Open Access Article
Open Access ArticleShape engineering of polystyrene particles from spherical to raspberry-like to hollow flower-like via one-step non-surfactant self-templating polymerization of styrene in ethanol–water mixtures†
Xuechen Xiang ,
Zhe Chen,
Dongfang Ren,
Jiaqiong Xu,
Xiaofeng Li,
Zixin Ye,
Ning Chen,
Qiming Chen* and
Shiyu Ma*
,
Zhe Chen,
Dongfang Ren,
Jiaqiong Xu,
Xiaofeng Li,
Zixin Ye,
Ning Chen,
Qiming Chen* and
Shiyu Ma*
Research Center of Water Resources and Interface Science, School of Chemistry and Molecular Engineering, East China Normal University, No. 500, Dongchuan Rd., Shanghai 200241, P. R. China. E-mail: qmchen@chem.ecnu.edu.cn; syma@chem.ecnu.edu.cn
First published on 20th March 2020
Abstract
We report a facile method for preparation of polystyrene (PS) particles with spherical, raspberry-like, and hollow flower-like structures by single-step non-surfactant self-templating polymerization of styrene in ethanol–water mixtures. PS particles with diverse morphologies could be easily obtained by simply adjusting the volume ratios of the styrene/water/ethanol mixture and initiator-ethanol–water mixture. By decreasing this ratio, the particles with spherical, raspberry-like, and hollow flower-like structures were obtained in sequence. The wettability of the coatings changing from hydrophilicity to hydrophobicity was easily tuned by the PS particles with different roughnesses. A competitive mechanism of interfacial polymerization and exudation was proposed to interpret the formation of PS particles with diverse morphologies.
1 Introduction
Synthesis of asymmetric non-spherical nanomaterials with controllable morphologies has become a hot topic of research.1 Non-spherical morphologies endow materials with enhanced physical and chemical properties, such as mechanical strength, thermal stability, good adsorptivity, catalytic activity,2 and rheological properties.3 To date, PS particles with different non-spherical shapes, such as bowl-like,4 raspberry-like,5 flower-like,2 peanut-like,6 snowman-like, and triple rod particles7 have been synthesized. Due to their unique hierarchical structures, designed surface properties, and high surface roughness, raspberry-like and flower-like particles have always been used to enhance Raman scattering, separation, and heterogeneous catalysis and also to enhance the efficiency of the drug delivery system. As the most promising building blocks, they have also been used for the fabrication of superhydrophobic coatings.5Recently, various approaches including surfactant-free emulsion polymerization,5,12 miniemulsion polymerization,11 seeded emulsion polymerization,13,14 electrostatic adsorption,9,10 layer-by-layer templating,16,41 and self-assembly methods8 have been developed to construct raspberry-like and flower-like hierarchical structures. Fan et al. prepared a series of raspberry-like poly(styrene-acrylic acid) particles by changing the molar ratios of styrene and acrylic acid in a soap-free emulsion polymerization process.5 Huang et al. prepared a series of raspberry-like polystyrene/carbon black composite microspheres by changing the π–π interactions during the mixing process.15 Dong et al. transformed the microspheres from raspberry-like to flower-like morphology by simply adjusting the stoichiometry of the silane precursor.17 Uniformly-sized hierarchical spheres could be obtained by these methods. And nowadays, a kind of ternary system such as monomer/water/methanol system has been used in polymer latex preparation.31,32 However, the mechanism of polymerization in ternary system is not clear and it is still a challenge to prepare PS particles with controlled morphologies in ternary system due to the existence of liquid/liquid and liquid/solid interfacial tension.
Herein, we demonstrate a facile, one-step route to synthesize single-component PS particles with diverse morphologies by altering the volume ratios of styrene/water/ethanol mixture to initiator-ethanol–water mixture. Using this non-surfactant self-templating polymerization process, PS particles with spherical, raspberry-like, and hollow flower-like structures were prepared. A further study was made on the range of proportions for each shape. The formation of PS particles with diverse morphologies might be determined by a competitive process of interfacial polymerization and exudation in oil droplets. The surface coated with these particles of hierarchical structures showed tunable wettability that could be changed from hydrophilicity (water contact angle, 62.5°) to hydrophobicity (water contact angle, 134.5°).
2 Experimental section
2.1 Materials
Styrene (St, 99.5%, AR, Sinopharm Chemical Reagent Co. Ltd) was purified by distillation under reduced pressure and stored in a refrigerator prior to use. Absolute ethanol (EtOH, 99.7%, AR), methanol (99.7%, GR), isopropanol (99.7%, AR), and tert-butanol (98%, CP) were procured from Sinopharm Chemical Reagent Co. Ltd Potassium persulfate (KPS, 99.5%, AR, Sinopharm Chemical Reagent Co. Ltd) and sodium bisulfite (NaHSO3, 99.99%, Shanghai Aladdin Biochemical Technology Co. Ltd) were used as a dual initiator system in this study. Ultrapure water (H2O) with resistivity higher than 18.2 MΩ cm was used throughout the study. Argon gas was bubbled into the water to remove dissolved oxygen.2.2 Preparation of PS particles with diverse morphologies
PS particles with diverse morphologies were prepared by a one-step non-surfactant self-templating polymerization process. The details of experimental procedures used in this paper are presented in Table 1. In a typical procedure, styrene (3.5 mL), water (40 mL), and ethanol (60 mL) (ratio of mass fraction (R) of St/H2O/EtOH = 2.95/44.34/52.71) were added into a flask, and stirred at 50 rpm in argon atmosphere till the mixture turned transparent. This formed the initial St/H2O/EtOH ternary system (called Monomer Mixture, MM, the volume of MM was represented as VM). The initiator solution (the volume of initiator solution was represented as VI) was a mixture of EtOH and H2O (VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 3
VH2O = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) containing radicals, which were obtained from redox initiator. Small aliquots of MM (ca. 10 mL) were taken in Erlenmeyer flasks and covered with a plug, and mixed with different volumes of initiator solution and reacted at 25 °C for 7 days. The PS particles with diverse morphologies were obtained. After washing with H2O and EtOH, PS particles were used to fabricate the hierarchical structured coatings.
