Open Access Article
Open Access ArticleDesign and evaluation of novel thrombin-based GLP-1 analogs with peptidic albumin binding domain for the controlled release of GLP-1
Xianli Niuab,
Shirong Nonga,
Xiaomin Zhangbc,
Xiangyang Libc,
Cheng Wangbc,
Wei Li *a and
Tianhong Zhoua
*a and
Tianhong Zhoua
aKey Laboratory of Genetic Engineering and Medicine, Key Laboratory of Viral Biology, Jinan University, Guangzhou, Guangdong 510632, P. R. China
bDepartment of Biochemistry and Molecular Biology, Zunyi Medical University, Zhuhai, Guangdong 519041, P. R. China. E-mail: Nzj_2020@zmu.edu.cn
cFifth Affiliated Hospital of Zunyi Medical University, Zhuhai, Guangdong 519100, P. R. China
First published on 29th January 2020
Abstract
Currently, the curative effects of polypeptide drugs are often restricted due to the short in vivo duration of action. In this study, we fused a series of heptapeptide tags with different length fatty chains to the N-terminus of mutated glucagon-like peptide-1 (GLP-1) using an intermediate sequence comprising a flexible linker (GGGGS)2 and thrombin (TBN)-cleavable site (FNPR), to develop promising prolonged GLP-1 receptor (GLP-1R) agonists. As a result, twenty-one fusion peptides, termed PES01–PES21, were designed and prepared. Surface plasmon resonance (SPR) measurements and plasma stability tests showed that PES14 exert better albumin binding affinity and in vitro plasma stability compared with the other ones. Preclinical assay in db/db mice proved that PES14 exert the hypoglycemic efficacies in a dose-dependent model within the range of 10–90 nmol kg−1. Furtherly, an enhanced glucose-lowering effect and significantly prolonged hypoglycemic duration of PES14 were exhibited in multiple oral glucose tolerance tests (OGTTs) and hypoglycemic duration test, compared with Liraglutide and Semaglutide, respectively. Moreover, the in vivo t1/2 of intact PES14 and released GLP-1 were approximately 95.1 h and 110.5 h in rhesus monkeys after a single subcutaneous injection of 90 nmol kg−1, respectively. Furthermore, long-term treatment with PES14 in db/db mice for 8 weeks obtained beneficial efficacies on body weight gain, food intake, fat% and hemoglobin A1c (HbA1c) reduction compared with the control and superior to those of Semaglutide treatment. Meanwhile, chronic treatment of PES14 also exhibited proper insulin immunoreactivity and effectively enhanced the improvement on hepatocyte damage. All these results suggested that PES14 has the potential to be developed as a once-weekly anti-diabetic drug.
Introduction
In recent years, therapeutic polypeptides have been investigated as potent agents due to the remarkable specificity, small molecular weight and low immunogenicity.1–3 Unfortunately, the potential advantages of peptide drugs could not be completely developed primarily due to the short in vivo action time which attributes to several mechanisms, including glomerular filtration and proteolysis, which means that the peptide-based drugs must be frequently administered to maintain the therapeutic level.4–6 Consequently, many strategies were performed to increase the in vivo therapeutic duration of peptide-based agents.7–9Enlarging the molecular size of peptides by protein fusions, PEGylation or glycosylation, could effectively reduce renal clearance, and further prolong the circulating exposure of the therapeutic peptides.10–12 However, large size of PEG chain and glycosyl inevitably affect the biological efficacies of peptides.13,14 Recently, human serum albumins (HSA) have been widely utilized to be drug carriers. The strategies to “hitchhike” albumin, which is to link a peptide agent with an albumin binding moiety, could induce the tight association of the conjugate with HSA and effectively prolong the in vivo lifespan compared with the dissociative ones.15–17
GLP-1 is a kind of endogenous hormones secreted from intestinal L cells which response to the nutrient intake, and the GLP-1-based therapies are considered as the most effective approaches for the treatment of patients with type 2 diabetes mellitus (T2DM).18–20 However, GLP-1 exerts a short in vivo active duration due to the dipeptidyl peptidase IV (DPP-IV) digestion and rapid renal filtration, which severely limit its application in diabetes therapies.21–23
According to our previous research,24 the strategy to obtain the extended half-lives of peptide drugs was performed by fusing the peptide drugs with a lipidated the heptapeptide tags exerting high affinity for HSA. In order to develop the GLP-1 receptor agonist with prolonged active duration, we re-optimized the previous tags and fused the tag to the mutated GLP-1(7-37) via a thrombin-cleavable linker to construct novel GLP-1R agonist pro-drugs. In particular the 8th alanine (Ala) of GLP-1(7-37), which is a DPP-IV digestion site, was replaced by 2-aminoisobutyric acid (Aib) to enhance the proteolytic resistance. As a result, our newly designed fusion peptides could bind to HSA which could effectively protect the modified peptides from rapid elimination action from glomerulus and proteolytic digestion when they entered into the bloodstream. The protease cleavable sequence (FNPR) will be digested by the hydrolysis of TBN, and the mutated GLP-1(A8Aib) will also be released subsequently.
