih, H. Poursoltani, A. Salah Nejad, A. A. Motallebi and H. Mahdavi, Food Control, 2008, 19, 495–498 CrossRef CAS.
- G. C. Brandao, G. D. Matos and S. L. C. Ferreira, Microchem. J., 2011, 98, 231–233 CrossRef CAS.
- Z. Güler, Small Rumin. Res., 2007, 71, 130–137 CrossRef.
- C. Benincasa, J. Lewis, G. Sindona and A. Tagarelli, Food Chem., 2008, 110, 257–262 CrossRef CAS PubMed.
- H. J. Kalkwarf, J. C. Khoury and B. P. Lanphear, Am. J. Clin. Nutr., 2003, 77, 257–265 CrossRef CAS PubMed.
- S. M. Shamsia, Int. J. Genet. Mol. Biol., 2009, 1, 052–058 CAS.
- X. Chi, G. Zhang, Y. Yang and F. Hu, Spectrosc. Lett., 2016, 49, 477–481 CrossRef CAS.
- I. R. do Nascimento, R. M. de Jesus, W. N. L. dos Santos, A. S. Souza, W. D. Fragoso and P. S. dos Reis, Microchem. J., 2010, 96, 37–41 CrossRef CAS.
- N. Herwig, K. Stephan, U. Panne, W. Pritzkow and J. Vogl, Food Chem., 2011, 124, 1223–1230 CrossRef CAS.
- L. Husakova, I. Urbanova, J. Sramkova, M. Konecna and J. Bohuslavova, Talanta, 2013, 106, 66–72 CrossRef CAS PubMed.
- A. Ataro, R. I. McCrindle, B. M. Botha, C. M. E. McCrindle and P. P. Ndibewu, Food Chem., 2008, 111, 243–248 CrossRef CAS.
- M. T. Osorio, A. Koidis and P. Papademas, Int. J. Dairy Technol., 2015, 68, 573–581 CrossRef.
- F. M. Al-Awadi and T. S. Srikumar, J. Dairy Res., 2001, 68, 463–469 CrossRef CAS PubMed.
- W. N. Sawaya, J. K. Khalil, A. Al-Shalhat and H. Al-Mohammad, J. Food Sci., 1984, 49, 744–747 CrossRef CAS.
- P. Licata, G. Di Bella, A. G. Potorti, V. Lo Turco, A. Salvo and G. M. Dugo, Food Addit. Contam., Part B, 2012, 5, 268–271 CrossRef CAS PubMed.
- N. Khan, I. S. Jeong, I. M. Hwang, J. S. Kim, S. H. Choi, E. Y. Nho, J. Y. Choi, K. S. Park and K. S. Kim, Food Chem., 2014, 147, 220–224 CrossRef CAS PubMed.
- I. Vllasaku, J. Tomovska, T. Stafilov, K. Kurteshi and M. Menkovska, UBT International Conference, 2017, pp. 42–46 Search PubMed.
- FAO/WHO, Codex Alimentarius, 1995 Search PubMed.
- Ministry of Health of the People's Republic of China (MHPRC), GB2762-2017 Maximum levels of contaminants in foods, National Standard, P. R. China, 2017 Search PubMed.
- O. Miedico, M. Tarallo, C. Pompa and A. E. Chiaravalle, Small Rumin. Res., 2016, 135, 60–65 CrossRef.
- F. Fantuz, S. Ferraro, L. Todini, R. Piloni, P. Mariani and E. Salimei, Int. Dairy J., 2012, 24, 143–145 CrossRef CAS.
- K. J. van Hulzen, R. C. Sprong, R. van der Meer and J. A. van Arendonk, J. Dairy Sci., 2009, 92, 5754–5759 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ab,
Xia Li†ab,
Zengmei Liab and
Ligang Deng*ab
ab,
Xia Li†ab,
Zengmei Liab and
Ligang Deng*ab
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 500
500 ± 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307 ± 289
307 ± 289