2) containing radicals, which were obtained from redox initiator. Small aliquots of MM (ca. 10 mL) were taken in Erlenmeyer flasks and covered with a plug, and mixed with different volumes of initiator solution and reacted at 25 °C for 7 days. The PS particles with diverse morphologies were obtained. After washing with H2O and EtOH, PS particles were used to fabricate the hierarchical structured coatings.
| Sample | Monomer mixture | VM/mL | Initiator solution | VI/mL | VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI VI |
Styrene/water/ethanol mass fraction ratio (R) | |||
|---|---|---|---|---|---|---|---|---|---|
| Styrene/mL | Ethanol–water mixture/mL | C(KPS)/g mL−1 | C(NaHSO3)/g mL−1 | Ethanol–water mixture/mL | |||||
| 1 | 0.34 | 9.66 | 10 | 6.32 × 10−4 | 4.10 × 10−4 | 1.5 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15 |
2.95/44.34/52.71 |
| 2 | 0.34 | 9.66 | 10 | 6.32 × 10−4 | 4.10 × 10−4 | 20 | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1.13/45.22/53.65 |
| 3 | 0.34 | 9.66 | 10 | 6.32 × 10−4 | 4.10 × 10−4 | 30 | 30 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.85/45.53/53.62 |
| 4 | 0.34 | 9.66 | 10 | 6.32 × 10−4 | 4.10 × 10−4 | 90 | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
0.34/45.58/54.08 |
| 5 | 0.34 | 9.66 | 10 | 6.32 × 10−4 | 4.10 × 10−4 | 120 | 120 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
0.26/45.62/54.12 |
| 6 | 0.34 | 9.66 | 10 | 6.32 × 10−4 | 4.10 × 10−4 | 130 | 130 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
0.24/45.63/54.13 |
| 7 | 0.34 | 9.66 | 10 | 6.32 × 10−4 | 4.10 × 10−4 | 150 | 150 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
0.21/45.64/54.12 |
| 8 | 0.34 | 9.66 | 10 | 6.32 × 10−4 | 4.10 × 10−4 | 190 | 190 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
0.17/45.66/54.17 |
2.3 Assembly of hierarchical structured coatings
Prior to experiment, glass slides with dimensions of 20 × 20 mm2 were treated with piranha solution (a mixture containing 98% concentrated sulfuric acid and 30% hydrogen peroxide in 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) at 80 °C for 2 h and rinsed thoroughly with water to remove any contaminants (Warning: the piranha solution has strong oxidizing properties and it reacts violently with organic materials). The obtained PS suspensions were centrifuged, washed with H2O and EtOH and diluted with water to obtain 0.2 wt% concentration, and sonicated to obtain uniform dispersions. A glass slide was vertically immersed into each of the PS suspensions in a vial.5 Then, the vials were vertically placed in a constant temperature chamber at 25 °C for 7 days to completely evaporate the water present in the suspensions. The coatings were obtained after water evaporation, leaving deposition of particles on the glass slides.
3 v/v) at 80 °C for 2 h and rinsed thoroughly with water to remove any contaminants (Warning: the piranha solution has strong oxidizing properties and it reacts violently with organic materials). The obtained PS suspensions were centrifuged, washed with H2O and EtOH and diluted with water to obtain 0.2 wt% concentration, and sonicated to obtain uniform dispersions. A glass slide was vertically immersed into each of the PS suspensions in a vial.5 Then, the vials were vertically placed in a constant temperature chamber at 25 °C for 7 days to completely evaporate the water present in the suspensions. The coatings were obtained after water evaporation, leaving deposition of particles on the glass slides.