Here, we aimed to optimize this acylated heptapeptide strategy, and evaluate the newly designed fusion peptides. In vitro assays including albumin binding affinity and plasma stability, and both the preliminary in vivo pharmacodynamics and pharmacokinetics characteristics of selected fusion peptide were also carefully studied.
Experimental
Materials and animals
Liraglutide and Semaglutide were obtained from GL biochem (Shanghai, China). Human serum albumin (HSA) was acquired from Sigma-Aldrich (Merck, Germany). The db/db mice and Goto–Kakizaki (GK) rats (male, 6–8 weeks) were purchased from WuXi AppTec (Shanghai, China). Male rhesus monkeys obtained from Xie Er Xin Co., Ltd (Beijing, China). The mice and rats were grouped in six/cage and housed in a humidity and temperature controlled room under a 12 h light–dark cycle. Glucose level was detected using a handheld one touch UltraEasy glucometer (Johnson & Johnson, USA). All animal studies were conducted in conformity to the Laboratory Animal Management Regulations in China and approved by the Jinan University Animal Ethics Committee.Design, preparation and identification of PES peptide
All the peptides were synthesized by using a solid phase method according to the previously reported standard method25 and were purified by HPLC using the specified conditions (Agilent C18 reversed-phase column; detection wavelength: 280 nm). Molecular weight of PES peptides were detected using an MALDITOF/MS (Bruker, Karlsruhe, Germany).Albumin binding affinity measurements
According to the user's manual, surface plasmon resonance (SPR) measurements were conducted to detect the binding affinity of PES peptides for albumins by using with BIAcoT200 system (General Electric Company, America).26Plasma stability assay
The human plasma without albumin was prepared by using a SwellGel Blue Albumin Removal Kit (Thermo Fisher Scientific, America) according to the manufacturer's instructions. PES13–15, at a final level of 1 mg L−1, were incubated in human plasma with or without HSA at 37 °C for 72 h. Then the mixture were collected at 0, 1, 2, 4, 6, 8, 12, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144 h time points, and were further detected by the methods of LC-MS/MS.Protease cleavage test
Protease cleavage test was performed in the pH 7.4 PBS containing the thrombin at the final concentration of 0.4 U mL−1.27,28 The reaction mixture was then incubated at 37 °C for 72 h shielded from light and was taken out at 0, 2, 4, 8, 12, 16, 24, 36, 48, 60, 72, 84 and 96 hours, respectively. Finally, detection were performed by using the commercially available ELISA kits (Millipore, USA), and LC-MS/MS method were used to analyze the released GLP-1 and different hydrolysed fragments, respectively.Oral glucose tolerance test
OGTT was performed to assess ability of PES14 to balance BGLs of male diabetic mice. In brief, overnight fasted diabetic mice were divided to five groups, which were subcutaneously injected with saline and PES14 at four doses of 10, 30, 90 and 150 nmol kg−1 half an hour before oral glucose load (2.0 g kg−1), respectively. Then the BGLs were detected before and 15, 30, 60 and 120 min after each glucose challenged. Multiple OGTTs were further performed at 30 hour-interval after administration of PES14 (10, 30 and 90 nmol kg−1) or Liraglutide (90 nmol kg−1) in GK rats to evaluate the glucose-lowering effects by calculating the area under the curve (AUC) from 0 to 2 h, 72 to 74 h and 144 to 146 h, respectively.Hypoglycemic duration test
With free food and water, db/db mice received a single subcutaneous administration of saline, Semaglutide (90 nmol kg−1), PES14 (10, 30 and 90 nmol kg−1). Blood samples were collected from tail vein incision at 0, 0.5, 1, 2, 4, 8, 24, 36, 48, 72, 120, 168, 216 h and the BGLs were determined by a glucometer.Pharmacokinetic study
Single s.c. administration of PES14 at 10, 30 and 90 nmol kg−1 were performed in the rhesus monkey. Blood samples were collected at pre-dose, 1, 2, 4, 8, 24, 36, 48, 72, 96, 120, 144 and 168 h after injection. The plasma concentration of intact PES14 and free GLP-1 (A8Aib) detected by using LC-MS/MS method according to previously reported.29Chronic in vivo study