2.4 Characterization
Scanning electron microscopic (SEM) and transmission electron microscopic (TEM) images were recorded using Hitachi S-4800 microscope and JEOLJEM-2100 microscope, respectively. The particle size distribution of the PS particles dispersed in water was determined by dynamic light scattering technique (DLS, Zetasizer Nano ZS, Malvern Panalytical Company). Atomic force microscopy (AFM) was conducted on Bruker Multimode 8. FT-IR spectra of the as-prepared PS particles were recorded by Nicolet Fourier transform infrared spectrometer (NEXUS 670) using the KBr technique. The images of water contact angles were obtained using JC2000D1 contact angle analyzer (Powereach, China) with ultrapure water droplet size of 8 μL at room temperature. All the contact angle values were determined as averages of measurements from at least five different points on each sample surface, using the Laplace–Young fitting mode.3 Results and discussion
3.1 Synthesis of PS particles with diverse morphologies
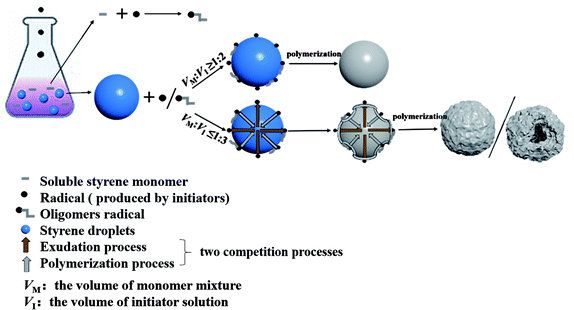
PS particles with smooth surfaces have been extensively fabricated for various purposes over the past few decades. However, rough surface particles with great potential applications require further research. Herein, a simple method of synthesis of PS particles with diverse morphologies is reported, which have been designed by adjusting the ratio of VM to VI.The process for the preparation of PS particles with diverse morphologies is shown in Scheme 1. Styrene, water, and ethanol were taken in a reaction flask and blended to obtain a uniform and transparent mixture. By keeping VM constant and changing VI, PS particles with spherical, raspberry-like, and hollow flower-like structures could be obtained.
 | ||
| Scheme 1 Schematic illustration of the synthesis process for PS particles with different morphologies. | ||
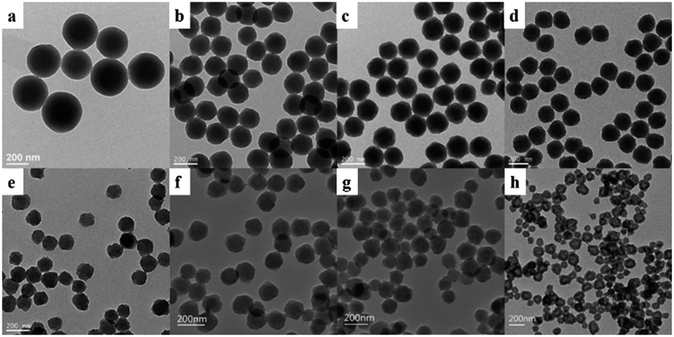
Fig. 1 presents TEM and SEM images of the obtained PS particles. By changing the ratio of VM to VI, three typical morphologies of PS particles could be prepared. Sample 1 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15, Fig. 1a and d) with smooth spherical structures were obtained. The diameters of the PS particles were about 250 nm (Fig. 2), which were much larger than expected. This might be due to the aggregation of the colliding particles in the absence of shear force.33 When the stirring speed used was set up at 250 rpm, the PS particles with 220 nm in diameter and good monodispersity were obtained (Fig. S1†). The surface of Sample 4 (VM
0.15, Fig. 1a and d) with smooth spherical structures were obtained. The diameters of the PS particles were about 250 nm (Fig. 2), which were much larger than expected. This might be due to the aggregation of the colliding particles in the absence of shear force.33 When the stirring speed used was set up at 250 rpm, the PS particles with 220 nm in diameter and good monodispersity were obtained (Fig. S1†). The surface of Sample 4 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, Fig. 1b and e) were rough and heterogeneous. The size of raspberry-like particles was reduced to 180 nm (Fig. 2). Sample 8 (VM
9, Fig. 1b and e) were rough and heterogeneous. The size of raspberry-like particles was reduced to 180 nm (Fig. 2). Sample 8 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19, Fig. 1c and f) with hollow flower-like structures were obtained. The particle size was only 132 nm (Fig. 2). The possible formation process of the hollow PS particles was as follows: the polystyrene on the surface of PS particle was more physically robust than that of the interiors. In other words, the interior of particles was “softer” and possessed a loose structure, due to lower degree of polymerization in the polymerization process. Whereas, the shell was “harder” with a dense structure, due to a higher degree of polymerization.18–23 St monomers and short oligomers in the interior of particles could be extracted when the EtOH–H2O mixture was added to the MM.
19, Fig. 1c and f) with hollow flower-like structures were obtained. The particle size was only 132 nm (Fig. 2). The possible formation process of the hollow PS particles was as follows: the polystyrene on the surface of PS particle was more physically robust than that of the interiors. In other words, the interior of particles was “softer” and possessed a loose structure, due to lower degree of polymerization in the polymerization process. Whereas, the shell was “harder” with a dense structure, due to a higher degree of polymerization.18–23 St monomers and short oligomers in the interior of particles could be extracted when the EtOH–H2O mixture was added to the MM.