The diabetic mice were grouped in five groups which were administrated with twice-weekly subcutaneous administration of saline, Semaglutide (90 nmol kg−1) and PES14 (10, 30 and 90 nmol kg−1), respectively, for eight consecutive weeks. Food intake body weight gains and of db/db mice were measured for twice a week. OGTT was performed at week 0 and 8 to detect the improvement on glucose-stabilizing ability of the db/db mice. Measurement of % HbA1c value and blood biochemical parameters level were performed by using automatic biochemical analyzer. At the end of the 8 week treatment, the pancreatic tissues were isolated and embedded in paraffin, and the sections were further stained using hematoxylin and eosin (H&E) according to previously reported standard histopathological procedures.30Data analysis
All the data were analyzed by using SPSS 21.0 (IBM, USA) and presented by mean ± SD. All parameter differences were tested using one-way ANOVA. P < 0.05 was considered as statistically significant. Interventionary studies involving animals or humans, and other studies require ethical approval must list the authority that provided approval and the corresponding ethical approval code.Results
Design, preparation and identification of PES peptides
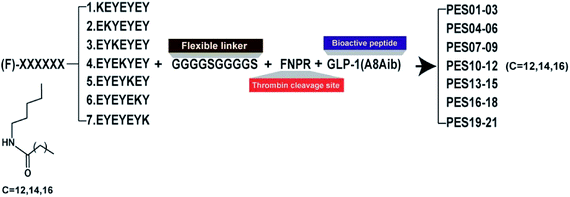
According to the previous research,24 the heptapeptide-palmitic tags were proved with high affinity for human serum albumin. To establish a better strategy for controlled release of therapeutic agents, we re-designed seven heptapeptide tags with different position of lysine (KEYEEYE, EKYEEYE, EYKEEYE, EYEKEYE, EYEEKYE, EYEEYKE and EYEEYEK) and then the lysine were modified with different lengths of fatty chain maleimide (Fig. 1). These albumin binding tags were further fused to the N-terminus of mutated GLP-1(7-37) utilizing a peptide fragment comprising flexible linker (GGGGS)2 and TBN-cleavable sites (FNPR) to generate fully new acylated heptapeptide tag-based GLP-1 pro-drugs. Particularly, the 8th Ala of natural GLP-1, a DPP-IV digestion site, was replaced by an unnatural amino acid, Aib, to enhance the in vivo proteolytic stability. Consequently, twenty-one fusion peptides (termed as PES01–PES21) with the different tags were successfully constructed (Fig. 1).Albumin binding affinity
To identify the albumin binding affinity of PES01–PES21, we further measured a series of related binding constants and evidenced in Table 1. PES13, PES14, and PES15 had similar albumin binding affinity for HSA and relatively higher than other conjugates (PES13, 3.83 × 10−7 M; PES14, 6.96 × 10−7 M, PES15, 5.12 × 10−7 M), which means that the fifth acylation site modified conjugates showed the higher potency to that of the others. As a result, PES13, PES14 and PES15 were selected to conduct the further protease cleavage assay.| Peptide | HSA | Peptide | HSA | ||||
|---|---|---|---|---|---|---|---|
| ka (M−1 s−1) | kd (s−1) | KD (M) | ka (M−1 s−1) | kd (s−1) | KD (M) | ||
| PES01 | 0.69 × 104 | 1.82 × 10−2 | 2.66 × 10−6 | PES12 | 2.17 × 104 | 5.31 × 10−2 | 2.45 × 10−6 |
| PES02 | 0.97 × 104 | 3.08 × 10−2 | 6.33 × 10−6 | PES13 | 1.10 × 104 | 4.22 × 10−3 | 3.83 × 10−7 |
| PES03 | 1.28 × 104 | 3.22 × 10−2 | 2.51 × 10−6 | PES14 | 1.45 × 104 | 1.01 × 10−2 | 6.96 × 10−7 |
| PES04 | 1.24 × 103 | 3.32 × 10−2 | 2.67 × 10−5 | PES15 | 2.18 × 104 | 1.12 × 10−2 | 5.12 × 10−7 |
| PES05 | 0.89 × 104 | 7.22 × 10−2 | 8.11 × 10−6 | PES16 | 1.10 × 104 | 1.92 × 10−2 | 1.74 × 10−6 |
| PES06 | 1.88 × 104 | 9.22 × 10−2 | 4.90 × 10−6 | PES17 | 1.20 × 104 | 6.27 × 10−2 | 5.21 × 10−6 |
| PES07 | 8.24 × 103 | 6.32 × 10−2 | 7.61 × 10−6 | PES18 | 1.14 × 104 | 3.60 × 10−2 | 3.16 × 10−6 |
| PES08 | 2.67 × 104 | 9.42 × 10−2 | 3.53 × 10−6 | PES19 | 0.25 × 104 | 2.22 × 10−2 | 8.73 × 10−6 |
| PES09 | 1.21 × 104 | 5.31 × 10−2 | 4.32 × 10−6 | PES20 | 3.18 × 103 | 5.34 × 10−2 | 1.68 × 10−5 |
| PES10 | 1.29 × 104 | 3.28 × 10−2 | 2.54 × 10−6 | PES21 | 1.14 × 104 | 3.60 × 10−2 | 3.16 × 10−6 |
| PES11 | 8.38 × 103 | 7.82 × 10−2 | 9.33 × 10−6 | ||||
Thrombin cleavage assay
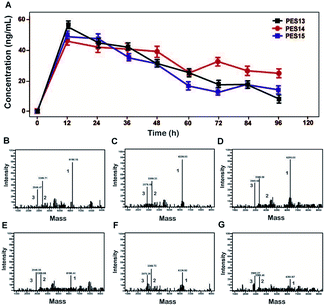
As illustrated in Fig. 2, proteolysis of plasma TBN can induce the release of transistant GLP-1 from PES peptide. The amount of mutated GLP-1(7-37) was monitored using ELISA method, and the concentration–time curves were shown in Fig. 2A. Furthermore, the transient existence of intact PES peptides, acylated heptapeptide tag-(GGGGS)2-FNPR, and mutated GLP-1 in the protease cleavage reaction were further identified by the method of LC-MS/MS (Fig. 2B–G).Plasma stability test
Plasma stability tests of PES peptides were conducted in human plasma with or without HSA. As is evidenced in Fig. 3, PES13, PES14 and PES15 showed the half-lives of 64.4, 60.1 and 48.5 h in human plasma (HSA-free) at 37 °C, respectively. Notably, PES14 exhibit significantly prolonged half-life (t1/2 > 72.0 h) in plasma (with HSA) compared with PES13 and PES15 (t1/2 = 68.7 h and 56.5 h, respectively). Therefore, PES14 was selected for further in vivo efficacy evaluation.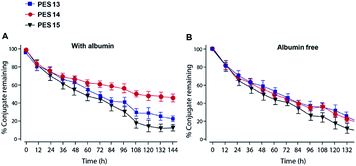 | ||
| Fig. 3 Degradation profiles of PES peptides in plasma stability test (A) with or (B) without HSA. All the data are showed as means ± SD (n = 6). | ||
Oral glucose tolerance test in diabetic mice
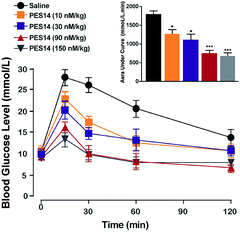
The in vivo glucoregulatory activities of PES14 (10, 30, 90 and 150 nmol kg−1) were first examined in db/db mice by oral glucose tolerance test (OGTT). As shown in Fig. 4, administration of PES14 at four dosages (10, 30, 90 and 150 nmol kg−1) all exert significantly hypoglycemic efficacies compared with the saline group, with reducing percentage of 25.9% for 10 nmol kg−1, 36.3% for 30 nmol kg−1, 57.8% for 90 nmol kg−1 and 62.7% for 150 nmol kg−1. Furthermore, the similar glucose AUC of PES14 was observed between the dosage of 90 and 150 nmol kg−1 (780.17 nmol L−1 min−1 vs. 738.56 nmol L−1 min−1) indicating that the highest effective does of PES14 is close to 90 nmol kg−1. The above results also suggest that single subcutaneous treatment of PES14 (10, 30 and 90 nmol kg−1) in diabetic mice exhibited a clear dose–effect relationship.Sustained glucose-stabilizing activity test in GK rats
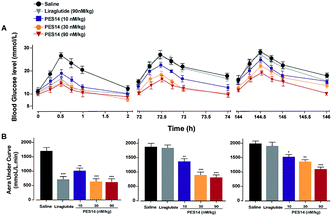
To evaluate hypoglycemic ability of PES14, a modified multiple OGTTs were performed at half an hour after the single subcutaneous administration of saline, PES14 (10, 30 and 90 nmol kg−1) and Liraglutide (90 nmol kg−1) in GK rats. After each round of glucose injection, the BGLs of saline treated mice quickly reached the peak at 30 min and then slowly drop to normal level more than 120 min (Fig. 5). The glucose-lowering effects of PES14 during the entire experimental period (0–144 h) were maintained and significant better than saline at the doses of all 10, 30 and 90 nmol kg−1. In third OGTT (144–146 h), PES14 at all three dosages still exert an AUC-lowering percentage of 22.1% for 10 nmol kg−1, 31.0% for 30 nmol kg−1 and 44.7% for 90 nmol kg−1, versus placebo treatment. In contrast, the treatment of Liraglutide was completely ineffective during the period of 72–73.5 h (Fig. 5).Hypoglycemic duration test
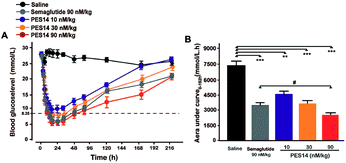
The hypoglycemic efficacities of PES14 (10, 30 and 90 nmol kg−1) were further investigated in diabetic mice, and used the Semaglutide (90 nmol kg−1) as the positive control. In this study, the time period during which the BGLs were under 8.35 mmol L−1 was regarded as the hypoglycemic duration. As is illuminated in Fig. 6A, BGLs of the db/db mice subcutaneously treated with PES14 (30 and 90 nmol kg−1) rapidly decreased and stayed at the normoglycemic value within 2 h, and the duration for BGLs rebound over 8.35 mmol L−1 were 22.3 h for 30 nmol kg−1 and 50.8 h for 90 nmol kg−1, respectively. In comparison, the saline treated group exhibited a hyperglycemic state (>20 mmol L−1) during the entire experiment (0–144 h). Interestingly, the treatment of PES14 at 90 nmol kg−1 showed the comparable hypoglycemic effects with that of Semaglutide at the same dose. Moreover, the hypoglycemic effects of PES14 at all 10, 30 and 90 nmol kg−1 maintained more than 120 h, 168 h and 216 h, respectively, while it was about 216 h in Semaglutide treated group. Furthermore, three dose of PES14 (10, 30 and 90 nmol kg−1) remarkably decreased the AUC of 38.4%, 49.3% and 65.0% for the treatment period of 0–192 h, respectively, compared with the saline group (Fig. 6B). It is worth to mention that the AUC0–192 h of PES14 (90 nmol kg−1) was significantly lower than that of Semaglutide at same dose (P < 0.05), indicating that PES14 exert better antidiabetic efficacy than Semaglutide.Pharmacokinetics of PES14 in rhesus monkey