 | ||
Fig. 1 TEM (a–c) and SEM (d–f) images of Sample 1 (a and d, VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15), Sample 4 (b and e, VM 0.15), Sample 4 (b and e, VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), and Sample 8 (c and f, VM 9), and Sample 8 (c and f, VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19). 19). | ||
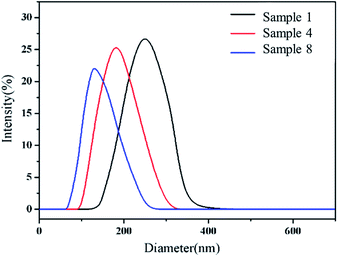 | ||
Fig. 2 DLS results of Sample 1 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15), Sample 4 (VM 0.15), Sample 4 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and Sample 8 (VM 9) and Sample 8 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19). 19). | ||
Fig. 3 shows TEM images of the PS particles prepared by different VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI ratios in detail. Sample 1 (VM
VI ratios in detail. Sample 1 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15, Fig. 3a) had a smooth surface. Sample 2 (VM
0.15, Fig. 3a) had a smooth surface. Sample 2 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Fig. 3b) showed poor surface smoothness compared to that of Sample 1. There are some depressions and bulges on the surface of Sample 3 (VM
2, Fig. 3b) showed poor surface smoothness compared to that of Sample 1. There are some depressions and bulges on the surface of Sample 3 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, Fig. 3c). Therefore, spherical particles could be prepared by maintaining the VM
3, Fig. 3c). Therefore, spherical particles could be prepared by maintaining the VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI less than or equal to 1
VI less than or equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. From Sample 3 (VM
2. From Sample 3 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, Fig. 3c) to Sample 5 (VM
3, Fig. 3c) to Sample 5 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, Fig. 3e), the lower the ratio of VM
12, Fig. 3e), the lower the ratio of VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI, the higher the roughness of surface. For Sample 6 (VM
VI, the higher the roughness of surface. For Sample 6 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, Fig. 3f), a very small hollow domain appeared in several particles. For Sample 7 (VM
13, Fig. 3f), a very small hollow domain appeared in several particles. For Sample 7 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, Fig. 3g), the hollow domains present in all particles. Thence, raspberry-like particles could be obtained when VM
15, Fig. 3g), the hollow domains present in all particles. Thence, raspberry-like particles could be obtained when VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI was between 1
VI was between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and 1
12 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, whereas hollow flower-like particles could be prepared by maintaining VM
3, whereas hollow flower-like particles could be prepared by maintaining VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI between 1
VI between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 and 1
19 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13. Moreover, particle size decreased as the ratio of VM to VI decreased (Fig. S2†). When the ratio of VM to VI decreased, the mass fraction of St monomer in the mixture decreased, along with a corresponding decrease in the size of oil droplets. Especially, the hollow area of the PS particles increased as the ratio decreased.
13. Moreover, particle size decreased as the ratio of VM to VI decreased (Fig. S2†). When the ratio of VM to VI decreased, the mass fraction of St monomer in the mixture decreased, along with a corresponding decrease in the size of oil droplets. Especially, the hollow area of the PS particles increased as the ratio decreased.
At the same time, it was noticed that as the ratio decreased, the roughness of the particle surface increased. In order to describe the differences in roughness, the products were characterized by AFM. Fig. 4 shows the AFM images processed by NanoScope Analysis 1.5 software, in which the section function was used to display the surface conditions of the two adjacent particles. Each curve was statistically derived from 50 sets of data points. The length of the smallest bump on the curve was included in the description of diverse roughness. For Sample 1 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15, Fig. 4a), the curve was smooth and the bump was around 250 nm in length, which was similar to the size of the spherical PS particles obtained. For Sample 4 (VM
0.15, Fig. 4a), the curve was smooth and the bump was around 250 nm in length, which was similar to the size of the spherical PS particles obtained. For Sample 4 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, Fig. 4b), a bump, approximately 100 nm in size, which was smaller than the particle size. For Sample 8 (VM
9, Fig. 4b), a bump, approximately 100 nm in size, which was smaller than the particle size. For Sample 8 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19, Fig. 4d), the curves were coarser and had more obvious bumps of about 45 nm size. For Sample 6 (VM
19, Fig. 4d), the curves were coarser and had more obvious bumps of about 45 nm size. For Sample 6 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, Fig. 4c), the particles had raspberry-like structures, but the bumps were about 75 nm in size, which were larger than that of Sample 8 (VM
13, Fig. 4c), the particles had raspberry-like structures, but the bumps were about 75 nm in size, which were larger than that of Sample 8 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19). Thus, when the ratio of VM to VI decreased, roughness of the particle surface increased.
19). Thus, when the ratio of VM to VI decreased, roughness of the particle surface increased.
 | ||
Fig. 4 AFM images and the surface structures of different samples: (a) Sample 1 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15); (b) Sample 4 (VM 0.15); (b) Sample 4 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9); (c) Sample 6 (VM 9); (c) Sample 6 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13); (d) Sample 8 (VM 13); (d) Sample 8 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19). 19). | ||
The effects of other soluble alcohols (methanol, isopropanol, and tert-butanol) on morphologies of PS particles were also investigated. Fig. S3 and S4† show TEM images of the PS particles prepared in styrene/water/methanol ternary system, styrene/water/isopropanol ternary system, and styrene/water/tert-butanol ternary system. The results showed that the roughness of PS particles increased as the ratio of VM to VI decreased, and different alcohols had different effects on the morphologies of PS particles.
In summary, PS particles with diverse morphologies could be easily prepared by altering the ratio of VM to VI. As the ratio of VM to VI decreased, the surface roughness of PS particles increased, and size of PS particles decreased. In St/H2O/EtOH ternary system, for VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI ≥ 1
VI ≥ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the spherical PS particles were obtained for 1
2, the spherical PS particles were obtained for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 ≤ VM
12 ≤ VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI ≤ 1
VI ≤ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the raspberry-like PS particles were prepared; for 1
3, the raspberry-like PS particles were prepared; for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 ≤ VM
19 ≤ VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI ≤ 1
VI ≤ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, the hollow flower-like PS particles were synthesized.
13, the hollow flower-like PS particles were synthesized.
3.2 Discussion on the mechanism of formation
Both experimental and theoretical studies evidenced that, ternary systems containing one hydrotrope (such as EtOH) and two immiscible fluids, both being soluble in the hydrotrope at any proportion, showed unexpected solubilization power and the presence of ‘detergentless’ microemulsions in such mixtures.31–33,46 On the basis of these results, we proposed a plausible mechanism as shown in Scheme 2.Generally, when the St/H2O/EtOH ternary system reaches equilibrium, St monomers mainly exist in two phases. One is the EtOH–H2O phase, in which St monomers exist mainly in a dissolved state.31 The other is the oil phase, where St monomers are present in the form of long-lived small oil droplets.24,28 The size of oil droplets is determined by the mass fraction of monomers in ternary systems.25,27 The DLS was employed to characterize the size of oil droplets in ternary systems.47 The oil droplets formed in the MM were 172 nm in diameter and had good monodispersity (Fig. 5a).
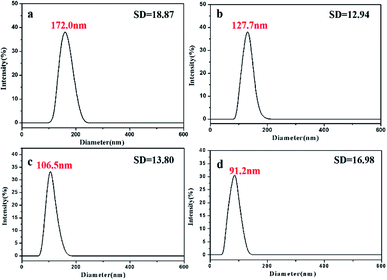 | ||
| Fig. 5 DLS results of oil droplets in ternary systems with different R: (a) 2.95/44.34/52.71; (b) 0.34/45.58/54.08; (c) 0.24/45.63/54.13; (d) 0.21/45.64/54.15. | ||
Based on thermodynamic theory, when the pressure, temperature and composition are constant, the final state of the system is completely determined. In order to observe the size evolution process of oil droplets in ternary systems, we developed two methods to obtain the ternary system with the same R. One is a direct mixing method, in which the EtOH was added to the styrene–water mixture. The other is an indirect mixing method, in which mixing MM with EtOH–H2O mixture (wherein the volume of EtOH–H2O mixture was represented as VE and VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 3
VH2O = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in this mixture). Fig. 5 shows the DLS results of oil droplets in ternary systems with different R obtained with the direct mixing method. For R = 0.34/45.58/54.08 (R is equal to that in the system of VM
2 in this mixture). Fig. 5 shows the DLS results of oil droplets in ternary systems with different R obtained with the direct mixing method. For R = 0.34/45.58/54.08 (R is equal to that in the system of VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), the droplet size was 127.7 nm (Fig. 5b). For R = 0.24/45.63/54.13 (R is equal to that in the system of VM
9), the droplet size was 127.7 nm (Fig. 5b). For R = 0.24/45.63/54.13 (R is equal to that in the system of VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13), the droplet size was 106.5 nm (Fig. 5c). For R = 0.21/45.64/54.15 (R is equal to that in the system of VM
13), the droplet size was 106.5 nm (Fig. 5c). For R = 0.21/45.64/54.15 (R is equal to that in the system of VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15), the droplet size was 91.2 nm (Fig. 5d). Thus, as the concentration of St in ternary systems decreased, the size of oil droplets also decreased.47
15), the droplet size was 91.2 nm (Fig. 5d). Thus, as the concentration of St in ternary systems decreased, the size of oil droplets also decreased.47
Fig. 6 shows the evolution of size and distribution of oil droplets in the ternary system obtained by the indirect mixing method during the aging process. Mixing 10 mL MM (R = 2.95/44.34/52.71) with 90 mL EtOH–H2O mixture (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) at 25 °C, we obtained a new ternary system (R = 0.34/45.58/54.08). After aging for 20 min, the DLS curves of oil droplets became broader with standard deviation (SD) of 35.78 (Fig. 6a), and the size of oil droplets became larger (211.9 nm) compared to that of the MM (172.0 nm, Fig. 5a). This result indicated that the change of oil droplets was happening. After aging for 200 min, a small peak appeared at 50.7 nm and it remained that way for at least 2.5 d (Fig. 6b). This phenomenon might be attributed to the exudate agglomeration.26 After aging for 3600 min, the size of mean peak of oil droplets decreased (228.7 nm, Fig. 6c) compared to that of oil droplets aging for 200 min (264.7 nm, Fig. 6b). After aging for 5040 min (Fig. 6d), the small peak of oil droplets disappeared, and the size of mean peak decreased continuously (225.5 nm, Fig. 6d). Finally, when the new ternary system reached equilibrium, the size of oil droplets would be reduced to 127.7 nm (R = 0.34/45.58/54.08, Fig. 5b).