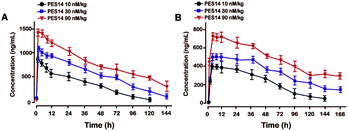
Pharmacokinetic tests of PES14 at three doses (10, 30 and 90 nmol kg−1) were performed in rhesus monkeys. As illustrated in Fig. 7, PES14 at 10 nmol kg−1, 30 nmol kg−1 or 90 nmol kg−1 had elimination half-lives of 62.6 h, 78.8 h or 95.1 h for intact PES14, and 74.0 h, 90.9 h or 110.5 h for released GLP-1. Combined with the results of hypoglycemic duration test and multiple OGTTs, a single dose of PES14 at 90 nmol kg−1 could sustain glucoregulatory ability over at least 168 h, suggesting that PES14 has potential to be developed as a weekly antidiabetic agent.Chronic studies
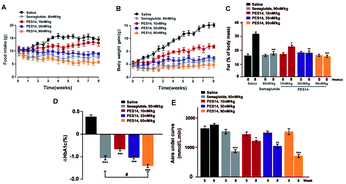
Chronic studies were conducted under the twice-weekly subcutaneous injection of PES14 (10, 30 and 90 nmol kg−1) in diabetic mice for 8 consecutive weeks to further evaluate its therapeutic potential. It was observed that saline treated group maintained a continuous upward trend in body weight gain, food intake and fat% of db/db mice during the 56 day treatment period, while the PES14 treatment at three doses exhibited a obviously reduction (Fig. 8A and B). It's worth noting that the dose-dependent reaction were shown in both weight and food intake changes in diabetic mice after the chronic treatment of PES14 at 10, 30 and 90 nmol kg−1.All three doses of PES14 significantly decreased the % HbA1c value, which was a well-known sensitive indicator of cumulative blood glucose than real-time glucose concentration, compared with the saline group (Fig. 8C).31 Particularly, PES14 treatment exerts an obviously improved the reducing effect of % HbA1c compared Semaglutide at the same dose (P < 0.05). Before and after the consecutive 8 week treatment, OGTT were performed to further evaluate whether a long-term treatment of PES14 effectively improved the glucose metabolism in diabetic mice. As is showed in the Fig. 8D, chronically treatment of PES14 at all three doses markedly decreased the OGTT AUC by 17.1% for 10 nmol kg−1, 28.9% for 30 nmol kg−1, 58.2% for 90 nmol kg−1, compared with those at day 0, while it was only 45.2% after the Semaglutide treatment, indicating that PES14 may had greater chronic improvement on the glucose tolerance than Semaglutide at same dose.
After a consecutive 8 weeks treatment, the blood biochemical indexes of db/db mice were detected and results were listed in Table 2. As we expected, a significant decline in serum TG, TC and LDL levels were observed in PES14 group compared with saline (P < 0.05). In addition, similar reduction trend was also observed in serum ALT and AST, which indicated that the hepatocyte damage was effectively improved compared with saline treated ones. The LDL/HDL ratio was considered as calculated index to quantize the increase magnitude of the two lipoproteins among different groups.32 After 8 week exposure, PES14 treatment decreased the LDL/HDL ratio up to 47% while the treatment of Semaglutide only exerts a 39% reduction.
| Parameters | Saline | Semaglutide | PES14 (10 nM kg−1) | PES14 (30 nM kg−1) | PES14 (90 nM kg−1) |
|---|---|---|---|---|---|
| TG (mmol L−1) | 1.2 ± 0.4 | 0.9 ± 0.3* | 1.1 ± 0.1 | 1.0 ± 0.7 | 0.8 ± 0.5 |
| TC (mmol L−1) | 5.0 ± 0.7 | 4.3 ± 0.2* | 4.8 ± 0.6 | 4.4 ± 0.3* | 3.7 ± 0.4** |
| LDL (mmol L−1) | 1.7 ± 0.2 | 1.1 ± 0.4** | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.0 ± 0.3** |
| HDL (mmol L−1) | 2.3 ± 0.7 | 2.4 ± 0.5 | 2.5 ± 0.6 | 2.5 ± 0.5 | 2.7 ± 0.3 |
| LDL/HDL | 0.66 ± 0.10 | 0.45 ± 0.18** | 0.64 ± 0.19 | 0.44 ± 0.15** | 0.35 ± 0.13*** |
| ALT (IU L−1) | 61.9 ± 4.4 | 44.1 ± 3.1** | 59.2 ± 2.8 | 55.0 ± 4.0 | 45.0 ± 3.5** |
| AST (IU L−1) | 162 ± 10.6 | 130 ± 6.9** | 158 ± 12.2 | 140 ± 9.9* | 126 ± 8.5** |
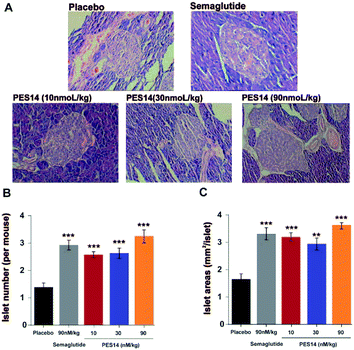
At last, the long-term physiological effects of PES14 at 30 and 90 nmol kg−1 on diabetic mice were assessed by conducting the H&E staining. As indicated in Fig. 9A, chronic treated with PES14 in db/db mice preserved proper insulin immunoreactivity. Furthermore, increased the area and number of pancreatic islets were distinctly showed in pancreases obtained from the diabetic ones treated with PES14, compared with those of the saline treated ones (Fig. 9B and C).
Discussion
A better understanding of the underlying physiological consequences of HSA, as a drug carrier attribute to its long lifespan and abundance in blood circulation, might offer more pathways for developing candidate peptide drugs with pleiotropic therapeutic values.33,34 According to the previous research,24 a heptapeptide tag, EYEKEYE, was preliminarily applied to three bioactive peptides and presented that the conjugates bind with the desired affinity to albumin, and the efficacies were also proved in mouse models.In this study, our intention is to design an albumin-binding fusion peptide that can associate with HSA and liberate the bioactive peptide. The human TBN, which was a natural protease at a stable level, was utilized as a highly regulated and specific scissor.35–37 We selected the GLP-1, a potential therapeutic peptide for the treatment of T2DM, as a model agent to demonstrate our re-designed strategy which could tightly bind to the HSA and perform a sustained release of the therapeutic peptide. As is showed in Fig. 1, the present study describes the design and characterization of a series of GLP-1 conjugates (termed PES01–PES21). Especially, we enhanced the enzyme tolerance capacity of the released GLP-1 by changed the 8th Ala to Aib.38
To further confirm the binding affinity of these conjugates to HSA, in vitro SPR measurements were performed and the results were listed in Table 1. As a result, PES13–PES15 had relatively higher albumin binding affinity than other conjugates, especially for the kd, which represent the dissociation rate from albumin. According to our speculation, the increased HSA binding stability of PES13–15 observed above might be attributed to the most suitable steric hindrance brought from the modification at the fifth lysine acylation site. Moreover, the protease cleavage assay revealed that PES13–PES15 can release the transient GLP-1 via plasma thrombin (Fig. 2A). Meanwhile, the TBN hydrolysis of PES13 (Fig. 2B, 2 hours; Fig. 2C, 72 hours), PES14 (Fig. 2D, 2 hours; Fig. 2E, 72 hours) and PES15 (Fig. 2F, 2 hours; Fig. 2G, 72 hours) were captured in the mass spectrometric profiles. Importantly, plasma stability tests of three candidate peptides indicated that PES14 exert the longer half-life in human plasma with albumin compared with PES13 and PES15 (>72.0 h vs. 68.7 h and 56.5 h) (Fig. 3). It is worth discussing that very fast release of GLP-1 were observed in the all three peptide groups in the first 12 h, and then the transient concentration of GLP-1 all slowly decreased in the follow-up 84 h, especially for the PES14, indicating that our newly designed molecules may not be fully bound to albumin when they enter the blood circulation, resulting in the rapid release of GLP-1 due to thrombin digestion. However, as the excess molecules are degraded, the residual ones are tightly bound to albumin and will not be easily cleaved by thrombin, resulting in a relatively slow release rate in the subsequent 84 hours. The above results can prove that PES14 has potential to develop into a therapeutic drug which controls blood glucose quickly, while maintaining continuous control of blood glucose in a relatively smooth manner after a single dose injection. Combined with the above results, PES14 was selected to assess its antidiabetic efficacies in vivo.
We further evaluate that whether the PES14 peptide, which exhibited the highest HSA binding affinity and the best in vitro plasma stability, would exert remarkable and long-acting hypoglycemic action in vivo. The following OGTT were performed to explore the dose dependency of glucose-lowering effects of PES14 in diabetic mice. As is showed in Fig. 4, PES14 at three dose (10, 30 and 90 nmol kg−1) all exhibit dose-glucose-lowering effects relationship in range from 10 nmol kg−1 to 90 nmol kg−1. It is worth noting that, there are similar glucose-lowering effects of PES14 between the dosage of 90 and 150 nmol kg−1, indicating that the highest effective does of PES14 is close to 90 nmol kg−1. So the peptides at the dosage of 10, 30 and 90 nmol kg−1 were selected for the further in vivo evaluation. Furthermore, the prolonged hypoglycemic effects of PES14 were confirmed by the modified multiple OGTTs, as well as hypoglycemic duration test in GK rats and diabetic mice, respectively (Fig. 5 and 6). As we expect, PES14 exerts a better glucose-lowering ability and sustained hypoglycemic effect than Liraglutide and Semaglutide at same dose, respectively, which probably attribute to its improved renal clearance and enzymic metabolism. Particularly, hypoglycemic effects of PES14 at 10, 30 and 90 nmol kg−1 were shown to be exerted via the glucose-dependent mechanisms due to the sustaining controlled release of GLP-1, indicating that PES14 can offer the advantage of increased drug safety in clinic and avoid hypoglycemia. To rationalize in vivo efficacy results of PES14, pharmacokinetic tests were then performed in rhesus monkeys As illustrated in Fig. 7, PES14 at 10 nmol kg−1, 30 nmol kg−1 or 90 nmol kg−1 had elimination half-lives of 62.6 h, 78.8 h or 95.1 h for intact PES14, and 74.0 h, 90.9 h or 110.5 h for released GLP-1. In short, PES14 has potential to be developed as a weekly anti-diabetic agent.