9) at 25 °C, we obtained a new ternary system (R = 0.34/45.58/54.08). After aging for 20 min, the DLS curves of oil droplets became broader with standard deviation (SD) of 35.78 (Fig. 6a), and the size of oil droplets became larger (211.9 nm) compared to that of the MM (172.0 nm, Fig. 5a). This result indicated that the change of oil droplets was happening. After aging for 200 min, a small peak appeared at 50.7 nm and it remained that way for at least 2.5 d (Fig. 6b). This phenomenon might be attributed to the exudate agglomeration.26 After aging for 3600 min, the size of mean peak of oil droplets decreased (228.7 nm, Fig. 6c) compared to that of oil droplets aging for 200 min (264.7 nm, Fig. 6b). After aging for 5040 min (Fig. 6d), the small peak of oil droplets disappeared, and the size of mean peak decreased continuously (225.5 nm, Fig. 6d). Finally, when the new ternary system reached equilibrium, the size of oil droplets would be reduced to 127.7 nm (R = 0.34/45.58/54.08, Fig. 5b).
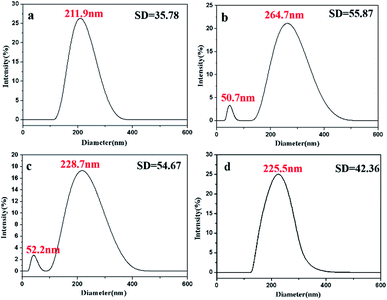 | ||
Fig. 6 DLS results of oil droplets in the ternary system prepared with VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1 VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 at different aging times: (a) 20 min; (b) 200 min; (c) 3600 min; (d) 5040 min. 9 at different aging times: (a) 20 min; (b) 200 min; (c) 3600 min; (d) 5040 min. | ||
Similar results were observed for Sample 6 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, Fig. S5†) and Sample 7 (VM
13, Fig. S5†) and Sample 7 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, Fig. S6†). Based on the results, we found that the exudation process was slow, and the exudation rate of substance in oil droplets were different. Taking the time corresponding to the appearance of the small peak in the curve as a reference (Fig. S7†), for VM
15, Fig. S6†). Based on the results, we found that the exudation process was slow, and the exudation rate of substance in oil droplets were different. Taking the time corresponding to the appearance of the small peak in the curve as a reference (Fig. S7†), for VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, the small peak appeared at 200 min, whereas for VM
9, the small peak appeared at 200 min, whereas for VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, the peak appeared at 100 min. Finally, for VM
13, the peak appeared at 100 min. Finally, for VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1
VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, the peak appeared at 30 min. Therefore, it can be considered that the ratio of VM to VE affected the rate of material exudation from oil droplets, and the rate of exudation increased as the ratio decreased. This might be the important factors for the formation of diverse morphologies and even hollow structures of PS particles.
15, the peak appeared at 30 min. Therefore, it can be considered that the ratio of VM to VE affected the rate of material exudation from oil droplets, and the rate of exudation increased as the ratio decreased. This might be the important factors for the formation of diverse morphologies and even hollow structures of PS particles.
According to the principle of phase equilibrium in physical chemistry,48 the chemical potential of St monomer in two phases is equal. When the EtOH–H2O mixture is added to St/H2O/EtOH ternary system which has already in equilibrium, the chemical potential of St in the oil phase do not change, but the chemical potential of St in the EtOH–H2O phase decreased as the concentration decreased. As the addition of EtOH–H2O mixture increases, the chemical potential of St decreases more significantly in the EtOH–H2O phase. St monomer in the oil phase will seep out from oil phase into the EtOH–H2O phase, bringing the ternary system to a new equilibrium (namely oil droplets can reach a new size).38,39,42–45 The function of interfacial layer of oil droplets was similar to a permeable membrane, which can support the exchange of internal materials.20 When the initiator solution was added to the MM instead of the EtOH–H2O mixture, both exudation and polymerization processes would take place simultaneously. KPS initiated St monomers dissolved in the mixture to form the short oligomers. The short oligomers could be captured by the surface oil droplets to stabilize oil droplets.34–37,40 The free radicals in the mixture could also be adsorbed onto the oil droplet surfaces. Therefore, the polymerization reaction could occur easily on the surface of oil droplets (Fig. 7).44 At this point, there was a competition between the exudation process and polymerization process, resulting in the as-prepared PS particles with diverse morphologies. The addition of different volumes of initiator solution to MM would alter the competitive behaviors of these two processes, which leading to the production of PS particles with diverse morphologies.
3.3 Wettability of coatings assembled from PS particles with diverse morphologies
Based on the as-prepared PS particles with diverse morphologies, coatings assembled with the as-prepared particles were fabricated by vertical deposition method at 25 °C. Coatings with different thicknesses could be prepared by controlling the parameters of vertical deposition method.29,30 However, investigations into the effect of thickness on the wettability of the coatings was not in the scope of this paper. Water contact angles of the coatings assembled with the as-prepared particles are presented in Fig. 8. For Sample 1 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15, Fig. 8a), the water contact angle was around 62.5°. For Sample 4 (VM
0.15, Fig. 8a), the water contact angle was around 62.5°. For Sample 4 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, Fig. 8b), the contact angle was increased to about 89.5°. For Sample 6 (VM
9, Fig. 8b), the contact angle was increased to about 89.5°. For Sample 6 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, Fig. 8c), the contact angle was about 123.0°and it would be increased to 134.5° for Sample 7 (VM
13, Fig. 8c), the contact angle was about 123.0°and it would be increased to 134.5° for Sample 7 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1
VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, Fig. 8d). Therefore, the water contact angles of the fabricated coatings increased as the ratio of VM to VI decreased. The wettability of the coatings could be successfully tuned from hydrophilicity (Sample 1, 62.5°) to hydrophobicity (Sample 7, 134.5°) by varying the particles with different roughnesses.
15, Fig. 8d). Therefore, the water contact angles of the fabricated coatings increased as the ratio of VM to VI decreased. The wettability of the coatings could be successfully tuned from hydrophilicity (Sample 1, 62.5°) to hydrophobicity (Sample 7, 134.5°) by varying the particles with different roughnesses.
4 Conclusions
In conclusion, PS particles with diverse morphologies were successfully synthesized by a simple, one-step, non-surfactant self-templating polymerization of St monomers in EtOH–H2O mixture. The premixing of three components and altering the addition of initiator solution (namely altering the ratio of VM to VI) played key roles in controlling the morphologies. The as-prepared particles could be categorized as follows: for VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI ≥ 1
VI ≥ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the particles were spherical in shape; for 1
2, the particles were spherical in shape; for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 ≤ VM
12 ≤ VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI ≤ 1
VI ≤ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the particles had raspberry-like structures; for 1
3, the particles had raspberry-like structures; for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 ≤ VM
19 ≤ VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI ≤ 1
VI ≤ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, the particles had hollow flower-like structures and controllable diameter (100–250 nm). The roughness of particles increased as the ratio of VM to VI decreased, whereas the size of the particles reduced correspondingly. The formation of as-prepared particles is determined by a competitive process of exudation and polymerization in oil droplets. Different volumes of initiator solution added to the monomer mixture would alter the competitive behaviors of these two processes, which leading to the as-prepared PS particles with diverse morphologies. The wettability of the coatings could be tuned from hydrophilicity to hydrophobicity by varying the particles with different roughnesses.
13, the particles had hollow flower-like structures and controllable diameter (100–250 nm). The roughness of particles increased as the ratio of VM to VI decreased, whereas the size of the particles reduced correspondingly. The formation of as-prepared particles is determined by a competitive process of exudation and polymerization in oil droplets. Different volumes of initiator solution added to the monomer mixture would alter the competitive behaviors of these two processes, which leading to the as-prepared PS particles with diverse morphologies. The wettability of the coatings could be tuned from hydrophilicity to hydrophobicity by varying the particles with different roughnesses.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Prof. Banglin Chen, Jianping Ge and Kun Zhang.Notes and references
- C. H. Chen, X. F. Li, D. Jiang, Z. Wang and Y. Wang, ChemSusChem, 2018, 11, 2540–2546 CrossRef CAS PubMed.
- F. P. Dong, H. B. Xie and Q. Zheng, RSC Adv., 2017, 7, 6685–6690 RSC.
- J.-W. Kim, R. J. Larse and D. A. Weitz, Adv. Mater., 2007, 19, 2005–2009 CrossRef CAS.
- J. B. Fan, Y. Song and H. Liu, Sci. Adv., 2017, 3, e1603203 CrossRef PubMed.
- X. L. Fan and X. K. Jia, Polym. Chem., 2015, 6, 703–713 RSC.
- S. Shi, T. Wang and Y. T. Tang, Chin. Chem. Lett., 2011, 22, 1127–1129 CrossRef CAS.
- J.-W. Kim, R. J. Larsen and D. A. Weitz, Adv. Mater., 2007, 19, 2005–2009 CrossRef CAS.
- X. M. Yang, T. Y. Dai and Y. Lu, Polymer, 2006, 47(1), 441–447 CrossRef CAS.
- M. Chen, S. Zhou and B. You, Macromolecules, 2005, 38, 6411–6417 CrossRef CAS.
- C. S. Wagner, S. Shehata and K. Henzler, J. Colloid Interface Sci., 2011, 355, 115–123 CrossRef CAS PubMed.
- Y. Zhang, H. Chen and X. Shu, Colloids Surf., A, 2009, 350, 26–32 CrossRef CAS.
- D. Nagao, T. Ueno and D. Oda, Colloid Polym. Sci., 2009, 287, 1051–1056 CrossRef CAS.
- A. Perro, S. Reculusa and E.-L. Bourgeat, Colloids Surf., A, 2006, 284–285, 78–83 CrossRef.
- S. Reculusa, P.-L. Céline and S. Ravaine, Chem. Mater., 2002, 14, 2354–2359 CrossRef CAS.
- J. Huang, Q. Li and Y. Bao, Colloid Polym. Sci., 2009, 287, 37–43 CrossRef CAS.
- H. J. Tsai and Y. L. Lee, Langmuir, 2007, 23, 12687–12692 CrossRef CAS PubMed.
- F. Dong, H. Xie, Q. Zheng and C. S. Ha, RSC Adv., 2017, 7, 6685–6690 RSC.
- A. R. Goodall, M. C. Wilkinson and J. Hearn, J. Polym. Sci., Part A: Polym. Chem., 2010, 15, 2193–2218 Search PubMed.
- R. A. Cox, M. C. Wilkinson and J. M. Creasey, J. Polym. Sci., Part A: Polym. Chem., 1977, 15, 2311–2319 CAS.