In order to comprehensively evaluate therapeutic potential of PES14, we then investigated the chronic in vivo effects on diabetic mice. It was found that a twice-weekly administration of PES14 at three doses in diabetic mice all obtained long-term beneficial effects on food intake lowering as well as body weight and % fat controlling at the end of 56 day treatment (Fig. 8A and B). Similarly, an obvious reduction of % HbA1c values in db/db mice were observed in PES14 treated groups, and better than Semaglutide treated ones at same dose (P < 0.05) (Fig. 8C). Remarkably, PES14 treatment (90 nmol kg−1) exerts a better OGTT AUC reducing effect than Semaglutide (90 nmol kg−1), suggesting its greater long-term glucose stabilizing capacity (Fig. 8D). Furthermore, the pancreases preserved the increased area and number of islet in diabetic mice received chronic treatment of PES14 at all three doses compared with those in saline treated ones (Fig. 9A and B).
Overall, PES14 performed comparable efficacies with Semaglutide in the acute glucose-lowering experiments, but was found to be significantly superior to Semaglutide in long-term efficacy tests. According to many previous reports,39,40 one reason for that the Semaglutide is currently the best GLP-1R agonist drug for the treatment of T2DM is due to its high sequence identity with native GLP-1. The other is the excellent in vivo stability of Semaglutide. Therefore, we speculate that the PES14 exert significantly better long-term efficacies than Semaglutide is mainly because of that the released mutated GLP-1 from PES14 owned a higher sequence homology with native GLP-1 compared with Semaglutide, leading to better control of % HAb1c, body weight, and improvement on islet function.
Conclusions
The present research demonstrates that PES14, as a GLP-1 derivative which was generated by fusing a mutated GLP-1 to heptapeptide tag with a C14 fatty chain maleimide through a thrombin cleavage linker, exert enhanced albumin binding affinity and prolonged hypoglycemic ability. Furthermore, evaluation of in vivo efficacy also showed that PES14 exert enhanced acute and chronic beneficial effects on db/db mice, compared with Liragutide and Semaglutide, and showed great promise to treat T2DM patients. Not only that, this strategy for controlled-release therapeutic peptide could also be applied to other short-acting peptides, not just to the GLP-1 for the treatment of T2DM.Author contributions
Conceptualization, Xianli Niu; data curation, Xianli Niu; funding acquisition, Tianhong Zhou; methodology, Xianli Niu and Shirong Nong; project administration, Wei Li; supervision, Xiaomin Zhang, Xiangyang Li and Cheng Wang; validation, Xiaomin Zhang, Xiangyang Li and Cheng Wang; writing-original draft, Xianli Niu; writing-review & editing, Shirong Nong.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We indeed appreciate the linguistic assistance from Mogoedit during the writing and revising this manuscript.Notes and references
- F. M. Veronese, A. Mero and G. Pasut, PEGylated Protein Drugs: Basic Science and Clinical Applications, 2009, pp. 11–31 Search PubMed.
- G. Pasut, A. Guiotto and F. Veronese, Expert Opin. Ther. Pat., 2004, 14, 859–894 CrossRef CAS.
- P. H. Maurer, J. Immunol., 1970, 105, 1011–1012 CAS.
- H. Chen, W. Guohao, L. Lixin, J. Orit, D. O. Kiesewetter, L. Yi, M. Ying, Z. Xianzhong, W. Hua and Z. Lei, Theranostics, 2016, 6, 243–253 CrossRef CAS PubMed.
- Y. Shechter, M. Mironchik, S. Rubinraut, A. Saul, H. Tsubery and M. Fridkin, Bioconjugate Chem., 2005, 16, 913–920 CrossRef CAS PubMed.
- L. Diao and B. Meibohm, Clin. Pharmacokinet., 2013, 52, 855–868 CrossRef CAS PubMed.
- D. Drucker, Curr. Pharm. Des., 2001, 7, 1399–1412 CrossRef CAS PubMed.
- J. S. Ryu, A. Y. Cho, W. S. Sang and H. Min, Methods Mol. Biol., 2014, 1088, 35–50 CrossRef CAS PubMed.
- W. Glaesner, A. M. Vick, R. Millican, B. Ellis, S.-H. Tschang, Y. Tian, K. Bokvist, M. Brenner, A. Koester and N. Porksen, Diabetes/Metab. Res. Rev., 2010, 26, 287–296 CrossRef CAS PubMed.
- F. M. Veronese and G. Pasut, Drug Discovery Today, 2005, 10, 1451–1458 CrossRef CAS PubMed.
- W. P. Sheffield, J. A. Marques, V. Bhakta and I. J. Smith, Thromb. Res., 2000, 99, 613–621 CrossRef CAS.
- A. J. Whitmarsh and D. P. Hornby, Protein fusions as an aid to purification, Springer Netherlands, 1994 Search PubMed.
- V. Gabercporekar, I. Zore, B. Podobnik and V. Menart, Curr. Opin. Drug Discovery Dev., 2008, 11, 242–250 CAS.