- A. R. Goodall, M. C. Wilkinson and J. Hearn, J. Colloid Interface Sci., 1975, 53, 327–331 CrossRef CAS.
- H. Lockie, R. Manica and R. F. Tabor, Langmuir, 2012, 28, 4259–4266 CrossRef CAS PubMed.
- T. Yamamoto and S. C. Kim, J. Polym. Res., 2017, 24, 149 CrossRef.
- Y. J. Wong, L. Zhu and W. S. Teo, J. Am. Chem. Soc., 2011, 133, 11422–11425 CrossRef CAS PubMed.
- G. François and J. L. Katz, Chemphyschem, 2010, 6, 209–216 Search PubMed.
- L. M. Prince, J. Colloid Interface Sci., 1967, 23, 165–173 CrossRef CAS.
- L. M. Prince, J. Colloid Interface Sci., 1969, 29, 216–221 CrossRef CAS PubMed.
- G. D. Smith, C. E. Donelan and R. E. Barden, J. Colloid Interface Sci., 1977, 60, 488–496 CrossRef CAS.
- N. L. Sitnikova, R. Sprik and G. Wegdam, Langmuir, 2005, 21, 7083–7089 CrossRef CAS PubMed.
- Y. G. Ko and D. H. Shin, J. Phys. Chem. B, 2007, 111, 1545–1551 CrossRef CAS PubMed.
- A. S. Dimitrov and K. Nagayama, Langmuir, 1996, 12, 1303–1311 CrossRef CAS.
- Z. Y. Li, H. Cheng and C. C. Han, Macromolecules, 2012, 45, 3231–3239 CrossRef CAS.
- G. J. Kim, S. Lim, B. H. Lee and S. E. Shim, Polymer, 2010, 51, 1197–1205 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- X. L. Fan, X. K. Jia, H. P. Zhang and Q. Y. Zhang, Langmuir, 2013, 29, 11730–11741 CrossRef CAS PubMed.
- X. P. Pei, K. K. Zhai, X. C. Liang and Y. K. Deng, J. Colloid Interface Sci., 2018, 512, 600–800 CrossRef CAS PubMed.
- W. C. Yan, M. W. Pan, J. F. Yuan and G. Liu, Polymer, 2017, 122, 139–147 CrossRef CAS.
- M. Chen, L. M. Wu, S. X. Zhou and B. You, Macromolecules, 2004, 37, 9613–9619 CrossRef CAS.
- R. Yan, Y. Y. Zhang, X. H. Wang and W. Q. Zhang, J. Colloid Interface Sci., 2012, 368, 220–225 CrossRef CAS PubMed.
- X. L. Fan, J. Liu, X. K. Jia and Q. Y. Zhang, Nano Res., 2017, 10, 2905–2922 CrossRef CAS.
- M. Chen, L. M. Wu, S. X. Zhou and B. You, Macromolecules, 2004, 37, 9613–9619 CrossRef CAS.
- G. L. Li, X. L. Yang, F. Bai and W. Q. Huang, J. Colloid Interface Sci., 2006, 297, 705–710 CrossRef CAS PubMed.
- G. L. Li, X. L. Yang and J. Wang, Colloids Surf., A, 2008, 322, 192–198 CrossRef CAS.
- Z. H. Nie, J. I. Park, W. Li and S. A. F. Bon, J. Am. Chem. Soc., 2008, 130, 16508–16509 CrossRef CAS PubMed.
- D. Z. Yin, X. Du, H. Liu, Q. Y. Zhang and L. Ma, Colloids Surf., A, 2012, 414, 289–295 CrossRef CAS.
- D. Z. Yin, Q. Y. Zhang, C. J. Yin and X. B. Zhao, Polym. Adv. Technol., 2012, 23, 273–277 CrossRef CAS.
- W. Hou and J. Xu, Curr. Opin. Colloid Interface Sci., 2016, 25, 67–74 CrossRef CAS.
- X. Yan, P. Alcouffe, G. Sudre, L. David, J. Bernard and F. Ganachaud, Chem. Commun., 2017, 53, 1401 RSC.
- P. W. Atkins and J. D. Paula, Physical Chemistry, Oxford University Press, 7th edn, 2002, ch. 6 Search PubMed.
Footnote |
† Electronic supplementary information (ESI) available: TEM images of PS particles prepared with R = 1/15.03/17.86 (VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15) at 250 rpm; DLS results of the as-prepared PS particles. TEM images of the PS particles prepared with VM 0.15) at 250 rpm; DLS results of the as-prepared PS particles. TEM images of the PS particles prepared with VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 in different system; TEM images of PS particles prepared with VM 9 in different system; TEM images of PS particles prepared with VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VI = 1 VI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 in different system; DLS results for oil droplets in the system of VM 15 in different system; DLS results for oil droplets in the system of VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1 VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 at different aging time; DLS results for oil droplets in the system of VM 13 at different aging time; DLS results for oil droplets in the system of VM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE = 1 VE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 at different aging time; FT-IR spectra of the as-prepared PS particles. See DOI: 10.1039/d0ra00005a 15 at different aging time; FT-IR spectra of the as-prepared PS particles. See DOI: 10.1039/d0ra00005a |
| This journal is © The Royal Society of Chemistry 2020 |