- F. M. Veronese, Biomaterials, 2001, 22, 405–417 CrossRef CAS PubMed.
- R. Tian, S. Zhu, Q. Zeng, L. Lang and X. Chen, Bioconjugate Chem., 2019, 30, 1711–1723 CrossRef CAS PubMed.
- M. T. Larsen, M. Kuhlmann, M. L. Hvam and K. A. Howard, Mol. Cell. Ther., 2016, 4, 3 CrossRef PubMed.
- D. Sleep, J. Cameron and L. R. Evans, Biochim. Biophys. Acta, 2013, 1830, 5526–5534 CrossRef CAS PubMed.
- D. K. Arulmozhi and B. Portha, Eur. J. Pharm. Sci., 2006, 28, 96–108 CrossRef CAS PubMed.
- A. Penfornis, S. Borot and D. Raccah, Diabetes Metab., 2008, 34(suppl. 2), S78–S90 CrossRef CAS.
- H. Jing, H. Yue, C. Xinyu, Z. Feng, F. Yingying and F. Junjie, Eur. J. Pharm. Sci., 2018, 123, 111–123 CrossRef PubMed.
- L. L. Nielsen, J. Am. Board Fam. Med., 2005, 10, 703–710 CAS.
- L. Lu, X. Su, Y. Wang, Y. Luo, J. Yang, L. Xie, X. Gao, Y. Ma, Y. Tian and F. Yuan, RSC Adv., 2017, 54178–54187 RSC.
- H. D. Gao, P. Liu, Y. Yang and F. Gao, RSC Adv., 2016, 6, 83438–83447 RSC.
- A. Zorzi, S. J. Middendorp, J. Wilbs, K. Deyle and C. Heinis, Nat. Commun., 2017, 8, 16092 CrossRef CAS PubMed.
- M. F. Songster and G. Barany, ChemInform, 2010, 29(12) DOI:10.1002/chin.199812278.
- M. A. Chun-Mei, X. X. Huang, Z. L. Cao, B. Zheng, L. Z. Meng and L. Liu, World Clinical Drugs, 2016 Search PubMed.
- M. C. Guillin, A. Bezeaud, M. C. Bouton and M. Jandrot-Perrus, Thromb. Haemostasis, 1995, 74, 129–133 CrossRef CAS.
- K. Borensztajn, M. P. Peppelenbosch and C. A. Spek, Trends Mol. Med., 2008, 14, 429–440 CrossRef CAS PubMed.
- L. P. Miranda, K. A. Winters, C. V. Gegg, A. Patel, J. Aral, J. Long, J. Zhang, S. Diamond, M. Guido and S. Stanislaus, J. Med. Chem., 2008, 51, 2758–2765 CrossRef CAS PubMed.
- X. Zhong, S. Yang, T. Liu, S. Ji, J. Hu and H. Li, Eur. J. Med. Chem., 2018, 841–850 CrossRef CAS.
- A. K. Saleh and M. A. A. Moussa, Clin. Chem., 1985, 31, 1872–1876 CrossRef CAS.
- F. Rasmussen, R. J. Hancox, P. Nair, H. S. Hansen, H. C. Siersted and M. Nybo, Clin. Respir. J., 2013, 7, 268–275 CrossRef PubMed.
- J. Zhou, X. Li, X. Zhu, J. Sun, Q. Qiu, W. Huang and H. Qian, Chem. Biol. Drug Des., 2016, 87, 936–945 CrossRef CAS PubMed.
- G. Stehle, A. Wunder, H. H. Schrenk, G. Hartung, D. L. Heene and H. r. Sinn, Anti-Cancer Drugs, 1999, 10, 785 CrossRef CAS.
- I. Aoki, K. Shimoyama, N. Aoki, M. Homori, A. Yanagisawa, K. Nakahara, Y. Kawai, S.-I. Kitamura and K. Ishikawa, J. Am. Coll. Cardiol., 1996, 27, 560–566 CrossRef CAS.
- S. Ludwig, S. Dharmalingam, S. Erickson-Nesmith, S. Ren, F. Zhu, G. M. Ma, R. Zhao, J. W. Fenton II, F. A. Ofosu and H. t. Velthuis, Diabetes Res. Clin. Pract., 2005, 70, 118 CrossRef.
- Z. Cohen, R. F. Gonzales, G. F. Davis-Gorman, J. G. Copeland and P. F. McDonagh, Thromb. Res., 2002, 107, 217–221 CrossRef CAS.
- J. Lau, P. Bloch, L. Schaffer, I. Pettersson, J. Spetzler, J. Kofoed, K. Madsen, L. B. Knudsen, J. McGuire and D. B. Steensgaard, J. Med. Chem., 2015, 7370–7380 CrossRef CAS PubMed.
- J. Lau, P. Bloch, L. Schäffer, I. Pettersson and T. Kruse, J. Med. Chem., 2015, 58, 7370 CrossRef CAS PubMed.
- T. C. Marbury, A. Flint, J. B. Jacobsen, J. Derving Karsbøl and K. Lasseter, Clin. Pharmacokinet., 2017, 56, 1381–1390 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |